Abstract
Little is known about the influence of rearing environments concurrent with voluntary intermittent access to ethanol on subsequent adult ethanol-related behaviors. Previous research has shown that adult rats reared in post-weaning, social isolation conditions (IC) respond more for operant ethanol compared to laboratory standard conditions (SC). Ethanol exposed adolescents tend to consume more ethanol in adulthood than rats exposed as adults. The current study examined voluntary ethanol consumption during adolescence between IC and SC rats, subsequent operant responding for ethanol, and extinction of responding in the same rats as adults. Differences in ethanol metabolism may alter the amount of reward value per unit of ethanol consumed. Therefore, the current study also examined Blood Ethanol Concentrations (BEC) between IC and SC rats. Ethanol-naïve Long-Evans rats arrived in the lab at postnatal day (PND) 21 and were separated into either IC or SC where they remained for the duration of the experiments. On PND 27, rats received intermittent access to 20% ethanol (3 days/week) for 4 or 6 weeks. Rats in the 6-week cohort were then trained to lever press for 20% ethanol in 30-min sessions followed by extinction. A separate cohort was reared in IC or SC, injected with 1.5 or 3.0 g/kg of ethanol (i.p.), followed by BEC measurement. Overall, IC rats had higher ethanol preference and consumption during adolescence/early-adulthood. IC and SC rats did not differ in their rates of operant responding for ethanol and SC rats responded more than IC rats during extinction. There were no differences in BEC between IC and SC rats. These findings highlight the importance of the environment during rat adolescent development with isolation conditions increasing binge-like drinking and ethanol preference after 3–4 weeks without differences in metabolism as a potential factor. Additionally, the findings indicate that intermittent adolescent access to ethanol may change typical differences in operant responding patterns between IC and SC rats in adulthood.
Keywords: Post Weaning Social Isolation, Intermittent Access to Ethanol, Ethanol Selfadministration, Adolescent, Ethanol Metabolism, Extinction
Introduction
Adolescents between the ages of 12 and 20 consume approximately 90% of their ethanol in binge drinking sessions (Centers for Disease Control and Prevention, 2015). While much of ethanol-related disease deaths occur among adults, the groundwork for the development of ethanol problems is often laid during childhood and adolescence. In humans, adolescents who use ethanol are significantly more likely to develop dependence than individuals who are exposed in adulthood (Bonomo, Bowes, Coffey, Carlin, & Patton, 2004) with earlier adolescent use (Hingson, Heeren, & Winter, 2006) and lower socioeconomic status associated with greater risk (Grittner, Kuntsche, Graham, & Bloomfield, 2012). Children from lower income levels tend to have more aversive environmental conditions and a higher likelihood of experimenting with ethanol (Escarce, 2003; McLoyd, 1998; Richardson, Radziszewska, Dent, & Flay, 1993). Given the mounting evidence for lasting changes to the brain caused by ethanol exposure during adolescence (Ehlers & Criado, 2010; Spear, 2014), it is imperative to understand the mechanisms of vulnerability and behavioral outcomes associated with these changes to develop treatments and inform preventative measures.
Animal models are widely used to advance our understanding of the relationship between adolescent and adult ethanol use. In rats, adolescents tend to voluntarily consume more ethanol than adults (Daoura, Haaker, & Nylander, 2011) and consume more ethanol during adulthood when exposed to ethanol during early adolescence (Alaux-Cantin et al., 2013; Spear, 2014). In addition, animals reared in a post-weaning social isolation environment (isolation condition; IC), are more vulnerable to developing addiction-like behaviors in adulthood (Bardo, Neisewander, & Kelly, 2013; Butler, Ariwodola, & Weiner, 2014; Karkhanis, Locke, McCool, Weiner, & Jones, 2014; Stairs & Bardo, 2009; Whitaker, Degoulet, & Morikawa, 2013). Despite a common thread during adolescence, little work has explored the role of these two factors concurrently, though both adolescent alcohol and post-weaning social isolation increase ethanol consumption later in life.
Many forms of isolation procedures and conditions exist in the literature. The current study uses post-weaning social isolation that requires the isolated rats to be singly housed in small, hanging cages with wire-mesh fronts and bottoms for 30 days starting on postnatal day (PND) 21. Rearing in post-weaning social isolation has many lasting effects including deficits in prepulse inhibition of acoustic startle response, poorer novel object discrimination, and decreased mPFC volume (for a review, see: Fone & Porkess, 2008). Indeed, the effects of rearing in the isolation condition depend not only on the lack of conspecific contact, but also on the environmental conditions during the rearing period. For example, isolation in cages with wire mesh floors increases basal corticosterone levels compared to cages with sawdust bedding (Heidbreder et al., 2000), but over-handling can completely mitigate the effect (Holson, Scallet, Ali, & Turner, 1991). Thus, the current study employed procedures consistent with the postweaning social isolation model (limited handling, wire cages, etc.), to ensure long-term neural and behavioral changes (Fone & Porkess, 2008).
Rat strain may be another important factor which can alter outcomes of post-weaning social isolation (Lukkes, Watt, Lowry, & Forster, 2009). Limited literature exists that formally compares post-weaning social isolation effects between rat strains. However, after post-weaning social isolation Lister Hooded rats exhibit hyperlocomotion in an open-field test – an effect which is inconsistent in Sprague-Dawley rats (Garcia, Haddon, Saucier, & Cain, 2017; Green, Cain, Thompson, & Bardo, 2003; Leng, Feldon, & Ferger, 2004; Lukkes, Mokin, Scholl, & Forster, 2009; Lukkes, Vuong, Scholl, Oliver, & Forster, 2009; Weiss, Pryce, Jongen-Rêlo, Nanz-Bahr, & Feldon, 2004). Thus, the current study used Long-Evans rats; a strain which tends to voluntarily consume alcohol under intermittent access conditions (Simms et al., 2008; Simms, Bito-Onon, Chatterjee, & Bartlett, 2010) and tends to achieve higher blood ethanol concentrations (BECs) when drinking similar amounts of ethanol compared to other outbred strains (Carnicella, Ron, & Barak, 2014). In addition, the use of Long-Evans rats allows for some comparison to similar work using other strains (e.g. Lesscher et al., 2015).
Given the various factors that may contribute to neural and behavioral changes associated with the isolation conditions, many types of comparison groups are also used with post-weaning isolation (Fone & Porkess, 2008). The standard condition (SC) comparison group used in the current report complies with the standard housing conditions defined in the National Institute of Health (NIH) guidelines (National Research Council, 2011). The standard condition does not account for all the neural or behavioral changes that accompany post-weaning social isolation. However, the standard condition does give a comparison to a known and widely-used laboratory environment that is used in the literature as a comparison group for post-weaning social isolation conditions in several cases.
Different rearing environments alter the response of rats to a variety of drugs of abuse (Bardo et al., 2013; Bardo & Dwoskin, 2004; Stairs & Bardo, 2009). However, there is a limited literature examining the effect of post-weaning isolation conditions on operant responding for ethanol. What has been found is that ICs respond for ethanol at higher rates (Deehan, Cain, & Kiefer, 2007; McCool & Chappell, 2009), consume more (Deehan, Palmatier, Cain, & Kiefer, 2011; McCool & Chappell, 2009), and have higher breakpoints (Deehan et al., 2011) compared to SCs. In addition, a greater proportion of ICs than SCs switch levers when ethanol becomes contingent on responding on a different lever (Deehan et al., 2007). All these studies used sucrose fading procedures to encourage drinking. The fading procedures are a potential confound (Augier et al., 2014; Simms et al., 2010) because both sucrose and saccharin are highly rewarding and overlap in neural activity patterns with drugs of abuse (Westwater, Fletcher, & Ziauddeen, 2016).
Isolation rearing also alters responding for voluntary access to ethanol, but there is some inconsistency within the literature. The majority of experiments report higher consumption and/or preference for ethanol by isolated rats compared to group (typically 4 animals per cage) or pair-housed animals (e.g. Deehan et al., 2007; Lesscher et al., 2015; Schenk, Gorman, & Amit, 1990; Skelly, Chappell, Carter, & Weiner, 2015), while a small number report no differences (Kazmaier, Butcher, Senter, & Stutz, 1973; Rockman & Gibson, 1992). The discrepancy is likely due to methodological differences including age of isolation initiation, age of consumption testing initiation, length of isolation, among others (for a review see: Cortés-Patiño & GarciaMijares, 2016). In general, isolation initiated during adolescence increases ethanol consumption during adulthood in a voluntary choice procedure and increases preference for more highly concentrated ethanol solutions than group-housed controls (Schenk et al., 1990). However, in IC rats that remain in isolation for the experiment duration, the characteristics of binge-like drinking and mechanisms of increased ethanol consumption have not been determined.
Additionally, both chronic and acute exercise are known to increase ethanol metabolism (Ardies, Morris, Erickson, & Farrar, 1989). Since SC rats have greater access to social play and greater amount of space for exercise, one might assume that they likely get more exercise than IC rats and, therefore, metabolize ethanol at a higher rate. Therefore, given the lack of literature exploring the effect of environment on ethanol metabolism in the rat, we measured BEC of SC and IC rats injected with ethanol.
Overall, previous findings align with other reports of increased reward and reward-related stimuli sensitivity among ICs (Beckmann & Bardo, 2012; Jones, Marsden, & Robbins, 1990; Kirkpatrick, Marshall, Clarke, & Cain, 2013; Rose, Love, & Dell, 1986; Stairs, Klein, & Bardo, 2006). To determine if ICs and SCs differ in the development of binge ethanol drinking during adolescence, Experiment 1 used a voluntary intermittent ethanol access paradigm over 4 weeks (Simms et al., 2008). As ICs have higher vulnerability to addiction (Karkhanis et al., 2014; Whitaker et al., 2013), it was hypothesized that ICs would drink more than SCs, and all rats would escalate their ethanol consumption with ICs escalating more rapidly than SCs. Experiment 2 had several objectives. To extend the results from Experiment 1 and observe the effects of a longer access period on binge consumption (Lesscher et al., 2015), the voluntary access period was increased to 6 weeks. Subsequent operant responding for ethanol among differentially reared animals was then measured. Given that ICs exhibit more operant responding for ethanol in the absence of prior ethanol exposure compared to SCs (Deehan et al., 2007; Deehan et al., 2011; McCool & Chappell, 2009), it was predicted that ICs would have higher amounts of lever pressing compared to SCs throughout acquisition and extinction. To determine whether differences in ethanol metabolism exist between IC and SC rats, Experiment 3 quantified BEC after an ethanol injection. Since SCs likely get more exercise than ICs and exercise increases ethanol metabolism (Ardies et al., 1989), it was hypothesized that SCs would have lower BECs than ICs.
Materials and Methods
Experiment 1
Animals and Rearing Conditions.
Thirty-one male Long-Evans rats arrived in the laboratory at postnatal day (PND) 21 from Charles River Laboratories. Rats were assigned randomly to one of two environmental conditions; either isolated (IC; n=15) or standard (SC; n=16) condition, where they lived for the duration of the study. ICs were housed alone in hanging metal cages (17×24×20cm) with steel sides and a wire mesh front and bottom. Previous literature indicates that handling during the isolation period can interfere with the isolation effect (Pritchard, Van Kempen, & Zimmerberg, 2013). ICs received no handling during the 1-week acclimation period and minimal handling for weighing once weekly during intermittent access to ethanol. IC rats did not have their cages changed between PND 21 and 51 to minimize handling. SCs, which served as a laboratory standard comparison group (National Research Council, 2011), were pair-housed in plastic shoebox cages (20×43× 20cm) with wire tops and bedding. SCs were handled weekly during both weighing and weekly cage and bedding changes. All rats were housed under a reversed 12-hour dark:light schedule at a temperature of 21 ±1°C and humidity between 30 and 50% along with ad libitum food and water access for the entire experiment. A dim red lamp was left on during the dark cycle. This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (2011) and was approved by the Institutional Animal Care and Use Committee of Kansas State University.
4-Week Intermittent Adolescent Access to Ethanol.
Intermittent adolescent access to 20% ethanol solution (v/v) in a 2-bottle choice paradigm (IAAE) occurred in the home cage consistent with methodology found in Simms et al. (2008). IAAE began on PND 27 with ethanol randomly placed on the left or right of the cage for each exposure period. Ethanol-exposed rats (n= 7 ICs; 8 SCs) were given concurrent access to water and a 20% ethanol solution every other day, 3 days a week for 4 weeks. On intervening days, both bottles contained only water. Bottles were weighed daily after 24 hours and consumption was recorded for both bottles. To enable comparisons across environmental groups, SC ethanol consumption was estimated by assigning half the total volume consumed in both bottles to each rat. Additionally, a group of water-only controls (n= 8 ICs; 8 SCs) received two bottles of water every day and were otherwise treated identically.
Experiment 2
Animals and Rearing Conditions.
Thirty-eight male Long-Evans rats arrived in the laboratory at PND 21 from Charles River Laboratories. Environmental conditions and condition assignment were identical to those described in Experiment 1 (IC: n=18; SC: n=20).
6-Week Intermittent Adolescent Access to Ethanol.
IAAE procedures were identical to Experiment 1 except that the duration of the procedure lasted 6 weeks. The day before initiation of IAAE, side bias was assessed based on consumption of water from two bottles (PND 26). Subsequently, the ethanol bottle was always placed on the side opposite the bias determined by the preliminary test. Additionally, a group of water-only controls (n= 3 ICs; 4 SCs) received two bottles of water every day and were otherwise treated identically.
Operant Chambers.
Rats were trained to lever press for ethanol in operant chambers (ENV-001, Med Associates, St. Albans, VT) that were operated via computer interface (MedPC; Med Associates) and enclosed within ventilated sound-attenuating compartments. Two metal retractable levers were positioned 7.3 cm above the metal grid floor on either side of the magazine. Centered above each lever was a 28-V, white cue-light 3cm in diameter. The magazine was located between the two levers 2.5 cm from the floor and outfitted with a photobeam for sensing head entries. The magazine contained a dipper cue-light (1 watt). Ethanol (20%) was delivered to the bottom of the magazine via a dipper cup (0.1ml).
Lever Press Training.
After six weeks of ethanol exposure and a 2-day rest period, all rats in the ethanol condition began operant training. One hour prior to training sessions, home cage water was removed to facilitate training. All rats were first given one 30-min session to acclimate to the operant chambers. Non-contingent, 8-sec presentations of ethanol and the dipper cue-light occurred on an increasing interval schedule until 20 presentations of the dipper cup had occurred. During the following session, rats received magazine training and the active lever was present. Responding on the active lever resulted in presentation of the dipper with ethanol and dipper cue-light for 8 sec. In the subsequent 5 training days, only the active lever was present and rats were shaped to respond on the active lever for access to ethanol on a fixed ratio 1 (FR1) schedule. In this phase, active lever responses resulted in 4 seconds of access to ethanol paired concurrently with the dipper cue-light. The house light remained on for all sessions. Criteria for completed training was a group mean of 30 or more active lever presses over the last 3 sessions for each environmental condition.
Oral Self-Administration of Ethanol.
After the training phase, rats were given unrestricted access to water in the home cage prior to operant sessions for the remainder of the experiment. Following acquisition of lever press behavior, all rats previously exposed to ethanol responded for ethanol (water only controls responded for water) on an FR1 schedule for 12 daily, 30-min sessions during which both levers (active and inactive) were presented. Responses on the active lever resulted in 4 seconds of access to ethanol or water. Dipper presentations were paired with illumination of both a cue-light and the dipper cue-light. Active lever presses occurring during dipper presentations were considered time out responses and did not result in extending the dipper presentation time. Inactive lever responses were recorded but had no programmed consequence. Active and inactive lever responses, and time out responding were measured during each FR1 session. Stability was defined as less than a 20% difference between the last 3 sessions of active lever responding for each group. Two rats (both SCs) failed to acquire operant ethanol (< 10 active lever responses for each of the first 5 FR1 sessions) and were removed from all analyses.
Extinction.
After stable responding for ethanol was attained, extinction sessions commenced. During the extinction sessions, conditions were identical to those present during FR1 sessions except that responses on the active lever had no programmed consequence. The criterion for extinction was at least 70% reduction in active lever responding compared to the average of the last 3 days of active lever responding on the FR1 schedule for each group.
Experiment 3
Animals and Rearing Conditions.
Twelve male Long-Evans rats arrived in the laboratory at PND 21 from Charles River Laboratories. Environmental conditions were identical to those in Experiments 1 and 2 (IC: n=6; SC: n=6). All rats received 2 intraperitoneal (i.p.) injections of 20% ethanol (w/v) in 0.9% saline separated by a week. The first dose was 1.5 g/kg on PND 52 and the second dose was 3 g/kg on PND 58.
Blood Collection and BEC Determination.
Blood samples (36 μL) were pipetted from the saphenous vein at 2 hours post injection directly into heparinized (4 μL) aliquots on ice. The samples were centrifuged for 10 minutes at 13,500 × g at 4°C. BEC was measured from plasma with the AM1 Analyzer (Analox Instruments, London, UK). One SC and one IC rat were excluded from the analysis at the 1.5 g/kg dose due to a high probability of a missed injection indicated by impossibly low BECs (below 12 mg/dL). One SC rat died prior to the second injection.
Data Analysis
All statistical analyses were performed using JMP Pro 12.1 (SAS, Cary, NC). All data were analyzed using factorial or mixed factorial ANOVAs using REML. The alpha-level was set at .05 for all analyses.
During the IAAE phase in Experiments 1 and 2, the rats were only weighed weekly to minimize handling of the IC rats. Therefore, to calculate the ethanol dose per day, the weight difference after each week was divided by seven and distributed cumulatively to each day. To enable comparisons across environmental groups, individual SC ethanol consumption was calculated as half the total volume consumed in both bottles for each rat. To account for fluid loss from bottle drips, 3 grams were subtracted from each consumption measure. Consumption of ethanol was calculated based on the number of grams of ethanol per gram of 20% ethanol solution consumed. Percent preference for ethanol was defined as the number of grams of 20% ethanol solution consumed divided by total grams of fluid consumed from both bottles on a given day, multiplied by 100.
Consumption (g/kg) and preference data were analyzed separately using a series of mixed factorial ANOVAs with Environment (IC/SC) and Exposure (ethanol access/water only control) as the between-subjects variables along with and Day as the within-subjects variable. Planned orthogonal contrasts were used to probe interactions. To determine escalation of consumption and preference across the IAAE phase, non-orthogonal contrasts comparing the first and last weeks per environmental group were also performed.
During the operant responding phase in Experiment 2, active, inactive, and time out responding data were each analyzed separately using either factorial or mixed factorial ANOVAs according to the operant schedule (FR1 or extinction). We performed a series of mixed factorial ANOVAs with Environment as the between-subjects variable and Session as the within-subjects variable. Planned orthogonal contrasts were used to probe interactions.
Blood ethanol concentration (mg/dL) data from Experiment 3 were analyzed using a mixed factorial ANOVA with Environment (IC/SC) as the between-subjects variable and Dose (1.5 or 3.0 g/kg i.p. ethanol) as the within-subjects variable.
Results
Experiment 1
4-Week Ethanol Consumption.
Ethanol consumed was measured in terms of grams of ethanol per kilogram body weight (no significant differences in bodyweight existed between groups across the studies). To determine if differences in ethanol consumption existed between environmental groups across the 4-week exposure period, a mixed factorial ANOVA was performed. The main effects of Day, F(11,142)=5.10, p<.001, and Environment, F(1,13)=6.68, p=.023, were significant along with their interaction, F(11,142)=3.83, p<.001. Planned contrasts revealed that ethanol consumption was significantly higher in ICs compared to SCs on exposure Days 1, 12, 15, 24, and 26, Fs(1,41)=4.82–17.78, all ps<.05 (Figure 1A). To determine whether ethanol intake escalated within each environmental group, planned contrasts between Week 1 and Week 4 for ICs and SCs were also performed. There was a significant increase in voluntary ethanol consumption for both groups from the beginning to end of the 4-week exposure: ICs, F(1,142)=15.53; SCs, F(1,142)=9.61, all ps<.01. Together with the contrasts between IC and SCs for each day, these results suggest ICs consume more ethanol especially during the latter half of the 4-week access period while both IC and SCs ethanol consumption escalated over time.
Figure 1.
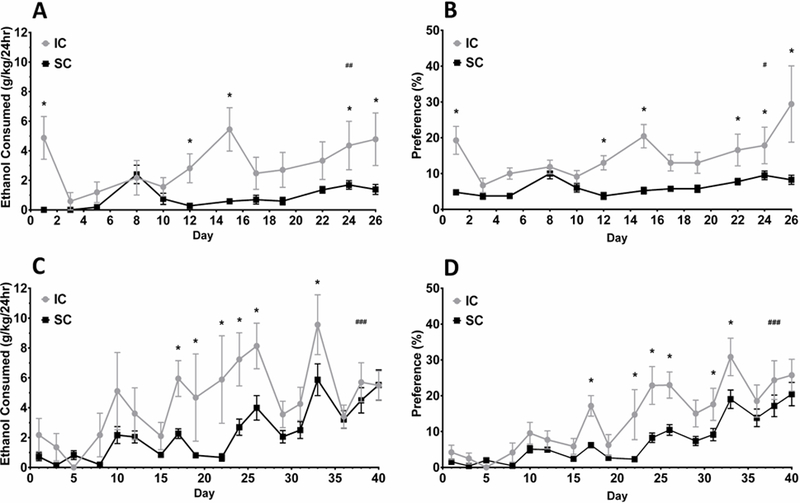
Mean ethanol consumption and preference (± S.E.M.) during intermittent adolescent access to ethanol. Adolescent rats rearing in either an IC (●) or SC (■) received 2 bottles each day in their home cages, with either a 20% ethanol solution (v/v) & a water bottle (M, W, F) or 2 bottles of water (T, R, S, U). (A) Mean ethanol consumption (g/kg) and (B) preference over 4 weeks in Experiment 1. (C) Mean ethanol consumption (g/kg) and (D) preference over 6 weeks in Experiment 2. In general, a pattern of escalating ethanol consumption and preference was observed with differences between IC and SCs greatest during the 3rd and 4th weeks. *p < .05 for IC versus SC for the same day. First to last week comparisons for each group: #p < .05 for ICs and SCs; ##p < .01 for ICs and SCs; ###p < .001 for IC and SCs.
To determine whether the environmental groups differed in preference for ethanol over time, a mixed factorial ANOVA was performed. The main effects of Day, F(11,143)=4.16, p<.001, and Environment, F(1,13)=15.43, p=.002, were significant along with their interaction, F(11,143)=2.5, p<.007. Planned contrasts showed ethanol preference was significantly higher in ICs compared to SCs on ethanol exposure Days 1, 12, 15, 22, 24, and 26, Fs(1,96)=4.15–26.65, all ps<.05 (Figure 1B). To determine whether each group escalated in preference for ethanol across the experiment, planned contrasts between Week 1 and Week 4 for ICs and SCs were also performed and both groups had a significant increase in ethanol preference from the beginning to end of the 4-week exposure period: ICs, F(1,143)=18.89; SCs, F(1,143)=4.88, all ps<.05. Similar to ethanol consumption, along with the contrasts between IC and SCs for each day, these results suggest IC rats had higher ethanol preference during the latter half of the 4-week access period while preference for ethanol escalated over time for both IC and SCs.
4-Week Water Consumption.
To assess whether the environmental or ethanol exposure conditions produced differences in water consumption (g/kg), a mixed factorial ANOVA on the water-only days was performed. The main effects of Day, F(11,297)=24.06, p<.001, and Environment, F(1,27)=27.30, p<.001, were significant along with their interaction, F(11,143)=2.5, p<.001. While the main effect of Exposure was not significant, the interaction between Day and Exposure, F(11,297)=2.22, p=.013, was significant. To determine whether each group decreased water consumption across the experiment, planned contrasts between the water only days’ data for Week 1 and Week 4 were performed for each group. All groups showed a significant decrease in water consumption from the first to last week, Fs(1,297)=18.06–95.94, all ps<.001 (Figure 2A).
Figure 2.
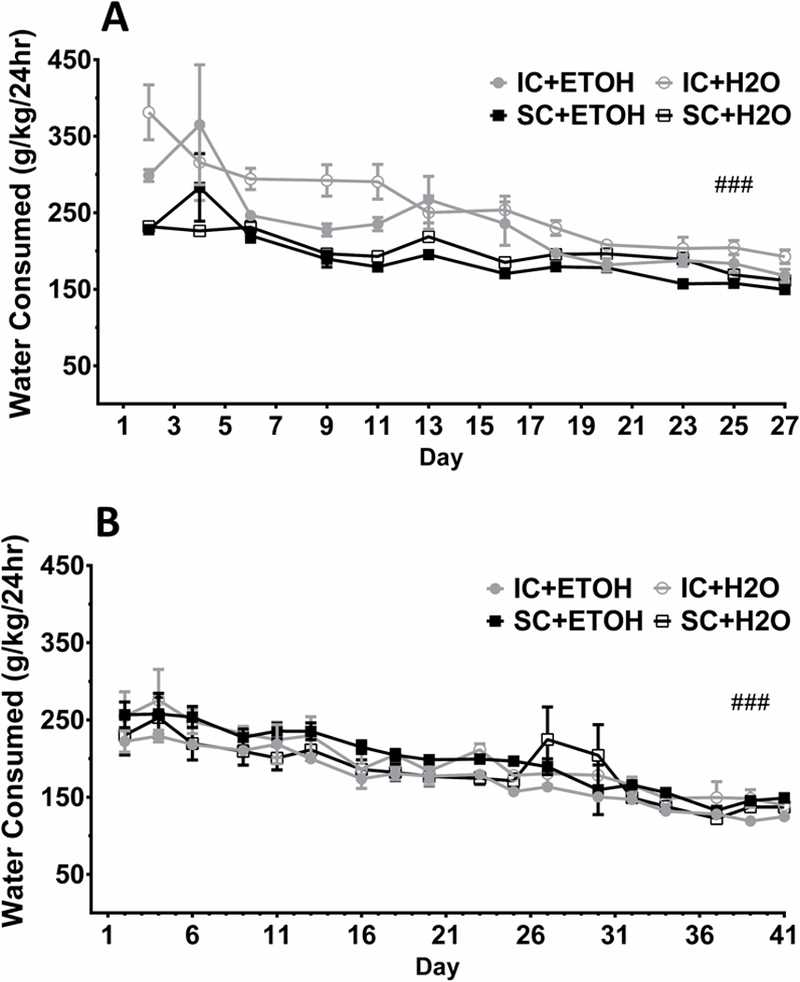
Mean water consumption (± S.E.M.) on water only days during (A) 4 weeks and (B) 6 weeks of access. IC and SC ethanol animals (IC+ETOH [●] and SC+ETOH [■]) were those who had access to 20% ethanol (v/v) on ethanol days while IC and SC water animals (IC+H2O [○] and SC+H2O [□]) never had access to ethanol during IAAE. Overall, all groups saw a significant decrease in water consumption over both 4 and 6 weeks. ###p < .001 for all groups between the first and last weeks for the water only days of access.
Experiment 2
6-Week Ethanol Consumption.
A mixed factorial ANOVA was performed on ethanol consumption across the 6-week access period. The main effect of Day, F(17,456)=8.66, p<.001 was significant. The main effect of Environment was marginally significant, F(1,27)=3.55, p=.070 along with the interaction of Day and Environment, F(17,456)=1.55, p=.075. Although the interaction was not significant, the contrasts between groups for each day may still be performed due the a priori nature of the contrasts (Abelson & Prentice, 1997; Myers & Well, 1995; Rosenthal & Rosnow, 1985). Planned contrasts showed ethanol consumption was significantly higher in IC rats compared to SC rats on ethanol exposure Days 17, 19, 22, 24, 26, and 33, Fs(1,126)=4.50–8.08, all ps<.05. These results suggest environment influenced consumption during the middle portion of the 6-week exposure period. To determine whether ethanol intake escalated within each environmental group, planned contrasts between Week 1 and Week 6 for ICs and SCs were also performed. There was a significant increase in voluntary ethanol consumption in both the IC and the SC group from the beginning to end of the 6-week exposure: ICs, F(1,456)=23.89; SCs, F(1,456)=24.11, all ps<.001 (Figure 1C). These comparisons suggest both groups increased their ethanol consumption over the 6-week exposure period.
To determine whether the environmental groups differed in ethanol preference over the 6week exposure period, a mixed factorial ANOVA was performed. The main effects of Day, F(17,456)=24.37, p<.001, and Environment, F(1,27)=4.79, p=.037, were significant along with their interaction, F(17,456)=2.08, p=.007. Planned contrasts showed ethanol preference was significantly higher in ICs compared to SCs on Days 17, 22, 24, 26, 31, and 33, Fs(1,105)=4.0511.80, all ps<.05 (Figure 1D). Similar to the 6-week consumption results above, these results suggest differences in preference between the two groups occurred mostly during the middle portion of the exposure period. Planned contrasts between Week 1 and Week 6 for ICs and SCs indicated whether groups escalated preference during the access period. Both groups significantly increased ethanol preference from the beginning to end of the 6-week exposure period: ICs, F(1, 456)=139.39; SCs, F(1, 456)=76.52, all ps<.001. These results suggest that both ICs’ and SCs’ preference for ethanol increased across the experiment.
6-Week Water Consumption.
Over the 6-week period, an overall decrease in water consumption was found, but was not influenced by environment or ethanol exposure. A 3-way mixed factorial ANOVA revealed only a main effect of Day, F(17,541)=25.14, p<.001. To determine whether each group decreased water consumption across the experiment, planned contrasts between the water only days’ data from Week 1 and Week 6 were performed for each group. All groups showed a significant decrease in water consumption from the first to last week, Fs(1,541)=47.29–219.99, all ps<.001 (Figure 2B).
FR1 Operant Responding.
Analysis of active lever responding revealed only a significant main effect of Session, F(11,297)=4.66, p<.001 (Figure 3A). Thus, environmental condition did not alter active lever responding for ethanol during the FR1 sessions. Similar results were found in the analysis of inactive lever responding: there was only a significant main effect of Session, F(11,297)=6.04, p<.001 (Figure 3A).
Figure 3.
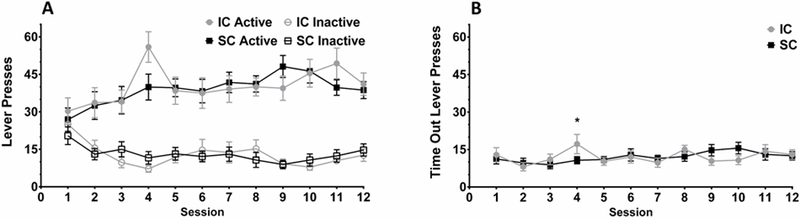
Fixed ratio 1 (FR1) operant ethanol self-administration in adult rats that underwent 6 weeks of intermittent access as adolescents. (A) Mean active, inactive, and (B) time out lever presses (± S.E.M.). Rats that underwent rearing in either an IC (●) or SC (■) concurrent with 6 weeks of IAAE were trained to lever press for 0.1ml presentations of 20% ethanol (v/v) with paired cues on a FR1 for daily 30 min sessions. Results suggest no persistent differences between environmental groups in FR1 responding. *p < .05 for IC versus SC for the same session.
Responding during the time out period was analyzed with a mixed factorial ANOVA and this revealed a significant main effect of Session, F(11,297)=2.03, p=.026, along with a significant interaction between Session and Environment, F(11,297)=1.85, p=.045. A set of planned contrasts was performed to probe the interaction. Only Session 4 showed a significant difference between the environmental conditions, F(1,113)=4.69, p=.034 (Figure 3B), thus suggesting no persisting differences between ICs and SCs on withholding responding during the time out periods.
Extinction.
Responding on the formerly active lever during extinction was analyzed with a mixed factorial ANOVA. This analysis revealed a significant main effect of Session, F(18,486)=25.81, p<.001, and Environment, F(1,27)=8.37, p=.007 (Figure 4). A set of planned contrasts was performed to examine differences between ICs and SCs for each session. ICs responded significantly less on the previously active lever than SCs during Sessions 3, 5, 6, 8 and 15, Fs(1,213)=4.33–10.99, all ps<.05. These results suggest that, whereas all rats extinguished, SCs responded more than ICs primarily during the extinction sessions early in training.
Figure 4.

Mean responding during extinction training (± S.E.M) in adult rats that underwent 6 weeks of ethanol access. During extinction, responses on the previously active lever did not result in presentation of ethanol or cues and responses on the formerly inactive lever also had no programmed consequence. SC rats had more responding on the previously active lever early in training when compared to IC rats. *p < .05 for formerly active lever presses of IC versus SC. #p < .05 for inactive lever presses of IC vs SC.
A mixed factorial ANOVA was used to analyze responding on the inactive lever during extinction. Significant main effects of Session, F(18,486)=4.84, p<.001, and Environment, F(1,27)=4.31, p=.047 (Figure 4) were found. Planned contrasts revealed that ICs responded significantly less on the inactive active lever than SCs during Sessions 1, 2, 6, and 8, Fs(1,84)=4.73–5.70, all ps<.05. Thus, ICs responded on the inactive lever significantly less than SCs primarily during the extinction sessions early in training.
Experiment 3
Blood ethanol concentrations were analyzed with a mixed factorial ANOVA. There was a significant main effect of Dose, F(1,17)=1153.30, p<0.001, with the 3.0 g/kg dose achieving higher BECs than the 1.5g/kg dose. These results indicate that IC and SC rats metabolize ethanol similarly (Figure 5).
Figure 5.
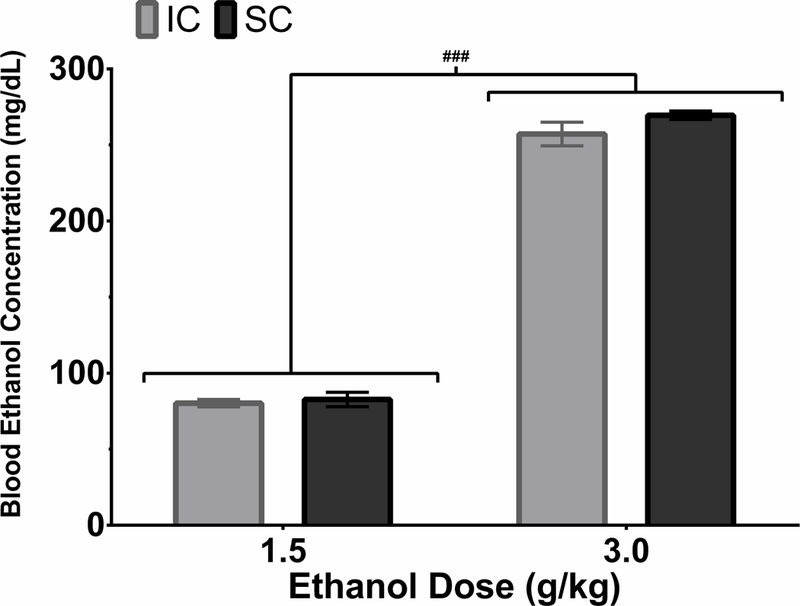
Mean blood ethanol concentrations (BECs; ± S.E.M.) in IC (light grey) and SC (dark grey) rats which reared from PND 21–51 and then received 1.5 g/kg of 20% ethanol (w/v) i.p. followed 6 days later by 3.0 g/kg of 20% ethanol i.p. Blood samples were collected 2 hours after each injection. The 3.0 g/kg dose induced higher BECs compared to the 1.5 g/kg dose. IC and SC rats had similar BECs at both doses. ###p < .001 between the two doses.
Discussion
The current study characterized voluntary intermittent adolescent access to ethanol and subsequent operant responding for ethanol during adulthood in differentially reared rats. Overall, there were higher levels of ethanol preference and consumption by IC rats compared to SC rats during the third and fourth weeks of access while consumption was escalating. Interestingly, SCs and ICs did not differ in their rates of operant responding for ethanol but SCs responded more than ICs during extinction. These effects were not driven by differences in ethanol metabolism as our results observed similar BECs between IC and SC rats across two ethanol doses.
Consumption
It was predicted that ICs would drink more and more rapidly escalate their ethanol consumption across each period of exposure compared to SCs. The current study suggests that environment influences consumption of 20% ethanol in adolescents most strongly during Weeks 3 and 4 of IAAE with ICs consuming more than SCs. After 4 weeks, differences in ethanol consumption diminish between ICs and SCs. In Experiment 2, with longer IAAE, SCs increased their consumption to levels similar to the ICs. Adults and adolescents tend to reach relatively stable ethanol consumption after 3–4 weeks of intermittent access (Carnicella et al., 2014; Schramm-Sapyta et al., 2014) which may explain the convergence of the groups’ consumptions after 4 weeks and suggests that the effects of isolation are most evident early in intermittent access to ethanol. Lesscher and colleagues (2015) found significantly greater overall consumption among ICs in both 7 and 24-hour intermittent ethanol exposures over 6 weeks in adults; however, the isolation procedures differed considerably from the current experiments. This suggests that, while post-weaning social isolation alters the escalation of ethanol consumption, several factors, including access to social play in adolescence (Lesscher et al., 2015) and age of drinking initiation, may influence the effect.
It is important to note that consumption was divided equally between the two SC cagemates drinking from a given bottle, which artificially reduces variability thereby increasing probability of a Type I error (Croon & van Veldhoven, 2007). Every time any variable is averaged into smaller increments or continuous variables are broken into categories before analysis, Type I error inflation occurs. Yet it is commonplace in the literature to see lower level data (e.g. amount drunk over time) aggregated into a higher level (e.g. “high drinker”) when there was a choice to do so (Young, 2016). This limitation was accepted from the outset of the study in order to allow us to examine the effects of different housing conditions during adolescence on voluntary ethanol consumption. As evident in our results, ICs’ ethanol consumption was consistently more than double that of the SCs’ average consumption in the first 4 weeks of intermittent exposure in experiments 1 and 2. The most extreme differences between ICs and SCs occurred during weeks 3 and 4 and these differences suggest that isolation increased ethanol consumption above and beyond potential artifacts created by reduced variability in the SC group during that 2 week period.
The current study also found a general pattern of increasing consumption that is atypical of adolescents and more like that of adults. Studies using the same access methods in adults found initially low levels of drinking early on and gradual escalation to more than 4 g/kg/24hr by around 3–4 weeks (Carnicella et al., 2014). There was also initially high drinking in the ICs in Experiment 1, but not in the SCs. Interestingly this finding seems to be inconsistent, as it did not replicate in Experiment 2 and it varies in previous studies using intermittent access in adolescents. For example, a few studies using IAAE methods similar to the present study found initially high levels of drinking early on and either a general decrease in consumption across adolescence (Fisher, Bright, Gallo, Pajser, & Pickens, 2017; Schramm-Sapyta et al., 2014), or a relatively flat pattern of consumption overall with high drinkers escalating in late adolescence and moderate drinkers decreasing monotonically (DiLeo, Wright, Mangone, & McDannald, 2015; DiLeo, Wright, & McDannald, 2016).
SCs’ average consumption did not reach levels typically seen in adolescents or adults at 4 weeks in either experiment. In previous literature, rats were typically caged individually during IAAE and this could also account for the lack of typical drinking levels seen at 4 weeks (Carnicella et al., 2014; Lesscher et al., 2015; Simms et al., 2008). Even with methods that use wire mesh to divide the home cage between rats during drinking (Broadwater, Varlinskaya, & Spear, 2011) there are issues with maintaining physical contact essential to rat social behavior during adolescence that arise. Importantly, the SCs in the current study escalated to levels typically produced by intermittent access to ethanol by 6 weeks (Carnicella et al., 2014; DiLeo et al., 2015; DiLeo et al., 2016; Schramm-Sapyta et al., 2014; but see: Fisher et al., 2017), suggesting that social contact during the first four weeks slowed escalation. While all groups decreased water consumption (g/kg) over time, in Experiment 1 IC rats consumed more water on water only days. This effect did not replicate in Experiment 2; thus, we do not think this is a consistent finding. We observed similar patterns of ethanol consumption across the two experiments and therefore the pattern of water consumption does not appear to alter ethanol consumption.
Due to the pair housing of the SC rats in the current experiments, repeated BEC measurements after voluntary consumption could be used to more accurately measure the amount of ETOH consumed per rat. However, we could underestimate peak values considering that rats who have not yet consumed at the time blood samples are taken would also be included in the mean (Spoelder, Tsutsui, Lesscher, Vanderschuren, & Clark, 2015). Further, the BEC measurements would have to be repeated several times and the rats observed because it would be difficult to distinguish one SC not drinking and one SC being prevented from doing so during the access timeframe due to the other rat drinking before BEC collection. Therefore, in Experiment 3 we injected two different doses of ethanol and then measured BEC. While this does not measure BEC during voluntary consumption, it does tightly control the ethanol dose and timing across environmental conditions to ensure the reported results accurately reflect ethanol metabolism. One would expect SCs to have a lower BEC because they likely get more exercise (Ardies et al., 1989), as was hypothesized; however, the results of the Experiment 3 indicate that IC and SC rats’ BECs do not differ from each other after 2 hours at either dose. While metabolic rate was not directly measured, it can be inferred that differences in rate would cause a difference in BEC after 2 hours of metabolism. Thus, IC and SC rats metabolize ethanol at a similar rate and should garner similar levels of intoxication effect and duration for equal amounts of ethanol consumed.
Operant Responding
Originally it was hypothesized that ICs would respond for ethanol and associated cues more than SCs based on previous literature (Deehan et al., 2007; Deehan et al., 2011; McCool & Chappell, 2009). FR1 responding for ethanol, however, was very similar between ICs and SCs. Interestingly, ICs had lower responding than SCs in the early sessions of extinction. Both findings are inconsistent with operant and extinction response patterns typically seen in ICs that responded for other substances (Gluck & Pearce, 1977; Jones, Marsden, & Robbins, 1991; Stairs et al., 2006), indicating an effect that may be unique to ethanol exposure during adolescence among the two environmental groups.
ICs responding for 10% ethanol under an FR1 schedule tends to be higher than SCs among adults selectively bred to prefer ethanol (Deehan et al., 2011) and in Long-Evans rats (Deehan et al., 2007). Deehan and colleagues’ results did not reach significance during 30minute sessions, similar to our results. However, differences did emerge during 60-minute sessions among Long-Evans rats (Deehan et al., 2007). The present findings are more consistent with that of Lesscher and colleagues (2015). However, there are considerable factors that differ from the current experiment such as resocialization of ICs, ethanol consumption initiated in adulthood, 4 rats per cage in the social housing condition, and 3 weeks of initial rearing. It is well established that the number of rats per SC cage, the size of the SC cage, and the amount of handling will alter both the behavioral and neurochemical profiles of SC rats (Renner and Rosenzweig, 1987). Most notably, neither Deehan’s nor Lesscher’s groups had rats that consumed ethanol during the critical neurodevelopmental period of early adolescence. Our current results suggest that after IAAE exposure, rats raised in an SC with two rats per cage are generally similar to IC rats when responding for ethanol on a FR-1 schedule of reinforcement.
There is limited literature combining IAAE and operant responding for ethanol. In adults, intermittent access to ethanol (IAE) for 7 weeks leads to stable ethanol self-administration (Barak, Carnicella, Yowell, & Ron, 2011; Carnicella, Kharazia, Jeanblanc, Janak, & Ron, 2008; Carnicella, Amamoto, & Ron, 2009). IAE increases the number of rats that acquire operant selfadministration compared to ethanol-naïve rats, but not the overall levels of ethanol selfadministration of those that do acquire. This led to the conclusion that IAE may affect drinking and seeking behaviors differently (Carnicella, Yowell, & Ron, 2011). There were differences between the current study and that of Carnicella et al. (2011) including operant training durations, FR schedule, and criteria for completed training.
Combined, the current findings lead to a few possible conclusions for the lack of differences between environmental groups on FR1 responding. The previous findings among differentially reared groups may be a result of using sucrose fading procedures to facilitate operant consumption or intermittent adolescent access procedures caused consumption to converge thereby eliminating expected group differences in operant responding.
Extinction
Research into extinction among isolated rats has consistently found cognitive rigidity marked by increased responding during extinction (Gluck & Pearce, 1977; Jones et al., 1991; Stairs et al., 2006) and longer time periods to extinguish drug-associated contexts (Whitaker et al., 2013). However, with extinction rates comparable to previous literature that used intermittent access to 20% ethanol before operant ethanol procedures (Haack et al., 2014), either a facilitation in extinction in ICs or an impairment in extinction in SCs was found. The reason for this effect may be the result of adolescent ethanol exposure itself.
Adolescent ethanol exposure produces lasting, dose-dependent changes in the activity of several brain regions involved in extinction and reinstatement of drug-seeking in adulthood including the nucleus accumbens and prefrontal cortex (Liu & Crews, 2015). In addition, impairment of extinction of ethanol self-administration occurs in adults intermittently exposed to ethanol in vapor chambers for 2 weeks during adolescence (Gass et al., 2014). Conversely, a recent study found that higher consumption during IAAE is correlated with lower amounts of commission errors in a go/no-go reversal learning task and faster extinction learning (Fisher et al., 2017). ICs in the current study consumed more during mid-adolescence and, consistent with Fisher and colleagues (2017), showed less responding during the early trials of extinction.
Together, our results suggest that adolescents reach levels of ethanol intake comparable to that of previous studies of IAE among adults. The current study also highlights the importance of the environment during intermittent access to ethanol in adolescence with isolation rearing increasing adolescent ethanol drinking and preference after 3–4 weeks of IAAE. Rearing in postweaning social isolation conditions does not alter ethanol metabolism, suggesting the differences in voluntary consumption are due to differences in motivation or other factors. The current findings also indicate IAAE may be changing typical differences in operant responding patterns between ICs and SCs in adulthood and may alter extinction learning.
Highlights.
Post-weaning social isolation rats voluntarily consumed more 20% ethanol
Environmental group differences in ethanol consumption were greatest at 3–4 weeks
Operant ethanol self-administration was similar between environmental groups
Isolated rats extinguished responding faster than the standard condition rats
Blood ethanol indicates no differences in metabolism of ethanol between groups
Acknowledgements
The authors would like to acknowledge Dr. Charles L Pickens for his help with the alcohol access model and the undergraduate researchers that made the current study possible.
Sources of support: Research reported in this publication was supported by Kansas State University and the Cognitive and Neurobiological Approaches to Plasticity (CNAP) Center of Biomedical Research Excellence (COBRE) of the National Institutes of Health under grant number P20GM113109. The funding source had no direct involvement in the conception, data collection, interpretation, or writing of the current report.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelson RP, & Prentice DA (1997). Contrast tests of interaction hypotheses. Psychological Methods, 2(4), 315–328. Retrieved from http://webcom.upmfgrenoble.fr/LIP/Perso/DMuller/M2R/ACM/articles/abelson_1997_PM.pdf [Google Scholar]
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, et al. (2013). Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology, 67, 521–531. doi: 10.1016/j.neuropharm.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Ardies CM, Morris GS, Erickson CK, & Farrar RP (1989). Both acute and chronic exercise enhance in vivo ethanol clearance in rats. Journal of Applied Physiology, 66(2), 555–560. doi: 10.1152/jappl.1989.66.2.555 [DOI] [PubMed] [Google Scholar]
- Augier E, Flanigan M, Dulman RS, Pincus A, Schank JR, Rice KC, et al. (2014). Wistar rats acquire and maintain self-administration of 20 % ethanol without water deprivation, saccharin/sucrose fading, or extended access training. Psychopharmacology, 231(23), 4561–4568. doi: 10.1007/s00213-014-3605-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S, Carnicella S, Yowell QV, & Ron D (2011). Glial cell line-derived neurotrophic factor reverses alcohol-induced allostasis of the mesolimbic dopaminergic system: Implications for alcohol reward and seeking. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(27), 9885–9894. doi: 10.1523/JNEUROSCI.1750-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, & Kelly TH (2013). Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacological Reviews, 65(1), 255–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, & Dwoskin LP (2004). Biological connection between novelty- and drugseeking motivational systems. Nebraska Symposium on Motivation, 50, 127–158. [PubMed] [Google Scholar]
- Beckmann JS, & Bardo MT (2012). Environmental enrichment reduces attribution of incentive salience to a food-associated stimulus. Behavioural Brain Research, 226(1), 331–334. doi: 10.1016/j.bbr.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomo YA, Bowes G, Coffey C, Carlin JB, & Patton GC (2004). Teenage drinking and the onset of alcohol dependence: A cohort study over seven years. Addiction, 99(12), 1520–1528. doi: 10.1111/j.1360-0443.2004.00846.x [DOI] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, & Spear LP (2011). Chronic intermittent ethanol exposure in early adolescent and adult male rats: Effects on tolerance, social behavior, and ethanol intake. Alcoholism: Clinical and Experimental Research, 35(8), 1392–1403. doi: 10.1111/j.1530-0277.2011.01474.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Ariwodola OJ, & Weiner JL (2014). The impact of social isolation on HPA axis function, anxiety-like behaviors, and ethanol drinking. Frontiers in Integrative Neuroscience, 7 doi: 10.3389/fnint.2013.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Kharazia V, Jeanblanc J, Janak PH, & Ron D (2008). GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proceedings of the National Academy of Sciences of the United States of America, 105(23), 8114–8119. doi: 10.1073/pnas.0711755105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Amamoto R, & Ron D (2009). Excessive alcohol consumption is blocked by glial cell line–derived neurotrophic factor. Alcohol, 43(1), 35–43. doi: 10.1016/j.alcohol.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, & Barak S (2014). Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol, 48(3), 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Yowell QV, & Ron D (2011). Regulation of operant oral ethanol selfadministration: A dose-response curve study in rats. Alcoholism, Clinical and Experimental Research, 35(1), 116–125. doi: 10.1111/j.1530-0277.2010.01328.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2015). Binge drinking. Retrieved March 19, 2017, from https://www.cdc.gov/alcohol/fact-sheets/binge-drinking.htm
- doi doi: 10.1037/pne0000037. [DOI] [Google Scholar]
- Croon MA, & van Veldhoven MJ (2007). Predicting group-level outcome variables from variables measured at the individual level: A latent variable multilevel model. Psychological Methods, 12(1), 45–57. doi: 10.1037/1082-989X.12.1.45 [DOI] [PubMed] [Google Scholar]
- Daoura L, Haaker J, & Nylander I (2011). Early environmental factors differentially affect voluntary ethanol consumption in adolescent and adult male rats. Alcoholism: Clinical and Experimental Research, 35(3), 506–515. doi: 10.1111/j.1530-0277.2010.01367.x [DOI] [PubMed] [Google Scholar]
- Deehan GA, Cain ME, & Kiefer SW (2007). Differential rearing conditions alter operant responding for ethanol in outbred rats. Alcoholism: Clinical and Experimental Research, 31(10), 1692–1698. doi: 10.1111/j.1530-0277.2007.00466.x [DOI] [PubMed] [Google Scholar]
- Deehan GA, Palmatier MI, Cain ME, & Kiefer SW (2011). Differential rearing conditions and alcohol-preferring rats: Consumption of and operant responding for ethanol. Behavioral Neuroscience, 125(2), 184. [DOI] [PubMed] [Google Scholar]
- DiLeo A, Wright KM, Mangone E, & McDannald MA (2015). Alcohol gains access to appetitive learning through adolescent heavy drinking. Behavioral Neuroscience, 129(4), 371–379. doi: 10.1037/bne0000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLeo A, Wright KM, & McDannald MA (2016). Subsecond fear discrimination in rats: Adult impairment in adolescent heavy alcohol drinkers. Learning & Memory, 23(11), 618–622. doi: 10.1101/lm.043257.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, & Criado JR (2010). Adolescent ethanol exposure: Does it produce long lasting electrophysiological effects? Alcohol, 44(1), 27. doi: 10.1016/j.alcohol.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escarce JJ (2003). Socioeconomic status and the fates of adolescents. Health Services Research, 38(5), 1229–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher H, Bright N, Gallo M, Pajser A, & Pickens CL (2017). Relationship of low doses of alcohol voluntarily consumed during adolescence and early adulthood with subsequent behavioral flexibility. Behavioural Pharmacology, 28(7), 531–544. doi: 10.1097/FBP.0000000000000331 [DOI] [PubMed] [Google Scholar]
- Fone KC, & Porkess MV (2008). Behavioural and neurochemical effects of post-weaning social isolation in rodents—relevance to developmental neuropsychiatric disorders. Neuroscience & Biobehavioral Reviews, 32(6), 1087–1102. [DOI] [PubMed] [Google Scholar]
- Garcia EJ, Haddon TN, Saucier DA, & Cain ME (2017). Differential housing and novelty response: Protection and risk from locomotor sensitization. Pharmacology, Biochemistry and Behavior, 154, 20–30. doi: 10.1016/j.pbb.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Glen WB, McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, et al. (2014). Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 39(11), 2570–2583. doi: 10.1038/npp.2014.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck JP, & Pearce HE (1977). Acquisition and extinction of an operant response in differentially reared rats. Developmental Psychobiology, 10(2), 143–149. doi: 10.1002/dev.420100207 [DOI] [PubMed] [Google Scholar]
- Green TA, Cain ME, Thompson M, & Bardo MT (2003). Environmental enrichment decreases nicotine-induced hyperactivity in rats. Psychopharmacology, 170(3), 235–241. doi: 10.1007/s00213-003-1538-3 [DOI] [PubMed] [Google Scholar]
- Grittner U, Kuntsche S, Graham K, & Bloomfield K (2012). Social inequalities and gender differences in the experience of alcohol-related problems. Alcohol and Alcoholism, 47(5), 597–605. doi: 10.1093/alcalc/ags040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack AK, Sheth C, Schwager AL, Sinclair MS, Tandon S, & Taha SA (2014). Lesions of the lateral habenula increase voluntary ethanol consumption and operant selfadministration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PloS One, 9(4), e92701. doi: 10.1371/journal.pone.0092701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, et al. (2000). Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience, 100(4), 749–768. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, & Winter MR (2006). Age at drinking onset and alcohol dependence: Age at onset, duration, and severity. Archives of Pediatrics & Adolescent Medicine, 160(7), 739–746. doi: 10.1001/archpedi.160.7.739 [DOI] [PubMed] [Google Scholar]
- Holson RR, Scallet AC, Ali SF, & Turner BB (1991). “Isolation stress” revisited: Isolation-rearing effects depend on animal care methods. Physiology & Behavior, 49(6), 1107–1118. [DOI] [PubMed] [Google Scholar]
- Jones GH, Marsden CA, & Robbins TW (1990). Increased sensitivity to amphetamine and reward-related stimuli following social isolation in rats: Possible disruption of dopamine-dependent mechanisms of the nucleus accumbens. Psychopharmacology, 102(3), 364–372. doi: 10.1007/BF02244105 [DOI] [PubMed] [Google Scholar]
- Jones GH, Marsden CA, & Robbins TW (1991). Behavioural rigidity and rule-learning deficits following isolation-rearing in the rat: Neurochemical correlates. Behavioural Brain Research, 43(1), 35–50. doi: 10.1016/S0166-4328(05)80050-6 [DOI] [PubMed] [Google Scholar]
- Karkhanis AN, Locke JL, McCool BA, Weiner JL, & Jones SR (2014). Social isolation rearing increases nucleus accumbens dopamine and norepinephrine responses to acute ethanol in adulthood. Alcoholism: Clinical and Experimental Research, 38(11), 27702779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmaier K, Butcher RE, Senter RJ, & Stutz RM (1973). Rearing conditions and ethanol consumption by rats. Quarterly Journal of Studies on Alcohol, 34(2), 520–524. [PubMed] [Google Scholar]
- Kirkpatrick K, Marshall AT, Clarke J, & Cain ME (2013). Environmental rearing effects on impulsivity and reward sensitivity. Behavioral Neuroscience, 127(5), 712–724. doi:http://dx.doi.org.er.lib.k-state.edu/10.1037/a0034124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng A, Feldon J, & Ferger B (2004). Long-term social isolation and medial prefrontal cortex: Dopaminergic and cholinergic neurotransmission. Pharmacology Biochemistry and Behavior, 77(2), 371–379. doi: 10.1016/j.pbb.2003.11.011 [DOI] [PubMed] [Google Scholar]
- Lesscher HM, Spoelder M, Rotte MD, Janssen MJ, Hesseling P, Lozeman-van’t Klooster JG, et al. (2015). Early social isolation augments alcohol consumption in rats. Behavioural Pharmacology, 26(7 Spec No), 673–680. doi: 10.1097/FBP.0000000000000165 [doi] [DOI] [PubMed] [Google Scholar]
- Liu W, & Crews FT (2015). Adolescent intermittent ethanol exposure enhances ethanol activation of the nucleus accumbens while blunting the prefrontal cortex responses in adult rat. Neuroscience, 293, 92–108. doi: 10.1016/j.neuroscience.2015.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, & Forster GL (2009). Adult rats exposed to earlylife social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Hormones and Behavior, 55(1), 248–256. doi: 10.1016/j.yhbeh.2008.10.014 [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Vuong S, Scholl J, Oliver H, & Forster G (2009). Corticotropin-releasing factor receptor antagonism within the dorsal raphe nucleus reduces social anxiety-like behavior after early-life social isolation. Journal of Neuroscience, 29(32), 9955–9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Watt MJ, Lowry CA, & Forster GL (2009). Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Frontiers in Behavioral Neuroscience, 3, 18. doi: 10.3389/neuro.08.018.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, & Chappell AM (2009). Early social isolation in male long-evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol selfadministration. Alcoholism: Clinical and Experimental Research, 33(2), 273–282. doi: 10.1111/j.1530-0277.2008.00830.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoyd VC (1998). Socioeconomic disadvantage and child development. The American Psychologist, 53(2), 185–204. [DOI] [PubMed] [Google Scholar]
- Myers JL, & Well AD (1995). Research design and statistical analysis [Google Scholar]
- National Research Council. (2011) Guide for the care and use of laboratory animals (8th ed.). Washington (DC): National Academies Press (US) Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK54050/ [Google Scholar]
- Pritchard LM, Van Kempen TA, & Zimmerberg B (2013). Behavioral effects of repeated handling differ in rats reared in social isolation and environmental enrichment. Neuroscience Letters, 536, 47–51. doi: 10.1016/j.neulet.2012.12.048 [DOI] [PubMed] [Google Scholar]
- Richardson JL, Radziszewska B, Dent CW, & Flay BR (1993). Relationship between after-school care of adolescents and substance use, risk taking, depressed mood, and academic achievement. Pediatrics, 92(1), 32–38. [PubMed] [Google Scholar]
- Rockman GE, & Gibson JE (1992). Effects of duration and timing of environmental enrichment on voluntary ethanol intake in rats. Pharmacology, Biochemistry, and Behavior, 41(4), 689–693. [DOI] [PubMed] [Google Scholar]
- Rose FD, Love S, & Dell PA (1986). Differential reinforcement effects in rats reared in enriched and impoverished environments. Physiology & Behavior, 36(6), 1139–1145. doi: 10.1016/0031-9384(86)90491-9 [DOI] [PubMed] [Google Scholar]
- Rosenthal R, & Rosnow RL (1985). Contrast analysis: Focused comparisons in the analysis of variance CUP Archive. [Google Scholar]
- Schenk S, Gorman K, & Amit Z (1990). Age-dependent effects of isolation housing on the self-administration of ethanol in laboratory rats. Alcohol, 7(4), 321–326. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta N, Francis R, MacDonald A, Keistler C, O’Neill L, & Kuhn C (2014). Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology, 231(8), 1831–1839. doi: 10.1007/s00213-013-3319-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Bito-Onon JJ, Chatterjee S, & Bartlett SE (2010). Long-evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 35(7), 1453–1463. doi: 10.1038/npp.2010.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. (2008). Intermittent access to 20% ethanol induces high ethanol consumption in Long–Evans and wistar rats. Alcoholism: Clinical and Experimental Research, 32(10), 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly MJ, Chappell AE, Carter E, & Weiner JL (2015). Adolescent social isolation increases anxiety-like behavior and ethanol intake and impairs fear extinction in adulthood: Possible role of disrupted noradrenergic signaling. Neuropharmacology, 97, 149–159. doi: 10.1016/j.neuropharm.2015.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2014). Adolescents and alcohol: Acute sensitivities, enhanced intake, and later consequences. Neurotoxicology and Teratology, 41, 51–59. doi: 10.1016/j.ntt.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelder M, Tsutsui KT, Lesscher HMB, Vanderschuren L, & Clark JJ (2015). Adolescent alcohol exposure amplifies the incentive value of reward-predictive cues through potentiation of phasic dopamine signaling. Neuropsychopharmacology, 40(13), 2873. doi: 10.1038/npp.2015.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DJ, & Bardo MT (2009). Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacology Biochemistry and Behavior, 92(3), 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DJ, Klein ED, & Bardo MT (2006). Effects of environmental enrichment on extinction and reinstatement of amphetamine self-administration and sucrose-maintained responding. Behavioural Pharmacology, 17(7), 597–604. doi: 10.1097/01.fbp.0000236271.72300.0e [DOI] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Rêlo AL, Nanz-Bahr NI, & Feldon J (2004). Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behavioural Brain Research, 152(2), 279–295. doi: 10.1016/j.bbr.2003.10.015 [DOI] [PubMed] [Google Scholar]
- Westwater ML, Fletcher PC, & Ziauddeen H (2016). Sugar addiction: The state of the science. European Journal of Nutrition, 55(Suppl 2), 55–69. doi: 10.1007/s00394-016-1229-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker LR, Degoulet M, & Morikawa H (2013). Social deprivation enhances VTA synaptic plasticity and drug-induced contextual learning. Neuron, 77(2), 335–345. doi: 10.1016/j.neuron.2012.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ME (2016). The problem with categorical thinking by psychologists. Behavioural Processes, 123, 43–53. doi: 10.1016/j.beproc.2015.09.009 [DOI] [PubMed] [Google Scholar]


