Abstract
Since the 2015 WHO classification was introduced into clinical practice, the importance of immunohistochemistry (IHC) has figured prominently in lung cancer diagnosis. In addition to distinction of small versus non-small cell carcinoma (NSCC), patients’ treatment of choice is directly linked to histological subtypes of NSCC, which pertains to IHC results, particularly for poorly-differentiated tumors. The use of IHC has improved diagnostic accuracy in the lung carcinoma classification, but the interpretation remains challenging in some instances. Also, pathologists must be aware of many interpretation pitfalls, and the use of IHC should be efficient to spare the tissue for molecular testing. The IASLC Pathology Committee received questions on practical application and interpretation of IHC in lung cancer diagnosis. After discussions in several IASLC Pathology Committee meetings, the issues and caveats were summarized as eleven key questions, which cover common and important diagnostic situations in a daily clinical practice with some relevant challenging queries. The questions included best IHC markers for distinguishing NSCLC subtypes, differences in TTF1 clones, utility of IHC in diagnosing uncommon subtypes of lung cancer and distinguishing primary from metastatic tumors.” This article provides answers and explanations for the key questions about the use of IHC in lung carcinoma diagnosis representing viewpoints of experts in thoracic pathology that should assist the community in the appropriate use of IHC in diagnostic pathology.
Keywords: Lung cancer, immunohistochemistry, TTF1, p40, neuroendocrine markers
Introduction
In the last decade, significant progress has been made in the field of immunohistochemistry (IHC). Higher sensitivity and specificity have been provided by staining enhancing techniques, such as signal amplification with and without a linker, development of monoclonal rabbit antibodies, and emerging novel markers. Under the current therapeutic strategy algorithm for lung cancer patients, the diagnosis of lung cancer, including subtyping, is now directly linked to treatment of choice. Accordingly, the 2015 WHO classification of lung cancer firstly introduced IHC in the classification schema to reflect biological features, and thus IHC is routinely used in clinical practice in diagnosing lung cancer particularly on small biopsy/cytology specimens and poorly-differentiated tumors. Currently, adenocarcinoma and squamous cell carcinoma are efficiently separated with TTF1 and p40 staining, respectively, even in the case of poorly-differentiated non-small cell carcinoma (NSCC) with small biopsies and cytologic specimens.1 However, as with any technique, there are pitfalls and disadvantages in selection of antibody panel, antibody clones, and interpretation of the staining. In response to these practical issues, the Pathology Committee of the International Association of Lung Cancer Study (IASLC) defined eleven questions, which are frequently encountered in daily practice, and achieved consensus based on literature review, personal experience of experts, and discussion among the committee members. Some questions remained challenging that consensus has not been achieved, but we tried to describe possible solutions with as many explanations as possible to benefit the practicing community. In this recommendation, we excluded IHC for predictive biomarkers, which has been established elsewhere.2, 3
Key questions of diagnostic IHC of lung cancer
Individual members submitted questions, based on their experience as experts. The eleven questions that summarized the most pressing issues with IHC were selected for discussion with the entire panel. The consensus has been established through three face-to-face committee meetings in 2016 and 2017. The eleven key questions are listed in Table 1.
Table 1.
Key questions and recommendations for diagnostic immunohistochemistry in lung cancer
| 1. What is the best combination of markers to use in daily practice? | When IHC is needed for the subtyping of non-small cell carcinoma (NSCC), TTF1 and p40 are the gold standard, and these two markers are usually sufficient in clinical practice if there are no morphological features of neuroendocrine differentiation. p40 is preferable to p63 to identify squamous cell carcinoma. |
| 2. What extent of TTF1 and p40 positive reactions should we consider to be positive? | Focal positivity for TTF1 is considered a positive reaction indicating pulmonary adenocarcinoma in the proper clinical context, whereas for p40 the cut-off rate should be positivity in more than 50% of tumor nuclei. Focal/weak positivity for p40 is not diagnostic of squamous cell carcinoma. |
| 3. Are there any staining differences in lung adenocarcinoma between among TTF1 clones (SPT24, SP141 and 8G7G3/1)? | The staining performance of TTF1 varies among the clones. Among the most commonly used antibodies, 8G7G3/1 is the most specific antibody to identify lung adenocarcinoma. |
| 4. Should a NSCC that is diffusely positive for CK7 but negative for TTF1 and p40 be regarded as “probably adenocarcinoma”? | CK7 is not specific for adenocarcinoma; the marker can be seen in squamous cell carcinoma. The use of CK7 is discouraged for subtyping of NSCC. |
| 5. When should NE markers be applied to a NSCC? | NE markers should only be applied in support of NE morphology. |
| 6. What is the best antibody panel to differentiate NE tumors from other types of NSCC and which one is the most reliable? | A panel of chromogranin A, synaptophysin and CD56 is the best combination to identify NE tumours. The staining significance of each antibody varies among the sample types, histological subtypes and extent/intensity of positive reactions. |
| 7. When should a proliferation marker be used in diagnosis? | The main established role of Ki-67in lung carcinomas is to help distinguish carcinoids from high grade NE carcinomas (large cell neuroendocrine carcinoma and small cell carcinomas), especially in small or crushed biopsy or cytology samples. The role of Ki-67 in separating typical from atypical carcinoids is not established and needs more investigation |
| 8. Is IHC useful to render a specific diagnosis of uncommon lung cancer subtypes (sarcomatoid carcinoma, salivary gland-type tumors, and NUT carcinoma)? | Currently, IHC, as well as molecular testing, are needed to achieve the definitive diagnoses of uncommon lung cancers and to distinguish from the mimics. |
| 9. What portion of the cytology sample is best for immunostaining: the cell block, the air-dried smears or the ethanol-fixed smears? Can destained smears be used adequately? | All cytology preparations including cell blocks, ethanol-fixed and air-dried slides can principally be used for immunostaining. Formalin fixed cell blocks are most straightforward, while rigorous protocol optimization, validation and quality control are required in immunostaining in cytology. |
| 10. Which IHC panel is recommended to differentiate lung mucinous adenocarcinoma from metastatic mimics? | There is no useful marker to differentiate pulmonary mucinous adenocarcinoma from metastatic mimics. Clinicopathological tumor board is crucial for this clinical context. |
| 11. Are there any IHC or other markers to differentiate between primary lung cancers and metastases; between squamous cell carcinomas of lung primary and metastases from thymic, head and neck, endocervical, and the other cancers; and between adenocarcinomas of primary and metastases from gynecologic, mammary, uroepithelial, nonpulmonary NE, prostate, and liver cancers? | In this clinical context, morphological comparison with prior tumor is crucial. There are no absolute IHC markers to make the differential diagnosis, and pathologists should be aware of IHC pitfalls. |
1. What is the best combination of markers to use in daily practice?
Short answer: When IHC is needed for the subtyping of non-small cell carcinoma (NSCC), TTF1 and p40 are the gold standard, and these two markers are usually sufficient in clinical practice if there are no morphological features of neuroendocrine differentiation (NE). p40 is preferable to p63 to identify squamous cell carcinoma.
IHC testing value depends on the probability of the proposed diagnosis, which is a combination of clinical findings and histology. The choice and number of markers are heavily dependent on these assessments. When focused on a tumor of likely lung origin for which the main question is subtyping of adenocarcinoma versus squamous carcinoma, recommendations of a limited panel include TTF1 and p40.
TTF1 is a critical single marker for adenocarcinoma as is p40 for squamous cell carcinoma,1 with Napsin-A also showing some diagnostic utility as a secondary marker for adenocarcinoma. When compared to corresponding surgical resection, TTF1 had slightly better performance than Napsin-A, whereas a combination of TTF1 and Napsin-A may yield greater sensitivity for adenocarcinoma.4 However, based on our experience, the majority of cases do not require both markers, thus TTF1 is an essential marker for adenocarcinoma in the routine case, while a larger panel can be used in challenging cases.
Some reports have shown better performance of Napsin-A compared to TTF15 with greater sensitivity6 but TTF1 as a nuclear stain can make interpretation more straightforward. Also, staining performances of Napsin-A are different between monoclonal and polyclonal antibodies as discussed in Key Question #10. In TTF1-positive non-small cell tumors, Napsin-A may play a role in neuroendocrine (NE) tumor classification as this may be positive in a subset of large cell neuroendocrine carcinomas molecularly similar to adenocarcinoma helping to separate these from high grade NE tumors such as small cell carcinoma, which are typically Napsin-A negative.7, 8 The use of surfactant protein A is discouraged as its performance is inferior.9, 10
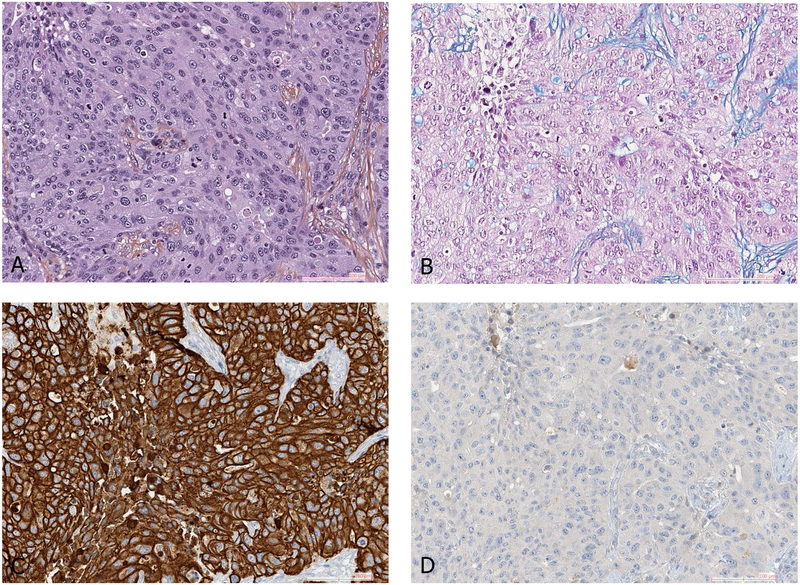
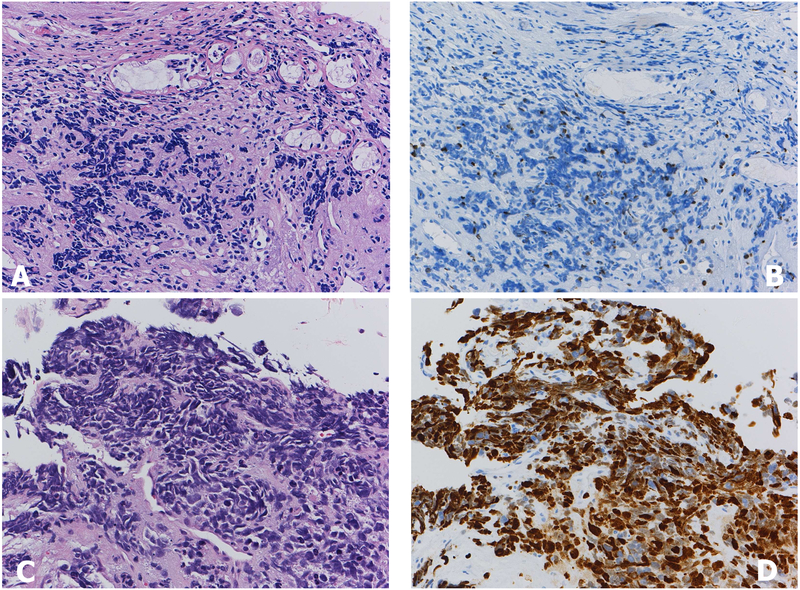
As a marker of squamous cell carcinoma, p63 IHC was more commonly used prior to the introduction of the p40 antibody. A number of studies showed that TTF1 and p63 were the most useful markers in distinguishing adenocarcinoma from squamous cell carcinoma.11, 12 However, the use of p40 IHC, which targets a splice variant of p63, is more specific and comparably sensitive to p63 in the determination of squamous histology.13, 14 For example, up to 20–30% of lung adenocarcinomas can be immunoreactive with p63. While this is usually weak to moderate in a minority of cells, rare cases show more diffuse staining, including ALK positive adenocarcinoma (Figure 1).15 p63 staining can also be seen in some sarcomas, myoepithelial tumors and lymphomas. p63 positive tumors that are TTF1 negative, even if the staining is diffusely positive, should not be assumed to be squamous cell carcinomas as subsequent p40 may be negative which would favor the diagnosis of NSCC not otherwise specified. It has been observed that p40 IHC is less likely to stain p63-positive lung adenocarcinoma, sarcomas and lymphomas, and that only an occasional adenocarcinoma shows weak and focal p40 staining. The extent of staining required to define positivity is discussed in the next section
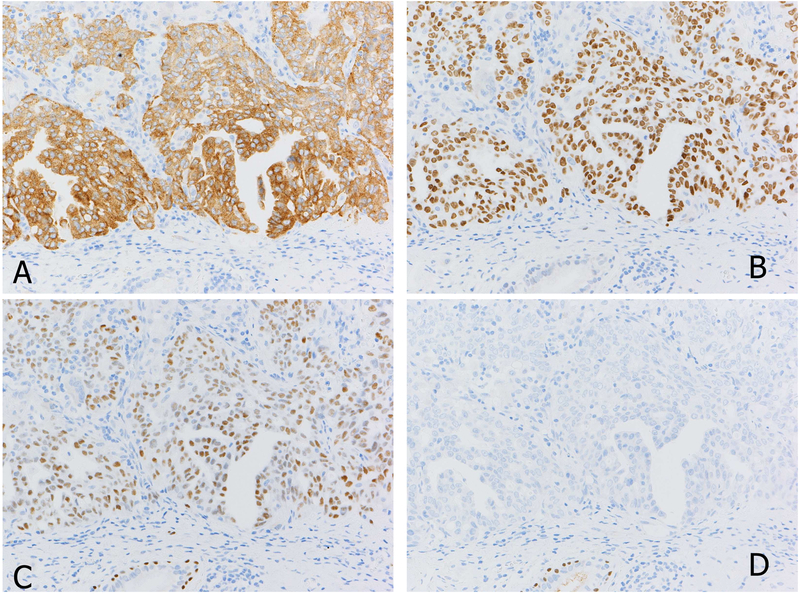
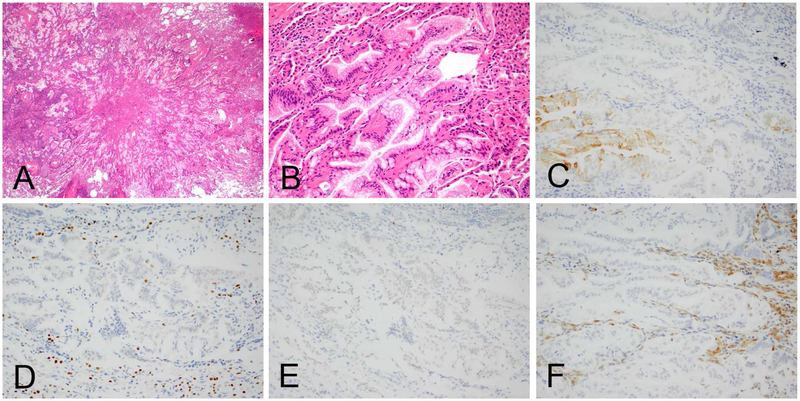
Figure 1.
ALK positive adenocarcinoma of the lung (A). A vast majority of ALK positive lung cancers are also positive for TTF1 as this case (B). Another characteristic of ALK positive tumor include discordant expression between p63 (C) and p40 (D), which can be a pitfall when p63 is used alone as squamous cell carcinoma marker.
In studies of diagnostically difficult biopsy cases with resection confirmation, p40 has higher sensitivity and specificity for squamous carcinoma when compared to CK5/6.16 Therefore, p40 IHC emerges as a critical marker in the classification of carcinomas of lung primary, with p63 as an alternative and CK5/6 in difficult cases (Table 2).
Table 2.
Commonly used antibodies in lung cancer
| A. Markers in the differential diagnosis of carcinoma of likely lung origin |
| TTF1, p40 - best for daily practice |
| CD56, synaptophysin, chromogranin A, when NE morphology is identified |
| B. Markers in the differential diagnosis of adenocarcinoma (e.g. TTF1 negative/unknown primary) |
| TTF1, PAX8, GATA3, CDX2, CK7, CK20, (PSMA or NKX3.1 for men) |
| C. Markers for epithelioid undifferentiated neoplasms |
| Pan-cytokeratin (AE1/AE3, CAM5.2), S100, desmin, SMA, CD34, CD31, CD45 |
| Calretinin, OCT4 (specific settings - tumor distribution, age) |
2. What extent of TTF1 and p40 positive reactions should we consider to be positive?
Short answer: Focal positivity for TTF1 is considered a positive reaction indicating pulmonary adenocarcinoma in the proper clinical context, whereas for p40 the cut-off rate should be positivity in more than 50% of tumor nuclei. Focal/weak positivity for p40 is not diagnostic of squamous cell carcinoma.
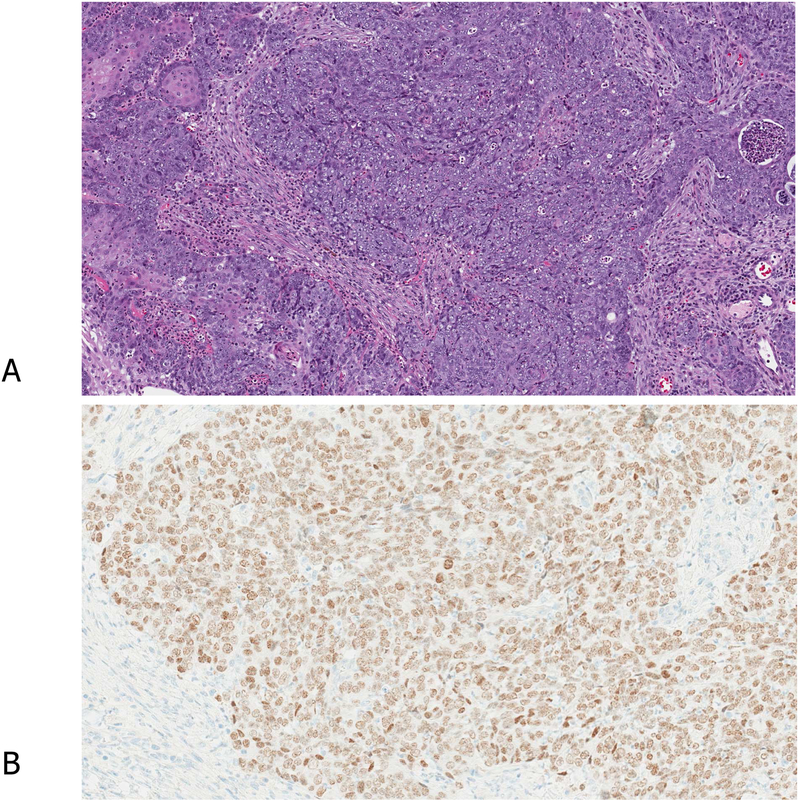
Similar to other diagnostic immunomarkers, the sensitivity and specificity of TTF1 are dependent upon the context in which they are applied as well as the clone used (see Key Question #3), the staining techniques and protocols. In most studies, approximately 75%−80% of adenocarcinomas are positive for TTF1, whereas adenocarcinoma with mucinous features tend to be negative.17, 18 Regarding TTF1 immunoreactivity, focal positivity is considered a positive reaction (Figure 2), indicative of adenocarcinoma in the proper clinical context. Indeed, it has been reported that a cut off value of >5% or more weak or strong positive reaction reached a sensitivity of 0.8 and a specificity of 0.9.19 In cases with only focal TTF1 tumor cell staining and a substantial TTF1-negative solid pattern component, a p40 stain should be performed to pursue possible adenosquamous differentiation.
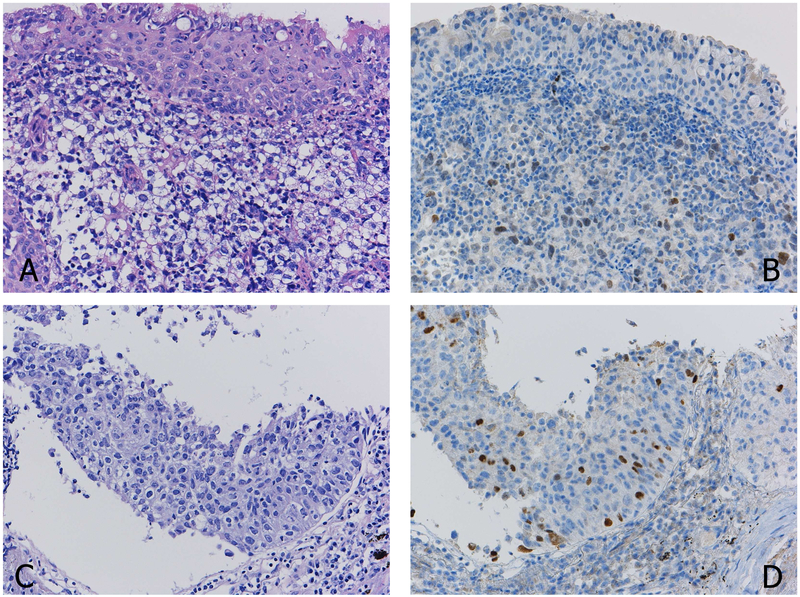
Figure 2.
A case with focal positivity of TTF1. Histologically, the tumor cells do not show clear morphologic differentiation (A). The displayed TTF1 staining (B) should be evaluated as positive, thus the tumor is diagnosed as “NSCC, favor adenocarcinoma”.
Another case with unclear morphologic differentiation (C). As the definition of a positive reaction with p40 is defined as 50% or more positive staining of the tumor cells, the widely scattered but sparse positive reaction with p40 (D) should not be considered as a definite diagnosis of squamous cell carcinoma (D).
In contrast to TTF1, focal and weak positivity for p40 is not diagnostic of squamous cell carcinoma, since focal positivity for p40 can be seen in adenocarcinomas and other tumor types. The cut-off value for p40 should be positivity in more than 50% of tumor nuclei. Positivity in less than 10% should not be used for diagnostic classification. A range of 10–50% positivity is a matter of discussion and dependent on the clinical context and the intensity of staining (Figure 2). Of note, the keratinizing component is often negative for p40 and therefore negative staining of the component does not exclude the diagnosis of squamous cell carcinoma. However, keratinization is a diagnostic criterion for squamous cell carcinoma, so if present, IHC is not required. In indeterminate cases, it is recommended to use the 2015 WHO terminology of NSCC-NOS, but the use of NSCC-NOS should be minimized.
Another important consideration is the criterion for adenosquamous carcinoma with respect to TTF1 and p40/p63 evaluation. First, this diagnosis cannot be made without a resection specimen, and in small biopsies, the possibility can be raised if two distinct cell populations are present. If each component is morphologically differentiated with glandular patterns for adenocarcinoma and keratinizing squamous cell carcinoma, immunohistochemistry may not be needed to suggest the diagnosis. However, if one or both components consist of solid patterns, immunoreactivity for each marker should be seen in different components/areas of the tumor. Conversely, double positivity (TTF1 and p40/p63) in the same cell does not define adenosquamous carcinoma. It has been reported that such tumors should probably be classified as NSCC, favor adenocarcinoma,20, 21 while selection of antibody clone may cause such reactions, as discussed later.
Another challenging situation is the recurrence of EGFR mutated adenocarcinomas following targeted therapy that results in a pure squamous cell carcinoma that may be p40 positive and TTF1 negative while retaining the original EGFR mutation, sometime with an additional T790M mutation.22, 23 This transition of histologic differentiation may represent a mechanism of resistance to tyrosine kinase inhibitors.
3. Are there any staining differences in lung adenocarcinoma between TTF1 clones (SPT24, SP141 and 8G7G3/1)?
Short answer: The staining performance of TTF1 varies among the clones. Among the most commonly used antibodies, 8G7G3/1 is the most specific antibody to identify lung adenocarcinoma.
A number of different TTF1 clones are commercially available including rabbit and goat polyclonal antibodies, mouse monoclonal antibodies including 8G7G3/1, SPT24, BGX-397A, SMP150 and 5S143 clones, and rabbit monoclonal antibodies including SP141, EP15844, C12-I and G21-G clones.24 However, the mouse monoclonal antibodies 8G7G3/1 and SPT24, and the more recently available rabbit monoclonal antibody, SP141, are the most widely used in clinical practice.4, 19, 24, 25
There are two clinical benefits of TTF1 staining: the differential diagnosis of lung adenocarcinoma from squamous cell carcinoma, and the distinction of primary lung adenocarcinoma from non-pulmonary carcinoma, both of which require specificity of staining. However, sensitivity and specificity are always part of a trade-off.
Focusing on TTF1 and the distinction between lung adenocarcinoma and squamous cell carcinoma, a review of the current literature24 revealed that the 8G7G3/1 clone was less sensitive for the detection of lung adenocarcinoma in comparison to the SPT24 clone (Table 3). However, when referring to TTF1 staining in lung squamous cell carcinoma, the specificity for adenocarcinoma is higher in 8G7G3/1 than SPT24 (Figure 3). It is noted that a certain percentage of squamous cell carcinoma are labelled with TTF1, particularly when applying a signal amplification system;26 the frequency of positivity in squamous cell carcinoma is higher with SPT24.
Table 3.
TTF1 expression in lung adenocarcinoma and squamous cell carcinoma.
| 8G7G3/1 | SPT24 | |||
|---|---|---|---|---|
| n= | Positive (%) | n= | Positive (%) | |
| Lung adenocarcinoma24 | 2614 | 2004 (76.7%) | 579 | 471 (81.3%) |
| Direct comparison with identical series of lung squamous cell carcinoma | ||||
| Kadota K, et al.191 | 449 | 0 (0%) | 448 | 27 (6.0%) |
| Kashima et al.26 | 38 | 1 (3%) with Envision | 38 | 5 (13%) with Envision |
| 38 | 4 (11%) with CSA-II | 38 | 20 (53%) with CSA-II | |
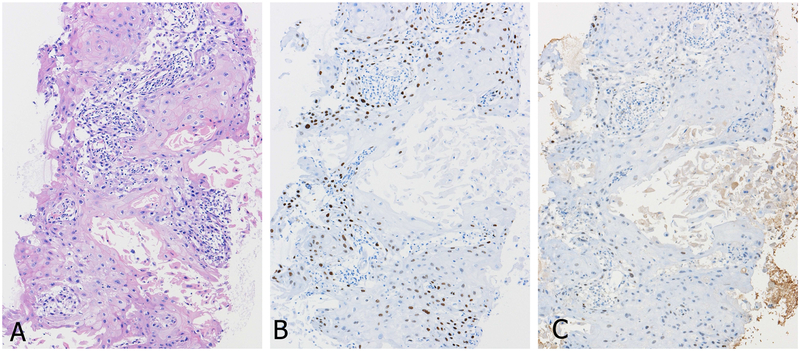
Figure 3.
TTF1 expression according to antibody clones in primary squamous cell carcinoma of the lung (A, H&E staining). Positive reactions with clone SPT24 staining (B) is contrasted to weak or negative with clone 8G7G3/1 (C).
When using TTF1 for the differential diagnosis between primary lung adenocarcinoma and other cancers, the characteristics of the antibody clone in use should be considered. It has been reported that a small percentage of non-pulmonary tumors can be positive for TTF1 (Table 4), and the SPT24 clone has lower specificity for the detection of lung adenocarcinoma than the 8G7G3/1 clone (Table 4). There is not as much literature examining the staining performance of the newer SP141 clone, but some reports suggested that SP141 has characteristics similar to the SPT24.25, 27 So even in TTF1 positive adenocarcinomas in the lung, it is important for pathologists to be aware of previous extrathoracic carcinomas and, particularly in tumors with unusual morphology, to pursue additional immunohistochemical markers to address other primary sites.
Table 4.
Results of TTF1 expression in tumors from primary sites including female genital tract, breast, colon and stomach in some of the published studies in the literature (reproduced from Ordonez NG. Value of thyroid transcription factor −1 immunostaining in tumor diagnosis: a review and update. Appl Immunohistochem Mol Morphol. 2012 Oct;20(5):4429–44).
| 8G7G3/1 | SPT24 | |||
|---|---|---|---|---|
| n= | positive (%) | n= | positive (%) | |
| Ovarian carcinoma | 615 | 22 (3.6%) | 161 | 16 (9.9%) |
| Endometrial adenocarcinoma | 215 | 17 (7.9%) | 68 | 19 (27.9%) |
| Uterine cervical adenocarcinoma | 92 | 3 (3.3%) | 39 | 6 (15.4%breast) |
| Uterine cervical squamous carcinoma | 7 | 0 (0%) | ||
| Breast adenocarcinoma | 297 | 4 (1.5%) | 580 | 13 (2.4%) |
| Colon adenocarcinoma | 594 | 11 (1.8%) | 258 | 15 (5.8%) |
| Gastric adenocarcinoma | 170 | 3 (1.8%) | 110 | 1 (0.9%) |
There are several pre-analytic considerations in regard to TTF1 immunostaining that deserve special mention. Gruchy et al. found reduced or absent TTF1 immunostaining in cytology specimens fixed in alcohol-based fixatives, including CytoLyt®, and in surgical pathology specimens subjected to decalcifying agents, such as formic or hydrochloric acid.28 This reduction in staining with TTF1 was not seen in specimens that were fixed only with routine 10% buffered formalin. It should be stressed that immunohistochemical protocols need to be validated on control tissues which undergo the same pre-analytic conditions as the test tissue, including fixation in alcohol-based fixatives and decalcification treatments even using gentler EDTA-based solutions.
4. Should a NSCC that is diffusely positive for CK7 but negative for TTF1 and p40 be regarded as “probably adenocarcinoma”?
Short answer: CK7 is not specific for adenocarcinoma; the marker can be seen in squamous cell carcinoma. The use of CK7 is discouraged for subtyping of NSCC.
CK7 is a 54 KDa protein present in simple epithelia and lung alveoli and expressed in 94 to 100% of lung adenocarcinoma. Almost all studies have demonstrated high sensitivity of CK7 for diagnosing lung adenocarcinoma, ranging from 92 to 100%, contrasting with its low specificity ranging from 50% to 93% when compared to other markers such as Napsin-A and TTF1 (Table 5). Indeed, 5 to 77% (mean 25%) of squamous cell carcinoma according to a recent series can display CK7 staining (Figure 4), often weaker than that observed in adenocarcinoma.29 Most adenosquamous carcinomas, large cell carcinomas and pleomorphic carcinomas, and large cell neuroendocrine carcinoma (LCNEC) can also express CK7.30–32 Of note, CK7 expression, in contrast with TTF1, is not restricted to adenocarcinoma arising from the lung and is widely expressed by other tumors such as breast carcinoma. For all these reasons, and to spare the tissue for potential molecular testing, the IASLC Pathology panel discourages the use of CK7 as a marker of glandular differentiation or of a lung primary.
Table 5.
Summary of CK7 expression in lung cancer
| Studies | Type of samples | Primary lung ADC |
Primary Lung SqCC |
Primary other NSCC | Sensitivity for ADC |
Specificity ADC |
PPV for ADC |
NPV for ADC |
|---|---|---|---|---|---|---|---|---|
| Lyda and Weiss192 | Surgical specimens | 31/33 (94%) |
8/37 (22%) | 2/6 LCNEC (33%), 5/9 LCC (56%) |
||||
| Johansson44 | Surgical specimens | 11/11 (100%) |
4/12 (33%) | 8/9 LCC/pleiomorphic Carcinoma |
||||
| Camilo et al193 | Surgical specimens | 16/17 (91%) |
1/18 (5,5%) | 3/5 LCC (60%) | ||||
| Mukhopadhyay et al39 | Biopsies versus surgical specimens |
19/19 (100%) |
9/15 (60%) | 3/4 LCC (75%) | 100% | 37% | 61% | 100% |
| Warth et al18 | TMA from surgical specimens |
509/530 (96%) |
96/456 (21%) |
47 ASC (89%), 60 LCC (71%), 31 sarcomatoid Carcinoma (68%) |
96% | 79% | 84% | 94% |
| Kimbrell et al29 | Cytology versus surgical specimens |
8/8 (100%) |
7/9 (77%) |
6/6 LCC (100%) 3/3 ASC (100%) |
||||
| Noh et al40 | TMA (poorly differentiated areas) |
32/36 (92%) |
5/38 (13%) | 6/8 LCC (75%) | 92% | 76% | 75% | 92% |
| Righi et al33 | Cellblocks (FNAC) versus surgical specimens |
66/66 (100%) |
15/24 (62%) |
10/13(1/1 ASC, 9/12 LCC and sarcomatoid carcinoma) |
100% | 92% | 92% | 100% |
| Koh et al42 | TMA from surgical specimens | 107/108 (99%) |
15/59 (25%) |
12/17 incl.4ASC, 4 pleiomorphic Carcinoma, 9 LCC |
99% | 65% | 81% | 98% |
| Gurda et al43 | Cellblocks (FNAC) | 45/48 (94%) |
7/14 (50%) | 94% | 50% | 86% | 70% | |
| Sekar et al41 | Cellblocks (FNAC) | 15/15 (100%) |
1/15 (6%) |
17/30 other NSCC (56%) | 100% | 93% | 94% | 100% |
PPV, positive predictive value (TP/TP+FP); NPV, negative predictive value (TN/TN+FN); ADC, adenocarcinoma; SqCC, Squamous cell carcinoma; ASC, Adenosquamous carcinoma; LCNEC, large cell neuroendocrine carcinoma; LCC, large cell carcinoma; TMA, tissue microarrays; FNAC, fine needle aspiration cytology
Figure 4.
Cytokeratin expression of squamous cell carcinoma in a bronchial biopsy specimen (A). Nuclear staining with p40 (BC28 Ab) supports the diagnosis (B). Both cytokeratin 7 (OVTL12/30 Ab, C) and cytokeratin 5/6 (D5/16B4 Ab, D) are positive.
According to the 2015 WHO classification, if there is no morphology, mucin stains or immunohistochemical markers supporting adenocarcinoma or squamous cell carcinoma, the tumor should be classified as NSCC, NOS in small biopsy specimens. In the absence of TTF1 or p40 expression, CK5/6 and CK7 cannot distinguish between adenocarcinoma and squamous cell carcinoma when used alone.33 NSCC with only CK7 expression and negative or equivocal for other markers, i.e. TTF1, Napsin-A and p40, should be considered as NSCC, NOS.34 However, some pulmonary large cell carcinomas lacking squamous differentiation (e.g., keratinization and/or intercellular bridge) and TTF1−/p40± or TTF1−/p40− were reported to be clinicopathologically and genetically indistinguishable from the solid-subtype adenocarcinoma.35–37 In a series of 315 surgical specimens, TTF1/p63/CK5/6-negative tumors accounted for 10% of adenocarcinoma.38 For these reasons, molecular testing is still recommended in the 2015 WHO classification in case of a CK5/6 (−), CK7 (+), TTF1 (−) p40 (−) and mucicarmine (−) NSCC.1 All suspected NSCC, NOS, should be tested for keratin to confirm carcinoma differentiation and if negative, worked up for metastatic melanoma, lymphoma, sarcoma and epithelioid hemangioendothelioma.
TTF1-negative adenocarcinoma is reported in the literature to range from 0 to 47%, with a mean percentage of 15% (Table 3).18, 33, 39–44 TTF1 negativity correlates with invasive mucinous adenocarcinoma and solid adenocarcinoma with mucin (Figure 5), with only 10% to 15% of mucinous adenocarcinoma being TTF1 positive.
Figure 5.
Solid adenocarcinoma of the lung (A), which is diagnosed by numerous intracytoplasmic vacuoles of mucus within the cytoplasm of tumor cells with Alcian blue staining (B). Diffuse cytokeratin 7 (OV-TL12/30, C) expression and absence of TTF1 (8G7G3/1 Ab, D) expression are noted.
5. When should NE markers be applied to a NSCC?
Short answer: NE markers should only be applied in support of NE morphology.
The current WHO classification recommends that NE markers should be performed only when NE morphologic features (organoid nesting, rosette-like structures, palisading patterns, etc.) are present.1, 45 Positive NE markers may be encountered in approximately 10–30% of NSCC without overt NE morphology by light microscopy. Such tumors may be termed “non-small cell carcinoma with NE differentiation”, however it is recommended that resected tumors be classified primarily as squamous cell carcinoma, adenocarcinoma or large cell carcinoma, as applicable, with a comment regarding the positive NE markers (Figure 6).1, 45 NE marker staining is not recommend for tumors lacking NE morphology based on the lack of consistent data supporting the clinical relevance of positive NE markers in the absence of NE morphologic features.46–48 In small biopsy specimens showing NSCC with NE morphology, NE markers should be performed and, if positive, the diagnosis of “non-small cell carcinoma, favor LCNEC” is recommended.1, 45 If NE morphology is present and the markers are negative, the terminology of “non-small cell carcinoma with NE morphology” should be used, with a comment that LCNEC is suspected but stains failed to demonstrate NE differentiation. Given that NE morphology may not be appreciated on a small biopsy or cytology sample, there is a potential for cases of LCNEC to be missed on small specimens. Even so, only when there is a suggestion of NE morphology and markers are positive should the prospect of LCNEC should be raised.1, 49 A discussion of issues with NE antibodies, sensitivities and specificities appears in Key Question #6.
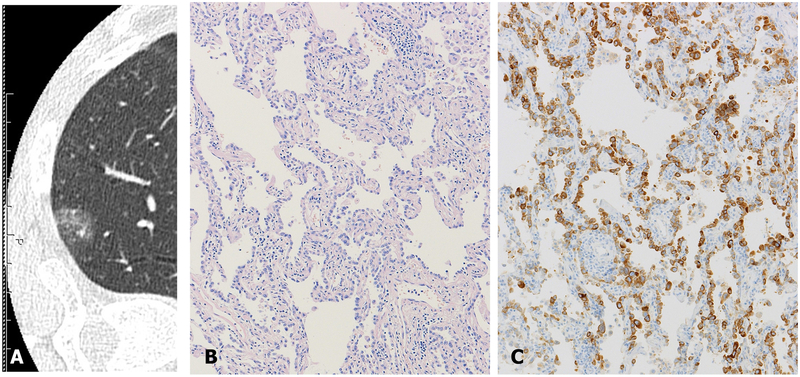
Figure 6.
A lesion of typical ground glass attenuation on CT image (A) was surgically removed and histologically shows adenocarcinoma (B). This tumor has diffuse synaptophysin expression (C). Despite the diffuse expression, the tumor should be diagnosed as adenocarcinoma, because the tumor does not have any morphological neuroendocrine features.
6. What is the best antibody panel to differentiate NE tumors from other types of NSCC and which one is the most reliable?
Short answer: A panel of chromogranin A, synaptophysin and CD56 is the best combination to identify NE tumors. The staining significance of each antibody varies among the sample types, histological subtypes and extent/intensity of positive reactions.
The 2015 WHO classification recognizes three markers for NE differentiation that include chromogranin A, synaptophysin and CD56.45 As there is no clear cut-off for any of these NE markers, the interpretation should be rendered in the context of morphological features, sample types (cytology, biopsy or surgical specimens) and extent of positive reactions.
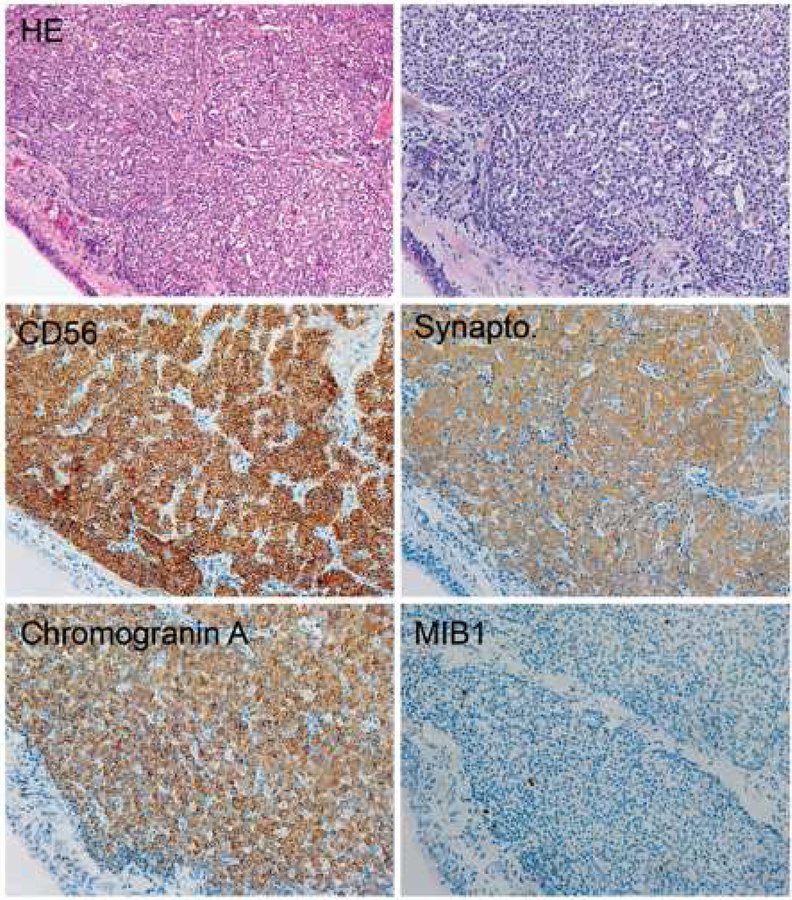
Chromogranin and synaptophysin50 are true markers of NE differentiation, as their epitopes are part of neurosecretory granules or of synaptic vescicles.51 More chromogranin A staining can be detected in carcinoid tumors than in LCNEC or small cell lung cancer (SCLC). In carcinoids, chromogranin A usually strongly and diffusely stains the cytoplasm (Figure 7). In contrast, in SCLC focal chromogranin A positivity may be present in some but not all tumor cells (Figure 8). This should still be called positive for chromogranin A in case of SCLC. This same trend is also seen with synaptophysin (Table 6). However, some SCLC and LCNEC will show diffuse and strong expression of multiple NE markers and this finding does not exclude these diagnoses.
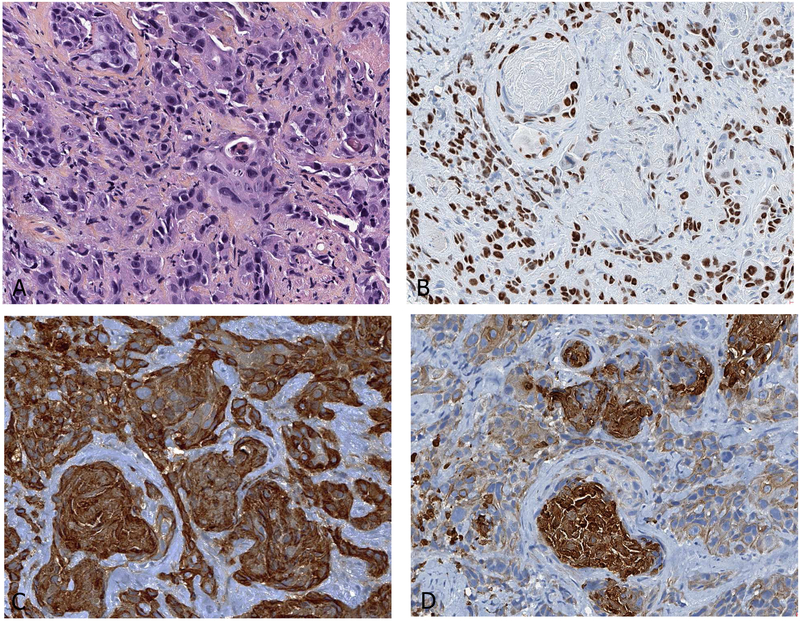
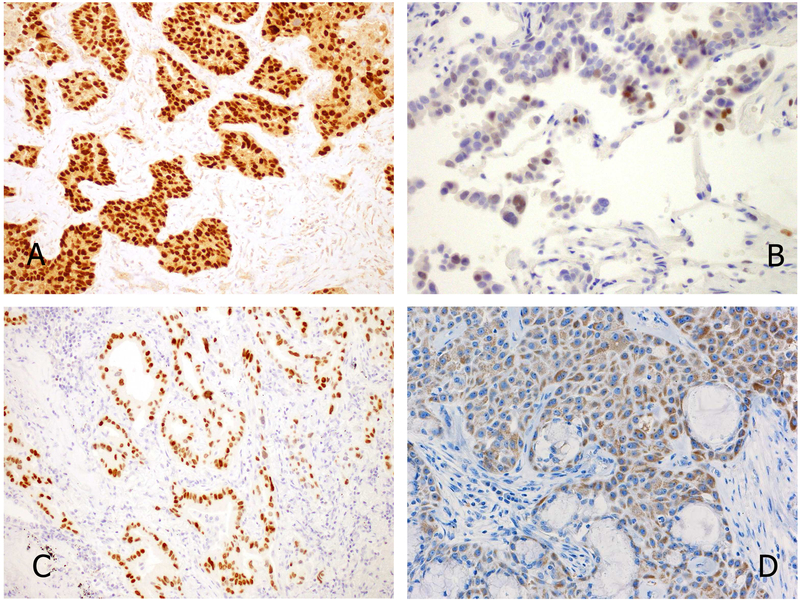
Figure 7.
Typical immunoprofile of carcinoid tumor. Note the homogenous distribution of tumor cells, strong staining for three neuroendocrine markers and low MIB1 immunoreactions.
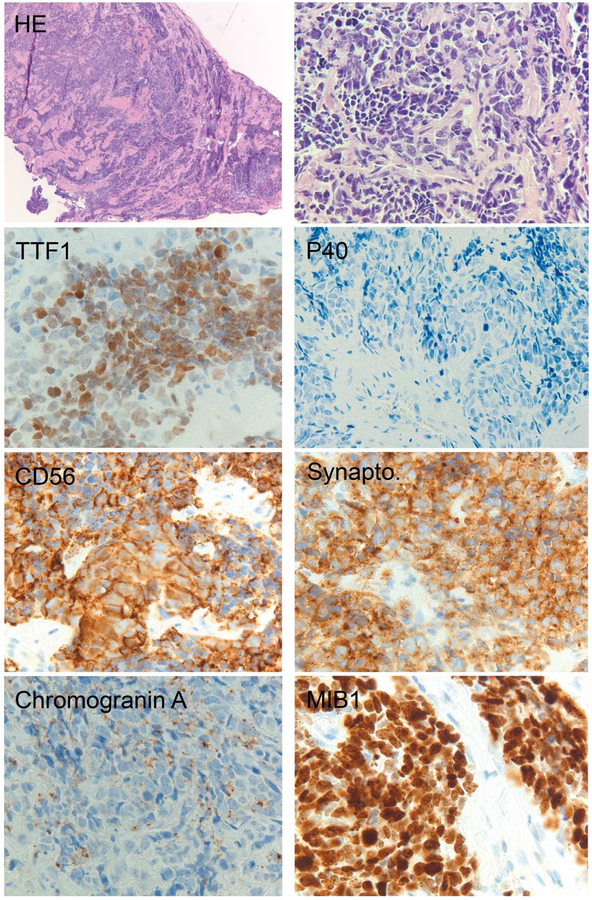
Figure 8.
Typical immunoprofile of small cell lung carcinoma. Note the irregular distribution of tumor cells, strong staining for two neuroendocrine markers and dot-like positivity with chromogranin A. Also, MIB1 labeling is high.
Table 6.
The sensitivity (median, range) for the three neuroendocrine IHC markers in the lung for small cell lung carcinomas (SCLC), large cell neuroendocrine carcinoma (LCNEC), typical carcinoids, atypical carcinoids, according to the references with 10 or more cases in a histologic type.
Conversely, CD56 (Neural cell adhesion molecule, NCAM) is the most sensitive for the diagnosis of SCLC, although 5–10% of SCLC can be negative for all three NE markers. However, the expression is not specific for NE differentiation as the protein is expressed on neurons, glia, hematopoietic cells (natural killer cells, γδ-T cells, activated CD8+ T cells, dendritic cells), and skeletal muscle. The lack of specificity implies that CD56 expression as a ‘NE’ marker should be interpreted in the context of NE morphological features with H&E. The IHC pattern for CD56 in most SCLCs is strong membranous staining in all tumor cells (Figure 8). In tumors suspected to be SCLC or LCNEC that are TTF1 negative, a p40 stain should be performed to exclude basaloid squamous cell carcinoma.
The combination of NE morphologic features and positivity for any of these NE markers are suggestive of the diagnosis of NE tumor. Currently, there is no consensus if 1, 2 or 3 markers should be used.52 It is noted that a range 10–20% of the NSCC are positive with one NE marker,46, 53 is not recommended to routinely perform NE immunohistochemical markers in poorly differentiated non-small cell carcinomas that lack NE morphology as there is no established clinical significance to this finding.1 Most cases of LCNEC and SCLC are positive for two or more NE markers out of these three.46, 53 Also, there are no clear cut off value of the extent of NE marker positive reactions that we should consider to be positive. Our panel of experts commonly accept any amount of positive staining of any of these NE markers if the NE morphology is apparent. Of note, utilization of IHC in conjunction with H&E morphology increases the concordance between pathologists and their diagnostic confidence.52
In addition to these three markers, other NE markers are also known. hASH1 is biologically considered as a lineage marker of NE cells,54, 55 and stains NE tumors specifically.53, 56, 57 However, the sensitivity is not sufficiently high similar to that of Leu7/CD57. Polyclonal neuron-specific enolase (NSE) has high sensitivity but is no longer used because of low specificity. Recent studies of insulinoma-associated protein 1 (INSM1) suggest this may be a promising addition to the available panel of stains, because of high specificity and sensitivity for labeling an entire spectrum of neuroendocrine tumors independent of originating organs and histological grades.58–60 While it is not clear that adding INSM1 improves the current recommended panel of CGA, SYN and CD56 in detecting NE differentiation, the nuclear staining pattern may enable more straightforward interpretation.58
7. When should a proliferation marker be used in diagnosis?
Short answer: The main established role of Ki-67in lung carcinomas is to help distinguish carcinoids from high grade NE carcinomas (large cell neuroendocrine carcinoma and small cell carcinomas), especially in small or crushed biopsy/cytology samples. The role of Ki-67 in separating typical from atypical carcinoids is not established and needs more investigation.
The cell proliferation Ki-67 protein (henceforth simply Ki-67) is the product of MKI67 gene mapping to the 10q26.2 gene, which is involved in all active stages of the cell cycle with a maximum in M phase, but not in resting or senescent cells.61, 62 Several antibodies to Ki-67 are available for paraffin-embedded sections, but the MIB-1 clone is ranked as the most widely used reagent after antigenicity recovery systems.63 Ki-67 expression may be scored semi-quantitatively as a percentage of positive cells (labeling index) upon manual counting of 5002000 tumors cells, 2 mm2-spanning areas or eyeballing estimation on hot spot areas, but a standard scoring method of Ki-67 has not been established for lung cancer.64
In lung NE tumor, the main diagnostic role for Ki-67 is in helping to distinguish carcinoids from the high-grade SCLC and LCNEC in small crushed biopsies (Figure 9). There are no clear thresholds for separation of typical [TC] and atypical [AC] carcinoid with some data suggesting TC has a range between 2.3 to 4.15% and AC ranges from 9 to 17.8%.63 The cutoff for distinguishing AC from high grade tumor has also not been firmly established,63 but a range of 2.5 to 30% have been considered as the cutoff for distinguishing AC from LCNEC. Although a cutoff of 20% was suggested as an upper limit for AC in the 2015 WHO classification, lower limits of 40 and 50% were suggested for LCNEC and SCLC, so more work is needed to determine how better to use Ki-67 to distinguish AC from high grade NE carcinomas.
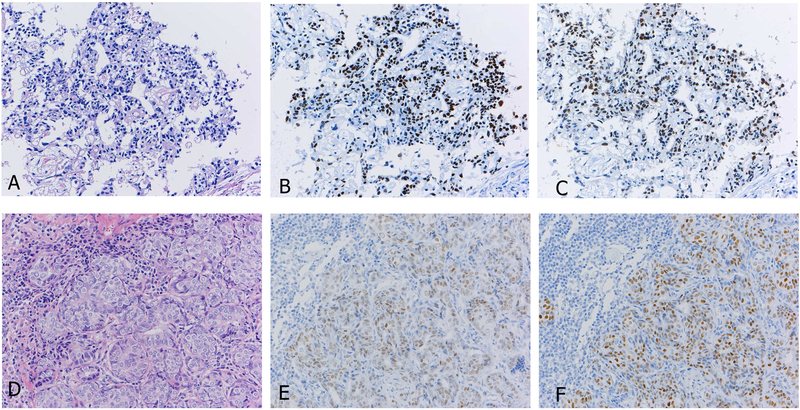
Figure 9.
Transbronchial biopsies often have crushed tumor cells as shown (A and C). In this situation, cytology specimens, if available, can be more useful for diagnosis, and Ki-67 staining (B and D) can help with the differential diagnosis between carcinoid tumor (A and B) and small cell carcinoma (C and D).
Ki-67 is not currently recommended for rendering a diagnosis or providing managerial information on NSCC. In general, Ki-67 has not consistently been shown to be a poor prognostic factor, although it tends to be increased in poorly differentiated tumors.65 There may be differences in prognostic impact according to histologic type where the labelling index has been shown to correlate with poor outcomes in adenocarcinoma.66, 67 However, it may not correlate with prognosis in squamous cell carcinoma.68
A new marker, anti–phosphohistone H3 (PHH3), has emerged as a mitosis-specific marker, which has been investigated in various types of human cancers,69, 70, 71 but large studies on lung cancer are lacking.
8. Is IHC useful to render a specific diagnosis of uncommon lung cancer subtypes (sarcomatoid carcinoma, salivary gland-type tumors, and NUT carcinoma)?
Short answer: Currently, IHC, as well as, molecular testing are needed to achieve the definitive diagnoses of uncommon lung cancers and to distinguish from the mimics.
Sarcomatoid carcinoma
“Sarcomatoid carcinomas” in the lung is an umbrella term encompassing spindle cell carcinomas, giant cell carcinomas, and pleomorphic carcinomas (spindle/giant cell carcinomas with one or more conventional carcinoma components) in addition to carcinosarcoma and pulmonary blastoma.72 The key role of IHC is to distinguish sarcomatoid carcinomas from sarcomatoid mesothelioma, primary or metastatic sarcomas, metastatic sarcomatoid carcinoma (e.g. renal) and to exclude mimics, such as metastatic melanoma. Keratin expression can be quite variable in sarcomatoid carcinomas. The expression is extremely weak and focal in most cases and may be inapparent in small biopsy specimens. Importantly, if a conventional carcinoma component, such as acinar adenocarcinoma or keratinizing squamous cell carcinoma is evident in association with a spindle/giant cell tumor, documentation of epithelial differentiation by IHC is not necessary. Markers of glandular and squamous differentiation, TTF1 and p40, respectively, are frequently negative in sarcomatoid carcinomas, but can be positive in a subset, even in cases with minimal keratin expression.30 It can be useful to utilize a panel of cytokeratins in suspected pleomorphic carcinomas as some cases show positivity with only one keratin antibody. CK18 is a sensitive marker for epithelial differentiation in sarcomatoid tumors.
The distinction of sarcomatoid carcinoma and sarcomatoid mesothelioma can be problematic as IHC for specific differentiation markers may be negative in both tumor types. Similar to sarcomatoid carcinomas, sarcomatoid mesotheliomas are commonly negative or only weakly/focally positive for mesothelial markers (WT1, Calretinin, D2–40). Although BAP1 loss is generally useful for distinguishing between reactive mesothelial proliferations and malignant mesothelioma, the loss occurs in 20% or less of sarcomatoid mesotheliomas73 and may occur in other tumor types such as sarcomatoid RCC.74, 75. Expression of carcinoma markers, including Claudin-4, BER-EP4 or B72.3, would support the diagnosis of sarcomatoid carcinoma over mesothelioma, but similar to keratins, expression of these markers may be extremely weak and focal. Recently, an excellent performance of GATA3 has been reported in this distinction, and 100% sensitivity for sarcomatoid/desmoplastic malignant mesothelioma in particular suggested that lack of GATA3 expression could be used to exclude the diagnosis of sarcomatoid mesothelioma.76 Of note, p16 FISH cannot be used in this differential diagnosis as both tumors can have homozygous deletions.77 In many cases, practically, IHC work-up may not be informative, and the final diagnosis requires incorporation of clinico-radiologic information and, molecular findings, if available.78–80
Distinction of sarcomatoid carcinoma from primary or metastatic sarcoma can be equally problematic as IHC profiles of these tumors can overlap. In particular, just as sarcomatoid carcinomas may be virtually negative for keratins, some high-grade sarcomas are known to express keratins, usually weakly and focally (Table 7). Thus, focal labeling for keratins should not be used as the sole criterion supporting the diagnosis of sarcomatoid carcinoma over sarcoma, and conversely, the lack of detectable keratins, particularly in a small sample, does not favor sarcoma over sarcomatoid carcinoma. It is important to remember that other than a few specific types of mesenchymal neoplasms (Table 7), primary pulmonary spindle/giant cell sarcomas are extremely rare, and even with minimal/absent keratins, such tumors are more likely to represent sarcomatoid carcinoma than primary sarcoma, particularly in a clinical context characteristic of lung cancer patients (e.g. older smokers). Molecular testing may support the diagnosis of sarcomatoid carcinoma by identifying alterations typical of non-small cell carcinoma, such as EGFR, KRAS or MET exon 14 splice site mutations, of which the latter are associated with sarcomatoid histology.81, 82
Table 7.
Specific sarcomas which mimic sarcomatoid carcinoma of the lung in thoracic regions
| Clinical context |
Sarcoma | Keratin expression* |
IHC markers | References |
|---|---|---|---|---|
| Primary | Malignant solitary fibrous tumor |
+/− | STAT6, CD34, BCL6 | 199–203 |
| Inflammatory Myofibroblastic tumor |
+/− | ALK, SMA, FISH for ALK, ROS1, RET, NTRK3 fusions |
204–206 | |
| Primary pleural synovial sarcoma |
+/− | TLE1, focal keratins, FISH for SSI8 fusions | 207 | |
| Metastatic | Uterine leiomyosarcoma | +/− | SMA, ER, desmin | |
| De-differentiated liposarcoma |
− | CDK4, MDM2 | ||
| Malignant peripheral nerve sheath tumor |
− | SOX 10, S100, H3K27me3 loss | ||
| Malignant melanoma | − | SOX 10, Melan A, HMB45, SI00 |
usually focal and weak, but diffuse positive reactions can occur
Salivary gland-type tumors
Salivary gland-type carcinomas can arise in the lung; in this setting, a metastasis from salivary gland primary must be excluded clinically. Their typical location is peribronchial/endobronchial. By far, the most common types of primary pulmonary salivary-type neoplasms include mucoepidermoid carcinoma and adenoid cystic carcinoma.83 Importantly, all salivary neoplasms lack the expression of lung lineage markers, TTF1 and Napsin-A; if these markers are detected, this would support primary lung adenocarcinoma over salivary-type neoplasms. Care must be taken to recognize entrapped TTF1 positive cells that may proliferate extensively in salivary gland tumors that infiltrate the interstitium.
The diagnosis of mucoepidermoid carcinoma can be supported by consistent labeling for p40/p63 in intermediate and squamous cells, positive intracytoplasmic mucin with mucin stains and the demonstration of MAML2 rearrangements by FISH with a range of 77% to 100% tumors.84–86 SOX10 is usually negative in mucoepidermoid carcinoma but can be positive in a subset.87 The main differential diagnosis in the lung is with adenosquamous carcinoma; expression of TTF1/Napsin-A in the glandular component would support the former, whereas MAML2 rearrangement would support the latter. In case without these features, the distinction remains quite challenging.
For adenoid cystic carcinoma, the main differential diagnosis is with pulmonary basaloid squamous cell carcinoma and other salivary-type tumors. Adenoid cystic carcinoma demonstrates dual luminal epithelial/abluminal myoepithelial composition, with luminal cells labeling for low-molecular weight keratins and c-Kit, while abluminal myoepithelial cells label with p63/p40, SMA and S100. The tumors are positive for SOX10.87 In addition, most adenoid cystic carcinoma are positive for MYB by IHC and harbor MYB fusions.88
Rarer types of salivary neoplasms have been documented in the lung. These include epithelial-myoepithelial carcinoma (p63/SMA/S100-positive outer myoepithelial cells),89 acinic cell carcinoma (SOX10, DOG1-positive),90, 91 hyalinizing clear cell carcinoma (recently renamed in head and neck sites as “clear cell carcinoma”; p63/p40-positive, EWSR1 fusions),92 myoepithelial carcinoma (SOX10-positive, co-expresses epithelial markers [keratins, EMA] + S100 + variable myogenic markers, p63, GFAP; EWSR1 or FUS fusions),93, 94 and mammary analogue secretory carcinoma (S100, mammaglobin, GCDFP-15, SOX10, GATA-3, ETV6–NTRK3 fusion).95, 96
Nut carcinoma
Nuclear protein in testis (NUT) carcinoma is defined by the presence NUT gene rearrangement on chromosome 15. A highly specific antibody for NUT protein is commercially available, which demonstrates a distinctive speckled nuclear positivity in NUT carcinoma (Figure 10).97, 98 The only other neoplasms that label for NUT are germ cell tumor tumors, particularly seminomas; however, their labeling is typically focal and lacks the speckled pattern.98 NUT carcinomas are usually positive for keratins, although rare cases can be negative.97, 99 p63/p40 are usually positive, supporting squamous differentiation. Notably, NUT carcinomas may be positive for CD34, which is important in the differential diagnosis with leukemic infiltrates.100 Other differential diagnosis includes SWI/SNF chromatin remodeling factor-deficient carcinoma or sarcoma, which also displays solid growth pattern of mildly discohesive epithelioid cells often partly with rhabdoid features. The tumors are reported as SMARCA4 or SMARCB1-deificient carcinoma or sarcoma in a small series.101–105 As the tumors have been recognized recently, a definite entity of this tumor has not been established in the current classification.
Figure 10.
Typical H&E appearance of NUT carcinoma: undifferentiated, primitive, but monomorphic features with focal abrupt squamous differentiation (A). NUT immunohistochemistry shows diffuse nuclear labeling with characteristic speckled pattern (B).
9. What portion of the cytology sample is best for immunostaining: the cell block, the airdried smears or the ethanol-fixed smears? Can de-stained smears be used adequately?
Short answer: All cytology preparations including cell blocks, ethanol-fixed and air-dried slides can principally be used for immunostaining. Formalin fixed cell blocks are most straightforward, while rigorous protocol optimization, validation and quality control are required in immunostaining in cytology.
The ability to perform highly accurate immunostaining in cytological specimens is crucial given the fact that up to 40% of all lung cancer diagnoses are made by cytology alone. The major difference and challenge in cytology relates to the greater variability of pre-analytical conditions and the lack of tissue architecture/contexture that might necessitate different scoring strategies. In principle, one can group cytological preparations into “cell block cytology” and “non-cell block cytology”. Cell blocks are the most easily accessible cytology format for immunostaining, since most immunostaining protocols are optimized for FFPE tissue/cell material (Figure 11 A, B). Principally, it should be possible to apply the same standardized protocols for FFPE fixed tissue on automated immunostainers. This assumption is supported by studies showing highly concordant results for different markers between cell blocks and matched histological specimens.106–109 However, lack of international standards for pre-fixation methods and preparation protocol is a major issue on cell blocks.110, 111 Currently, more than 10 methods for cell block preparation are in use, the most common ones in the United States being plasma thrombin (33%), Histogel (Thermo Fisher Scientific; 27%), Cellient automated cell block system (Hologic, 27%),112 and modifications of these.113 Almost all protocols share the final step of fixing the pellet in 10% buffered formalin and processing it to an FFPE block. The large spectrum of (pre-) fixation ranges from fixing the cell material in 10% buffered formalin right to pre-fixation in ethanol or methanol-based solution before formalin fixation, or even pure fixation in 95% ethanol.
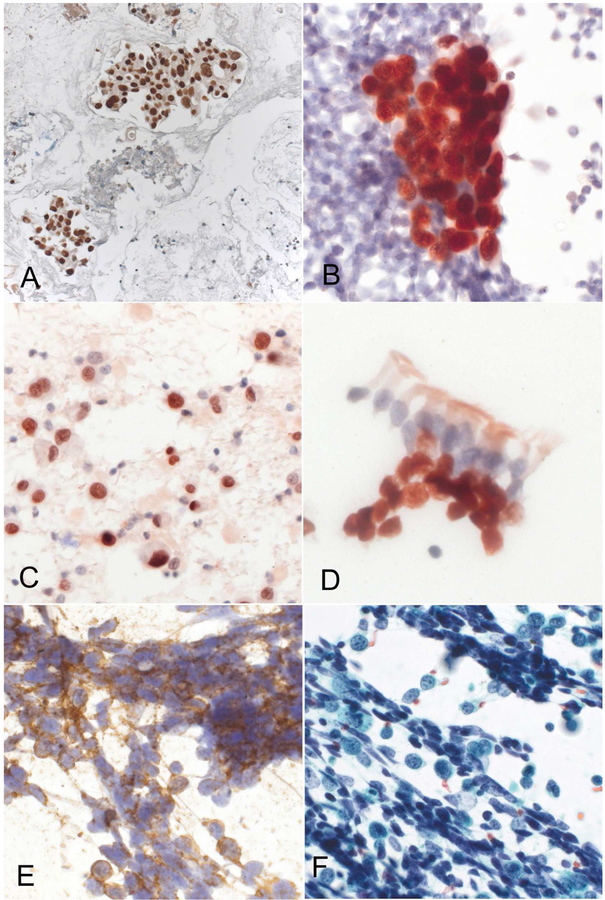
Figure 11.
Immunostaining of cytology specimens. A) TTF1 positive adenocarcinoma in cell block specimen (by brown 3,3’-diamonobenzidine, Ventana Benchmark XT immunostainer), BE) immunostaining on Papanicolaou stained, ethanol fixed, non-cell block specimens (Leica Bond automated immunostainer); B) TTF1 positive adenocarcinoma (detection by red 3-amino-9 ethylcarbazole), C) p40 positive non-keratinizing squamous cell carcinoma, D) p40 positive benign hyperplastic basal cells underlying ciliated respiratory cells (bronchial brush cytology), E: CD56 positive small cell carcinoma with corresponding Papanicolaou stained specimen (F).
Although the large variety of transport media, pre-fixatives and cell block protocols do not appears to cause systematic problems on immunostaining according to a previous survey,114 recent analyses pinpoint to specific challenges related to pre-analytical factors in cell blocks, especially with ethanol or methanol pre-fixation.28, 115 In addition to absent or near absent expression of TTF1 with CytoLyt® fixative,28 nearly half (43%) of 30 antibodies tested on the Cellient (Hologic) cell block system failed initial validation using conditions established for FFPE tissue specimens on the Ventana Benchmark XT immunostainer.116
Non-cell block cytology specimens consist of a variety of preparations, which include air-dried and alcohol-fixed smears, Cytospins (Thermo Fisher Scientific, Waltham, Massachusetts), ThinPrep (Hologic, Marlborough, Massachusetts), or SurePath (Becton Dickinson, Franklin Lakes, New Jersey) liquid-based preparations. The large variety of pre-analytical conditions and preparation methods makes standardization of immunostaining on non-cell block specimens more challenging than in cell blocks. Nevertheless, immunostaining on ethanol-fixed smears or cytospins is principally possible and widely practiced; many laboratories that apply immunostaining to non-cell block specimens use the diagnostic Papanicolaou-stained slides (Figure 11 C–E).114, 117–119 Prior Papanicolaou staining, which does not negatively interfere with the immunostaining reaction, allows triaging the available slides for immunostaining and marking of areas of special interest. Alternatively, air-dried and unstained extra slides with postfixation by acetone or formalin are also used for immunostaining, but require extra material not used for primary morphological diagnosis.120, 121 When slides containing tumor are available, before the initiation of immunostaining, it may well be prudent to photograph or scan stained neoplastic elements for documentation purposes.
In the practical application of immunostaining to either cell block or non-cell block specimens, careful protocol validation and continuous quality control is essential, especially in ethanol-fixed non-cell block preparations, because of the high variability of pre-analytical factors and the current lack of standardization. External quality assessment is also important to maintain a high immunostaining quality not only in histological but also in cytological specimens. In fact, UK NEQAS has an external quality assessment program in place to help standardize and improve the quality of immunostaining in cytology.122
10. Which immunohistochemistry panel is recommended to differentiate lung mucinous adenocarcinoma from metastatic mimics?
Short answer: There is no useful marker to differentiate pulmonary mucinous adenocarcinoma from metastatic mimics. Clinicopathological tumor board is crucial for this clinical context.
When adenocarcinomas of the GI and pancreatobiliary tracts metastasize to the lung, they may exhibit prominent mucinous features. In addition, mucinous carcinomas of the ovary, breast and other organs may metastasize to the lung. Given that the differentiation of lung adenocarcinomas with mucinous features (pulmonary mucinous adenocarcinomas) from metastatic lesions is often challenging on a morphologic basis alone, multiple groups have studied the role of immunohistochemistry in this context.123–143 Particularly, distinguishing a metastasis from a pancreatic primary from invasive mucinous adenocarcinoma of the lung is far more challenging, given the similar immunoprofiles (focal CDX2, and CK20, see Table 8); furthermore, a lepidic growth pattern, characteristic of invasive mucinous adenocarcinoma (Figure 12), is also often identified in pancreatic ductal adenocarcinoma metastatic to the lung (Figure 13).144 Even with molecular testing, a complete solution for the differential diagnosis remains unsolved,145 unless direct comparison of molecular profiles between the lung and non-lung tumors can be made.138, 144 Notably, a significant proportion of pulmonary mucinous adenocarcinomas including invasive mucinous adenocarcinomas, are not reactive to TTF1 and/or Napsin-A. Rather, these immunoreactions typically highlight normal type II pneumocytes entrapped in the tumor (Figures 13 and 14), possibly leading to false positive interpretation in the setting of metastasis. It is also worth mentioning that the high specificity of Napsin-A for lung origin may not be achieved when a polyclonal antibody is used. One study revealed Napsin-A expression in 92% of 13 non-pulmonary mucinous adenocarcinomas and 100% of 8 pulmonary mucinous adenocarcinomas by immunohistochemistry with a polyclonal antibody, compared to none of the 13 non-pulmonary and 38% of the 8 pulmonary mucinous adenocarcinomas with Napsin-A expression when a monoclonal antibody was used.140 Interestingly, other studies using a polyclonal antibody reported Napsin-A expression in none of 49 non-pulmonary mucinous adenocarcinomas;138, 139 thus, the low specificity reported in the former study may be attributed to its particular immunohistochemistry platform.140, 146 Further, nonspecific labeling with polyclonal Napsin-A in mucinous adenocarcinomas appears to have peculiar supranuclear localization opposed to the pan-cytoplasmic granular staining present with monoclonal Napsin-A, possibly due to cross-reaction with pan-mucin antigen by the polyclonal antibody (Figure 14).140, 147
Table 8.
Immunoprofiles of pulmonary mucinous adenocarcinomas and their mimics*
| TTF1 | Napsin-A6) | CK7 | CK20 | CDX2 | |
|---|---|---|---|---|---|
| Pulmonary adenocarcinomas | |||||
| Invasive mucinous adenocarcinoma1) | −/+ | −/+ | ++ | +/− | +/− |
| Colloid adenocarcinoma | +/− | +/− | + | +/− | + |
| Signet ring cell carcinoma2) | + | +/− | ++ | − | − |
| Solid adenocarcinoma with mucin | + | +/− | ++ | − | − |
| Mucinous adenocarcinoma of the lung, NOS3) | +/− | −/+ | ++ | −/+ | −/+ |
| Non-pulmonary adenocarcinomas | |||||
| GI tract all | − | − | +/− | + | + |
| Lower GI tract4) | − | − | −/+ | ++ | ++ |
| Upper GI tract5) | − | − | + | +/− | +/− |
| Pancreas | − | − | ++ | +/− | +/− |
| Breast, mucinous | − | − | ++ | − | − |
| Ovary, mucinous | − | − | ++ | +/− | +/− |
-: <10%, −/+: 10–40%, +/−: 40–70%, +: 70–90%, ++: >90% of examined tumors exhibited positive expression
including mixed mucinous sand non-mucinous adenocarcinoma
adenocarcinoma with signet ring cell features
NOS: not otherwise specified
colorectum and appendix
esophagus, stomach and ampulla
immunohistochemistry with monoclonal Napsin-A antibodies
Figure 12.
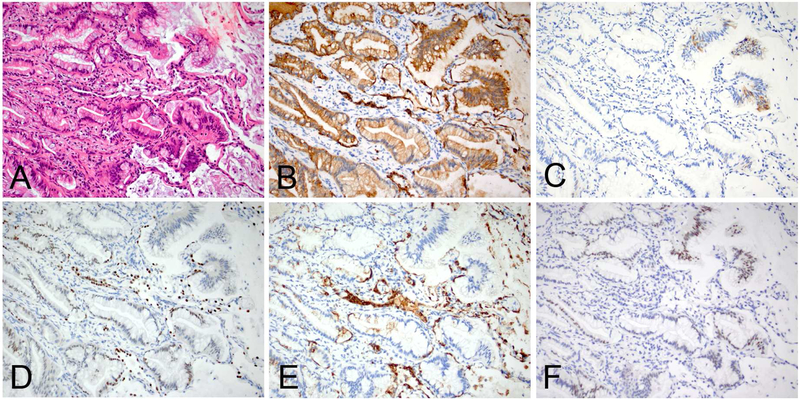
An example of invasive mucinous adenocarcinoma of the lung demonstrating lepidic and acinar patterns (A), diffuse CK7 expression (B), focal CK20 expression (C), scattered foci with weak TTF1 (D) and/or Napsin-A (E) expressions, and weak to moderate CDX2 expression (F). Of note, the entrapped type II pneumocytes are reactive to CK7 (B), TTF1 (D) and Napsin-A (E).
Figure 13.
A pancreatic ductal adenocarcinoma metastatic to the lung exhibiting a lepidic pattern (A, at a low-power magnification & B, at a high-power magnification), focal CK7 expression (C), negative TTF1 (D), diffuse weak CDX2 expression (E), and loss of SMAD4 (F). Of note, strong nuclear expression of TTF1 in the entrapped pneumocytes may give a false positive impression. Also, loss of SMAD4 expression has been reported as useful marker for the diagnosis of pancreatic adenocarcinoma, but a significant proportion of invasive mucinous adenocarcinomas of the lung harbors this alteration.138
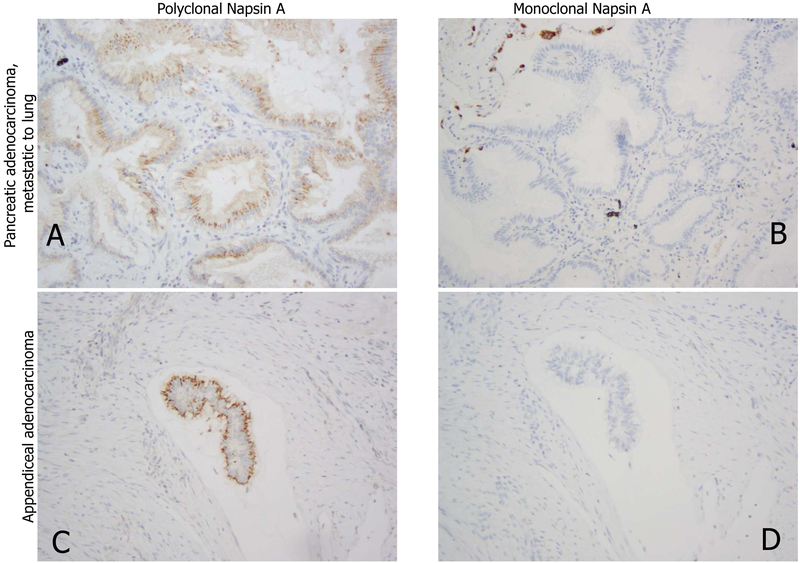
Figure 14.
Apical granular reactions of polyclonal Napsin-A are shown in non-pulmonary carcinoma (A, metastasis of pancreatic duct carcinoma and C, appendiceal adenocarcinoma) in contrast to negative reaction with monoclonal Napsin-A (clone IP64, B, D).
The majority of invasive mucinous adenocarcinomas and other pulmonary adenocarcinomas with mucinous features, in particular, those that lack TTF1 expression, react to HNF4α.135, 148 However, HNF4α is a differentiation transcription factor of the primary gut, including hepatobiliary and GI tracts, which universally express this transcription factor. Therefore, HNF4α will not help with differentiating between pulmonary mucinous adenocarcinomas and GI and pancreatic primaries.148
Among other metastatic mucinous adenocarcinomas, breast and ovarian primaries can be differentiated from a lung primary by their specific markers, including GATA3 and ER for breast colloid carcinoma and PAX8 for ovarian mucinous carcinoma.136 Despite encouraging results in literature, however, the markers cannot be relied upon in this situation, because not all metastatic tumors are positive for these markers. Only 40% of ovarian mucinous tumors express PAX8, suggesting low sensitivity for this differential diagnosis.
11. Are there any IHC or other markers to differentiate between primary lung cancers and metastases; between squamous cell carcinomas of lung primary and metastases from thymic, head and neck, endocervical, and the other cancers; and between adenocarcinomas of primary and metastases from gynecologic, mammary, uroepithelial, nonpulmonary NE, prostate, and liver cancers?
Short answer: In this clinical context, morphological comparison with prior tumor is crucial. There are no absolute IHC markers to make the differential diagnosis, and pathologists should be aware of IHC pitfalls.
Differentiating primary lung carcinoma and metastasis from extrinsic sites is an important practice in diagnostic service. The gold standard for the decision is based on the morphological comparison with prior tumors; however, IHC provides strong support for this interpretation, particularly when previous materials are unavailable for review or when morphological assessment results in equivocal findings.
Squamous cell carcinoma
Distinguishing primary lung squamous cell carcinoma versus metastasis is challenging particularly when the nodule is solitary. Prior materials should be reviewed whenever possible, because growth pattern and the degree of keratinization may provide clues for the decision. In some instances, it should be kept in mind that metastatic tumors may change their morphological features, particularly following chemo/radiation therapy. Identifying an in situ component may support the primary nature of the tumor, but primary squamous cell carcinoma arising in the peripheral lung may not have such a component. Further, in small samples, an in situ component may not be present or recognized morphologically. Ancillary stains are usually of limited utility, except for the few instances which follow.
Thymic squamous cell carcinoma labels for CD117 (85%) and CD5 (70%), whereas primary lung squamous cell carcinoma is only uncommonly positive for these markers.149–151 Notably, CD117 and CD5 are expressed in ~15% of lung adenocarcinoma, and their expression in adenocarcinoma does not suggest a thymic primary. Although PAX8 can be positive for thymic carcinomas when using a polyclonal antibody, this likely results from cross-reactivity to another PAX gene product, which is not reproduced when using a monoclonal antibody.152 More than immunohistochemical staining, clinical and radiologic correlation is important to confirm whether a tumor is arising in the lung or the thymus.
High-risk HPV detection is helpful when the differential diagnoses include metastatic squamous cell carcinoma from head and neck (especially oropharynx), endocervix, vulva, anus, and penis. Detecting HPV in tumor tissue strongly favors metastasis from these sites,153, 154 because HPV infection is considered exceptional in lung squamous cell carcinoma with the caveat that some geographic difference may exist with regard to the HPV detection rate reported in lung cancers.155 Although diffuse p16 immunostaining is an accepted surrogate for high-risk HPV infection in the cervix and oropharynx, about 20% of primary lung NSCC demonstrate similar p16 positivity despite the lack of HPV infection.154, 156 So, in case of a diffuse positive result with p16 staining, further molecular testing is recommended to confirm the presence of the HPV genome.
Gynecologic organs
The immunoprofiles of adenocarcinomas arising from the female genital tract (cervix, endometrium, fallopian tube, and ovary) differ depending on the tumor histotype and primary sites. Cervical adenocarcinoma is often associated with high-risk HPV infection and accordingly characterized by confluent p16 expression; thus, this marker can be utilized for the differential diagnosis as discussed. Serous carcinomas of the ovary and uterus are often positive for WT1, which is usually negative in lung adenocarcinoma. WT1 expression must be nuclear in this context, as cytoplasmic WT1 expression is non-specific. PAX8 recognizes most adenocarcinomas in the female genital tract, in contrast with lung adenocarcinoma, and thus is of high utility (Figure 15A).152, 157 TTF1 can be positive in a subset of uterine and ovarian carcinomas (Figure 16).158–160 Napsin-A is highly expressed in most clear cell carcinoma of the ovary and endometrium, which also frequently express HNF-1β.160
Figure 15.
Useful antibodies and pitfalls for some of the more common differential diagnosis of metastatic lung cancer.
PAX8 (monoclonal) positivity in metastatic serous adenocarcinoma from the uterine corpus (A). Focal ER positivity in primary lung adenocarcinoma (B). GATA3 positivity in metastatic adenocarcinoma from the breast (C). MGB1 expression in salivary-type adenocarcinoma (mucoepidermoid carcinoma) of the lung (D).
Figure 16.
TTF1 expression in non-pulmonary carcinoma.
A-C: Metastasis of ovarian endometrioid carcinoma to the lung (A) expresses PAX8 (B) and TTF1 (clone SPT24, C). The diagnosis was confirmed with identical KRAS mutation (G12A) between lung and ovarian cancer.
D-F: Lymph node metastasis of mammary invasive ductal carcinoma (D) displays dual expression of ER (E) and TTF1 (clone SPT24, F).
Other organs
Breast:
Common breast cancer markers include ER, GATA3, mammaglobin, and GCDFP15, which are expressed in 80%, >90%,161 40–60%,162–164 and 20–40%162, 164 of breast carcinomas, respectively. ER expression does not necessarily support breast primary, as a wide range of ER positivity is reported in lung adenocarcinoma depending on different staining protocols (Figure 15B).165–167 Nuclear GATA3 staining, usually of a diffuse and strong quality, favors a breast primary (Figure 15C), because its expression is rare to uncommon (0–8%) in lung adenocarcinoma, with the different results most likely a consequence of the different antibody clones utilized.161, 166, 168 Triple negative breast cancers may stain with SOX10.169, 170 GCDFP15 is uncommonly positive in lung adenocarcinoma (0–5.2%).89, 166, 171 Mammaglobin is usually negative in lung adenocarcinoma.162–164, 166, 167 However, GATA3 and mammaglobin can be positive in salivary-type carcinomas in the bronchopulmonary tree (Figure 15D). Breast carcinoma rarely expresses TTF1, which occurs more commonly with the SPT24 clone, but rarely with the 8G7G3/1 clone (Figure 16).172–174 Similarly, Napsin-A is usually negative in breast carcinoma but some labeling has been reported.6 Utilization of theses immunohistochemical markers needs to be made whenever possible in the context of morphological comparison with the primary breast cancer specimen.
Urothelial carcinoma:
Metastatic urothelial carcinoma may be histologically indistinguishable from poorly differentiated squamous cell carcinoma of the lung. The differentiation can be facilitated by specific urothelial markers, such as uroplakin III and more sensitive uroplakin II.175, 176 GATA3, which is positive in about 80% of urothelial carcinomas, can be positive in primary lung squamous cell carcinoma with a range of 0–20%.161, 167, 175, 177
Neuroendocrine tumors:
High-grade NE tumors can express TTF1, regardless of the primary site, and its reactivity should not be interpreted as evidence of pulmonary origin.178 Other cellular lineage markers, including CDX2, PSX1, ISL1 and NKX2.2, are useful for separating carcinoid tumors from metastatic well-differentiated neuroendocrine tumors of non-pulmonary origin,179–181 but not for high-grade neuroendocrine tumors of the lung.
Renal carcinoma:
Renal cell carcinomas express PAX8, while PAX8 expression is rare (0–2%) in lung adenocarcinoma.152, 157 Monoclonal PAX8 antibody is more specific than the polyclonal reagent.152, 182 Napsin-A can be positive in renal cell carcinomas (about 80% in papillary carcinoma and about 40% in clear cell carcinoma).6, 183
Prostate cancer:
Prostatic adenocarcinoma metastatic to the lung may be mistaken for lung adenocarcinoma, and sometimes for LCNEC. Prostatic adenocarcinomas express PSA, PMSA and NKX3.1, and lack CK7 expression, unlike lung adenocarcinomas.184, 185
Liver cancer:
Metastatic hepatocellular carcinoma should be distinguished from lung adenocarcinoma with hepatoid morphology.186 Hepatoid adenocarcinoma of the lung may express alpha fetoprotein, HepPar1,186 and arginase-1,187 and careful clinicopathological correlation is required for diagnosis.
Summary
We selected eleven key questions on IHC to be most relevant to current practice. IHC is now an indispensable tool for diagnostic pathology, but has many pitfalls, as discussed. As aberrant TTF1 expression in schwannoma was recently reported,188 we do not still recognize all of them. Therefore, morphology should be served as a basis of our pathology diagnosis, and all recommendations above are valid only in the proper clinical context. In particular, pathologists should keep in mind that clinical findings, including age, sex, smoking status, also provide an important diagnostic clue.189, 190
In the next couple of years, development of new technology and emergence of new antibodies may change the current diagnostic situation, and accordingly our recommendations may not be appropriate at that time. We feel that periodical updates are necessary in collaboration with other related organizations. Similar to the predictive biomarker testing landscape, diagnostic IHC is also directly linked to patients’ treatment of choice. All professionals in this field should ensure that all patients receive appropriate diagnoses with the aid of IHC.
Acknowledgements
Major contributors to draft this article were as follows: Alain Borczuk for KQ1, Arne Warth for KQ2, Prudence A. Russell for KQ3, Sylvie Lantuejoul for KQ4, Mary Beth Beasley for KQ5, Erik Thunnissen for KQ6, Giuseppe Pelosi for KQ7, Natasha Rekhtman for KQ8, Lukas Bubendorf for KQ9, Mari Mino-Kenudson for KQ10, Akihiko Yoshida for KQ11.
This work was supported in part by JSPS KAKENHI Grant Number 16H05167 (for YY), and Cancer Center Support Grant from the US National Institutes of Health/National Cancer Institute (grant P30CA008748, for NR).
Thank you to Dr. Justin A. Bishop for reviewing the section on salivary tumors in Key Question #8.
DISCLOSURES:
Yasushi Yatabe eceived honoraria (each less than USD10,000 per year) fees from MSD, AstraZeneca, Chugai-Roche, Novartis, Dako and Ventana-Roche.
Sanja Dacic reports personal fees from Astra-Zeneca, personal fees from Roche-Genentech, personal fees from Bayer, outside the submitted work.
Lukas Bubendorf reports grants and personal fees from Roche, from Astra Zeneca, from Pfizer, from Boehringer Ingelheim, grants and personal fees from MSD, personal fees from Takeda, grants from Eli Lilly, grants from Bayer, outside the submitted work.
Mari Mino-Kenudson reports personal fees from Merrimack Pharmaceuticals, personal fees from H3 Biomedicine, outside the submitted work.
Kim R. Geisinger worked as an expert witness for Womble, an international law firm based in Winston-Salem, NC, USA. As in past, this involved review of pathology specimens to determine the presence of cancer and then what type it was. I last reviewed for them over a year ago. In the last 3 years I have made no more than US$10,000.
Lucian R. Chirieac reports fees from Merck Sharp & Dohme and medicolegal work related to mesothelioma and lung cancer. The financial disclosures do not apply to the current study. Johan Bolting reports personal fees from Astra Zeneca, personal fees from Merck Sharp & Dohme, personal fees from Roche, personal fees from Pfizer, personal fees from Boehringer Ingelheim, personal fees from Novartis, grants and personal fees from Bristol-Myers Squibb, outside the submitted work.
Fernando Lopez-Rios reports grants and personal fees from Ventana Medical Systems, outside the submitted work.
Mauro Papotti reports personal fees from AstraZeneca, personal fees from Roche, personal fees from Pfizer, personal fees from AbbVie, personal fees from MSD, outside the submitted work.
Lynette M. Sholl reports personal fees from Foghorn Therapeutics, outside the submitted work.
Fred R. Hirsch has participated in several scientific advisory boards (Roche/Genenetech, AstraZeneca, BMS, Bayer, Loxo, Ventana, Helsinn, Abbvie) and has research funding to my lab research from Clovis, Merck, Biodesix, Rain Therapeutics, Cetya Therapeutics, Amgen- all through University of Colorado
Keith M. Kerr has received consultation fees or lecture fees from the following: AstraZeneca, Abbvie, BMS, Bayer, Eli Lilly, MSD, Merck Serono, Novartis, Pfizer, Roche, Ventana. Ming Sound Tsao reports grants and personal fees from AstraZeneca, personal fees from BMS, grants and personal fees from Merck, personal fees from Pfizer, personal fees from Abbvie, personal fees from Bayer, outside the submitted work.
Andrew G. Nicholson
Ignacio Wistuba reports grants and personal fees from Genentech/Roche, grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Astra Zeneca/Medimmune, grants and personal fees from Pfizer, grants and personal fees from HTG Molecular, personal fees from MSD, grants and personal fees from Asuragen, personal fees from Merck, personal fees from GlaxoSmithKline, from DepArray, grants from Adaptive, grants from EMD Serono, grants from Johnson & Johnson, grants from Bayer, grants from Takeda, grants from Amgen, grants from Karus, grants from Bayer, outside the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
NOTHING TO DISCLOSE:
Alain C. Borczuk
Claudia Poleri
Mary Beth Beasley
Jin-Haeng Chung
Andre L. Moreira
Giuseppe Pelosi
Prudence A. Russell
Anja C. Roden
Sylvie Lantuejoul Teh-Ying Chou
William D. Travis
Arne Warth
Akihiko Yoshida
Gang Chen
Erik Thunnissen
Masayuki Noguchi
Natasha Rekhtman
References
- 1.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243–1260. [DOI] [PubMed] [Google Scholar]
- 2.Tsao MS, Hirsch FR, Yatabe Y. IASLC ALTALS of ALK and ROS1 Testing in Lung Cancer. Notrth Fort Mers, FL: an IASLC publication; 2016. [Google Scholar]
- 3.Tsao MS, Kerr K, Dacic S, et al. IASLC Atlas of PD-L1 Testing in Lung Cancer Available at https://www.iaslc.org/publications/iaslc-atlas-pd-l1-testing-lung-cancer.
- 4.Tran L, Mattsson JS, Nodin B, et al. Various Antibody Clones of Napsin A, Thyroid Transcription Factor 1, and p40 and Comparisons With Cytokeratin 5 and p63 in Histopathologic Diagnostics of Non-Small Cell Lung Carcinoma. Appl Immunohistochem Mol Morphol 2016;24:648–659. [DOI] [PubMed] [Google Scholar]
- 5.Whithaus K, Fukuoka J, Prihoda TJ, et al. Evaluation of napsin A, cytokeratin 5/6, p63, and thyroid transcription factor 1 in adenocarcinoma versus squamous cell carcinoma of the lung. Arch Pathol Lab Med 2012;136:155–162. [DOI] [PubMed] [Google Scholar]
- 6.Turner BM, Cagle PT, Sainz IM, et al. Napsin A, a new marker for lung adenocarcinoma, is complementary and more sensitive and specific than thyroid transcription factor 1 in the differential diagnosis of primary pulmonary carcinoma: evaluation of 1674 cases by tissue microarray. Arch Pathol Lab Med 2012;136:163–171. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, Schmidt LA, Hatanaka K, et al. Evaluation of napsin A, TTF-1, p63, p40, and CK5/6 immunohistochemical stains in pulmonary neuroendocrine tumors. Am J Clin Pathol 2014;142:320–324. [DOI] [PubMed] [Google Scholar]
- 8.Rekhtman N, Pietanza CM, Sabari J, et al. Pulmonary large cell neuroendocrine carcinoma with adenocarcinoma-like features: napsin A expression and genomic alterations. Mod Pathol 2018;31:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueno T, Linder S, Elmberger G. Aspartic proteinase napsin is a useful marker for diagnosis of primary lung adenocarcinoma. Br J Cancer 2003;88:1229–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki A, Shijubo N, Yamada G, et al. Napsin A is useful to distinguish primary lung adenocarcinoma from adenocarcinomas of other organs. Pathol Res Pract 2005;201:579–586. [DOI] [PubMed] [Google Scholar]
- 11.Xu XY, Yang GY, Yang JH, et al. Analysis of clinical characteristics and differential diagnosis of the lung biopsy specimens in 99 adenocarcinoma cases and 111 squamous cell carcinoma cases: utility of an immunohistochemical panel containing CK5/6, CK34betaE12, p63, CK7 and TTF-1. Pathol Res Pract 2014;210:680–685. [DOI] [PubMed] [Google Scholar]
- 12.Ocque R, Tochigi N, Ohori NP, et al. Usefulness of immunohistochemical and histochemical studies in the classification of lung adenocarcinoma and squamous cell carcinoma in cytologic specimens. Am J Clin Pathol 2011;136:81–87. [DOI] [PubMed] [Google Scholar]
- 13.Nonaka D A study of DeltaNp63 expression in lung non-small cell carcinomas. Am J Surg Pathol 2012;36:895–899. [DOI] [PubMed] [Google Scholar]
- 14.Bishop JA, Teruya-Feldstein J, Westra WH, et al. p40 (DeltaNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol 2012;25:405–415. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida A, Tsuta K, Nakamura H, et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol 2011;35:1226–1234. [DOI] [PubMed] [Google Scholar]
- 16.Zhao W, Wang H, Peng Y, et al. DeltaNp63, CK5/6, TTF-1 and napsin A, a reliable panel to subtype non-small cell lung cancer in biopsy specimens. Int J Clin Exp Pathol 2014;7:4247–4253. [PMC free article] [PubMed] [Google Scholar]
- 17.Lau SK, Luthringer DJ, Eisen RN. Thyroid transcription factor-1: a review. Appl Immunohistochem Mol Morphol 2002;10:97–102. [DOI] [PubMed] [Google Scholar]
- 18.Warth A, Muley T, Herpel E, et al. Large-scale comparative analyses of immunomarkers for diagnostic subtyping of non-small-cell lung cancer biopsies. Histopathology 2012;61:1017–1025. [DOI] [PubMed] [Google Scholar]
- 19.Smits AJ, Vink A, Tolenaars G, et al. Different cutoff values for thyroid transcription factor-1 antibodies in the diagnosis of lung adenocarcinoma. Appl Immunohistochem Mol Morphol 2015;23:416–421. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi T, Takamochi K, Yanai Y, et al. Non-small cell lung carcinoma with diffuse coexpression of thyroid transcription factor-1 and DeltaNp63/p40. Hum Pathol 2018;78:177–181. [DOI] [PubMed] [Google Scholar]
- 21.Pelosi G, Fabbri A, Tamborini E, et al. Challenging Lung Carcinoma with Coexistent DeltaNp63/p40 and Thyroid Transcription Factor-1 Labeling Within the Same Individual Tumor Cells. J Thorac Oncol 2015;10:1500–1502. [DOI] [PubMed] [Google Scholar]
- 22.Longo L, Mengoli MC, Bertolini F, et al. Synchronous occurrence of squamous-cell carcinoma “transformation” and EGFR exon 20 S768I mutation as a novel mechanism of resistance in EGFR-mutated lung adenocarcinoma. Lung Cancer 2017;103:24–26. [DOI] [PubMed] [Google Scholar]
- 23.Jukna A, Montanari G, Mengoli MC, et al. Squamous Cell Carcinoma “Transformation” Concurrent with Secondary T790M Mutation in Resistant EGFR-Mutated Adenocarcinomas. J Thorac Oncol 2016;11:e49–51. [DOI] [PubMed] [Google Scholar]
- 24.Ordonez NG. Value of thyroid transcription factor-1 immunostaining in tumor diagnosis: a review and update. Appl Immunohistochem Mol Morphol 2012;20:429–444. [DOI] [PubMed] [Google Scholar]
- 25.Klebe S, Swalling A, Jonavicius L, et al. An immunohistochemical comparison of two TTF-1 monoclonal antibodies in atypical squamous lesions and sarcomatoid carcinoma of the lung, and pleural malignant mesothelioma. J Clin Pathol 2016;69:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kashima K, Hashimoto H, Nishida H, et al. Significant expression of thyroid transcription factor1 in pulmonary squamous cell carcinoma detected by SPT24 monoclonal antibody and CSA-II system. Appl Immunohistochem Mol Morphol 2014;22:119–124. [DOI] [PubMed] [Google Scholar]
- 27.Bae JM, Kim JH, Park JH, et al. Clinicopathological and molecular implications of aberrant thyroid transcription factor-1 expression in colorectal carcinomas: an immunohistochemical analysis of 1319 cases using three different antibody clones. Histopathology 2018;72:423–432. [DOI] [PubMed] [Google Scholar]
- 28.Gruchy JR, Barnes PJ, Dakin Hache KA. CytoLyt(R) fixation and decalcification pretreatments alter antigenicity in normal tissues compared with standard formalin fixation. Appl Immunohistochem Mol Morphol 2015;23:297–302. [DOI] [PubMed] [Google Scholar]
- 29.Kimbrell HZ, Gustafson KS, Huang M, et al. Subclassification of non-small cell lung cancer by cytologic sampling: a logical approach with selective use of immunocytochemistry. Acta Cytol 2012;56:419–424. [DOI] [PubMed] [Google Scholar]
- 30.Weissferdt A, Kalhor N, Rodriguez Canales J, et al. Spindle cell and pleomorphic (“sarcomatoid”) carcinomas of the lung: an immunohistochemical analysis of 86 cases. Hum Pathol 2017;59:1–9. [DOI] [PubMed] [Google Scholar]
- 31.Weissferdt A, Kalhor N, Correa AM, et al. “Sarcomatoid” carcinomas of the lung: a clinicopathological study of 86 cases with a new perspective on tumor classification. Hum Pathol 2017;63:14–26. [DOI] [PubMed] [Google Scholar]
- 32.Rossi G, Mengoli MC, Cavazza A, et al. Large cell carcinoma of the lung: clinically oriented classification integrating immunohistochemistry and molecular biology. Virchows Arch 2014;464:61–68. [DOI] [PubMed] [Google Scholar]
- 33.Righi L, Graziano P, Fornari A, et al. Immunohistochemical subtyping of nonsmall cell lung cancer not otherwise specified in fine-needle aspiration cytology: a retrospective study of 103 cases with surgical correlation. Cancer 2011;117:3416–3423. [DOI] [PubMed] [Google Scholar]
- 34.Montezuma D, Azevedo R, Lopes P, et al. A panel of four immunohistochemical markers (CK7, CK20, TTF-1, and p63) allows accurate diagnosis of primary and metastatic lung carcinoma on biopsy specimens. Virchows Arch 2013;463:749–754. [DOI] [PubMed] [Google Scholar]
- 35.Hwang DH, Szeto DP, Perry AS, et al. Pulmonary large cell carcinoma lacking squamous differentiation is clinicopathologically indistinguishable from solid-subtype adenocarcinoma. Arch Pathol Lab Med 2014;138:626–635. [DOI] [PubMed] [Google Scholar]
- 36.Pelosi G, Fabbri A, Papotti M, et al. Dissecting Pulmonary Large-Cell Carcinoma by Targeted Next Generation Sequencing of Several Cancer Genes Pushes Genotypic-Phenotypic Correlations to Emerge. J Thorac Oncol 2015;10:1560–1569. [DOI] [PubMed] [Google Scholar]
- 37.Rekhtman N, Tafe LJ, Chaft JE, et al. Distinct profile of driver mutations and clinical features in immunomarker-defined subsets of pulmonary large-cell carcinoma. Mod Pathol 2013;26:511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rekhtman N, Ang DC, Sima CS, et al. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod Pathol 2011;24:1348–1359. [DOI] [PubMed] [Google Scholar]
- 39.Mukhopadhyay S, Katzenstein AL. Subclassification of non-small cell lung carcinomas lacking morphologic differentiation on biopsy specimens: Utility of an immunohistochemical panel containing TTF-1, napsin A, p63, and CK5/6. Am J Surg Pathol 2011;35:15–25. [DOI] [PubMed] [Google Scholar]
- 40.Noh S, Shim H. Optimal combination of immunohistochemical markers for subclassification of non-small cell lung carcinomas: A tissue microarray study of poorly differentiated areas. Lung Cancer 2012;76:51–55. [DOI] [PubMed] [Google Scholar]
- 41.Sekar A, Gupta N, Rajwanshi A, et al. The role of the cytopathologist in subtyping and epidermal growth factor receptor testing in non-small cell lung cancer: An institutional experience. Cytopathology 2017;28:371–377. [DOI] [PubMed] [Google Scholar]
- 42.Koh J, Go H, Kim MY, et al. A comprehensive immunohistochemistry algorithm for the histological subtyping of small biopsies obtained from non-small cell lung cancers. Histopathology 2014;65:868–878. [DOI] [PubMed] [Google Scholar]
- 43.Gurda GT, Zhang L, Wang Y, et al. Utility of five commonly used immunohistochemical markers TTF-1, Napsin A, CK7, CK5/6 and P63 in primary and metastatic adenocarcinoma and squamous cell carcinoma of the lung: a retrospective study of 246 fine needle aspiration cases. Clin Transl Med 2015;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansson L Histopathologic classification of lung cancer: Relevance of cytokeratin and TTF-1 immunophenotyping. Ann Diagn Pathol 2004;8:259–267. [DOI] [PubMed] [Google Scholar]
- 45.Travis WD, Brambilla E, Burke A, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer; 2015. [DOI] [PubMed] [Google Scholar]
- 46.Ionescu DN, Treaba D, Gilks CB, et al. Nonsmall cell lung carcinoma with neuroendocrine differentiation--an entity of no clinical or prognostic significance. Am J Surg Pathol 2007;31:2632. [DOI] [PubMed] [Google Scholar]
- 47.Howe MC, Chapman A, Kerr K, et al. Neuroendocrine differentiation in non-small cell lung cancer and its relation to prognosis and therapy. Histopathology 2005;46:195–201. [DOI] [PubMed] [Google Scholar]
- 48.Sterlacci W, Fiegl M, Hilbe W, et al. Clinical relevance of neuroendocrine differentiation in nonsmall cell lung cancer assessed by immunohistochemistry: a retrospective study on 405 surgically resected cases. Virchows Arch 2009;455:125–132. [DOI] [PubMed] [Google Scholar]
- 49.Travis WD, Brambilla E, Nicholson AG. Testing for Neuroendocrine Immunohistochemical Markers Should Not Be Performed in Poorly Differentiated NSCCs in the Absence of Neuroendocrine Morphologic Features according to the 2015 WHO Classification. J Thorac Oncol 2016;11:e26–27. [DOI] [PubMed] [Google Scholar]
- 50.Chejfec G, Falkmer S, Grimelius L, et al. Synaptophysin. A new marker for pancreatic neuroendocrine tumors. Am J Surg Pathol 1987;11:241–247. [PubMed] [Google Scholar]
- 51.Loy TS, Darkow GV, Quesenberry JT. Immunostaining in the diagnosis of pulmonary neuroendocrine carcinomas. An immunohistochemical study with ultrastructural correlations. Am J Surg Pathol 1995;19:173–182. [DOI] [PubMed] [Google Scholar]
- 52.Thunnissen E, Borczuk AC, Flieder DB, et al. The Use of Immunohistochemistry Improves the Diagnosis of Small Cell Lung Cancer and Its Differential Diagnosis. An International Reproducibility Study in a Demanding Set of Cases. J Thorac Oncol 2017;12:334–346. [DOI] [PubMed] [Google Scholar]
- 53.Ye B, Cappel J, Findeis-Hosey J, et al. hASH1 is a specific immunohistochemical marker for lung neuroendocrine tumors. Hum Pathol 2016;48:142–147. [DOI] [PubMed] [Google Scholar]
- 54.Borges M, Linnoila RI, van de Velde HJ, et al. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature 1997;386:852–855. [DOI] [PubMed] [Google Scholar]
- 55.Linnoila RI, Zhao B, DeMayo JL, et al. Constitutive achaete-scute homologue-1 promotes airway dysplasia and lung neuroendocrine tumors in transgenic mice. Cancer Res 2000;60:4005–4009. [PubMed] [Google Scholar]
- 56.Jiang SX, Kameya T, Asamura H, et al. hASH1 expression is closely correlated with endocrine phenotype and differentiation extent in pulmonary neuroendocrine tumors. Mod Pathol 2004;17:222–229. [DOI] [PubMed] [Google Scholar]
- 57.Altree-Tacha D, Tyrrell J, Li F. mASH1 is Highly Specific for Neuroendocrine Carcinomas: An Immunohistochemical Evaluation on Normal and Various Neoplastic Tissues. Arch Pathol Lab Med 2017;141:288–292. [DOI] [PubMed] [Google Scholar]
- 58.Mukhopadhyay S, Dermawan JK, Lanigan CP, et al. Insulinoma-associated protein 1 (INSM1) is a sensitive and highly specific marker of neuroendocrine differentiation in primary lung neoplasms: an immunohistochemical study of 345 cases, including 292 whole-tissue sections. Mod Pathol 2018. [DOI] [PubMed] [Google Scholar]
- 59.Rooper LM, Sharma R, Li QK, et al. INSM1 Demonstrates Superior Performance to the Individual and Combined Use of Synaptophysin, Chromogranin and CD56 for Diagnosing Neuroendocrine Tumors of the Thoracic Cavity. Am J Surg Pathol 2017;41:1561–1569. [DOI] [PubMed] [Google Scholar]
- 60.Rosenbaum JN, Guo Z, Baus RM, et al. INSM1: A Novel Immunohistochemical and Molecular Marker for Neuroendocrine and Neuroepithelial Neoplasms. Am J Clin Pathol 2015;144:579–591. [DOI] [PubMed] [Google Scholar]
- 61.Sobecki M, Mrouj K, Camasses A, et al. The cell proliferation antigen Ki-67 organises heterochromatin. Elife 2016;5:e13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sobecki M, Mrouj K, Colinge J, et al. Cell-Cycle Regulation Accounts for Variability in Ki-67 Expression Levels. Cancer Res 2017;77:2722–2734. [DOI] [PubMed] [Google Scholar]
- 63.Pelosi G, Rindi G, Travis WD, et al. Ki-67 antigen in lung neuroendocrine tumors: unraveling a role in clinical practice. J Thorac Oncol 2014;9:273–284. [DOI] [PubMed] [Google Scholar]
- 64.Rindi G, Klimstra DS, Abedi-Ardekani B, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin B, Paesmans M, Mascaux C, et al. Ki-67 expression and patients survival in lung cancer: systematic review of the literature with meta-analysis. Br J Cancer 2004;91:2018–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kadota K, Suzuki K, Kachala SS, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Mod Pathol 2012;25:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yim J, Zhu LC, Chiriboga L, et al. Histologic features are important prognostic indicators in early stages lung adenocarcinomas. Mod Pathol 2007;20:233–241. [DOI] [PubMed] [Google Scholar]
- 68.Kadota K, Nitadori J, Woo KM, et al. Comprehensive pathological analyses in lung squamous cell carcinoma: single cell invasion, nuclear diameter, and tumor budding are independent prognostic factors for worse outcomes. J Thorac Oncol 2014;9:1126–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun A, Zhou W, Lunceford J, et al. Level of phosphohistone H3 among various types of human cancers. BMJ Open 2012;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsuta K, Liu DC, Kalhor N, et al. Using the mitosis-specific marker anti-phosphohistone H3 to assess mitosis in pulmonary neuroendocrine carcinomas. Am J Clin Pathol 2011;136:252–259. [DOI] [PubMed] [Google Scholar]
- 71.Voss SM, Riley MP, Lokhandwala PM, et al. Mitotic count by phosphohistone H3 immunohistochemical staining predicts survival and improves interobserver reproducibility in well-differentiated neuroendocrine tumors of the pancreas. Am J Surg Pathol 2015;39:13–24. [DOI] [PubMed] [Google Scholar]
- 72.Travis WD. Sarcomatoid neoplasms of the lung and pleura. Arch Pathol Lab Med 2010;134:1645–1658. [DOI] [PubMed] [Google Scholar]
- 73.Hwang HC, Pyott S, Rodriguez S, et al. BAP1 Immunohistochemistry and p16 FISH in the Diagnosis of Sarcomatous and Desmoplastic Mesotheliomas. Am J Surg Pathol 2016;40:714–718. [DOI] [PubMed] [Google Scholar]
- 74.Davidson B, Totsch M, Wohlschlaeger J, et al. The diagnostic role of BAP1 in serous effusions. Hum Pathol 2018;79:122–126. [DOI] [PubMed] [Google Scholar]
- 75.Kapur P, Christie A, Raman JD, et al. BAP1 immunohistochemistry predicts outcomes in a multiinstitutional cohort with clear cell renal cell carcinoma. J Urol 2014;191:603–610. [DOI] [PubMed] [Google Scholar]
- 76.Berg KB, Churg A. GATA3 Immunohistochemistry for Distinguishing Sarcomatoid and Desmoplastic Mesothelioma From Sarcomatoid Carcinoma of the Lung. Am J Surg Pathol 2017;41:1221–1225. [DOI] [PubMed] [Google Scholar]
- 77.Tochigi N, Attanoos R, Chirieac LR, et al. p16 Deletion in sarcomatoid tumors of the lung and pleura. Arch Pathol Lab Med 2013;137:632–636. [DOI] [PubMed] [Google Scholar]
- 78.Prins JB, Williamson KA, Kamp MM, et al. The gene for the cyclin-dependent-kinase-4 inhibitor, CDKN2A, is preferentially deleted in malignant mesothelioma. International journal of cancer 1998;75:649–653. [DOI] [PubMed] [Google Scholar]
- 79.Illei PB, Ladanyi M, Rusch VW, et al. The use of CDKN2A deletion as a diagnostic marker for malignant mesothelioma in body cavity effusions. Cancer 2003;99:51–56. [DOI] [PubMed] [Google Scholar]
- 80.Marchevsky AM, LeStang N, Hiroshima K, et al. The differential diagnosis between pleural sarcomatoid mesothelioma and spindle cell/pleomorphic (sarcomatoid) carcinomas of the lung: evidence-based guidelines from the International Mesothelioma Panel and the MESOPATH National Reference Center. Hum Pathol 2017;67:160–168. [DOI] [PubMed] [Google Scholar]
- 81.Liu X, Jia Y, Stoopler MB, et al. Next-Generation Sequencing of Pulmonary Sarcomatoid Carcinoma Reveals High Frequency of Actionable MET Gene Mutations. J Clin Oncol 2016;34:794–802. [DOI] [PubMed] [Google Scholar]
- 82.Schrock AB, Li SD, Frampton GM, et al. Pulmonary Sarcomatoid Carcinomas Commonly Harbor Either Potentially Targetable Genomic Alterations or High Tumor Mutational Burden as Observed by Comprehensive Genomic Profiling. J Thorac Oncol 2017;12:932–942. [DOI] [PubMed] [Google Scholar]
- 83.Falk N, Weissferdt A, Kalhor N, et al. Primary Pulmonary Salivary Gland-type Tumors: A Review and Update. Adv Anat Pathol 2016;23:13–23. [DOI] [PubMed] [Google Scholar]
- 84.Roden AC, Garcia JJ, Wehrs RN, et al. Histopathologic, immunophenotypic and cytogenetic features of pulmonary mucoepidermoid carcinoma. Mod Pathol 2014;27:1479–1488. [DOI] [PubMed] [Google Scholar]
- 85.Achcar Rde O, Nikiforova MN, Dacic S, et al. Mammalian mastermind like 2 11q21 gene rearrangement in bronchopulmonary mucoepidermoid carcinoma. Hum Pathol 2009;40:854–860. [DOI] [PubMed] [Google Scholar]
- 86.Salem A, Bell D, Sepesi B, et al. Clinicopathologic and genetic features of primary bronchopulmonary mucoepidermoid carcinoma: the MD Anderson Cancer Center experience and comprehensive review of the literature. Virchows Arch 2017;470:619–626. [DOI] [PubMed] [Google Scholar]
- 87.Hsieh MS, Lee YH, Chang YL. SOX10-positive salivary gland tumors: a growing list, including mammary analogue secretory carcinoma of the salivary gland, sialoblastoma, low-grade salivary duct carcinoma, basal cell adenoma/adenocarcinoma, and a subgroup of mucoepidermoid carcinoma. Hum Pathol 2016;56:134–142. [DOI] [PubMed] [Google Scholar]
- 88.Roden AC, Greipp PT, Knutson DL, et al. Histopathologic and Cytogenetic Features of Pulmonary Adenoid Cystic Carcinoma. J Thorac Oncol 2015;10:1570–1575. [DOI] [PubMed] [Google Scholar]
- 89.Nguyen CV, Suster S, Moran CA. Pulmonary epithelial-myoepithelial carcinoma: a clinicopathologic and immunohistochemical study of 5 cases. Hum Pathol 2009;40:366–373. [DOI] [PubMed] [Google Scholar]
- 90.Fechner RE, Bentinck BR, Askew JB, Jr. Acinic cell tumor of the lung. A histologic and ultrastructural study. Cancer 1972;29:501–508. [DOI] [PubMed] [Google Scholar]
- 91.Zhu S, Schuerch C, Hunt J. Review and updates of immunohistochemistry in selected salivary gland and head and neck tumors. Arch Pathol Lab Med 2015;139:55–66. [DOI] [PubMed] [Google Scholar]
- 92.Garcia JJ, Jin L, Jackson SB, et al. Primary pulmonary hyalinizing clear cell carcinoma of bronchial submucosal gland origin. Hum Pathol 2015;46:471–475. [DOI] [PubMed] [Google Scholar]
- 93.Savera AT, Sloman A, Huvos AG, et al. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. Am J Surg Pathol 2000;24:761–774. [DOI] [PubMed] [Google Scholar]
- 94.Leduc C, Zhang L, Oz B, et al. Thoracic Myoepithelial Tumors: A Pathologic and Molecular Study of 8 Cases With Review of the Literature. Am J Surg Pathol 2016;40:212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang T, McHugh JB, Berry GJ, et al. Primary mammary analogue secretory carcinoma of the lung: a case report. Hum Pathol 2018;74:109–113. [DOI] [PubMed] [Google Scholar]
- 96.Bishop JA. Unmasking MASC: bringing to light the unique morphologic, immunohistochemical and genetic features of the newly recognized mammary analogue secretory carcinoma of salivary glands. Head Neck Pathol 2013;7:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sholl LM, Nishino M, Pokharel S, et al. Primary Pulmonary NUT Midline Carcinoma: Clinical, Radiographic, and Pathologic Characterizations. J Thorac Oncol 2015;10:951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haack H, Johnson LA, Fry CJ, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol 2009;33:984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Evans AG, French CA, Cameron MJ, et al. Pathologic characteristics of NUT midline carcinoma arising in the mediastinum. Am J Surg Pathol 2012;36:1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.French CA, Kutok JL, Faquin WC, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol 2004;22:4135–4139. [DOI] [PubMed] [Google Scholar]
- 101.Yoshida A, Kobayashi E, Kubo T, et al. Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities. Mod Pathol 2017;30:797–809. [DOI] [PubMed] [Google Scholar]
- 102.Sauter JL, Graham RP, Larsen BT, et al. SMARCA4-deficient thoracic sarcoma: a distinctive clinicopathological entity with undifferentiated rhabdoid morphology and aggressive behavior. Mod Pathol 2017;30:1422–1432. [DOI] [PubMed] [Google Scholar]
- 103.Le Loarer F, Watson S, Pierron G, et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nature genetics 2015;47:1200–1205. [DOI] [PubMed] [Google Scholar]
- 104.Kimura N, Hasegawa M, Hiroshima K. SMARCB1/INI1/BAF47-deficient pleural malignant mesothelioma with rhabdoid features. Pathology international 2018;68:128–132. [DOI] [PubMed] [Google Scholar]
- 105.Agaimy A The expanding family of SMARCB1(INI1)-deficient neoplasia: implications of phenotypic, biological, and molecular heterogeneity. Adv Anat Pathol 2014;21:394–410. [DOI] [PubMed] [Google Scholar]
- 106.Skov BG, Skov T. Paired Comparison of PD-L1 Expression on Cytologic and Histologic Specimens From Malignancies in the Lung Assessed With PD-L1 IHC 28–8pharmDx and PD-L1 IHC 22C3pharmDx. Appl Immunohistochem Mol Morphol 2017;25:453–459. [DOI] [PubMed] [Google Scholar]
- 107.Wang W, Tang Y, Li J, et al. Detection of ALK rearrangements in malignant pleural effusion cell blocks from patients with advanced non-small cell lung cancer: a comparison of Ventana immunohistochemistry and fluorescence in situ hybridization. Cancer Cytopathol 2015;123:117–122. [DOI] [PubMed] [Google Scholar]
- 108.Vohra P, Buelow B, Chen YY, et al. Estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 expression in breast cancer FNA cell blocks and paired histologic specimens: A large retrospective study. Cancer Cytopathol 2016;124:828–835. [DOI] [PubMed] [Google Scholar]
- 109.Gorman BK, Kosarac O, Chakraborty S, et al. Comparison of breast carcinoma prognostic/predictive biomarkers on cell blocks obtained by various methods: Cellient, formalin and thrombin. Acta Cytol 2012;56:289–296. [DOI] [PubMed] [Google Scholar]
- 110.Jain D, Mathur SR, Iyer VK. Cell blocks in cytopathology: a review of preparative methods, utility in diagnosis and role in ancillary studies. Cytopathology 2014;25:356–371. [DOI] [PubMed] [Google Scholar]
- 111.Saqi A The State of Cell Blocks and Ancillary Testing: Past, Present, and Future. Arch Pathol Lab Med 2016;140:1318–1322. [DOI] [PubMed] [Google Scholar]
- 112.Crapanzano JP, Heymann JJ, Monaco S, et al. The state of cell block variation and satisfaction in the era of molecular diagnostics and personalized medicine. Cytojournal 2014;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rekhtman N, Buonocore DJ, Rudomina D, et al. Novel Modification of HistoGel-Based Cell Block Preparation Method: Improved Sufficiency for Molecular Studies. Arch Pathol Lab Med 2018;142:529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fischer AH, Schwartz MR, Moriarty AT, et al. Immunohistochemistry practices of cytopathology laboratories: a survey of participants in the College of American Pathologists Nongynecologic Cytopathology Education Program. Arch Pathol Lab Med 2014;138:1167–1172. [DOI] [PubMed] [Google Scholar]
- 115.Zhou F, Moreira AL. Lung Carcinoma Predictive Biomarker Testing by Immunoperoxidase Stains in Cytology and Small Biopsy Specimens: Advantages and Limitations. Arch Pathol Lab Med 2016;140:1331–1337. [DOI] [PubMed] [Google Scholar]
- 116.Sauter JL, Grogg KL, Vrana JA, et al. Young investigator challenge: Validation and optimization of immunohistochemistry protocols for use on cellient cell block specimens. Cancer Cytopathol 2016;124:89–100. [DOI] [PubMed] [Google Scholar]
- 117.Kalhor N, Zander DS, Liu J. TTF-1 and p63 for distinguishing pulmonary small-cell carcinoma from poorly differentiated squamous cell carcinoma in previously pap-stained cytologic material. Mod Pathol 2006;19:1117–1123. [DOI] [PubMed] [Google Scholar]
- 118.Savic S, Bode B, Diebold J, et al. Detection of ALK-positive non-small-cell lung cancers on cytological specimens: high accuracy of immunocytochemistry with the 5A4 clone. J Thorac Oncol 2013;8:1004–1011. [DOI] [PubMed] [Google Scholar]
- 119.Schmitt F, Cochand-Priollet B, Toetsch M, et al. Immunocytochemistry in Europe: results of the European Federation of Cytology Societies (EFCS) inquiry. Cytopathology 2011;22:238–242. [DOI] [PubMed] [Google Scholar]
- 120.Metzgeroth G, Kuhn C, Schultheis B, et al. Diagnostic accuracy of cytology and immunocytology in carcinomatous effusions. Cytopathology 2008;19:205–211. [DOI] [PubMed] [Google Scholar]
- 121.Roh MH, Schmidt L, Placido J, et al. The application and diagnostic utility of immunocytochemistry on direct smears in the diagnosis of pulmonary adenocarcinoma and squamous cell carcinoma. Diagn Cytopathol 2012;40:949–955. [DOI] [PubMed] [Google Scholar]
- 122.Kirbis IS, Maxwell P, Flezar MS, et al. External quality control for immunocytochemistry on cytology samples: a review of UK NEQAS ICC (cytology module) results. Cytopathology 2011;22:230–237. [DOI] [PubMed] [Google Scholar]
- 123.Goldstein NS, Thomas M. Mucinous and nonmucinous bronchioloalveolar adenocarcinomas have distinct staining patterns with thyroid transcription factor and cytokeratin 20 antibodies. Am J Clin Pathol 2001;116:319–325. [DOI] [PubMed] [Google Scholar]
- 124.Castro CY, Moran CA, Flieder DG, et al. Primary signet ring cell adenocarcinomas of the lung: a clinicopathological study of 15 cases. Histopathology 2001;39:397–401. [DOI] [PubMed] [Google Scholar]
- 125.Merchant SH, Amin MB, Tamboli P, et al. Primary signet-ring cell carcinoma of lung: immunohistochemical study and comparison with non-pulmonary signet-ring cell carcinomas. Am J Surg Pathol 2001;25:1515–1519. [DOI] [PubMed] [Google Scholar]
- 126.Lau SK, Desrochers MJ, Luthringer DJ. Expression of thyroid transcription factor-1, cytokeratin 7, and cytokeratin 20 in bronchioloalveolar carcinomas: an immunohistochemical evaluation of 67 cases. Mod Pathol 2002;15:538–542. [DOI] [PubMed] [Google Scholar]
- 127.Simsir A, Wei XJ, Yee H, et al. Differential expression of cytokeratins 7 and 20 and thyroid transcription factor-1 in bronchioloalveolar carcinoma: an immunohistochemical study in fineneedle aspiration biopsy specimens. Am J Clin Pathol 2004;121:350–357. [DOI] [PubMed] [Google Scholar]
- 128.Stenhouse G, Fyfe N, King G, et al. Thyroid transcription factor 1 in pulmonary adenocarcinoma. J Clin Pathol 2004;57:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rossi G, Murer B, Cavazza A, et al. Primary mucinous (so-called colloid) carcinomas of the lung: a clinicopathologic and immunohistochemical study with special reference to CDX-2 homeobox gene and MUC2 expression. Am J Surg Pathol 2004;28:442–452. [DOI] [PubMed] [Google Scholar]
- 130.Saad RS, Cho P, Silverman JF, et al. Usefulness of Cdx2 in separating mucinous bronchioloalveolar adenocarcinoma of the lung from metastatic mucinous colorectal adenocarcinoma. Am J Clin Pathol 2004;122:421–427. [DOI] [PubMed] [Google Scholar]
- 131.Mazziotta RM, Borczuk AC, Powell CA, et al. CDX2 immunostaining as a gastrointestinal marker: expression in lung carcinomas is a potential pitfall. Appl Immunohistochem Mol Morphol 2005;13:55–60. [DOI] [PubMed] [Google Scholar]
- 132.Tsuta K, Ishii G, Nitadori J, et al. Comparison of the immunophenotypes of signet-ring cell carcinoma, solid adenocarcinoma with mucin production, and mucinous bronchioloalveolar carcinoma of the lung characterized by the presence of cytoplasmic mucin. J Pathol 2006;209:78–87. [DOI] [PubMed] [Google Scholar]
- 133.Wislez M, Antoine M, Baudrin L, et al. Non-mucinous and mucinous subtypes of adenocarcinoma with bronchioloalveolar carcinoma features differ by biomarker expression and in the response to gefitinib. Lung Cancer 2010;68:185–191. [DOI] [PubMed] [Google Scholar]
- 134.Stoll LM, Johnson MW, Gabrielson E, et al. The utility of napsin-A in the identification of primary and metastatic lung adenocarcinoma among cytologically poorly differentiated carcinomas. Cancer Cytopathol 2010;118:441–449. [DOI] [PubMed] [Google Scholar]
- 135.Kunii R, Jiang S, Hasegawa G, et al. The predominant expression of hepatocyte nuclear factor 4alpha (HNF4alpha) in thyroid transcription factor-1 (TTF-1)-negative pulmonary adenocarcinoma. Histopathology 2011;58:467–476. [DOI] [PubMed] [Google Scholar]
- 136.Chu PG, Chung L, Weiss LM, et al. Determining the site of origin of mucinous adenocarcinoma: an immunohistochemical study of 175 cases. Am J Surg Pathol 2011;35:1830–1836. [DOI] [PubMed] [Google Scholar]
- 137.Wu J, Chu PG, Jiang Z, et al. Napsin A expression in primary mucin-producing adenocarcinomas of the lung: an immunohistochemical study. Am J Clin Pathol 2013;139:160–166. [DOI] [PubMed] [Google Scholar]
- 138.Krasinskas AM, Chiosea SI, Pal T, et al. KRAS mutational analysis and immunohistochemical studies can help distinguish pancreatic metastases from primary lung adenocarcinomas. Mod Pathol 2014;27:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rossi G, Cavazza A, Righi L, et al. Napsin-A, TTF-1, EGFR, and ALK Status Determination in Lung Primary and Metastatic Mucin-Producing Adenocarcinomas. International journal of surgical pathology 2014;22:401–407. [DOI] [PubMed] [Google Scholar]
- 140.Rekhtman N, Kazi S. Nonspecific reactivity of polyclonal napsin a antibody in mucinous adenocarcinomas of various sites: a word of caution. Arch Pathol Lab Med 2015;139:434–436. [DOI] [PubMed] [Google Scholar]
- 141.Geles A, Gruber-Moesenbacher U, Quehenberger F, et al. Pulmonary mucinous adenocarcinomas: architectural patterns in correlation with genetic changes, prognosis and survival. Virchows Arch 2015;467:675–686. [DOI] [PubMed] [Google Scholar]
- 142.Duruisseaux M, Antoine M, Rabbe N, et al. Lepidic predominant adenocarcinoma and invasive mucinous adenocarcinoma of the lung exhibit specific mucin expression in relation with oncogenic drivers. Lung Cancer 2017;109:92–100. [DOI] [PubMed] [Google Scholar]
- 143.Yatabe Y, Koga T, Mitsudomi T, et al. CK20 expression, CDX2 expression, K-ras mutation, and goblet cell morphology in a subset of lung adenocarcinomas. J Pathol 2004;203:645–652. [DOI] [PubMed] [Google Scholar]
- 144.Rosenblatt MB, Lisa JR, Collier F. Primary and metastatic bronciolo-alveolar carcinoma. Dis Chest 1967;52:147–152. [DOI] [PubMed] [Google Scholar]
- 145.Ritterhouse LL, Vivero M, Mino-Kenudson M, et al. GNAS mutations in primary mucinous and non-mucinous lung adenocarcinomas. Mod Pathol 2017;30:1720–1727. [DOI] [PubMed] [Google Scholar]
- 146.Heymann JJ, Hoda RS, Scognamiglio T. Polyclonal napsin A expression: a potential diagnostic pitfall in distinguishing primary from metastatic mucinous tumors in the lung. Arch Pathol Lab Med 2014;138:1067–1071. [DOI] [PubMed] [Google Scholar]
- 147.Mukhopadhyay S, Katzenstein AL. Comparison of monoclonal napsin A, polyclonal napsin A, and TTF-1 for determining lung origin in metastatic adenocarcinomas. Am J Clin Pathol 2012;138:703–711. [DOI] [PubMed] [Google Scholar]
- 148.Sugano M, Nagasaka T, Sasaki E, et al. HNF4alpha as a marker for invasive mucinous adenocarcinoma of the lung. Am J Surg Pathol 2013;37:211–218. [DOI] [PubMed] [Google Scholar]
- 149.Hishima T, Fukayama M, Fujisawa M, et al. CD5 expression in thymic carcinoma. The American journal of pathology 1994;145:268–275. [PMC free article] [PubMed] [Google Scholar]
- 150.Nakagawa K, Matsuno Y, Kunitoh H, et al. Immunohistochemical KIT (CD117) expression in thymic epithelial tumors. Chest 2005;128:140–144. [DOI] [PubMed] [Google Scholar]
- 151.Kriegsmann M, Muley T, Harms A, et al. Differential diagnostic value of CD5 and CD117 expression in thoracic tumors: a large scale study of 1465 non-small cell lung cancer cases. Diagn Pathol 2015;10:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Toriyama A, Mori T, Sekine S, et al. Utility of PAX8 mouse monoclonal antibody in the diagnosis of thyroid, thymic, pleural and lung tumours: a comparison with polyclonal PAX8 antibody. Histopathology 2014;65:465–472. [DOI] [PubMed] [Google Scholar]
- 153.Weichert W, Schewe C, Denkert C, et al. Molecular HPV typing as a diagnostic tool to discriminate primary from metastatic squamous cell carcinoma of the lung. Am J Surg Pathol 2009;33:513–520. [DOI] [PubMed] [Google Scholar]
- 154.Bishop JA, Ogawa T, Chang X, et al. HPV analysis in distinguishing second primary tumors from lung metastases in patients with head and neck squamous cell carcinoma. Am J Surg Pathol 2012;36:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ragin C, Obikoya-Malomo M, Kim S, et al. HPV-associated lung cancers: an international pooled analysis. Carcinogenesis 2014;35:1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chang SY, Keeney M, Law M, et al. Detection of human papillomavirus in non-small cell carcinoma of the lung. Hum Pathol 2015;46:1592–1597. [DOI] [PubMed] [Google Scholar]
- 157.Laury AR, Perets R, Piao H, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol 2011;35:816–826. [DOI] [PubMed] [Google Scholar]
- 158.Zhang PJ, Gao HG, Pasha TL, et al. TTF-1 expression in ovarian and uterine epithelial neoplasia and its potential significance, an immunohistochemical assessment with multiple monoclonal antibodies and different secondary detection systems. Int J Gynecol Pathol 2009;28:10–18. [DOI] [PubMed] [Google Scholar]
- 159.Fujiwara S, Nawa A, Nakanishi T, et al. Thyroid transcription factor 1 expression in ovarian carcinomas is an independent prognostic factor. Hum Pathol 2010;41:560–565. [DOI] [PubMed] [Google Scholar]
- 160.Iwamoto M, Nakatani Y, Fugo K, et al. Napsin A is frequently expressed in clear cell carcinoma of the ovary and endometrium. Hum Pathol 2015;46:957–962. [DOI] [PubMed] [Google Scholar]
- 161.Miettinen M, McCue PA, Sarlomo-Rikala M, et al. GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol 2014;38:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bhargava R, Beriwal S, Dabbs DJ. Mammaglobin vs GCDFP-15: an immunohistologic validation survey for sensitivity and specificity. Am J Clin Pathol 2007;127:103–113. [DOI] [PubMed] [Google Scholar]
- 163.Sasaki E, Tsunoda N, Hatanaka Y, et al. Breast-specific expression of MGB1/mammaglobin: an examination of 480 tumors from various organs and clinicopathological analysis of MGB1positive breast cancers. Mod Pathol 2007;20:208–214. [DOI] [PubMed] [Google Scholar]
- 164.Takeda Y, Tsuta K, Shibuki Y, et al. Analysis of expression patterns of breast cancer-specific markers (mammaglobin and gross cystic disease fluid protein 15) in lung and pleural tumors. Arch Pathol Lab Med 2008;132:239–243. [DOI] [PubMed] [Google Scholar]
- 165.Raso MG, Behrens C, Herynk MH, et al. Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin Cancer Res 2009;15:5359–5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yang M, Nonaka D. A study of immunohistochemical differential expression in pulmonary and mammary carcinomas. Mod Pathol 2010;23:654–661. [DOI] [PubMed] [Google Scholar]
- 167.Hattori Y, Yoshida A, Yoshida M, et al. Evaluation of androgen receptor and GATA binding protein 3 as immunohistochemical markers in the diagnosis of metastatic breast carcinoma to the lung. Pathology international 2015;65:286–292. [DOI] [PubMed] [Google Scholar]
- 168.Schnitt SJ, Wang HH, Owings DV, et al. Sampling grossly benign breast biopsy specimens. Lancet 1989;2:1038. [DOI] [PubMed] [Google Scholar]
- 169.Harbhajanka A, Chahar S, Miskimen K, et al. Clinicopathological, immunohistochemical and molecular correlation of neural crest transcription factor SOX10 expression in triple-negative breast carcinoma. Hum Pathol 2018;80:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nelson ER, Sharma R, Argani P, et al. Utility of Sox10 labeling in metastatic breast carcinomas. Hum Pathol 2017;67:205–210. [DOI] [PubMed] [Google Scholar]
- 171.Striebel JM, Dacic S, Yousem SA. Gross cystic disease fluid protein-(GCDFP-15): expression in primary lung adenocarcinoma. Am J Surg Pathol 2008;32:426–432. [DOI] [PubMed] [Google Scholar]
- 172.Sakurai A, Sakai Y, Yatabe Y. Thyroid transcription factor-1 expression in rare cases of mammary ductal carcinoma. Histopathology 2011;59:145–148. [DOI] [PubMed] [Google Scholar]
- 173.Robens J, Goldstein L, Gown AM, et al. Thyroid transcription factor-1 expression in breast carcinomas. Am J Surg Pathol 2010;34:1881–1885. [DOI] [PubMed] [Google Scholar]
- 174.Ni YB, Tsang JY, Shao MM, et al. TTF-1 expression in breast carcinoma: an unusual but real phenomenon. Histopathology 2014;64:504–511. [DOI] [PubMed] [Google Scholar]
- 175.Gruver AM, Amin MB, Luthringer DJ, et al. Selective immunohistochemical markers to distinguish between metastatic high-grade urothelial carcinoma and primary poorly differentiated invasive squamous cell carcinoma of the lung. Arch Pathol Lab Med 2012;136:1339–1346. [DOI] [PubMed] [Google Scholar]
- 176.Hoang LL, Tacha D, Bremer RE, et al. Uroplakin II (UPII), GATA3, and p40 are Highly Sensitive Markers for the Differential Diagnosis of Invasive Urothelial Carcinoma. Appl Immunohistochem Mol Morphol 2015;23:711–716. [DOI] [PubMed] [Google Scholar]
- 177.Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol 2012;36:1472–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Agoff SN, Lamps LW, Philip AT, et al. Thyroid transcription factor-1 is expressed in extrapulmonary small cell carcinomas but not in other extrapulmonary neuroendocrine tumors. Mod Pathol 2000;13:238–242. [DOI] [PubMed] [Google Scholar]
- 179.Srivastava A, Hornick JL. Immunohistochemical staining for CDX-2, PDX-1, NESP-55, and TTF-1 can help distinguish gastrointestinal carcinoid tumors from pancreatic endocrine and pulmonary carcinoid tumors. Am J Surg Pathol 2009;33:626–632. [DOI] [PubMed] [Google Scholar]
- 180.Wang YC, Iezza G, Zuraek MB, et al. Lack of NKX2.2 expression in bronchopulmonary typical carcinoid tumors: implications for patients with neuroendocrine tumor metastases and unknown primary site. J Surg Res 2010;163:47–51. [DOI] [PubMed] [Google Scholar]
- 181.Yang Z, Klimstra DS, Hruban RH, et al. Immunohistochemical Characterization of the Origins of Metastatic Well-differentiated Neuroendocrine Tumors to the Liver. Am J Surg Pathol 2017;41:915–922. [DOI] [PubMed] [Google Scholar]
- 182.Liau JY, Tsai JH, Jeng YM, et al. The Diagnostic Utility of PAX8 for Neuroendocrine Tumors: An Immunohistochemical Reappraisal. Appl Immunohistochem Mol Morphol 2016;24:57–63. [DOI] [PubMed] [Google Scholar]
- 183.Ordonez NG. Napsin A expression in lung and kidney neoplasia: a review and update. Adv Anat Pathol 2012;19:66–73. [DOI] [PubMed] [Google Scholar]
- 184.Gurel B, Ali TZ, Montgomery EA, et al. NKX3.1 as a marker of prostatic origin in metastatic tumors. Am J Surg Pathol 2010;34:1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Seipel AH, Samaratunga H, Delahunt B, et al. Immunohistochemical profile of ductal adenocarcinoma of the prostate. Virchows Arch 2014;465:559–565. [DOI] [PubMed] [Google Scholar]
- 186.Haninger DM, Kloecker GH, Bousamra Ii M, et al. Hepatoid adenocarcinoma of the lung: report of five cases and review of the literature. Mod Pathol 2014;27:535–542. [DOI] [PubMed] [Google Scholar]
- 187.Chandan VS, Shah SS, Torbenson MS, et al. Arginase-1 is frequently positive in hepatoid adenocarcinomas. Hum Pathol 2016;55:11–16. [DOI] [PubMed] [Google Scholar]
- 188.Wang DZ, Liu P, Yao L, et al. Aberrant expression of thyroid transcription factor-1 in schwannomas. Hum Pathol 2018;71:84–90. [DOI] [PubMed] [Google Scholar]
- 189.Nackaerts K, Park K, Sun JM, et al. Clinical Presentation and Prognostic Factors in Lung Cancer In: Pass HI, Ball D, Scagliotti GV, eds. IASLC Thoracic Oncology. Philadelphia, PA: Elsevier; 2018. [Google Scholar]
- 190.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nature reviews 2007;7:778–790. [DOI] [PubMed] [Google Scholar]
- 191.Kadota K, Nitadori J, Rekhtman N, et al. Reevaluation and reclassification of resected lung carcinomas originally diagnosed as squamous cell carcinoma using immunohistochemical analysis. Am J Surg Pathol 2015;39:1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lyda MH, Weiss LM. Immunoreactivity for epithelial and neuroendocrine antibodies are useful in the differential diagnosis of lung carcinomas. Hum Pathol 2000;31:980–987. [DOI] [PubMed] [Google Scholar]
- 193.Camilo R, Capelozzi VL, Siqueira SA, et al. Expression of p63, keratin 5/6, keratin 7, and surfactant-A in non-small cell lung carcinomas. Hum Pathol 2006;37:542–546. [DOI] [PubMed] [Google Scholar]
- 194.Nicholson SA, Beasley MB, Brambilla E, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol 2002;26:1184–1197. [DOI] [PubMed] [Google Scholar]
- 195.Zheng G, Ettinger DS, Maleki Z. Utility of the quantitative Ki-67 proliferation index and CD56 together in the cytologic diagnosis of small cell lung carcinoma and other lung neuroendocrine tumors. Acta Cytol 2013;57:281–290. [DOI] [PubMed] [Google Scholar]
- 196.Yeh YC, Chou TY. Pulmonary neuroendocrine tumors: study of 90 cases focusing on clinicopathological characteristics, immunophenotype, preoperative biopsy, and frozen section diagnoses. Journal of surgical oncology 2014;109:280–286. [DOI] [PubMed] [Google Scholar]
- 197.Hiroshima K, Iyoda A, Shida T, et al. Distinction of pulmonary large cell neuroendocrine carcinoma from small cell lung carcinoma: a morphological, immunohistochemical, and molecular analysis. Mod Pathol 2006;19:1358–1368. [DOI] [PubMed] [Google Scholar]
- 198.Maleki Z Diagnostic issues with cytopathologic interpretation of lung neoplasms displaying high-grade basaloid or neuroendocrine morphology. Diagn Cytopathol 2011;39:159–167. [DOI] [PubMed] [Google Scholar]
- 199.Lecoutere E, Creytens D. Multifocal cytokeratin expression in pleural and abdominal malignant solitary fibrous tumors: an unusual diagnostic pitfall. Virchows Arch 2015;467:119–121. [DOI] [PubMed] [Google Scholar]
- 200.Hanau CA, Miettinen M. Solitary fibrous tumor: histological and immunohistochemical spectrum of benign and malignant variants presenting at different sites. Hum Pathol 1995;26:440–449. [DOI] [PubMed] [Google Scholar]
- 201.Yoshida A, Tsuta K, Ohno M, et al. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol 2014;38:552–559. [DOI] [PubMed] [Google Scholar]
- 202.Doyle LA, Vivero M, Fletcher CD, et al. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol 2014;27:390–395. [DOI] [PubMed] [Google Scholar]
- 203.Walters MP, McPhail ED, Law ME, et al. BCL-6 expression in mesenchymal tumours: an immunohistochemical and fluorescence in situ hybridisation study. J Clin Pathol 2011;64:866–869. [DOI] [PubMed] [Google Scholar]
- 204.Freeman A, Geddes N, Munson P, et al. Anaplastic lymphoma kinase (ALK 1) staining and molecular analysis in inflammatory myofibroblastic tumours of the bladder: a preliminary clinicopathological study of nine cases and review of the literature. Mod Pathol 2004;17:765–771. [DOI] [PubMed] [Google Scholar]
- 205.Antonescu CR, Suurmeijer AJ, Zhang L, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 gene fusions and rare novel RET rearrangement. Am J Surg Pathol 2015;39:957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lovly CM, Gupta A, Lipson D, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov 2014;4:889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lino-Silva LS, Flores-Gutierrez JP, Vilches-Cisneros N, et al. TLE1 is expressed in the majority of primary pleuropulmonary synovial sarcomas. Virchows Arch 2011;459:615–621. [DOI] [PubMed] [Google Scholar]