Abstract
Teeth are richly supported by blood vessels and peripheral nerves. The aim of this study was to describe in detail the developmental time‐course and localization of blood vessels during early tooth formation and to compare that to innervation, as well as to address the putative role of vascular endothelial growth factor (VEGF), which is an essential regulator of vasculature development, in this process. The localization of blood vessels and neurites was compared using double immunofluorescence staining on sections at consecutive stages of the embryonic (E) and postnatal (PN) mandibular first molar tooth germ (E11‐PN7). Cellular mRNA expression domains of VEGF and its signaling receptor VEGFR2 were studied using sectional radioactive in situ hybridization. Expression of VEGF mRNA and the encoded protein were studied by RT‐PCR and western blot analysis, respectively, in the cap and early bell stage tooth germs, respectively. VEGFR2 was immunolocalized on tooth tissue sections. Smooth muscle cells were investigated by anti‐alpha smooth muscle actin (αSMA) antibodies. VEGF showed developmentally regulated epithelial and mesenchymal mRNA expression domains including the enamel knot signaling centers that correlated with the growth and navigation of the blood vessels expressing Vegfr2 and VEGFR2 to the dental papilla and enamel organ. Developing blood vessels were present in the jaw mesenchyme including the presumptive dental mesenchyme before the appearance of the epithelial dental placode and dental neurites. Similarly, formation of a blood vessel plexus around the bud stage tooth germ and ingrowth of vessels into dental papilla at E14 preceded ingrowth of neurites. Subsequently, pioneer blood vessels in the dental papilla started to receive smooth muscle coverage at the early embryonic bell stage. Establishment and patterning of the blood vessels and nerves during tooth formation are developmentally regulated, stepwise processes that likely involve differential patterning mechanisms. Development of tooth vascular supply is proposed to be regulated by local, tooth‐specific regulation by epithelial–mesenchymal tissue interactions and involving tooth target expressed VEGF signaling. Further investigations on tooth vascular development by local VEGF signaling, as well as how tooth innervation and development of blood vessels are integrated with advancing tooth organ formation by local signaling mechanisms, are warranted.
Keywords: innervation, patterning, tooth development, vascularization, VEGF, VEGFR2
Introduction
Teeth are organs that serve distinct specialized masticatory functions and are essential for the majority of vertebrates. The developing tooth germ is also a beneficial model system for the investigation of developmental mechanisms in three‐dimensional organ formation, development and function of peripheral innervation and stem cells, as well as in the study of the mechanisms underlying human dental genetic anomalies (Fried et al. 2007; Cobourne & Sharpe, 2013; Klein et al. 2013; Kaukua et al. 2014; Luukko & Kettunen, 2014, 2016; Thesleff, 2014; Yu et al. 2015). In order to carry out its lifelong functions, the tooth is supplied and supported by the peripheral sensory nerves, which mediate sensory functions as well as blood vessels, and supply oxygen and nutrients and dispose of waste products. In addition, blood flow in the dental blood vessels is under the control of sympathetic nerves (Olgart, 1996).
The development of tooth germ is characterized by epithelial folding morphogenesis, which leads to the formation of the tooth‐specific crown shape as well as gradual determination and differentiation of tooth‐specific hard tissue secreting cells, followed by root formation and eruption of the tooth into the oral cavity (Kollar & Lumsden, 1979; Thesleff & Nieminen, 2005). Classical developmental biology research has established that odontogenesis is regulated by local, reciprocal and sequential epithelial–mesenchymal tissue interactions between ectodermal epithelium and neural crest‐derived ectomesenchymal cells (Mina & Kollar, 1987; Lumsden, 1988). Conserved signaling molecular pathways, which mediate dental tissue interactions, are integrated into signaling networks controlling odontogenesis (Cobourne & Sharpe, 2010; Jussila & Thesleff, 2012). Many of the important signals are expressed in the epithelial signaling centers involved in regulation of tooth budding morphogenesis and ultimate shape of the crown, finally determined by deposition of enamel (Jernvall et al. 1994; Thesleff et al. 2001; Luukko et al. 2003).
Developmental investigations using the mouse mandibular first molar tooth germ as a model system have demonstrated that growth, navigation and patterning of dental sensory trigeminal nerves occurs in a strict, developmentally regulated manner that is intimately linked with advancing crown morphogenesis and cell differentiation (Mohamed & Atkinson, 1983; Kettunen et al. 2005, 2007; Moe et al. 2008). Local interactions between the dental epithelial and mesenchymal tissue components (Luukko et al. 2008; Luukko & Kettunen, 2014, 2016) and tooth target expressed members of signaling molecule families such as transforming growth factor β (TGFβ), WNT, hedgehog (HH) and fibroblast growth factor (FGF)‐families (Kettunen & Thesleff, 1998; Kettunen et al. 1998, 2007; Cobourne & Sharpe, 2013; Thesleff, 2014) integrate tooth organogenesis in the development of its peripheral nerve supply (Kettunen et al. 2005, 2007). In addition, besides serving sensory functions, there is evidence that nerves serve broader, non‐neuronal roles during odontogenesis (Tuisku & Hildebrand, 1994; Kaukua et al. 2014; Zhao et al. 2014).
The cardiovascular and neuronal systems are among the earliest organ systems to develop (Lohela et al. 2009). Blood vessels and nerves are frequently arranged in an orderly pattern, and often are located alongside one another, and are functionally and physically interdependent (Carmeliet & Tessier‐Lavigne, 2005). Development of vascular and nervous systems is regulated by members of various signaling molecular families such as semaphorin and ephrin, netrin, slit and vascular endothelial growth factor (VEGF) as well as their receptors (Carmeliet & Tessier‐Lavigne, 2005; Carmeliet & Jain, 2011; Chung & Ferrara, 2011; Erskine et al. 2011; Ruiz de Almodovar et al. 2011). In the adult tooth, blood vessels and nerves are often located in the vicinity of each other (Hildebrand et al. 1995; Rodd & Boissonade, 2003; Steiniger et al. 2013). Especially myelinated nerve bundles display a tight association with arterioles in the root pulp and in the central region of the crown pulp (Steiniger et al. 2013). Because neurites and blood vessels have been located in the same areas of the developing tooth in separate studies, this raises the question of their intimate association and developmental interdependence during development (Gaunt, 1959; Decker, 1967; Mohamed & Atkinson, 1983; Luukko, 1997; Kettunen et al. 2005; Nait Lechguer et al. 2008; Rothova et al. 2011; Yuan et al. 2014).
Although localization of dental blood vessels has been investigated earlier in different species and by using different methods, studies in the mouse mandibular first molar, a central model system for developmental dental studies, have been limited to restricted stages (Gaunt, 1959; Decker, 1967; Nait Lechguer et al. 2008; Rothova et al. 2011; Yuan et al. 2014). In particular, comparison between the localization of neurites and blood vessels during tooth development has not been performed. Most molecular discoveries regarding formation of the tooth and its supporting tissues have, hitherto, been based on work with mouse teeth, in particular the mandibular first molar (Jheon et al. 2013; Luukko & Kettunen, 2014; Thesleff, 2014). Here, we performed systematic, intricate analysis of the developmental time‐course and localization of blood vessels in the mouse molar tooth germ prior to histological commencement of tooth germ development to after the inception of enamel formation in the crown postnatally, and compared their localization with growing neurites on the same sections using double immunofluorescence. VEGFA is critical for both vasculogenesis and angiogenesis, and for the development of large blood vessels (Carmeliet et al. 1996; Ferrara et al. 1996; Yancopoulos et al. 2000; Ferrara, 2004). Thus, expression of VEGF mRNA and its signaling receptor VEGFR2, as well as development of smooth muscle coverage of blood vessels, were investigated systematically during embryonic and early postnatal stages of mandibular first molar development. Our results show that, like tooth innervation, formation of blood vessels in the tooth is a developmentally regulated process that is intimately integrated with advancing tooth histomorphogenesis. Moreover, rather than being dependent on, or regulated by, neurites, blood vessel development is suggested to be controlled by local tissue interactions and developmentally regulated VEGF signaling, involving the activity of the enamel knot signaling center.
Material and methods
Animals
The use of CD1 and C57BL/6 mice in the present study was approved by the Department of Biomedicine, Faculty of Medicine and Dentistry, University of Bergen, under the surveillance of the Norwegian Animal Research authority. Breeding pairs of both mouse strains were kept together for over 3 nights. The appearance of a vaginal plug was taken as day 0 of embryogenesis and the day of parturition as postnatal day 0. Embryos and postnatal mice were collected at embryonic days (E) 10–14 and 16–18, as well as postnatal days (PN) 0, 2, 4, 7 and 11.
Immunohistochemistry
VEGFR2 (KDR/FLK1) is a specific marker for forming embryonic blood vessels arising by both vasculogenesis and angiogenesis; an antibody against it has earlier been used to detect developing blood vessels in tooth germ (Quinn et al. 1993; Yamaguchi et al. 1993; Nait Lechguer et al. 2008). The heads of embryos and the mandibles of postnatal pups were embedded in Tissue‐Tek OCT (Sakura Finetek, USA) and stored at −80 °C. Fresh frozen frontal and sagittal sections of the mandibular first molar tooth germ were cut serially into 16‐ and 30‐μm‐thick sections for the early embryonic and other stages, respectively. Tissue sections were placed on slides and dried for 30 min, and stored at −80 °C before use. For immunohistochemistry, tissue sections were first fixed in 4% paraformaldehyde (PFA) for 15 min and subsequently incubated in cold methanol for 30 min and blocked with 10% donkey serum (D9663, Sigma‐Aldrich Corporation, St. Louis, MO, USA) for 30 min at room temperature. Sections were then incubated in polyclonal goat anti‐mouse VEGFR2 (AF644, R&D Systems, Abingdon, UK; 1 : 20 dilution) primary‐conjugated donkey anti‐goat (705‐066‐147, Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA; 1 : 250 dilution) secondary antibody at 37 °C for 1 h. The avidin biotin peroxidase complex method (PK 4000, Vectastain Elite ABS kit; Thermo Fisher Scientific Inc., Waltham, MA, USA) was used according to the instructions of the manufacturer. 3‐Amino‐9‐ethylcarbazole (AEC; A6926, Sigma‐Aldrich Corporation) was applied as a chromogen. Sections were observed in a Zeiss Axioskop 2 Plus microscope (Oberkochen, Germany). Digital photomicrographs were taken using a Spot Insight digital camera (Diagnostic Instruments, Sterling Heights, MI, USA). The image plates were prepared using Adobe photoshop CS5 software (Adobe Systems Incorporated, San Jose, CA, USA).
Double immunofluorescence staining
The heads of embryos and the mandibles of postnatal pups were embedded in Tissue‐Tek OCT (Sakura Finetek, USA) and stored at −80 °C. Fresh frozen frontal, horizontal and sagittal sections of the mandibular first molar tooth germ were cut serially, and identically, into 16‐μm‐thick sections for the early embryonic stages and 30‐μm‐thick sections for the other stages. Embryonic tissues were cut into serial sections covering the whole tooth germ. Postnatal lower jaws were cut into identical sections. Tissue sections were placed on slides and dried for 30 min, and stored at −80 °C. Sections were first fixed in 4% PFA for 15 min and then placed in cold methanol for 30 min, and blocked with 10% donkey serum (D9663, Sigma‐Aldrich Corporation) for 30 min at room temperature. The slides were incubated at 4 °C overnight with the primary polyclonal goat anti‐mouse VEGFR2 (1 : 20 dilution) and polyclonal rabbit anti‐rat peripherin (AB1530, Chemicon International, Temecula, CA, USA; 1 : 100 dilution) antibodies. Peripherin antibody detects developing neurites during early tooth formation in the mouse and has been used in several studies (Loes et al. 2002; Kettunen et al. 2005, 2007; Shrestha et al. 2014). Sections were subsequently incubated in secondary fluorescein (FITC) donkey anti‐goat antibody (for VEGFR2; 1 : 50 dilution) and then washed three times in 1× phosphate‐buffered saline (PBS), which was followed by blocking in 10% normal goat serum for 30 min at room temperature. Thereafter, the slides were incubated in Cy3 goat anti‐rabbit antibody (for peripherin; 1 : 100 dilution) for 1 h at 37 °C. In addition, polyclonal rabbit anti‐human alpha smooth muscle actin (‐αSMA; AB5694, Abcam, UK; 1 : 100 dilution) and mouse monoclonal antibody against neurofilament 200 (NF200; N0142, Sigma‐Aldrich, USA; 1 : 100 dilution) were employed to visualize smooth muscle cells covering endothelial cells and neurites, respectively. Staining was performed on horizontal tooth sections. Cy3 donkey anti‐rabbit (for ‐αSMA; 1 : 50 dilution) and FITC donkey anti‐mouse (for NF200; 1 : 50 dilution) were used as secondary antibodies, which were incubated for 1 h at 37 °C. Sections were mounted using Vectashield mounting medium (Vector H‐1200; Vector Laboratories, Burlingame, CA, USA). Sections were observed sequentially with 4′,6‐diamidino‐2‐phenylindole (DAPI), Cy3 and FITC filter sets in a Zeiss Axioplan microscope (Carl Zeiss AG, Germany), and three channel images were taken with a Zeiss Axiocam digital camera.
cDNA and in situ hybridization
For in situ hybridization the heads of embryos and the mandibles of postnatal pups were fixed in 4% PFA overnight. Subsequently, the tissues were dehydrated in ethanol and embedded in paraffin, and serial or identical 7‐μm‐thick sections were cut. Tissue sections were stored at 4 °C until used. Sectional radioactive in situ hybridization was performed as described earlier (Luukko et al. 1996; Kettunen & Thesleff, 1998). The following plasmids were used to generate the probes: 1.8 kb mouse Vegf (covers 3′ two‐thirds of the coding region, as well as the entire 3‐untranslated region) subcloned in pBluescript (Stratagene, La Jolla, CA, USA) plasmid (Shweiki et al. 1992; Dumont et al. 1995), and 726 bp mouse Vegfr2 (partial cDNA spanning the transmembrane domain and part of the extracellular ligand‐binding domain) in pGEM7 plasmid (Promega; Yamaguchi et al. 1993; Dumont et al. 1995). Each plasmid was linearized by the appropriate restriction endonuclease and applied in in vitro transcription of 35S‐UTP‐labeled antisense and sense RNA probes using RNA polymerase. Hybridization was carried out for 15–20 h. For autoradiography, the slides were coated with NTB‐2 emulsion (Eastman Kodak, Rochester, NY, USA). After 3–4 weeks’ exposure, the sections were developed in Kodak D‐19 and fixed with Unifix (Eastman Kodak) fixative. The slides were counterstained with hematoxylin, and mounted with Depex (Electron Microscopy Sciences, Hatfield, PA, USA). Sections were observed using a Zeiss Axioskop 2 Plus microscope with high and low magnification objectives and representative digital bright‐ and dark‐field images were taken with 5 0.15 NA and 10 0.3 NA objectives and a Spot Insight camera. Image plates were constructed using Adobe photoshop CS4 software. No specific hybridization signal was observed in sections hybridized with control sense probes (not shown).
RT‐PCR analysis
Total RNA was isolated from E14 mouse molar tooth germs using GenElute Mammalian total RNA Miniprep Kit (Sigma cat no. RTN70‐1KT) and reverse‐transcribed with RevertAid M‐MuLV reverse transcriptase for RT‐PCR (Fermentas, cat no. EP0441). VEGF and GAPDH (glyceraldehyde 3‐phosphate dehydrogenase) genes were amplified using Taq DNA polymerase (VWR cat no. 5101600‐0100) for 40 cycles using various concentrations of MgCl2 (1.5–2.5 mm). The PCR products were fractionated by electrophoresis in 2% agarose gel. The following primer pairs were used for PCR amplification: GAPDH‐F (5′‐GCTGAGTATGTCGTGGAGTC‐3′/GAPDH‐R (5′‐TTGGTGGTGCAGGATGCATT‐3′) and VEGF‐F (5′‐GACCCTGGTGGACATCTTCCAGGA‐3′/VEGF‐R (5‐GGT GAG AGG TCT GGT TCC CGA‐3). The primers have been used earlier (Mukouyama et al. 2002; Ruhrberg et al. 2002).
Western blot
Mouse E16 mandibular molar tooth germs were homogenized using a manual homogenizer in 200 μL loading buffer [2% sodium dodecyl sulfate (SDS), 10% glycerol, 50 mm Tris‐HCl, pH 6.8 and 0.1% Bromophenol blue]. The tissues were subsequently vortexed and centrifuged. A 50‐μL sample was loaded and proteins were separated by 12% acrylamide gel (SDS‐PAGE). Electroblotting of proteins to a nitrocellulose membrane was performed overnight. The membrane was blocked with 5% dry milk in PBS‐0.1% Tween 20. Polyclonal goat anti‐mouse VEGF164 (R&D Systems, cat no. AF493; 0.1 μg mL−1) was added and incubation performed overnight. This was followed by a PBS‐0.1% Tween 20 wash and incubation in HRP‐conjugated anti‐goat antibody (Dako 1 : 2000). The Bio‐Rad Kaleidoscope ladder (cat no. 161‐0375) was used as a molecular weight standard. Bound antibody was visualized using the ECL detection system (enhanced Super Signal Chemiluminescence Kit, 34096 Pierce, USA) and an Image Reader (Las‐3000 version 2.0W).
Results
Comparison of the development of the blood vessels and peripheral nerves in the mouse mandibular first molar primordium
Before initial histological signs of the mandibular first molar tooth formation at embryonic day 10 (E10), many VEGFR2‐immunoreactive blood vessels were broadly scattered in the jaw mesenchyme, including the presumptive area of the dental mesenchyme (Fig. 1A). It was noteworthy that many blood vessels were present close to, and possibly even in contact with, the oral epithelium (Fig. 1A, an arrow). In contrast, the trigeminal mandibular neurites have been earlier demonstrated in the deep core area of mandibular process mesenchyme, located far from the presumptive first molar region itself (Lumsden, 1982; Loes et al. 2002; Kettunen et al. 2005, 2007).
Figure 1.
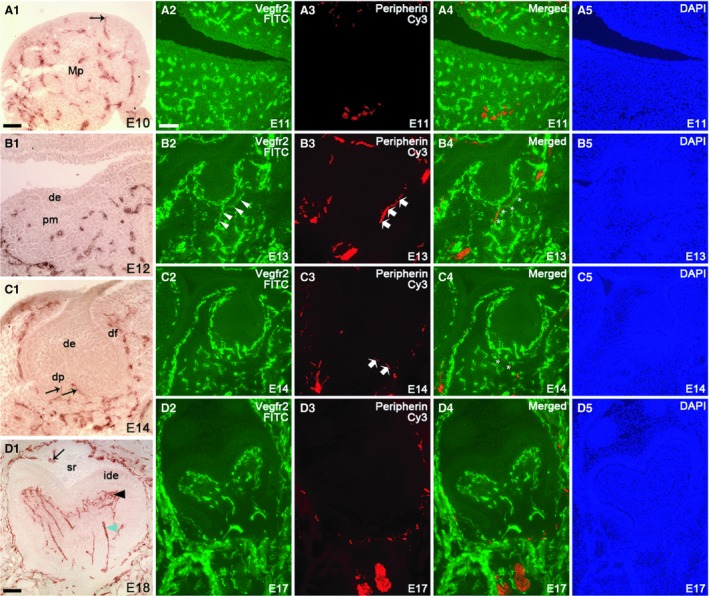
Distribution of VEGFR2‐positive blood vessels in the mandibular process and tooth germ at selected embryonic stages (E10, E12, E14 and E18) detected by immunohistochemistry and comparison of the localization of blood vessels and nerve fibers in the mouse mandibular first molar tooth germ at selected embryonic stages (E11, E13, E14 and E17), detected by immunofluorescence staining using anti‐VEGFR2 and anti‐peripherin antibodies (frontal sections). Immunofluorescence images are shown in the FITC channel (A2, B2, C2, D2), Cy3 channel (A3, B3, C3, D3), merged channel (A4, B4, C4, D4) and DAPI channel (A5, B5, C5, D5). (A1) At E10, blood vessels were spread all over the mandibular process and some vessels were in contact with oral epithelium (black arrow). (A2–A5) At E11, blood vessels were detected within the mandibular mesenchyme covering also the presumptive dental mesenchyme. Nerves were located far away from the tooth area. (B1) At E12, blood vessels were detected at a considerable distance from the dental epithelium. (B2–B5) At E13, blood vessels were present in the outer border of the condensed dental mesenchyme. The first neurites grew into the target area and extended to the buccal side of the tooth germ. (C1–C5) At E14, pioneer branches of blood vessels started to enter the dental papilla (black arrow). A new neurite branch grew to the lingual side of the tooth germ. (D2–D5) At E17, blood vessels were abundant in the papilla, but dental neurites remain in the dental follicle. (D1) At E18, blood vessels entered the enamel organ as a result of accompanying the outer enamel invaginations (black arrow). Differentiation of blood vessel plexus (black arrowhead) next to the inner dental epithelium and larger diameter vessels (green arrowhead) in the deeper dental papilla is clearly discernible at E18. de, dental epithelium; df, dental follicle; dp, dental papilla mesenchyme; ide, inner dental epithelium; Mp, mandibular process; pm, presumptive dental mesenchyme; sr, stellate reticulum cells. Scale bar in (A1): 100 μm; applies to A1–D5.
At the epithelial thickening stage (E11) numerous dispersed blood vessels were evident in the mandibular mesenchyme (Fig. 1A2,A4). Although they also persisted in the presumptive dental mesenchyme area, they were no longer present in close proximity to the oral or dental epithelium. At the early bud stage (E12) of tooth development, blood vessels appeared in the outer edge of the dental mesenchyme and in surrounding peridental mesenchyme (Fig. 1B1). The inferior alveolar nerve located in the deep core part of the mandibular mesenchyme was evident in E11 embryos (Fig. 1A3,A4). At E12 the molar nerve emerging from this major nerve branch has been reported to head towards the tooth target (Kettunen et al. 2005).
At E13, the dental mesenchyme had condensed around the invaginated bud‐shaped dental epithelium (Fig. 1B2,B4,B5). Blood vessels were localized in the mesenchyme around the tooth germ. On the buccal side of the tooth germ, two parallel blood vessels followed the contour of the tooth germ (Fig. 1B2,B4). At this stage, the first neurites had branched from the molar nerve, encountered the presumptive dental follicle target area, and extended to the buccal side of the tooth germ (Luukko et al. 2005; Fig. 1B3,B4). These axons appeared to accompany blood vessels to the tooth target area and thereafter follow them to the buccal side of the tooth (Fig. 1B4; white stars).
One day later (E14), the enamel organ had acquired a cap shape and surrounded the innermost area of the condensed dental mesenchyme, defined as the dental papilla; the dental mesenchymal cells, which surround the enamel organ and dental papilla, had formed the dental follicle (Fig. 1C1,C5). A higher number of blood vessels were found encircling the dental epithelium and dental papilla in the dental follicle area. Similarly, an increased number of nerve fibers were seen in the dental follicle target field area around the tooth germ on both buccal and lingual sides. Of note, whereas the first blood vessels, emerging from the blood vessel plexus at the base of the tooth germ, entered the dental papilla (future dental pulp; Fig. 1C1,C2,C4), no nerves were seen inside the dental papilla (Fig. 1C2,C4), in line with earlier reports (Mohamed & Atkinson, 1983; Kettunen et al. 2005, 2007).
During subsequent development, when the molar tooth germ had reached the early bell stage (E16 and 17; Fig. 1D2–D5), numerous blood vessels were observed in the dental follicle. Even though a heavy concentration of blood vessels were seen in contact with the outer enamel epithelium, they were absent from the enamel organ. An increased number of blood vessels were also evident within the dental papilla. Large diameter blood vessels were seen in the middle core area of the papilla mesenchyme and appeared to run towards the inner dental epithelium. An apparent plexus of thin blood vessels with many ramifications was seen to develop next to the preodontoblast layer (Fig. 1D2,D4). Many neurites were seen in the mesenchymal dental follicle target field surrounding the tooth germ, but they were still absent from the dental papilla or enamel organ (Fig. 1D3,D4).
In the later embryonic bell stage and in newborn tooth germs (E18 and PN0), a large number of blood vessels persisted in the dental follicle and clusters of blood vessels were evident in the dental papilla (Figs 1D1 and 2A1,A3). Relatively large‐diameter vessels were observed running from the primary apical foramen area towards the coronal, subodontoblastic area, where a plexus of small diameter blood vessels were present. Interestingly, at E18, blood vessels were seen in the epithelial enamel organ for the first time (Fig. 1D1; black arrow). At the newborn stage, blood vessels were relatively close to the stratum intermedium cells (Fig. 2A1,A3). Similar to the cap and early bell stages, nerve fibers were seen around the tooth germ, but they were absent from the dental pulp and enamel organ (Fig. 2A2,A3).
Figure 2.
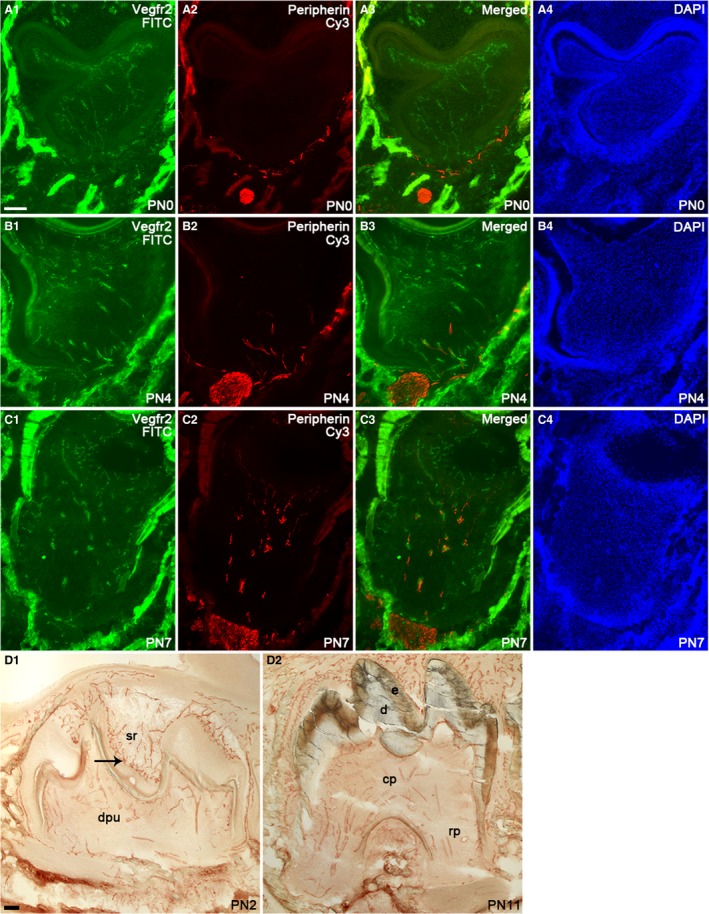
Localization of blood vessels and nerve fibers in the mouse mandibular first molar tooth germ at postnatal stages PN0, PN4 and PN7 detected by immunofluorescence using anti‐VEGFR2 and anti‐peripherin antibodies, respectively (A1–C4). At PN2 and PN11, blood vessel distribution in the tooth germ is displayed by immunohistochemistry with anti‐VEGFR2 antibody (D1–D2). Images are shown in the FITC channel (A1, B1, C1), Cy3 channel (A2, B2, C2), merged channels (A3, B3, C3) and DAPI channel (A4, B4, C4). Scale bar in (A1): 100 μm; applies to A1–D2.
At PN2, dentin deposition in the tooth crown commences and is followed by enamel production, which had become more evident at PN4. Similar to earlier stages, many blood vessels and nerve fibers were apparent around the tooth germ (Figs 1D1 and 2B1–B4). Similarly, blood vessels were evident in the dental pulp with an apparent blood vessel plexus in the subodontoblastic area. Some blood vessels also appeared to have reached the odontoblast layer. In the epithelial compartment of the tooth, the number of blood vessels had increased and they were now located next to the stratum intermedium cells adjacent to the ameloblast layer, forming a plexus‐like structure (Fig. 2D1). At PN4, branches of nerve fibers were found to have grown into the dental pulp for the first time, following the initiation of enamel deposition (Fig. 2B2,B3). It was also observed that some of the nerve fibers entered the pulp were associated with the larger blood vessels (Fig. 2B3). At PN7, the formation of the mesial and distal roots of the molar tooth had been initiated, followed by their further growth and elongation as seen at PN11 (Fig. 2D2). More blood vessels were seen inside the dental pulp, and similar feature was also observed in the enamel organ. Thick bundles of vessels had entered the dental pulp through the mesial and distal root areas and, in particular, the thicker nerve bundles appeared to follow larger blood vessels. Smaller diameter blood vessels were detected in the coronal pulp, especially next to the odontoblast layer (Fig. 2C1,C3).
Some nerve fibers have been documented to accompany blood vessels but many also lie without vascular contact in the adult tooth pulp (Uddman et al. 1999). We therefore studied the association between growing neurites and blood vessels during early postnatal pulp development. We performed double immunofluorescence (IF) staining using anti‐VEGFR2 and anti‐peripherin antibodies, as well as anti‐NF200 and anti‐αSMA antibodies. Peripherin and NF200 (neurofilament 200) were used as markers for nerve fibers (Debus et al. 1983; Kettunen et al. 2005), VEGFR2 for endothelial cells (Yamaguchi et al. 1993) and αSMA for vascular smooth muscle cells (Skalli et al. 1986). Previously, nerve‐associated blood vessels have been reported to be covered by αSMA‐immunopositive smooth muscle cells in skin (Li et al. 2013). Localization of NF200 and αSMA should therefore reveal association between nerve fibers with blood vessels covered with smooth muscle cells. After the nerve fiber ingrowth into the dental pulp, the thicker neurite bundles, but not the thin ones or individual neurites, were found to associate with blood vessels at PN4 (not shown), PN7 and PN11 (Fig. 3A1–B1). Double IF staining with anti‐NF200 and anti‐αSMA antibodies revealed that blood vessels the majority of the neurites were closely aligned with, were covered by smooth muscle cells especially at PN7 and PN11 (Fig. 3A2‐B2). Because pioneer blood vessels were found to grow into the dental papilla already at E14, this prompted us to investigate when these blood vessels start to receive smooth muscle coverage. Double immunoreactivity staining with anti‐VEGFR2 and anti‐αSMA antibodies showed αSMA immunoreactivity in mandibular artery at E14, whereas no such reactivity was observed in the dental blood vessels (Fig. 3C1–C4). At E16 early bell stage, some of the blood vessels, which had grown into the dental papilla, and those located in the dental follicle, were αSMA‐immunopositive (Fig. 3D1‐D4). It appears, therefore, that initiation of smooth muscle coverage of dental papilla blood vessels in the molar tooth germ takes place at the early bell stage about 2 days after appearance of the pioneer blood vessels in the dental papilla.
Figure 3.
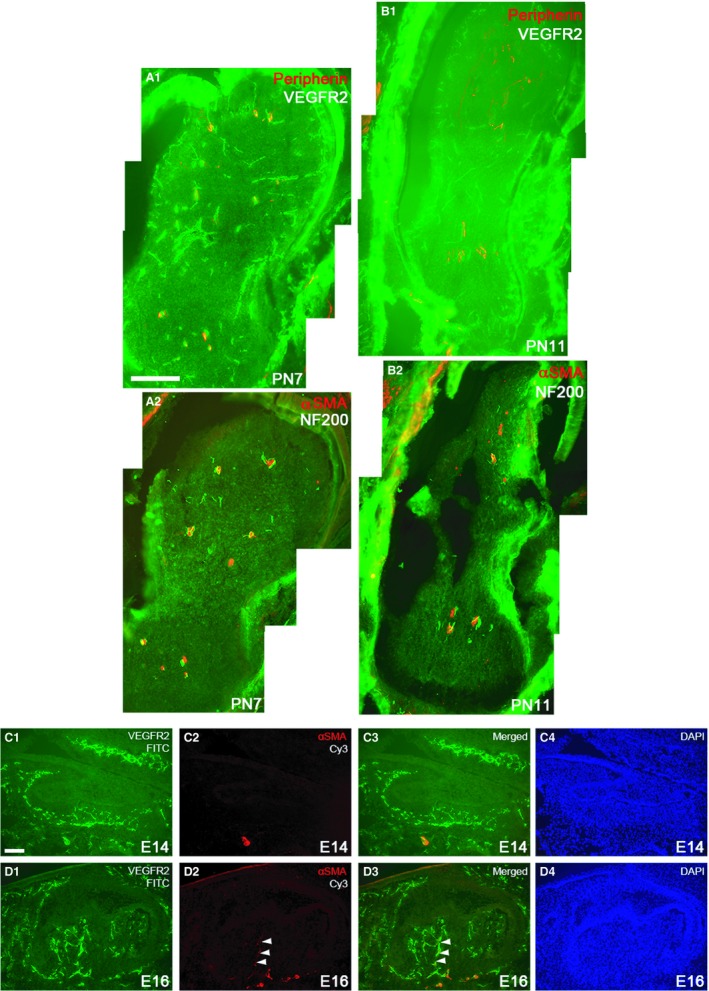
Comparison of the localization of blood vessels and neurites in the mandibular first molar tooth germ at selected postnatal stages (PN7, PN11) detected by immunofluorescence using anti‐VEGFR2 and anti‐peripherin antibodies (A1, B1). Anti‐αSMA and anti‐NF200 antibodies were used to display association of neurites with blood vessels with αSMA‐immunopositive mural cells (A2, B2). Sections are approximately from the level of the future enamel‐cementum junction. (Horizontal sections) Scale bar in (A1): 100 μm; applies to (A1–C2). (D1–E4) Blood vessels and adjoining smooth muscle cells in sagittal sections of mandibular first molar tooth germs at E14 and E16, detected by immunofluorescence using anti‐VEGFR2 (C1, D1) and anti‐αSMA antibodies, respectively (C2,D2). (C4, D4) DAPI staining to display cell nuclei. C4 and D4 show merged channels. Blood vessels with no smooth muscle cells accompanying them are seen in the E14 dental follicle and papilla. The inferior alveolar artery is enveloped by smooth muscle cells (C1–C3). Two blood vessels with aligned smooth muscle cells are observed to invade E16 dental pulp (white arrowheads) (D1–D3). Scale bar in (C1): 100 μm; applies to (C1–D4).
Developmentally regulated cellular expression of VEGF mRNA in the developing tooth
As VEGF is a critical regulator of blood vessel development (Carmeliet et al. 1996; Ferrara et al. 1996; Yancopoulos et al. 2000), spatio‐temporal cellular expression patterns of VEGF mRNA were investigated using sectional in situ hybridization during embryonic and postnatal development of the mouse mandibular first molar during E11‐PN1 (Fig. 4). At the epithelial thickening (E11) stage, VEGF mRNA exhibited a positive, though weak and diffuse, hybridization signal in the presumptive dental mesenchyme, as well as in deep mandibular and maxillary mesenchyme (Fig. 4A1,A2), whereas the developing heart, in line with earlier reports, (Ferrara et al. 1996; Lagercrantz et al. 1998; Lymboussaki et al. 1999) showed a notable expression of Vegf (Fig. 4A1,A2). At E12 early bud stage, weak expression of Vegf was observed in the upper molar dental mesenchyme and epithelium, whereas a notable hybridization signal was detected in the buccal peridental mesenchyme in the lower first molar (Fig. 4B1,B2). Two days later, at the early cap stage (E14), Vegf expression was prominent in the epithelial primary enamel knot and adjacent dental papilla mesenchyme (Fig. 4C1,C2). During the bell stage, the secondary enamel knots, which define the sites and number of the molar cusps, develop, and tooth‐specific crown morphogenesis takes place (Jernvall et al. 1994; Luukko et al. 2003). During the early bell stage at E15 and later bell stage at E18, the enamel knots as well as part of the inner and outer dental epithelium, stratum intermedium and stellate reticulum cells, displayed prominent Vegf expression (Fig. 4D1,D2,E1,E2). The final stages of the first molar tooth crown formation take place postnatally by formation of dentin and enamel hard tissues. Postnatally, at PN1, Vegf expression continued in the enamel organ. The most intense expression was seen in the stratum intermedium and stellate reticulum in the areas of future mesial slopes of the future cusps (Fig. 4F1,F2).
Figure 4.
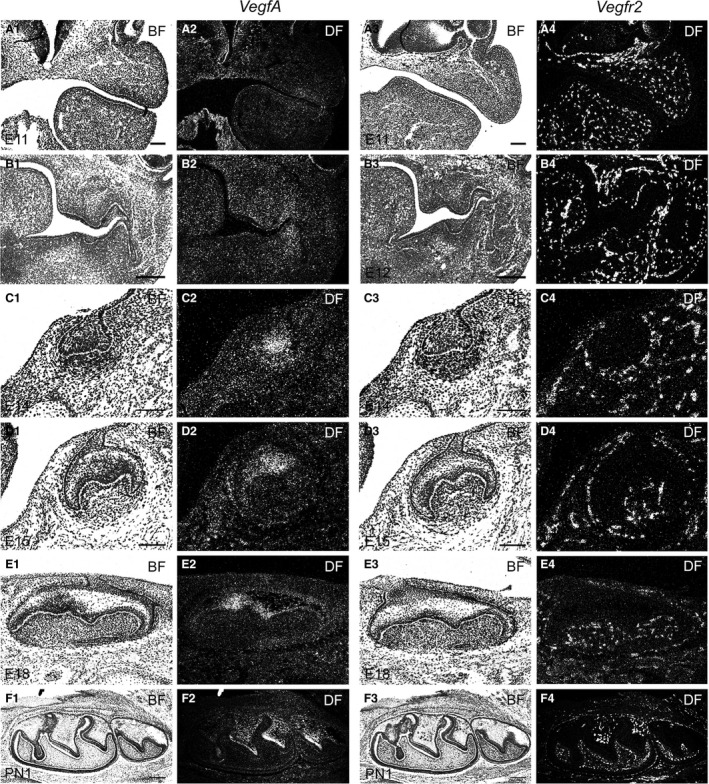
Bright‐ (A1, A3, B1, B3, C1, C3, D1, D3, E1, E3, F1, F3) and dark‐field (A2, A4, B2, B4, C2, C4, D2, D4, E2, E4, F2, F4) images of E11, E12, E14, E15, E18 and PN1 tooth germs showing VEGF and VEGFR2 mRNA expression in the developing mandibular first molar tooth germ and neighbouring tissues. Coronal (A1–D4) and sagittal (E1–F4) sections. Scale bars: (A1–A4,C1–D4) 100 μm, (B1–B4,F1–F4) 200 μm.
Expression of Vegf splicing variants and VEGF protein in the developing tooth germ
The VEGFA gene has eight exons, alternative splicing of which gives rise to at least four isoforms in the mouse, namely VEGF120, VEGF144, VEGF164 and VEGF188 (Ferrara, 2004). A shorter form, VEGF115, has been identified in immortal mouse embryonic fibroblasts (Sugihara et al. 1998). A protein product of exon six is responsible for binding of VEGF188 and VEGF144 to heparan‐sulfate proteoglycans in extracellular matrix. Exon seven in VEGF165 is responsible for moderate diffusibility of this isoform. VEGF120 lacks exons six and seven and is consequently highly soluble (Ferrara, 2004). VEGF120, VEGF144, VEGF165 and VEGF188 isoforms are expressed during mouse embryogenesis (Ng et al. 2001; Mukouyama et al. 2002; Ruhrberg et al. 2002). As the Vegf probe used in in situ hybridization potentially recognizes all isoforms, we investigated which isoforms are present in the developing tooth. RT‐PCR analysis with specific primers showed that E14 cap stage tooth germ mainly expresses VEGF120, 144 and 164 isoforms (Fig. 5A). Two additional weak bands, likely VEGF188 and VEGF115 isoforms, were also seen. VEGF protein is secreted from cells as a double homodimer with a molecular weight of 45 kDa (Ferrara & Henzel, 1989; Mukouyama et al. 2002; Ferrara, 2004). Western blot analysis of microdissected E16 molars, which was employed under non‐reducing conditions, showed a faint band of about 45 kDa VEGF protein (Fig. 5B).
Figure 5.
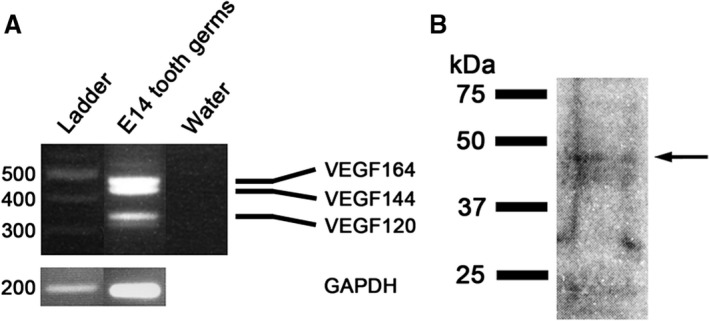
(A) RT‐PCR analysis of cDNA derived from E14 tooth germs using primers specific for Vegf or Gapdh. Water was used as a negative control. The predominant Vegf isoforms the E14 tooth germ expresses are Vegf164, Vegf144 and Vegf120. (B) Western blot analysis shows VEGF protein with a molecular mass of about 45 kDa present in E16 tooth germs under non‐reducing conditions.
Cellular expression of VEGFR2 mRNA during tooth development
VEGFR2 is a cell membrane bound protein and the major receptor for VEGF. To investigate expression of VEGFR2 mRNA during early tooth histomorphogenesis, and to compare its expression with that of Vegf, its cellular localization was investigated using sectional in situ hybridization during embryonic and postnatal development of the mouse mandibular first molar (E11‐PN1). In addition, localization and expression intensity of VEGFR2 mRNAs were compared with that of VEGFR2 protein in histological tooth sections. Vegfr2 hybridization signal was prominent in the blood vessels and showed high correlation to VEGFR2 immunoreaction during E11 to PN1 (Fig. 4A4,B4,C4,D4,E4,F3). Based on the in situ hybridization localization data, it appears that Vegfr2 is predominantly, if not exclusively, produced in the developing blood vessels during tooth formation. The intensity of mRNA expression was generally high and homogeneous throughout the stages studied. In PN1 tooth, however, Vegfr2 expression appeared to be elevated in blood vessels in the vicinity of the stratum intermedium cell layer next to the preameloblasts, before the onset of enamel production (Fig. 4F4).
Discussion
During odontogenesis, development of key tooth supporting tissues, nerves and blood vessels take place concomitantly with advancing tooth morphogenesis. To address putative developmental relationships between dental blood vessels and peripheral neurites, the time‐course and localization of blood vessels and their differentiation were analyzed and compared with the growth and distribution of nerve fibers during embryonic and postnatal development of the mandibular first molar tooth germ using immunofluorescence staining on sections. Moreover, cellular expression of VEGF, an essential regulator of blood vessel formation, and its signaling receptor VEGFR2 were investigated during odontogenesis.
Formation of tooth vascular supply is developmentally regulated and spatio‐temporally coordinated with tooth morphogenesis and precedes tooth innervation
Comparison of the localization of developing blood vessels and neurites showed that the development of tooth vasculature preceded that of innervation. Before and during initial stages of tooth histomorphogenesis at E10 and E11, numerous blood vessels were dispersed throughout the mandibular process mesenchyme, including the presumptive dental mesenchyme that preceded the appearance of the single trigeminal molar nerve at E12, which is the first one to innervate the tooth germ (Luukko, 1997; Kettunen et al. 2005, 2007). Later at the bud stage, dental blood vessels formed a plexus, whereas the first neurites had just reached the tooth target at this stage (Luukko, 1997; Kettunen et al. 2005, 2007). The leading neurite(s) heading to the E13 bud stage tooth germ and turning buccally appears to follow the preexisting blood vessels (Fig. 1B1‐B3). Interestingly, the first blood vessels, emerging from the plexus, entered the dental papilla (future dental pulp) at the early cap stage (E14), slightly earlier than reported in the previous study (Rothova et al. 2011). Later, at the bell stage, preceding the final differentiation of the odontoblasts, small‐diameter blood vessels formed a plexus under the preodontoblast layer in the future cusp area. The first neurites did not invade the dental pulp before the postnatal day 4. Some thicker nerve fibers appeared to follow, to some extent, preexisting blood vessels in the center of the pulp. At PN7 and PN11, thicker nerve bundles were associated with smooth muscle‐covered blood vessels likely representing thin arteries. Finally, before differentiation of the ameloblasts started, blood vessels grew, following the outer enamel epithelium invaginations (Decker, 1967; Yoshida et al. 1985) to the stratum intermedium area. The present data show that tooth vascularization precedes tooth innervation. The development and patterning of embryonic and postnatal tooth blood supply, as shown earlier for the development of tooth trigeminal innervation (Luukko et al. 2005, 2008), is a distinct, stepwise process that takes place in a developmentally regulated, well‐defined manner and is intimately coupled with advancing histomorphogenesis of the tooth germ proper. In particular, the finding that blood vessels were not allowed to enter the dental papilla before the cap stage, suggests that there occurs a shift of net influence apparently by down‐ and up‐regulation of locally produced molecules that mediate a repellent and/or attractive influence on developing blood vessels, allowing the ingrowth of the blood vessels into the future dental pulp, as has previously shown for the ingrowing dental trigeminal nerve fibers. Ingrowth of blood vessels into the enamel organ appears to involve similar local control. Local tissue interactions regulate tooth morphogenesis and tooth innervation (Mina & Kollar, 1987; Lumsden, 1988; Kettunen et al. 2005; Thesleff & Nieminen, 2005; Luukko & Kettunen, 2016). We propose that they regulate tooth vascularization and its integration with advancing tooth formation.
Tooth vasculogenesis and innervation is suggested to take place in an independent and non‐independent manner
In the adult, blood vessels and nerves are frequently arranged in an orderly pattern and are located alongside one another, which is defined as neurovascular congruency (Martin & Lewis, 1989; Hildebrand et al. 1995; Rodd & Boissonade, 2003; Oh & Gu, 2013; Steiniger et al. 2013). In several organs such as limbs, skin, hair follicles and intestine, neurovascular congruency is established during embryogenesis through different mechanisms (Bates et al. 2002; Mukouyama et al. 2002; Oh & Gu, 2013; Hatch & Mukouyama, 2015). In skin, sensory nerves are able to direct blood vessels to align along the nerves (Mukouyama et al. 2002) and blood vessels, in turn, may instruct sympathetic nerves to follow the vessels (Honma et al. 2002; Glebova & Ginty, 2005; Damon et al. 2007; Nam et al. 2013; Brunet et al. 2014; Hatch & Mukouyama, 2015). It is not always the endothelial cells, but the aligning αSMA immunoreactive mural cells that produce neurite attractive molecules, including artemin, neurotrophin 3 (NT3), NGF, netrin1 and endothelin involved in sympathetic axon guidance (Glebova & Ginty, 2005; Makita 2008; Nam et al. 2013; Brunet et al. 2014). In addition to establishing neurovascular congruence through interaction between nerves and blood vessels, neurovascular congruence may be directed by tooth‐organ itself as earlier proposed for other tissues such as whisker follicle (Oh & Gu, 2013).
Development of both neural and vascular systems has been shown to involve signaling activity of multiple proteins and receptors such as VEGF, VEGFR, semaphorins, plexins, neuropilin, Eph/ephrins, slit/Roundabouts, Sonic Hedgehog(SHH) and netrin (Gelfand et al. 2009; Adams & Eichmann, 2010; Bellon et al. 2010; Ruiz de Almodovar et al. 2011). Based on the present findings that dental blood vessels appear before dental sensory neurites, it is proposed that navigation and patterning of the sensory neurites are partially influenced by blood vessels. The pioneer axons of the molar nerve appeared to follow blood vessels to the tooth target and around the tooth. Similarly, axons appeared to follow preexisting blood vessels as they penetrated the dental pulp. However, in later developmental stages, many neurites did not appear to associate with blood vessels in the pulp. Thus, development of tooth vasculature and sensory innervation appears to be partially interrelated and takes place both independently and dependently of the blood vessels according to the developmental stage. Ingrowing sympathetic axons that enter the pulp later may follow blood vessels, as shown in other organs (Honma et al. 2002; Glebova & Ginty, 2005; Damon et al. 2007; Moe et al. 2008). This apparently is dependent on local interactions between the blood vessels, neurites and dental cells and tissues.
Vascular Endothelial Growth Factor(VEGF) is suggested to mediate local, tooth organ‐blood vessel interactions and regulate development of tooth vascularization
Development of organ vasculature involves instructions from the organ (Oh & Gu, 2013), and the development of blood vessels and nerves involves shared molecular mechanisms (Martin & Lewis, 1989; Adams et al. 1999; Bates et al. 2002; Carmeliet & Tessier‐Lavigne, 2005; Gelfand et al. 2009; Adams & Eichmann, 2010; Oh & Gu, 2013). This prompted us to address putative developmental functions of VEGF by studying its expression during tooth development. VEGF is a secreted protein, which is an important regulator of embryonic blood vessel formation and regulates vasculogenesis and angiogenic sprouting by affecting proliferation and cell migration (Carmeliet et al. 1996; Ferrara et al. 1996; Gelfand et al. 2009). Mouse VEGF has three principal isoforms, defined as VEGF120, VEGF164 and VEGF188, which are generated by alternative splicing (Mackenzie & Ruhrberg, 2012). Each isoform has a distinct affinity for heparan sulfate, and consequently they form a VEGF gradient in the tissues (Poltorak et al. 1997; Ferrara, 2004; Carmeliet & Tessier‐Lavigne, 2005). RT‐PCR analysis shows that the cap stage tooth germ expresses several Vegf isoforms, such as mRNA of freely soluble VEGF120 as well as VEGF144 and VEGF164, both of which bind cell surface and extracellular matrix (Poltorak et al. 1997; Ruhrberg et al. 2002; Ferrara, 2004). Sectional in situ hybridization revealed that Vegf exhibits spatio‐temporally regulated cellular expression domains in both epithelial and mesenchymal tissue components during tooth development that showed apparent correlation with the development and localization of dental blood vessels. In particular, at E14 cap stage tooth, a prominent Vegf expression appeared in the enamel knot signaling center and dental papilla mesenchyme before the onset of blood vessel ingrowth into the papilla. We propose that VEGF may initiate and attract the ingrowth of the blood vessels into the dental papilla from the blood vessel plexus located in the base of the dental follicle, and further guide vessel branches to the developing cuspal areas by creating a protein gradient. Similarly, Vegf expression in the stellate reticulum and stratum intermedium cells preceded ingrowth of blood vessels into the enamel organ, i.e. before terminal differentiation of ameloblasts and enamel production, suggesting related functions. In support of this, similar findings have been reported in the retina, where intense Vegf expression was seen in front of the vascular plexus (Stone et al. 1995; Gerhardt et al. 2003).
We also found VEGF signaling receptor VEGFR2 in the developing dental blood vessels. In addition, its co‐receptor NPN1, which binds VEGF165 and enhances binding of VEGF165 to VEGFR2 (Kitsukawa et al. 1995, 1997; Simons et al. 2016), is expressed in the dental blood vessels during molar tooth formation (Loes et al. 2001; Lillesaar & Fried, 2004) and NPN1 molars do not develop beyond the early bud stage, apparently due to general embryonic defects in vascular remodeling (Kawasaki et al. 1999; Kettunen et al. 2005). Thus, we suggest that the tooth target expressed VEGF, through binding VEGFR2, and its NPN1 co‐receptor, acts as a critical regulator of the development and patterning of tooth vasculature by mediating local signaling interactions between the tooth target and blood vessels (Vieira et al. 2007). Moreover, sonic hedgehog (SHH) signaling has been reported earlier to be necessary for tooth vascularization (Gritli‐Linde et al. 2002). The function of SHH may be indirect. as it stimulates VEGF mRNA expression in mesenchymal cells (Pola et al. 2001).
VEGF signaling may serve neuronal and non‐neuronal functions during tooth formation
Vascular endothelial growth factor signaling serves multiple neuronal and developmental functions (Carmeliet and Ruiz de Almodovar, 2013). VEGF, by binding directly NPN1, has been shown to direct neuronal guidance, survival and patterning (Schwarz et al. 2004; Cariboni et al. 2011; Tillo et al. 2015) and to promote guidance of commissural axons (Erskine et al. 2011). VEGF acts also as a chemoattractant to commissural axons and stimulates growth of peripheral axons through VEGFR2 (Sondell et al. 1999, Sondell et al. 2000, Ruiz de Almodovar et al. 2011). Trigeminal dental nerve fibers show NPN1 immunoreactivity during mouse molar development (Kawakami et al. 1996; Kolodkin et al. 1997; Lillesaar & Fried, 2004; Kettunen et al. 2005; Sijaona et al. 2011) and Npn1‐deficient mouse embryos have defects in tooth innervation (Kettunen et al. 2005). Thus, it is possible that VEGF signaling is involved in regulation of tooth innervation by acting directly on developing axons. Moreover, VEGF expression in the primary enamel knot may even suggest functions for VEGF in morphogenesis. Previously VEGF has been proposed also to regulate cell differentiation, namely chondrocyte and osteoblast differentiation (Carlevaro et al. 2000; Duan et al. 2015).
Conclusions
In the present study, it was found that the development and patterning of the dental blood vessels take place in a distinct, spatio‐temporally regulated manner that is intimately coupled with advancing tooth morphogenesis and cell differentiation. Tooth innervation appears to occur by vasculogenesis in an independent and a non‐independent manner. Development of the tooth vascular supply is proposed to involve local, tooth‐specific regulation by epithelial–mesenchymal tissue interactions and tooth target expressed VEGF signaling that is developmentally regulated. Future investigations are warranted to elucidate further the regulatory mechanisms of integration of the tooth‐supporting tissues with the tooth histomorphogenesis.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
P.K. and K.L. designed the research. All immunohistological and immunofluorescence experiments were carried out by O.S. and R.C. In situ hybridization experiments were performed by K.L. with the assistance of Kjellfrid Haukanes (K.H.). RT‐PCR and western blot were executed by K.H. with contributions from P.K. P.K. and K.L. authored the manuscript, with comments and editing from O.S. and R.C.
Acknowledgements
Kjellfrid Haukanes is acknowledged for skillful technical assistance. The authors also thank the staff of the animal facility for careful mouse husbandry. This work was supported by the University of Bergen and Norwegian Research Council.
References
- Adams RH, Eichmann A (2010) Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol 2, a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RH, Wilkinson GA, Weiss C, et al. (1999) Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev 13, 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Taylor GI, Newgreen DF (2002) The pattern of neurovascular development in the forelimb of the quail embryo. Dev Biol 249, 300–320. [DOI] [PubMed] [Google Scholar]
- Bellon A, Luchino J, Haigh K, et al. (2010) VEGFR2 (KDR/Flk1) signaling mediates axon growth in response to semaphorin 3E in the developing brain. Neuron 66, 205–219. [DOI] [PubMed] [Google Scholar]
- Brunet I, Gordon E, Han J, et al. (2014) Netrin‐1 controls sympathetic arterial innervation. J Clin Invest 124, 3230–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariboni A, Davidson K, Dozio E, et al. (2011) VEGF signalling controls GnRH neuron survival via NRP1 independently of KDR and blood vessels. Development 138, 3723–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlevaro MF, Cermelli S, Cancedda R, et al. (2000) Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto‐paracrine role during endochondral bone formation. J Cell Sci 113(Pt 1), 59–69. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Ruiz de Almodovar C (2013) VEGF ligands and receptors: implications in neurodevelopment and neurodegeneration. Cell Mol Life Sci 70, 1763–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Tessier‐Lavigne M (2005) Common mechanisms of nerve and blood vessel wiring. Nature 436, 193–200. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, et al. (1996) Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380, 435–439. [DOI] [PubMed] [Google Scholar]
- Chung AS, Ferrara N (2011) Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol 27, 563–584. [DOI] [PubMed] [Google Scholar]
- Cobourne MT, Sharpe PT (2010) Making up the numbers: the molecular control of mammalian dental formula. Semin Cell Dev Biol 21, 314–324. [DOI] [PubMed] [Google Scholar]
- Cobourne MT, Sharpe PT (2013) Diseases of the tooth: the genetic and molecular basis of inherited anomalies affecting the dentition. Wiley Interdiscip Rev Dev Biol 2, 183–212. [DOI] [PubMed] [Google Scholar]
- Damon DH, Teriele JA, Marko SB (2007) Vascular‐derived artemin: a determinant of vascular sympathetic innervation? Am J Physiol Heart Circ Physiol 293, H266–H273. [DOI] [PubMed] [Google Scholar]
- Debus E, Weber K, Osborn M (1983) Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation 25, 193–203. [DOI] [PubMed] [Google Scholar]
- Decker JD (1967) The development of a vascular supply to the rat molar enamel organ. An electron microscopic study. Arch Oral Biol 12, 453–458. [DOI] [PubMed] [Google Scholar]
- Duan X, Murata Y, Liu Y, et al. (2015) Vegfa regulates perichondrial vascularity and osteoblast differentiation in bone development. Development 142, 1984–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont DJ, Fong GH, Puri MC, et al. (1995) Vascularization of the mouse embryo: a study of flk‐1, tek, tie, and vascular endothelial growth factor expression during development. Dev Dyn 203, 80–92. [DOI] [PubMed] [Google Scholar]
- Erskine L, Reijntjes S, Pratt T, et al. (2011) VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron 70, 951–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25, 581–611. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Henzel WJ (1989) Pituitary follicular cells secrete a novel heparin‐binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 161, 851–858. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver‐Moore K, Chen H, et al. (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380, 439–442. [DOI] [PubMed] [Google Scholar]
- Fried K, Lillesaar C, Sime W, et al. (2007) Target finding of pain nerve fibers: neural growth mechanisms in the tooth pulp. Physiol Behav 92, 40–45. [DOI] [PubMed] [Google Scholar]
- Gaunt WA (1959) The vascular supply to the dental lamina during early development. Acta Anat (Basel) 37, 232–252. [DOI] [PubMed] [Google Scholar]
- Gelfand MV, Hong S, Gu C (2009) Guidance from above: common cues direct distinct signaling outcomes in vascular and neural patterning. Trends Cell Biol 19, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, et al. (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161, 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD (2005) Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci 28, 191–222. [DOI] [PubMed] [Google Scholar]
- Gritli‐Linde A, Bei M, Maas R, et al. (2002) Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development 129, 5323–5337. [DOI] [PubMed] [Google Scholar]
- Hatch J, Mukouyama YS (2015) Spatiotemporal mapping of vascularization and innervation in the fetal murine intestine. Dev Dyn 244, 56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand C, Fried K, Tuisku F, et al. (1995) Teeth and tooth nerves. Prog Neurobiol 45, 165–222. [DOI] [PubMed] [Google Scholar]
- Honma Y, Araki T, Gianino S, et al. (2002) Artemin is a vascular‐derived neurotropic factor for developing sympathetic neurons. Neuron 35, 267–282. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Kettunen P, Karavanova I, et al. (1994) Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non‐dividing cells express growth stimulating Fgf‐4 gene. Int J Dev Biol 38, 463–469. [PubMed] [Google Scholar]
- Jheon AH, Seidel K, Biehs B, et al. (2013) From molecules to mastication: the development and evolution of teeth. Wiley Interdiscip Rev Dev Biol 2, 165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jussila M, Thesleff I (2012) Signaling networks regulating tooth organogenesis and regeneration, and the specification of dental mesenchymal and epithelial cell lineages. Cold Spring Harb Perspect Biol 4, a008425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaukua N, Shahidi MK, Konstantinidou C, et al. (2014) Glial origin of mesenchymal stem cells in a tooth model system. Nature 513, 551–554. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Kitsukawa T, Takagi S, et al. (1996) Developmentally regulated expression of a cell surface protein, neuropilin, in the mouse nervous system. J Neurobiol 29, 1–17. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Kitsukawa T, Bekku Y, et al. (1999) A requirement for neuropilin‐1 in embryonic vesse formation. Development 126, 4895–4902. [DOI] [PubMed] [Google Scholar]
- Kettunen P, Thesleff I (1998) Expression and function of FGFs‐4, ‐8, and ‐9 suggest functional redundancy and repetitive use as epithelial signals during tooth morphogenesis. Dev Dyn 211, 256–268. [DOI] [PubMed] [Google Scholar]
- Kettunen P, Karavanova I, Thesleff I (1998) Responsiveness of developing dental tissues to fibroblast growth factors: expression of splicing alternatives of FGFR1, ‐2, ‐3, and of FGFR4; and stimulation of cell proliferation by FGF‐2, ‐4, ‐8, and ‐9. Dev Genet 22, 374–385. [DOI] [PubMed] [Google Scholar]
- Kettunen P, Loes S, Furmanek T, et al. (2005) Coordination of trigeminal axon navigation and patterning with tooth organ formation: epithelial‐mesenchymal interactions, and epithelial Wnt4 and Tgfbeta1 regulate semaphorin 3a expression in the dental mesenchyme. Development 132, 323–334. [DOI] [PubMed] [Google Scholar]
- Kettunen P, Spencer‐Dene B, Furmanek T, et al. (2007) Fgfr2b mediated epithelial‐mesenchymal interactions coordinate tooth morphogenesis and dental trigeminal axon patterning. Mech Dev 124, 868–883. [DOI] [PubMed] [Google Scholar]
- Kitsukawa T, Shimono A, Kawakami A, et al. (1995) Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development 121, 4309–4318. [DOI] [PubMed] [Google Scholar]
- Kitsukawa T, Shimizu M, Sanbo M, et al. (1997) Neuropilin‐semaphorin III/D‐mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron 19, 995–1005. [DOI] [PubMed] [Google Scholar]
- Klein OD, Oberoi S, Huysseune A, et al. (2013) Developmental disorders of the dentition: an update. Am J Med Genet C Semin Med Genet 163C, 318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollar EJ, Lumsden AG (1979) Tooth morphogenesis: the role of the innervation during induction and pattern formation. J Biol Buccale 7, 49–60. [PubMed] [Google Scholar]
- Kolodkin AL, Levengood DV, Rowe EG, et al. (1997) Neuropilin is a semaphorin III receptor. Cell 90, 753–762. [DOI] [PubMed] [Google Scholar]
- Lagercrantz J, Farnebo F, Larsson C, et al. (1998) A comparative study of the expression patterns for vegf, vegf‐b/vrf and vegf‐c in the developing and adult mouse. Biochim Biophys Acta 1398, 157–163. [DOI] [PubMed] [Google Scholar]
- Li W, Kohara H, Uchida Y, et al. (2013) Peripheral nerve‐derived CXCL12 and VEGF‐A regulate the patterning of arterial vessel branching in developing limb skin. Dev Cell 24, 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillesaar C, Fried K (2004) Neurites from trigeminal ganglion explants grown in vitro are repelled or attracted by tooth‐related tissues depending on developmental stage. Neuroscience 125, 149–161. [DOI] [PubMed] [Google Scholar]
- Loes S, Kettunen P, Kvinnsland IH, et al. (2001) Expression of class 3 semaphorins and neuropilin receptors in the developing mouse tooth. Mech Dev 101, 191–194. [DOI] [PubMed] [Google Scholar]
- Loes S, Kettunen P, Kvinnsland H, et al. (2002) Mouse rudimentary diastema tooth primordia are devoid of peripheral nerve fibers. Anat Embryol (Berl) 205, 187–191. [DOI] [PubMed] [Google Scholar]
- Lohela M, Bry M, Tammela T, et al. (2009) VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol 21, 154–165. [DOI] [PubMed] [Google Scholar]
- Lumsden AGS (1982) The developing innervation of the lower jaw and its relation to the formation of tooth germs in mouse embryo In: Teeth: Form: Function, and Evolution. (ed. Kurten B.), pp. 32–43. New York: Columbia University Press. [Google Scholar]
- Lumsden AG (1988) Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development 103(Suppl), 155–169. [DOI] [PubMed] [Google Scholar]
- Luukko K (1997) Immunohistochemical localization of nerve fibres during development of embryonic rat molar using peripherin and protein gene product 9.5 antibodies. Arch Oral Biol 42, 189–195. [DOI] [PubMed] [Google Scholar]
- Luukko K, Kettunen P (2014) Coordination of tooth morphogenesis and neuronal development through tissue interactions: lessons from mouse models. Exp Cell Res 325, 72–77. [DOI] [PubMed] [Google Scholar]
- Luukko K, Kettunen P (2016) Integration of tooth morphogenesis and innervation by local tissue interactions, signaling networks, and semaphorin 3A. Cell Adh Migr 10, 618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luukko K, Moshnyakov M, Sainio K, et al. (1996) Expression of neurotrophin receptors during rat tooth development is developmentally regulated, independent of innervation, and suggests functions in the regulation of morphogenesis and innervation. Dev Dyn 206, 87–99. [DOI] [PubMed] [Google Scholar]
- Luukko K, Loes S, Furmanek T, et al. (2003) Identification of a novel putative signaling center, the tertiary enamel knot in the postnatal mouse molar tooth. Mech Dev 120, 270–276. [DOI] [PubMed] [Google Scholar]
- Luukko K, Kvinnsland IH, Kettunen P (2005) Tissue interactions in the regulation of axon pathfinding during tooth morphogenesis. Dev Dyn 234, 482–488. [DOI] [PubMed] [Google Scholar]
- Luukko K, Moe K, Sijaona A, et al. (2008) Secondary induction and the development of tooth nerve supply. Ann Anat 190, 178–187. [DOI] [PubMed] [Google Scholar]
- Lymboussaki A, Olofsson B, Eriksson U, et al. (1999) Vascular endothelial growth factor (VEGF) and VEGF‐C show overlapping binding sites in embryonic endothelia and distinct sites in differentiated adult endothelia. Circ Res 85, 992–999. [DOI] [PubMed] [Google Scholar]
- Mackenzie F, Ruhrberg C (2012) Diverse roles for VEGF‐A in the nervous system. Development 139, 1371–1380. [DOI] [PubMed] [Google Scholar]
- Makita T, Sucov HM, Gariepy CE, et al. (2008) Endothelins are vascular‐derived axonal guidance cues for developing sympathetic neurons. Nature 452, 759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Lewis J (1989) Origins of the neurovascular bundle: interactions between developing nerves and blood vessels in embryonic chick skin. Int J Dev Biol 33, 379–387. [PubMed] [Google Scholar]
- Mina M, Kollar EJ (1987) The induction of odontogenesis in non‐dental mesenchyme combined with early murine mandibular arch epithelium. Arch Oral Biol 32, 123–127. [DOI] [PubMed] [Google Scholar]
- Moe K, Kettunen P, Kvinnsland IH, et al. (2008) Development of the pioneer sympathetic innervation into the dental pulp of the mouse mandibular first molar. Arch Oral Biol 53, 865–873. [DOI] [PubMed] [Google Scholar]
- Mohamed SS, Atkinson ME (1983) A histological study of the innervation of developing mouse teeth. J Anat 136(Pt 4), 735–749. [PMC free article] [PubMed] [Google Scholar]
- Mukouyama YS, Shin D, Britsch S, et al. (2002) Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 109, 693–705. [DOI] [PubMed] [Google Scholar]
- Nait Lechguer A, Kuchler‐Bopp S, Hu B, et al. (2008) Vascularization of engineered teeth. J Dent Res 87, 1138–1143. [DOI] [PubMed] [Google Scholar]
- Nam J, Onitsuka I, Hatch J, et al. (2013) Coronary veins determine the pattern of sympathetic innervation in the developing heart. Development 140, 1475–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng YS, Rohan R, Sunday ME, et al. (2001) Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn 220, 112–121. [DOI] [PubMed] [Google Scholar]
- Oh WJ, Gu C (2013) Establishment of neurovascular congruency in the mouse whisker system by an independent patterning mechanism. Neuron 80, 458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olgart L (1996) Neural control of pulpal blood flow. Crit Rev Oral Biol Med 7, 159–171. [DOI] [PubMed] [Google Scholar]
- Pola R, Ling LE, Silver M, et al. (2001) The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med 7, 706–711. [DOI] [PubMed] [Google Scholar]
- Poltorak Z, Cohen T, Sivan R, et al. (1997) VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J Biol Chem 272, 7151–7158. [DOI] [PubMed] [Google Scholar]
- Quinn TP, Peters KG, De Vries C, et al. (1993) Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci U S A 90, 7533–7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd HD, Boissonade FM (2003) Immunocytochemical investigation of neurovascular relationships in human tooth pulp. J Anat 202, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothova M, Feng J, Sharpe PT, et al. (2011) Contribution of mesoderm to the developing dental papilla. Int J Dev Biol 55, 59–64. [DOI] [PubMed] [Google Scholar]
- Ruhrberg C, Gerhardt H, Golding M, et al. (2002) Spatially restricted patterning cues provided by heparin‐binding VEGF‐A control blood vessel branching morphogenesis. Genes Dev 16, 2684–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz de Almodovar C, Fabre PJ, Knevels E, et al. (2011) VEGF mediates commissural axon chemoattraction through its receptor Flk1. Neuron 70, 966–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Q, Gu C, Fujisawa H, et al. (2004) Vascular endothelial growth factor controls neuronal migration and cooperates with Sema3A to pattern distinct compartments of the facial nerve. Genes Dev 18, 2822–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha A, Moe K, Luukko K, et al. (2014) Sema3A chemorepellant regulates the timing and patterning of dental nerves during development of incisor tooth germ. Cell Tissue Res 357, 15–29. [DOI] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, et al. (1992) Vascular endothelial growth factor induced by hypoxia may mediate hypoxia‐initiated angiogenesis. Nature 359, 843–845. [DOI] [PubMed] [Google Scholar]
- Sijaona A, Luukko K, Kvinnsland IH, et al. (2011) Expression patterns of Sema3F, PlexinA4, ‐A3, Neuropilin1 and ‐2 in the postnatal mouse molar suggest roles in tooth innervation and organogenesis. Acta Odontol Scand 70, 140–148. [DOI] [PubMed] [Google Scholar]
- Simons M, Gordon E, Claesson‐Welsh L (2016) Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol 17, 611–625. [DOI] [PubMed] [Google Scholar]
- Skalli O, Ropraz P, Trzeciak A, et al. (1986) A monoclonal antibody against alpha‐smooth muscle actin: A new probe for smooth muscle differentiation. J Cell Biol 103, 2787–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondell M, Lundborg G, Kanje M (1999) Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci 19, 5731–5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondell M, Sundler F, Kanje M (2000) Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk‐1 receptor. Eur J Neurosci 12, 4243–4254. [DOI] [PubMed] [Google Scholar]
- Steiniger BS, Bubel S, Bockler W, et al. (2013) Immunostaining of pulpal nerve fibre bundle/arteriole associations in ground serial sections of whole human teeth embedded in technovit(R) 9100. Cells Tissues Organs 198, 57–65. [DOI] [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, et al. (1995) Development of retinal vasculature is mediated by hypoxia‐induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci 15, 4738–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara T, Wadhwa R, Kaul SC, et al. (1998) A novel alternatively spliced form of murine vascular endothelial growth factor, VEGF 115. J Biol Chem 273, 3033–3038. [DOI] [PubMed] [Google Scholar]
- Thesleff I (2014) Current understanding of the process of tooth formation: transfer from the laboratory to the clinic. Aust Dent J 59(Suppl 1), 48–54. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Nieminen P (2005) Tooth induction. Encyclopedia of life sciences, http://www.els.net, 1–8.
- Thesleff I, Keranen S, Jernvall J (2001) Enamel knots as signaling centers linking tooth morphogenesis and odontoblast differentiation. Adv Dent Res 15, 14–18. [DOI] [PubMed] [Google Scholar]
- Tillo M, Erskine L, Cariboni A, et al. (2015) VEGF189 binds NRP1 and is sufficient for VEGF/NRP1‐dependent neuronal patterning in the developing brain. Development 142, 314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuisku F, Hildebrand C (1994) Evidence for a neural influence on tooth germ generation in a polyphyodont species. Dev Biol 165, 1–9. [DOI] [PubMed] [Google Scholar]
- Uddman R, Katob J, Lindgren P, et al. (1999) Expression of calcitonin gene‐related peptide‐1 receptor mRNA in human tooth pulp and trigeminal ganglion. Arch Oral Biol 44, 1–6. [DOI] [PubMed] [Google Scholar]
- Vieira JM, Schwarz Q, Ruhrberg C (2007) Role of the neuropilin ligands VEGF164 and SEMA3A in neuronal and vascular patterning in the mouse. Novartis Found Symp 283, 230–235; discussion 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Dumont DJ, Conlon RA, et al. (1993) flk‐1, an flt‐related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development 118, 489–498. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, et al. (2000) Vascular‐specific growth factors and blood vessel formation. Nature 407, 242–248. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Takeuchi K, Hoshino M, et al. (1985) Ultra‐ structure research on the vascularization of the enamel organ in the developing molar teeth of the rat. Jpn J Oral Biol 27, 549–561. [Google Scholar]
- Yu T, Volponi AA, Babb R, et al. (2015) Stem cells in tooth development, growth, repair, and regeneration. Curr Top Dev Biol 115, 187–212. [DOI] [PubMed] [Google Scholar]
- Yuan G, Zhang L, Yang G, et al. (2014) The distribution and ultrastructure of the forming blood capillaries and the effect of apoptosis on vascularization in mouse embryonic molar mesenchyme. Cell Tissue Res 356, 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Feng J, Seidel K, et al. (2014) Secretion of Shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell 14, 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]


