Abstract
Parasitic helminth infection elicits a type 2 cytokine-mediated inflammatory response. During type 2 inflammation, damaged or stimulated epithelial cells exposed to helminths and their products produce alarmins and cytokines including IL-25, IL-33, and thymic stromal lymphopoietin. These factors promote innate immune cell activation that supports the polarization of CD4+ T helper type 2 (Th2) cells. Activated innate and Th2 cells produce the cytokines IL-4, −5, −9, and −13 that perpetuate immune activation and act back on the epithelium to drive goblet cell hyperplasia and increased epithelial cell turnover. Together, these events drive worm expulsion and wound healing processes. While the role of Th2 cells in this context has been heavily studied, recent work has revealed that epithelial cell-derived cytokines are drivers of key innate immune responses that are critical for type 2 anti-helminth responses. Cutting-edge studies have begun to fully assess how other factors and pathways, including lipid mediators, chemokines, Fc receptor signaling, danger-associated molecular pattern molecules, and direct cell-cell interactions, also participate in shaping innate cell-mediated type 2 inflammation. In this review, we discuss how these pathways intersect and synergize with pathways controlled by epithelial cell-derived cytokines to coordinate innate immune responses that drive helminth-induced type 2 inflammation.
Keywords: innate immune cell, helminth, epithelial cell-derived cytokine, eicosanoid, Notch
INTRODUCTION
Cytokines direct the mammalian immune response to an array of pathogens, including viruses, single-celled prokaryotes and eukaryotes, and multicellular eukaryotic organisms [1-4]. A diversity of mammalian cytokines has evolved, with specific groups of cytokines mediating distinct host immune responses to different pathogen types [1-4]. Infection with large, multicellular parasitic helminths that reside in and on host tissues elicits a unique type 2 cytokine response [3,5-7]. Type 2 cytokines such as IL-4, −5, −9, and −13 are produced by innate immune cells and polarized CD4+ T helper type 2 (Th2) cells to coordinate epithelial cell responses including goblet cell hyperplasia, increased mucin production, enhanced smooth muscle contractility, and increased epithelial cell turnover [5-8]. Together, these activities drive worm expulsion and wound healing responses that control worm-induced tissue damage [3,5-9] (Fig. 1).
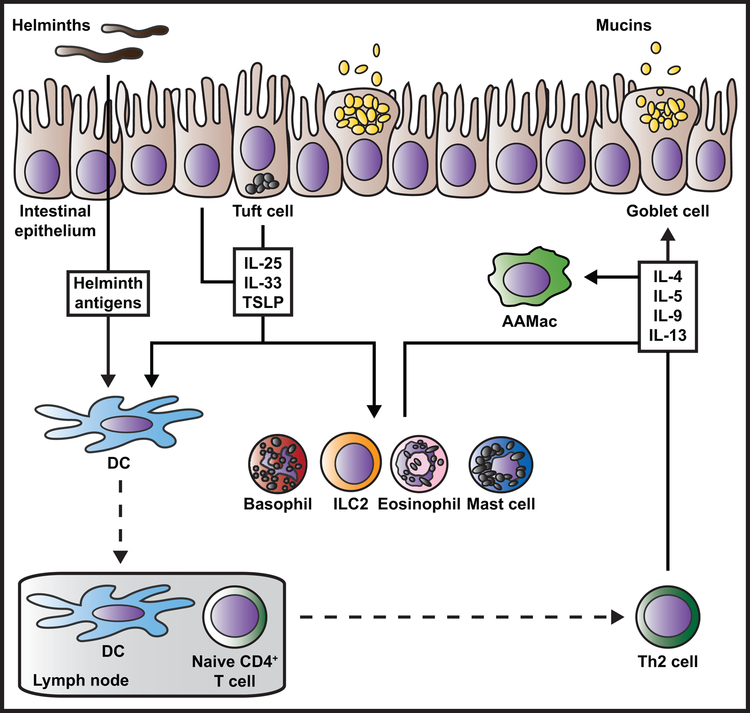
Figure 1. Current paradigm for the regulation of type 2 inflammation during helminth infection.
Damaged, stimulated, or dying intestinal epithelial cells produce cytokines and alarmins such as IL-25, IL-33, and TSLP in response to helminth parasite infection. Tuft cells are a rich source of IL-25. Epithelial cell-derived cytokines act on a variety of innate immune cells including basophils, ILC2s, eosinophils, and mast cells, delivering potent activation, proliferation, recruitment, and/or survival signals. Epithelial cell-derived cytokines also act on DCs that take up and process helminth antigens, grooming these cells to travel to the draining lymph nodes where they present antigen to naïve CD4+ T cells and promote Th2 polarization. In the tissue site, activated innate immune cells and recruited Th2 cells produce large amounts of the type 2 cytokines IL-4, −5, −9, and −13. Different cell types differentially produce these cytokines (not depicted here). Type 2 cytokines amplify innate and adaptive immune cell activation and contribute to wound repair (not depicted here), with IL-4 serving as a key activator of AAMac polarization. IL-4 and IL-13 act back on the damaged epithelium and non-hematopoietic cells to cause goblet cell hyperplasia, tuft cell mobilization, increased intestinal permeability and contractility, and increased epithelial cell turnover that promote worm expulsion.
Recent studies have demonstrated the importance of innate immune cells in promoting helminth-induced type 2 inflammation [3,5-11]. Group 2 innate lymphoid cells (ILC2s), basophils, dendritic cells (DCs), alternatively activated macrophages (AAMacs), eosinophils, and mast cells are rich sources of type 2 and other cytokines that promote effector responses and tissue repair [3,5-11]. Intensive study has revealed that the epithelial cell-derived cytokines IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) activate type 2 innate immune responses [3,6-8,11-13] (Fig. 1). However, gaps remain in our understanding of how cytokines intersect with other host and pathogen-derived molecules to control these key innate immune responses.
The effect of IL-4, −9 and −13 on innate cells, particularly AAMacs, has recently been extensively reviewed [6,7,9,14-17]. Thus, this review will discuss how epithelial cell-derived cytokines, lipids, chemokines, antibodies, danger-associated molecular patterns (DAMPs), and cell-cell interactions control innate immune responses during type 2 inflammation (Table 1). While we will focus on studies conducted in murine models of helminth infection, we will also refer to the literature on innate immune activities during type 2 allergic inflammation in mice. Finally, we will highlight emerging evidence that shows that effects of epithelial cell-derived cytokines synergize with the activities of other mediators to orchestrate helminth-induced innate immune responses.
Table 1: Regulators of innate immune cell responses during type 2 inflammation.
Factors discussed in the text (list not exhaustive) that regulate basophil, mast cell, ILC2, DC, or eosinophil functions during type 2 inflammation. Cellular targets and supporting references cited in the text are highlighted, with possible cellular sources indicated.
| Factor | Function during type 2 inflammation | Known or proposed direct cellular targets and supporting references | Cellular sources (secreted factors only) |
|---|---|---|---|
| Adenosine | Promotes IL-33 production | Unclear in vivo [140] | Damaged tissue stromal cells? |
| β7 integrin | Promotes cell accumulation in the small intestine | Mast cells [122] | NA |
| CCR3 ligands (ie. eotaxin) | Elicits cell migration | Mast cells, eosinophils [47,48] | DCs, macrophages |
| GM-CSF | Supports cell survival (effect in vivo remains unclear) | Eosinophils [61] | Activated T cells, mast cells, macrophages, ILC2s |
| IgG | Promotes anti-parasite effector responses | Mast cells [129] | B cells |
| IgE | Elicits degranulation and promotes type 2 cytokine production | Basophils, mast cells [58,124-126 (reviews)] | B cells |
| IL-2 | Supports cell proliferation and survival | ILC2s [63,69,70] | T cells, ILCs |
| IL-3 | Promotes cell differentiation, survival, and activation | Basophils, mast cells [55-57; 50,52,58 (reviews)] | T cells |
| IL-5 | Promotes cell accumulation in tissues, survival, and type 2 cytokine production |
Eosinophils [61; 59,60 (reviews)] | Th2 cells, eosinophils, mast cells |
| IL-7 | Supports cell differentiation and survival | ILC2s [62,63,65,66,68; 51,67 (reviews)] | Stromal cells |
| IL-18 | Suppresses cell survival and type 2 cytokine production | Mast cells [41; 42 (review)] | Macrophages, DCs, epithelial cells (active or precursor forms) |
| IL-25 | Promotes cell accumulation in tissues, activation, and type 2 cytokine production and potentiates degranulation | Basophils, ILC2s, eosinophils [18-21,27,36,79,107,109,117,149; 8 (review)] | Tuft cells, granulocytes |
| IL-33 | Promotes cell accumulation in tissues, activation, survival, and type 2 cytokine and prostaglandin production or release | Basophils, mast cells, ILC2s, DCs, eosinophils [22,23,25,35-38,54,78,79,88,107,144,146,148,153-155; 8,13,141 (reviews)] | Epithelial and myeloid cells |
| Leukotrienes (LTs) | Promote cell accumulation in tissues and type 2 cytokine production | ILC2s, eosinophils [80,82,84,88,146; 86,87 (reviews)] | Mast cells, basophils, eosinophils |
| MHC II interactions | Support cell proliferation, enable interactions with T cells, and promote type 2 cytokine production | Basophils (?), ILC2s, DCs [63,103-105,109; 98,99 (reviews)] | NA |
| NMU | Promotes cell accumulation in tissues, activation, and type 2 cytokine production | Mast cells, ILC2s, eosinophils [130-132,135,136] | Neurons |
| Notch signaling | Controls cell differentiation and tissue localization and promotes type 2 cytokine production | Basophils, mast cells, ILC2s [114-119] | NA |
| PGD2 | Promotes cell accumulation in tissues, activation, chemotaxis, and type 2 cytokine production | Basophils, mast cells, ILC2s, eosinophils [74,79,81,85,90,91,93; 67,75,92 (reviews)] | Mast cells |
| PGE2 | Supports Th2 polarizing capacity and promotes IL-33 production | DCs [89,145,153,154] | Mast cells |
| Sphingosine 1 phosphate | Promotes chemotaxis | ILC2s [95] | Platelets, erythrocytes, endothelium, hepatocytes |
| TL1A | Promotes cell accumulation in tissues, activation, survival, and type 2 cytokine production | ILC2s [39,40] | T cells, myeloid, epithelial, and endothelial cells |
| TSLP | Supports Th2 polarizing capacity and promotes type 2 cytokine, chemokine, and prostaglandin production or release | Basophils, mast cells, ILC2s, DCs [26,31,143,144,150-152; 12 (review)] | Epithelial and myeloid cells |
CYTOKINE PATHWAYS THAT REGULATE INNATE IMMUNE RESPONSES DURING HELMINTH INFECTION
Epithelial Cell-derived Cytokines and Alarmins
Epithelial cells are one of the first cell types exposed to intestinal helminths [3,6-8,11-13]. Thus, cytokines including IL-25, IL-33, and TSLP that are released from stimulated, injured, or dying epithelial cells are critical for the induction of innate immune responses that drive the type 2 inflammatory process [3,6-8,11-13]. In the intestine, IL-25 is largely produced by tuft cells, rare chemosensory cells that become prominent during helminth infection [18-20]. Single cell RNA sequencing analysis of small intestinal epithelial cells showed that a subset of CD45-expressing tuft cells may also be a major TSLP source [21], establishing tuft cells as central cytokine producers in the inflamed epithelium [18-21]. IL-33, on the other hand, is produced in response to damage by a range of epithelial cell types during helminth infection [8,13,22,23]. Whether a specific epithelial cell lineage has a higher propensity to produce IL-33 is unclear and the subject of ongoing studies. Notably, mast cells [24,25], inflammatory DCs [23], basophils, and eosinophils [13,26] may also produce IL-25, IL-33, and TSLP, but the significance of hematopoietic sources of these cytokines during helminth infection is not fully understood.
Epithelial damage appears to be a key event that leads to the release of IL-25, IL-33, and TSLP [3,6-8,11-13]; however, the pathways that control the transcription, production, and secretion of these cytokines during helminth infection are not fully described. A recent study demonstrated that the intestinal metabolite succinate can promote IL-25 release from tuft cells that activates ILC2s during infection with Nippostrongylus brasiliensis and Heligmosomoides polygyrus, parasites used as models of hookworm infection in mice [27]. Some allergens have enzymes that can proteolytically activate IL-33 [28] or induce TSLP secretion [29], and helminth-derived proteases may play a similar role in the induction of epithelial cell-derived cytokine responses during worm infection [8,30-32]. However, how changes in diet, microbiota-derived intestinal metabolites [6,27,33,34], or helminth proteases [8,30-32] regulate innate immune responses during helminth-induced type 2 inflammation is not fully elucidated.
IL-25, IL-33, and TSLP can mediate the recruitment, expansion, activation, and/or cytokine producing capacity of innate cells that express their cognate receptors [3,6-8,11-13]. The effects of these cytokines on different innate immune cell types are infection- and tissue-dependent, suggesting that these cytokines play non-redundant roles in helminth species-specific immune responses (in-depth coverage of this topic can be found in [3,6-8,11-13]). However, despite intensive study, questions remain regarding how epithelial cell-derived cytokines control innate immune responses in the complex tissue microenvironment. For example, the exact role of IL-33 in granulocyte activation during helminth infection remains to be elucidated. IL-33 deficient mice had more Mcpt8 (a basophil-specific protease) and more mast cells during N. brasiliensis infection compared to wild type controls [35], suggesting that IL-33 does not promote basophil or mast cell population expansion, or that compensatory mast cell hyperplasia and basophilia occurs in response to IL-33 deficiency. Similarly, despite eosinophil expression of the IL-33 receptor [13,36], impaired eosinophil accumulation in IL-33 deficient mice [35] may be due to a decrease in IL-5, eotaxin, or IL-13 produced by IL-33-activated ILC2s rather than a direct IL-33 effect on eosinophils [37,38]. Importantly, the type 2 inflammatory roles and functions of epithelial- and immune cell-derived alarmins and cytokines outside of IL-25, IL-33, and TSLP, including the tumor necrosis factor family member TL1A [39,40] and endogenous DAMPs [3,8], are not clear. In this vein, a number of studies have revealed tissue- and cell-specific effects of IL-1 family members such as IL-18 during type 2 inflammation [8,41,42], but these findings remain to be fully investigated, specifically during helminth infection. Employing reporter and transgenic mouse strains for in vivo studies in helminth infection will increase our understanding of the novel effects and functional redundancies of various epithelial cell-derived cytokines on innate immune cells.
Chemokines and Chemokine Receptors
Chemokines are cytokines that ligate their cognate receptors to promote cell migration and positioning between and within tissues [43]. Various in vitro and in vivo models show that migration of cells can be controlled by soluble mediators that orchestrate transient and temporal cell movement or by immobilized factors that facilitate directed movement and spatial positioning of cells [43]. Type 2 inflammation-associated innate immune cells express a variety of chemokine receptors in the steady-state and during type 2 inflammation [43-45], suggesting that chemokines coordinate innate immune cell movements that control type 2 inflammatory responses. In support of this idea, human epidemiological data and murine studies have shown that levels of eotaxin, CCL17, CCL22, CCL5 (RANTES), and CCL24 are increased during helminth infection [46,47]. CCR3, a receptor for eotaxin and a number of other chemokines, has a role in the recruitment of eosinophils during helminth infection [47]. In addition, mast cells are responsive to CCL3 and CXCL2 produced by DCs during exposure to Fasciola hepatica fluke antigen [48]. While ILC2s and basophils express some of the important chemokine receptors associated with type 2 inflammation (reviewed in [44,45]), how chemokines control the migration of these cells into tissues and their spatial positioning in the tissue site during helminth infection has not been fully explored.
Growth Factors/Survival Cytokines
Growth factors and survival cytokines such as IL-2, −3, −5, −7 and granulocyte-macrophage colony-stimulating factor (GM-CSF) promote the survival, differentiation, and activation of innate immune cells during helminth infection [3,5,6,49-51]. IL-3, IL-5, and GM-CSF are secreted by activated T cells, mast cells, macrophages, and ILC2s and drive increases in numbers of granulocytes [37,38,49,50,52-55]. IL-3 is a potent promoter of basophil and mast cell responses, mediating the mobilization, survival, and activation of IL-4-producing basophils [50,52,55-58], and eliciting mast cell development and responses during helminth infection [55]. Conversely, GM-CSF and IL-5 play important roles in eosinophil biology, with GM-CSF promoting the in vitro survival of eosinophils [54], and IL-5 acting as a key eosinophil survival factor both in vitro and in vivo [59]. Notably, the importance of GM-CSF and IL-5, and of eosinophils in general, during helminth infection remains unclear and may be dependent on the species of parasite [60]. For example, GM-CSF was not critical for protection against N. brasiliensis, but mice lacking the common β chain (and thus signaling by both GM-CSF and IL-5) were less resistant [61], suggesting that IL-5 promotes type 2 inflammation in this context. However, eosinophils are not required for primary resistance to infection [60] so the relevant cellular targets of IL-5 in N. brasiliensis infection and during infection with other species remain unclear.
IL-2 and IL-7 act predominantly on cells of the lymphoid lineage, in particular ILC2s, and in doing so act as critical mediators of ILC2-dependent type 2 inflammation [5,51,62-68]. IL-2 derived from T cells promotes the survival and expansion of IL-13-producing ILC2s and Th2 cells in the protective response against N. brasiliensis [63,69], though IL-2 is not required for ILC2 function in H. polygyrus infection [70]. Likewise, stromal cell-derived IL-7 delivers a potent anti-apoptotic, proliferative survival signal to ILC progenitors and mature ILC2s, directing ILC development, ILC2 lineage determination, and lymphoid organogenesis, though how IL-7 affects other aspects of ILC2 functionality in vivo is less clear [5,51,62,63,65-68].
NON-CYTOKINE PATHWAYS THAT REGULATE INNATE IMMUNE RESPONSES DURING HELMINTH INFECTION
Bioactive Lipid Mediators
Bioactive lipid mediators such as the eicosanoid prostaglandins (PGs) and leukotrienes (LTs) are released under type 2 and other inflammatory conditions [71-75]. They play numerous crucial roles in the promotion, suppression, and regulation of type 2 inflammation [71-76]. Eicosanoids are synthesized by cyclic oxidation of polyunsaturated fatty acids such as arachidonic acids and linoleic acids in the diet or released from membrane phospholipids [71-73]. These lipids are produced by mast cells, macrophages, and other cell types in response to epithelial cell-derived cytokines, damage signals, and crosslinking of Fc receptors [71-78] (Fig. 2). ILC2s [79-83], eosinophils [84,85], basophils [85,86], and mast cells [86,87] express eicosanoid receptors and respond to their cognate ligands (Fig. 2). While we understand more about how eicosanoids function during allergic inflammation, their roles during helminth infection have recently been explored. New studies show that LTs promote anti-helminth ILC2 functions during H. polygyrus infection, and during N. brasiliensis infection, LTs activated ILC2s in an NFAT-dependent manner [88] and promoted eosinophil accumulation [84]. In addition, PGE2 licensed DCs to induce Th2 polarization in mice in response to egg antigen from Schistosoma mansoni, a trematode parasite that can infect both mice and humans [89]. Numerous studies have focused on how PGD2 and its receptor CRTH2 (chemoattractant receptor homologous molecule expressed on Th2 cells) can promote production of type 2 cytokines and accumulation of eosinophils, ILC2s, basophils, mast cells, and Th2 cells during type 2 inflammation [67,74,75,79,81,85,90-93]. Only one study has investigated the role of the PGD2-CRTH2 pathway during helminth infection, showing that ILC2 accumulation in the lung was impaired in CRTH2 deficient mice in a model of chronic type 2 pulmonary inflammation induced by N. brasiliensis infection [81]. Further studies will be needed to assess how eicosanoids control innate immune responses in the intestine during helminth infection, particularly as regards the potential suppressive or pro-resolving properties of the eicosanoid family [71,73,76] (Fig. 2).
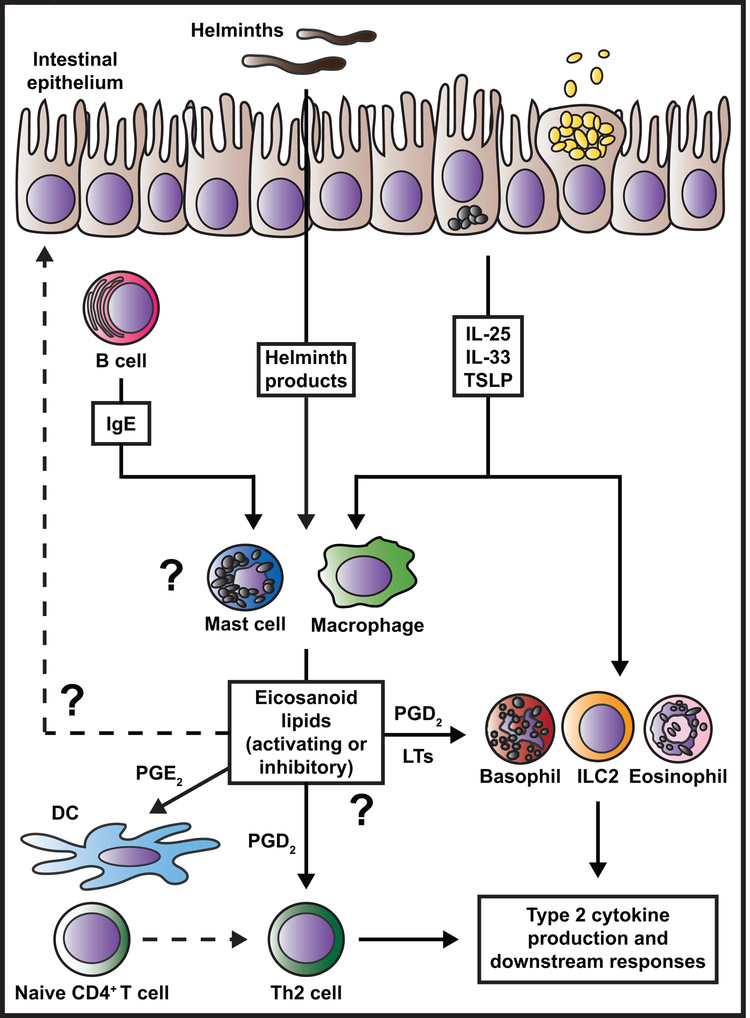
Figure 2. Proposed model for eicosanoid regulation of innate immune responses during helminth-induced type 2 inflammation.
IgE crosslinking of FcεRI, helminth products, or epithelial cell-derived cytokines elicit production or release of eicosanoids including PGs and LTs from mast cells and macrophages during helminth infection. PGE2 can act on DCs to promote their ability to polarize naïve CD4+ T cells to the Th2 fate. PGD2 and LT species activate various innate immune cells and Th2 cells to produce type 2 cytokines and induce accumulation of these cells in tissues. These eicosanoid-mediated effects occur simultaneously or in sequence with events precipitated by epithelial cell-derived cytokines, driving synergistic and highly coordinated spatial and temporal regulation of innate immune cell activities. Open questions remain regarding 1) whether eicosanoids act on the epithelium to promote or suppress anti-helminth effector responses, 2) how different eicosanoid family members promote, suppress, or resolve innate immune cell effector functions, and 3) the identity of key eicosanoid-producing cell types in the intestine.
Finally, how lipids other than eicosanoids, including steroids and sphingolipids, regulate innate immune responses during helminth infection is largely unexplored. One study has shown a role for sex hormones in DC and Th2 responses that control sex-specific differences in resistance to Trichuris muris, a whipworm parasite of mice [94]. Regarding sphingolipids, sugar-containing glycolipids, a recent study showed that sphingosine 1 phosphate-mediated chemotaxis controlled redistribution of inflammatory ILC2s during N. brasiliensis infection [95]. However, there is much work to be done to determine how sex hormones, naturally occurring corticosteroids such as cortisol, other steroid lipids, and various glycolipids impact innate immune function in helminth infection.
Direct Cellular Interactions
While many signals that control type 2 inflammation are released into the tissue microenvironment, others involve direct cell-cell interactions that modulate target cell gene expression and function [3,7,96]. For instance, the interaction that occurs between antigen presenting cells and naïve T cells drives the acquisition of critical type 2 inflammatory effector functions in CD4+ T cells that culminates in Th2 polarization [97-102]. During helminth-induced type 2 inflammation, this interaction is critically dependent on classical DCs that express MHC II and provision of co-stimulation through interactions between CD40 and OX40L and their receptors [97-102]. This topic has been reviewed extensively in [98,99] and thus our discussion will focus on other cell-cell interactions of note.
Innate immune cells such as eosinophils, basophils, and ILC2s express MHC II and costimulatory molecules [63,65,103-108]. While these molecules are classically thought of as important for the activation and differentiation of T cells, innate cell function can also be modulated via these pathways [3,5-7], particularly for ILC2s [5,67]. Engagement of MHC II and CD80 and CD86 on ILC2s can elicit cytokine production and proliferation and facilitate T cell interactions [63,109], though the full significance of MHC II expression on ILC2s is unclear. Interactions between ICOS and ICOSL also facilitate ILC2 survival and cytokine production [106]. Basophils express MHC II, and some early studies suggested that these cells can present antigen to T cells [103-105]. More recent work using new tools to dissect basophil biology has shown that antigen presentation in the lymph node is likely not a critical function of basophils in vivo [100-102,110-112], though an interesting recent study has shown that basophils can acquire peptide-MHC II complexes from DCs through trogocytosis that allows them to present antigen [113]. Basophil MHC II expression could facilitate interactions between basophils and Th2 cells in the tissue that serve to amplify Th2 cell cytokine production. In addition to the ongoing inquiry related to the role of MHC II expression on granulocytes, how expression of various co-stimulatory molecules by basophils, mast cells, and eosinophils affects their function is not yet clear.
Other direct cellular interactions that occur in the context of the Notch signaling pathway and integrin pathways are important in the regulation of type 2 innate immune cells. In Notch signaling, interaction of a Notch receptor-bearing cell with a ligand-bearing cell leads to release and nuclear translocation of the Notch intracellular domain, where it forms a transcriptional activating complex with the transcription factor recombining binding protein suppressor of hairless (RBPJ) that binds DNA, resulting in changes in target gene expression [96]. Notch signaling drives development of mast cells and ILC2s [114-116], the differentiation of KLRG1+ inflammatory ILC2s [117], cytokine production by bone marrow-derived basophils in vitro [118], and localization of mast cells within the intestine during helminth infection [119] (Fig. 3). Notch signaling in CD4+T cells controls polarization to the Th2 fate [96,115,120,121], but the full significance of Notch signaling in innate cells remains to be fully described (Fig. 3). Integrin expression is key for the appropriate localization and accumulation of innate immune cells during helminth infection, with impaired mast cell recruitment and worm clearance observed during infection with the nematode Trichinella spiralis in β7 integrin-deficient mice [122]. Basophils upregulate integrins during N. brasiliensis infection in mice, suggesting that these molecules play a role in regulating an array of innate immune cells [123], but the full scope of integrin-mediated pathways that orchestrate innate immune responses during infection with various helminth species also requires further inquiry.
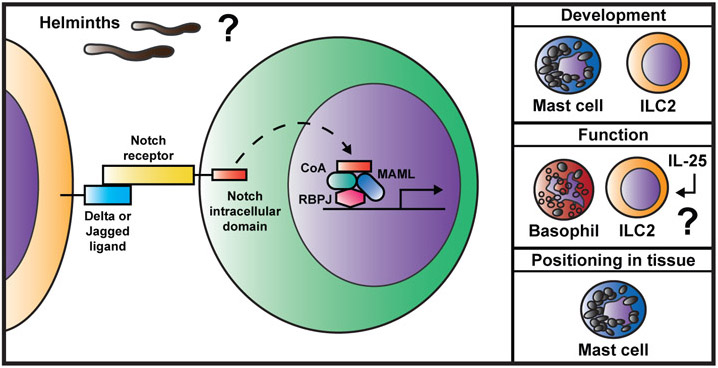
Figure 3. Notch signaling affects innate immune cell responses during type 2 inflammation.
Notch signaling occurs when a ligand-bearing cell interacts with a cell expressing a Notch receptor. This leads to cleavage of the Notch intracellular domain in the receiving cells and translocation to the nucleus. In the nucleus, the Notch intracellular domain forms a transcriptional activating complex along with Mastermind-like protein (MAML), various co-activators (CoA), and the transcription factor RBPJ. The complex binds to DNA and regulates expression of target genes. Notch signaling can regulate the development of innate immune cells (mast cells and ILC2s), their differentiation and function (basophils and ILC2s), and their positioning in tissues (mast cells). How Notch signaling intersects with epithelial cell-derived cytokine-mediated pathways and specifically how innate immune cell-intrinsic Notch affects type 2 inflammation in vivo during helminth infection remains unclear.
Antibodies and Fc Receptors
A hallmark of the immune response to parasite infection is immunoglobulin (Ig) E binding to Fc receptors on the surface of mast cells and basophils, leading to degranulation and secretion of inflammatory mediators [58,124,125]. This interaction, which bridges antigen specific and innate immunity, is mediated largely by the high affinity IgE receptor (FcεRI) that is constitutively expressed on mast cells and basophils [58,111,124-126]. Class-switched IgE signals through a complex network including FcεRI, the low affinity IgE receptor CD23, the IgE and FcεRI binding protein galectin-3, complement receptors, and integrins [126]. IgG binding to the inhibitory Fc receptor FcγRII-B or activating Fc receptors FcγRI, FcγRII-A, FcγRIII, or FcγRIV also impacts the function of innate immune cells in inflammation [127], and Fcγ receptors play a role in trapping of H. polygyrus larvae during secondary infection [128]. FcεR-and FcγR-dependent pathways may intersect, synergizing to facilitate worm expulsion during murine infection with the roundworm Strongyloides venezuelensis [129]. However, further studies are needed to dissect the complex interplay between Ig types and their respective activating or inhibitory Fc receptors on innate cells during helminth infection.
Neurotrophic Factors
A burst of interest in neuroimmunology has led to a number of recent studies that show that interactions between the nervous system and innate immune cells control type 2 inflammation. ILC2s localize close to neurons in the intestine and accumulate and produce type 2 cytokines in response to the neuropeptide neuromedin U (NMU), promoting worm clearance in N. brasiliensis infection [130,131] and allergic lung inflammation [132]. Further, ILC2s in the lung respond to other neurotrophic factors produced by pulmonary neuroendocrine cells during allergy [133]. A very recent study has shown that ILC2s that expressed the β2-adrenergic receptor were inhibited following receptor agonism, and β2-adrenergic receptor deficient mice had increased resistance to N. brasiliensis and H. polygyrus infections, suggesting that sympathetic nervous system signals can dampen ILC2 responses [134]. Earlier work showed that other type 2 innate cells also have connections to neurons, similar to ILC2s. Mast cells and eosinophils become activated and home to tissues in response to NMU [135,136]. Further, mast cells produce factors that stimulate neurons directly, including serotonin, histamine, and neurotrophin 4 that induces smooth muscle innervation [137]. While the study of the crosstalk between the nervous system and the immune system during helminth infection is still in early days, the nervous system clearly plays an important role in directing innate immune functions that support the type 2 inflammatory response.
DAMPs and Other Alarmins
Tissue damage caused by helminth migration, feeding, or secreted proteases drives the release of a wide array of alarmins including high mobility group box 1 protein, matrix metalloproteinases, S100 family proteins, uric acid crystals, and extracellular adenosine derivatives that are strong activators of anti-helminth and wound healing responses (reviewed in [6,8,138]). For example, extracellular purine-nucleoside adenosine released by damaged tissue is a potent regulator of type 2 inflammation [139,140]. Mice lacking the A2B adenosine receptor had impaired Th2 cell development, tissue eosinophilia, AAMac formation, ILC2 activation, and H. polygyrus and N. brasiliensis expulsion in vivo [140]. Notably however, in vitro ligation of different adenosine receptors had differential effects on ILC2 cytokine production [139], suggesting that the effects of adenosine on innate immune cells may be complex. Likewise, mast cells respond to danger-associated extracellular ATP via the P2X7 receptor to drive downstream ILC2 activation and worm expulsion [25]. While roles for other DAMPs have been described in the context of allergic inflammation [8], less is known about these alarmins during helminth infection, and the mechanisms by which these molecules activate or suppress antihelminth type 2 inflammatory responses are still unclear.
CROSSTALK BETWEEN EPITHELIAL CELL-DERIVED CYTOKINES AND OTHER INNATE IMMUNE CELL REGULATORS
Biochemical Synergy
In vitro approaches and in vivo studies of helminth infection in single-gene knockout or transgenic mice have allowed us to understand many mechanisms that control innate immune responses during type 2 inflammation [3,5-11] (Fig. 1). However, these approaches can lead to oversimplification of the complex in vivo environment, in which scores of biochemical factors are produced concurrently or in tightly regulated spatial and temporal circuits. Excitingly, recent studies have addressed how various novel host, pathogen, and microbiota-derived factors synergize to orchestrate type 2 inflammation [6,7]. In this final section, we will focus on recent work that explores the intersections between epithelial cell-derived cytokine pathways and other mediators of innate immune cells that coordinate type 2 immune responses. It is important to note that some of these studies have been conducted in the context of allergic inflammation, and it remains to be seen whether similar results will be observed in helminth infection.
A Web of Regulation
There is significant evidence for crosstalk between epithelial cell-derived cytokine pathways and type 2 innate immune functions that depend on soluble mediators, including chemokines and bioactive lipids [6-8,141]. For instance, epithelial cell-derived cytokines can act back on epithelial cells to induce release of chemotactic factors during N. brasiliensis infection]. TSLP exposure promoted chemokine production and release from basophils during T. muris infection [143], and IL-33 elicited chemokine release from numerous innate immune cell types in the context of allergic inflammation [141]. Similarly, in vitro studies demonstrated that IL-33 and TSLP can induce release of PGD2 from mast cells [78,144], and PGE2 can conversely induce IL-33 production from DCs and macrophages [145]. Together, these studies suggest that epithelial cell-derived cytokines promote downstream accumulation of other biochemical species, and vice versa. In addition, exposure to PGs and LTs can potentiate IL-33-mediated activation of ILC2s during N. brasiliensis infection [88,146], suggesting that proper exposure to different signals in the correct order can lead to optimal type 2 inflammatory responses (Fig. 2).
Signals downstream of antibodies binding to Fc receptors can also intersect with pathways mediated via epithelial cell-derived cytokines during type 2 inflammation [58,124,125]. FcεR and FcγR signaling activated the production of epithelial-derived cytokines in various myeloid cell types in vitro and during type 2 allergic inflammation in the lung [24,147,148]. Interestingly, epithelial-derived cytokines can also prime specific Fc receptor-dependent effector functions in innate immune cells. For example, IL-25 increased IgE-mediated degranulation of allergic human basophils without affecting the release of IL-4, IL-8 and IL-13 [149]. In atopic dermatitis, signaling via FcγRI increased expression of the TSLP receptor on monocyte-derived DCs [150]. These data show that intersection of Fc receptor and epithelial cell-derived cytokine pathways may be important in bridging innate and adaptive responses in type 2 inflammation.
Type 2 innate immune cells must also integrate the signals received from epithelial cell-derived cytokines and from direct interactions with other cell types, with exposure to epithelial cell-derived cytokines often facilitating these cell-cell interactions [6,7,96,115]. TSLP induced expression of OX40L on human and mouse DCs, facilitating their capacity to prime Th2 cells in vitro and during allergic sensitization [151,152]. IL-33 could also drive activation of DCs and ILC2s during allergy, highlighted by upregulation of OX40L and CD40 [153,154]. Likewise, IL-33 may upregulate MHC II on bone marrow-derived mast cells in vitro [155], though the functional significance of this remains to be determined. Similarly, IL-25 and IL-33 regulated the expression of OX40L in lung ILC2s, which promoted downstream activation of the Th2 response during helminth infection [107]. Finally, cell-cell interactions can also prime a cell to receive signals delivered via cytokine receptors. For example, exposure of ILC2s to Notch signals in combination with IL-25 enhanced their functional plasticity during allergic airway inflammation, allowing them to produce the effector cytokines IL-5 and IL-13 as well as IL-17 [117] (Fig. 3).
DISCUSSION AND FUTURE DIRECTIONS
Exciting ongoing research continues to reveal new aspects of innate immune cell regulation and function during helminth-induced type 2 inflammation [3,5-11]. We now understand many effects of epithelial cell-derived cytokines, lipids, Fc receptor signaling, and direct cell-cell interactions on innate immune responses during type 2 inflammation [3,6-8,11-13]. Notably, we are beginning to unravel how networks of these factors synergize spatially and temporally during helminth infection to promote innate immune-dependent type 2 inflammation, worm expulsion, and wound-healing responses. However, significant work remains to be done in this area. Some of the studies discussed here have been conducted in vitro or in the context of allergic disease, and these findings should be tested in vivo during helminth infection. Systems immunology approaches and mouse models that allow for cell lineage-specific and inducible deletion of players in innate immune regulatory networks in vivo during infection will be needed to better understand how innate immune cell activities are controlled in the tissue. In addition, studying how epithelial cell-derived cytokine and other pathways integrate intracellularly on the molecular level, through the use of common signaling molecules, pathways, or transcription factors, will require cutting-edge in vivo biochemical tools that could leverage optogenetic approaches and live imaging. Thinking more broadly, more studies are needed to dissect how epithelial cell-derived cytokine responses integrate signals from microbiota- and diet-derived factors to shape innate immune responses in health and inflammation [6,33,34]. Finally, we understand little about how the diversity of soluble biochemical factors present in the human intestine during helminth infection can modulate innate immune-dependent type 2 inflammatory responses. Studies that address this gap and bridge work in murine models and in helminth-infected human patients will inform the development of critical new strategies to manage, treat, or prevent helminth infection in humans.
ACKNOWLEDGEMENTS
This work was supported by the NIH NIAID (K22 AI116729, R01 AI132708, and R01 AI130379 to E.D.T.W). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors thank members of the Tait Wojno lab for their feedback during manuscript development. The authors declare no competing financial interests.
Abbreviations:
- AAMac:
alternatively activated macrophage
- CRTH2:
chemoattractant receptor homologous molecule expressed on Th2 cells
- DAMP:
danger-associated molecular pattern
- DC:
dendritic cell
- GM-CSF:
granulocyte-macrophage colony-stimulating factor
- ILC2:
group 2 innate lymphoid cell
- Ig:
immunoglobulin
- LT:
leukotriene
- NMU:
neuromedin U
- PG:
prostaglandin
- RBPJ:
recombining binding protein suppressor of hairless
- Th2:
CD4+ T helper type 2
- TSLP:
thymic stromal lymphopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Karupiah G Cytokines and chemokines in infectious diseases handbook. Immunol Cell Biol 2003;81:496. [DOI] [PubMed] [Google Scholar]
- [2].Onyiah JC, Colgan SP. Cytokine responses and epithelial function in the intestinal mucosa. Cell Mol Life Sci 2016;73(22):4203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pulendran B, Artis D. New paradigms in type 2 immunity. Science 2012;337(6093):431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2014;74(1):5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol 2013;14(6):536–42. [DOI] [PubMed] [Google Scholar]
- [6].Harris NL, Loke P. Recent advances in type-2-cell-mediated immunity: Insights from helminth infection. Immunity 2017;47(6):1024–36. [DOI] [PubMed] [Google Scholar]
- [7].Webb LM, Tait Wojno ED. The role of rare innate immune cells in type 2 immune activation against parasitic helminths. Parasitology 2017;144(10):1288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hammad H, Lambrecht B. Barrier epithelial cells and the control of type 2 immunity. Immunity 2015;43(1):29–40. [DOI] [PubMed] [Google Scholar]
- [9].Gieseck R, Wilson M, Wynn T. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol 2018;18(1):62–76. [DOI] [PubMed] [Google Scholar]
- [10].Maizels RM, Hewitson JP. Myeloid cell phenotypes in susceptibility and resistance to helminth parasite infections. Microbiol Spectr 2016;4(6). [DOI] [PubMed] [Google Scholar]
- [11].Sorobetea D, Svensson-Frej M, Grencis R. Immunity to gastrointestinal nematode infections. Mucosal Immunol 2018;11(2):304–15. [DOI] [PubMed] [Google Scholar]
- [12].Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol 2010;11(4):289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Molofsky A, Savage A, Locksley R. Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity 2015;42(6):1005–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: Roles in homeostasis and disease. Annu Rev Immunol 2013;31:317–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shea-Donohue T, Sun R, Bohl JA, McLean LP, Zhao A. Enteric nematodes and the path to up-regulation of type 2 cytokines IL-4 and IL-13. Cytokine 2015;75(1):62–7. [DOI] [PubMed] [Google Scholar]
- [16].Licona-Limon P, Arias-Rojas A, Olguin-Martinez E. IL-9 and Th9 in parasite immunity. Semin Immunopathol 2017;39(1):29–38. [DOI] [PubMed] [Google Scholar]
- [17].Nair MG, Herbert DR. Immune polarization by hookworms: Taking cues from T helper type 2, type 2 innate lymphoid cells and alternatively activated macrophages. Immunology 2016; 148(2): 115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].von Moltke J, Ji M, Liang H, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016;529(7585):221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 2016;351(6279):1329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 2016;529(7585):226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, et al. A single-cell survey of the small intestinal epithelium. Nature 2017;551(7680):333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol 2008; 180(4):2443–9. [DOI] [PubMed] [Google Scholar]
- [23].Wills-Karp M, Rani R, Dienger K, Lewkowich I, Fox JG, Perkins C, et al. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med 2012;209(3):607–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ikeda K, Nakajima H, Suzuki K, Kagami S, Hirose K, Suto A, et al. Mast cells produce interleukin-25 upon FcεRI-mediated activation. Blood 2003;101(9):3594–6. [DOI] [PubMed] [Google Scholar]
- [25].Shimokawa C, Kanaya T, Hachisuka M, Ishiwata K, Hisaeda H, Kurashima Y, et al. Mast cells are crucial for induction of group 2 innate lymphoid cells and clearance of helminth infections. Immunity 2017;46(5):863–874.e4. [DOI] [PubMed] [Google Scholar]
- [26].Wang Y, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med 2007;204(8):1837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schneider C, O’Leary CE, von Moltke J, Liang H, Ang QY, Turnbaugh PJ, et al. A metabolite-triggered tuft cell-ILC2 circuit drives small intestinal remodeling. Cell 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Scott IC, Majithiya JB, Sanden C, Thornton P, Sanders PN, Moore T, et al. Interleukin-33 is activated by allergen- and necrosis-associated proteolytic activities to regulate its alarmin activity during epithelial damage. Sci Rep 2018;8(1):3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Moniaga CS, Jeong SK, Egawa G, Nakajima S, Hara-Chikuma M, Jeon JE, et al. Protease activity enhances production of thymic stromal lymphopoietin and basophil accumulation in flaky tail mice. Am J Pathol 2013;182(3):841–51. [DOI] [PubMed] [Google Scholar]
- [30].Clair P, Coward WR, Pritchard DI, Hewitt CR. Basophils express a type 2 cytokine profile on exposure to proteases from helminths and house dust mites. J Leukoc Biol 2003;73(1):165–71. [DOI] [PubMed] [Google Scholar]
- [31].Massacand JC, Stettler RC, Meier R, Humphreys NE, Grencis RK, Marsland BJ, et al. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc Natl Acad Sci USA 2009;106(33):13968–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Park MK, Cho MK, Kang SA, Park H, Kim YS, Kim KU, et al. Protease-activated receptor 2 is involved in Th2 responses against trichinella spiralis infection. Korean J Parasitol 2011;49(3):235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zaiss M, Rapin A, Lebon L, Dubey L, Mosconi I, Sarter K, et al. The intestinal microbiota contributes to the ability of helminths to modulate allergic inflammation. Immunity 2015;43(5):998–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Reynolds LA, Finlay BB, Maizels RM. Cohabitation in the intestine: Interactions among helminth parasites, bacterial microbiota, and host immunity. J Immunol 2015;195(9):4059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hung L, Lewkowich IP, Dawson LA, Downey J, Yang Y, Smith DE, et al. IL-33 drives biphasic IL-13 production for noncanonical type 2 immunity against hookworms. Proc Natl Acad Sci USA 2013;110(1):282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tang W, Smith SG, Beaudin S, Dua B, Howie K, Gauvreau G, et al. IL-25 and IL-25 receptor expression on eosinophils from subjects with allergic asthma. Int Arch Allergy Immunol 2014;163(1):5–10. [DOI] [PubMed] [Google Scholar]
- [37].Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013;502(7470):245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Molofsky AB, Nussbaum JC, Liang H, Van Dyken SJ, Cheng LE, Mohapatra A, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med 2013;210(3):535–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yu X, Pappu R, Ramirez-Carrozzi V, Ota N, Caplazi P, Zhang J, et al. TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol 2014;7(3):730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Meylan F, Hawley ET, Barron L, Barlow JL, Penumetcha P, Pelletier M, et al. The TNF-family cytokine TL1A promotes allergic immunopathology through group 2 innate lymphoid cells. Mucosal Immunol 2014;7(4):958–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Helmby H, Takeda K, Akira S, Grencis RK. Interleukin (il)-18 promotes the development of chronic gastrointestinal helminth infection by downregulating IL-13. J Exp Med 2001;194(3):355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Novick D, Kim S, Kaplanski G, Dinarello CA. Interleukin-18, more than a Th1 cytokine. Semin Immunol 2013;25(6):439–48. [DOI] [PubMed] [Google Scholar]
- [43].Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu Rev Immunol 2014;32(1):659–702. [DOI] [PubMed] [Google Scholar]
- [44].Sokol CL, Luster AD. The chemokine system in innate immunity. Cold Spring Harb Perspect Biol 2015;7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Soriani A, Stabile H, Gismondi A, Santoni A, Bernardini G. Chemokine regulation of innate lymphoid cell tissue distribution and function. Cytokine Growth Factor Rev 2018. [DOI] [PubMed] [Google Scholar]
- [46].Chuah C, Jones MK, Burke ML, McManus DP, Gobert GN. Cellular and chemokine-mediated regulation in schistosome-induced hepatic pathology. Trends Parasitol 2014;30(3):141–50. [DOI] [PubMed] [Google Scholar]
- [47].Turner JD, Pionnier N, Furlong-Silva J, Sjoberg H, Cross S, Halliday A, et al. Interleukin-4 activated macrophages mediate immunity to filarial helminth infection by sustaining CCR3-dependent eosinophilia. PLoS Pathog 2018; 14(3):e1006949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Vukman KV, Adams PN, Dowling D, Metz M, Maurer M, O’Neill SM. The effects of fasciola hepatica tegumental antigens on mast cell function. Int J Parasitol 2013;43(7):531–9. [DOI] [PubMed] [Google Scholar]
- [49].Broughton SE, Urmi D, Hercus TR, Nero TL, Grimbaldeston MA, Bonder CS, et al. The GM–CSF/IL-3/IL-5 cytokine receptor family: From ligand recognition to initiation of signaling. Immunol Rev 2012;250(1):277–302. [DOI] [PubMed] [Google Scholar]
- [50].David V Basophil modulation by cytokine instruction. Eur J Immunol 2012;42(10):2544–50. [DOI] [PubMed] [Google Scholar]
- [51].Vonarbourg C, Diefenbach A. Multifaceted roles of interleukin-7 signaling for the development and function of innate lymphoid cells. Semin Immunol 2012;24(3): 165–74. [DOI] [PubMed] [Google Scholar]
- [52].Siracusa MC, Wojno ED, Artis D. Functional heterogeneity in the basophil cell lineage. Adv Immunol 2012;115:141–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schwartz C, Willebrand R, Huber S, Rupec RA, Wu D, Locksley R, et al. Eosinophil-specific deletion of IκBα in mice reveals a critical role of NF-κB–induced bcl-xL for inhibition of apoptosis. Blood 2015; 125(25):3896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Willebrand R, Voehringer D. IL-33-induced cytokine secretion and survival of mouse eosinophils is promoted by autocrine GM-CSF. PLoS One 2016;11(9):e0163751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature 1998;392(6671):90–3. [DOI] [PubMed] [Google Scholar]
- [56].Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med 2004;200(4):507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lantz CS, Min B, Tsai M, Chatterjea D, Dranoff G, Galli SJ. IL-3 is required for increases in blood basophils in nematode infection in mice and can enhance IgE-dependent IL-4 production by basophils in vitro. Lab Invest 2008;88(11):1134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Eberle JU, Voehringer D. Role of basophils in protective immunity to parasitic infections. Semin Immunopathol 2016;38(5):605–13. [DOI] [PubMed] [Google Scholar]
- [59].Furuta GT, Atkins FD, Lee NA, Lee JJ. Changing roles of eosinophils in health and disease. Ann Allergy Asthma Immunol 2014;113(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Huang L, Appleton JA. Eosinophils in helminth infection: Defenders and dupes. Trends Parasitol 2016;32(10):798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Shim DS, Schilter HC, Knott ML, Almeida RA, Short RP, Mackay CR, et al. Protection against nippostrongylus brasiliensis infection in mice is independent of GM-CSF. Immunol Cell Biol 2012;90(5):553–8. [DOI] [PubMed] [Google Scholar]
- [62].Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 2010;463(7280):540–4. [DOI] [PubMed] [Google Scholar]
- [63].Oliphant C, Hwang Y, Walker J, Salimi M, Wong S, Brewer J, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4+ T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 2014;41(2):283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Roediger B, Kyle R, Tay SS, Mitchell AJ, Bolton HA, Guy TV, et al. IL-2 is a critical regulator of group 2 innate lymphoid cell function during pulmonary inflammation. J Allergy Clin Immunol 2015; 136(6): 1653,1663.e7. [DOI] [PubMed] [Google Scholar]
- [65].Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010;464(7293):1367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Price AE, Liang H, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13–expressing cells in type 2 immunity. Proc Natl Acad Sci USA 2010; 107(25): 11489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tait Wojno ED, Artis D. Emerging concepts and future challenges in innate lymphoid cell biology. J Exp Med 2016;213(11):2229–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 2011; 12(11): 1045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bouchery T, Kyle R, Camberis M, Shepherd A, Filbey K, Smith A, et al. ILC2s and T cells cooperate to ensure maintenance of M2 macrophages for lung immunity against hookworms. Nat Commun 2015;6:6970. [DOI] [PubMed] [Google Scholar]
- [70].Pelly VS, Kannan Y, Coomes SM, Entwistle LJ, Ruckerl D, Seddon B, et al. IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol 2016;9(6):1407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol 2002;23(3):144–50. [DOI] [PubMed] [Google Scholar]
- [72].Kubata BK, Duszenko M, Martin KS, Urade Y. Molecular basis for prostaglandin production in hosts and parasites. Trends Parasitol 2007;23(7):325–31. [DOI] [PubMed] [Google Scholar]
- [73].Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol 2015;15(8):511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Fajt ML, Gelhaus SL, Freeman B, Uvalle CE, Trudeau JB, Holguin F, et al. Prostaglandin D2 pathway upregulation: Relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol 2013;131(6):1504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Doherty TA, Broide DH. Lipid regulation of group 2 innate lymphoid cell function: Moving beyond epithelial cytokines. J Allergy Clin Immunol 2018;141(5):1587–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Buckley C, Gilroy D, Serhan C. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 2014;40(3):315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lewis RA, Soter NA, Diamond PT, Austen KF, Oates JA, Roberts LJ. Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J Immunol 1982;129(4):1627–31. [PubMed] [Google Scholar]
- [78].Moulin D, Donzé O, Talabot-Ayer D, Mézin F, Palmer G, Gabay C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine 2007;40(3):216–25. [DOI] [PubMed] [Google Scholar]
- [79].Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen C,M, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol 2011;12(11):1055–62. [DOI] [PubMed] [Google Scholar]
- [80].Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol 2013;132(1):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Tait Wojno ED, Monticelli LA, Tran SV, Alenghat T, Osborne LC, Thome JJ, et al. The prostaglandin D2 receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal Immunol 2015;8(6):1313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Salimi M, Stöger L, Liu W, Go S, Pavord I, Klenerman P, et al. Cysteinyl leukotriene E4 activates human group 2 innate lymphoid cells and enhances the effect of prostaglandin D2 and epithelial cytokines. J Allergy Clin Immunol 2017;140(4):1090–1100.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zhou W, Toki S, Zhang J, Goleniewksa K, Newcomb DC, Cephus JY, et al. Prostaglandin I2 signaling and inhibition of group 2 innate lymphoid cell responses. Am J Respir Crit Care Med 2016;193(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Patnode ML, Bando JK, Krummel MF, Locksley RM, Rosen SD. Leukotriene B4 amplifies eosinophil accumulation in response to nematodes. J Exp Med 2014;211(7):1281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Nagata K, Hirai H, Tanaka K, Ogawa K, Aso T, Sugamura K, et al. CRTH2, an orphan receptor of T-helper-2-cells, is expressed on basophils and eosinophils and responds to mast cell-derived factor(s). FEBS Lett 1999;459(2): 195–9. [DOI] [PubMed] [Google Scholar]
- [86].Theron AJ, Steel HC, Tintinger GR, Gravett CM, Anderson R, Feldman C. Cysteinyl leukotriene receptor-1 antagonists as modulators of innate immune cell function. J Immunol Res 2014;2014:608930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: Cellular distribution and function in immune and inflammatory responses. J Immunol 2004;173(3):1503–10. [DOI] [PubMed] [Google Scholar]
- [88].von Moltke J, O’Leary CE, Barrett NA, Kanaoka Y, Austen KF, Locksley RM. Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s. J Exp Med 2017;214(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kaisar MMM, Ritter M, Del Fresno C, Jonasdottir HS, van der Ham AJ, Pelgrom LR, et al. Dectin-1/2-induced autocrine PGE2 signaling licenses dendritic cells to prime Th2 responses. PLoS Biol 2018;16(4):e2005504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor Crth2. J Exp Med 2001;193(2):255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Monneret G, Gravel S, Diamond M, Rokach J, Powell WS. Prostaglandin D2 is a potent chemoattractant for human eosinophils that acts via a novel DP receptor. Blood 2001;98(6):1942–8. [DOI] [PubMed] [Google Scholar]
- [92].Pettipher R The roles of the prostaglandin D(2) receptors DP(1) and CRTH2 in promoting allergic responses. Br J Pharmacol 2007;153:S191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Xue L, Salimi M, Panse I, Mjösberg JM, McKenzie ANJ, Spits H, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol 2014; 133(4): 1184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hepworth MR, Hardman MJ, Grencis RK. The role of sex hormones in the development of Th2 immunity in a gender-biased model of trichuris muris infection. Eur J Immunol 2010;40(2):406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Huang Y, Mao K, Chen X, Sun M, Kawabe T, Li W, et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 2018;359(6371):114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Maillard I, Adler SH, Pear WS. Notch and the immune system. Immunity 2003;19(6):781–91. [DOI] [PubMed] [Google Scholar]
- [97].MacDonald AS, Straw AD, Dalton NM, Pearce EJ. Cutting edge: Th2 response induction by dendritic cells: A role for CD40. J Immunol 2002;168(2):537–40. [DOI] [PubMed] [Google Scholar]
- [98].Sher A, Pearce E, Kaye P. Shaping the immune response to parasites: Role of dendritic cells. Curr Opin Immunol 2003;15(4):421–9. [DOI] [PubMed] [Google Scholar]
- [99].Lucas C, Jie S, Colleen K, Fraser M, Connie K, Pearce EJ. Review series on helminths, immune modulation and the hygiene hypothesis: Mechanisms underlying helminth modulation of dendritic cell function. Immunology 2009;126(1):28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Phythian-Adams AT, Cook PC, Lundie RJ, Jones LH, Smith KA, Barr TA, et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med 2010;207(10):2089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Smith KA, Harcus Y, Garbi N, Hämmerling GJ, MacDonald AS, Maizels RM. Type 2 innate immunity in helminth infection is induced redundantly and acts autonomously following CD11c+ cell depletion. Infect and Immun 2012;80(10):3481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Lundie RJ, Webb LM, Marley AK, Phythian-Adams AT, Cook PC, Jackson-Jones LH, et al. A central role for hepatic conventional dendritic cells in supporting Th2 responses during helminth infection. Immunol Cell Biol 2016;94(4):400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote TH2 cytokine-dependent immunity. Nat Immunol 2009;10(7):697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Sokol CL, Chu N, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol 2009;10(7):713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol 2009;10(7):706–12. [DOI] [PubMed] [Google Scholar]
- [106].Maazi H, Patel N, Sankaranarayanan I, Suzuki Y, Rigas D, Soroosh P, et al. ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity 2015;42(3):538–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Halim TYF, Rana BMJ, Walker JA, Kerscher B, Knolle MD, Jolin HE, et al. Tissue-restricted adaptive type 2 immunity is orchestrated by expression of the costimulatory molecule OX40L on group 2 innate lymphoid cells. Immunity 2018;48(6):1195–1207.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Padigel UM, Lee JJ, Nolan TJ, Schad GA, Abraham D. Eosinophils can function as antigen-presenting cells to induce primary and secondary immune responses to strongyloides stercoralis. Infect Immun 2006;74(6):3232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Angkasekwinai P, Sodthawon W, Jeerawattanawart S, Hansakon A, Pattanapanyasat K, Wang Y-. ILC2s activated by IL-25 promote antigen-specific Th2 and Th9 functions that contribute to the control of trichinella spiralis infection. PLoS One 2017;12(9):e0184684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood 2009;113(12):2816–25. [DOI] [PubMed] [Google Scholar]
- [111].Schwartz C, Turqueti-Neves A, Hartmann S, Yu P, Nimmerjahn F, Voehringer D. Basophil-mediated protection against gastrointestinal helminths requires IgE-induced cytokine secretion. Proc Natl Acad Sci U S A 2014;111(48):E5169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Sullivan BM, Liang H, Bando JK, Wu D, Cheng LE, McKerrow JK, et al. Genetic analysis of basophil function in vivo. Nat Immunol 2011;12(6):527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Miyake K, Shiozawa N, Nagao T, Yoshikawa S, Yamanishi Y, Karasuyama H. Trogocytosis of peptide-MHC class II complexes from dendritic cells confers antigen-presenting ability on basophils. Proc Natl Acad Sci USA 2017;114(5):1111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Sakata-Yanagimoto M, Nakagami-Yamaguchi E, Saito T, Kumano K, Yasutomo K, Ogawa S, et al. Coordinated regulation of transcription factors through Notch2 is an important mediator of mast cell fate. Proc Natl Acad Sci USA 2008;105(22):7839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Radtke F, MacDonald HR, Tacchini-Cottier F. Regulation of innate and adaptive immunity by notch. Nat Rev Immunol 2013;13(6):427–37. [DOI] [PubMed] [Google Scholar]
- [116].Yang Q, Monticelli L, Saenz S, Chi A, Sonnenberg G, Tang J, et al. T cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity 2013;38(4):694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Zhang K, Xu X, Pasha MA, Siebel CW, Costello A, Haczku A, et al. Cutting edge: Notch signaling promotes the plasticity of group-2 innate lymphoid cells. J Immunol 2017;198(5):1798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Qu S, Lin J, Zhang J, Song L, Yang X, Wu C. Notch signaling pathway regulates the growth and the expression of inflammatory cytokines in mouse basophils. Cell Immunol 2017;318:29–34. [DOI] [PubMed] [Google Scholar]
- [119].Sakata-Yanagimoto M, Sakai T, Miyake Y, Saito TI, Maruyama H, Morishita Y, et al. Notch2 signaling is required for proper mast cell distribution and mucosal immunity in the intestine. Blood 2011;117(1):128–34. [DOI] [PubMed] [Google Scholar]
- [120].Tu L, Fang TC, Artis D, Shestova O, Pross SE, Maillard I, et al. Notch signaling is an important regulator of type 2 immunity. J Exp Med 2005;202(8): 1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Bailis W, Yashiro-Ohtani Y, Fang T, Hatton R, Weaver C, Artis D, et al. Notch simultaneously orchestrates multiple helper T cell programs independently of cytokine signals. Immunity 2013;39(1):148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Artis D, Humphreys NE, Potten CS, Norbert W, Müller W, McDermott JR, et al. B7 integrin-deficient mice: Delayed leukocyte recruitment and attenuated protective immunity in the small intestine during enteric helminth infection. Eur J Immunol 2000;30(6): 1656–64. [DOI] [PubMed] [Google Scholar]
- [123].Bakocevic N, Claser C, Yoshikawa S, Jones LA, Chew S, Goh CC, et al. CD41 is a reliable identification and activation marker for murine basophils in the steady state and during helminth and malarial infections. Eur J Immunol 2014;44(6):1823–34. [DOI] [PubMed] [Google Scholar]
- [124].Wu LC, Zarrin AA. The production and regulation of IgE by the immune system. Nat Rev Immunol 2014;14(4):247–59. [DOI] [PubMed] [Google Scholar]
- [125].Hepworth MR, Maurer M, Hartmann S. Regulation of type 2 immunity to helminths by mast cells. Gut Microbes 2012;3(5):476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med 2012;18(4):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Nimmerjahn F, Ravetch JV. Fcγ receptors: Old friends and new family members. Immunity 2006;24(1): 19–28. [DOI] [PubMed] [Google Scholar]
- [128].Esser-von Bieren J, Mosconi I, Guiet R, Piersgilli A, Volpe B, Chen F, et al. Antibodies trap tissue migrating helminth larvae and prevent tissue damage by driving IL-4Rα-independent alternative differentiation of macrophages. PLoS Pathog 2013;9(11):e1003771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Matsumoto M, Sasaki Y, Yasuda K, Takai T, Muramatsu M, Yoshimoto T, et al. IgG and IgE collaboratively accelerate expulsion of strongyloides venezuelensis in a primary infection. Infect Immun 2013;81(7):2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Klose CSN, Mahlakõiv T, Moeller JB, Rankin LC, Flamar A, Kabata H, et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 2017;549(7671:282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Cardoso V, Chesne J, Ribeiro H, Garcia-Cassani B, Carvalho T, Bouchery T, et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 2017;549(7671):277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour RE, Nyman J, Dionne D, et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 2017;549(7672):351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sui P, Wiesner DL, Xu J, Zhang Y, Lee J, Van Dyken S, et al. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science 2018;360(6393). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Moriyama S, Brestoff JR, Flamar A, Moeller JB, Klose CSN, Rankin LC, et al. B2-adrenergic receptor–mediated negative regulation of group 2 innate lymphoid cell responses. Science 2018;359(6379): 1056–61. [DOI] [PubMed] [Google Scholar]
- [135].Moriyama M, Sato T, Inoue H, Fukuyama S, Teranishi H, Kangawa K, et al. The neuropeptide neuromedin U promotes inflammation by direct activation of mast cells. J Exp Med 2005;202(2):217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Moriyama M, Fukuyama S, Inoue H, Matsumoto T, Sato T, Tanaka K, et al. The neuropeptide neuromedin U activates eosinophils and is involved in allergen-induced eosinophilia. Am J Physiol Lung Cell Mol Physiol 2006;290(5):L971–7. [DOI] [PubMed] [Google Scholar]
- [137].Patel KR, Aven L, Shao F, Krishnamoorthy N, Duvall MG, Levy BD, et al. Mast cell-derived neurotrophin 4 mediates allergen-induced airway hyperinnervation in early life. Mucosal Immunol 2016;9(6): 1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: Evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol 2013;13(8):607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Csoka B, Selmeczy Z, Koscsó B, Németh ZH, Pacher P, Murray PJ, et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J 2012;26(1):376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Patel N, Wu W, Mishra P, Chen F, Millman A, Csóka B, et al. A2B adenosine receptor induces protective antihelminth type 2 immune responses. Cell Host Microbe 2014;15(3):339–50. [DOI] [PubMed] [Google Scholar]
- [141].Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: The new kid in the IL-1 family. Nat Rev Immunol 2010;10(2):103–10. [DOI] [PubMed] [Google Scholar]
- [142].Kang Z, Swaidani S, Yin W, Wang C, Barlow J, Gulen M, et al. Epithelial cell-specific Act1 adaptor mediates interleukin-25-dependent helminth expulsion through expansion of lin–c-kit+ innate cell population. Immunity 2012;36(5):821–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 2011;477(7363):229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Buchheit KM, Cahill KN, Katz HR, Murphy KC, Feng C, Lee-Sarwar K, et al. Thymic stromal lymphopoietin controls prostaglandin D2 generation in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2016;137(5):1566,1576.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Yanagawa Y, Suzuki M, Matsumoto M, Togashi H. Prostaglandin E2 enhances IL-33 production by dendritic cells. Immunol Lett 2011; 141(1):55–60. [DOI] [PubMed] [Google Scholar]
- [146].Lund SJ, Portillo A, Cavagnero K, Baum RE, Naji LH, Badrani JH, et al. Leukotriene C4 potentiates IL-33–Induced group 2 innate lymphoid cell activation and lung inflammation. J Immunol 2017; 199(3):1096–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Okayama Y, Okumura S, Sagara H, Yuki K, Sasaki T, Watanabe N, et al. FcϵRI-mediated thymic stromal lymphopoietin production by IL-4-primed human mast cells. Eur Respir J 2009;34(2):425–35. [DOI] [PubMed] [Google Scholar]
- [148].Tjota MY, Williams JW, Lu T, Clay BS, Byrd T, Hrusch CL, et al. IL-33-dependent induction of allergic lung inflammation by FcγRIII signaling. J Clin Invest 2013;123(5):2287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Wang H, Mobini R, Fang Y, Barrenäs F, Zhang H, Xiang Z, et al. Allergen challenge of peripheral blood mononuclear cells from patients with seasonal allergic rhinitis increases IL-17RB, which regulates basophil apoptosis and degranulation. Clin Exp Allergy 2010;40(8): 1194–202. [DOI] [PubMed] [Google Scholar]
- [150].Hulse KE, Reefer AJ, Engelhard VH, Patrie JT, Ziegler SF, Chapman MD, et al. Targeting allergen to FcγRI reveals a novel TH2 regulatory pathway linked to thymic stromal lymphopoietin receptor. J Allergy Clin Immunol 2010;125(1):247,256.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Ito T, Wang Y, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med 2005;202(9): 1213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Seshasayee D, Lee WP, Zhou M, Shu J, Suto E, Zhang J, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest 2007;117(12):3868–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Besnard A-G, Togbe D, Guillou N, Erard F, Quesniaux V, Bernhard R. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol 2011;41 (6):1675–86. [DOI] [PubMed] [Google Scholar]
- [154].Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol 2013;131(1):187,200.e8. [DOI] [PubMed] [Google Scholar]
- [155].Tomonobu I, Chizu E, Tatsuo M, Takafumi N, Nobuhiro N, Chiharu N, et al. IL-33 promotes MHC class II expression in murine mast cells. Immun Inflamm Dis 2015;3(3): 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]