Abstract
Severe cutaneous wounds expose the body to the external environment, which may lead to impairments in bodily functions and increased risk of infection. There is a need to develop skin substitutes which could effectively promote complete skin regeneration following an injury. Murine models are used to test such skin substitutes, but their healing involves contraction of the dermis not found in human wounds. We have previously described a device called a dome, which comes in two models, that is used to prevent skin contraction in mice. One model provides a physical barrier to minimize contraction, and the other model has additional perforations in the barrier to allow cellular contribution from the surrounding intact skin. Taking advantage of an enhanced version of these two models, we compared granulation tissue formation, the extent of vascularization, and the transition to myofibroblastic phenotype between the models. We enhanced the dome by developing a twist open cap dome and applied the two models of the dome into the excisional wound biopsy in mice. We demonstrate that the dome can be used to prevent skin contraction in mice. The control model prevented skin contraction while barricading the contribution of surrounding intact skin. When not barricaded, the intact skin enhances wound healing by increasing the number of myofibroblasts and neovascularization. Using a novel model of inhibition of skin contraction in rodents, we examined the contribution from the surrounding intact skin to granulation tissue formation, myofibroblastic differentiation, and neovascularization during the course of skin healing in mice.
Keywords: cell contribution, dome, murine model, myofibroblasts, wound contraction, wound healing
Introduction
Wound repair is an intricate process that involves multiple interconnected pathways important for adequate healing. Disruption in these pathways and impaired wound healing continue to be clinical challenges (Bielefeld et al. 2013; Amini‐Nik et al. 2017). Some of the challenges include limited skin grafts availability, high costs, and large wound sizes. Thus, skin substitutes are used for extensive wounds such as those found in burn injuries. Skin substitutes can provide quick wound coverage while decreasing the extent of scarring (Lee, 2000; Shores et al. 2007; Jeong et al. 2017; Monsuur et al. 2017; Sheikholeslam et al. 2018). However, there are still limitations to current skin substitutes due to prolonged inflammatory phases and inadequate regeneration in larger wounds (Bello et al. 2001; Nyame et al. 2014; Shahrokhi et al. 2014). Furthermore, although skin substitutes are more available than skin grafts, they are costly and can be more expensive than cadaveric allografts (Jones et al. 2002; Debels et al. 2015; Nicholas et al. 2016; Jeschke et al. 2017; Hakimi et al. 2018). Thus, novel skin substitutes are being developed and tested in order to improve the outcomes of healing while maintaining lower costs.
Studying wound healing in animal models is crucial to understanding the mechanisms of skin regeneration and comparing the effectiveness of various treatments, including newly developed skin substitutes. Murine models are inexpensive and are readily available but show different wound healing to that of humans, mainly due to rapid skin contraction not seen in humans via an ancillary muscle called the panniculus carnosus (Lorenz et al. 1992; Sorg et al. 2007; Amini‐Nik et al. 2011; Wong et al. 2011; Naldaiz‐Gastesi et al. 2018; Zomer & Trentin, 2018). Due to this muscle, quick wound closure occurs with minimal scarring or deformities. In humans, reepithelization and granulation tissue formation are the main processes behind wound closure, which render wound‐healing studies in rodents troublesome due to potential confounding factors (Sullivan et al. 2001). In addition, rapid contraction seen in murine models prevent tested skin substitutes from allowing regeneration to occur in the excised area, as closure often occurs before the skin substitute has time to integrate into the wound. Porcine models are widely used in wound repair studies as they have been shown closely to mimic the mechanisms of healing found in humans, and have similar skin compositions and thickness (Sullivan et al. 2001; Seaton et al. 2015; Hakimi et al. 2018). However, these models tend to be expensive to maintain, have high risks of infection, and are not easily accessible. Therefore, there is a need for easily accessible animal models such as murine models with minimal wound contraction which better resemble wound healing found in humans.
We have previously described and validated a surgical device, a dome, that is used to prevent skin contraction while allowing the housing of a synthetic skin graft (Jeschke et al. 2017). When using skin substitutes on wounds in humans, cells and other factors from the surrounding intact skin and the wound bed infiltrate the skin substitute for wound closure (Poon et al. 2009; Bielefeld et al. 2011, 2013; Sadiq et al. 2018). However, the extent of contribution from the neighboring skin to wound healing is not yet clear. Here, we present for the first time an enhanced version of the dome device where the dome has a cap, allowing the healing area to be opened and skin substitutes to be added, monitoring their integration. Using this enhanced model of the dome and by comparing the two models, we evaluate the interaction between the wound and the surrounding intact skin found around the wound. We characterize and verify the effect of surrounding skin on myofibroblast differentiation, vascularization, and granulation tissue formation in comparison with the barricaded model.
Materials and methods
Manufacturing enhanced version of the dome
The dome was developed at the Sunnybrook Research Institute Department of Design and Machining Services. The new version allows researchers to open the dome cap to apply skin substitutes or the desired molecules for further evaluation (Fig. 1).
Figure 1.
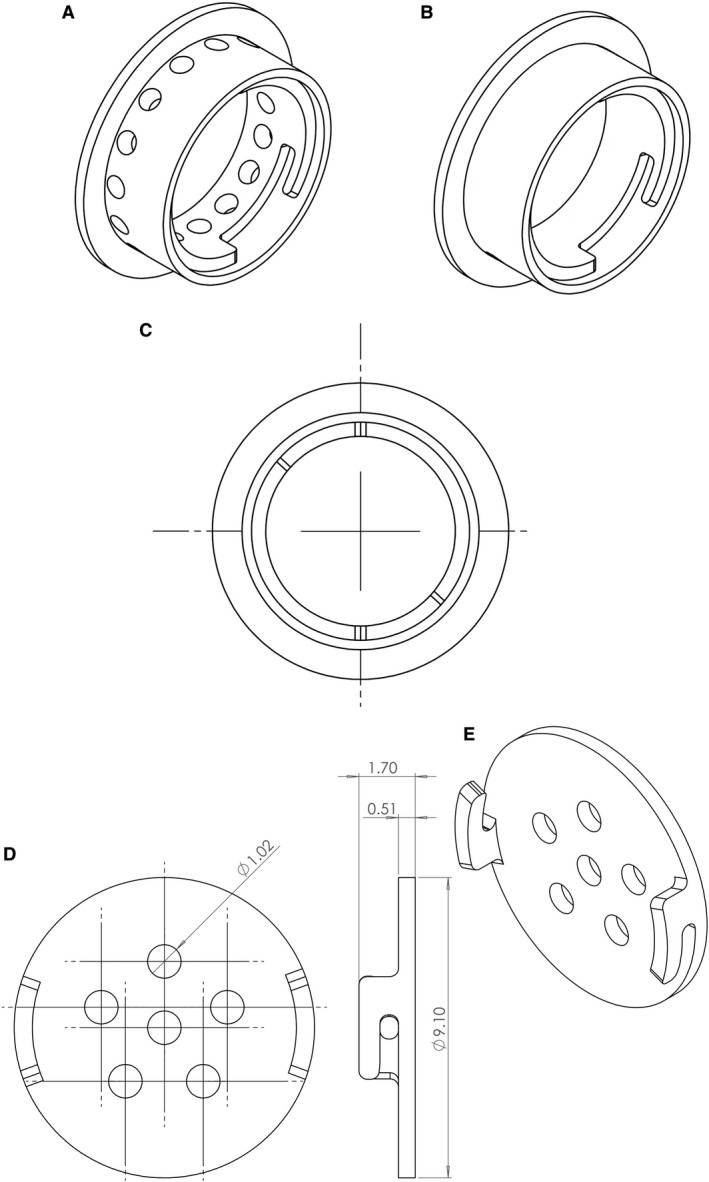
Enhanced dome with twist open cap. The dome was manufactured using Viper SI from 3D Systems, designed using SolidWorks. (A) Side view of the perforated dome model is shown (there are 15 holes, diameter = 1.02 mm) and (B) non‐perforated dome model. (C) Top view of the dome showing where materials and skin substitutes could be delivered (diameter of dome = 8 mm). (D,E) The perforated dome cap is shown (six holes, diameter = 1.02 mm).
Physical properties of the dome
The domes used are made of Accura® ClearVue™, which is a clear plastic developed using Viper SI by 3D Systems. The two types of domes used in the study were either perforated or non‐perforated as previously shown (Jeschke et al. 2017). The domes are used to prevent wound healing in order to test the effects of the grafted skin substitutes alone. The new domes were modified from the previous study where bigger holes on top and the sides of the dome were designed (1.02 mm each) using SolidWorks. The dome included 15 perforations on the sides of the dome instead of 30 holes seen in the previous models, and the cap included six holes. Furthermore, the new design had a removable cap to allow for skin substitute insertions or delivery of growth factors or drugs.
Experiment
Dome preparation
The domes were fabricated in the Department of Design and Machining Services at the Sunnybrook Research Institute. To prepare the dome for the experiments, the domes were incubated in 70% ethanol for 5 min and air‐dried in a tissue culture biosafety cabinet. The dome was sterilized by UV radiation for 30 min before usage.
Wound excisions and placing the dome
The preparation of wound was performed as previously described (Jeschke et al. 2017). Briefly, the mice were anesthetized with 1–2% isofluorane + oxygen until unconscious, and the eyes were protected using eye gel. The hair on the back of the mouse was shaved, and the skin in the middle of the back was pinched so that a biopsy punch through the pinched skin penetrated through both skin layers to make two holes of equal size. Perforated and nonperforated domes were inserted under the wounded skin on the left and right sides, respectively. The sides of both dome prototypes were embedded into the dermal area to separate the surrounding skin from the wound area (Fig. 2). The wounds were then harvested with the dome after 11 days and fixed in 10% formalin.
Figure 2.
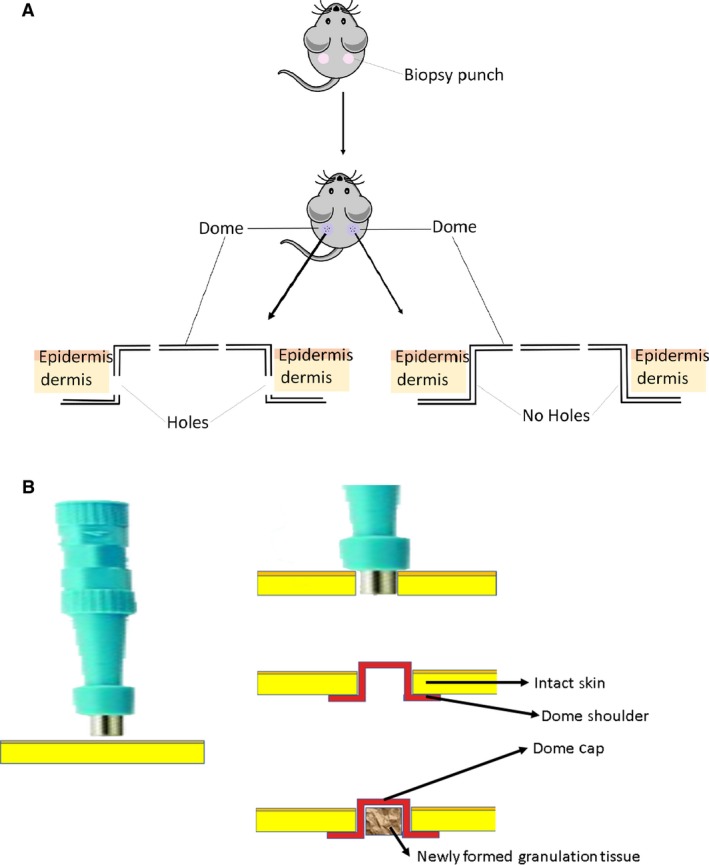
Infliction of wound and administration of dome. (A) Excisional biopsy punches were inflicted to produce one wound on each side of the mouse of equal diameter size. The nonperforated dome was added to the right‐side wound while the perforated domes were added to the left‐side wound on each mouse. The shoulders of the dome were placed for 11 days under the surrounding skin, preventing contraction. The wounds were harvested for analysis. (B) Insertion of the dome into the skin after punch biopsy and formation of the granulation tissue, which mainly comprises vascular connective tissue.
Animal model ethical approval
Mice were obtained from Jackson Laboratory under the guidelines of the Sunnybrook Research Institute and Sunnybrook Health Sciences Animal Policy and Welfare Committee of the University of Toronto. Animal procedures were reviewed and approved by Sunnybrook Research Institute and Sunnybrook Health Sciences Centre at the University of Toronto animal care and use committee.
Imaging and quantification
The samples were embedded in paraffin and placed on slides. Masson's trichrome staining was performed for histological studies as previously described (Jeschke et al. 2017). Immunohistochemistry was performed to characterize the cellular composition of the harvested wounds. The slides were heated at 60 °C for 30 min and treated with citrosol to deparaffinize the samples. The samples were then rehydrated using 100, 95, and 70% ethanol. Biocare Medical Antigen decloaker solution and the decloaking Chamber™ NxGen were used for heat‐induced epitope retrieval (HIER). For imaging, five different hpf areas from each sample at 40× magnification were used to quantify the ratio of positive cells to total cell number (~ 70–100 cells per hpf).
Results
Development of twist open cap dome for studying skin substitutes in rodents
It is important to monitor the grafted skin substitutes in wound healing studies and, therefore, a clear top with twist‐open cap is desirable. Moreover, it would be beneficiary to be able to apply skin substitutes after dome insertion when the surgical procedure is complete. We, therefore, advanced the previous version of the dome and developed twist‐open cap domes for both models of perforated and non‐perforated domes. Moreover, we have modified our previous design of the dome models to make bigger holes to prevent the holes from clogging. The new design has six holes (1.02 mm diameter) on top and 15 openings on the side (in the perforated model). Furthermore, the new design included a removable cap to monitor wound closure and to allow for potential delivery of materials or skin substitutes. Both models have twist‐open caps allowing air exchange and liquid discharge (Fig. 1).
Excisional wound biopsy was used to inflict wounds in wild‐type mice. Domes were administered to the wound to prevent and minimize the effect of contraction on wound healing. Thus, we were able to study alternative methods of recovery of granulation and epithelization of the wound (Galiano et al. 2004; Fahie & Shettko, 2007). We have developed two models of the dome, perforated and nonperforated, the latter of which prevents contribution of the surrounding tissue to the healing wound. Histological studies were performed to validate the two dome models.
Surrounding intact skin contributes to and enhances wound healing
It is believed that cells from the wound bed area and the surrounding intact skin migrate to the void excised skin (Bielefeld et al. 2011, 2013; Yousuf & Amini‐Nik, 2017). However, the extent of this contribution is not yet verified. This is important to verify because, for large wounds, healing mainly relies on the wound bed contribution. To understand this process, we subjected mice to excisional wound biopsy and used two models of domes, a perforated model to allow for the contribution of surrounding intact skin and a non‐perforated model to prevent this interaction as a control group (Fig. 2). The harvested wounds were sectioned onto slides and Masson's trichrome staining was performed for histological analysis (Fig. 3). Wounds with perforated domes showed increased skin thickness (701.0 ± 13.75 μm, P < 0.01) compared with non‐perforated domes (477.6 ± 6.625 μm) 11 days post‐surgery, demonstrating an increase in granulation tissue formation in domes with holes. In the focused area of the formed granulation tissue, we did not observe any significant differences in the extent of cellularity. These data show that wounds start to heal and form granulation tissue without the contribution of surrounding intact skin, but the wounds that could communicate with intact skin showed a larger granulation tissue formation and thicker tissue compared with control.
Figure 3.
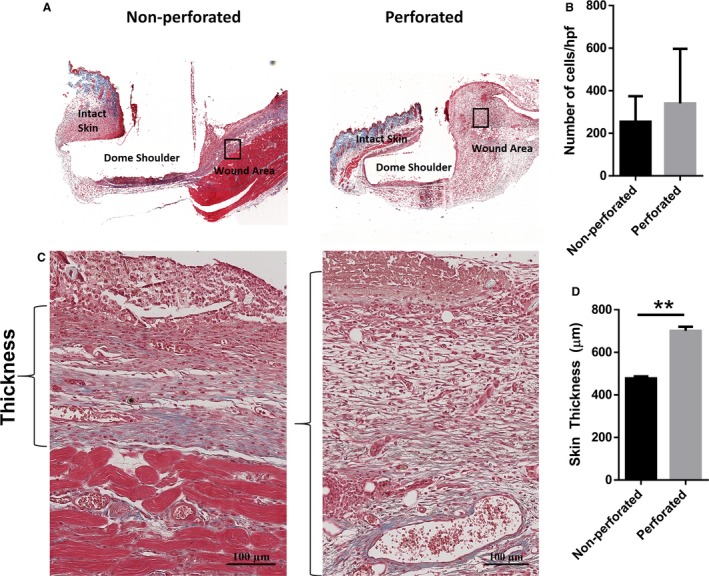
Newly formed granulation tissue is thicker in wounds with a perforated dome. (A) Masson's trichrome staining was performed on cross‐sectional slices of the harvested wounds. Representative images of the formed granulation tissue after 11 days can be seen in (C). Brackets indicate the measured thickness of the skin. (B) No significant differences in cellularity were observed between the non‐perforated and perforated dome groups. (D) Skin thickness was significantly higher in the perforated group compared to the barricaded model (n = 3, P < 0.05, unpaired t‐test).
Surrounding intact skin contributes to the increased number of myofibroblasts in the wound
During the wound healing process, fibroblasts differentiate into myofibroblasts, which function to contract the edges of the wound for proper wound closure, a distinct phenomenon from the wound contraction observed in mice due to panniculus carnosus (Skalli & Gabbiani, 1988; Grinnell, 1994; Berry et al. 1998; Nedelec et al. 2000; Desmoulière et al. 2005). At the end of wound healing, these myofibroblasts are lost by undergoing apoptosis and the failure of this process is associated with hypertrophic scarring and/or keloid formation (Desmouliere et al. 1995; Moulin et al. 2004). Although there have been previous indications that myofibroblasts differentiate from local fibroblasts in the skin, the extent of the contribution of the intact skin surrounding the wound is still unclear (Chang et al. 2002; Darby & Hewitson, 2007). Using our two models of domes, we were able to test the effect of the surrounding skin on myofibroblastic differentiation compared with the effects of the wound bed. Immunohistochemistry staining for α‐smooth muscle actin positive (α‐SMA+) cells, which is a marker for myofibroblasts, was performed on the wound sections showing a higher number of α‐SMA+ cells in the wounds provided with perforated domes in zone 4 (center of the wound), as indicated in Fig. 4. However, no differences were seen in the number of α‐SMA+ cells adjacent to the wound in zone 1, as shown in Fig. 4. These findings suggest that the intact skin play a role in signaling for myofibroblastic differentiation and migration into the wound.
Figure 4.
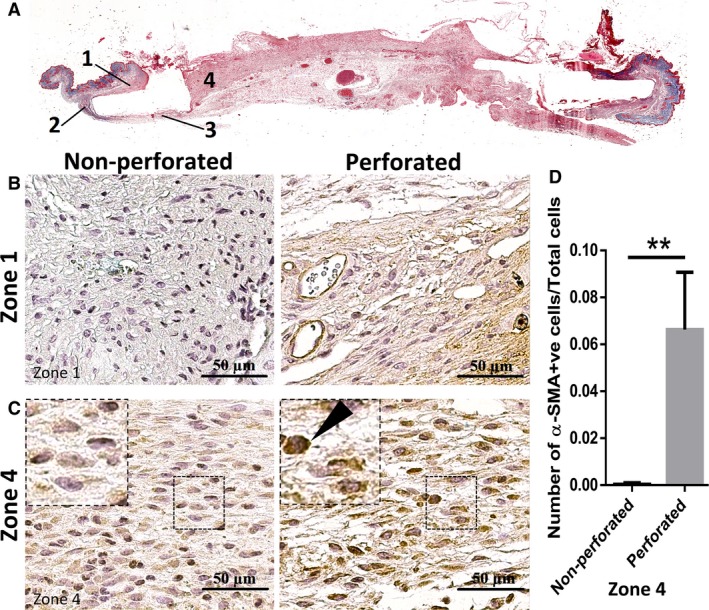
Cells in newly formed granulation tissue showed higher levels of myofibroblastic cells when the perforated dome was used. Different zones of the tissue as shown in (A) were compared for number of myofibroblasts. Unlike zone 4, which makes up the wound area, no significant differences were seen in zone 1 (B), which comprises the surrounding intact skin (similar results were found for zones 2 and 3). (C,D) A higher number of myofibroblasts were seen in the perforated dome group than in the non‐perforated dome. The arrow indicates an example of α‐SMA + cells (n = 3, P < 0.05, unpaired t‐test).
Surrounding intact skin contributes to an increase in vascularization of the wound
In the proliferative phase of wound healing, neovascularization occur, where endothelial cells migrate into the wound to form blood vessels in the wound, allowing for healthy healing of the tissue (Martin, 1997; Demidova‐Rice et al. 2012). Experiments were performed with the two dome models to examine the effect of the neighboring intact skin on vascularization of the wound. Tissue samples collected from the wound areas were stained with CD31, a marker for endothelial cells (Fig. 5). In wounds where perforated domes were placed, more blood vessel formation was observed compared with non‐perforated domes, suggesting that cellular and/or cellular cues that initiate neovascularization (e.g. migration of endothelial progenitor cells) are enhanced when the surrounding intact skin contributes. The area of blood vessels in the perforated domes was slightly higher, although not significant, compared with domes with no holes, suggesting that surrounding cues has less effect on maturation of blood vessel. These results suggest that the surrounding skin likely plays an important role in the initiation of vascularization of the wound either by a direct cellular contribution or through signaling cues beneficiary in neovascularization.
Figure 5.
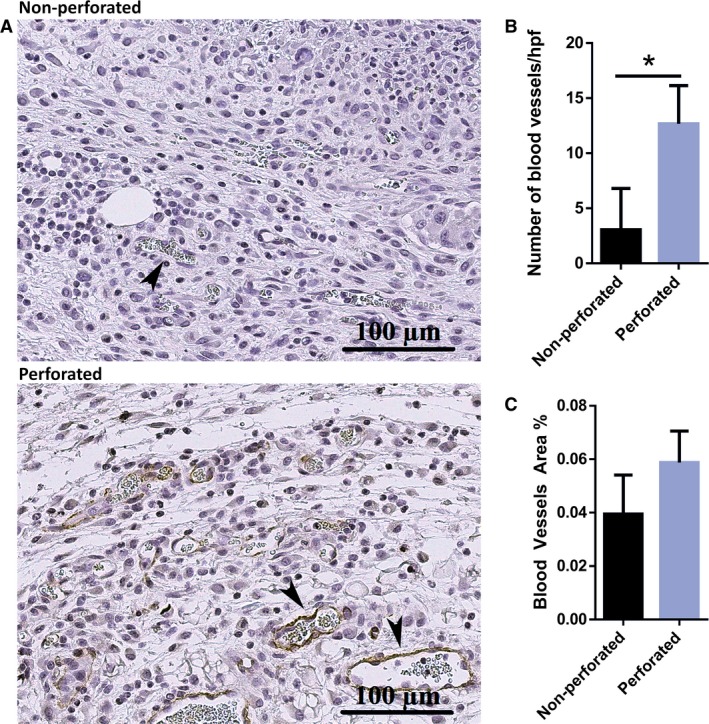
Cells in newly formed granulation tissue have a higher number of blood vessels in the non‐perforated dome group. (A) Representative image of the vascularization formed in the healing tissue in wounds harvested with perforated vs. non‐perforated dome (the arrows indicate blood vessels in the tissue). (B) Graphical representation showing a higher number of blood vessels in perforated domes compared with non‐perforated domes. (C) A trending increase, though not significant, can be seen in the area of blood vessels (n = 3, P < 0.05, unpaired t‐test).
Discussion
The use of animal models is crucial for studies on wound healing, and the effects of treatments and therapeutic technologies such as skin substitutes. However, rapid skin contraction in murine models may prevent skin substitutes from adhering to the wound bed, creating challenges in testing the efficacy of newly developed substitutes. Our enhanced dome models allow for the prevention of the inflicted wounds from rapid contraction while simultaneously allowing for the evaluation of the contribution of the surrounding skin to wound healing.
The role of myofibroblasts in wound closure and contracting the wound edges has already been shown, and several hypotheses have been proposed on the source of these cells, which are not typically found in the healthy intact skin (Grinnell, 1994; Darby & Hewitson, 2007). Some of the proposed sources of myofibroblasts include local fibroblasts, circulating fibrocytes, and resident cells which have undergone epithelial‐mesenchymal (EMT) transition (Hinz et al. 2004, 2007; Mori et al. 2005; Zeisberg et al. 2007; Moeller et al. 2009; Acharya et al. 2012). However, the extent of the contribution of each source is unclear, and several of these experiments were not focused on skin wound healing. We took advantage of our dome models to show that the surrounding skin plays a significant role in the contribution of wound healing to several key stages such as myofibroblast differentiation, neovascularization, and granulation tissue formation. The dome model with perforations, which allowed for interactions with the neighboring skin, showed a larger granulation tissue formation and thicker dermal component, a higher number of myofibroblastic cells, and blood vessel formation. These findings suggest that the healing wound most likely receives signaling cues from the periwound to increase myofibroblastic differentiation, which assists in wound contraction for wound closure (Amini‐Nik et al. 2011, 2014; Shah & Amini‐Nik, 2017; Yousuf & Amini‐Nik, 2017; Sadiq et al. 2018). Future studies are needed to determine whether this primary contribution of the surrounding skin is through direct myofibroblastic migration from the periwound or through the sprouting of blood vessels into the wound area.
The observed increased number of blood vessels might also be due to either signaling cues from the surrounding skin or to the higher chance for the cells to migrate into the wound from the periphery of the wound. Neovascularization is a complex phenomenon and pinpointing a single factor that contributes to the enhanced neovascularization is not possible with current models (Thomas et al. 2017). However, our data highlight the extent of the contribution of surrounding skin and suggest that surrounding intact skin is essential for initiation of vascularization. It would be ideal to use transgenic reporter animals where endothelial cells are permanently labeled with fluorescent markers (Kisanuki et al. 2001; Amini‐Nik et al. 2011, 2014; Sarkar et al. 2012) in order to evaluate the extent of contribution, their lineage, and fate during healing and to compare the two models. These findings raise the possibility that the cells contributing to wound healing primarily come from the surrounding skin rather than from the wound bed or the cells in circulation. These two models allow researchers to determine whether the grafted skin substitute is able to initiate the process of neovascularization without the contribution of surrounding intact skin. This is essential because, for large wounds, the extent of the contribution is limited, as wounds rely mainly on their bed rather than surrounding skin. Our studies do not discuss the mechanisms by which these diverse types of cells affect wound healing. Cell lineage studies using reporter mice (for the host animal) or applying labeled cells to the skin substitutes are necessary to address the contribution of each of these different cell types to wound healing and tissue repair.
Conflict of interest
The authors have no conflict of interest to declare.
Author contribution
M.E.A. performed the experiments, analyzed the data, and wrote the first draft of the manuscript. M.G.J. contributed to the design of the project and raised funds. S.A.N. designed the dome and the experiments, supervised analysis, and read the article.
Acknowledgements
We thank Sunnybrook Machine Shop and Michael Pozzobon for their help in manufacturing the dome models used in this study. We acknowledge Medicine by Design (2016/2) and Toronto Hydro for their generous funding.
References
- Acharya A, Baek ST, Huang G, et al. (2012) The bHLH transcription factor Tcf21 is required for lineage‐specific EMT of cardiac fibroblast progenitors. Development 139, 2139–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini‐Nik S, Glancy D, Boimer C, et al. (2011) Pax7 expressing cells contribute to dermal wound repair, regulating scar size through a beta‐catenin mediated process. Stem Cells 29, 1371–1379. [DOI] [PubMed] [Google Scholar]
- Amini‐Nik S, Cambridge E, Yu W, et al. (2014) beta‐Catenin‐regulated myeloid cell adhesion and migration determine wound healing. J Clin Invest 124, 2599–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini‐Nik S, Yousuf Y, Jeschke MG (2017) Scar management in burn injuries using drug delivery and molecular signaling: current treatments and future directions. Adv Drug Deliv Rev 123, 135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello YM, Falabella AF, Eaglstein WH (2001) Tissue‐engineered skin. Current status in wound healing. Am J Clin Dermatol 2, 305–313. [DOI] [PubMed] [Google Scholar]
- Berry DP, Harding KG, Stanton MR, et al. (1998) Human wound contraction: collagen organization, fibroblasts, and myofibroblasts. Plast Reconstr Surg 102, 124–131; discussion 132–134. [DOI] [PubMed] [Google Scholar]
- Bielefeld KA, Amini‐Nik S, Whetstone H, et al. (2011) Fibronectin and β‐catenin act in a regulatory loop in dermal fibroblasts to modulate cutaneous healing. J Biol Chem 286, 27687–27697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeld KA, Amini‐Nik S, Alman BA (2013) Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell Mol Life Sci 70, 2059–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Chi JT, Dudoit S, et al. (2002) Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A 99, 12877–12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby IA, Hewitson TD (2007) Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol 257, 143–179. [DOI] [PubMed] [Google Scholar]
- Debels H, Hamdi M, Abberton K, et al. (2015) Dermal matrices and bioengineered skin substitutes: a critical review of current options. Plast Reconstr Surg Glob Open 3, e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidova‐Rice TN, Durham JT, Herman IM (2012) Wound healing angiogenesis: innovations and challenges in acute and chronic wound healing. Adv Wound Care (New Rochelle) 1, 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmouliere A, Redard M, Darby I, et al. (1995) Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 146, 56. [PMC free article] [PubMed] [Google Scholar]
- Desmoulière A, Chaponnier C, Gabbiani G (2005) Tissue repair, contraction, and the myofibroblast. Wound Repair Regen 13, 7–12. [DOI] [PubMed] [Google Scholar]
- Fahie MA, Shettko D (2007) Evidence‐based wound management: a systematic review of therapeutic agents to enhance granulation and epithelialization. Vet Clin North Am Small Anim Pract 37, 559–577. [DOI] [PubMed] [Google Scholar]
- Galiano RD, Michaels V, Dobryansky M, et al. (2004) Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen 12, 485–492. [DOI] [PubMed] [Google Scholar]
- Grinnell F (1994) Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol 124, 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi N, Cheng R, Leng L, et al. (2018) Handheld skin printer: in situ formation of planar biomaterials and tissues. Lab Chip 18, 1440–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Pittet P, Smith‐Clerc J, et al. (2004) Myofibroblast development is characterized by specific cell‐cell adherens junctions. Mol Biol Cell 15, 4310–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, et al. (2007) The myofibroblast: one function, multiple origins. Am J Pathol 170, 1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W, Yang CE, Roh TS, et al. (2017) Scar prevention and enhanced wound healing induced by polydeoxyribonucleotide in a rat incisional wound‐healing model. Int J Mol Sci 18, E1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke MG, Sadri A‐R, Belo C, et al. (2017) A surgical device to study the efficacy of bioengineered skin substitutes in mice wound healing models. Tissue Eng Part C Methods 23, 237–242. [DOI] [PubMed] [Google Scholar]
- Jones I, Currie L, Martin R (2002) A guide to biological skin substitutes. Br J Plast Surg 55, 185–193. [DOI] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, et al. (2001) Tie2‐Cre transgenic mice: a new model for endothelial cell‐lineage analysis in vivo . Dev Biol 230, 230–242. [DOI] [PubMed] [Google Scholar]
- Lee KH (2000) Tissue‐engineered human living skin substitutes: development and clinical application. Yonsei Med J 41, 774–779. [DOI] [PubMed] [Google Scholar]
- Lorenz HP, Longaker MT, Perkocha LA, et al. (1992) Scarless wound repair: a human fetal skin model. Development 114, 253–259. [DOI] [PubMed] [Google Scholar]
- Martin P (1997) Wound healing – aiming for perfect skin regeneration. Science 276, 75–81. [DOI] [PubMed] [Google Scholar]
- Moeller A, Gilpin SE, Ask K, et al. (2009) Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 179, 588–594. [DOI] [PubMed] [Google Scholar]
- Monsuur HN, van den Broek LJ, Jhingoerie RL, et al. (2017) Burn eschar stimulates fibroblast and adipose mesenchymal stromal cell proliferation and migration but inhibits endothelial cell sprouting. Int J Mol Sci 18, E1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori L, Bellini A, Stacey MA, et al. (2005) Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res 304, 81–90. [DOI] [PubMed] [Google Scholar]
- Moulin V, Larochelle S, Langlois C, et al. (2004) Normal skin wound and hypertrophic scar myofibroblasts have differential responses to apoptotic inductors. J Cell Physiol 198, 350–358. [DOI] [PubMed] [Google Scholar]
- Naldaiz‐Gastesi N, Bahri OA, Lopez de Munain A, et al. (2018) The panniculus carnosus muscle: an evolutionary enigma at the intersection of distinct research fields. J Anat 233, 275–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelec B, Ghahary A, Scott PG, et al. (2000) Control of wound contraction. Basic and clinical features. Hand Clin 16, 289–302. [PubMed] [Google Scholar]
- Nicholas MN, Jeschke MG, Amini‐Nik S (2016) Cellularized bilayer pullulan‐gelatin hydrogel for skin regeneration. Tissue Eng Part A 22, 754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyame TT, Chiang HA, Orgill DP (2014) Clinical applications of skin substitutes. Surg Clin North Am 94, 839–850. [DOI] [PubMed] [Google Scholar]
- Poon R, Nik SA, Ahn J, et al. (2009) β‐catenin and transforming growth factor β have distinct roles regulating fibroblast cell motility and the induction of collagen lattice contraction. BMC Cell Biol 10, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadiq A, Shah A, Jeschke MG, et al. (2018) The role of serotonin during skin healing in post‐thermal injury. Int J Mol Sci 19, E1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar K, Rey S, Zhang X, et al. (2012) Tie2‐dependent knockout of HIF‐1 impairs burn wound vascularization and homing of bone marrow‐derived angiogenic cells. Cardiovasc Res 93, 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaton M, Hocking A, Gibran NS (2015) Porcine models of cutaneous wound healing. ILAR J 56, 127–138. [DOI] [PubMed] [Google Scholar]
- Shah A, Amini‐Nik S (2017) The role of serotoninergic system in skin healing. Int J Drug Res Technol 7, 8. [Google Scholar]
- Shahrokhi S, Arno A, Jeschke MG (2014) The use of dermal substitutes in burn surgery: acute phase. Wound Repair Regen 22, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikholeslam M, Wright MEE, Jeschke MG, et al. (2018) Biomaterials for skin substitutes. Adv Healthc Mater 7, 1700897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shores JT, Gabriel A, Gupta S (2007) Skin substitutes and alternatives: a review. Adv Skin Wound Care 20, 493–508; quiz 509–510. [DOI] [PubMed] [Google Scholar]
- Skalli O, Gabbiani G (1988) The biology of the myofibroblast relationship to wound contraction and fibrocontractive diseases In: The Molecular and Cellular Biology of Wound Repair. (eds. Clark RAF, Henson PM.), pp. 373–402. Boston, MA: Springer. [Google Scholar]
- Sorg H, Krueger C, Vollmar B (2007) Intravital insights in skin wound healing using the mouse dorsal skin fold chamber. J Anat 211, 810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan TP, Eaglstein WH, Davis SC, et al. (2001) The pig as a model for human wound healing. Wound Repair Regen 9, 66–76. [DOI] [PubMed] [Google Scholar]
- Thomas H, Cowin AJ, Mills SJ (2017) The importance of pericytes in healing: wounds and other pathologies. Int J Mol Sci 18, E1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VW, Sorkin M, Glotzbach JP, et al. (2011) Surgical approaches to create murine models of human wound healing. J Biomed Biotechnol 2011, 969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf Y, Amini‐Nik S (2017) The role of myeloid lineage cells on skin healing and skin regeneration. J Tissue Sci Eng 8, 2. [Google Scholar]
- Zeisberg EM, Tarnavski O, Zeisberg M, et al. (2007) Endothelial‐to‐mesenchymal transition contributes to cardiac fibrosis. Nat Med 13, 952–961. [DOI] [PubMed] [Google Scholar]
- Zomer HD, Trentin AG (2018) Skin wound healing in humans and mice: challenges in translational research. J Dermatol Sci 90, 3–12. [DOI] [PubMed] [Google Scholar]


