Abstract
BACKGROUND
Nigeria bears the greatest burden of diabetes prevalence in Sub-Saharan Africa. Diabetic foot ulcer (DFU) is a serious and potentially life-threatening complication of diabetes. Significant improvements in diabetic foot incidence and outcomes have been recorded in many Western countries in the past decade. However, the current burden of DFU in Nigeria is largely unknown.
AIM
To evaluate the patients’ profile, ulcer characteristics, associated co-morbidities and outcome of patients with DFU in Nigeria.
METHODS
Multicenter evaluation of diabetic foot ulcer in Nigeria was a one year multicenter observational study of patients hospitalized for DFU in six tertiary health institutions in Nigeria from March 2016 to March 2017. Demographic and diabetes information, ulcer characteristics and associated co-morbidities were assessed. Relevant laboratory and imaging studies were performed. All patients received appropriate multi-disciplinary care and were followed up until discharge or death. Outcome variables of interest were ulcer healing, lower extremity amputation (LEA), duration of hospitalization and mortality.
RESULTS
A total of 336 patients (55.1% male) with mean age of 55.9 ± 12.5 years were enrolled into this study. Majority (96.1%) had type 2 diabetes. Only 25.9% of the subjects had prior foot care knowledge. Most of the subjects presented late to the hospital and median (IQR) duration of ulcer at presentation was 39 (28-54) d. Ulcers were already advanced (Wagner grades ≥ 3) in 79.2% of the subjects while 76.8% of the ulcers were infected at the time of admission. The commonest co-morbidities were systemic hypertension, anemia and hyperglycemic emergencies. One hundred and nineteen subjects (35.4%) suffered LEA while 10.4% left against medical advice. The median (IQR) duration of hospitalization was 52.0 (29-66) d with case fatality rate of 20.5%.
CONCLUSION
The burden of DFU in Nigeria is very high. The major gaps include low level of foot care knowledge among diabetic patients, overdependence on self-medication and unorthodox medicine following development of foot ulceration, late hospital presentation, and high amputation and mortality rates. Extensive foot care education within the framework of a multi-disciplinary foot care team is highly desirable.
Keywords: Burden, Diabetes, Epidemiology, Foot ulcer, Amputation, Mortality, Multicenter evaluation of diabetic foot ulcer in Nigeria, Nigeria, Africa
Core tip: The multicenter evaluation of diabetic foot ulcer in Nigeria was a one year observational study of 336 adults who were hospitalized for diabetic foot ulcer in six tertiary hospitals in Nigeria. The subjects were managed by multi-disciplinary diabetic foot care teams and were followed up until discharge or death. This study demonstrated a high burden of diabetic foot ulcer in Nigeria which accounted for about a quarter of diabetes related hospital admissions over the study period. The study recorded high amputation and mortality rates of 35.4% and 20.5% respectively. Major challenges in diabetic foot care identified in this study include low level of foot care knowledge among the patients, poor health-seeking behavior and late hospital presentation.
INTRODUCTION
Although diabetes mellitus (DM) prevalence is rising globally, Sub-Saharan Africa appears to be the worst-hit[1]. In the last two decades, Nigeria, for instance has witnessed more than a 100% increase in the prevalence of the disease, from 2.2% in 1997 to nearly 6% in 2015[2]. It is generally reported that Nigeria which is the most populous country in Africa has the greatest burden of diabetes within the Sub-Saharan sub-continent[3]. This disproportionate increase in diabetes prevalence has largely been blamed on changing demographic dynamics including increasing urbanization and adoption of unhealthy lifestyles. Paralleling this increase in disease burden is also an upsurge in the prevalence of diabetes-related complications and death. One of the most devastating of these complications is diabetic foot ulcer (DFU), a costly, disabling but preventable complication of diabetes that is associated with significant morbidity and mortality.
DFU refers to a breech in the continuity of the skin epithelium involving its full thickness or beyond, distal to the ankle joints, in a person living with DM[4]. Foot ulceration is common in patients with DM with current global prevalence of about 6.3%[5]. It is estimated that a person with diabetes has a 25% lifetime risk of developing DFU[6]. In Africa with constrained resources and fractured health systems, the prevalence of DFU is higher at about 7.2%[5]. The burden of DFU in Africa is substantial, constituting a major source of hospitalization and mortality[7]. With the rapidly rising diabetes prevalence in Africa, the burden of DFU in this region is expected to be on the increase.
Many of the predisposing factors for DFU are well established and include advancing age, long duration of diabetes, poor glycemic control, presence of neuropathy and peripheral vascular disease[8,9]. However, majority of DFUs are usually as a result of interplay among an at-risk foot, repeated micro-trauma and super-imposed infection[4]. Managing DFUs is usually very challenging especially in resource-constrained settings. The cost of managing DFU is substantial, and DFUs account for up to 40% of diabetes-related expenditures, making it one of the most expensive diabetes complications to deal with[10]. DFUs often heal very slowly resulting in prolonged hospitalization, or may fail to heal completely. They are also very prone to infection with resultant tissue necrosis and gangrene. Consequently, foot ulcerations are the commonest cause of lower extremity amputation (LEA) in persons with diabetes, accounting for up to 85% of LEAs in this population[4]. The International Diabetes Federation estimates that at least one limb are lost to DFU somewhere in the world every 30 s[1]! Lower limb amputation is associated with significant disabilities including loss of productivity and reduced quality of life[11]. Furthermore, it has been observed that 5-year survival after an LEA is worse than many cancers[12]. Foot ulceration in diabetic patients is therefore a medical, economic and psychosocial issue requiring serious attention.
The burden of DFU in Nigeria has been reportedly high, with prevalence rates ranging from 11%-32% among hospitalized patients[13,14]. At about half a decade ago, amputation rate from DFU in Nigeria was as high as 52%[15]. Furthermore, DFU is the commonest cause of diabetes-related mortality in Nigeria after hyperglycemic emergencies[2]. Diabetic foot ulceration is therefore a matter of serious public health concern in Nigeria. Contemporary data on the actual burden of DFU in Nigeria are however very scanty, and available studies on this subject were single-centered and mostly retrospective. In order to fill this gap, we sought to evaluate the current burden of DFU in a larger population across multiple centers in Nigeria.
MATERIALS AND METHODS
Study areas and design
The multicenter evaluation of diabetic foot ulcer in Nigeria (MEDFUN) was an observational study conducted in six tertiary healthcare institutions in Nigeria, between March 2016 and April 2017. These centers include Enugu State University Teaching Hospital located in South-Eastern Nigeria, Lagos State University Teaching Hospital in the South-West, Aminu Kano Teaching Hospital in the North-West, Ahmadu Bello University Teaching Hospital Zaria also in the North-West, Federal Medical Center Keffi in the North-Central and Federal Medical Center Umuahia also located in the South-East. The locations and geographic spread of these study sites are indicated in a map of Nigeria hereby presented (Supplementary). All the centers render specialized tertiary health care and serve as referral centers for primary and secondary health facilities within and outside their geopolitical zone. The Research and Ethics committee of each of the participating centers approved the study protocol while verbal informed consent was obtained from each patient prior to recruitment.
Subjects and recruitment
In the present study, subjects with type 1 DM (T1DM) or type 2 DM (T2DM) hospitalized for DFU in any of the participating centers were consecutively enrolled after obtaining verbal consent. Distinction between T1DM and T2DM was made clinically as follows: Subjects who reported dependence on insulin for diabetes control since the time of diagnosis were classified as having T1DM while those who had been controlled on oral anti-diabetic drugs with or without insulin were adjudged to have T2DM. Pregnant women, subjects with diabetes other than types 1 and 2, and those with wounds limited to above the ankle joints were excluded.
Data collection and clinical measurements
Using a specially designed structured proforma, relevant socio-demographic and diabetes-related information such as gender, age, occupation, cigarette smoking status, diabetes type and duration, as well as the type of healthcare facility where the patient was receiving diabetes care prior to development of foot ulcer were obtained and documented. Knowledge of foot care was assessed and patients were interviewed on whether they had received foot care education prior to foot ulceration. History of development and progression of ulcer including mechanism of ulceration, site of ulcer, duration of ulcer and prior ulcer treatment methods were also assessed. Clinical wound infection was determined according to the International Working Group on Diabetes Foot (IWGDF) guideline by the presence of purulent exudates or any two or more of the following: Periwound edema, periwound redness, local warmth, foul smell, pain or tenderness on palpation and fever[4]. Commonly known risk factors for DFU were also evaluated, including history of previous DFU, barefoot walking, improper foot wear, visual impairment, foot deformity, peripheral neuropathy and peripheral artery disease (PAD). Peripheral neuropathy was diagnosed by loss of pressure perception to Semmes-Weinstein 10 g monofilament test or diminished vibration sense using the 128 Hz tunning fork. PAD was diagnosed based on impalpable dorsalis pedis and/or posterior tibial artery pulsations on manual palpation or significant arterial narrowing (> 50%) on Doppler ultrasonography of the lower limbs. The severity of ulcer was graded using two different ulcer classification systems, namely, the Wagner’s grading system and the University of Texas wound classification system[16,17].
Relevant laboratory and imaging studies were performed for each subject including urine protein using dipstick detection, full blood count, erythrocyte sedimentation rate, glycated hemoglobin (HbA1c), blood culture, ulcer specimen culture, lipid profile, plain radiograph of the foot and Doppler ultrasonography of both lower limbs. Co-morbid complications including hypertension, anemia, shock, hyperglycemic emergency, hypoglycemia, stroke, kidney disease and cardiac failure were explored and documented.
Patient management and outcome indicators
Every patient received appropriate multi-disciplinary care including bed rest, wound debridement, daily wound dressing, antibiotic therapy, skin grafting and limited amputation in addition to control of blood glucose and treatment of associated co-morbidities. All the primary investigators who led the multidisciplinary team were endocrinologists. Other relevant specialists including nutritionists, plastic surgeons, orthopedic surgeons and vascular surgeons were co-opted based on need and availability. None of the centers had a podiatrist, an important foot care specialist that is grossly in short supply in Nigeria. The decision to amputate or not was an exclusive prerogative of the multi-disciplinary footrace team at each study center. All the enrollees were followed up until discharge or death. Outcome variables of interest included ulcer healing, amputation, duration of hospitalization and mortality. We defined amputation above the mid-tarsal bone or involving the big toe as major amputation, otherwise it was minor. At the stoppage of data collection, records of medical admissions over the study period were reviewed retrospectively in all the centers to determine the total number of medical admissions and diabetes-related admissions.
Statistical analysis
Data were collated in all the six participating centers and analyzed using the Statistical Package for Social Sciences (IBM version 23.0; SPSS Inc., Chicago, IL, United States). Categorical variables were presented as numbers and percentages while continuous variables were presented as means and standard deviations or medians and interquartile ranges as appropriate. Analysis at this stage was mainly descriptive. Data were presented in frequency tables, bar charts, pie charts and line graphs as deemed appropriate. The Chi-Square test was used to test differences in categorical proportions while continuous variables were compared between two or more groups of interest using the Student’s t-test. Statistical significance was established at P < 0.05.
RESULTS
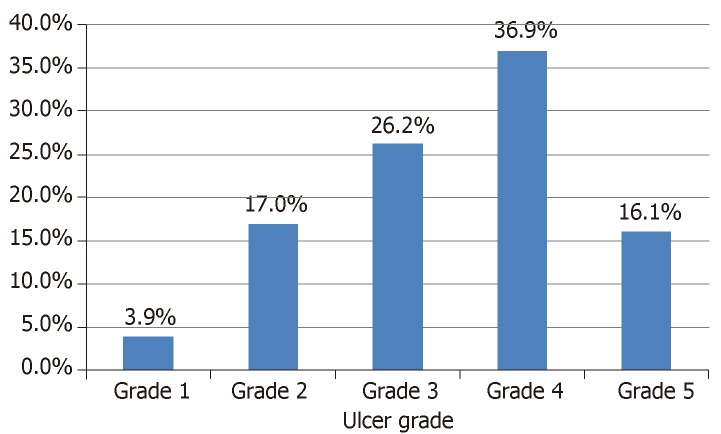
There were 9778 total and 1350 (13.8%) diabetes related admissions in the medical wards over the study period. Out of this number, 336 patients with a male: female ratio of 1:0.8 had DFU, and this number accounted for 24.9% of DM-related admissions. Majority of the DFU subjects (96.1%) had type 2 diabetes. The mean ± SD age and mean ± SD duration of DM were 55.9 ± 12.5 years and 8.5 ± 5.7 years respectively. Most of the patients (71.7%) were not accessing diabetes care at the study centers but were referred because of the foot ulcer. Glycemic control was generally poor with mean HbA1c of 9.6 ± 1.9%. Only 87 subjects (20.4%) had received foot care education prior to development of ulcer. Neuropathic and neuro-ischemic ulcers predominated in 37.2% and 40.2% of the subjects respectively. Ulcers were adjudged advanced (Wagner grade ≥ 3) in 79.2% of the subjects and majority were already infected. The commonest co-morbidities were systemic hypertension (56.8%), anemia (53.6%) and hyperglycemic emergencies (36.6%). Table 1 shows the clinical profile of the study participants while the ulcer grades are shown in Figure 1.
Table 1.
Clinical profile of the patients with diabetic foot ulcers
| Variable | n (%) | mean ± SD |
| Age (yr) | 55.9 ± 12.5 | |
| < 45 yr | 48 (14.3) | |
| 45-64 yr | 200 (59.5) | |
| ≥ 65 yr | 88 (26.2) | |
| Gender (male) | 185 (55.1) | |
| Occupation | ||
| Civil servants | 61(18.2) | |
| Traders | 137 (40.8) | |
| Artisans | 12 (3.6) | |
| Farmers | 40 (11.9) | |
| Unemployed | 86 (25.6) | |
| Cigarette smoking (current smokers) | 17 (5.1) | |
| Diabetes type (type 2) | 323 (96.1) | |
| Diabetes duration (yr) | 8.5 ± 5.7 | |
| ≤ 10 yr | 250 (74.4) | |
| 11-20 yr | 79 (23.5) | |
| > 20 yr | 7 (2.1) | |
| Newly diagnosed diabetes | 49 (14.6) | |
| Glycated hemoglobin (%) (n = 296) | 9.6 ± 1.9 | |
| HbA1c < 7% | 17 (5.7) | |
| Referred from outside the study centers | 241 (71.7) | |
| Ever had foot care education | 87 (25.9) | |
| Type of Ulcer | ||
| Neuropathic | 125 (37.2) | |
| Ischemic | 42 (12.5) | |
| Neuro-ischemic | 135 (40.2) | |
| Non-neuropathic, non-ischemic | 34 (10.1) | |
| Duration of ulcer before admission (d) | 39 (28-54)1 | |
| Ulcer > 30 d duration | 237 (70.5) | |
| Previous history of ulcer | 96 (28.6) | |
| Advanced ulcer (Wagner grade ≥ 3) | 266 (79.2) | |
| Presence of wound infection | 258 (76.8) | |
| Co-morbid complications | ||
| Hypertension | 191 (56.8) | |
| Shock | 40 (11.9) | |
| Anemia | 180 (53.6) | |
| Hyperglycemic emergency | 123 (36.6) | |
| Hypoglycemia | 33 (9.8) | |
| Cardiac failure | 23 (6.8) | |
| Renal impairment | 66 (19.6) | |
| Stroke | 32 (9.5) |
Data presented as median (interquartile range).
Figure 1.
Distribution of diabetic foot ulcer severity by Wagner grading system.
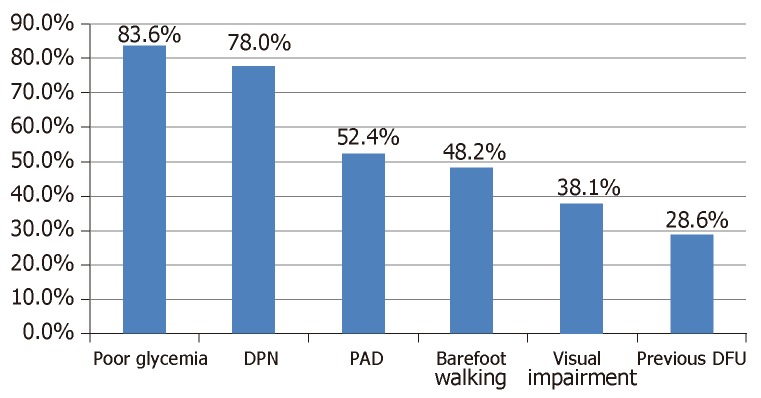
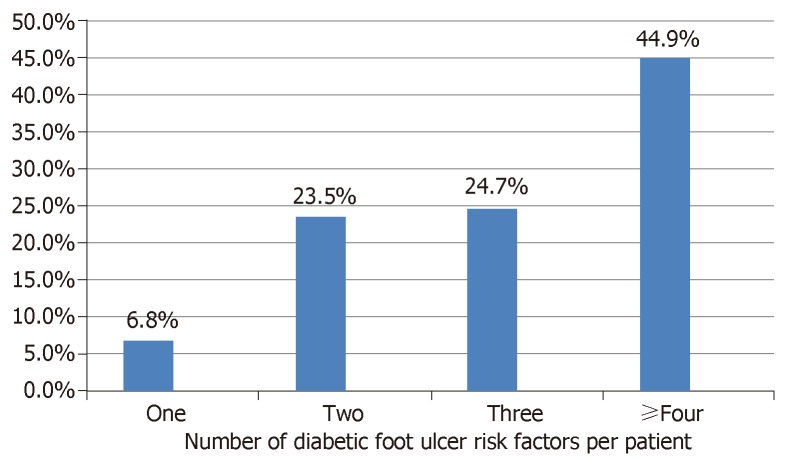
Identifiable factors that probably predisposed the patients to developing DFU are presented in Figure 2. Diabetic peripheral neuropathy (DPN) and PAD were present in 78.0% and 52.4% of the participants respectively. About 48.2% of the subjects admitted to barefoot walking while 28.6% have had previous foot ulceration. As shown in Figure 3, majority of the subjects have multiple risk factors such that up to 44.9% have four or more risk factors operating simultaneously.
Figure 2.
Prevalence of risk factors for diabetic foot ulcers in the study population. DFU: Diabetic foot ulcer; DPN: Diabetic peripheral neuropathy; PAD: Peripheral artery disease.
Figure 3.
Per patient burden of diabetic foot ulcer risk factors.
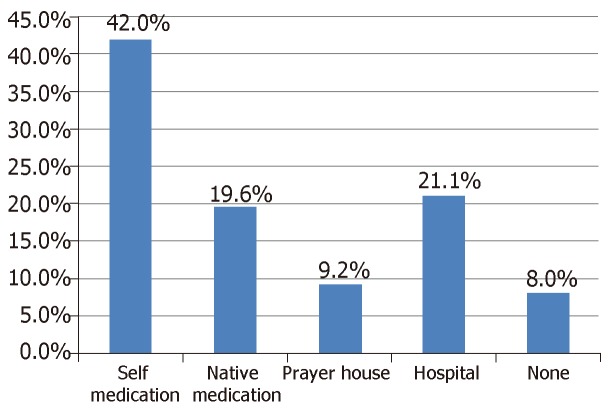
Only 21.1% of our subjects sought treatment in hospital as their first option following development of foot ulcer. The most preferred initial treatment option was self-medication which was practiced by 42.0% of the patients. 19.6% of the subjects patronized traditional healers/herbalists while 9.2% relied on prayer houses. These are summarized in Figure 4.
Figure 4.
Preferred initial treatment options for patients with diabetic foot ulcers in Nigeria.
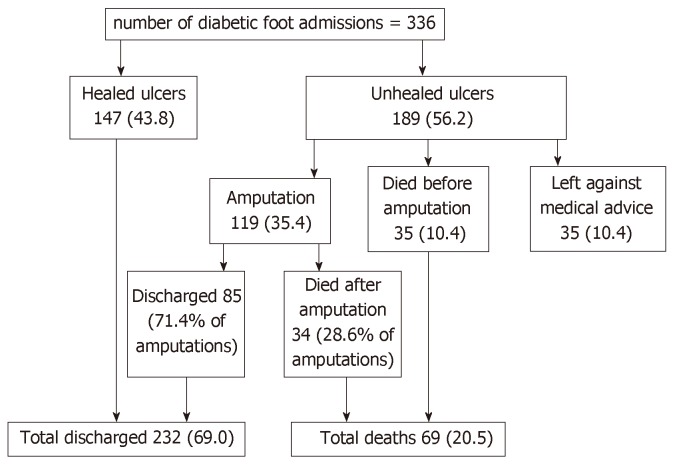
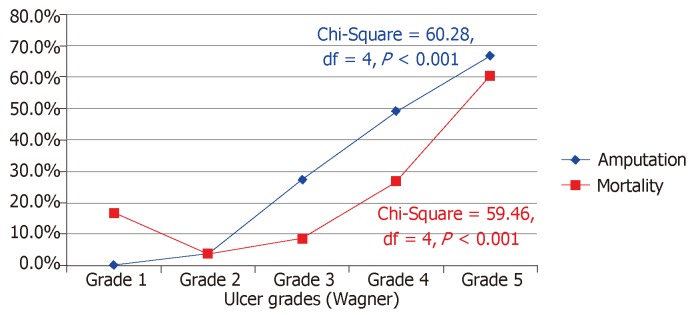
Figure 5 shows the admission outcomes of the patients studied. Of the 336 subjects hospitalized for DFU, satisfactory wound healing occurred in 147 subjects (43.8%). One hundred and nineteen subjects (35.4%) underwent LEA of which 75.6% were major amputations. Thirty-five subjects (10.4%) left against medical advice, mainly due to refusal of amputation (48.6%) and financial constraint (42.9%). Sixty-nine deaths (20.5%) were recorded, including 34 deaths post LEA. The median time between admission and death was 16 d (interquartile range 10-33 d). The median (IQR) duration of hospitalization for the study population excluding those who discharged against medical advice was 52.0 (29-66) d. Both amputation and mortality rates significantly increased with higher ulcer grades (Figure 6).
Figure 5.
Outcomes of diabetic foot ulcer admissions in Nigeria.
Figure 6.
Amputation and mortality rates by ulcer grades.
DISCUSSION
With a population estimated at about 200 million people, Nigeria is the most populous black nation. And with diabetes prevalence of nearly 6% in adults, representing about 5-7 million adults, Nigeria currently harbors the largest number of people living with diabetes in the West African sub-region[1]. Understanding the burden of diabetes and its complications in Nigeria is therefore a reliable sneak peek into the rest of Africa. Diabetic foot ulceration is one of the most challenging complications of DM. Due to absence of national data, prevalence rates of DFU in Nigeria from several single center studies vary widely from 11.7%-32%[13,14]. Similar wide variations have also been reported for DFU outcomes with amputation rates ranging from 12.6%-52%[14,15] and mortality rates ranging from as low as 8.7% to above 40%[13,14,18]. The need to have a current and more representative national data on the outlook of DFU in Nigeria therefore became the driving force that birthed the MEDFUN study.
Our data shows that DFU constitutes about a quarter of diabetes related hospital admissions in Nigeria. This represents a much higher burden than what is obtainable in developed nations where DFU generally accounts for less than 10% of medical admissions[19,20]. Our findings closely mirror the scenarios in some other African settings where the burden of DFU is also reportedly high[21,22]. Worrisome too is the fact that the bulk of our patients (73.8%) belonged to the young and middle-age categories and nearly three-quarter have had diabetes for less than 10 years duration. This finding is supported by previous local studies and suggests that in Nigeria, DFU affects predominantly the actively-working segment of the population who are often their family bread winners[14,15,18]. In contrast, majority of patients with DFU in Netherlands and Thailand for instance are above the age of 60 years and have had diabetes for longer duration[20,23]. The socio-economic consequences of this scenario on a people already groaning under poverty and many communicable diseases could be better imagined.
The prevalence of diabetic foot disease largely reflects the quality of diabetes care as this complication of DM is largely preventable through proper diabetes management[24]. Approximately 83% of our study subjects had HbA1c above 7%, a reflection of the poor quality of diabetes care in our locality especially at primary and secondary healthcare levels where the bulk of our patients came from. Poor glucose control has been widely reported in Nigeria even among subjects attending tertiary health institutions[25,26]. Factors that may be responsible for this include poverty, poor drug compliance, poor access to healthcare, shortage of trained diabetes care manpower and diabetes status unawareness. It is noteworthy that 14.6% of the subjects in this study were unaware of their diabetes status until they presented with foot ulcer. Chronic hyperglycemia is generally known to predispose to many diabetes-related complications including peripheral neuropathy, and the latter is a potent risk factor for development of DFU[8,9,27]. Up to 78% of our study subjects presented with DPN which we also identified as a major risk factor for DFU. DPN predisposes to DFU by causing loss of protective sensation in the feet as well as foot deformities, resulting in abnormal weight bearing, recurrent micro-trauma, callous formation and eventual ulceration[8,9].
Our study uncovered very low levels of foot care knowledge among the participants. We observed that nearly three-quarter of the patients had never received foot care education since diagnosis of diabetes. This finding is of great concern owing to the strategic importance of proper foot care knowledge in the prevention of DFU and amputation. It has been demonstrated that diabetic patients who are knowledgeable about foot care are 3 times less likely to develop DFU and to suffer LEA[28,29]. Patients who have adequate foot care knowledge are less likely to engage in harmful foot practices that could predispose to ulceration. They are also more likely to present earlier to the hospital following ulceration thereby reducing the likelihood of amputation. Other authors in Nigeria have observed low level of foot care knowledge both among the general diabetic population and those with DFU[14,30]. In a recent multi-center study, 78.4% of the 352 diabetic patients surveyed had poor knowledge of foot care and the authors lamented that high risk behaviors such as bare foot walking and improper foot wear were rampant among the patients[30]. Anumah et al[14] recently reported that 84.7% of patients who were hospitalized for DFU at a tertiary hospital had no prior foot care education. Certified diabetes educators are grossly in short supply in Nigeria and almost non-existent in rural and semi-urban areas. This manpower shortage may largely explain this serious gap in diabetes care in our locality.
The poor health-seeking behavior of patients with DFU in Nigeria was also brought to bear in this study. Our data show that the practice of self-medications and patronage of unorthodox treatment outlets including native/herbal homes and prayer houses were common initial treatment options among the patients. Although this attitude may be partly attributable to poverty and poor access to healthcare, it may not be totally unconnected with the negative illness perceptions that are pervasive in Africa. In many traditional African cultures, diseases are often ascribed to diabolism and spiritual etiologies[31-33]. In Lagos, Nigeria, as many as 46% of diabetic patients take alternative herbal medicines[34]. The presence of a non-healing wound may therefore be misinterpreted as the outcome of “stepping on poison” or “spiritual attack” and orthodox care is usually not sought until the disease is advanced. This may partly account for the late hospital presentation which was observed among our study subjects. The ulcer had lasted more than 1 mo in over 70% of our subjects prior to hospitalization, with 79.3% of patients presenting with at least Wagner grade 3 ulcers. Delayed hospital presentation is also a common denominator in many previous studies of DFU in Nigeria[13-15].
Amputation and mortality rates of 35.4% and 20.5% respectively that were observed in this study are unacceptably high and not in tandem with the trends in the civilized world. In Australia for instance, LEA rate from DFU is less than 2% with over 70% being minor amputation[19]. Very low amputation and mortality rates were also reported in Netherlands, Thailand, and Scotland[20,23,35]. Differences in the quality of healthcare systems are likely to be responsible for these discrepancies. We hypothesize that the poor glucose control and delay in hospital presentation following development of DFU contributed significantly to these unpleasant outcomes. Lavery et al[36] demonstrated that duration of ulcer more than 30 d increased the probability of wound infection by nearly 5 times and that amputation was 154 times more likely in infected wounds. This is probably due to the higher propensity of accelerated tissue necrosis and gangrene in such wounds especially in a limb with compromised vascular supply. Wound infection was present in 76.8% of our patients while over half had developed some form of gangrene. Such patients are expected to suffer more amputation and death from overwhelming sepsis. Not surprisingly therefore, we observed significant associations between ulcer severity as measured by Wagner grading, and amputation as well as mortality (P < 0.001 respectively). Our data agree with many other previous studies in Nigeria that also reported high amputation and mortality rates among patients with DFU[13,18,37]. Nigeria is therefore in dire need of total overhaul of diabetes care to stem this ugly tide. Importantly, appropriate multi-disciplinary care team approach led by an endocrinologist has been found to drastically improve diabetic foot outcomes and is hereby advocated[38].
Conclusion
The results from this study revealed that the burden of DFU in Nigeria is still alarming even in this 21st century. This study has exposed several treatment gaps including poor knowledge of foot care among patients, high patronage of self-medications and unorthodox treatment, and delayed hospital presentation with advanced foot ulcers, resulting in prolonged hospitalization, high LEA rate and high mortality. Bridging these gaps through intensive public enlightenment programmes, foot care education of diabetic patients and establishment of well-trained diabetic foot care team may go a long way in reversing this ugly trend.
Strenghts and limitations
To our knowledge, MEDFUN is the largest, most extensive and the only multi-center study on DFU both in Nigeria and the West-African sub-region. The limitations of this study however need to be highlighted. Firstly, the study centers covered only 4 out of the six geo-political zones of Nigeria. However, the 2 geo-political zones that were not included in this study share common characteristics with one or more of the other 4 zones. It is therefore arguable that our results are largely generalizable as a true reflection of the burden of DFU in Nigeria. Secondly, each of the participating centers adopted its own DFU management protocol based on availability of manpower. Clinical decisions were therefore dependent on the clinicians at each center. It is not unlikely that this lack of uniformity might have affected the outcome of this study. This is also applicable to the clinical measurements which are prone to inter-observer bias and laboratory tests which might have been influenced by performance variations of diagnostic equipments at the different study centers. However, this lack of uniformity is common in studies of this nature, including the widely cited Eurodiale study which was the largest multi-center DFU study in Europe[30].
ARTICLE HIGHLIGHTS
Research background
Diabetic foot ulcer (DFU) is a serious and costly complication of diabetes that is associated with high morbidity and mortality. However, DFU-related lower extremity amputation (LEA) and death are both preventable through appropriate healthcare measures.
Research motivation
The prevalence of diabetes in Nigeria is steadily rising with the country currently harboring the largest burden of diabetes in Sub-Saharan Africa. Evaluation of a disease burden helps in identifying healthcare gaps that need to be addressed. However, the current burden of DFU in Nigeria is largely unknown.
Research objectives
We evaluated the patient and ulcer characteristics as well as the outcomes of patients hospitalized for DFU in six tertiary healthcare centers in Nigeria over a one year period.
Research methods
In an observational study design, we followed up a total of 336 type 1 and type 2 diabetic patients who were hospitalized for DFU until they exited the hospital. Then we documented their baseline profile, clinical progress, disease outcomes and mode of exit.
Research results
The study revealed that DFU accounted for about a quarter of diabetes related hospitalization in Nigeria. It further showed that most of the affected patients lacked knowledge of foot care and resorted to self-medications or alternative medicine approaches following development of foot ulcer. Consequently, over three-quarter of the patients presented late to the hospital with advanced ulcer. The study revealed a high LEA and mortality rates of 35.4% and 20.5% respectively.
Research conclusions
We concluded that the burden of DFU in Nigeria is still substantial and decried the high degree of foot care ignorance and poor health-seeking behavior among patients with DFU in our country.
Research perspectives
We advocate for massive public enlightenment programmes about diabetic foot with emphasis on its prevention and timely treatment. Massive training of diabetes educators and podiatrists in Nigeria to improve foot care knowledge and foot care practice is strongly recommended.
Footnotes
Institutional review board statement: Approval for the study was given by the local Research and Ethics committee of each of the participating centers.
Informed consent statement: Participation in this study was voluntary. Verbally granted informed consent was obtained from each patient prior to enrollment into the study. Confidentiality was ensured at all stages by means of unique coding system consisting of patients’ initials and assigned numbers.
Conflict-of-interest statement: All authors declare no conflict of interest. This study did not receive funding from any external source.
Manuscript source: Unsolicited manuscript
Peer-review started: January 11, 2019
First decision: January 25, 2019
Article in press: March 8, 2019
Specialty type: Endocrinology and metabolism
Country of origin: Nigeria
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: Beltowski J, Hosseinpour-Niazi S, Jiang L, Reggiani GM, Senol MG S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
Contributor Information
Ejiofor Ugwu, Department of Medicine, Enugu State University of Science and Technology Enugu, Enugu 400001, Nigeria. ofornet@yahoo.com.
Olufunmilayo Adeleye, Department of Medicine, Lagos State University Lagos, Lagos 100001, Nigeria.
Ibrahim Gezawa, Department of Medicine, Bayero University Kano, Kano 700001, Nigeria.
Innocent Okpe, Department of Medicine, Ahmadu Bello University Zaria, Kaduna 800001, Nigeria.
Marcelina Enamino, Department of Medicine, Federal Medical Center Keffi, Nasarawa 961101, Nigeria.
Ignatius Ezeani, Department of Medicine, Federal Medical Center Umuahia, Abia 440001, Nigeria.
References
- 1.International Diabetes Federation. 2017. Diabetes atlas 8th Edition. International Diabetes Federation. Available from: http://www.diabetesatlas.org. [Google Scholar]
- 2.Adeloye D, Ige JO, Aderemi AV, Adeleye N, Amoo EO, Auta A, Oni G. Estimating the prevalence, hospitalisation and mortality from type 2 diabetes mellitus in Nigeria: a systematic review and meta-analysis. BMJ Open. 2017;7:e015424. doi: 10.1136/bmjopen-2016-015424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mbanya JC, Motala AA, Sobngwi E, Assah FK, Enoru ST. Diabetes in sub-Saharan Africa. Lancet. 2010;375:2254–2266. doi: 10.1016/S0140-6736(10)60550-8. [DOI] [PubMed] [Google Scholar]
- 4.Bakker K, Apelqvist J, Lipsky BA, Van Netten JJ International Working Group on the Diabetic Foot. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence-based global consensus. Diabetes Metab Res Rev. 2016;32 Suppl 1:2–6. doi: 10.1002/dmrr.2694. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. 2017;49:106–116. doi: 10.1080/07853890.2016.1231932. [DOI] [PubMed] [Google Scholar]
- 6.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 7.Abbas ZG, Archibald LK. Epidemiology of the diabetic foot in Africa. Med Sci Monit. 2005;11:RA262–RA270. [PubMed] [Google Scholar]
- 8.Clayton W, Elasy TA. A review of the pathophysiology, classification, and treatment of foot ulcers in diabetic patients. Clin Diabetes. 2009;22:52–58. [Google Scholar]
- 9.Agale SV. Chronic Leg Ulcers: Epidemiology, Aetiopathogenesis, and Management. Ulcers. 2013 [Google Scholar]
- 10.Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care. 2014;37:651–658. doi: 10.2337/dc13-2176. [DOI] [PubMed] [Google Scholar]
- 11.Desmond D, Gallagher P. Quality of life in people with lower limb amputation. Handbook of Disease burdens and quality of life measures. VR Preedy and RR Watson Eds. 2010:3785–3796. [Google Scholar]
- 12.Armstrong DG, Wrobel J, Robbins JM. Guest Editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J. 2007;4:286–287. doi: 10.1111/j.1742-481X.2007.00392.x. [DOI] [PubMed] [Google Scholar]
- 13.Ogbera AO, Fasanmade O, Ohwovoriole AE, Adediran O. An assessment of the disease burden of foot ulcers in patients with diabetes mellitus attending a teaching hospital in Lagos, Nigeria. Int J Low Extrem Wounds. 2006;5:244–249. doi: 10.1177/1534734606294538. [DOI] [PubMed] [Google Scholar]
- 14.Anumah FO, Mshelia-Reng R, Abubakar A, Sough T, Asudo F, Jamda MA, Omonua O, Odumodu KC, Shaibu R. Management outcome of diabetic foot ulcers in a teaching hospital in Abuja, Nigeria. J Diabetes Complicat. 2017;9:15–20. [Google Scholar]
- 15.Edo AE, Edo GO, Ezeani IU. Risk factors, ulcer grade and management outcome of diabetic foot ulcers in a Tropical Tertiary Care Hospital. Niger Med J. 2013;54:59–63. doi: 10.4103/0300-1652.108900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner FW., Jr The diabetic foot. Orthopedics. 1987;10:163–172. doi: 10.3928/0147-7447-19870101-28. [DOI] [PubMed] [Google Scholar]
- 17.Lavery LA, Armstrong DG, Harkless LB. Classification of diabetic foot wounds. J Foot Ankle Surg. 1996;35:528–531. doi: 10.1016/s1067-2516(96)80125-6. [DOI] [PubMed] [Google Scholar]
- 18.Ekpebegh CO, Iwuala SO, Fasanmade OA, Ogbera AO, Igumbor E, Ohwovoriole AE. Diabetes foot ulceration in a Nigerian hospital: in-hospital mortality in relation to the presenting demographic, clinical and laboratory features. Int Wound J. 2009;6:381–385. doi: 10.1111/j.1742-481X.2009.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baba M, Davis WA, Norman PE, Davis TM. Temporal changes in the prevalence and associates of diabetes-related lower extremity amputations in patients with type 2 diabetes: the Fremantle Diabetes Study. Cardiovasc Diabetol. 2015;14:152. doi: 10.1186/s12933-015-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoekenbroek RM, Lokin JLC, Nielen MM, Stroes ESG, Koelemay MJW. How common are foot problems among individuals with diabetes? Diabetic foot ulcers in the Dutch population. Diabetologia. 2017;60:1271–1275. doi: 10.1007/s00125-017-4274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almobarak AO, Awadalla H, Osman M, Ahmed MH. Prevalence of diabetic foot ulceration and associated risk factors: an old and still major public health problem in Khartoum, Sudan? Ann Transl Med. 2017;5:340. doi: 10.21037/atm.2017.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiwanga FS, Njelekela MA. Diabetic foot: prevalence, knowledge, and foot self-care practices among diabetic patients in Dar es Salaam, Tanzania - a cross-sectional study. J Foot Ankle Res. 2015;8:20. doi: 10.1186/s13047-015-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thewjitcharoen Y, Krittiyawong S, Porramatikul S, Parksook W, Chatapat L, Watchareejirachot O, Sripatpong J, Himathongkam T. Outcomes of hospitalized diabetic foot patients in a multi-disciplinary team setting: Thailand's experience. J Clin Transl Endocrinol. 2014;1:187–191. doi: 10.1016/j.jcte.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bus SA, van Netten JJ. A shift in priority in diabetic foot care and research: 75% of foot ulcers are preventable. Diabetes Metab Res Rev. 2016;32 Suppl 1:195–200. doi: 10.1002/dmrr.2738. [DOI] [PubMed] [Google Scholar]
- 25.Uloko AE, Ofoegbu EN, Chinenye S, Fasanmade OA, Fasanmade AA, Ogbera AO, Ogbu OO, Oli JM, Girei BA, Adamu A. Profile of Nigerians with diabetes mellitus - Diabcare Nigeria study group (2008): Results of a multicenter study. Indian J Endocrinol Metab. 2012;16:558–564. doi: 10.4103/2230-8210.98011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chinenye S, Young EE. State of diabetes care in Nigeria: A review. Niger Health J. 2011;11:101–109. [Google Scholar]
- 27.Boulton AJ. Diabetic neuropathy and foot complications. Handb Clin Neurol. 2014;126:97–107. doi: 10.1016/B978-0-444-53480-4.00008-4. [DOI] [PubMed] [Google Scholar]
- 28.Monami M, Zannoni S, Gaias M, Nreu B, Marchionni N, Mannucci E. Effects of a Short Educational Program for the Prevention of Foot Ulcers in High-Risk Patients: A Randomized Controlled Trial. Int J Endocrinol. 2015;2015:615680. doi: 10.1155/2015/615680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malone JM, Snyder M, Anderson G, Bernhard VM, Holloway GA, Jr, Bunt TJ. Prevention of amputation by diabetic education. Am J Surg. 1989;158:520–3; discussion 523-4. doi: 10.1016/0002-9610(89)90183-9. [DOI] [PubMed] [Google Scholar]
- 30.Desalu OO, Salawu FK, Jimoh AK, Adekoya AO, Busari OA, Olokoba AB. Diabetic foot care: self reported knowledge and practice among patients attending three tertiary hospital in Nigeria. Ghana Med J. 2011;45:60–65. doi: 10.4314/gmj.v45i2.68930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feyisetan BJ, Asa S, Ebigbola JA. Mothers' management of childhood diseases in Yorubaland: the influence of cultural beliefs. Health Transit Rev. 1997;7:221–234. [PubMed] [Google Scholar]
- 32.Sabuni LP. Dilemma with the local perception of causes of illnesses in central Africa: muted concept but prevalent in everyday life. Qual Health Res. 2007;17:1280–1291. doi: 10.1177/1049732307307864. [DOI] [PubMed] [Google Scholar]
- 33.Abubakar A, Van Baar A, Fischer R, Bomu G, Gona JK, Newton CR. Socio-cultural determinants of health-seeking behaviour on the Kenyan coast: a qualitative study. PLoS One. 2013;8:e71998. doi: 10.1371/journal.pone.0071998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogbera AO, Dada O, Adeyeye F, Jewo PI. Complementary and alternative medicine use in diabetes mellitus. West Afr J Med. 2010;29:158–162. doi: 10.4314/wajm.v29i3.68213. [DOI] [PubMed] [Google Scholar]
- 35.Kennon B, Leese GP, Cochrane L, Colhoun H, Wild S, Stang D, Sattar N, Pearson D, Lindsay RS, Morris AD, Livingstone S, Young M, McKnight J, Cunningham S. Reduced incidence of lower-extremity amputations in people with diabetes in Scotland: a nationwide study. Diabetes Care. 2012;35:2588–2590. doi: 10.2337/dc12-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care. 2006;29:1288–1293. doi: 10.2337/dc05-2425. [DOI] [PubMed] [Google Scholar]
- 37.Adeleye JO. Diabetic foot disease: The perspective of a Nigerian tertiary health centre. Pract Diabetes Int. 2005;22:211–214. [Google Scholar]
- 38.Rubio JA, Aragón-Sánchez J, Jiménez S, Guadalix G, Albarracín A, Salido C, Sanz-Moreno J, Ruiz-Grande F, Gil-Fournier N, Álvarez J. Reducing major lower extremity amputations after the introduction of a multidisciplinary team for the diabetic foot. Int J Low Extrem Wounds. 2014;13:22–26. doi: 10.1177/1534734614521234. [DOI] [PubMed] [Google Scholar]