Abstract
Sphingolipids, such as sphingomyelins, ceramides, glycosphingolipids, and sphingosine-1-phosphates (S1P) are a large group of structurally and functionally diverse molecules. Some specific species are found associated with atherogenesis and provide novel therapeutic targets. Herein, we briefly review how sphingolipids are implicated in the progression of atherosclerosis and related diseases, and then we discuss the potential therapy options by targetting several key enzymes in sphingolipid metabolism.
Keywords: Atherosclerosis, Ceramide, Sphingolipids
Sphingolipids metabolism
Overview of sphingolipid biosynthesis
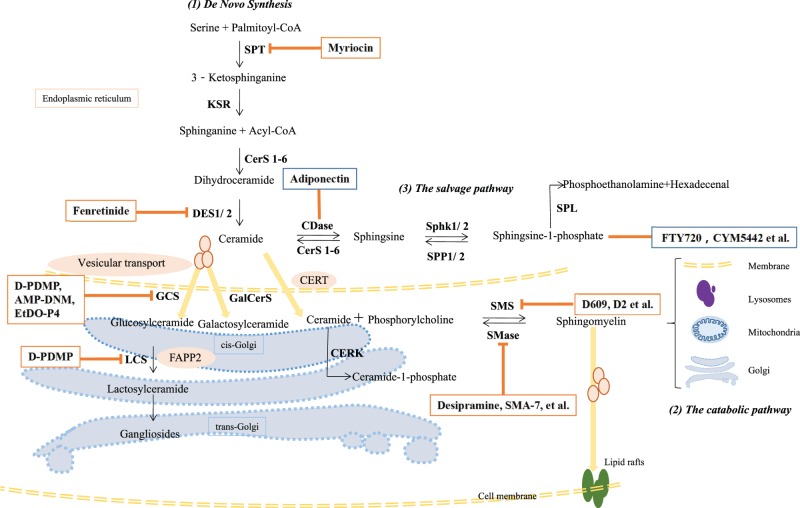
Sphingolipids are based on a ceramide parent structure. Ceramides are composed of a hydrophobic sphingoid backbone and a fatty acyl chain, linked to the backbone via an amino bond [1]. Three metabolic pathways are involved in ceramide production (Figure 1). (1) De novo synthesis begins in the cytosolic layer of the endoplasmic reticulum (ER) with the condensation of the amino acid serine and palmitoyl-coenzyme A (CoA) via serine palmitoyltransferase (SPT), generating 3-ketosphinganine. 3-ketosphinganine is then reduced to sphinganine, the 18-carbon backbone, via 3-ketosphinganine reductase (KSR). Finally, N-acylation of sphinganine by ceramide synthases (CerS) generates dihydroceramide, which is subsequently converted into ceramide via dihydroceramide desaturase (DES) [2]. This de novo pathway is the major source of ceramide in cells, and all eukaryotic cells have the capacity to produce sphingolipids in this way. (2) A catabolic pathway occurs in lysosomes, including hydrolysis of sphingomyelin via sphingomyelinase (SMase) and catabolism of glycosphingolipids via glycosidases hydrolyzing glycosidic bonds [3]. (3) A salvage pathway generates ceramides by recycling sphingosine via CerS, as the sphingosine is produced by the hydrolysis of ceramide catalyzed by ceramidase (CDase) [4]. At least half of the sphingosine enters this reutilization pathway, playing an important role in sphingolipid homeostasis [3].
Figure 1. Sphingolipid biosynthesis and sphingolipid-centric theraputics.
(1) De novo sphingolipid synthesis starts in the ER with the decarboxylation of a serine residue and condensation with a palmitoyl-CoA catalyzed by SPT. Sequential reactions lead to the production of ceramides, which are precursors for the biosynthesis of sphingomyelins and glycosphingolipids. In the ER, ceramides can be deacylated by CDase to form sphingosine. Sphingosine can be phosphorylated to form sphingosine-1-phosphate (S1P) by SphK1/2. In the Golgi, ceramides transferred by CERT are predestined to synthesize sphingomyelins by the addition of phosphocholine head group or be phosphorylated to form ceramide-1-phosphate. Ceramides transferred by vesicular transport can be glycosylated to form glucosylceramides or galactosylceramides. FAPP2 can transfer glucosylceramides from the cis-Golgi to the trans-Golgi, where they are converted into lactosylceramides. (2) Bidirectionally, in the plasma membrane, lysosome, mitochondria, and Golgi, sphingomyelins can be converted into ceramides by SMSases. Similarly, ceramide-1-phosphate and glycosphingolipids can be hydrolyzed to form ceramides (not shown). (3) Sphingosine can be recycled to generate ceramides by CerSs. Myriocin is a SPT inhibitor; Fenretinide plays a role in DES1 inhibition; Adiponectin exerts its metabolic improvement functions through CDase signaling; FTY720 and CYM5442 are S1P analogs; D609 and D2-series are SMS inhibitors; Desipramine and SMA-7 et al. are inhibitors of SMase. D-PDMP inhibits both GCS and LCS; AMP-DNM and EtDO-P4 are specific GCS inhibitors. Abbreviations: AMP-DNM, N-(5-adamantane-1-yl-methoxy)-pentyl-1-deoxynoijirimycin; CDase, ceramidase; CERK, ceramide kinase; CerS1-6, ceramide synthase1-6; CERT, ceramide transfer protein; DES1/2, dihydroceramide desaturase1/2; D-PDMP, d-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol; EtDO-P4, d-threo-1-ethylendioxyphenyl-2-palmitoylamino-3-pyrrolidino-propanol; FAPP2, four-phosphate adaptor protein 2; FTY720, 2-amino-2-[2-(4-octylphenyl)ethyl] propane-1, 3-diolhydrochloride; GalCerS, galactosylceramide synthase; GCS, glucosylceramide synthase; KSR, 3-ketosphinganine reductase; LCS, lactose CerS; SMS, sphingomyelin synthase; SphK1/2, sphingosine kinase1/2; SPP1/2, S1P phosphatase1/2; SPL, S1P lyase.
Ceramides have three main ways to be incorporated into various sphingolipids. (1) In the ER, ceramide can be deacetylated to form sphingosine, which in turn is phosphorylated to serine palmitoyltransferase (S1P) via sphingosine kinase (SphK) [5]. (2) In the Golgi, after being transported from the ER by ceramide transfer protein (CERT) in a non-vesicular pathway, ceramide acquires a phosphorylcholine moiety from phosphatidylcholine to form sphingomyelin and diacylglycerol via sphingomyelin synthase (SMS); when transported from the ER via transport vesicles, ceramide add a glucose/galactose to form glucosylceramide (GluCer)/galactosylceramide (GalCer) via glucosylceramide synthase (GCS, also named UDP-glucose ceramide glucosyltransferase) or galactosylceramide synthase (GalCerS, also named 2-hydroxyacylsphingosine 1-β-galactosyltransferase). Then, GluCer is transferred from the cis-Golgi to the trans-Golgi by vesicular transport or a carrier protein, four-phosphate adapter protein 2 (FAPP2), to generate lactosylceramide (LacCer) catalyzed by lactose ceramide synthase (LCS), also named glucosylceramide β1→4 galactosyltransferase (GalT-2) [6]. In the trans-Golgi, LacCer can further produce complex globosides and gangliosides [7]. (3) In the Golgi, ceramides can also be phosphorylated to form ceramide-1-phosphate (C1P) via ceramide kinase (CERK).
Regulation in sphingolipid metabolism
The abundance and species of sphingolipids can be regulated by the availability of different substrates and the activity of various enzymes. It was proven that feeding rodents and rabbits a diet enriched in saturated fats increased their levels of various sphingolipids. In humans, different diets also affect the serum levels of ceramides. Thus, the oversupply of substrate palmitate and serine may promote de novo ceramide biosynthesis [8].
In addition, many key enzymes not only influence the synthetic rate but also introduce variations into the basic structure. SPT, acting as a rate-limiting enzyme, can generate a multitude of sphingoid bases by altering the substrate specificity. More specifically, SPT can utilize alanine or glycine instead of serine and prefer myristate or stearate as a fatty acid substrate, instead of the canonical palmitate. The sphingoid bases can be further compounded by an additional double-bond via DES1 and an OH via DES2 [9]. The N-linked fatty acid chains also display wide variations with various chain lengths, unsaturation levels, and hydroxylation levels. Distinct CerS isoforms prefer specific fatty acyl-CoAs with different chain lengths, such as the CerS1 mainly involved in the synthesis of C18:0 ceramides [10].
Distribution and transport of sphingolipids
Plasma sphingolipids are very rare, mainly consisting of the most prevalent sphingomyelins (∼87%), complex glycosphingolipids (9-10%), and ceramides (∼3%) [7]. Insoluble lipids are associated with apolipoprotein (apo), forming lipoproteins for transport in circulation and metabolism. According to their flotation density, lipoproteins are classified as chylomicrons, very-low-density lipoproteins (VLDL), low-density lipoproteins (LDL), or high-density lipoproteins (HDL). Approximately, sphingomyelins are distributed into VLDL/LDL (63–75%) and HDL (25–35%); the most abundant glycosphingolipids, GluCer and LacCer, are present as VLDL (8–14%), LDL (46–60%), and HDL (28–44%), while ceramides are distributed equally as VLDL, LDL, and HDL [11]. How sphingolipids are incorporated into lipoprotein particles is not very clear. Recently, it was demonstrated that microsomal triglyceride transfer protein (MTP), by helping apoB lipoproteins with assembly, plays a crucial role in the plasma levels of sphingomyelin and ceramides, along with GluCer concentrations [12].
Intracellular sphingolipids have specific compartmentalizations and can be transported between different membranes via two routes, as mentioned above: vesicular transport and non-vesicle transporters. Apart from CERT for ceramide transport and FAPP2 for GluCer transport, there are other identified transfer proteins, such as protein spinster homolog 2 (SPNS2) for S1P, C1P transfer protein (CPTP) for C1P, and glycolipid transfer protein (GLTP) for LacCer [9].
Sphingolipids associated with metabolic disease
The metabolic syndrome, mainly driven by obesity, defines a multiplex risk factor for atherosclerotic vascular disease and type 2 diabetes [13]. It is a growing epidemic, composed of dyslipidemia, insulin resistance, hypertension, a pro-thrombotic state, and a pro-inflammatory state. Also, non-alcoholic fatty liver disease (NAFLD), which progresses from steatosis alone to ultimate cirrhosis, is a common metabolic disease. Countless studies have shown that subjects with the above metabolic disorders exhibit greater plasma or tissue levels of one or more of the sphingolipid species [14–16]. Some specific sphingolipids are even emerging as biomarkers and prognostic indicators, such as for cardiovascular disease [17]. Sphingolipid metabolism is strongly associated with the pathogenesis of a repertoire of metabolic diseases. Great efforts have been exerted in identifying the critical sphingolipids, modulating sphingolipid synthesis and catabolism, recognizing the biological functions, identifying the transporting mode, and locating the sphingolipid-dependent signal pathways in diverse pathologies. More importantly, disrupting sphingolipid metabolism has proven to provide novel therapeutic avenues for metabolic disorders, which is the ultimate goal.
The sphingolipidome is extremely diverse and complex, so in this brief review, we focus on relationships between specific sphingolipids and atherosclerosis, a leading cause of worldwide morbidity and mortality, and summarize how metabolic pathways are being regulated for anti-atherosclerosis effects.
Sphingomyelins and atherosclerosis
Human studies investigating the role of sphingomyelins
Employing a novel high-throughput enzymatic method for plasma lipid determination, Jiang et al. [18] systematically assessed plasma sphingomyelin for the first time. Higher plasma sphingomyelin level was found in coronary artery disease (CAD) patients, and proposed as an independent risk factor for CAD. Also, the arterial tissues obtained by coronary artery bypass grafting (CABG) surgery had a higher concentration than normal vascular tissues [19]. Subsequently, the relationships of plasma sphingomyelin with left ventricle systolic dysfunction and clinical cardiac events were investigated [20,21]. What is more, Nelson et al. [22] found the sphingomyelin level was positively correlated with earlier, subclinical atherosclerotic disease, such as carotid intimal–medial wall thickness.
The question as to whether higher plasma sphingomyelin concentration is risk factor for CAD and indicates worse prognosis remains controversial. Yeboah et al. [23] assessed the predictive value in a cohort study of participants free of clinical CAD at baseline. After 5 years of follow-up, the data showed no association between plasma sphingomyelin levels and incident CAD events (myocardial infarction, definite angina, coronary revascularization, resuscitated cardiac arrest, and cardiovascular death). Sigruener et al. [24] found long chain saturated sphingomyelins (23:0, 24:0) seemed to be associated with a protective effect on cardiovascular mortality. Therefore, more studies are needed to determine how sphingomyelins are implicated in cardiovascular diseases and to illuminate the mechanisms involved.
SMSs as potential therapeutic targets for atherosclerosis
SMSs act on the last step of sphingomyelin synthesis; three homologs have been identified: SMS1, SMS2, and SMSr (SMS-related protein) [25]. SMS1 and SMS2 both possess SMS activity, albeit with distinguishable subcellular localization. SMS1 is mostly located in the Golgi apparatus and SMS2 is mainly found in plasma membranes. SMSr serves as monofunctional ceramide phosphoethanolamine (CPE, a sphingomyelin analog) synthase in the ER.
It was reported that overexpression of SMS1 and SMS2 would increase plasma sphingomyelin level and aggravate atherosclerosis in mice models [26,27]. Conversely, inhibiting SMS1 and SMS2 activity in vitro could lower sphingomyelin concentrations [28] and cause blunt NFκB activation responding to inflammatory stimuli [29]. Further in vivo experiments showed that atherosclerotic lesions were efficiently decreased and inflammatory responses were lowered in the SMS2 deficient (SMS2−/−) mice models [30–32]. Besides, SMS2 deficiency protected mice against tissue and whole-body insulin resistance [33,34], which might be associated with less liver steatosis [35].
Although SMS1 deficiency in macrophages showed similar anti-atherosclerotic effects in a mice model [36], SMS1 is indeed not an appropriate therapeutic target. To date, it has been reported that SMS1−/− mice exhibited several severe abnormalities, including defective insulin secretion [37], progressive hearing loss at a low frequency range [38], CD4+ T-cell dysfunction [39], adipocyte lipid storage dysfunction [40], male spermatogenesis defects [41], and mesenchymal transition of epithelial cells derived from the renal papillary collecting duct [42]. Disruption of SMSr in mice resulted in marginal changes in the plasma levels of sphingomyelin, ceramide, and S1P [43,44]. Thus, compared with SMS1 and SMSr, SMS2 is a more promising therapeutic target for atherosclerosis, but it is a challenge to develop highly specific SMS2 inhibitors without cross-reaction with SMS1.
Tricyclodecan-9-yl-xanthogenate
Tricyclodecan-9-yl-xanthogenate (D-609) was the first compound reported as an inhibitor against sphingomyelin synthesis from Bacillus cereus [45,46]. Some undesired properties of D609 and its prodrugs, e.g. instability, low specificity, and week potency hindered them from being practical drugs (Table 1) [47].
Table 1. Human studies investigating the role of diverse sphingolipid species in atherosclerosis related diseases.
| Study population | n | Correlating sphingolipids | Clinical end points | Reference |
|---|---|---|---|---|
| Human studies investigating the role of sphingomyelins | ||||
| African American and whites with CAD | 279 cases and 277 controls | Higher plasma sphingomyelins | CAD | Jiang et al. (2000) [18] |
| CABG patients | 32 CABG patients | Higher concentration of sphingomyelins in arterial tissues | Coronary arteries obtained on CABG surgery | Kummerow et al. (2001) [19] |
| CAD patients | 1102 CAD patients and 444 controls | Higher plasma sphingomyelins | 6-year, a predictor for myocardial infarction (MI) and cardiovascular death | Schlitt et al. (2006) [20] |
| Asymptomatic adults, MESA study | 6814 adults | Higher plasma sphingomyelins | Subclinical atherosclerosis (carotid intimal–medial wall thickness, ankle-arm blood pressure, and Agatston coronary artery calcium score) | Nelson et al. (2006) [22] |
| Adults free of clinical CAD in MESA | 6809 adults | Plasma sphingomyelins, not associated with risk of incident CAD | 5-year, incident CAD events (MI, resuscitated cardiac arrest, angina, cardiovascular death and revascularization) | Yeboah et al. (2009) [23] |
| Chinese, participants underwent coronary angiography for chest pain | 732 adults | Plasma sphingomyelins | CAD, left ventricle systolic dysfunction | Chen et al. (2011) [21] |
| Caucasian, LURIC study | 2538 CAD patients and 733 controls | Protetive sphingomyelins (23:0; 24:0); risky sphingomyelin species (16:0; 24:1) and risky ceramides (16:0; 24:1) | 8-year, total and/ or CAD mortality | Sigruener et al. (2014) [24] |
| Human studies investigating the role of ceramides on T2D | ||||
| Obese T2D, adults | 13 patients and 14 lean controls | Higher ceramide species (C18:1, 18:0, 20:0, 24:1, and 24:0) | T2D | Haus et al. (2009) [59] |
| Obese adults, T2D | 13 lean, 5 obese, 12 T2D | Higher total ceramides | T2D | Boon et al. (2013) [62] |
| Obese female T2D, children, and adolescents | 14 patients and 14 lean controls | Higher ceramide species (C18:0, 20:0, and 22:0), higher dihydroceramide (C24:1) | Obese T2D | Lopez et al. (2013) [60] |
| T2D, athletes, adults | 15 T2D, 15 athletes, and 14 obese controls | Higher ceramide species (C18:0, 20:0, and 24:1) and total dihydroceramide | T2D | Bergman et al. (2015) [61] |
| Two cohorts: DESIR, western France; CoLaus, Switzerland | 298, 300 participants without T2D | Higher dihydroceramides, higher ceramide species (C18:0, 20:0, and 22:0) | 9-year, 5-year, incident T2D | Wigger et al. (2017) [65] |
| 1557 multi-ethnic adults, the Dallas Heart Study | 1557 participants without T2D | Short-chain saturated ceramide (C16:0, 18:0), longer chain polyunsaturated ceramides (C24:2, 30:10, and 32:11) | 7-year, incident diabetes | Neeland et al. (2018) [64] |
| American Indian in SHFS | 2086 participants without diabetes | Higher ceramide species (C16:0, 18:0, 20:0, and 22:0); sphingomyelin; GluCer; LacCer | 5.4-year, pre-diabetes | Lemaitre et al. (2018) [63] |
| Human studies investigating the role of ceramides on CAD | ||||
| CAD patients | 33 CAD patients | Higher ceramides | CAD | Mello et al. (2009) [135] |
| Chinese, CAD patients | 304 CAD patients and 52 controls | Higher ceramides, higher sphingomyelins | ACS | Pan et al. (2014) [69] |
| German, CAD patients, LURIC | 258 CAD patients and 187 controls | Higher ceramide species (C16:0 and 18:0); LacCer, GluCer, globotriaosylceramide | 3-year, cardiovascular death | Tarasov et al. (2014) [71] |
| European, CAD patients, ATHEROREMO-IVUS | 581 CAD patients | Higher ceramide species (C16:0, 24:0, and 16:0/24:0 ratio); LacCer (C18:0) | 1-year, vulnerable plaque characteristics, MACE | Cheng et al. (2015) [66] |
| Chinese, CHF patients | 423 CHF patients | Higher ceramides | 4.4-year, mortality | Yu et al. (2015) [70] |
| Healthy volunteers, BLSA | 433 participants | Higher ceramide species (C18:0, 20:0, and 24:1); higher dihydroceramides | Lower aerobic capacity | Fabbri et al. (2016) [72] |
| Three CAD cohorts: Corogene (Finnish); BECAC (Norway); PUM-ACS (Swiss) | 80 stable CAD and 80 controls; 51 stable CAD and 1586 controls; 81 ACS and 1506 controls | Higher ceramide species (C16:0, 18:0, 24:1, and 16:0/24:0 ratio) | 2.5-year; 4.6-year; 1-year. Cardiovascular death | Laaksonen et al. (2016) [67] |
| Finnish, healthy individuals | 8101 | Higher ceramide species (C16:0, 18:0, 24:1 and ratios with 24:0) | 13-year, MACE | Havulinna et al. (2016) [73] |
| European Caucasians, the PREDIMED trial | 980 participants | Higher ceramide species (C16:0, C22:0, C24:0 and C24:1) | 4.5-year, non-fatal AMI, non-fatal stroke, or cardiovascular death | Wang et al. (2017) [74] |
| Participants before nonurgent coronary angiography | 265 CAD and 230 No CAD | Higher ceramide species (C16:0, 18:0, 24:1 and ratios with 24:0) | 12.8-year, MACE | Meeusen et al. (2018) [68] |
| Two cohorts: FHS and SHIP participants | 2642 and 3134 | Lower plasma C24:0/C16:0, C22:0/C16:0 ceramide ratios | 6-year and 8.24-year, incident CAD and total mortality | Peterson et al. (2018) [15] |
| Human studies investigating the role of glycosphingolipids | ||||
| Autopsy (died with atherosclerosis) | 3 | Higher concentration of GluCer and LacCer in arterial tissues | Atherosclerotic plaque | Chatterjee et al. (1997) [105] |
| CAD patients | 140 CAD patients and 80 controls | Higher dihexosylceramide | Unstable CAD | Meikle et al. (2011) [109] |
| Human studies investigating the role of S1P | ||||
| CAD patients | 126 mild, 102 intermediate, and 90 severe CAD | Higher S1P | CAD | Deutschman et al. (2003) [136] |
| MI patients | 22 MI patients and 21 controls | Lower S1P | MI | Knapp et al. (2009) [125] |
| CAD patients | 83 MI, 95 stable CAD, and 85 healthy controls | Lower HDL-bound S1P, higher non-HDL-bound S1P | Stable CAD and MI | Sattler et al. (2010) [126] |
| Danes, CCHS | 95 CAD and 109 No CAD | Lower HDL-bound S1P, dihydro-S1P and ceramide (C24:1) | CAD | Argraves et al. (2011) [127] |
| MI patients | 32 MI and 32 controls | Lower S1P | MI | Knapp et al. (2013) [128] |
| CAD patients | 59 | Lower HDL-bound S1P | 0.5-year, CAD | Katherine Sattler et al. (2014) [129] |
| Patients with ischemic heart disease | 74 | Lower S1P and sphingomyelins | Reduced left ventricular ejection fraction | Polzin et al. (2017) [130] |
Abbreviations: ACS, acute coronary syndrome; ATHEROREMO-IVUS, Atherosclerosis Intravascular Ultrasound Study; BECAC, Bergen Coronary Angiography Cohort; BLSA, Baltimore Longitudinal Study of Aging study; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCHS, Copenhagen City Heart Study; CHF, chronic heart failure; CoLaus, Cohorte Lausannoise study; DESIR, Data from the Epidemiological Study on the Insulin Resistance Syndrome; FHS, Framingham Heart Study; LURIC, Ludwigshafen Risk and Cardiovascular Health; MACE, major adverse cardiac events (defined as all-cause mortality, ACS and unplanned coronary revascularization); MESA, Multi-Ethnic Study of Atherosclerosis; MI, myocardial infarction; PREDIMED, the Prevencion con Dieta Mediterranea; SHFS, Strong Heart Family Study; SHIP, Study of Health in Pomerania; SPUM-ACS, Special Program University Medicine-Inflammation in Acute Coronary Syndrome; T2D, type 2 diabetes.
D2 group inhibitors
The D2 group inhibitors with more potent and better performance on inhibiting SMS2 than D-609 were found by applying structure-based virtual screening [48]. However, D2-series are still not applicable drugs as they possess a toxic α-aminonitrile group [25].
2-Quinolone SMS2 inhibitors
Recently, Adachi et al. [25] established a high throughput screening-compatible assay condition and identified a 2-quinolone derivative as an SMS2 selective inhibitor. There was no further specific experimental data to evaluate their safety and efficacy.
4-benzyloxybenzo[d]isoxazole-3-amine
Very recently, 4-benzyloxybenzo[d]isoxazole-3-amine derivatives were identified as potent SMS2 selective inhibitors [49]. What was more, one of the compounds were proved to significantly attenuate chronic inflammation in db/db mice after oral dosing for 6 weeks. Undoubtedly, the study provides robust evidence of developing selective SMS2 inhibitors to prevent inflammation-associated diseases, e.g. atherosclerosis.
SMases as potential therapeutic targets for atherosclerosis
There are three types of SMases depending on the optimum pH value: acid, neutral, and alkaline. The secretory form of acid SMase could convert sphingomyelins in lipoproteins into ceramide. The acid SMase was believed to be one potent inducer of subendothelial lipoprotein aggregation and foam cell formation [50]. It remains controversial whether the level of circulating acid SMase activity affects atherosclerosis development. In 2008, Devlin et al. [51] compared acid SMase gene-deficient (Asm−/−) mice and non-deficient (Asm+/+) mice on the ApoE−/− and LDLr−/− backgrounds, and found the absence of acid SMase strikingly contributed to reductions in lipoprotein retention within early lesions. But in 2011, Leger et al. [52] found no expected accelerated or exacerbated lesions in ApoE−/− mice which concurrently overexpressed acid SMase by injecting recombinant adeno-associated virus. Several compounds have been tested as acid SMase inhibitors, such as the tricyclic antidepressants (desipramine, imipramine, and amitryptiline), SMA-7, and siramesine [53,54]. To date, no references were found to demonstrate that any kind of SMase inhibitors has definite effects on anti-atherosclerosis.
Ceramides and atherosclerosis
Human studies investigating the role of ceramides
Ceramides are found accumulated in atherosclerotic lesions and in obesity [55]. They are involved in insulin resistance [56], lipoprotein uptake and aggregation [57], vascular tone [58], inflammation, oxidative stress, and apoptosis. Moreover, circulating ceramides are correlated strongly with diabetes and some specific species have served as predictive biomarkers of future adverse cardiovascular events [16]. Here, we summarize the related evidence.
Since 2009, several small cross-sectional studies showed diabetic patients had elevated plasma ceramide levels [59–62]. Lately, prospective studies based on large population were reported, further revealed that higher concentrations of several ceramide species (e.g. C16:0, 18:0, and dihydroceramides) were associated with fasting insulin levels [63,64] and an increased risk of future diabetes in individuals without diabetes [65]. However, after adjustment for age, sex, and race, none of the ceramide species was independently associated with incident type 2 diabetes [64].
In studies of patients with CAD, ceramide species (C16:0, 18:0, 22:0, 22:0, 24:0, and 24:1) were quantitated, and also used in ratios to perform risk estimation for plaque instability [66], adverse CAD incidents [67–69], and future mortality [15,70,71]. In studies of healthy individuals, serum ceramides were strongly associated with lower aerobic capacity [72] and could also forecast adverse cardiovascular outcomes [73,74]. Research findings from different groups were not totally consistent, and whether C24-ceramides were cardioprotective remained controversial [24]. The differences may relate to patient selection and different quantitation methods. In general, these strongly supportive evidence of plasma ceramides driving cardiometabolic dysfunction provided the basis for developing ceramide-reducing interventions.
SPT as a potential therapeutic target for atherosclerosis
Myriocin, a commonly used SPT inhibitor (also known as thermozymocidin) inhibits the first step in the de novo synthesis pathway, originating from a traditional Chinese medicine called Isaria sinclairii, classified as a fungal species. Park et al. first investigated the beneficial effects of myriocin in ApoE−/− mice [75]. Myriocin administration could dramatically prevent the progression of atherosclerotic lesions and even regress the pre-existing plaques, with lower plasma lipid levels, including total cholesterols (TC), triglycerides (TG), ceramides, sphingomyelins, S1P, sphingosine, and glycosphingolipids [76–79]. Besides, myriocin was found to improve insulin sensitivity [80–82], ameliorate hepatic lipid accumulation and further reverse NAFLD [83,84]. Because myriocin inhibits the initial step in the synthesis of a number of sphingolipids, the identity of the critical species is unknown. Nevertheless, the experimental results supported the hypothesis that myriocin, an SPT inhibitor, could be a novel therapeutic drug for atherosclerosis and related diseases.
CerSs as potential therapeutic targets for atherosclerosis
Six fatty acyl selective CerSs (CerS1–6) exist in mammals, distributed in distinct tissues [85]. The regulation of CerSs is elaborate at multiple levels, and the enzyme activity may present inconsistently with the mRNA or protein expression levels. Mutations in CerSs genes or deregulation in the CerSs’ contents and enzyme activity are all correlated with human disease. Over the past few years, each CerS knockout was established in mice, showing that specific CerS deactivation may cause serious impacts and may be lethal, such as CerS1-null mice exhibiting Purkinje cell death [86], CerS2-null mice generating myelin sheath defects and hepatocellular carcinomas [87], CerS3-null mice dying shortly after birth [88], and CerS4-null mice developing alopecia [89]. Relatively, CerS5/6-null mice showed a mild phenotype, presenting some behavioral abnormalities [90].
Turpin et al. [91] measured the gene expression of CerS1, 2, 4, 5, and 6 in human adipose tissues and identified that only CerS6 expression was positively correlated with obesity. Further, they generated conventional CerS6-deficient mice, as well as specific brown adipose tissue and liver CerS6 deletion mice, and then demonstrated CerS6 ablation could up-regulate β-oxidation and increase lipid utilization [91]. But so far, there are no conclusive data proving that targetting specifically unique CerS could benefit atherosclerotic degression, and no pharmacological inhibitors with a high degree selectivity for one certain CerS are available. Given that inhibiting CerS6 is good for obesity and diabetes, it will probably restrain atherosclerosis development, but needs further novel studies to provide favorable evidence.
DESs as potential therapeutic targets for atherosclerosis
DESs catalyze the last step in de novo ceramide biosynthesis, which is responsible for the conversion of dihydroceramide into ceramide. The dominant isoform is DES1, distributed in most tissues. In the last few years, multiple publications have demonstrated that dihydroceramides are implicated in a far wider spectrum of biological functions than previously thought [92]. Heterozygous deletion of DES1 in mice was also demonstrated to prevent diet-induced vascular dysfunction and hypertension in mice [93]. Importantly, pharmacological inhibition of DES1 protected humans from obesity and insulin resistance. The most notable inhibitory compound is fenretinide, which has been tested in several clinical trials [94]. Fenretinide treatment could positively balance the metabolic profile by improving insulin sensitivity in overweight premenopausal women [95]. Also, long-term therapy with fenretinide could alleviate diet-induced adiposity and dyslipidemia and prevent hepatic steatosis in mice [96–98]. Altogether, although there was no direct evidence on inhibited Des1 preventing atherosclerosis, it is reasonable to hypothesize DES1 as a effective target for normalizing vascular homeostasis by controlling ceramide production.
CDase as a potential therapeutic target for atherosclerosis
Since inhibitors targetting ceramide biosynthesis are potential means for the treatment of metabolic syndrome, promoting ceramide degradation may provide similar benefits. Deacylation of ceramide species is initiated by the family of enzymes called CDase. Chavez et al. [99] demonstrated that overexpression CDase negated the inhibitory effects of exogenous free fatty acids on muscle insulin sensitivity through blocking ceramide accumulation in vitro. Holland et al. [100] found that adiponectin, a protein hormone has antidiabetic and cardioprotective properties, could stimulate CDase activity and further lower cellular ceramides. CDase was found to have some homology with the adiponectin receptors, AdipoR1 and AdipoR2. In vivo studies, targetted induction of ceramide degradation in adipose tissue or liver by overexpressing transgenic CDase was found sufficient to recapitulate most adiponectin actions [14]. Moreover, overexpression of AdipoR1 or AdipoR2 in either the adipocyte or hepatocyte revealed enhanced CDase activation, improved hyperglycemia and glucose intolerance, while opposing hepatic steatosis [101]. Together, adiponectin exerted its metabolic improvement functions through CDase signaling [102]. These findings support the strategy of CDase replacement as a potential treatment for atherosclerosis.
Glycosphingolipids and atherosclerosis
Human studies investigating the role of glycosphingolipids
Glycosphingolipids are extremely diverse, composed of hydrophobic ceramide scaffolds and hydrophilic sugar chains. Glycosyl groups are different, such as d-glucose, d-galactose, d-acetylglucosamine, d-acetylgalactosamine, l-fucose, d-mannose, and sialic acid. Sphingoglycolipids can be generally divided into cerebrosides, sulphatides, globosides, and gangliosides. According to the number of glycosides, they can be divided into monohexosylceramide (MHC), dihexosylceramide (DHC), trihexosylceramide (THC), and tetrahexosylceramide.
Associating glycosphingolipids with atherosclerosis was based on the following observations: gangliosides [103], GluCer and LacCer accumulate in the atherosclerotic plaques [104,105]; GluCer and LacCer stimulate the proliferation of aortic smooth muscle [106]; GluCer and LacCer suppress apoE production in macrophages and cholesterol-loaded foam cells [107] and LacCer stimulates the recruitment of monocytes to the endothelium [108]. Recently, specific plasma glycosphingolipids were identified as discriminatory risk-associated lipids for unstable CAD and CAD mortality, such as DHC [109], GluCer and LacCer [66,71]. Our research team successfully separated GalCer and GluCer, a pair of isomers [110]. We discovered that the plasma GalCer levels were increasing in atherosclerotic patients, rather than GluCer. Although the enormous number of distinct glycosphingolipid species has made it difficult to determine which one is critical, it is suggested that inhibiting glycosphingolipid synthesis may be an effective approach for the treatment of atherosclerosis.
Glycosphingolipid synthase inhibitors
d-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol
d-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (D-PDMP), an analog of GluCer, could inhibit both GCS and LCS activity [111]. Chatterjee et al. [112] confirmed that oral D-PDMP could dose-dependently ameliorate atherosclerosis and vascular stiffness in both ApoE−/− mice and rabbits fed a high-fat diet. They further proposed that D-PDMP could slow the progression of cardiac hypertrophy in ApoE−/− mice by inhibiting mitogen-activated protein kinase (MAPK) phosphorylation [113,114]. Thus, D-PDMP could be accepted as a potential desirable compound for treating cardiovascular diseases.
N-(5-adamantane-1-yl-methoxy)-pentyl-1-deoxynoijirimycin
The iminosugar N-(5-adamantane-1-yl-methoxy)-pentyl-1-deoxynoijirimycin (AMP-DNM) is another inhibitor, specifically inhibiting the activity of GCS. Aerts et al. [115] found this small molecule inhibitor could improve both muscle and hepatic insulin sensitivity in rodent models. Subsequent studies reported that AMP-DNM treatment could also reverse hepatic steatosis [116], improve adipocyte function, and reduce inflammation in leptin-deficient obese mice [117]. Bietrix et al. [118] evaluated the beneficial effect of AMP-DNM on atherosclerosis development in both APOE*3 Leiden mice and LDLr−/− mice. Collectively, AMP-DNM can be suggested as a possible valid approach for the prevention or treatment of atherosclerosis.
d-threo-1-ethylendioxyphenyl-2-palmitoylamino-3-pyrrolidino-propanol
d-threo-1-ethylendioxyphenyl-2-palmitoylamino-3-pyrrolidino-propanol (EtDO-P4), another specific GCS inhibitor, can reduce plasma and tissue glycosphingolipid concentrations [119]. Glaros et al. [120] assessed the impact of EtDO-P4 on atherosclerosis in apoE−/− mice. Unexpectedly, EtDO-P4 administration did not result in decreased lesion areas, although the plasma GluCer and LacCer concentrations were reduced. Unlike D-PDMP and AMP-DNM, EtDO-P4 did not affect plasma cholesterol or TG levels. At present, it is not clear whether one or more glycosphingolipids take part in atherosclerosis, and whether inhibition of glycosphingolipid synthesis per se has an antiatherogenic impact.
S1P and atherosclerosis
Human studies investigating the role of S1P
S1P is a bioactive lipid, primarily carried by apoM on HDL, and signals its G protein-coupled receptors, named S1PR1-5 [5], S1P is degraded by two pathways: dephosphorylation by S1P phosphatases (SPP1/2) and irreversible cleavage by S1P lyase (SPL). S1P has dual nature in the pathogenesis of atherosclerosis: S1P preserves endothelium via S1PR1/3 [121]; inhibits smooth muscle cells migration via S1PR2; and possesses anti-inflammatory properties via S1PR4 [122]; while S1P also promotes inflammatory monocyte/macrophage recruitment through S1PR2/3 [123,124]. Although it is yet not concensus, several clinical data reported that plasma S1P concentrations were negatively associated with prevalence and severity of CAD and myocardial infarction [125–130].
S1P receptor agonists
Nofer et al. [131] reported that 2-amino-2-[2-(4-octylphenyl)ethyl] propane-1, 3-diolhydrochloride (FTY720), a synthetic S1P analog targetting S1PR1, S1PR3, S1PR4, and S1PR5, could dose-dependently retard the progression of atherosclerosis in LDLr−/− mice. Another study team reached a similar conclusion with ApoE−/− mice [132]. As FTY720 is a non-selective S1P analog, Nofer et al. [131] further investigated the antiatherogenic effects of S1PR1-selective agonists, such as CYM5442 and KRP-203, and demonstrated that activating S1PR1 at least partially mediated atheroprotective effects [133,134]. Thus, S1P analogs may be promising bullets against atherosclerosis.
Conclusions
The worldwide burden of metabolic diseases, especially atherosclerotic disorder, is staggering. Understanding the precise roles of sphingolipid metabolites and related enzymes on the development of atherosclerosis will invite new available treatments. This brief review mainly focussed on seeking therapeutic targets for atherosclerosis from the complicated sphingolipids metabolism. The above possible targets or inhibitors shed significant light on those patients suffering from atherosclerosis as well as related diseases, although further investigation and refining is necessary.
Abbreviations
- AMP-DNM
N-(5-adamantane-1-yl-methoxy)-pentyl-1-deoxynoijirimycin
- Apo
apolipoprotein
- CABG
coronary artery bypass grafting
- CAD
coronary artery disease
- CDase
ceramidase
- CERK
ceramide kinase
- CerS
ceramide synthase
- CERT
ceramide transfer protein
- CoA
coenzyme A
- CPE
ceramide phosphoethanolamine
- CPTP
C1P transfer protein
- C1P
ceramide-1-phosphate
- DES
dihydroceramide desaturase
- DHC
dihexosylceramide
- D-PDMP
d-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol
- D-609
tricyclodecan-9-yl-xanthogenate
- ER
endoplasmic reticulum
- EtDO-P4
d-threo-1-ethylendioxyphenyl-2-palmitoylamino-3-pyrrolidino-propanol
- FAPP2
four-phosphate adapter protein 2
- FTY720
2-amino-2-[2-(4-octylphenyl)ethyl] propane-1, 3-diolhydrochloride
- GalCer
galactosylceramide synthase
- GalT-2
glucosylceramide β1→4 galactosyltransferase
- GCS
glucosylceramide synthase
- GLTP
glycolipid transfer protein
- GluCer
glucosylceramide
- HDL
high-density lipoprotein
- KSR
3-ketosphinganine reductase
- LacCer
lactosylceramide
- LCS
lactose ceramide synthase
- LDL
low-density lipoprotein
- MAPK
mitogen-activated protein kinase
- MHC
monohexosylceramide
- MTP
microsomal triglyceride transfer protein
- NAFLD
non-alcoholic fatty liver disease
- SMase
sphingomyelinase
- SMS
sphingomyelin synthase
- SMSr
sphingomyelin synthase-related protein
- SphK
sphingosine kinase
- SPL
S1P lyase
- SPNS2
spinster homolog 2
- SPP
S1P phosphatase
- SPT
serine palmitoyltransferase
- S1P
sphingosine-1-phosphate
- TC
total cholesterol
- TG
triglyceride
- THC
trihexosylceramide
- VLDL
very-low-density lipoprotein
Funding
This work was supported by the Interdisciplinary Medicine Seed Fund of Peking University [grant number BMU2017MC005].
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Maula T., Al Sazzad M.A. and Slotte J.P. (2015) Influence of hydroxylation, chain length, and chain unsaturation on bilayer properties of ceramides. Biophys. J. 109, 1639–1651 10.1016/j.bpj.2015.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castro B.M., Prieto M. and Silva L.C. (2014) Ceramide: a simple sphingolipid with unique biophysical properties. Prog. Lipid Res. 54, 53–67 10.1016/j.plipres.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 3.Maceyka M. and Spiegel S. (2014) Sphingolipid metabolites in inflammatory disease. Nature 510, 58–67 10.1038/nature13475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galadari S., Rahman A., Pallichankandy S. and Thayyullathil F. (2015) Tumor suppressive functions of ceramide: evidence and mechanisms. Apoptosis 20, 689–711 10.1007/s10495-015-1109-1 [DOI] [PubMed] [Google Scholar]
- 5.Hla T. and Dannenberg A.J. (2012) Sphingolipid signaling in metabolic disorders. Cell Metab. 16, 420–434 10.1016/j.cmet.2012.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meikle P.J. and Summers S.A. (2017) Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat. Rev. Endocrinol. 13, 79–91 10.1038/nrendo.2016.169 [DOI] [PubMed] [Google Scholar]
- 7.Iqbal J., Walsh M.T., Hammad S.M. and Hussain M.M. (2017) Sphingolipids and lipoproteins in health and metabolic disorders. Trends Endocrinol. Metab. 28, 506–518 10.1016/j.tem.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaurasia B. and Summers S.A. (2015) Ceramides - lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab. 26, 538–550 10.1016/j.tem.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 9.Hannun Y.A. and Obeid L.M. (2018) Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 19, 175–191 10.1038/nrm.2017.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chavez J.A. and Summers S.A. (2012) A ceramide-centric view of insulin resistance. Cell Metab. 15, 585–594 10.1016/j.cmet.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 11.Hammad S.M., Pierce J.S., Soodavar F., Smith K.J., Al Gadban M.M., Rembiesa B.. et al. (2010) Blood sphingolipidomics in healthy humans: impact of sample collection methodology. J. Lipid Res. 51, 3074–3087 10.1194/jlr.D008532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iqbal J., Walsh M.T., Hammad S.M., Cuchel M., Tarugi P., Hegele R.A.. et al. (2015) Microsomal triglyceride transfer protein transfers and determines plasma concentrations of ceramide and sphingomyelin but not glycosylceramide. J. Biol. Chem. 290, 25863–25875 10.1074/jbc.M115.659110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundy S.M. (2016) Metabolic syndrome update. Trends Cardiovasc. Med. 26, 364–373 10.1016/j.tcm.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 14.Xia J.Y., Holland W.L., Kusminski C.M., Sun K., Sharma A.X., Pearson M.J.. et al. (2015) Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 22, 266–278 10.1016/j.cmet.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson L.R., Xanthakis V., Duncan M.S., Gross S., Friedrich N., Volzke H.. et al. (2018) Ceramide remodeling and risk of cardiovascular events and mortality. J. Am. Heart Assoc. 7, 10.1161/JAHA.117.007931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Summers S.A. (2018) Could ceramides become the new cholesterol? Cell Metab. 27, 276–280 10.1016/j.cmet.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 17.Holland W.L. and Summers S.A. (2018) Strong heart, low ceramides. Diabetes 67, 1457–1460 10.2337/dbi18-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang X.C., Paultre F., Pearson T.A., Reed R.G., Francis C.K., Lin M.. et al. (2000) Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 20, 2614–2618 10.1161/01.ATV.20.12.2614 [DOI] [PubMed] [Google Scholar]
- 19.Kummerow F.A., Cook L.S., Wasowicz E. and Jelen H. (2001) Changes in the phospholipid composition of the arterial cell can result in severe atherosclerotic lesions. J. Nutr. Biochem. 12, 602–607 10.1016/S0955-2863(01)00181-4 [DOI] [PubMed] [Google Scholar]
- 20.Schlitt A., Blankenberg S., Yan D., von Gizycki H., Buerke M., Werdan K.. et al. (2006) Further evaluation of plasma sphingomyelin levels as a risk factor for coronary artery disease. Nutr. Metab. 3, 5 10.1186/1743-7075-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X., Sun A., Zou Y., Ge J., Lazar J.M. and Jiang X.C. (2011) Impact of sphingomyelin levels on coronary heart disease and left ventricular systolic function in humans. Nutr. Metab. 8, 25 10.1186/1743-7075-8-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson J.C., Jiang X.C., Tabas I., Tall A. and Shea S. (2006) Plasma sphingomyelin and subclinical atherosclerosis: findings from the multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 163, 903–912 10.1093/aje/kwj140 [DOI] [PubMed] [Google Scholar]
- 23.Yeboah J., McNamara C., Jiang X.C., Tabas I., Herrington D.M., Burke G.L.. et al. (2009) Association of plasma sphingomyelin levels and incident coronary heart disease events in an adult population: multi-ethnic study of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 30, 628–633 10.1161/ATVBAHA.109.199281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sigruener A., Kleber M.E., Heimerl S., Liebisch G., Schmitz G. and Maerz W. (2014) Glycerophospholipid and sphingolipid species and mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. PLoS ONE 9, e85724 10.1371/journal.pone.0085724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adachi R., Ogawa K., Matsumoto S.I., Satou T., Tanaka Y., Sakamoto J.. et al. (2017) Discovery and characterization of selective human sphingomyelin synthase 2 inhibitors. Eur. J. Med. Chem. 136, 283–293 10.1016/j.ejmech.2017.04.067 [DOI] [PubMed] [Google Scholar]
- 26.Wang X., Dong J., Zhao Y., Li Y. and Wu M. (2011) Adenovirus-mediated sphingomyelin synthase 2 increases atherosclerotic lesions in ApoE KO mice. Lipids Health Dis. 10, 7 10.1186/1476-511X-10-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y.R., Dong J.B., Li Y. and Wu M.P. (2012) Sphingomyelin synthase 2 over-expression induces expression of aortic inflammatory biomarkers and decreases circulating EPCs in ApoE KO mice. Life Sci. 90, 867–873 10.1016/j.lfs.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 28.Li Z., Hailemariam T.K., Zhou H., Li Y., Duckworth D.C., Peake D.A.. et al. (2007) Inhibition of sphingomyelin synthase (SMS) affects intracellular sphingomyelin accumulation and plasma membrane lipid organization. Biochim. Biophys. Acta 1771, 1186–1194 10.1016/j.bbalip.2007.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hailemariam T.K., Huan C., Liu J., Li Z., Roman C., Kalbfeisch M.. et al. (2008) Sphingomyelin synthase 2 deficiency attenuates NFkappaB activation. Arterioscler. Thromb. Vasc. Biol. 28, 1519–1526 10.1161/ATVBAHA.108.168682 [DOI] [PubMed] [Google Scholar]
- 30.Liu J., Zhang H., Li Z., Hailemariam T.K., Chakraborty M., Jiang K.. et al. (2009) Sphingomyelin synthase 2 is one of the determinants for plasma and liver sphingomyelin levels in mice. Arterioscler. Thromb. Vasc. Biol. 29, 850–856 10.1161/ATVBAHA.109.185223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J., Huan C., Chakraborty M., Zhang H., Lu D., Kuo M.S.. et al. (2009) Macrophage sphingomyelin synthase 2 deficiency decreases atherosclerosis in mice. Circ. Res. 105, 295–303 10.1161/CIRCRESAHA.109.194613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan Y., Shi F., Liu J., Dong J., Bui H.H., Peake D.A.. et al. (2010) Selective reduction in the sphingomyelin content of atherogenic lipoproteins inhibits their retention in murine aortas and the subsequent development of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 30, 2114–2120 10.1161/ATVBAHA.110.213363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z., Zhang H., Liu J., Liang C.P., Li Y., Li Y.. et al. (2011) Reducing plasma membrane sphingomyelin increases insulin sensitivity. Mol. Cell. Biol. 31, 4205–4218 10.1128/MCB.05893-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugimoto M., Shimizu Y., Zhao S., Ukon N., Nishijima K., Wakabayashi M.. et al. (2016) Characterization of the role of sphingomyelin synthase 2 in glucose metabolism in whole-body and peripheral tissues in mice. Biochim. Biophys. Acta 1861, 688–702 10.1016/j.bbalip.2016.04.019 [DOI] [PubMed] [Google Scholar]
- 35.Li Y., Dong J., Ding T., Kuo M.S., Cao G., Jiang X.C.. et al. (2013) Sphingomyelin synthase 2 activity and liver steatosis: an effect of ceramide-mediated peroxisome proliferator-activated receptor gamma2 suppression. Arterioscler. Thromb. Vasc. Biol. 33, 1513–1520 10.1161/ATVBAHA.113.301498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z., Fan Y., Liu J., Li Y., Huan C., Bui H.H.. et al. (2012) Impact of sphingomyelin synthase 1 deficiency on sphingolipid metabolism and atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 32, 1577–1584 10.1161/ATVBAHA.112.251538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yano M., Watanabe K., Yamamoto T., Ikeda K., Senokuchi T., Lu M.. et al. (2011) Mitochondrial dysfunction and increased reactive oxygen species impair insulin secretion in sphingomyelin synthase 1-null mice. J. Biol. Chem. 286, 3992–4002 10.1074/jbc.M110.179176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu M.H., Takemoto M., Watanabe K., Luo H., Nishimura M., Yano M.. et al. (2012) Deficiency of sphingomyelin synthase-1 but not sphingomyelin synthase-2 causes hearing impairments in mice. J. Physiol. 590, 4029–4044 10.1113/jphysiol.2012.235846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong L., Watanabe K., Itoh M., Huan C.R., Tong X.P., Nakamura T.. et al. (2012) CD4+ T-cell dysfunctions through the impaired lipid rafts ameliorate concanavalin A-induced hepatitis in sphingomyelin synthase 1-knockout mice. Int. Immunol. 24, 327–337 10.1093/intimm/dxs008 [DOI] [PubMed] [Google Scholar]
- 40.Yano M., Yamamoto T., Nishimura N., Gotoh T., Watanabe K., Ikeda K.. et al. (2013) Increased oxidative stress impairs adipose tissue function in sphingomyelin synthase 1 null mice. PLoS ONE 8, e61380 10.1371/journal.pone.0061380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wittmann A., Grimm M.O., Scherthan H., Horsch M., Beckers J., Fuchs H.. et al. (2016) Sphingomyelin synthase 1 is essential for male fertility in mice. PLoS ONE 11, e0164298 10.1371/journal.pone.0164298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brandan Y.R., Guaytima E.D.V., Favale N.O., Pescio L.G., Sterin-Speziale N.B. and Marquez M.G. (2018) The inhibition of sphingomyelin synthase 1 activity induces collecting duct cells to lose their epithelial phenotype. Biochim. Biophys. Acta 1865, 309–322 10.1016/j.bbamcr.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 43.Ding T., Kabir I., Li Y., Lou C., Yazdanyar A., Xu J.. et al. (2015) All members in the sphingomyelin synthase gene family have ceramide phosphoethanolamine synthase activity. J. Lipid Res. 56, 537–545 10.1194/jlr.M054627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bickert A., Ginkel C., Kol M., vom Dorp K., Jastrow H., Degen J.. et al. (2015) Functional characterization of enzymes catalyzing ceramide phosphoethanolamine biosynthesis in mice. J. Lipid Res. 56, 821–835 10.1194/jlr.M055269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adibhatla R.M., Hatcher J.F. and Gusain A. (2012) Tricyclodecan-9-yl-xanthogenate (D609) mechanism of actions: a mini-review of literature. Neurochem. Res. 37, 671–679 10.1007/s11064-011-0659-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez-Roura A., Casas J. and Llebaria A. (2002) Synthesis and phospholipase C inhibitory activity of D609 diastereomers. Lipids 37, 401–406 10.1007/s1145-002-0908-0 [DOI] [PubMed] [Google Scholar]
- 47.Taniguchi M. and Okazaki T. (2014) The role of sphingomyelin and sphingomyelin synthases in cell death, proliferation and migration-from cell and animal models to human disorders. Biochim. Biophys. Acta 1841, 692–703 10.1016/j.bbalip.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 48.Deng X., Lin F., Zhang Y., Li Y., Zhou L., Lou B.. et al. (2014) Identification of small molecule sphingomyelin synthase inhibitors. Eur. J. Med. Chem. 73, 1–7 10.1016/j.ejmech.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 49.Mo M., Yang J., Jiang X.C., Cao Y., Fei J., Chen Y.. et al. (2018) Discovery of 4-Benzyloxybenzo[d]isoxazole-3-amine derivatives as highly selective and orally efficacious human sphingomyelin synthase 2 inhibitors that reduce chronic inflammation in db/db mice. J. Med. Chem. 61, 8241–8254 10.1021/acs.jmedchem.8b00727 [DOI] [PubMed] [Google Scholar]
- 50.Marathe S., Choi Y., Leventhal A.R. and Tabas I. (2000) Sphingomyelinase converts lipoproteins from apolipoprotein E knockout mice into potent inducers of macrophage foam cell formation. Arterioscler. Thromb. Vasc. Biol. 20, 2607–2613 10.1161/01.ATV.20.12.2607 [DOI] [PubMed] [Google Scholar]
- 51.Devlin C.M., Leventhal A.R., Kuriakose G., Schuchman E.H., Williams K.J. and Tabas I. (2008) Acid sphingomyelinase promotes lipoprotein retention within early atheromata and accelerates lesion progression. Arterioscler. Thromb. Vasc. Biol. 28, 1723–1730 10.1161/ATVBAHA.108.173344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leger A.J., Mosquea L.M., Li L., Chuang W., Pacheco J., Taylor K.. et al. (2011) Adeno-associated virus-mediated expression of acid sphingomyelinase decreases atherosclerotic lesion formation in apolipoprotein E(-/-) mice. J. Gene Med. 13, 324–332 10.1002/jgm.1575 [DOI] [PubMed] [Google Scholar]
- 53.Adada M., Luberto C. and Canals D. (2016) Inhibitors of the sphingomyelin cycle: sphingomyelin synthases and sphingomyelinases. Chem. Phys. Lipids 197, 45–59 10.1016/j.chemphyslip.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 54.Petersen N.H., Olsen O.D., Groth-Pedersen L., Ellegaard A.M., Bilgin M., Redmer S.. et al. (2013) Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell 24, 379–393 10.1016/j.ccr.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 55.Schissel S.L., Tweedie-Hardman J., Rapp J.H., Graham G., Williams K.J. and Tabas I. (1996) Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J. Clin. Invest. 98, 1455–1464 10.1172/JCI118934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hla T. and Kolesnick R. (2014) C16:0-ceramide signals insulin resistance. Cell Metab. 20, 703–705 10.1016/j.cmet.2014.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walters M.J. and Wrenn S.P. (2008) Effect of sphingomyelinase-mediated generation of ceramide on aggregation of low-density lipoprotein. Langmuir 24, 9642–9647 10.1021/la800714w [DOI] [PubMed] [Google Scholar]
- 58.Sasset L., Zhang Y., Dunn T.M. and Di Lorenzo A. (2016) Sphingolipid de novo biosynthesis: a rheostat of cardiovascular homeostasis. Trends Endocrinol. Metab. 27, 807–819 10.1016/j.tem.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haus J.M., Kashyap S.R., Kasumov T., Zhang R., Kelly K.R., Defronzo R.A.. et al. (2009) Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 58, 337–343 10.2337/db08-1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez X., Goldfine A.B., Holland W.L., Gordillo R. and Scherer P.E. (2013) Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J. Pediatric Endocrinol. Metab. 26, 995–998 10.1515/jpem-2012-0407 [DOI] [PubMed] [Google Scholar]
- 61.Bergman B.C., Brozinick J.T., Strauss A., Bacon S., Kerege A., Bui H.H.. et al. (2015) Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am. J. Physiol. Endocrinol. Metab. 309, E398–408 10.1152/ajpendo.00134.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boon J., Hoy A.J., Stark R., Brown R.D., Meex R.C., Henstridge D.C.. et al. (2013) Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 62, 401–410 10.2337/db12-0686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lemaitre R.N., Yu C., Hoofnagle A., Hari N., Jensen P.N., Fretts A.M.. et al. (2018) Circulating sphingolipids, insulin, HOMA-IR, and HOMA-B: The Strong Heart Family Study. Diabetes 67, 1663–1672 10.2337/db17-1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neeland I.J., Singh S., McGuire D.K., Vega G.L., Roddy T., Reilly D.F.. et al. (2018) Relation of plasma ceramides to visceral adiposity, insulin resistance and the development of type 2 diabetes mellitus: the Dallas Heart Study. Diabetologia 61, 2570–2579 10.1007/s00125-018-4720-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wigger L., Cruciani-Guglielmacci C., Nicolas A., Denom J., Fernandez N., Fumeron F.. et al. (2017) Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Rep. 18, 2269–2279 10.1016/j.celrep.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 66.Cheng J.M., Suoniemi M., Kardys I., Vihervaara T., de Boer S.P., Akkerhuis K.M.. et al. (2015) Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Atherosclerosis 243, 560–566 10.1016/j.atherosclerosis.2015.10.022 [DOI] [PubMed] [Google Scholar]
- 67.Laaksonen R., Ekroos K., Sysi-Aho M., Hilvo M., Vihervaara T., Kauhanen D.. et al. (2016) Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 37, 1967–1976 10.1093/eurheartj/ehw148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meeusen J.W., Donato L.J., Bryant S.C., Baudhuin L.M., Berger P.B. and Jaffe A.S. (2018) Plasma ceramides. Arterioscler. Thromb. Vasc. Biol. 38, 1933–1939 10.1161/ATVBAHA.118.311199 [DOI] [PubMed] [Google Scholar]
- 69.Pan W., Yu J., Shi R., Yan L., Yang T., Li Y.. et al. (2014) Elevation of ceramide and activation of secretory acid sphingomyelinase in patients with acute coronary syndromes. Coron. Artery Dis. 25, 230–235 [DOI] [PubMed] [Google Scholar]
- 70.Yu J., Pan W., Shi R., Yang T., Li Y., Yu G.. et al. (2015) Ceramide is upregulated and associated with mortality in patients with chronic heart failure. Can. J. Cardiol. 31, 357–363 10.1016/j.cjca.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 71.Tarasov K., Ekroos K., Suoniemi M., Kauhanen D., Sylvanne T., Hurme R.. et al. (2014) Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J. Clin. Endocrinol. Metab. 99, E45–E52 10.1210/jc.2013-2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fabbri E., Yang A., Simonsick E.M., Chia C.W., Zoli M., Haughey N.J.. et al. (2016) Circulating ceramides are inversely associated with cardiorespiratory fitness in participants aged 54-96 years from the Baltimore Longitudinal Study of Aging. Aging Cell 15, 825–831 10.1111/acel.12491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Havulinna A.S., Sysi-Aho M., Hilvo M., Kauhanen D., Hurme R., Ekroos K.. et al. (2016) Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 Cohort. Arterioscler. Thromb. Vasc. Biol. 36, 2424–2430 10.1161/ATVBAHA.116.307497 [DOI] [PubMed] [Google Scholar]
- 74.Wang D.D., Toledo E., Hruby A., Rosner B.A., Willett W.C., Sun Q.. et al. (2017) Plasma ceramides, Mediterranean diet, and incident cardiovascular disease in the PREDIMED Trial (Prevencion con Dieta Mediterranea). Circulation 135, 2028–2040 10.1161/CIRCULATIONAHA.116.024261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park T.S., Panek R.L., Mueller S.B., Hanselman J.C., Rosebury W.S., Robertson A.W.. et al. (2004) Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation 110, 3465–3471 10.1161/01.CIR.0000148370.60535.22 [DOI] [PubMed] [Google Scholar]
- 76.Park T.S., Panek R.L., Rekhter M.D., Mueller S.B., Rosebury W.S., Robertson A.. et al. (2006) Modulation of lipoprotein metabolism by inhibition of sphingomyelin synthesis in ApoE knockout mice. Atherosclerosis 189, 264–272 10.1016/j.atherosclerosis.2005.12.029 [DOI] [PubMed] [Google Scholar]
- 77.Park T.S., Rosebury W., Kindt E.K., Kowala M.C. and Panek R.L. (2008) Serine palmitoyltransferase inhibitor myriocin induces the regression of atherosclerotic plaques in hyperlipidemic ApoE-deficient mice. Pharmacol. Res. 58, 45–51 10.1016/j.phrs.2008.06.005 [DOI] [PubMed] [Google Scholar]
- 78.Hojjati M.R., Li Z., Zhou H., Tang S., Huan C., Ooi E.. et al. (2005) Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J. Biol. Chem. 280, 10284–10289 10.1074/jbc.M412348200 [DOI] [PubMed] [Google Scholar]
- 79.Glaros E.N., Kim W.S., Wu B.J., Suarna C., Quinn C.M., Rye K.A.. et al. (2007) Inhibition of atherosclerosis by the serine palmitoyl transferase inhibitor myriocin is associated with reduced plasma glycosphingolipid concentration. Biochem. Pharmacol. 73, 1340–1346 10.1016/j.bcp.2006.12.023 [DOI] [PubMed] [Google Scholar]
- 80.Dekker M.J., Baker C., Naples M., Samsoondar J., Zhang R., Qiu W.. et al. (2013) Inhibition of sphingolipid synthesis improves dyslipidemia in the diet-induced hamster model of insulin resistance: evidence for the role of sphingosine and sphinganine in hepatic VLDL-apoB100 overproduction. Atherosclerosis 228, 98–109 10.1016/j.atherosclerosis.2013.01.041 [DOI] [PubMed] [Google Scholar]
- 81.Zabielski P., Daniluk J., Hady H.R., Markowski A.R., Imierska M., Gorski J.. et al. (2019) The effect of high-fat diet and inhibition of ceramide production on insulin action in liver. J. Cell. Physiol. 234, 1851–1861 10.1002/jcp.27058 [DOI] [PubMed] [Google Scholar]
- 82.Campana M., Bellini L., Rouch C., Rachdi L., Coant N., Butin N.. et al. (2018) Inhibition of central de novo ceramide synthesis restores insulin signaling in hypothalamus and enhances beta-cell function of obese Zucker rats. Mol. Metab. 8, 23–36 10.1016/j.molmet.2017.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kurek K., Piotrowska D.M., Wiesiolek-Kurek P., Lukaszuk B., Chabowski A., Gorski J.. et al. (2014) Inhibition of ceramide de novo synthesis reduces liver lipid accumulation in rats with nonalcoholic fatty liver disease. Liver Int. 34, 1074–1083 10.1111/liv.12331 [DOI] [PubMed] [Google Scholar]
- 84.Kasumov T., Li L., Li M., Gulshan K., Kirwan J.P., Liu X.. et al. (2015) Ceramide as a mediator of non-alcoholic fatty liver disease and associated atherosclerosis. PLoS ONE 10, e0126910 10.1371/journal.pone.0126910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park J.W., Park W.J. and Futerman A.H. (2014) Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim. Biophys. Acta 1841, 671–681 10.1016/j.bbalip.2013.08.019 [DOI] [PubMed] [Google Scholar]
- 86.Zhao L., Spassieva S.D., Jucius T.J., Shultz L.D., Shick H.E., Macklin W.B.. et al. (2011) A deficiency of ceramide biosynthesis causes cerebellar purkinje cell neurodegeneration and lipofuscin accumulation. PLoS Genet. 7, e1002063 10.1371/journal.pgen.1002063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Imgrund S., Hartmann D., Farwanah H., Eckhardt M., Sandhoff R., Degen J.. et al. (2009) Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J. Biol. Chem. 284, 33549–33560 10.1074/jbc.M109.031971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jennemann R., Rabionet M., Gorgas K., Epstein S., Dalpke A., Rothermel U.. et al. (2012) Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum. Mol. Genet. 21, 586–608 10.1093/hmg/ddr494 [DOI] [PubMed] [Google Scholar]
- 89.Ebel P., Imgrund S., Vom Dorp K., Hofmann K., Maier H., Drake H.. et al. (2014) Ceramide synthase 4 deficiency in mice causes lipid alterations in sebum and results in alopecia. Biochem. J. 461, 147–158 10.1042/BJ20131242 [DOI] [PubMed] [Google Scholar]
- 90.Ebel P., Vom Dorp K., Petrasch-Parwez E., Zlomuzica A., Kinugawa K., Mariani J.. et al. (2013) Inactivation of ceramide synthase 6 in mice results in an altered sphingolipid metabolism and behavioral abnormalities. J. Biol. Chem. 288, 21433–21447 10.1074/jbc.M113.479907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Turpin S.M., Nicholls H.T., Willmes D.M., Mourier A., Brodesser S., Wunderlich C.M.. et al. (2014) Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20, 678–686 10.1016/j.cmet.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 92.Rodriguez-Cuenca S., Barbarroja N. and Vidal-Puig A. (2015) Dihydroceramide desaturase 1, the gatekeeper of ceramide induced lipotoxicity. Biochim. Biophys. Acta 1851, 40–50 10.1016/j.bbalip.2014.09.021 [DOI] [PubMed] [Google Scholar]
- 93.Zhang Q.J., Holland W.L., Wilson L., Tanner J.M., Kearns D., Cahoon J.M.. et al. (2012) Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes 61, 1848–1859 10.2337/db11-1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mody N. and McIlroy G.D. (2014) The mechanisms of Fenretinide-mediated anti-cancer activity and prevention of obesity and type-2 diabetes. Biochem. Pharmacol. 91, 277–286 10.1016/j.bcp.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 95.Johansson H., Gandini S., Guerrieri-Gonzaga A., Iodice S., Ruscica M., Bonanni B.. et al. (2008) Effect of fenretinide and low-dose tamoxifen on insulin sensitivity in premenopausal women at high risk for breast cancer. Cancer Res. 68, 9512–9518 10.1158/0008-5472.CAN-08-0553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Preitner F., Mody N., Graham T.E., Peroni O.D. and Kahn B.B. (2009) Long-term Fenretinide treatment prevents high-fat diet-induced obesity, insulin resistance, and hepatic steatosis. Am. J. Physiol. Endocrinol. Metab. 297, E1420–E1429 10.1152/ajpendo.00362.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koh I.U., Jun H.S., Choi J.S., Lim J.H., Kim W.H., Yoon J.B.. et al. (2012) Fenretinide ameliorates insulin resistance and fatty liver in obese mice. Biol. Pharm. Bull. 35, 369–375 10.1248/bpb.35.369 [DOI] [PubMed] [Google Scholar]
- 98.Bikman B.T., Guan Y., Shui G., Siddique M.M., Holland W.L., Kim J.Y.. et al. (2012) Fenretinide prevents lipid-induced insulin resistance by blocking ceramide biosynthesis. J. Biol. Chem. 287, 17426–17437 10.1074/jbc.M112.359950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chavez J.A., Holland W.L., Bar J., Sandhoff K. and Summers S.A. (2005) Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J. Biol. Chem. 280, 20148–20153 10.1074/jbc.M412769200 [DOI] [PubMed] [Google Scholar]
- 100.Holland W.L., Miller R.A., Wang Z.V., Sun K., Barth B.M., Bui H.H.. et al. (2011) Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 17, 55–63 10.1038/nm.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Holland W.L., Xia J.Y., Johnson J.A., Sun K., Pearson M.J., Sharma A.X.. et al. (2017) Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol. Metab. 6, 267–275 10.1016/j.molmet.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reibe-Pal S. and Febbraio M.A. (2017) Adiponectin serenades ceramidase to improve metabolism. Mol. Metab. 6, 233–235 10.1016/j.molmet.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Breckenridge W.C., Halloran J.L., Kovacs K. and Silver M.D. (1975) Increase of gangliosides in atherosclerotic human aortas. Lipids 10, 256–259 10.1007/BF02532490 [DOI] [PubMed] [Google Scholar]
- 104.Mukhin D.N., Chao F.F. and Kruth H.S. (1995) Glycosphingolipid accumulation in the aortic wall is another feature of human atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 15, 1607–1615 10.1161/01.ATV.15.10.1607 [DOI] [PubMed] [Google Scholar]
- 105.Chatterjee S.B., Dey S., Shi W.Y., Thomas K. and Hutchins G.M. (1997) Accumulation of glycosphingolipids in human atherosclerotic plaque and unaffected aorta tissues. Glycobiology 7, 57–65 10.1093/glycob/7.1.57 [DOI] [PubMed] [Google Scholar]
- 106.Bhunia A.K., Han H., Snowden A. and Chatterjee S. (1997) Redox-regulated signaling by lactosylceramide in the proliferation of human aortic smooth muscle cells. J. Biol. Chem. 272, 15642–15649 10.1074/jbc.272.25.15642 [DOI] [PubMed] [Google Scholar]
- 107.Garner B., Mellor H.R., Butters T.D., Dwek R.A. and Platt F.M. (2002) Modulation of THP-1 macrophage and cholesterol-loaded foam cell apolipoprotein E levels by glycosphingolipids. Biochem. Biophys. Res. Commun. 290, 1361–1367 10.1006/bbrc.2002.6356 [DOI] [PubMed] [Google Scholar]
- 108.Gong N., Wei H., Chowdhury S.H. and Chatterjee S. (2004) Lactosylceramide recruits PKCalpha/epsilon and phospholipase A2 to stimulate PECAM-1 expression in human monocytes and adhesion to endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 101, 6490–6495 10.1073/pnas.0308684101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meikle P.J., Wong G., Tsorotes D., Barlow C.K., Weir J.M., Christopher M.J.. et al. (2011) Plasma lipidomic analysis of stable and unstable coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 31, 2723–2732 10.1161/ATVBAHA.111.234096 [DOI] [PubMed] [Google Scholar]
- 110.Li M., Tong X., Lv P., Feng B., Yang L., Wu Z.. et al. (2014) A not-stop-flow online normal-/reversed-phase two-dimensional liquid chromatography–quadrupole time-of-flight mass spectrometry method for comprehensive lipid profiling of human plasma from atherosclerosis patients. J. Chromatogr. A 1372, 110–119 10.1016/j.chroma.2014.10.094 [DOI] [PubMed] [Google Scholar]
- 111.Chatterjee S. and Ghosh N. (1996) Oxidized low density lipoprotein stimulates aortic smooth muscle cell proliferation. Glycobiology 6, 303–311 10.1093/glycob/6.3.303 [DOI] [PubMed] [Google Scholar]
- 112.Chatterjee S., Bedja D., Mishra S., Amuzie C., Avolio A., Kass D.A.. et al. (2014) Inhibition of glycosphingolipid synthesis ameliorates atherosclerosis and arterial stiffness in apolipoprotein E-/- mice and rabbits fed a high-fat and -cholesterol diet. Circulation 129, 2403–2413 10.1161/CIRCULATIONAHA.113.007559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mishra S., Bedja D., Amuzie C., Avolio A. and Chatterjee S. (2015) Prevention of cardiac hypertrophy by the use of a glycosphingolipid synthesis inhibitor in ApoE-/- mice. Biochem. Biophys. Res. Commun. 465, 159–164 10.1016/j.bbrc.2015.07.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mishra S., Bedja D., Amuzie C., Foss C.A., Pomper M.G., Bhattacharya R.. et al. (2015) Improved intervention of atherosclerosis and cardiac hypertrophy through biodegradable polymer-encapsulated delivery of glycosphingolipid inhibitor. Biomaterials 64, 125–135 10.1016/j.biomaterials.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aerts J.M., Ottenhoff R., Powlson A.S., Grefhorst A., van Eijk M., Dubbelhuis P.F.. et al. (2007) Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes 56, 1341–1349 10.2337/db06-1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bijl N., Sokolovic M., Vrins C., Langeveld M., Moerland P.D., Ottenhoff R.. et al. (2009) Modulation of glycosphingolipid metabolism significantly improves hepatic insulin sensitivity and reverses hepatic steatosis in mice. Hepatology 50, 1431–1441 10.1002/hep.23175 [DOI] [PubMed] [Google Scholar]
- 117.van Eijk M., Aten J., Bijl N., Ottenhoff R., van Roomen C.P., Dubbelhuis P.F.. et al. (2009) Reducing glycosphingolipid content in adipose tissue of obese mice restores insulin sensitivity, adipogenesis and reduces inflammation. PLoS ONE 4, e4723 10.1371/journal.pone.0004723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bietrix F., Lombardo E., van Roomen C.P., Ottenhoff R., Vos M., Rensen P.C.. et al. (2010) Inhibition of glycosphingolipid synthesis induces a profound reduction of plasma cholesterol and inhibits atherosclerosis development in APOE*3 Leiden and low-density lipoprotein receptor-/- mice. Arterioscler. Thromb. Vasc. Biol. 30, 931–937 10.1161/ATVBAHA.109.201673 [DOI] [PubMed] [Google Scholar]
- 119.Abe A., Gregory S., Lee L., Killen P.D., Brady R.O., Kulkarni A.. et al. (2000) Reduction of globotriaosylceramide in Fabry disease mice by substrate deprivation. J. Clin. Invest. 105, 1563–1571 10.1172/JCI9711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Glaros E.N., Kim W.S., Rye K.A., Shayman J.A. and Garner B. (2008) Reduction of plasma glycosphingolipid levels has no impact on atherosclerosis in apolipoprotein E-null mice. J. Lipid Res. 49, 1677–1681 10.1194/jlr.E800005-JLR200 [DOI] [PubMed] [Google Scholar]
- 121.Kurano M. and Yatomi Y. (2018) Sphingosine 1-phosphate and atherosclerosis. J. Atherosclerosis Thromb. 25, 16–26 10.5551/jat.RV17010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fettel J., Kuhn B., Guillen N.A., Surun D., Peters M., Bauer R.. et al. (2019) Sphingosine-1-phosphate (S1P) induces potent anti-inflammatory effects in vitro and in vivo by S1P receptor 4-mediated suppression of 5-lipoxygenase activity. FASEB J. 33, 1711–1726 10.1096/fj.201800221R [DOI] [PubMed] [Google Scholar]
- 123.Michaud J., Im D.S. and Hla T. (2010) Inhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammation. J. Immunol. 184, 1475–1483 10.4049/jimmunol.0901586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Keul P., Lucke S., von Wnuck Lipinski K., Bode C., Graler M., Heusch G.. et al. (2011) Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ. Res. 108, 314–323 10.1161/CIRCRESAHA.110.235028 [DOI] [PubMed] [Google Scholar]
- 125.Knapp M., Baranowski M., Czarnowski D., Lisowska A., Zabielski P., Gorski J.. et al. (2009) Plasma sphingosine-1-phosphate concentration is reduced in patients with myocardial infarction. Med. Sci. Monit. 15, CR490–CR493 [PubMed] [Google Scholar]
- 126.Sattler K.J., Elbasan S., Keul P., Elter-Schulz M., Bode C., Graler M.H.. et al. (2010) Sphingosine 1-phosphate levels in plasma and HDL are altered in coronary artery disease. Basic Res. Cardiol. 105, 821–832 10.1007/s00395-010-0112-5 [DOI] [PubMed] [Google Scholar]
- 127.Argraves K.M., Sethi A.A., Gazzolo P.J., Wilkerson B.A., Remaley A.T., Tybjaerg-Hansen A.. et al. (2011) S1P, dihydro-S1P and C24:1-ceramide levels in the HDL-containing fraction of serum inversely correlate with occurrence of ischemic heart disease. Lipids in Health Dis. 10, 70 10.1186/1476-511X-10-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Knapp M., Lisowska A., Zabielski P., Musial W. and Baranowski M. (2013) Sustained decrease in plasma sphingosine-1-phosphate concentration and its accumulation in blood cells in acute myocardial infarction. Prostaglandins Other Lipid Mediat. 106, 53–61 10.1016/j.prostaglandins.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 129.Sattler K., Lehmann I., Graler M., Brocker-Preuss M., Erbel R., Heusch G.. et al. (2014) HDL-bound sphingosine 1-phosphate (S1P) predicts the severity of coronary artery atherosclerosis. Cell. Physiol. Biochem. 34, 172–184 10.1159/000362993 [DOI] [PubMed] [Google Scholar]
- 130.Polzin A., Piayda K., Keul P., Dannenberg L., Mohring A., Graler M.. et al. (2017) Plasma sphingosine-1-phosphate concentrations are associated with systolic heart failure in patients with ischemic heart disease. J. Mol. Cell Cardiol. 110, 35–37 10.1016/j.yjmcc.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 131.Nofer J.R., Bot M., Brodde M., Taylor P.J., Salm P., Brinkmann V.. et al. (2007) FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 115, 501–508 10.1161/CIRCULATIONAHA.106.641407 [DOI] [PubMed] [Google Scholar]
- 132.Keul P., Tolle M., Lucke S., von Wnuck Lipinski K., Heusch G., Schuchardt M.. et al. (2007) The sphingosine-1-phosphate analogue FTY720 reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 27, 607–613 10.1161/01.ATV.0000254679.42583.88 [DOI] [PubMed] [Google Scholar]
- 133.Poti F., Costa S., Bergonzini V., Galletti M., Pignatti E., Weber C.. et al. (2012) Effect of sphingosine 1-phosphate (S1P) receptor agonists FTY720 and CYM5442 on atherosclerosis development in LDL receptor deficient (LDL-R(-)/(-)) mice. Vasc. Pharmacol. 57, 56–64 10.1016/j.vph.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 134.Poti F., Gualtieri F., Sacchi S., Weissen-Plenz G., Varga G., Brodde M.. et al. (2013) KRP-203, sphingosine 1-phosphate receptor type 1 agonist, ameliorates atherosclerosis in LDL-R-/- mice. Arterioscler. Thromb. Vasc. Biol. 33, 1505–1512 10.1161/ATVBAHA.113.301347 [DOI] [PubMed] [Google Scholar]
- 135.de Mello V.D., Lankinen M., Schwab U., Kolehmainen M., Lehto S., Seppanen-Laakso T.. et al. (2009) Link between plasma ceramides, inflammation and insulin resistance: association with serum IL-6 concentration in patients with coronary heart disease. Diabetologia 52, 2612–2615 10.1007/s00125-009-1482-9 [DOI] [PubMed] [Google Scholar]
- 136.Deutschman D.H., Carstens J.S., Klepper R.L., Smith W.S., Page M.T., Young T.R.. et al. (2003) Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am. Heart J. 146, 62–68 10.1016/S0002-8703(03)00118-2 [DOI] [PubMed] [Google Scholar]