Abstract
Background and Aims
Vitamin D deficiency has been linked to a higher risk of ischemic stroke. We therefore explored the relationship between serum 25‐hydroxyvitamin D [25(OH)D] levels and early neurological deterioration (END) after acute ischemic stroke in a hospital‐based prospective study.
Methods
From June 2016 to June 2018, patients with ischemic stroke within 48 hr from symptom onset were consecutively recruited. Serum 25(OH)D levels were measured at admission. END was defined as an increase of ≥1 point in motor power or ≥2 points in the total National Institute of Health Stroke Scale score within 7 days after admission. Multiple logistic regression models were performed to calculate the odds ratio (OR) and confidence intervals (CI) of 25(OH)D levels in predicting END.
Results
A total of 478 subjects were enrolled, of which 136 (28.5%) patients developed END. The mean 25(OH)D levels were 49.5 ± 15.8 nmol/L. Univariate logistic regression analysis showed that advanced age, white matter lesions, high level of body mass index, diastolic blood pressure, fasting blood glucose and homocysteine, and low 25(OH)D levels were associated with END. Furthermore, multivariate regression analysis demonstrated that the first quartile of 25(OH)D concentrations [OR, 2.628; 95% CI,1.223–5.644; p = 0.013] was independently risk factor for END.
Conclusions
This study illustrated that lower 25(OH)D levels might be associated with an increasing risk of END in acute ischemic stroke patients.
Keywords: 25‐hydroxyvitamin D, early neurological deterioration, ischemic stroke
1. INTRODUCTION
Stroke is the leading cause of mortality and permanent disability, contributing to a tremendous burden on health resources in China (Liu et al., 2007). Although most ischemic stroke patients tend to improve over the subsequent few days after symptoms onset, a sizeable fraction does not substantially recover but even deteriorates, which has been identified as early neurological deterioration (END) (Seners, Turc, Oppenheim, & Baron, 2015). Previous studies reported that END after acute ischemic stroke is observed in 5%–40% patients and often leads to an increasing risk of functional disability and mortality (Helleberg, Ellekjær, Rohweder, & Indredavik, 2014; Kwon, Lee, Bae, & Kang, 2014; Seners et al., 2015; Sun et al., 2014; Zhang et al., 2016). Accordingly, more precise understanding of the potential mechanisms involved in END could provide valuable insights for improving stroke outcomes.
Vitamin D is recognized as a hormone that mainly regulates calcium metabolism, and possesses a strong anti‐inflammatory effect (Buell & Dawson‐Hughes, 2008). The inactive vitamin D is hydroxylated in the liver to form 25‐hydroxyvitamin D [25(OH)D], which is widely viewed as the superior biomarker for assessing vitamin D status (Hossein‐nezhad & Holick, 2012). Nowadays, vitamin D deficiency has been implicated as a potentially modifiable risk factor for ischemic cerebrovascular disease. A population‐based study nested in Hong Kong Chinese indicated that lower 25(OH)D levels might increase the risk of incident ischemic stroke (Leung et al., 2017). Recently, several studies have reported the significant correlation between 25(OH)D status and functional outcome after acute ischemic stroke (Park et al., 2015; Tu, Zhao, Xu, & Chen, 2014; Wang, Ji, Tong, & Zhang, 2014). Nevertheless, whether 25(OH)D levels are associated with acute ischemic stroke complications, such as END, has not been well clarified. We therefore performed this prospective study to evaluate the relationship between 25(OH)D levels and the development of END in patients with acute ischemic stroke.
2. MATERIALS AND METHODS
2.1. Subjects
This was a hospital‐based prospective study screening patients in Anhui Provincial Hospital from June 2016 to June 2018. Patients diagnosed as first‐ever ischemic stroke and hospitalized within 48 hr of symptoms onset were initially considered for inclusion in this study. Patients with age less than 18 years old, renal insufficiency, severe hepatic disease, severe heart failure, malignant tumor, acute or chronic inflammatory disease, autoimmune diseases, and early discharge were not included. We also excluded those received intravenous thrombolysis or an emergent endovascular procedure. This study was approved by the institutional review board of Anhui Provincial Hospital. Informed consent was obtained from participants or legal representatives.
2.2. Clinical data collection
Demographic characteristics and clinical data were collected at the time of enrollment. Variables were recorded as follows: (a) demographic characteristics, hypertension, diabetes mellitus, hyperlipidemia, current smoking and drinking, atrial fibrillation and medical history; (b) clinical parameters including systolic and diastolic blood pressure level, body mass index, stroke severity and stroke subtypes. Stroke subtypes was classified according to TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria (Ir, 1999). Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS) score. Functional outcome was evaluated at discharge using the modified Rankin scale. We also performed laboratory tests for hyper‐sensitive C‐reactive protein (immunoturbidimetry), fasting blood glucose (glucose oxidase assay), lipid profile (ALBK assay), homocysteine (immunoturbidimetry), and 25(OH)D level (chemiluminescent immunoassay).
2.3. Definition of END
Stroke severity was assessed using NIHSS at admission and continued 1–3 times a day within the 7 days after admission. The neurologist who performed serial neurologic examinations was certified in the NIHSS scoring and blinded to clinical information. In accordance with previous studies, we collected END cases using the following criteria: an increment of ≥1 point in motor power or ≥2 points in the total NIHSS score within 7 days (Kwon, Lim, Park, & Lee, 2011; Kwon et al., 2014; Zhang et al., 2016).
2.4. Measurement of serum 25(OH)D
Blood sample was collected from all patients within 24 hr after admission. After at least 30 min of clotting, the specimens were centrifuged at 1500 g for 10 min and the isolated serum were stored at −80°C until analysis. The 25(OH)D levels were measured by chemiluminescent immunoassay (Siemens Healthcare Diagnostics, Inc, NY). The intra‐ and inter‐assay coefficients of variation were 5.1% and 4.4%, respectively, with a standard concentration in these kits ranging from 10 to 275 nmol/L. All procedures were conducted in strict accordance to manufacturers' instructions.
2.5. Statistical analysis
All statistical analyses were performed using SPSS version 23.0 for Windows (SPSS IBM Inc, Chicago, IL). Continuous variables were presented as the means (standard deviation, SD) or median (interquartile range), and categorical variables were expressed as n (%). Differences in baseline characteristics according to quartile of 25(OH)D concentrations were determined using χ2, analysis of variance, or Kruskal–Wallis where appropriate. Binary logistic regression analysis was used to assess the association between 25(OH)D levels and END. Multivariable analysis was adjusted for the factors with a p < 0.1 in the univariable analysis (including age, white matter lesions, body mass index, diastolic blood pressure, fasting blood glucose and homocysteine levels). Kaplan–Meier curves were performed to estimate the probability of END stratified by 25(OH)D quartile. We also used the receiver operating characteristic (ROC) curves to measure the diagnostic performance of 25(OH)D concentrations. All tests were two‐tailed and statistical significance was established at p < 0.05.
3. RESULTS
We recruited 478 patients for the final analysis (Figure 1). Mean age of patients was 62.8 years (SD 9.9) and 52.3% were men. Among these patients, 66.1% had hypertension, 22.0% had diabetes mellitus and 16.9% had hyperlipidemia. The mean 25(OH)D levels were 49.5 ± 15.8 nmol/L, with quartile level as follows: first quartile (<40.5 nmol/L), second quartile (40.5–51.9 nmol/L), third quartile (52.0–60.4 nmol/L), and fourth quartile (>60.4 nmol/L).
Figure 1.
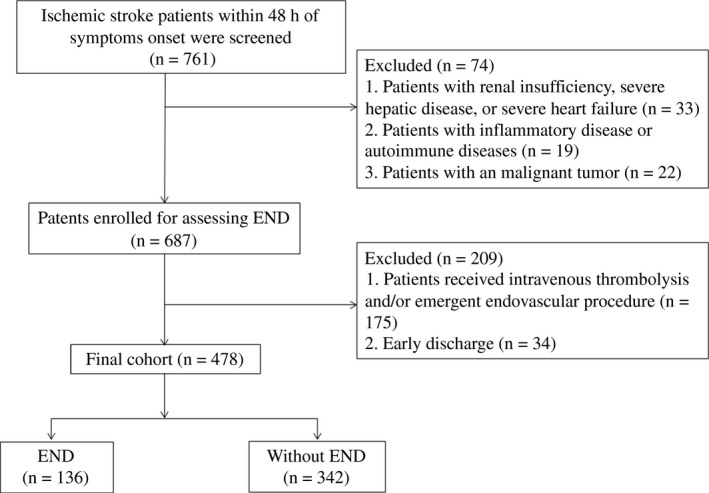
Flowchart of patient inclusion
Table 1 demonstrated the main demographic, clinical and imaging characteristics of the study population stratified by the 25(OH)D quartile. In general, decreased 25(OH)D level showed a significant correlation with age (p = 0.014), female sex (p = 0.003), diabetes mellitus (p = 0.006), white matter lesions (p = 0.001), END (p = 0.001), died at discharge (p = 0.042), body mass index (p = 0.028), systolic blood pressure (p = 0.002), fasting blood glucose (p = 0.027) and total cholesterol level (p = 0.027). However, there was no significant difference between 25(OH)D levels and NIHSS score (p = 0.339), and stroke subtypes (p = 0.293).
Table 1.
Baseline characteristics of the study population stratified by the 25(OH)D quartile
| Variable | 25(OH)D quartile | p value | |||
|---|---|---|---|---|---|
| First (n = 118) | Second (n = 124) | Third (n = 120) | Fourth (n = 116) | ||
| Demographic characteristics | |||||
| Age, year | 65.8 ± 11.0 | 63.1 ± 10.8 | 61.8 ± 8.9 | 60.4 ± 8.1 | 0.014 |
| Female, % | 60 (50.8) | 76 (61.3) | 40 (33.3) | 52 (44.8) | 0.003 |
| Vascular risk factors, % | |||||
| Hypertension | 84 (71.2) | 88 (71.0) | 76 (63.3) | 68 (58.6) | 0.113 |
| Diabetes mellitus | 36 (30.5) | 31 (25.0) | 24 (20.0) | 14 (12.1) | 0.006 |
| Hyperlipidemia | 24 (20.3) | 27 (21.8) | 14 (11.7) | 16 (13.8) | 0.102 |
| Current drinking | 22 (18.6) | 29 (23.4) | 30 (25.0) | 28 (24.1) | 0.658 |
| Current smoking | 33 (27.9) | 44 (35.4) | 37 (30.8) | 29 (25.0) | 0.330 |
| Atrial fibrillation | 20 (16.9) | 18 (14.5) | 14 (11.7) | 20 (17.2) | 0.600 |
| Clinical data | |||||
| Previous antiplatelet, % | 22 (18.6) | 26 (20.9) | 16 (13.3) | 20 (17.2) | 0.461 |
| Previous statin, % | 11 (9.3) | 14 (11.3) | 10 (8.3) | 14 (12.1) | 0.784 |
| Body mass index, kg/m2 | 25.3 ± 3.4 | 24.8 ± 3.8 | 24.8 ± 3.1 | 23.8 ± 2.6 | 0.028 |
| Systolic blood pressure, mmHg | 141.8 ± 22.6 | 138.8 ± 17.9 | 136.1 ± 17.6 | 133.0 ± 15.6 | 0.002 |
| Diastolic blood pressure, mmHg | 82.2 ± 9.9 | 81.5 ± 11.3 | 80.4 ± 10.5 | 81.6 ± 10.7 | 0.627 |
| Silent lacunar infarction, % | 50 (42.4) | 48 (38.7) | 53 (45.7) | 47 (37.3) | 0.308 |
| White matter lesions, % | 52 (44.1) | 46 (37.1) | 30 (25.0) | 20 (17.2) | 0.001 |
| NIHSS, score | 4.0 (2.0, 8.0) | 4.0 (2.0, 8.5) | 4.0 (2.0, 6.0) | 4.0 (2.0, 7.0) | 0.339 |
| END, % | 44 (37.3) | 42 (33.9) | 32 (26.7) | 18 (15.5) | 0.001 |
| Died at discharge, % | 17 (14.4) | 18 (14.5) | 6 (5.0) | 10 (8.6) | 0.042 |
| mRS at discharge, % | 0.056 | ||||
| 0‐2 | 63 (53.4) | 74 (59.7) | 83 (69.2) | 77 (66.4) | |
| 3‐6 | 55 (46.6) | 50 (40.3) | 37 (30.8) | 39 (33.6) | |
| Stroke subtype, % | 0.293 | ||||
| Large artery atherosclerosis | 41 (34.7) | 50 (40.3) | 47 (39.2) | 31 (26.7) | |
| Cardioembolism | 22 (18.6) | 20 (16.1) | 20 (16.7) | 23 (19.8) | |
| Small artery occlusion | 36 (30.5) | 41 (33.1) | 34 (28.3) | 50 (43.1) | |
| Other determined etiology | 9 (7.6) | 6 (4.8) | 5 (4.2) | 5 (4.3) | |
| Undetermined etiology | 10 (8.5) | 7 (5.6) | 14 (11.7) | 7 (6.0) | |
| Laboratory data | |||||
| Total cholesterol, mmol/L | 4.7 ± 1.1 | 4.5 ± 0.9 | 4.2 ± 1.1 | 4.2 ± 1.3 | 0.027 |
| Triglyceride, mmol/L | 1.3 (1.0, 1.8) | 1.3 (1.0, 2.0) | 1.2 (1.0, 1.6) | 1.4 (0.9, 2.1) | 0.476 |
| High‐density lipoprotein, mmol/L | 1.3 (1.2, 1.6) | 1.3(1.1, 1.5) | 1.2 (1.1, 1.5) | 1.4 (1.2, 1.6) | 0.243 |
| Low‐density lipoprotein, mmol/L | 2.5 ± 1.0 | 2.5 ± 0.8 | 2.4 ± 0.7 | 2.7 ± 1.0 | 0.229 |
| Hs‐CRP, mg/L | 3.0 (1.0, 6.0) | 4.0 (1.0, 8.0) | 3.0 (1.0, 6.5) | 3.0 (1.0, 5.8) | 0.108 |
| Fasting blood glucose, mmol/L | 6.4 ± 2.1 | 6.1 ± 1.9 | 5.8 ± 1.7 | 5.8 ± 1.8 | 0.027 |
| Homocysteine, mmol/L | 14.1 ± 9.7 | 14.7 ± 8.6 | 13.4 ± 5.4 | 13.3 ± 4.8 | 0.532 |
Note. 25(OH)D: 25‐hydroxyvitamin D; END: early neurological deterioration; Hs‐CRP: hyper‐sensitive C‐reactive protein; mRS: modified Rankin scale; NIHSS: national institute of health stroke scale.
END was observed in 136 patients, which accounted for 28.5% [95% confidence interval (CI) 25.6%–31.4%] of the cohort. As shown in Figure 2, patients with lower 25(OH)D levels portended an increasing risk of END, which mostly developed within the 48 hr after admission. Table 2 summarized the results of the binary logistic regression of the END. Univariate logistic regression analysis showed that advanced age, white matter lesions, high level of body mass index, diastolic blood pressure, fasting blood glucose and homocysteine, and lower 25(OH)D levels were associated with END. Furthermore, after controlling for potential confounders, the first quartile of 25(OH)D concentrations [odds ratio (OR), 2.622; 95% CI, 1.226–5.641; p = 0.015] was an independent risk factor for END.
Figure 2.
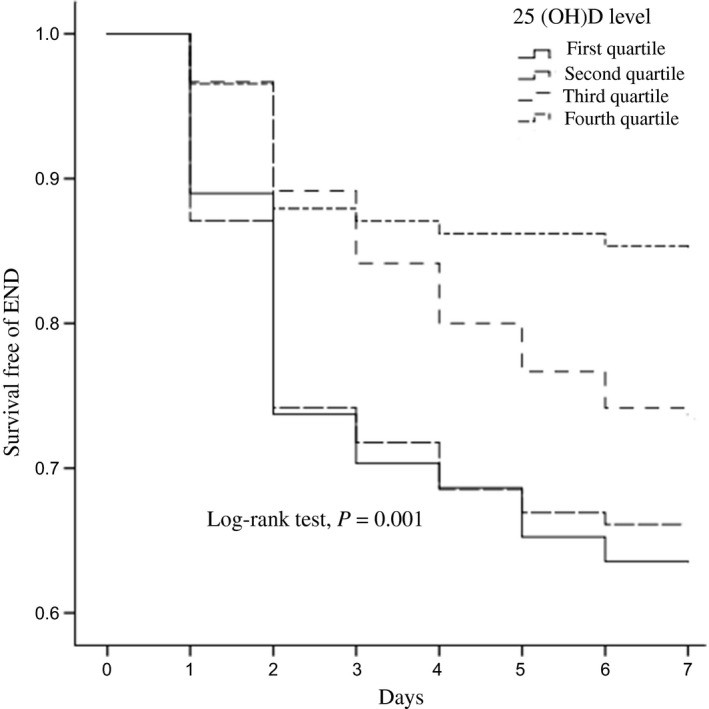
Kaplan–Meier curves estimates the probability of END stratified by 25(OH)D quartiles. 25(OH)D indicates 25‐hydroxyvitamin D; and END, early neurological deterioration
Table 2.
Univariate and multivariate logistic regression analysis for END
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Select variables | ||||
| Age | 1.024 (1.008–1.142) | 0.044 | ||
| Female | 1.322 (0.901–1.996) | 0.148 | ||
| Hypertension | 0.801 (0.529–1.212) | 0.294 | ||
| Diabetes mellitus | 1.069 (0.664–1.721) | 0.783 | ||
| Hyperlipidemia | 0.676 (0.383–1.190) | 0.175 | ||
| Current drinking | 1.059 (0.622–1.695) | 0.811 | ||
| Current smoking | 1.353 (0.885–2.070) | 0.163 | ||
| Atrial fibrillation | 0.962 (0.550–1.681) | 0.891 | ||
| Previous antiplatelet | 1.157 (0.693–1.932) | 0.576 | ||
| Previous statin | 1.123 (0.590–2.136) | 0.724 | ||
| Body mass index | 1.083 (1.019–1.151) | 0.012 | ||
| Systolic blood pressure | 1.007 (0.996–1.017) | 0.201 | ||
| Diastolic blood pressure | 1.019 (1.000–1.038) | 0.049 | ||
| NIHSS score | 1.027 (0.986–1.069) | 0.203 | ||
| Silent lacunar infarction | 1.206 (0.811–1.796) | 0.355 | ||
| White matter lesions | 1.737 (1.144–2.638) | 0.010 | ||
| Hs‐CRP | 1.004 (0.980–1.020) | 0.992 | ||
| Fasting blood glucose | 1.117 (1.011–1.234) | 0.030 | ||
| Homocysteine | 1.037 (1.007–1.067) | 0.016 | ||
| 25(OH)D level | ||||
| As continuous variable | 0.976 (0.961–0.987) | 0.001 | 0.973 (0.958–0.991) | 0.005 |
| As categorical variable | ||||
| Fourth quartile | Reference | Reference | ||
| Third quartile | 1.248 (0.938–3.774) | 0.108 | 1.188 (0.540–2.615) | 0.664 |
| Second quartile | 2.789 (1.492–5.211) | 0.006 | 1.925 (0.922–4.021) | 0.081 |
| First quartile | 3.237 (1.731–6.054) | 0.001 | 2.622 (1.226–5.641) | 0.015 |
Notes. 25(OH)D: 25‐hydroxyvitamin D; CI: confidence interval; END: early neurological deterioration; Hs‐CRP: hyper‐sensitive C‐reactive protein; NIHSS: national institute of health stroke scale; OR: odds ratio.
Multivariate analysis adjusted for age, sex, white matter lesions, body mass index, diastolic blood pressure, fasting blood glucose and homocysteine level.
The ROC curves showed the optimal cutoff value of serum 25(OH)D levels as an END indicator was estimated to be 42.5 nmol/L, which yielded a sensitivity of 71.9% and a specificity of 60.3%, with the area under curve of 0.635 (95% CI 0.580–0.689).
4. DISCUSSION
In this hospital‐based prospective study, END was observed in 136 (28.5%) patients, which is similar to previous data ranging from 5% to 40% (Kwon et al., 2014; Seners et al., 2015; Sun et al., 2014; Zhang et al., 2016). We also found that lower 25(OH)D serum levels were associated with the presence of END in patients with acute ischemic stroke. This association was independent of other well‐known predictors of neurological worsening such as age, cardiovascular risk factors, and neurological severity on admission.
Vitamin D refers to a group of fat‐soluble secosteroids hormones, and is typically ingested in dietary sources or produced in the skin as a result of sunlight exposure (Holick, 2007; Hossein‐nezhad & Holick, 2012). Its serum level is significantly decreased in various chronic diseases. In humans, vitamin D deficiency is considered to be related with the following disorders: hypertension, diabetes mellitus, metabolic syndrome, arterial stiffening, left ventricular hypertrophy, vascular dysfunction, and renin‐angiotensin system activation (Al Mheid & Quyyumi, 2017; Michos & Melamed, 2008). Over the past few years, accumulating epidemiological evidence illustrated that decreased 25(OH)D levels have been linked to an increased risk of coronary heart disease, cognitive decline and ischemic stroke (Balion et al., 2012; Giovannucci, Liu, Hollis, & Rimm, 2008; Poole et al., 2006). Currently, several studies have focused on the association between vitamin D levels with functional outcomes after ischemic stroke. In previous studies based on Caucasian stroke population, low 25(OH)D levels have been reported to be an indicator of unfavorable functional outcome at discharge (OR 2.06; 95% CI 1.06–3.94, p = 0.03) and 1‐year mortality (Harzad Ratio 1.95; 95% CI 1.14–3.32, p = 0.014) (Daubail et al., 2013, 2014). Furthermore, serum 25(OH)D level was a predictor of both severity at admission (r = −0.363, p < 0.001) and functional outcome at discharge (OR 0.79; 95% CI 0.73–0.85; p = 0.005) in a cohort of Chinese ischemic stroke patients (Wang et al., 2014). However, we did not detect an apparent association between 25(OH)D levels and stroke severity (r = −0.056, p = 0.225). This discrepancy might be explained at least in part by differences concerning the study design, especially the study population and sample size. Furthermore, on the contrary to previous results (Wat, Leung, Tam, & Kung, 2007), our study found that female have a higher prevalence of vitamin D deficiency than male. Nevertheless, this hypovitaminosis D does not appear to be associated with increased END risk among female. Future clinical studies with large sample sizes and multicenter data are needed to confirm our findings.
Emerging data from large clinical trials have suggested that vitamin D may be important for neurovascular protection. Thus far, no precise data are available regarding the association of Vitamin D deficiency with END. Serum 25(OH)D concentrations were reported to be associated with blood pressure level, glucose and lipid metabolism, which may exacerbate the development of END (Alvarez‐Sabín et al., 2003; Buell & Dawson‐Hughes, 2008; Pezzini et al., 2011). In our study, we performed a fully adjusted model after adjusting for age, sex, diastolic blood pressure and fasting blood glucose, and found that the association between 25(OH)D levels and risk of END remained robust. Several plausible biological mechanisms might be proposed for this relationship between the 25(OH)D levels and neurological worsening during the acute phase of stroke. There is a growing body of evidence that vitamin D possesses an anti‐inflammatory property and vitamin D deficiency may contribute to overall increased inflammatory activity (Takeda et al., 2010; Wong, Man, & Vanhoutte, 2010). In return, chronic inflammation is a pathological condition characterized by tissue destruction, promotes oxidative stress and attenuates cellular antioxidant capacity, which may propagate brain damage after acute stroke (Cruz‐Álvarez et al., 2017; Vila, Castillo, Dávalos, & Chamorro, 2000; Vila et al., 2003). In a rat model of local cerebral ischemia induced by ligation of the middle cerebral artery, 1,25(OH)2D3 administration was correlated to a significant reduction in brain ischemic infarct size (Wang et al., 2000). Also, rat models fed with a vitamin D‐deficient diet had higher risk of infarct volume growth compared with the controls (Balden, Selvamani, & Sohrabji, 2012). As infarct volume growth has been proposed as the main cause of neurological deterioration (Seners et al., 2015; Terasawa et al., 2008), we further hypothesized that the association of lower 25(OH)D levels with an increased risk of END may be partially explained by vitamin D deficiency‐induced inflammatory neuronal death and infarct volume increasing. Unfortunately, the infarct volume growth was not evaluated in our study, and consequently, we were not able to confirm this assumption.
Strengths of our study include using a standardized research method, large study size and the availability of a large array of information for covariate selection. However, some limitations of this observational study need to be discussed. First, the cross‐sectional design of our analyses limits our ability to determine a causal relationship between 25(OH)D levels and END. Second, 25(OH)D levels were only measured at admission, which yielded no data regarding the change of level in ischemic stroke patients. Third, the data on Vitamin D receptor gene polymorphisms, vitamin D supplementation, outdoor physical activity, sun exposure, health education, social level, and parathyroid hormone level were not collected in our study. Therefore, we could not adjust these variables in multivariable analysis, although their role in the regulating 25(OH)D levels has not been elucidated yet. In addition, the study was performed in one center with Chinese population, which might not be generalizable to other ethnic populations.
In conclusion, our study showed that lower 25(OH)D levels might be associated with a higher risk of END developing amongst acute ischemic stroke patients. Future randomized clinical controlled trials are therefore urgently needed to assess whether vitamin D supplementation could prevent neurological deterioration in the acute phase of ischemic stroke.
DISCLOSURES
The authors have no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (NO. 81573807).
Hu W, Liu D, Li Q, Wang L, Tang Q, Wang G. Decreasing serum 25‐hydroxyvitamin D levels and risk of early neurological deterioration in patients with ischemic stroke. Brain Behav. 2019;9:e01227 10.1002/brb3.1227
Contributor Information
Wei Hu, Email: hewei_anhui@126.com.
Guoping Wang, Email: guoping_wang@yeah.net.
REFERENCES
- Al Mheid, I. , & Quyyumi, A. A. (2017). Vitamin D and cardiovascular disease: Controversy unresolved. Journal of the American College of Cardiology., 70, 89–100. 10.1016/j.jacc.2017.05.031 [DOI] [PubMed] [Google Scholar]
- Alvarez‐Sabín, J. , Molina, C. A. , Montaner, J. , Arenillas, J. F. , Huertas, R. , Ribo, M. , … Quintana, M. (2003). Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator–treated patients. Stroke, 34, 1235–1240. 10.1161/01.STR.0000068406.30514.31 [DOI] [PubMed] [Google Scholar]
- Balden, R. , Selvamani, A. , & Sohrabji, F. (2012). Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia‐induced inflammation in adult rats. Endocrinology, 153, 2420–2435. 10.1210/en.2011-1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balion, C. , Griffith, L. E. , Strifler, L. , Henderson, M. , Patterson, C. , Heckman, G. , … Raina, P. (2012). Vitamin D, cognition, and dementia A systematic review and meta‐analysis. Neurology, 79, 1397–1405. 10.1212/WNL.0b013e31826c197f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell, J. S. , & Dawson‐Hughes, B. (2008). Vitamin D and neurocognitive dysfunction: Preventing “D” ecline? Molecular aspects of medicine., 29, 415–422. 10.1016/j.mam.2008.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz‐Álvarez, S. , Santana‐Martínez, R. , Avila‐Chávez, E. , Barrera‐Oviedo, D. , Hernández‐Pando, R. , Pedraza‐Chaverri, J. , & Maldonado, P. D. (2017). Apocynin protects against neurological damage induced by quinolinic acid by an increase in glutathione synthesis and Nrf2 levels. Neuroscience, 350, 65–74. 10.1016/j.neuroscience.2017.03.011 [DOI] [PubMed] [Google Scholar]
- Daubail, B. , Jacquin, A. , Guilland, J. C. , Hervieu, M. , Osseby, G. V. , Rouaud, O. , … Béjot, Y. (2013). Serum 25‐hydroxyvitamin D predicts severity and prognosis in stroke patients. European journal of neurology., 20, 57–61. 10.1111/j.1468-1331.2012.03758.x [DOI] [PubMed] [Google Scholar]
- Daubail, B. , Jacquin, A. , Guilland, J.‐C. , Khoumri, C. , Aboa‐Eboulé, C. , Giroud, M. , & Béjot, Y. (2014). Association between serum concentration of vitamin D and 1‐year mortality in stroke patients. Cerebrovascular Diseases, 37, 364–367. 10.1159/000362534 [DOI] [PubMed] [Google Scholar]
- Giovannucci, E. , Liu, Y. , Hollis, B. W. , & Rimm, E. B. (2008). 25‐hydroxyvitamin D and risk of myocardial infarction in men: A prospective study. Archives of internal medicine., 168, 1174–1180. 10.1001/archinte.168.11.1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helleberg, B. , Ellekjær, H. , Rohweder, G. , & Indredavik, B. (2014). Mechanisms, predictors and clinical impact of early neurological deterioration: The protocol of the Trondheim early neurological deterioration study. BMC Neurology, 14, 201 10.1186/s12883-014-0201-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick, M. F. (2007). Vitamin D deficiency. New England Journal of Medicine, 2007, 266–281. 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- Hossein‐nezhad, A. , & Holick, M. (2012). Optimize dietary intake of vitamin D: An epigenetic perspective. Current Opinion in Clinical Nutrition and Metabolic Care, 15, 567–579. 10.1097/MCO.0b013e3283594978 [DOI] [PubMed] [Google Scholar]
- Ir, O. (1999). The National Institutes of Health Stroke Scale and its importance in acute stroke management. Physical Medicine and Rehabilitation Clinics of North America, 10, 787–800. [PubMed] [Google Scholar]
- Kwon, H.‐M. , Lee, Y.‐S. , Bae, H.‐J. , & Kang, D.‐W. (2014). Homocysteine as a predictor of early neurological deterioration in acute ischemic stroke. Stroke, 45, 871–873. 10.1161/STROKEAHA.113.004099 [DOI] [PubMed] [Google Scholar]
- Kwon, H.‐M. , Lim, J.‐S. , Park, H.‐K. , & Lee, Y.‐S. (2011). Hypertriglyceridemia as a possible predictor of early neurological deterioration in acute lacunar stroke. Journal of the neurological sciences., 309, 128–130. 10.1016/j.jns.2011.06.057 [DOI] [PubMed] [Google Scholar]
- Leung, R. Y. , Han, Y. , Sing, C.‐W. , Cheung, B. M. , Wong, I. C. , Tan, K. C. , … Cheung, C. L. (2017). Serum 25‐hydroxyvitamin D and the risk of stroke in Hong Kong Chinese. Thrombosis and haemostasis., 117, 158–163. [DOI] [PubMed] [Google Scholar]
- Liu, M. , Wu, B. , Wang, W.‐Z. , Lee, L.‐M. , Zhang, S.‐H. , & Kong, L.‐Z. (2007). Stroke in China: Epidemiology, prevention, and management strategies. The Lancet Neurology., 6, 456–464. 10.1016/S1474-4422(07)70004-2 [DOI] [PubMed] [Google Scholar]
- Michos, E. D. , & Melamed, M. L. (2008). Vitamin D and cardiovascular disease risk. Current Opinion in Clinical Nutrition & Metabolic Care., 11, 7–12. 10.1097/MCO.0b013e3282f2f4dd [DOI] [PubMed] [Google Scholar]
- Park, K.‐Y. , Chung, P.‐W. , Kim, Y. B. , Moon, H. S. , Suh, B. C. , Won, Y. S. , … Kwon, O. S. (2015). Serum vitamin D status as a predictor of prognosis in patients with acute ischemic stroke. Cerebrovascular Diseases, 40, 73–80. 10.1159/000434691 [DOI] [PubMed] [Google Scholar]
- Pezzini, A. , Grassi, M. , Del Zotto, E. , Volonghi, I. , Giossi, A. , Costa, P. , … Padovani, A. (2011). Influence of acute blood pressure on short‐and mid‐term outcome of ischemic and hemorrhagic stroke. Journal of neurology., 258, 634–640. 10.1007/s00415-010-5813-z [DOI] [PubMed] [Google Scholar]
- Poole, K. E. , Loveridge, N. , Barker, P. J. , Halsall, D. J. , Rose, C. , Reeve, J. , & Warburton, E. A. (2006). Reduced vitamin D in acute stroke. Stroke, 37, 243–245. 10.1161/01.STR.0000195184.24297.c1 [DOI] [PubMed] [Google Scholar]
- Seners, P. , Turc, G. , Oppenheim, C. , & Baron, J.‐C. (2015). Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: A systematic review with pathophysiological implications. Journal of Neurology, Neurosurgery and Psychiatry, 86, 87–94. 10.1136/jnnp-2014-308327 [DOI] [PubMed] [Google Scholar]
- Sun, W. , Liu, W. , Zhang, Z. , Xiao, L. , Duan, Z. , Liu, D. , … Liu, X. (2014). Asymmetrical cortical vessel sign on susceptibility‐weighted imaging: A novel imaging marker for early neurological deterioration and unfavorable prognosis. European journal of neurology., 21, 1411–1418. 10.1111/ene.12510 [DOI] [PubMed] [Google Scholar]
- Takeda, M. , Yamashita, T. , Sasaki, N. , Nakajima, K. , Kita, T. , Shinohara, M. , … Hirata, K. I. (2010). Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arteriosclerosis, thrombosis, and vascular biology., 30, 2495–2503. 10.1161/ATVBAHA.110.215459 [DOI] [PubMed] [Google Scholar]
- Terasawa, Y. , Iguchi, Y. , Kimura, K. , Kobayashi, K. , Aoki, J. , Matsumoto, N. , … Kaji, R. (2008). Neurological deterioration in small vessel disease may be associated with increase of infarct volume. Journal of the neurological sciences., 269, 35–40. 10.1016/j.jns.2007.12.014 [DOI] [PubMed] [Google Scholar]
- Tu, W.‐J. , Zhao, S.‐J. , Xu, D.‐J. , & Chen, H. (2014). Serum 25‐hydroxyvitamin D predicts the short‐term outcomes of Chinese patients with acute ischaemic stroke. Clinical Science., 126, 339–346. 10.1042/CS20130284 [DOI] [PubMed] [Google Scholar]
- Vila, N. , Castillo, J. , Dávalos, A. , & Chamorro, A. (2000). Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke, 31, 2325–2329. 10.1161/01.STR.31.10.2325 [DOI] [PubMed] [Google Scholar]
- Vila, N. , Castillo, J. , Dávalos, A. , Esteve, A. , Planas, A. M. , & Chamorro, Á. (2003). Levels of anti‐inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke, 34, 671–675. 10.1161/01.STR.0000057976.53301.69 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Chiang, Y.‐H. , Su, T.‐P. , Hayashi, T. , Morales, M. , Hoffer, B. J. , & Lin, S. Z. (2000). Vitamin D 3 attenuates cortical infarction induced by middle cerebral arterial ligation in rats. Neuropharmacology, 39, 873–880. 10.1016/S0028-3908(99)00255-5 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Ji, H. , Tong, Y. , & Zhang, Z.‐B. (2014). Prognostic value of serum 25‐hydroxyvitamin D in patients with stroke. Neurochemical research, 39, 1332–1337. 10.1007/s11064-014-1316-0 [DOI] [PubMed] [Google Scholar]
- Wat, W. Z. , Leung, J. Y. , Tam, S. , & Kung, A. W. (2007). Prevalence and impact of vitamin D insufficiency in southern Chinese adults. Annals of Nutrition and Metabolism, 51, 59–64. 10.1159/000100822 [DOI] [PubMed] [Google Scholar]
- Wong, M. S. , Man, R. Y. , & Vanhoutte, P. M. (2010). Calcium‐independent phospholipase A 2 plays a key role in the endothelium‐dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. American Journal of Physiology‐Heart and Circulatory Physiology, 298, H1260–H1266. 10.1152/ajpheart.01068.2009 [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Sun, Z. , Ding, C. , Tang, Y. , Jiang, X. , Xie, Y. , … Xu, G. (2016). Metabolic syndrome augments the risk of early neurological deterioration in acute ischemic stroke patients independent of inflammatory mediators: A hospital‐based prospective study. Oxidative medicine and cellular longevity, 2016, 8346301. [DOI] [PMC free article] [PubMed] [Google Scholar]


