Abstract
This study aimed to investigate the spatial distribution and redistribution of lumbar erector spinae (ES) activity during a lumbar extension endurance task in pain‐free participants and how this is modified in people with low back pain (LBP). High density surface electromyography (HDEMG) was recorded using 13 × 5 electrode grids placed over the lumbar ES in 13 LBP and 13 control participants while completing an Ito test to task failure. The root mean square of the HDEMG signals was computed, a topographical map of the EMG amplitude generated and the centre of the activity (centroid) determined throughout the task. The centroid of the EMG amplitude map was systematically more cranial (F = 6.09, P = 0.022) for the LBP participants compared with the control subjects. Regression analysis showed that the extent of redistribution of ES activity was associated with longer endurance. These results show that LBP participants utilised a different motor strategy to perform the endurance task, characterised by greater activation of more cranial regions of the ES and less redistribution of ES activity throughout the task. This study provides new insight into the functional activation of the lumbar ES and how it is modified when people have pain.
Keywords: erector spinae, functional muscle activity, high density EMG, Ito test
Introduction
Previous anatomical and biomechanical research on the lumbar erector spinae (ES) has focused on the relations and the structure of different portions of this muscle group (Bogduk, 1980, 2005; Christophy et al. 2012). Bogduk (2005), via several dissection studies, described the origins, insertions and functions of the portions of the lumbar ES, work which was adapted by Christophy and colleagues to produce a biomechanical model of the lumbar musculature (Bogduk, 1980; Christophy et al. 2012). These descriptions concurred that although the portions of the muscle group have different origins and insertions, all play important roles in extending the lumbar spine during functional movements. The structures described for the portions of the ES indicate a broad bilateral muscular region lateral to the lumbar spine extending from L5 into the thoracic region (Bogduk, 2005; Christophy et al. 2012). To extend the lumbar spine, the most effective motor strategy would be to recruit fibres especially from the caudal portions of the ES, creating a longer lever arm and conferring a biomechanical advantage to the movement (Bogduk, 2005).
Surface electromyography (EMG) is used to measure muscle activity and can be applied as a means to understand variations in neuromuscular control in individuals with musculoskeletal pain (Fabian et al. 2005; Gallina et al. 2011; Abboud et al. 2014; Falla et al. 2014, 2017; Gizzi et al. 2015). More recent studies have utilised high‐density surface electromyography (HDEMG) to understand and quantify changes in the spatial distribution of muscle activity, which was not previously possible with classic bipolar surface EMG. Existing research utilising HDEMG has also commonly evaluated changes in the distribution of muscle activity during either sustained or dynamic contractions by quantifying a shift in the centroid of the HDEMG amplitude map, the point which defines the barycentre of muscle activation (Madeleine et al. 2006; Farina et al. 2008; Gallina et al. 2013; Falla et al. 2014, 2017). HDEMG studies on healthy asymptomatic volunteers have shown that the centre of muscle activity shifts during contraction (Falla & Farina, 2008b; Farina et al. 2008; Tucker et al. 2009) and that this redistribution of muscle activity has the physiological significance of minimising muscle fatigue and prolonging endurance (Farina et al. 2008; Gallina et al. 2013; Falla et al. 2014), possibly by preventing overload on the muscle fibres active at the beginning of the task.
Previous HDEMG investigations have described an association between endurance time and the redistribution of muscle activity in the trapezius in asymptomatic participants (Farina et al. 2008). More recently, HDEMG was applied to evaluate changes in lumbar ES activity in a low back pain (LBP) population (Abboud et al. 2014). Participants completed a force‐matching modified Sørenson test (lifting of the unsupported upper body with the legs affixed to a plinth), resisting a load cell around their shoulders which simulated 30% of their maximum voluntary contraction (MVC). Increased variability in the position of the centroid of the EMG amplitude map was observed in the healthy controls compared with the LBP group.
Despite these observations, the functional relevance of a change in the distribution of muscular activity remains unclear. We hypothesised that people with LBP would engage different regions of the lumbar ES during isometric back extension, reflecting less efficient activation of the ES, and that people with LBP would show less redistribution of ES activity, which would be associated with significantly lower endurance in this group.
Thus, the aim of this study was to investigate the spatial distribution of lumbar ES activity and redistribution of activity during an endurance task in participants with chronic LBP and pain‐free controls. Moreover, we evaluate the relationship between the extent of redistribution of activity and endurance time, with the hypothesis that those who display a larger redistribution of activity would be able to sustain the contraction for longer. This study may provide new insight into the functional activation of the lumbar ES and how it is modified when people have pain.
Methods
This study was an observational, cross‐sectional case–control study using a convenience sample of participants from the staff, students and community of the University of Birmingham, UK. Data collection took place in a laboratory within the Centre of Precision Rehabilitation for Spinal Pain, University of Birmingham.
Participants
LBP participants aged 20–55 were recruited via posters and social media accounts related to the University of Birmingham. Due to the nature of the fatiguing task, it was decided that 55 would be the maximum age of participants eligible for this study. Eligibility criteria included non‐specific LBP which had persisted for at least half the days of the previous 6 months, exceeding the minimum definition for LBP (Dionne et al. 2008). Consistent with previous studies, age‐ and gender‐matched control participants (CON) were recruited in the same way and were included if they had no history of LBP or lower limb disorders. Exclusion criteria for both groups comprised concurrent systemic issues including rheumatic and neuromuscular disorders, a history of chronic respiratory or neurological problems, spinal deformity or surgery, cardiovascular conditions, pregnancy and healthcare management for LBP in the previous 6 months (a requirement of the University ethics committee). To support a normal distribution for statistical analysis, a planned sample size of 30 participants (15 LBP and 15 CON) was chosen, consistent with previous HDEMG studies comparing symptomatic and asymptomatic participants.
Ethical approval was granted by the University of Birmingham ethics committee (ERN_17‐0782). Participants gave written informed consent prior to data collection and all procedures were completed in accordance with the Declaration of Helsinki.
Questionnaires
Prior to testing, participants from both groups were required to complete several questionnaires to gather population statistics, including the level of disability, intensity of pain and current level of activity. Participants were asked to complete the Oswestry Disability Index (ODI), as it has previously been shown to be a reliable measure of disability relating to spinal pain (Fairbank & Pynsent, 2000). The Tampa Scale for Kinesiophobia was used to assess any fear surrounding movement related to pain (Miller et al. 1991). A Pain Numeric Rating Scale (0–10) (PNRS) was used to assess current pain at the time of testing and pain over the prior week (Breivik et al. 2008). Information on the general health of participants at the time of testing was collected using the RAND 36‐item health survey, which has been shown to be effective and reliable as a measure of health across cultures and gender (Hays et al. 1993; VanderZee et al. 1996). Throughout the endurance task (see below), the rate of perceived exertion (RPE) was recorded at 30‐s intervals and immediately following task failure using the Borg RPE scale (Borg, 1998). This measure was used to assess the perceived exertion of the participants throughout the task and ensure that the task was appropriate for LBP participants.
Experimental set‐up
Surface EMG signals were recorded from the lumbar ES using 13 × 5 semi‐disposable 2D electrode grids (OT Bioelettronica, Turin, Italy). Electrodes with a 3‐mm diameter were spaced evenly at an 8‐mm inter‐electrode distance; one corner electrode was missing in each grid to provide directional reference. Electrodes were positioned over the lumbar ES on the right‐hand side in control participants and the most painful side in the LBP group. Where equal pain was reported bilaterally, participants were randomly allocated a side.
Prior to the application of electrodes, the skin in the region lateral to the lumbar spine was prepared by shaving the area if needed and then applying an abrasive paste (SPES Medica, Italy), and finally washing and drying the region. The electrodes were prepared by applying a thin custom double‐sided adhesive foam pad to the electrode grid (SPES Medica, Genoa, Italy). The cavities of the electrode grids were then filled with an electroconductive paste (SPES Medica). As there is no way of differentiating different portions of the ES in vivo, the electrode was placed on the ES in accordance with EMG guidelines and previous studies (Barbero et al. 2012; Falla et al. 2014). The grids were applied to the skin approximately 2 cm lateral to the lumbar spinous processes, starting at the level of the L5 and extending to approximately the level of L3, as described previously (Falla et al. 2014). Reference electrodes were placed on prepared skin over the right anterior superior iliac spine and on the spinous process of the vertebra prominens (Fig. 1).
Figure 1.
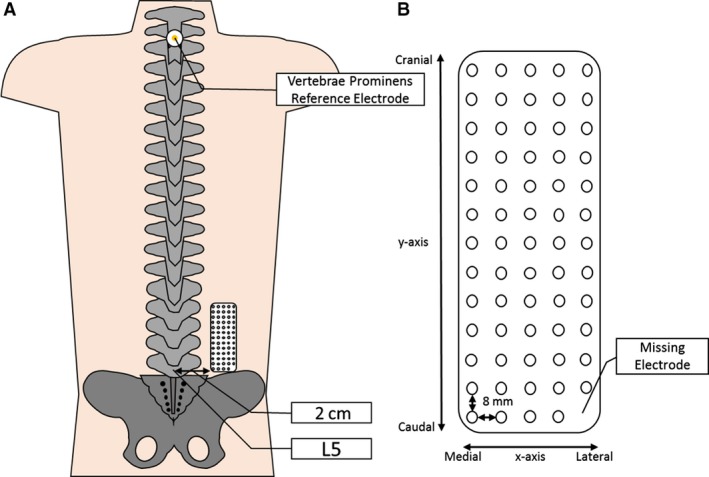
Depicting (A) the approximate positioning of the HDEMG grid 2 cm lateral to the L5 spinous process on the lumbar ES of the participant and (B) a schematic of the electrode grid showing the x‐ and y‐axes, reference electrode and inter‐electrode distance (not to scale).
A twin‐axis SG150B digital goniometer (Biometrics Ltd., Gwent, UK) was applied to the right mid‐axillary line of the participant. Only one axis (sagittal plane) was used for analysis. The lower sensor was attached to the centre of the iliac crest, with the midline of the sensor in line with the greater trochanter of the femur. With the participant positioned prone on the plinth, the resting angle was calibrated as 0°, with trunk deviation measured from this point. EMG signals and angular data were sampled at 2048 Hz and amplified (400‐channel EMG amplifier Quattrocento, OT Bioelettronica; −3 dB, bandwidth 10–500 Hz) by a factor of 150 and converted to digital form by a 16‐bit analogue‐to‐digital converter. Collected signals were stored on a computer hard drive and later analysed using a custom code on matlab (The Mathworks Inc., USA).
As described previously by Falla et al. (2014), each grid of electrodes recorded 64 monopolar signals. These signals were then processed offline to form horizontal derivatives across the grids. This was achieved by filtering the monopolar signals using a 20–350 Hz band‐pass filter and then processing adjacent signals to produce 59 bipolar EMG signals. The amplitude (RMS) and mean spectral power frequency (MNF) for each bipolar derivation were then calculated. The individual RMS and MNF values for each bipolar signal were averaged to produce the mean RMS and MNF values across the grid. The RMS values for each bipolar signal were used to create a topographical map of ES activity. This map was used to determine the location of the x‐ and y‐coordinates of the centroid as described previously (Madeleine et al. 2006; Farina et al. 2008; Tucker et al. 2009; Abboud et al. 2014; Falla et al. 2014, 2017). The location of the centroid was averaged across the 10‐s and 10% epochs for further use in analysis.
The values for the x‐ and y‐coordinates of the centroid were analysed as an absolute shift in mm from the start point quantified in the first 10% epoch (Falla et al. 2014). As movement of the centroid was both cranial and caudal in both groups, to allow for comparison between groups of the absolute shift in the y‐coordinate of the centroid, both positive and negative movements were made positive.
Experimental procedure
To complete the endurance task, participants were required to maintain an Ito test, as described by Ito et al. (1996) and Muller et al. (2010), until task failure or until 300 s. Participants were first asked to lie prone on a plinth, with a firm semi‐circular foam pad (18‐cm diameter) centred below the anterior superior iliac spines. To complete the endurance task, participants were asked to lift their sternum from the plinth, raising their upper body by ~15°. While maintaining this position, participants were asked to keep their arms in line with the body axis and not in contact with the plinth; participants were also required to contract their gluteal muscles and retain a neutral neck position. Prior to beginning the task, an investigator demonstrated the correct position for completion of the Ito test, and participants were permitted to complete a short 5‐s contraction to ensure they had the correct technique.
Throughout the task, the angle of the body axis was monitored visually and participants were alerted if their body axis was approaching the upper or lower acceptable limits (± 10°; Demoulin et al. 2007). Task failure was determined by a drop in the angle of trunk of greater than 10° at any point. While completing the contraction, participants were timed using a stopwatch; the time was recorded until task failure or until the maximum contraction duration was reached (300 s). Throughout the task, participants were given verbal encouragement and at 30‐s increments were provided with feedback on how long they had sustained the contraction.
Statistical analysis
Statistical analysis was performed using spss 24 (IBM, USA) with an alpha level set at 0.05. Regression analysis and analysis of covariance (ancova) tests were performed using prism (GraphPad, USA). Where P‐values were reported on spss as 0.000, they are here given as P < 0.001. Effect sizes have been reported where appropriate with anova results, based on guidance by Lakens (2013) in the format of generalised η2 (), alongside η2 values. For interpretation of these values, effect sizes are defined as small ( = 0.01), medium ( = 0.06) or large ( = 0.14) (Cohen, 1988; Lakens, 2013).
A Student t‐test was performed to identify any differences in endurance times between groups. The questionnaires used to gather sample characteristics were interpreted according to their respective guidelines (Miller et al. 1991; Hays et al. 1993; Fairbank & Pynsent, 2000; Childs et al. 2005). Student t‐tests were performed for each group to identify differences between the samples at baseline. To determine whether the failure of the task was influenced by fear of movement, the endurance time for each participant was correlated to their respective TSK score.
No direct comparison of the values reported for perceived exertion could be made between groups, as the time to task failure varied between groups. Therefore, the initial value after 30 s, the value at the mid‐point of endurance, and the level of exertion at task failure were determined for each participant. Significant differences between groups were investigated using a repeated measures analysis of variance (anova).
To make comparisons between groups with different times to task failure, the total contraction time for each participant was normalised into 10% epochs of the total endurance time (Farina et al. 2008). Repeated measures anova, with factors of group (CON and LBP) and time (10 epochs), was used to compare differences in EMG variables between groups. Newman–Keuls post‐hoc tests were also conducted where appropriate.
To identify trends in the displacement of the y‐coordinate of the centroid between groups, a linear regression was performed. To ensure that the results were not affected by the normalisation of time, this regression was performed using absolute endurance times and y‐coordinate displacement values calculated from the position of the centroid in the first 10‐s epoch. The regression lines for the CON and LBP groups were compared for statistical significance using an ancova.
Finally, to assess myoelectric manifestations of muscle fatigue, linear regressions were performed on RMS and MNF variables (Larivière et al. 2002). For each participant, the relations between RMS and time to task failure, and MNF and time to task failure were computed. In this analysis, both absolute values for RMS and MNF across time, and normalised values (using the using the first 10‐s epoch as a reference) were considered and the resulting slopes extracted. Independent samples t‐tests were then performed on the slopes for each condition to identify the mean slope for each group and identify any differences between these means (Roy et al. 1995; Pagé & Descarreaux, 2012).
Results
Participants
In all, 13 LBP and 13 CON participants successfully completed data collection; population characteristics are reported in Table 1. No significant anthropometric differences were found between groups for body mass index (BMI), height or weight and the BMI for both groups was within the ‘normal weight’ range (Stenholm et al. 2017). However, as anticipated, the LBP group presented with higher levels of disability (ODI −13.16%) and lower general and emotional health (RAND 36‐item health survey). Prior to data collection, LBP participants reported a current pain level of 1.92 out of 10, but a usual pain of 2.92, characterising the pain within the group as mild or low severity (Breivik et al. 2008). No significant correlation was found between scores on the TSK and the endurance time (R = −0.281, P = 0.165).
Table 1.
Mean participant characteristics separated by group, showing the standard deviation where appropriate
| Characteristic | LBP | Control | P‐value |
|---|---|---|---|
| Age (years) | 27.39 ± 9.7 | 26.46 ± 5.0 | – |
| Gender (# males) | 6 | 7 | – |
| Height (cm) | 168.75 ± 9.7 | 170.38 ± 6.7 | – |
| Weight (kg) | 70.97 ± 12.4 | 69.11 ± 12.7 | – |
| BMI | 24.78 | 23.78 | – |
| ODI (%)* | 13.16% ± 8% | 0.00% | < 0.001 |
| TSK | 25.31 ± 4.89 | 22.31 ± 7.20 | – |
| PNRS | |||
| Current pain* | 1.92 ± 1.44 | 0 | < 0.001 |
| Usual pain* | 2.92 ± 1.98 | 0 | < 0.001 |
| RAND 36‐item health survey | |||
| Physical functioning* | 82.52 ± 10.64 | 99.30 ± 2.52 | < 0.001 |
| Emotional wellbeing* | 69.85 ± 17.33 | 82.46 ± 7.58 | 0.024 |
| Pain* | 68.46 ± 16.79 | 95.00 ± 8.6 | < 0.001 |
| General health* | 64.62 ± 20.15 | 82.31 ± 10.53 | 0.010 |
Where significant differences occur, the characteristic is marked with an asterisk and a P‐value is displayed.
Endurance
Significantly lower endurance times were recorded for the LBP group (F = 8.4, P < 0.001) than for the control group (186.3 ± 72.3 s and 283.0 ± 33.0 s, respectively). With a 96.7‐s difference, this equates to the LBP group maintaining the contraction for 65.8% of the total time for the CON group on average. The mean values for initial, middle and final perceived exertion are shown in Fig. 2. No significant differences in exertion were found between the groups at any point (F = 1.42, P = 0.216).
Figure 2.
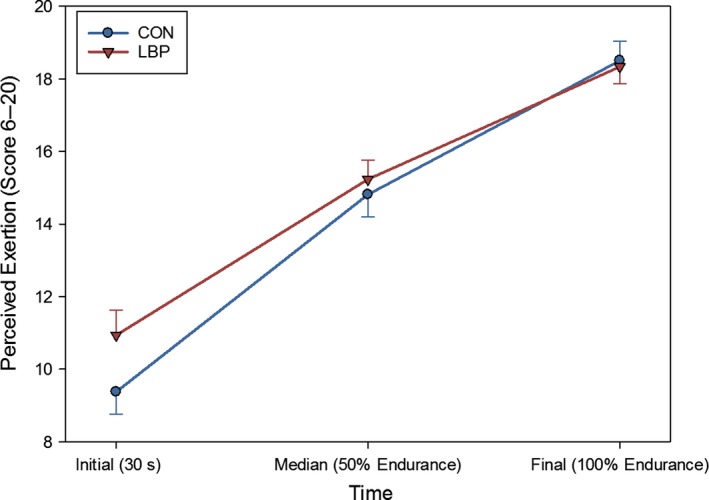
The mean values for the initial, mid‐point and final values (and SE) for the RPE as reported by participants during the endurance task. No significant differences were found between groups for exertion during the task.
Electromyographical changes
EMG amplitude and mean frequency
Across the duration of the contraction, the RMS was found to be systematically higher for CON than for LBP participants (main effect of group; F = 6.09, P = 0.022, = 0.18, η2 = 0.18; Fig. 3). This higher activation of the ES was visible in the topographical maps of the EMG amplitude (Fig. 4). On average, the CON participants showed a larger distribution of the activity throughout the entire muscle, whereas LBP participants showed a less diffused activation which tended to be more cranial. When this was quantified, an even distribution across the entire grid was observed in eleven CON and four LBP participants. Distribution was weighted cranially in one CON and eight LBP participants, and distribution was focused in the middle of the grid for one Con and one LBP participant.
Figure 3.
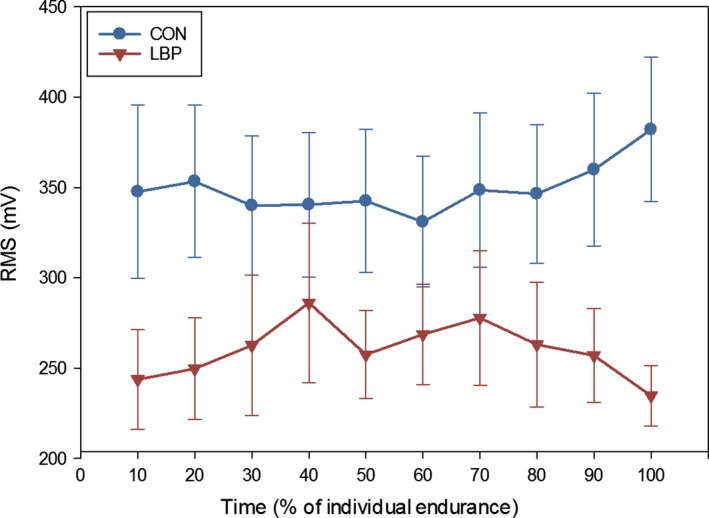
Average RMS values for LBP and CON participants across the duration of the endurance contractions (and SE), shown in 10% epochs of the participants’ total endurance times. No interactions or differences in shift were found between groups, but the CON group was found to be systematically higher throughout the contraction.
Figure 4.
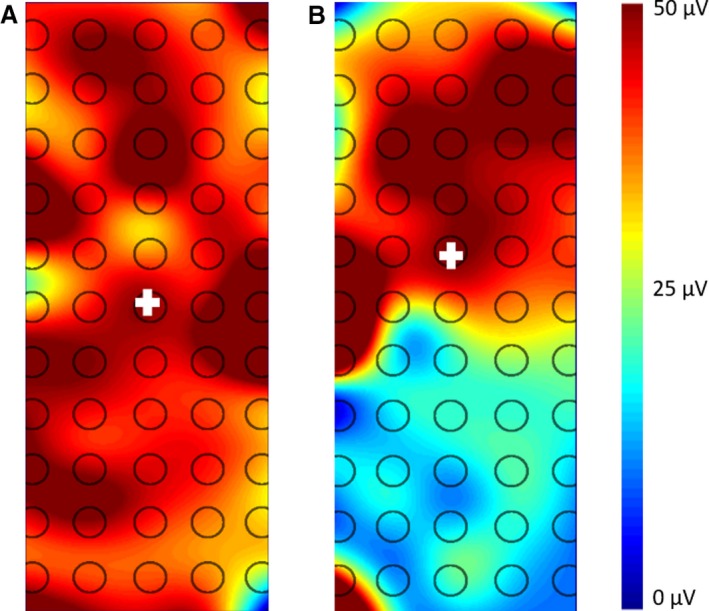
Representative RMS topographical maps for CON (A) and LBP (B) participants during the endurance task. The centroid is depicted by the crosshair and the scale is indicated in μV.
There were no significant differences between groups for the change in RMS throughout the task (F = 1.42, P = 0.216). There were also no significant increases or decreases in the mean RMS recorded for either group at any point during the endurance task (F = 0.929, P = 0.344). No significant differences between groups were observed for the mean MNF at any point during the contraction (F = 1.118, P = 0.334).
Centroid of the EMG RMS map
No significant differences were found between groups for the position of the x‐coordinate of the centroid (medial‐lateral direction) throughout the task (initial position F = 2.27, P = 0.77; shift over the duration of the contraction F = 2.27, P = 0.77).
The y‐coordinate of the centroid (cranial‐caudal direction) in CON participants was found to be systematically more caudal than in the LBP group (main effect for group F = 44.00, P < 0.001, = 0.64, η2 = 0.65). The y‐coordinate was found to be approximately 42.0 mm (± 4.99 mm) cranial of the reference electrode in CON participants, but approximately 53.6 mm (± 3.64 mm) cranial of the reference electrode in the LBP participants. Throughout the endurance task, there was a mean difference between the LBP and CON in the y‐coordinate position of 11.6 mm (Fig. 5).
Figure 5.
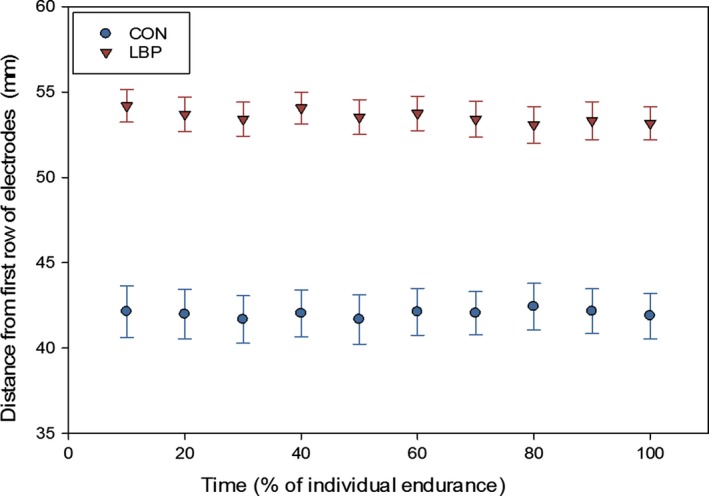
Absolute mean locations (and standard error, SE) of the y‐coordinate of the centroid for CON and LBP group throughout the endurance contraction.
Using the location of the y‐coordinate of the centroid in the 1st epoch as a reference point, the displacement was calculated for each 10% epoch. To achieve this, the shift in mm was measured from the position of the y‐coordinate in the 1st epoch; this was either a positive (cranial movement) or a negative (caudal) value. No clear direction of shift was found (cranially or caudally), as groups showed both cranial and caudal movements (CON six cranial, seven caudal; LBP nine cranial, four caudal). To better understand the movement of the centroid, all values for displacement were made positive and therefore net displacement was used for all y‐coordinate shift results. At task failure, the mean y‐coordinate displacement for the CON group was 2.10 ± 0.45 mm, whereas for the LBP group it was 1.40 ± 0.29 mm. Both groups showed a significant displacement of the centroid in the y‐axis over time (F = 2.5, P = 0.004, = 0.22, η2 = 0.30) and a significant displacement within each group (F = 9.9, P = 0.01; Fig. 6). There was no interaction between groups for the displacement of the y‐coordinate in the data which had been normalised to task failure (F = 1.709, P = 0.134).
Figure 6.
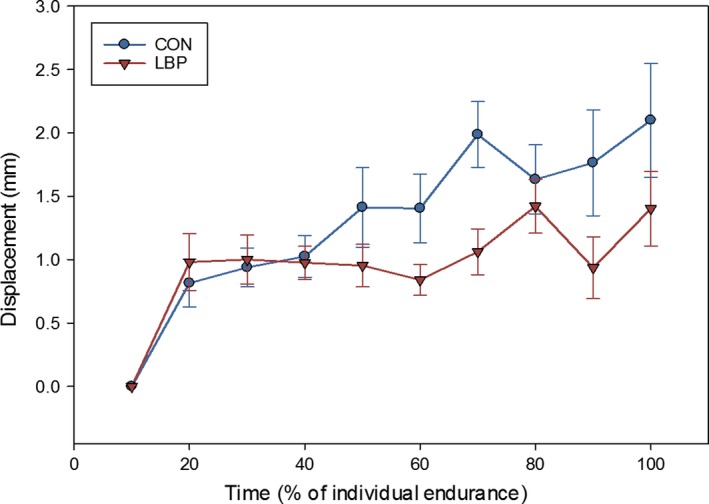
Displacement of the y‐coordinate of the centroid from the position in the first 10% epoch (and SE), showing a significant displacement of the y‐coordinate for both the CON and the LBP group.
The regression analysis performed using absolute values for time showed a significant relationship between the shift in the y‐coordinate of the centroid and the time to task failure (Fig. 7) for both groups (CON r 2 = 0.142, P < 0.0001; LBP r 2 = 0.053, P = 0.0004). Additionally, ancova analysis showed that there was a significant difference between the regression lines for each group (F = 5.597, P = 0.0183), indicating that the relationship between y‐coordinate shift and time was significantly different between groups (LBP/CON; Zar, 2010).
Figure 7.

Linear regression analysis of the shift in y‐coordinate of the centroid, showing significant variation in the shift of the y‐coordinate over the length of the endurance contraction (F = 5.597, P = 0.0183). Two CON points where shift was more than 6 mm not shown.
Myoelectric manifestations of muscle fatigue showed no differences under any condition. There were no differences in the slopes between groups for absolute RMS (P = 0.71), normalised RMS (P = 0.37), absolute MNF (P = 0.48) or normalised MNF (P = 0.79).
Discussion
This is the first study to assess muscle activation behaviour using HDEMG during a functional position‐matching lumbar endurance task in people with and without LBP. The results revealed an altered motor control strategy to a standardised endurance task in people with LBP with evidence of activation of more cranial regions of the lumbar ES with respect to asymptomatic people. Moreover, a relationship was also demonstrated between the extent of redistribution of muscle activity and endurance time which has important implications for the understanding of the neurophysiological responses to fatigue, additionally the physiological significance of these findings are supported by large effect sizes.
Distribution of activity
Throughout the endurance contraction, the RMS was found to be significantly higher in the CON group than in the LBP group. One possible explanation for this disparity in amplitude could be quantified from the systematic differences in the position of the centroid along the y‐axis. Throughout the task, the y‐coordinate of the centroid for the CON group was 12 mm caudal to that of the LBP group. Previous studies which have induced pain via injection of hypertonic saline, have shown that areas with greater pain show reduced activity, and that in an acute painful condition, the muscle activation can shift outside of the painful region (Madeleine et al. 2006; Falla & Farina, 2008a; Falla et al. 2017). Although somewhat speculative, it is likely that a more caudal centre of contraction could indicate a more biomechanically favourable contraction by activating a greater number of fibres. In this instance, those with pain appear to have shifted the activity in the ES more cranially. A more caudal contraction, which is distributed over a larger area of the muscle, would be able to utilise the larger volume of muscles from lower lumbar vertebrae and spread the load more effectively across a greater number of muscle fibres, creating a longer lever arm (Bogduk, 2005). The longer lever arm would act to minimise the force needed to sustain the contraction and the diffuse activation would reduce localised fatigue, facilitating sustained endurance.
Redistribution of lumbar ES activity
During the Ito test, the CON participants showed a greater shift of the centroid of the EMG amplitude map indicating a greater redistribution of lumbar ES activity than the LBP group. It was also shown that the amount of redistribution increased progressively over the duration of the task and that there was an association between the extent of redistribution of activity and endurance time. As previously described by Falla et al. (2014), a redistribution of activity likely prevents localised muscle fatigue through the build‐up of metabolic factors and overload on specific regions of the muscle. The task used in Falla et al. (2014) was dynamic and consisted of periodic contractions, whereas the contraction used here is static and so the tissue would be under further strain due to decreased blood flow and ischaemia (Masuda et al. 1999).
The results of this study do not support a direction of shift for this task, as there was no clear preference for a direction in either group. However, this study differs from previous studies which used HDEMG to examine the lumbar muscles, as it does not involve an external force. Gallina et al. (2013) investigated the significance of the shift in the trapezius muscle and determined that the direction of shift was task‐dependent. Russ et al. (2018) and Thomas et al. (2011) showed that there were specific differences in lumbar endurance between force‐ and position‐matching tasks but they were unable to give the reasons for these differences. The lack of a clear direction of shift seen in this study may imply a focus of muscle activity in a more biomechanically favourable point for each participant. As there was no specific point to ‘push’ against, the centre of activity for each participant was likely determined by individual anthropomorphic features, for example a greater trunk length to leg length ratio. In this study, it is speculated that as participants were not secured to the plinth or pushing against a point, the impact of the relative size and weight of the legs compared with the upper body would impact on the stability of the participant while contracting. Thus the participant might be likely to sustain a contraction which affords them the optimal stability for their individual anthropomorphic characteristics.
Muscular activity
Biomechanical and anatomical models of the lumbar musculature indicate that the shared insertions of portions the ES cause a diagonal slight overlapping of successive superficial fibres (Bogduk, 1980, 2005). According to anatomical studies, the portions of the ES which are likely to be muscular in the region beginning 2 cm lateral to L5 include the iliocostalis lumborum pars lumborum and the iliocostalis lumborum pars thoracis, with the muscular portions of the longissimus being too medial or too cranial to be covered by the electrode grid. In the pars lumborum, the deepest and most lateral fibres are from L5 and the most superficial and medial fibres from L1; each successive lamina of fibres slightly overlaps the previous layer (Bogduk, 2005; Christophy et al. 2012). The Ito test used in this study is designed to achieve relative isolation of the lumbar musculature, so the distribution and redistribution of activity in this portion of the muscle is thought to be key to understanding endurance in this task (Muller et al. 2010). It is therefore suspected and proposed that due to pain in the lumbar region, LBP participants utilised a motor control strategy which preferentially activated different portions of the muscle, such as the more cranial iliocostalis lumborum pars thoracis and thus led to a shorter time to task failure compared with the CON group. As no imaging was used in this study, the exact distribution of activity among portions of the ES, and what effect any individual variations in muscle architecture or fibre distribution could have on the activation pattern, remain unknown (Mannion et al. 1997, 2000).
Endurance and fatigue
The LBP group demonstrated endurance which fell significantly short of the CON group. Similar findings have been demonstrated in previous studies investigating lumbar endurance to task failure; however, the absolute endurance times reported here were significantly higher than those reported following a Sørenson test (Abboud et al. 2014; Jubany et al. 2017). This difference could be attributed in part to the differences between the force‐matching tasks previously used and the position‐matching tasks such as one the Ito test used here (Russ et al. 2018). However, Muller et al. (2010) reported lower endurance times for a position‐matching Sørenson test when compared directly with an Ito test. In this instance, it may also be relevant to consider the biomechanical and myoelectrical differences between the Ito and Sørenson positions. As discussed previously, muscle activation in the Ito test is focused on the lumbar region, whereas the Sørenson test has shared activation between the lumbar and hip extensors, possibly contributing to differences in endurance time (Muller et al. 2010). Additionally, although both tasks are measures of lumbar endurance, each requires a different position to be held; the Ito test requires spinal extension to be sustained and the Sørenson requires an unsupported neutral spine to be maintained against gravity (Muller et al. 2010). Due to this, it is likely that the point at which the participant's centre of mass is supported may be lower in the Ito test, producing a lower moment.
At task failure, both LBP and CON participants reported a mean RPE of between 18.3 and 18.5, indicating that both groups reached a similar level of exertion. Analysis of the MNF results and the indices measuring the myoelectric manifestations of fatigue revealed that there were no significant differences between the CON and LBP groups. Previous HDEMG studies evaluating fatigue of the lumbar ES have shown greater myoelectric manifestations of fatigue than these results suggest; however, other studies also did not find significant differences between groups (Tucker et al. 2009; Abboud et al. 2014). This could be explained partly by recent studies which have demonstrated that frequency variables, including MNF, do not accurately predict motor unit recruitment during contractions (Merletti & Farina, 2016; Vecchio et al. 2017). Additionally, it has been shown in the knee extensors that myoelectric manifestations of fatigue are only seen when the exertion exceeds 40% of the MVC (de Ruiter et al. 2012). Two exercises in a study by Plamondon et al. (2002) were similar in position and function to the Ito test; in that study, these exercises were found to be between 26 and 32% of a participant's MVC. As the current study did not assess the functional capacity of the participants, the results for MNF may be affected by the task being below 40% of an MVC for some participants.
The results of this study coalesce to indicate that the LBP participants utilise a different motor control strategy to complete the task. This strategy was characterised by a reduced activation of the ES which was focused more cranially and which throughout the task showed less redistribution of activity. It appears that participants used less favourable portions of the ES to complete the task, resulting in shorter endurance times.
Strengths and limitations
A strength of this study was its use of HDEMG to present a more comprehensive characterisation of ES activity during an endurance test, a test which can be easily replicated in a clinical environment. In addition, the Ito test presented here has previously been found to isolate the lumbar musculature better than the Sørenson test does (Muller et al. 2010). No significant differences were found in the RPE between groups, supporting the suitability of the Ito test in this population. However, it should be considered that as no clear guidelines for task failure have been validated for the Ito test, the task failure criteria of ± 10° could be perceived to affect the redistribution of activity during the task. Nonetheless, as systematic differences were seen between groups for all values related to the RMS, we are confident that the differences between groups are valid, although they could hinder comparisons with other lumbar endurance tasks. To mitigate this effect, where possible, generalised effect sizes have been reported with anova results, which have been interpreted in line with the guidelines suggested by Cohen (1988), and reiterated by Lakens (2013), whereby effect sizes are defined as small (η2 = 0.01), medium (η2 = 0.06), or large (η2 = 0.14). However, it has been suggested that these benchmarks for η2 may not be as accurate in repeated measures conditions. Therefore, we also included the values, which have been proposed to allow better comparisons between studies (Lakens, 2013).
As participants could not be under current active management by a healthcare professional (a requirement of the University Ethical Committee), the LBP group presented with low levels of current pain and mild disability. Although the sample size was relatively small and the LBP participants presented with relatively mild LBP, significant group differences were revealed; even greater group differences may be expected when testing patients with even greater pain severity or longer pain duration (Mannion et al. 2000; Arendt‐Nielsen & Graven‐Nielsen, 2008). Finally, synergistic muscles were not covered by the HDEMG grid. This limitation was imposed in an attempt to reduce the effect of crosstalk between overlapping muscles of different architecture which may have confounded the results (Martinez‐Valdes et al. 2018). Further studies using mixed methodologies, including intramuscular electrodes and motor unit decomposition, may provide clearer information about individual muscle contributions to this task.
Conclusion
Asymptomatic people display a spatial redistribution of lumbar ES activity during an endurance task and this adaptation is reduced in people with LBP. Moreover, people with LBP engage more cranial regions of the lumbar ES during trunk extension, likely reflecting an inefficient motor strategy.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
Conflict of interest
There are no competing interests to declare.
Author contributions
AS, EMV and DF contributed to study conception and design. AS and CM acquired the data. AS, EMV and DF performed the data analysis. All authors contributed to interpretation of the data. Drafting of the manuscript was performed by AS, EMV and DF. All authors participated in revising the manuscript and approving the final submission.
References
- Abboud J, Nougarou F, Page I, et al. (2014) Trunk motor variability in patients with non‐specific chronic low back pain. Eur J Appl Physiol 114, 2645–2654. [DOI] [PubMed] [Google Scholar]
- Arendt‐Nielsen L, Graven‐Nielsen T (2008) Muscle pain: sensory implications and interaction with motor control. Clin J Pain 24, 291–298. [DOI] [PubMed] [Google Scholar]
- Barbero M, Merletti R, Rainoldi A (2012) Atlas of Muscle Innervation Zones. Milan: Springer‐Verlag. [Google Scholar]
- Bogduk N (1980) A reappraisal of the anatomy of the human lumbar erector spinae. J Anat 131, 525–540. [PMC free article] [PubMed] [Google Scholar]
- Bogduk N (2005) Clinical Anatomy of the Lumbar Spine and Sacrum. Edinburgh: Elsevier/Churchill Livingstone. [Google Scholar]
- Borg G (1998) Borg's Perceived Exertion and Pain Scales. Champaign: Human Kinetics. [Google Scholar]
- Breivik H, Borchgrevink PC, Allen SM, et al. (2008) Assessment of pain. Br J Anaesth 101, 17–24. [DOI] [PubMed] [Google Scholar]
- Childs JD, Piva SR, Fritz JM (2005) Responsiveness of the numeric pain rating scale in patients with low back pain. Spine (Phila Pa 1976) 30, 1331–1334. [DOI] [PubMed] [Google Scholar]
- Christophy M, Faruk Senan NA, Lotz JC, et al. (2012) A musculoskeletal model for the lumbar spine. Biomech Model Mechanobiol 11, 19–34. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988) Statistical Power Analysis for the Behavioral Sciences. New York: Routledge Academic. [Google Scholar]
- Demoulin C, Crielaard JM, Vanderthommen M (2007) Spinal muscle evaluation in healthy individuals and low‐back‐pain patients: a literature review. Joint Bone Spine 74, 9–13. [DOI] [PubMed] [Google Scholar]
- Dionne CE, Dunn KM, Croft PR, et al. (2008) A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine (Phila Pa 1976) 33, 95–103. [DOI] [PubMed] [Google Scholar]
- Fabian S, Hesse H, Grassme R, et al. (2005) Muscular activation patterns of healthy persons and low back pain patients performing a functional capacity evaluation test. Pathophysiology 12, 281–287. [DOI] [PubMed] [Google Scholar]
- Fairbank JC, Pynsent PB (2000) The Oswestry Disability Index. Spine (Phila Pa 1976) 25, 2940–2952; discussion 2952. [DOI] [PubMed] [Google Scholar]
- Falla D, Farina D (2008a) Neuromuscular adaptation in experimental and clinical neck pain. J Electromyogr Kinesiol 18, 255–261. [DOI] [PubMed] [Google Scholar]
- Falla D, Farina D (2008b) Non‐uniform adaptation of motor unit discharge rates during sustained static contraction of the upper trapezius muscle. Exp Brain Res 191, 363–370. [DOI] [PubMed] [Google Scholar]
- Falla D, Gizzi L, Tschapek M, et al. (2014) Reduced task‐induced variations in the distribution of activity across back muscle regions in individuals with low back pain. Pain 155, 944–953. [DOI] [PubMed] [Google Scholar]
- Falla D, Cescon C, Lindstroem R, et al. (2017) Muscle pain induces a shift of the spatial distribution of upper trapezius muscle activity during a repetitive task: a mechanism for perpetuation of pain with repetitive activity? Clin J Pain 33, 1006–1013. [DOI] [PubMed] [Google Scholar]
- Farina D, Leclerc F, Arendt‐Nielsen L, et al. (2008) The change in spatial distribution of upper trapezius muscle activity is correlated to contraction duration. J Electromyogr Kinesiol 18, 16–25. [DOI] [PubMed] [Google Scholar]
- Gallina A, Merletti R, Vieira TM (2011) Are the myoelectric manifestations of fatigue distributed regionally in the human medial gastrocnemius muscle? J Electromyogr Kinesiol 21, 929–938. [DOI] [PubMed] [Google Scholar]
- Gallina A, Merletti R, Gazzoni M (2013) Uneven spatial distribution of surface EMG: what does it mean? Eur J Appl Physiol 113, 887–894. [DOI] [PubMed] [Google Scholar]
- Gizzi L, Muceli S, Petzke F, et al. (2015) Experimental muscle pain impairs the synergistic modular control of neck muscles. PLoS ONE 10, e0137844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays RD, Sherbourne CD, Mazel RM (1993) The RAND 36‐item health survey 1.0. Health Econ 2, 217–227. [DOI] [PubMed] [Google Scholar]
- Ito T, Shirado O, Suzuki H, et al. (1996) Lumbar trunk muscle endurance testing: an inexpensive alternative to a machine for evaluation. Arch Phys Med Rehabil 77, 75–79. [DOI] [PubMed] [Google Scholar]
- Jubany J, Marina M, Angulo‐Barroso R (2017) Electromyographic and kinematic analysis of trunk and limb muscles during a holding task in individuals with chronic low back pain and healthy controls. PM R 9, 1106–1116. [DOI] [PubMed] [Google Scholar]
- Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t‐tests and ANOVAs. Frontiers in Psychology 4, 863 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larivière C, Arsenault AB, Gravel D, et al. (2002) Evaluation of measurement strategies to increase the reliability of EMG indices to assess back muscle fatigue and recovery. J Electromyogr Kinesiol 12, 91–102. [DOI] [PubMed] [Google Scholar]
- Madeleine P, Leclerc F, Arendt‐Nielsen L, et al. (2006) Experimental muscle pain changes the spatial distribution of upper trapezius muscle activity during sustained contraction. Clin Neurophysiol 117, 2436–2445. [DOI] [PubMed] [Google Scholar]
- Mannion AF, Dumas GA, Cooper RG, et al. (1997) Muscle fibre size and type distribution in thoracic and lumbar regions of erector spinae in healthy subjects without low back pain: normal values and sex differences. J Anat 190, 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannion AF, Kaser L, Weber E, et al. (2000) Influence of age and duration of symptoms on fibre type distribution and size of the back muscles in chronic low back pain patients. Eur Spine J 9, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Valdes E, Negro F, Falla D, et al. (2018) Surface electromyographic amplitude does not identify differences in neural drive to synergistic muscles. J Appl Physiol (1985) 124, 1071–1079. [DOI] [PubMed] [Google Scholar]
- Masuda K, Masuda T, Sadoyama T, et al. (1999) Changes in surface EMG parameters during static and dynamic fatiguing contractions. J Electromyogr Kinesiol 9, 39–46. [DOI] [PubMed] [Google Scholar]
- Merletti R, Farina D (2016) Surface Electromyography: Physiology, Engineering, and Applications. Hoboken: John Wiley & Sons. [Google Scholar]
- Miller RP, Kori SH, Todd DD (1991) The Tampa Scale: a measure of kinesiophobia. Clin J Pain 7, 51–52. [Google Scholar]
- Muller R, Strassle K, Wirth B (2010) Isometric back muscle endurance: an EMG study on the criterion validity of the Ito test. J Electromyogr Kinesiol 20, 845–850. [DOI] [PubMed] [Google Scholar]
- Pagé I, Descarreaux M (2012) Trunk muscle fatigue during a lateral isometric hold test: what are we evaluating? Chiropr Man Therap 20, 12 10.1186/2045-709X-20-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamondon A, Serresse O, Boyd K, et al. (2002) Estimated moments at L5/S1 level and muscular activation of back extensors for six prone back extension exercises in healthy individuals. Scand J Med Sci Sports 12, 81–89. [DOI] [PubMed] [Google Scholar]
- Roy SH, De‐Luca CJ, Emley M, et al. (1995) Spectral electromyographic assessment of back muscles in patients with low back pain undergoing rehabilitation. Spine (Phila Pa 1976) 20, 38–48. [DOI] [PubMed] [Google Scholar]
- de Ruiter CJ, Maas EA, Wesseling MG, et al. (2012) Knee extensor fatigue threshold is related to whole‐body VO2max. Med Sci Sports Exerc 44, 1366–1374. [DOI] [PubMed] [Google Scholar]
- Russ DW, Ross AJ, Clark BC, et al. (2018) The effects of task type on time to task failure during fatigue: a modified Sorensen test. J Mot Behav 50, 96–103. [DOI] [PubMed] [Google Scholar]
- Stenholm S, Head J, Aalto V, et al. (2017) Body mass index as a predictor of healthy and disease‐free life expectancy between ages 50 and 75: a multicohort study. International Journal of Obesity 41, 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JS, Ross AJ, Russ DW, et al. (2011) Time to task failure of trunk extensor muscles differs with load type. J Mot Behav 43, 27–29. [DOI] [PubMed] [Google Scholar]
- Tucker K, Falla D, Graven‐Nielsen T, et al. (2009) Electromyographic mapping of the erector spinae muscle with varying load and during sustained contraction. J Electromyogr Kinesiol 19, 373–379. [DOI] [PubMed] [Google Scholar]
- VanderZee KI, Sanderman R, Heyink JW, et al. (1996) Psychometric qualities of the RAND 36‐item health survey 1.0: a multidimensional measure of general health status. Int J Behav Med 3, 104–122. [DOI] [PubMed] [Google Scholar]
- Vecchio AD, Negro F, Felici F, et al. (2017) Associations between motor unit action potential parameters and surface EMG features. J Appl Physiol (1985) 123, 835–843. [DOI] [PubMed] [Google Scholar]
- Zar JH (2010) Biostatistical Analysis. Upper Saddle River: Prentice Hall. [Google Scholar]


