From the Authors:
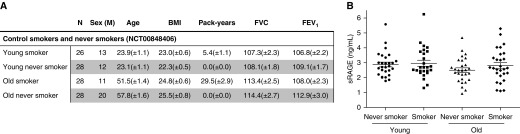
In our recent research letter focusing on the acute effects of smoking on the serum levels of sRAGE (soluble receptor for advanced glycation end products), we showed that smoking three cigarettes within 1 hour significantly decreases serum sRAGE levels within 2 hours (1). In addition, we also determined the effect of chronic cigarette smoke exposure on serum sRAGE levels by comparing smokers with never smokers (originally reported as “data not shown”). Here, we did not find any difference in serum sRAGE levels, which is in line with previous studies (2). In contrast, as rightfully mentioned in the response to our research letter, Biswas and colleagues previously reported that serum sRAGE levels were increased in smokers compared with nonsmokers (3). Biswas explains the discrepancies between their study and other studies by noting that most of the studies were not specifically designed to explore the effect of smoking on sRAGE in healthy individuals, whereas their study was (4). Further, in his original paper, he states that the differences may also be explained by the fact that his study population was of overall younger age compared with those in the other studies (3). Indeed, characteristics of the study population may affect the outcomes of sRAGE measurements; however, other factors, including the method and timing of serum preparation, the method of sRAGE quantification, and, most importantly, the timing of the last smoked cigarette before blood sampling may also drive the observed differences in serum sRAGE levels. Although our initial research letter only showed data of serum sRAGE levels in healthy control subjects versus patients with chronic obstructive pulmonary disease (COPD) (1), our study was also designed to investigate the chronic effects of smoking in healthy individuals. Specifically, to investigate the effect of chronic smoke exposure on the serum levels of sRAGE, we used a well-controlled cohort (ClinicalTrials.gov Identifier: NCT00848406) of young (18–40 yr old) and old (>40 yr old) smokers and never smokers without airway obstruction (Figure 1A) (5). To adequately measure serum sRAGE levels, we used the highly sensitive and selective simplified immunoprecipitation in 96-well ELISA format–coupled liquid chromatography–mass spectrometry assay, which we recently demonstrated to be superior to the commonly used sRAGE ELISA (6). When we focused on the healthy control subjects, we found that there were no differences in serum sRAGE levels between smokers and never smokers, whether old or young (Figure 1B). Of note, the definition of “nonsmokers” in our study and the one used by Biswas are not exactly the same. Whereas our nonsmokers had never smoked, the nonsmokers in Biswas’s study included subjects who had not smoked during the last 5 consecutive years. Moreover, the age of the study subjects does not influence the serum sRAGE levels. Indeed, our data show no differences in serum sRAGE levels between young (average 23.5 yr) and old (average 54.7 yr) subjects in either the never-smokers group or the smokers group (Figure 1B). In addition, our young subjects were even younger than the study population of Biswas, which had an average age of 34.1 years. Our data therefore indicate that neither age nor chronic smoke exposure affects serum sRAGE levels. The discrepancy in study results when comparing the serum sRAGE levels in smokers and nonsmokers may be explained by our finding that smoking before blood sampling acutely decreases serum sRAGE levels (1). Therefore, controlling or monitoring smoking behavior before blood sampling may be used as a precautionary measure to decrease the variability between measurements and increase the value of sRAGE as a biomarker for COPD. Lastly, we agree with Biswas that more research is needed regarding the effect of smoking on serum sRAGE levels and the underlying mechanisms before sRAGE can be clinically used as a biomarker for COPD.
Figure 1.
Serum sRAGE (soluble receptor for advanced glycation end products) levels in never smokers and smokers. (A) Patient characteristics. BMI = body mass index (kg/m2); N = number of group participants; sex (M) = number of males in a group. Data are shown as mean ± SEM. (B) Levels of sRAGE were measured in serum of young (18–40 yr old) smokers (n = 26) and nonsmokers (n = 28), and age-matched old (>40 yr old) smokers (n = 28) and nonsmokers (n = 28) without airway obstruction using immunoprecipitation in 96-well ELISA format–coupled liquid chromatography–mass spectrometry. Data are shown as individual measurements and mean ± SEM.
Supplementary Material
Footnotes
Supported by the Netherlands Organization for Scientific Research NWO (Domain Applied and Engineering Sciences; Perspectief program P12-04; projects 13541 and 13544), Lung Foundation Netherlands (project 6.2.15.044JO), and Noordelijke CARA Stichting (project: 2016/01).
Author Contributions: Conception and design of the study: S.D.P., F.K., M.K., P.H., R.B., and N.H.T.t.H. Acquisition of data: S.D.P., F.K., M.K., V.R.W., and A.F. Analysis of data: S.D.P., F.K., M.K., V.R.W., and A.F. Drafting of the manuscript: S.D.P. and F.K. Manuscript revision: S.D.P., F.K., M.K., V.R.W., A.F., M.v.d.B., P.H., R.B., and N.H.T.t.H.
Originally Published in Press as DOI: 10.1164/rccm.201812-2257LE on December 27, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Pouwels SD, Klont F, Kwiatkowski M, Wiersma VR, Faiz A, van den Berge M, et al. Cigarette smoking acutely decreases serum levels of the chronic obstructive pulmonary disease biomarker sRAGE. Am J Respir Crit Care Med. 2018;198:1456–1458. doi: 10.1164/rccm.201807-1249LE. [DOI] [PubMed] [Google Scholar]
- 2.Prasad K, Dhar I, Caspar-Bell G. Role of advanced glycation end products and its receptors in the pathogenesis of cigarette smoke-induced cardiovascular disease. Int J Angiol. 2015;24:75–80. doi: 10.1055/s-0034-1396413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas SK, Mudi SR, Mollah FH, Bierhaus A, Arslan MI. Serum soluble receptor for advanced glycation end products (sRAGE) is independently associated with cigarette smoking in non-diabetic healthy subjects. Diab Vasc Dis Res. 2013;10:380–382. doi: 10.1177/1479164113479618. [DOI] [PubMed] [Google Scholar]
- 4.Biswas SK. What does cigarette smoking do to the circulating level of soluble receptor for advanced glycation end products? Int J Angiol. 2016;25:137–138. doi: 10.1055/s-0036-1579690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imkamp K, Berg M, Vermeulen CJ, Heijink IH, Guryev V, Kerstjens HAM, et al. Nasal epithelium as a proxy for bronchial epithelium for smoking-induced gene expression and expression Quantitative Trait Loci. J Allergy Clin Immunol. 2018;142:314–317.e15, e15. doi: 10.1016/j.jaci.2018.01.047. [DOI] [PubMed] [Google Scholar]
- 6.Klont F, Pouwels SD, Hermans J, van de Merbel NC, Horvatovich P, Ten Hacken NHT, et al. A fully validated liquid chromatography-mass spectrometry method for the quantification of the soluble receptor of advanced glycation end-products (sRAGE) in serum using immunopurification in a 96-well plate format. Talanta. 2018;182:414–421. doi: 10.1016/j.talanta.2018.02.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.