Abstract
In most species, fertilization induces Ca2+ transients in the egg. In mammals, the Ca2+ rises are triggered by phospholipase Cζ (PLCζ) released from the sperm; IP3 generated by PLCζ induces Ca2+ release from the intracellular Ca2+ store through IP3 receptor, termed IP3-induced Ca2+ release. Here, we developed new fluorescent IP3 sensors (IRIS-2s) with the wider dynamic range and higher sensitivity (Kd = 0.047–1.7 μM) than that we developed previously. IRIS-2s employed green fluorescent protein and Halo-protein conjugated with the tetramethylrhodamine ligand as fluorescence resonance energy transfer (FRET) donor and acceptor, respectively. For simultaneous imaging of Ca2+ and IP3, using IRIS-2s as the IP3 sensor, we developed a new single fluorophore Ca2+ sensor protein, DYC3.60. With IRIS-2s and DYC3.60, we found that, right after fertilization, IP3 concentration ([IP3]) starts to increase before the onset of the first Ca2+ wave. [IP3] stayed at the elevated level with small peaks followed after Ca2+ spikes through Ca2+ oscillations. We detected delays in the peak of [IP3] compared to the peak of each Ca2+ spike, suggesting that Ca2+-induced regenerative IP3 production through PLC produces small [IP3] rises to maintain [IP3] over the basal level, which results in long lasting Ca2+ oscillations in fertilized eggs.
Introduction
In most species, rises in cytosolic Ca2+ concentration ([Ca2+]) trigger the egg-embryo transition. Unfertilized eggs, which are arrested at different stages of meiosis in different species, are “activated” and released from the arrest by fertilization1,2. In mammals, egg activation is triggered by a periodic series of Ca2+ transients, known as Ca2+ oscillations3,4. The response in mammalian eggs lasts for several hours and involves relatively low frequency, large amplitude Ca2+ increases5. The multiple increases in [Ca2+] are essential for completion of all the events of egg activation in mammals5,6.
The first Ca2+ transient occurs some minutes after sperm-egg fusion7. The Ca2+ oscillations in mammalian eggs appear to be a result of Ca2+ release via the inositol 1,4,5-trisphosphate (IP3) receptor/Ca2+ release channel (IP3R) located on the intracellular Ca2+ stores8. A sperm-specific phospholipase Cζ (PLCζ)9, which produces IP3 via hydrolysis of phosphatidyl 4,5-bisphosphate (PIP2), is reported as an egg-activating sperm factor10 in mammalian species. The microinjection of complementary RNA (cRNA) encoding PLCζ11 or recombinant PLCζ proteins12 into unfertilized mouse eggs triggers characteristic Ca2+ oscillations like those observed at fertilization. Sperm from transgenic mice with significantly reduced expression of PLCζ display a premature termination of Ca2+ oscillations following in vitro fertilization13. PLCζ shows extremely high Ca2+ sensitivity for its enzymatic activity compared with other PLC isoforms, with 70% maximal activity at 100 nM Ca2+ 12. Therefore, it has been considered that basal cytosolic Ca2+ in the fertilized egg can stimulate PLCζ to produce an amount of IP3 sufficient to trigger the initial release of Ca2+, which has not been confirmed experimentally, since single cell imaging using fluorescent IP3 indicators, such as green fluorescent protein (GFP)-fused to pleckstrin homology domain (GFP-PHD)14 and fretino-215, failed to clearly detected IP3 concentration ([IP3]) changes evoked in fertilized mouse eggs16,17.
Because all PLC isoforms including PLCζ are activated by Ca2+ 12,18–20, there will be further increase in IP3 production when [Ca2+] start to increase. This positive feedback has been proposed to play a central role for the generation of the upstroke of Ca2+ transients21,22. Except for PLCζ, members of each of the PLC families are expressed in eggs, and PLCβ1 is reported to contribute generation of Ca2+ transients23 and in theory any of these could be involved in modulating Ca2+ oscillations. On the other hand, the positive feedback regulation of Ca2+ acting directly on the IP3R has been proposed to drive regenerative Ca2+ increases24. Simultaneous detection of [Ca2+] and [IP3] is necessary to figure out the contributions of Ca2+-induced IP3 production from PLCs and Ca2+ release form IP3R for the generation of fertilization-induced Ca2+ transients.
In the present study, we developed novel fluorescent resonant energy transfer (FRET)-based IP3 sensor proteins, designated as IRIS-2s, to visualize IP3 dynamics in fertilized mouse eggs. The novel IP3 sensors possess an improved dynamic range compared with the previous sensor, IRIS-125. A high IP3 binding affinity variant, IRIS-2.3, can be successfully used to monitor [IP3] changes naturally induced in fertilized mouse eggs. IRIS-2s contain enhanced green fluorescent protein (EGFP) and Halo-protein with tetramethylrhodamine (TMR) ligand as FRET donor and acceptor, respectively. To monitor [Ca2+] and [IP3] changes simultaneously, we also developed a new Ca2+ sensor protein, designated as DYC3.60, which has enhanced cyan fluorescent protein (ECFP) as a solo fluorophore. The pair of IRIS-2s and DYC3.60 contains a new set of fluorophores for dual-FRET imaging, and real time monitoring with IRIS-2s and DYC3.60 provide us insights into the mechanism underlying the generation of Ca2+ oscillations in mouse fertilized eggs.
Results
Construction of IRIS-2s
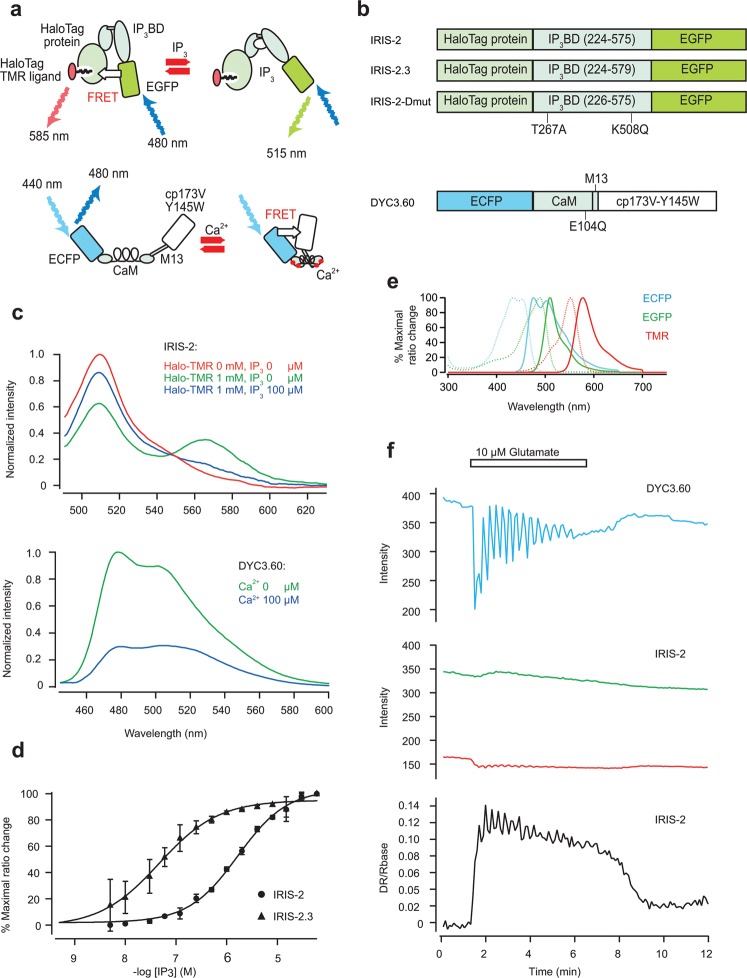
We constructed novel IP3 sensors composed of HaloTag protein (Promega), IP3 binding domain (IP3BD) of mouse IP3R125, and mEGFP (upper panel in Fig. 1a). HaloTag protein is an engineered, catalytically inactive derivative of a hydrolase that forms a covalent bond with commercially available HaloTag ligands. We used HaloTag® tetramethylrhodamine (TMR) ligand (Promega) as a FRET acceptor for mEGFP. Amino acid residues 224–575 and 224–579 of mouse IP3R1 were used for IRIS-2 and IRIS-2.3, respectively (upper panel in Fig. 1b), to manipulate the IP3 binding affinity of the sensors. We constructed IRIS-2-Dmut, in which two critical amino acid residues (Thr267 and Lys508) for IP3 binding have been replaced in IRIS-2, as a negative control25 (upper panel in Fig. 1b). The upper panel in Fig. 1c shows emission spectrum of IRIS-2 when excited at 480 nm. Purified IRIS-2 with addition of HaloTag TMR ligand (IRIS-2TMR) showed greater TMR emission (565 nm) and lesser EGFP emission (510 nm) (green line in Fig. 1c) compared with those of untreated IRIS-2 (red line in Fig. 1c), indicating that FRET between EGFP and TMR occurs in IRIS-2TMR. The addition of 100 μM IP3 increased the EGFP emission and decreased the TMR emission (blue line in Fig. 1c), indicating that the FRET efficiency of IRIS-2TMR decreases upon IP3 binding (Fig. 1a). The relative change in the EGFP/TMR emission ratio of IRIS-2TMR monitored with zero and 100 μM IP3 (155 ± 26%; n = 3) was three times larger than that of IRIS-1 (55.2 ± 2.7%; n = 3) (Fig. 1c and Supplementary Fig. 1). The high dynamic range achieved in IRIS-2 was preserved in IRIS-2.3 treated with HaloTag TMR ligand (IRIS-2.3TMR) (117 ± 3%; n = 3). Figure 1d shows the IP3 dependence of the emission ratio of IRIS-2TMR and IRIS-2.3TMR. The Kd value of IRIS-2.3TMR (0.047 ± 0.006 μM; n = 3; Fig. 1d) was 36-times smaller than that of IRIS-2TMR (1.7 ± 0.2 μM; n = 3; Fig. 1d). The Kd value of IRIS-2TMR was 3-times larger than that of IRIS-1 (0.55 ± 0.06 μM)25.
Figure 1.
Construction and characterization of IRIS-2s and DYC3.60. (a) Schematic drawing of IRIS-2s and DYC3.60. IRIS-2s are composed of the IP3BD of mouse IP3R1, EGFP and HaloTag protein. HaloTag TMR ligand was used for the acceptor in IRIS-2s. DYC3.60 is composed of calmodulin (CaM), M13 peptide, ECFP and a non-fluorescent mutant of circular permutated Venus (cp173V-Y145W). (b) Domain structures of IRIS-2 proteins and DYC3.60. (c) Emission spectra of IRIS-2 (upper panel) and DYC3.60 (lower panel) excited at 480 and 440 nm, respectively. Spectra of purified IRIS-2 (red line), IRIS-2TMR (in the presence of 1 μM HaloTag TMR ligand) (green line), and IRIS-2TMR in the presence of 100 μM IP3 (blue line). Spectra of lysate of DYC3.60-expressing COS7 cells were measured with 0.1 mM of CaCl2 (blue line) or without CaCl2 (green line). (d) Apparent IP3 affinities of purified IRIS-2TMR (circles) and IRIS-2.3TMR (triangles). Data were obtained from three independent measurements. Error bars correspond to the SD. (e) Excitation (broken lines) and emission (continuous lines) spectra of ECFP, EGFP, and TMR. (f) Time courses of emission changes of DYC3.60 (blue line), EGFP (green line) and TMR (red) of IRIS-2TMR in mGluR5 expressing HeLa cells stimulated with 10 μM glutamate (horizontal bars). The ratio of EGFP and TMR is drawn in the bottom panel (black line).
As a partner of IRIS-2s, we developed a FRET based Ca2+ indicator with single fluorophore to avoid fluorescent overlapping with IRIS-2s. We introduced a non-fluorescent mutation (Y145W26) into a yellow fluorescent protein, cp173Venus, of YC3.6027 (lower panels in Fig. 1a,b). The resultant protein have a fluorescent spectrum as same as ECFP, and addition of 100 μM Ca2+ decreased its emission by FRET quenching. The peak fluorescent amplitude was 71 ± 3% (n = 3) reduced after addition of Ca2+ in DYC3.60 (lower panel in Fig. 1c). Fluorescence from the three fluorophores used in IRIS-2s and DYC3.60 can be easily separated (Fig. 1e). Figure 1f shows time course changes of fluorescence from DYC3.60 and IRIS-2 in glutamate stimulated mGluR5-expressing HeLa cells. Less overlaps of excitation and emission spectra of IRIS-2 and DYC3.60 allowed dual-FRET imaging of Ca2+ and IP3 even without spectral unmixing28 (Fig. 1f).
Characterization of IRIS-2s and DYC3.60 expressed in cultured mammalian cells
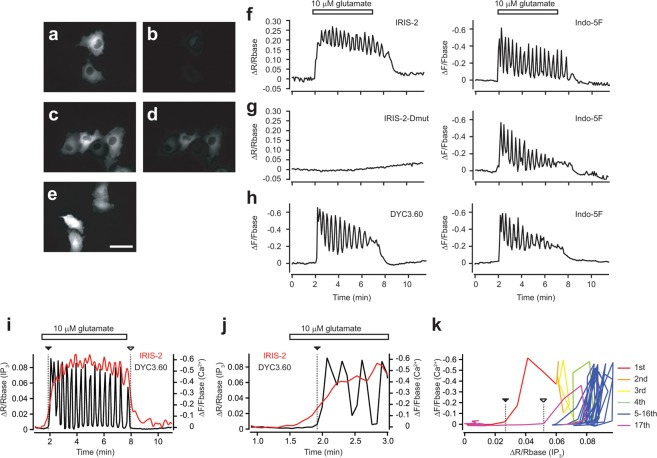
IRIS-2TMR and DYC3.60 were uniformly distributed within the cytosol when expressed in HeLa cells (Fig. 2a–e). Halo-TMR staining increased fluorescent signal detected by a 573–613-nm emission filter (Fig. 2b,d). The frequency of Ca2+ oscillations monitored with Indo-5F in mGluR5-expressing HeLa cells stimulated with 10 μM glutamate were not significantly different among IRIS-2-, IRIS-2-Dmut-, and DYC3.60-expressing cells (50 ± 16 mHz for IRIS-2, n = 9; 55 ± 6 mHz for IRIS-2-Dmut, n = 4; 47 ± 10 mHz, n = 6 for DYC3.60) (Fig. 2f–h). IRIS-2TMR signals did not return to its basal level during the intervals between Ca2+ transients, and its fluctuation was synchronous with Ca2+ oscillations (Fig. 2f). These characteristic IP3 dynamics monitored with IRIS-2TMR in HeLa cells are almost same as those recorded with other FRET-based IP3 sensors15,25,29,30.
Figure 2.
Effect of FRET sensors to [Ca2+] dynamics in HeLa cells. (a–d) Fluorescent images of IRIS-2 expressing cells without (a,b) and with HaloTag TMR staining (c,d). Bar, 50 μm. Emissions detected by filters for GFP are shown in (a,c). Emissions detected by filters for TMR are shown in (b,d). (e) A fluorescent image of DYC3.60 expressing cells. (f–h) Effect of FRET sensors to [Ca2+] dynamics in HeLa cells. Cells expressing IRIS-2 (f), IRIS-2-Dmut (g), or DYC3.60 (h) were stained with HaloTag TMR and Indo-5F. Left panels are time courses of emission ratio (f,g) or emission (h) changes of FRET sensors in mGluR5 expressing HeLa cells stimulated with 10 μM glutamate (horizontal bars). Right panels are time courses of emission changes of Indo-5F in the same cells of left panels. (i–k) [IP3] and [Ca2+] changes were imaged with the dual FRET probes. Initiation of the first Ca2+ spike and the termination of Ca2+ oscillations were marked with dashed line with closed and open triangles, respectively. (j) [IP3] and [Ca2+] changes around the rise of first Ca2+ spike. (k) A phase plane trajectory is drawn with [IP3] and [Ca2+] imaging data.
Initiation, maintenance, and termination of Ca2+ oscillations in HeLa cells
Figure 2i–k show imaging data of IRIS-2 and DYC3.60 in mGluR5-expressing HeLa cells. The dual FRET imaging clearly showed [IP3] increase precedes [Ca2+] rise as same as our previous report with IRIS-125 (Fig. 2i,j). Figure 2k shows a phase plane trajectory of [IP3] and [Ca2+] imaging data. [IP3] gradually increased from 1st to 4th Ca2+ spikes, and then, repeated Ca2+ spikes occurred in the certain range of [IP3] (Fig. 2k). In the range of [IP3], the trajectory cycled at almost the same orbit, suggesting that the trajectory is in a limit cycle (Fig. 2k). After termination of agonist stimulation, [IP3] decreased below the range of limit cycle maintenance, which resulted in the termination of Ca2+ oscillations (Fig. 2i,k). In the initial phase of Ca2+ oscillations, [IP3] increase precedes Ca2+ spikes (Fig. 2k), suggesting that [IP3] increases induce Ca2+ spikes. In the limit cycle phase, Ca2+ spikes occur without marked [IP3] increases (Fig. 2k), suggesting that Ca2+ induced positive and negative feedbacks to IP3R autonomously induce Ca2+ spikes24. Ca2+ oscillations last as long as [IP3] maintained in the range of limit cycle. Termination of agonist stimulation induces [IP3] decrease below to the range maintaining the limit cycle. [IP3] necessary to induce Ca2+ spike should be different at the initial state and later state of Ca2+ oscillations because Ca2+ directly or indirectly inactivates IP3R28,31. Thus, Ca2+ disappears even [IP3] above the basal level at the termination of Ca2+ oscillations (Fig. 2i).
Characterization of IRIS-2 in UV-uncaging experiments
Next, we checked the compatibility of IP3 sensors with UV-uncaging. Caged-compounds are light-sensitive probes that functionally encapsulated biomolecules in an inactive form. The active compounds can be released from caged-compounds with UV light in most of caged-compounds. IRIS-1 or IRIS-2 were expressed in HeLa cells and irradiated by UV pulses (Supplementary Fig. 2a,b). We found UV irradiation caused temporal reduction of fluorescence of both ECFP and Venus in IRIS-1 expressing cells (Supplementary Fig. 2a). Because of the difference of the signal reduction between those fluorescent proteins, the fluorescent ratio of IRIS-1 was significantly reduced (−1.9 ± 0.7%, n = 22). In contrast, the fluorescent signals from EGFP and HaloTag-TMR were stable after the UV irradiation (Supplementary Fig. 2b), which resulted in successful detection of [IP3] changes after UV-uncaging of caged-IP3 (Supplementary Fig. 2c).
Detection of IP3 concentration changes in fertilized mouse eggs
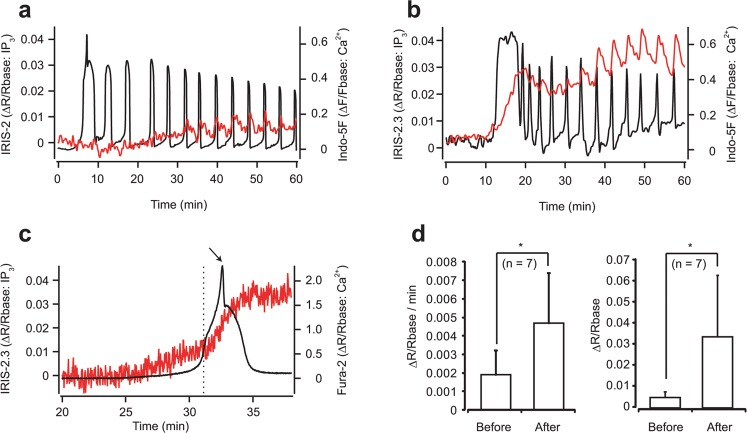
To detect IP3 dynamics in fertilized mouse eggs, IRIS-1, IRIS-2, or IRIS-2.3 was expressed in eggs by cRNA injection. For the simultaneous monitoring of [Ca2+] changes, we first used Indo-5F as a Ca2+ indicator according to the method described previously25. As shown in Supplementary Figure 3, we did not detect any changes of IRIS-1 signals in fertilized eggs. Not only the fails of the detection of IP3 changes, it was difficult to detect [Ca2+] changes after addition of sperm into the culturing media. Even in the experiments with successful detection of fertilization-induced [Ca2+] changes, the number of Ca2+ transients was less compared to IRIS-2-Dmut (number of Ca2+ spikes during 30 min after the first Ca2+ spikes: IRIS-1: 1.91 ± 0.13 (n = 3); IRIS-1-Dmut: 3.75 ± 0.5 (n = 4); p = 0.008, Student’s t-test), suggesting that IRIS-1 works as a significant IP3 buffer. We also tested IRIS-2 expressing eggs for in-vitro fertilization assay and found IRIS-2 expressing eggs had normal Ca2+ spikes after fertilization (Fig. 3a). However, it was also hard to detect clear increases in FRET signals in IRIS-2-expressing eggs during the first Ca2+ transient evoked after fertilization, while small repetitive transients of IRIS-2TMR signals synchronous with Ca2+ oscillations were observed approximately 30 min after the onset of the first Ca2+ transient (Fig. 3a). On the other hand, we clearly detected IP3 increases during the all Ca2+ transients, including the first Ca2+ transient, in IRIS-2.3-expressing eggs (Fig. 3b). During the first large Ca2+ transient, [IP3] continues to increase, and all the following Ca2+ transients accompanied with a rapid increase and a following slow decline on the elevated level of [IP3] (Fig. 3b). Three independent experimental results of [IP3] and [Ca2+] imaging with IRIS-2.3 and Indo-5F at the onset of first Ca2+ spike were shown in Supplementary Figure 4. We did not find significant difference of numbers of Ca2+ spikes during 30 min after 1st Ca2+ spike between IRIS-2 and IRIS-2.3 expressing eggs (IRIS-2: 5.17 ± 1.72, n = 6; IRIS-2.3: 6.33 ± 5.72, n = 9; p = 0.58, student’s t-test).
Figure 3.
IRIS-2 TMR and IRIS-2.3 TMR signals in fertilized mouse eggs. (a,b) [IP3] and [Ca2+] dynamics detected by IRIS-2s and Indo-5F. Normalized emission ratio changes (∆R/Rbase) of IRIS-2TMR (a) and IRIS-2.3TMR (b) are plotted with red lines. [Ca2+] changes detected with Indo-5F are shown with black lines (a,b). Sperm was added at time zero (a,b). Fluorescent images were acquired each 4 sec in (a) and each 10 sec in (b). (c) Time courses of emission ratio changes of Fura-2 (black) and IRIS-2.3TMR (red) at a first Ca2+ spike after fertilization. The average values from whole egg were plotted against time. A peak of the first Ca2+ spike is shown by an arrow. The time point of the change in the rate of rise in the IRIS-2.3TMR signal is shown by a vertical broken line. (d) Left panel shows the rates of [IP3] increases before and after the shoulder point of the first Ca2+ spike. Right panel shows peak [IP3] change before [Ca2+] rise and first [IP3] peak after [Ca2+] rise. n = 7. *p < 0.05, Student’s t-test.
Initiation of [IP3] and [Ca2+] changes
Next, we investigated the temporal order of the onset of increase between [IP3] changes and [Ca2+] changes during the first Ca2+ transient evoked after fertilization. To detect the initial [Ca2+] changes experimentally, we used Fura-2, whose affinity is higher than that of Indo-5F (Fura-2: Kd = 135 nM; Indo-5F: Kd = 470 nM), as a Ca2+ indicator to detect the timing of the onset of the first Ca2+ transient as precise as possible. As shown in Fig. 3c, [IP3] rise preceded the onset of the initial step of the first Ca2+ transient for 2.7 ± 2.4 min in 11 of 13 eggs. The initial [IP3] increase should initiate Ca2+ release from IP3R. IP3 and Ca2+ are the co-agonist of IP3R, and open probability of IP3R markedly increase with Ca2+ in the presence of IP332. Thus, Ca2+-induced Ca2+ release (CICR) from IP3R should have major role to produce initial Ca2+ spike. The first Ca2+ transient observed in IRIS-2.3-expressing eggs was composed of two steps separated by a shoulder point (dashed line in Fig. 3c) as reported previously33. The peak amplitude and the rising speed of [IP3] increased after the shoulder point of the first Ca2+ transient (Fig. 3c,d), suggesting acceleration of IP3 production via Ca2+-induced activation of PLC isozymes.
Positive feedback loop to produce rising phase of Ca2+ spikes
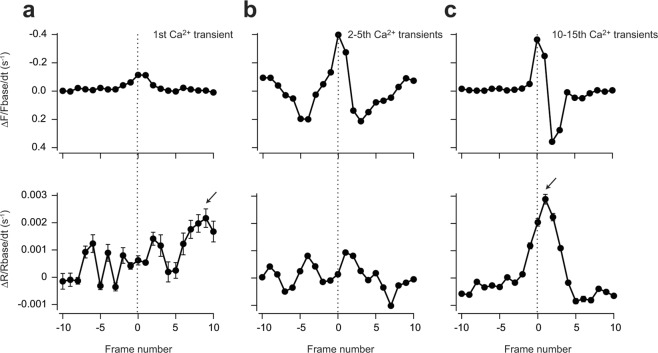
Each Ca2+ spike of Ca2+ oscillations usually form as a result of an initial slow pacemaker rise in [Ca2+] followed by a rapid rise in [Ca2+]34–36. The accelerated rise of [Ca2+] is suggested that a regenerative process is involved in the generation of the abrupt upstroke35. Such regenerative processes require a positive-feedback element22, and CICR from IP3R and Ca2+-induced IP3 production through PLC have been proposed as candidates of the positive feedback element. In the previous study, we compared rate of [IP3] and [Ca2+] rises at the onset of Ca2+ spikes and found each Ca2+ spike is not accompanied by acceleration in the rate of increase in IP3 in HeLa cells25. As same as HeLa cells, if the regenerative IP3 production mediated by PLC activated by cytosolic Ca2+ drives the rising phase of Ca2+ spikes, the rate of [IP3] rise should accelerate when the rate of [Ca2+] rise accelerate. To test this possibility, the fluorescent signals of both Indo-5F and IRIS-2.3TMR were differentiated and aligned at the time when the rate of [Ca2+] rise reached its maximum (Fig. 4). In the early phase (from first to 5th transients) of fertilization-induced Ca2+ oscillations, the amplitudes of IP3 fluctuations were relatively small (Fig. 3b), and the rate of [IP3] rise did not increase during the rising phase of the Ca2+ transients, as found in cultured HeLa cells25 (Fig. 4a,b). The amplitudes of IP3 fluctuations were gradually increased during the later phase of Ca2+ oscillations (Fig. 3a,b), and contrary to the early phase, the onset of the rate of [IP3] rise precedes that of [Ca2+] (Fig. 4c). The result suggests that Ca2+-induced IP3 production through PLC may work as a part of the positive feedback loop to produce abrupt [Ca2+] rise at Ca2+ spikes in later phase of Ca2+ oscillations. However, the peak of the rate of [IP3] rise always delayed from that of [Ca2+] (Fig. 4c), suggesting that CICR from IP3R has major role to produce the rising phase of Ca2+ spikes and [IP3] rises.
Figure 4.
Rate of [Ca2+] and [IP3] changes at each Ca2+ spike. Rate of [Ca2+] and [IP3] changes are shown as differentiated signals of Indo-5F (Em. 460–510 nm) (upper panel) and IRIS-2.3TMR (lower panel) aligned by the time when the differentiated Indo-5F signal was at its maximum (frame 0, broken line) during first Ca2+ transients after fertilization (n = 9) (a), from 2nd to 5th Ca2+ transients (n = 20) (b) and from 10th to 15th Ca2+ transients (n = 20) (c). Error bars correspond to the SD. Arrowheads indicate the peak of the rate of [IP3] rise. Broken vertical lines indicate the peaks of differentiated Indo-5F signals.
[IP3] stayed at the elevated level and did not return to the basal level through Ca2+ oscillations (Fig. 3b).
Dual-FRET imaging of [IP3] and [Ca2+] in fertilized mouse eggs
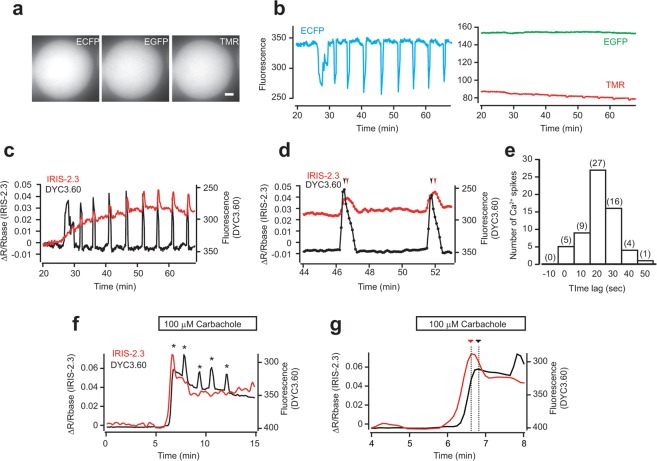
We also test our dual-FRET pair for [IP3] and [Ca2+] at fertilization of mouse eggs. We microinjected cRNAs of DYC3.60 and IRIS-2.3 into the eggs and stained the eggs with TMR. As shown in Fig. 5a, these fluorescent probes were distributed evenly in the egg. Well separation of excitation and emission spectra of these fluorophores enabled simultaneous detection of these fluorescence (Figs 1e, 5a,b). As same as the results we obtained with the pair of Indo-5F and IRIS-2.3TMR, we successfully detected fertilization-induced [Ca2+] and [IP3] changes with DYC3.60 and IRIS-2.3TMR (Fig. 5b and Supplementary video 1). As same as HeLa cells, [IP3] at the termination was higher than that at the initiation of Ca2+ oscillations in fertilized mouse eggs (Fig. 2i and Supplementary Fig. 5).
Figure 5.
Delayed IP3 pulses during Ca2+ oscillations in fertilized mouse eggs visualized by dual-FRET sensors. (a) Fluorescence images of DYC3.60 (ECFP) and IRIS-2.3TMR (EGFP and TMR) in a single mouse egg. DYC3.60 was illuminated with 425–445 nm light, and IRIS-2.3TMR was illuminated with 460–490 nm light. Scale bar, 10 μm. (b) Dual-FRET imaging of [IP3] and [Ca2+] in a fertilized mouse egg. Signals from DYC3.60 (ECFP) and IRIS-2.3TMR (EGFP and TMR) from single fertilized mouse egg are shown in the left and right panels, respectively. (c) Emission changes in DYC3.60 (black line) and ratio changes of IRIS-2.3TMR (red line) are shown. Sperm was added at time zero. (d) Data shown in (c) on an enlarged time scale. The arrowheads indicate the time of peaks in DYC3.60 signal (black) and IRIS-2.3TMR signal (red). (e) A histogram of the peak time difference between DYC3.60 signals and IRIS-2.3TMR signals (n = 61). The positive value indicates that the peak of DYC3.60 signals precedes that of IRIS-2.3TMR signals (17 ± 11 sec). (f) Emission ratio changes of DYC3.60 (black line) and IRIS-2.3TMR (red line) in an unfertilized egg stimulated with 100 μM carbachole. Asterisks show the peak of each Ca2+ spike. Carbachole was added during the time indicated by the horizontal bar. (g) Data shown in (f) on an enlarged time scale around the rise of first Ca2+ spike. The dashed lines with red and black triangles indicate the time of peaks in IRIS-2.3TMR signal and DYC3.60 signal, respectively.
Ca2+-induced regenerative IP3 production
We also detected delays in the peak of [IP3] compared to the peak of each Ca2+ spike (17 ± 11 sec, n = 63, Fig. 5c,d), suggesting that Ca2+-induced regenerative IP3 production through PLC produces small [IP3] rises at each Ca2+ spike to maintain [IP3] over the basal level, which results in long lasting Ca2+ oscillations in fertilized eggs. Which PLC isoforms contribute to this regenerative process? Eight PLC isoforms are known to express in the mouse egg: PLCβ137, PLCβ337, PLCβ423, PLCγ137, PLCγ237, PLCδ123, PLCδ423, and PLCε23. From these isoforms, knockout mice of PLCβ4, PLCδ1, PLCδ4, and PLCε are born normally23,38–40. On the other hand, knockout mice of PLCβ141, PLCβ342, PLCγ138, and PLCγ243 have problems on development of the embryo. However, dominant-negative experiments employing recombinant SH2 domain to inhibit PLCγ1 and γ2 did not inhibit the Ca2+ oscillatory pattern during fertilization44. Based on these findings, Igarashi et al. found that reduced expression of PLCβ1 by RNAi resulted in a significant decrease in Ca2+ transients and overexpression of PLCβ1 by cRNA injection resulted in perturbed duration and frequency of Ca2+ oscillations23. Thus, Ca2+ induced activation of PLCβ isozymes are the strong candidates which play a pivotal role to the accelerated production of IP3 during Ca2+ spikes in fertilized mouse eggs. To determine the role of PLCβ in the mouse egg, we stimulate unfertilized mouse eggs with 100 μM carbachole (Fig. 5f,g). The stimulation caused Ca2+ spikes and a monotonic [IP3] rise (Fig. 5f). At fertilization, [IP3] changes always follow after Ca2+ spikes. On the other hand, Ca2+ spikes did not accompany with delayed [IP3] rises in carbachole stimulated unfertilized eggs (Fig. 5f). Particularly, IP3 peak at the first Ca2+ spike preceded Ca2+ peak (Fig. 5g). These data showed that Ca2+-induced IP3 producing activity is not strong in unfertilized eggs, suggesting that sperm derived PLCζ should participate Ca2+-induced IP3 production. As we showed in Figs 3a,b and 4, Ca2+-induced [IP3] rises increased later phase of fertilization-induced Ca2+ oscillations, suggesting that fertilization induces quantitative or qualitative changes of PLC in later phase of Ca2+ oscillations.
Discussion
In this study, we developed a dual-FRET pair of biosensors for the detection of [IP3] and [Ca2+] in mammalian cells. The uniqueness of our dual-FRET pair is using single fluorophore for one of the pairs. Replacement of Y145 to W in EYFP is known to produce a non-fluorescent chromoprotein that retains its absorption of emission light26. Introduction of the Y145W mutation into cp173Venus of YC3.6027 resulted to produce single fluorophore with fluorescent quencher in the Ca2+ FRET sensor, DYC3.60. Usually, four fluorophores are necessary for dual-FRET imaging. Most of FRET sensors have cyan and yellow fluorescent proteins45, and these fluorescent proteins cover a broad spectral profile. Thus, using FRET sensor with cyan and yellow proteins, it is difficult to find a partner FRET sensor for dual-FRET imaging without using spectral unmixing to distinguish each fluorescent signal mathematically from significantly overlapped fluorescent signals46,47. We offer a dual-FRET imaging with three fluorophores, which gives easier detection and separation of fluorescent signals.
The new FRET sensors enabled imaging of [IP3] and [Ca2+] at fertilization of mouse eggs. We have succeeded to detect [IP3] changes in fertilized mouse eggs using a second-generation fluorescent IP3 sensor, IRIS-2.3, which has an improved dynamic range and a high IP3 sensitivity. Simultaneous monitoring of both Ca2+ and IP3 in fertilized mouse eggs showed that the [IP3] increase was detected approximately 3 min before the onset of the first Ca2+ transient. The result is consistent with the expectation that highly Ca2+ sensitive PLCζ produces IP3 at the basal level of [Ca2+] in the egg cytosol after sperm-egg fusion12. Mehlmann and Kline reported microinjection of small amount of IP3 (8.6 nM) is able to induce Ca2+ spike in unfertilized mouse eggs48. Our measurements showed the same results that the amount of IP3 produced in mouse eggs is small even after the fertilization because only IRIS-2.3, which shows the highest IP3 sensitivity (Kd = 47 nM) among the IP3 sensors developed, could detect [IP3] increases at the onset of the first Ca2+ transients.
IP3R has a bell-shaped calcium response curve: the open probability of IP3R is activated by low [Ca2+] and inhibited by high [Ca2+]32. Based on this finding, De Young and Keizer reported a mathematical model to reproduce Ca2+ oscillations with constant [IP3]24. In this and previous reports, we showed sustained [IP3] increase during Ca2+ oscillations in HeLa cells and fertilized mouse eggs25 (Figs 3 and 4), and the same results were obtained with other IP3 sensor proteins29,30. Consistently with our results, Mehlmann and Kline reported single microinjection of IP3 induces Ca2+ oscillations in unfertilized mouse eggs48. Jones et al. also reported Ca2+ oscillations with continuous low level caged-IP3 photolysis in unfertilized mouse eggs49. PLCζ is a smallest and simplest PLC isoform9. The activity of PLCζ is regulated by Ca2+ and localization into nucleus after pronuclear formation, and other regulations are not known50. PLCζ has highest Ca2+ sensitivity compared to the other PLC isoforms and is 70% active at the basal level [Ca2+] in cells12. Thus, PLCζ should be continuously active after fertilization until pronuclear formation51, which should sustain continuous [IP3] increase during fertilization-induced Ca2+ oscillations (Figs 3 and 4). We previously found that CICR dominantly work as a positive feedback loop to produce the rising phase of Ca2+ spikes in HeLa cells25. Our data suggest that the mechanism elicits the rising phase of Ca2+ spikes in fertilized mouse eggs is more complex. Initially, CICR dominantly works as the positive feedback loop, and Ca2+-induced IP3 production gradually participates to produce Ca2+ spikes cooperatively with CICR in the later phase of Ca2+ oscillations. Ca2+-induced IP3 production through PLC produces [IP3] rises at each Ca2+ spike to help keeping [IP3] over the basal level, which results in long lasting Ca2+ oscillations in fertilized eggs.
In conclusion, we produced FRET sensors with new choices of fluorophores for dual-FRET imaging of [IP3] and [Ca2+]. Less overlaps of excitation and emission spectrum of IRIS-2s and DYC3.60 allowed dual-FRET imaging of Ca2+ and IP3 even without spectral unmixing. Because of the smaller number of fluorophores, our dual-FRET approach can reduce the effort to detect each fluorescent signal separately. The wider dynamic range and higher sensitivity achieved by IRIS-2.3 will enable the detection of subtle [IP3] changes associated with [Ca2+] changes at egg fertilization to local [Ca2+] increase events.
Materials and Methods
Animals
Experiments used ddY mice for preparation of oocytes and sperm. All animal experiments were performed in accordance with the guidelines approved by the Animal Experiments Committee of RIKEN Brain Science Institute. All experiments were carried out in accordance with the approved ethical guidelines and regulations.
Gene construction
The FRET donor and acceptor of IRIS-1 were replaced with mEGFP and Halo-protein (Promega), respectively, to produce IRIS-2. Amino acid residues 224–575 of mouse IP3R1 in IRIS-2 were replaced with amino acid residues 224–579 of mouse IP3R1 to produce IRIS-2.3. The Y145W mutant26 of circular permutated Venus (cp173V-Y145W)27 was generated using the site-directed mutagenesis. The FRET acceptor of YC3.6027 was replaced with cp173Venus-Y145W to produce DYC3.60. IRIS-2, IRIS-2.3 and DYC3.60 cDNAs were cloned into the NheI and XbaI sites of pcDNA3.1 zeo(+) (Invitrogen) for the expression in HeLa cells. The cDNAs were cloned into the XbaI site of pTNTTM (Promega) with extended poly(A) tail (57 residues) and synthesized cRNAs were injected into mouse oocytes.
Protein expression and purification
The full-length cDNA of IRIS-2 was isolated from pcDNA3.1 zeo(+)-IRIS-2 by using NheI and XbaI sites and was cloned into the XbaI site of baculovirus transfer vector pFastBac1 (Invitrogen). The recombinant baculovirus was used for the large-scale expression of IRIS-2 in Sf9 cells as described previously52. The expressed proteins were purified on a HiTrap heparin HP column (GE Healthcare Life Sciences) as described previously53.
Cell culture and transfection
HeLa cells were cultured in Dulbecco’s modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum. HeLa cells were transfected with expression vectors by transfection reagent (Mirus TransIT). One day after the transfection, cells were used for imaging experiments.
Preparation of RNA
Plasmids carrying IRISs or DYC3.60 were digested by NdeI, and linearized DNA fragments were purified with Wizard SV Gel and PCR clean-up Kit (Promega). They were used as the templates for RNA transcription by T7 polymerase using T7 mMESSAGEmMACHINE Kit (Ambion). RNA was purified using RNeasy MinElute Cleanup Kit (Qiagen) and stored at −80 °C until use.
Preparation of gametes
Full grown immature oocytes were collected from the follicles in the ovaries of female mice 47–49 h after the injection of pregnant mare serum gonadotropin. Isolated oocytes were freed from cumulus cells mechanically by pipetting in M2 medium, and then cRNAs of IRIS-1, IRIS-2, IRIS-2.3, DYC3.60 or dKeima570 were injected as described below. Sperm was collected from the caudal epididymides and were incubated in M16 medium54 supplemented with 4 mg/ml BSA (Sigma) at 37 °C (5% CO2) for >5 h for capacitation and acrosome reaction55.
Microinjection and insemination
RNA solutions were diluted to 130 ng/μl with the intracellular medium (150 mM KCl, 5 mM Tris-KOH, pH 7.0). Immature oocytes were injected with 20 pl of RNA solutions and incubated in the M16 medium for 16 h at 37 °C with 5% CO2. Only eggs maturated normally to metaphase II with the first polar body were used in the following experiments. After loaded with 2 μM of Indo-5F or Fura-2 for 30 min in the M2 medium, eggs were freed from the zona pellucida by brief treatment with acidic Tyrode’s solution (pH 2.5)56 for insemination. Sperm was added during imaging experiments.
Imaging
After loading HeLa cells with 10 μM Indo-5F-AM (AnaSpec), imaging was performed under the constant flow (2 ml/min) of the balanced salt solution containing 20 mM Hepes, pH 7.4, 115 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 1.3 mM CaCl2, and 10 mM glucose as an imaging media at 37 °C through an inverted microscope (IX71 or IX81; Olympus) with a cooled charge-coupled device (CCD) camera (ORCA-ER; Hamamatsu Photonics) and a 40x, 1.35 NA, oil-immersion objective (Olympus). For the fluorescent images of IRIS-1 and Indo-5F, an emission splitter (W-view; Hamamatsu Photonics) was used with a light source exchanger (DG-4; Sutter Instrument Co.) on the IX71 inverted microscope. Sequential excitation of IRIS-1 and Indo-5F was performed by using a 450-nm dichroic mirror and two excitation filters (a 425–445 nm filter for IRIS-1 and a 360-nm filter for Indo-5F). Emissions from IRIS-1 and Indo-5F were split with a 460–510-nm filter (for IRIS-1 and Indo-5F), a long-path 520-nm (for IRIS-1) barrier filter, and two 505-nm dichroic mirrors equipped in W-view.
Eggs were incubated with M2 buffer at 37 °C on IX81 inverted microscope. Ca2+ and IP3 were visualized with sets of Indo-5F and IRIS-1, Indo-5F and IRIS-2, Indo-5F and IRIS-2.3, Fura-2 and IRIS-2.3, or DYC3.60 and IRIS-2.3, respectively. Sequential excitation of Ca2+ and IP3 indicators was performed by using dichroic mirrors (a 400-nm mirror for Indo-5F and a 450-nm mirror for IRIS-1 and DYC3.60 and a 505-nm mirror for IRIS-2 and IRIS-2.3) and excitation filters (a set of 340 and 380-nm filters for Fura-2 and a 380-nm filter for Indo-5F and a 425–445 nm filter for IRIS-1 and DYC3.60 and a 460–490-nm filter for IRIS-2 and IRIS-2.3). Emissions from Ca2+ and IP3 indicators were split with emission filters (a set of 400–420 and 460–510-nm filters for Indo-5F and a set of 460–510 and 525–565 filters for IRIS-1 and DYC3.60 and a 510–550 filter for Fura-2 and a set of 510–550 and 573–613-nm filters for IRIS-2 and IRIS-2.3TMR), and three filter exchangers (Lamda 10; Sutter Instruments, IX2-RFACA; Olympus).
Image acquisition was performed with MetaFluor (Molecular Devices). Data analysis was performed with MetaFluor and Igor Pro (WaveMetrics) softwares. The EGFP/TMR emission ratio (IRIS-2s), the ECFP/Venus emission ratio (IRIS-1s), the dKeima570/ECFP emission ratio (DYC3.60), the 420–440 nm/460–510 nm emission ratio (Indo-1) and the ratio of 510–550 nm emission excited at 340 nm and 510–550 nm emission excited at 380 nm (Fura-2) were defined as R. ∆R was defined as R - Rbase, where Rbase is the basal level of R. Baseline drift in each experiment was corrected with subtracting the trend line which is calculated with the line around the beginning of each experiment.
Uncaging of caged-IP3
HeLa cells transfected with IRIS-2 and DYC3.60 were loaded with 10 μM membrane permeable caged-IP3 (iso-Ins(1,4,5)P3/PM (caged), Enzo Life Science). The uncaging stimulation was done with extra light source (mercury lamp) equipped in IX81, filtered by a 333–348-nm filter and a 400-nm dichroic mirror, illuminated the cells through 20x, 0.50 NA, water-immersion objective (Olympus).
Supplementary information
Acknowledgements
We are grateful to Drs. Atsushi Miyawaki at Riken and Takeharu Nagai at Osaka University for donating YC3.60. We thank Mr. Akio Suzuki at Riken for technical help on plasmid constructions. We thank Dr. Sachiko Ishida at Riken for technical help on expression in SF-9 cells and purification of IP3 sensors. We thank Dr. Tooru Takahashi at University of Electro-Communications for technical supports on microinjection experiments into mouse oocytes and in vitro fertilizations. This work was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan to T. Matsu-ura (22770227), T. Michikawa (20370054), and K.M. (2022007).
Author Contributions
T. Matsu-ura, K. Suzuki, K. Sugiura and A.K. invented IRIS-2 variants. T. Matsu-ura and A.M. invented DYC3.60. T. Matsu-ura and H.S. established the method of the expression of IP3 sensors in mouse eggs. T. Matsu-ura performed other experiments. T. Matsu-ura and H.S. analyzed data. T. Matsu-ura, H.S., T. Michikawa, K. Suzuki, A.K. and K.M. wrote the manuscript. K.M. supervised the study.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-40931-w.
References
- 1.Schultz RM, Kopf GS. Molecular basis of mammalian egg activation. Curr. Top. Dev. Biol. 1995;30:21–62. doi: 10.1016/S0070-2153(08)60563-3. [DOI] [PubMed] [Google Scholar]
- 2.Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev. Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- 3.Jones KT. Ca2+ oscillations in the activation of the egg and development of the embryo in mammals. Int. J. Dev. Biol. 1998;42:1–10. [PubMed] [Google Scholar]
- 4.Miyazaki S, Shirakawa H, Nakada K, Honda Y. Essential role of the inositol 1,4,5-trisphosphate receptor/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev. Biol. 1993;158:62–78. doi: 10.1006/dbio.1993.1168. [DOI] [PubMed] [Google Scholar]
- 5.Swann K, Yu Y. The dynamics of calcium oscillations that activate mammalian eggs. Int. J. Dev. Biol. 2008;52:585–594. doi: 10.1387/ijdb.072530ks. [DOI] [PubMed] [Google Scholar]
- 6.Ducibella T, Schultz RM, Ozil JP. Role of calcium signals in early development. Semin. Cell Dev. Biol. 2006;17:324–332. doi: 10.1016/j.semcdb.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence Y, Whitaker M, Swann K. Sperm-egg fusion is the prelude to the initial Ca2+ increase at fertilization in the mouse. Development. 1997;124:233–241. doi: 10.1242/dev.124.1.233. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki S, et al. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science. 1992;257:251–255. doi: 10.1126/science.1321497. [DOI] [PubMed] [Google Scholar]
- 9.Saunders CM, et al. PLC zeta: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 10.Swann K. A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development. 1990;110:1295–1302. doi: 10.1242/dev.110.4.1295. [DOI] [PubMed] [Google Scholar]
- 11.Cox LJ, et al. Sperm phospholipase Czeta from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction. 2002;124:611–623. doi: 10.1530/rep.0.1240611. [DOI] [PubMed] [Google Scholar]
- 12.Kouchi Z, et al. Recombinant phospholipase Czeta has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J. Biol. Chem. 2004;279:10408–10412. doi: 10.1074/jbc.M313801200. [DOI] [PubMed] [Google Scholar]
- 13.Knott JG, et al. Interference reveals role for mouse sperm phospholipase Czeta in triggering Ca2+ oscillations during fertilization. Biol. Reprod. 2005;72:992–996. doi: 10.1095/biolreprod.104.036244. [DOI] [PubMed] [Google Scholar]
- 14.Hirose K, Kadowaki S, Tanabe M, Takeshima H, Iino M. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science. 1999;284:1527–1530. doi: 10.1126/science.284.5419.1527. [DOI] [PubMed] [Google Scholar]
- 15.Sato M, Ueda Y, Shibuya M, Umezawa Y. Locating inositol 1,4,5-trisphosphate in the nucleus and neuronal dendrites with genetically encoded fluorescent indicators. Anal. Chem. 2005;77:4751–4758. doi: 10.1021/ac040195j. [DOI] [PubMed] [Google Scholar]
- 16.Halet G, Tunwell R, Balla T, Swann K, Carroll J. The dynamics of plasma membrane PtdIns(4,5)P(2) at fertilization of mouse eggs. J. Cell Sci. 2002;115:2139–2149. doi: 10.1242/jcs.115.10.2139. [DOI] [PubMed] [Google Scholar]
- 17.Shirakawa H, Ito M, Sato M, Umezawa Y, Miyazaki S. Measurement of intracellular IP3 during Ca2+ oscillations in mouse eggs with GFP-based FRET probe. Biochem. Biophys. Res. Commun. 2006;345:781–788. doi: 10.1016/j.bbrc.2006.04.133. [DOI] [PubMed] [Google Scholar]
- 18.Kelley GG, Reks SE, Ondrako JM, Smrcka AV, Phospholipase C. epsilon): a novel Ras effector. EMBO J. 2001;20:743–754. doi: 10.1093/emboj/20.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol. Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- 20.Rhee SG, Bae YS. Regulation of phosphoinositide-specific phospholipase C isozymes. J. Biol. Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- 21.Harootunian AT, et al. Cytosolic Ca2+ oscillations in REF52 fibroblasts: Ca2+-stimulated IP3 production or voltage-dependent Ca2+ channels as key positive feedback elements. Cell Calcium. 1991;12:153–164. doi: 10.1016/0143-4160(91)90017-9. [DOI] [PubMed] [Google Scholar]
- 22.Meyer T, Stryer L. Molecular model for receptor-stimulated calcium spiking. Proc. Natl. Acad. Sci. USA. 1988;85:5051–5055. doi: 10.1073/pnas.85.14.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Igarashi H, Knott JG, Schultz RM, Williams CJ. Alterations of PLCbeta1 in mouse eggs change calcium oscillatory behavior following fertilization. Dev. Biol. 2007;312:321–330. doi: 10.1016/j.ydbio.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Young GW, Keizer J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc. Natl. Acad. Sci. USA. 1992;89:9895–9899. doi: 10.1073/pnas.89.20.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsu-ura T, et al. Cytosolic inositol 1,4,5-trisphosphate dynamics during intracellular calcium oscillations in living cells. J. Cell Biol. 2006;173:755–765. doi: 10.1083/jcb.200512141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganesan S, Ameer-Beg SM, Ng TT, Vojnovic B, Wouters FS. A dark yellow fluorescent protein (YFP)-based Resonance Energy-Accepting Chromoprotein (REACh) for Forster resonance energy transfer with GFP. Proc. Natl. Acad. Sci. USA. 2006;103:4089–4094. doi: 10.1073/pnas.0509922103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc. Natl. Acad. Sci. USA. 2004;101:10554–10559. doi: 10.1073/pnas.0400417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shinohara T, et al. Mechanistic basis of bell-shaped dependence of inositol 1,4,5-trisphosphate receptor gating on cytosolic calcium. Proc. Natl. Acad. Sci. USA. 2011;108:15486–15491. doi: 10.1073/pnas.1101677108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nezu A, Tanimura A, Morita T, Shitara A, Tojyo Y. A novel fluorescent method employing the FRET-based biosensor “LIBRA” for the identification of ligands of the inositol 1,4,5-trisphosphate receptors. Biochim. Biophys. Acta. 2006;1760:1274–1280. doi: 10.1016/j.bbagen.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Remus TP, et al. Biosensors to measure inositol 1,4,5-trisphosphate concentration in living cells with spatiotemporal resolution. J. Biol. Chem. 2006;281:608–616. doi: 10.1074/jbc.M509645200. [DOI] [PubMed] [Google Scholar]
- 31.Taylor CW, Tovey SC. IP(3) receptors: toward understanding their activation. Cold Spring Harb. Perspect. Biol. 2010;2:a004010. doi: 10.1101/cshperspect.a004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 33.Deguchi R, Shirakawa H, Oda S, Mohri T, Miyazaki S. Spatiotemporal analysis of Ca2+ waves in relation to the sperm entry site and animal-vegetal axis during Ca2+ oscillations in fertilized mouse eggs. Dev. Biol. 2000;218:299–313. doi: 10.1006/dbio.1999.9573. [DOI] [PubMed] [Google Scholar]
- 34.Jacob R, Merritt JE, Hallam TJ, Rink TJ. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988;335:40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- 35.Thomas AP, Renard DC, Rooney TA. Spatial and temporal organization of calcium signalling in hepatocytes. Cell Calcium. 1991;12:111–126. doi: 10.1016/0143-4160(91)90013-5. [DOI] [PubMed] [Google Scholar]
- 36.Bootman MD, Berridge MJ. Subcellular Ca2+ signals underlying waves and graded responses in HeLa cells. Curr. Biol. 1996;6:855–865. doi: 10.1016/S0960-9822(02)00609-7. [DOI] [PubMed] [Google Scholar]
- 37.Dupont G, McGuinness OM, Johnson MH, Berridge MJ, Borgese F. Phospholipase C in mouse oocytes: characterization of beta and gamma isoforms and their possible involvement in sperm-induced Ca2+ spiking. Biochem. J. 1996;316(Pt 2):583–591. doi: 10.1042/bj3160583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura Y, et al. Phospholipase Cdelta1 is required for skin stem cell lineage commitment. EMBO J. 2003;22:2981–2991. doi: 10.1093/emboj/cdg302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukami K, et al. Requirement of phospholipase Cdelta4 for the zona pellucida-induced acrosome reaction. Science. 2001;292:920–923. doi: 10.1126/science.1059042. [DOI] [PubMed] [Google Scholar]
- 40.Bai Y, et al. Crucial role of phospholipase Cepsilon in chemical carcinogen-induced skin tumor development. Cancer Res. 2004;64:8808–8810. doi: 10.1158/0008-5472.CAN-04-3143. [DOI] [PubMed] [Google Scholar]
- 41.Kim D, et al. Phospholipase C isozymes selectively couple to specific neurotransmitter receptors. Nature. 1997;389:290–293. doi: 10.1038/38508. [DOI] [PubMed] [Google Scholar]
- 42.Wang S, et al. Targeted disruption of the mouse phospholipase C beta3 gene results in early embryonic lethality. FEBS Lett. 1998;441:261–265. doi: 10.1016/S0014-5793(98)01518-X. [DOI] [PubMed] [Google Scholar]
- 43.Hashimoto A, et al. Cutting edge: essential role of phospholipase C-gamma 2 in B cell development and function. J. Immunol. 2000;165:1738–1742. doi: 10.4049/jimmunol.165.4.1738. [DOI] [PubMed] [Google Scholar]
- 44.Mehlmann LM, Carpenter G, Rhee SG, Jaffe LA. SH2 domain-mediated activation of phospholipase Cgamma is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev. Biol. 1998;203:221–232. doi: 10.1006/dbio.1998.9051. [DOI] [PubMed] [Google Scholar]
- 45.Hochreiter B, Garcia AP, Schmid JA. Fluorescent proteins as genetically encoded FRET biosensors in life sciences. Sensors (Basel) 2015;15:26281–26314. doi: 10.3390/s151026281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niino Y, Hotta K, Oka K. Simultaneous live cell imaging using dual FRET sensors with a single excitation light. PLoS One. 2009;4:e6036. doi: 10.1371/journal.pone.0006036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao M, Wan X, Li Y, Zhou W, Peng L. Multiplexed 3D FRET imaging in deep tissue of live embryos. Sci. Rep. 2015;5:13991. doi: 10.1038/srep13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehlmann LM, Kline D. Regulation of intracellular calcium in the mouse egg: calcium release in response to sperm or inositol trisphosphate is enhanced after meiotic maturation. Biol. Reprod. 1994;51:1088–1098. doi: 10.1095/biolreprod51.6.1088. [DOI] [PubMed] [Google Scholar]
- 49.Jones KT, Nixon VL. Sperm-induced Ca2+ oscillations in mouse oocytes and eggs can be mimicked by photolysis of caged inositol 1,4,5-trisphosphate: evidence to support a continuous low level production of inositol 1,4,5-trisphosphate during mammalian fertilization. Dev. Biol. 2000;225:1–12. doi: 10.1006/dbio.2000.9826. [DOI] [PubMed] [Google Scholar]
- 50.Kashir J, Nomikos M, Lai FA. Phospholipase C zeta and calcium oscillations at fertilisation: The evidence, applications, and further questions. Adv. Biol. Regul. 2018;67:148–162. doi: 10.1016/j.jbior.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 51.Larman MG, Saunders CM, Carroll J, Lai FA, Swann K. Cell cycle-dependent Ca2+ oscillations in mouse embryos are regulated by nuclear targeting of PLCzeta. J. Cell Sci. 2004;117:2513–2521. doi: 10.1242/jcs.01109. [DOI] [PubMed] [Google Scholar]
- 52.Iwai M, et al. Molecular cloning of mouse type 2 and type 3 inositol 1,4,5-trisphosphate receptors and identification of a novel type 2 receptor splice variant. J. Biol. Chem. 2005;280:10305–10317. doi: 10.1074/jbc.M413824200. [DOI] [PubMed] [Google Scholar]
- 53.Natsume T, Hirota J, Yoshikawa F, Furuichi T, Mikoshiba K. Real time analysis of interaction between inositol 1,4, 5-trisphosphate receptor type I and its ligand. Biochem. Biophys. Res. Commun. 1999;260:527–533. doi: 10.1006/bbrc.1999.0905. [DOI] [PubMed] [Google Scholar]
- 54.Whittingham DG. Culture of mouse ova. J. Reprod. Fertil. Suppl. 1971;14:7–21. [PubMed] [Google Scholar]
- 55.Kumakiri J, Oda S, Kinoshita K, Miyazaki S. Involvement of Rho family G protein in the cell signaling for sperm incorporation during fertilization of mouse eggs: inhibition by Clostridium difficile toxin B. Dev. Biol. 2003;260:522–535. doi: 10.1016/S0012-1606(03)00273-2. [DOI] [PubMed] [Google Scholar]
- 56.Behringer, R., Gertsenstein, M., Nagy, K. & Nagy, A. Manipulating the mouse embryo. (Cold Spring Harbor Laboratory Press, 1986).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.