Abstract
Rationale: MUC5AC and MUC5B are the predominant gel-forming mucins in the mucus layer of human airways. Each mucin has distinct functions and site-specific expression. However, the regional distribution of expression and cell types that secrete each mucin in normal/healthy human airways are not fully understood.
Objectives: To characterize the regional distribution of MUC5B and MUC5AC in normal/healthy human airways and assess which cell types produce these mucins, referenced to the club cell secretory protein (CCSP).
Methods: Multiple airway regions from 16 nonsmoker lungs without a history of lung disease were studied. MUC5AC, MUC5B, and CCSP expression/colocalization were assessed by RNA in situ hybridization and immunohistochemistry in five lungs with histologically healthy airways. Droplet digital PCR and cell cultures were performed for absolute quantification of MUC5AC/5B ratios and protein secretion, respectively.
Measurements and Main Results: Submucosal glands expressed MUC5B, but not MUC5AC. However, MUC5B was also extensively expressed in superficial epithelia throughout the airways except for the terminal bronchioles. Morphometric calculations revealed that the distal airway superficial epithelium was the predominant site for MUC5B expression, whereas MUC5AC expression was concentrated in proximal, cartilaginous airways. RNA in situ hybridization revealed MUC5AC and MUC5B were colocalized with CCSP-positive secretory cells in proximal superficial epithelia, whereas MUC5B and CCSP-copositive cells dominated distal regions.
Conclusions: In normal/healthy human airways, MUC5B is the dominant secretory mucin in the superficial epithelium and glands, with distal airways being a major site of expression. MUC5B and MUC5AC expression is a property of CCSP-positive secretory cells in superficial airway epithelia.
Keywords: airway mucins, MUC5AC, MUC5B, club cells, distal airways
At a Glance Commentary
Scientific Knowledge on the Subject
MUC5AC and MUC5B are the major gel-forming mucins in the mucus layer of human airways. Each mucin has a distinct regional expression. However, a systematic study of the site- and cell-specific expression of MUC5B and MUC5AC in normal/healthy human airways is not available.
What This Study Adds to the Field
This study provides a comprehensive description of secretory mucin expression in the normal/healthy human lung essential for understanding how abnormal regulation of secretory mucin expression contributes to the pathogenesis of mucoobstructive lung diseases. RNA in situ hybridization and immunohistochemistry characterized regional MUC5AC and MUC5B expression and cell types expressing these mucins along the proximal–distal axis of the normal/healthy human lung. MUC5B is the dominant secretory mucin in not only submucosal glands but also superficial airway epithelia. The distal airways are a major site of MUC5B expression and constitute a source of MUC5B production equivalent in importance to submucosal glands. The predominant region for MUC5AC production is the proximal (cartilaginous) airway superficial epithelium. MUC5B and MUC5AC production are properties of club cell secretory protein–expressing cells in both proximal and distal superficial airway epithelia.
Mucociliary clearance is a critical innate defense system for maintenance of lung health. Failed mucus clearance is a common feature of mucoobstructive lung diseases, yet how abnormalities in mucus properties produce intrapulmonary mucus accumulation remains unclear. Part of this uncertainty relates to the absence of a comprehensive formulation that describes all elements of mucus transport in health.
MUC5AC and MUC5B are the predominant gel-forming mucins in human airways (1–4). The classic paradigm describes MUC5AC secretion in human airways as a feature of superficial epithelial goblet cells, whereas MUC5B is predominantly secreted from submucosal glands (SMG) (2, 5–8). However, previous data generated from mice have demonstrated that the superficial epithelial secretory club cells in large and small airways produce Muc5b, which is rapidly secreted in the absence of Alcian Blue and periodic acid–Schiff (AB-PAS)-definable mucin granules or goblet cell morphology (9–13). Moreover, studies with Muc5b knockout mice demonstrated that Muc5b was required for normal lung defense and mucociliary clearance (14), despite the fact that SMG in the mice are restricted to the proximal trachea (15).
Recent studies of normal and diseased human lungs suggest the murine paradigm may also be pertinent to the human lung. Most mucoobstructive diseases (e.g., cystic fibrosis and chronic obstructive pulmonary disease) are associated with small airway mucus plugs that are composed of MUC5B and MUC5AC (16–18). Importantly, recent studies of normal and diseased tissues have established the presence of MUC5B expression in superficial epithelia, suggesting distal airway superficial epithelia may locally secrete the MUC5B associated with plugging (19–21). Of particular relevance to small airway MUC5B expression is the discovery of the link between MUC5B overexpression in peripheral airways and idiopathic pulmonary fibrosis (IPF) (22, 23).
These findings led us to perform a comprehensive study of region- and cell-specific expression of MUC5B and MUC5AC with an emphasis on comparisons of large airways with superficial epithelia and SMG versus small airways lined exclusively with superficial epithelia. To achieve this aim, multiple complementary approaches, including RNA in situ hybridization (ISH), immunohistochemistry, droplet digital PCR (ddPCR) quantification, and primary cell cultures, were used to quantitate the regional distribution of MUC5B and MUC5AC expression along the proximal–distal axis of the normal/healthy human lung. Based on murine data (10–14), we also explored the hypothesis that club cell secretory protein (CCSP) (also termed secretoglobin 1A member 1)-expressing secretory cells produce MUC5B, and MUC5AC, in human superficial airway epithelia. Some of the results of this study have been previously reported in the form of abstracts (24, 25).
Methods
For further details on the applied methods, see the online supplement.
Subjects and Tissue Collection
Lungs that provided airways for study were obtained from “normal” subjects, defined as no history of cigarette smoking or lung disease, who had been maintained on mechanical ventilation less than or equal to 7 days as potential lung transplant donors. Sixteen consecutive lungs (May 2016–March 2018) that were not eligible for transplant were studied. Table E1 in the online supplement shows demographics of the subjects studied. The protocol for lung dissection was approved by University of North Carolina Institutional Review Board. MUC5B promoter variant rs35705950 genotypes were determined using TaqMan (Life Technologies) genotyping (26).
Tissue Preparation, Immunohistochemistry, and RNA ISH
Excised lungs, obtained from 10 of the 16 normal subjects studied, were dissected to obtain tracheas, primary bronchi, segmental bronchi, and lung parenchyma containing distal bronchi and bronchioles (see Figure E1). Tissue specimens were fixed, 5-μm sections cut, and serial sections stained by hematoxylin and eosin (H&E) and AB-PAS. Because mechanical ventilation can induce goblet cell metaplasia and/or hyperplasia (GMH) in lungs of normal subjects (i.e., a ventilator-induced unhealthy state) (27–31), airways were histologically assessed for “health” by a pathologist. Five of the 10 lungs were selected as histologically “healthy” as defined by volume densities occupied by mucin-expressing cells in an airway epithelial region. We term these five lungs without airway GMH “normal/healthy.” This definition of normal/healthy is presented in detail in the online supplement. MUC5AC, MUC5B, and CCSP mRNA localization was assessed by RNA ISH using RNAscope (Advanced Cell Diagnostics) (see Figure E2) (32). Protein localization was assessed by immunohistochemistry as described (33). Antibodies and RNA ISH probes are shown in Table E2.
Morphometric Analysis
The airways were classified as: 1) trachea; 2) primary bronchi; 3) segmental bronchi; 4) distal bronchi (>2 mm in diameter); 5) proximal bronchioles (1–2 mm in diameter); 6) distal bronchioles (<1 mm in diameter); and 7) terminal bronchioles, based on airway diameters and morphology. All histologic sections were digitally scanned using an Olympus VS120 microscope. Table E3 shows the number of airways and the lengths of basement membrane studied for each airway region. Volume densities for mucous glycoproteins, MUC5AC, MUC5B, and CCSP mRNAs and proteins within airway superficial epithelia, obtained from AB-PAS staining, RNA ISH, or immunohistochemistry, were quantified by morphometric methods (13, 34–36) (see Figure E3). The number of cells expressing target genes was manually counted to assess gene colocalization and normalized to airway basement membrane lengths (see Figure E4). Calculations of region-specific total mucins and CCSP mRNA–stained volumes, using Weibel stereologic parameters (37, 38), were performed as described in the Methods section of the online supplement (see Table E4).
Absolute Quantification of MUC5AC and MUC5B Transcript Copy Numbers in Human Airway Epithelia
Designated airway regions, including trachea, segmental bronchi, bronchioles, and peripheral lung parenchyma obtained from 6 of the 16 normal subjects studied, were examined for absolute quantification of MUC5AC and MUC5B transcript copy numbers by ddPCR (Bio-Rad) (39).
Large and Small Airway Epithelial Cell Culture
Matched large and small airway epithelial (LAE and SAE, respectively) cells, isolated from four lifelong nonsmoker lungs, were expanded using a modified conditional reprogramming cell method (40, 41) to generate well-differentiated air–liquid interface cultures (see Figure E5) (42).
Mass Spectrometry
Quantification of MUC5B, MUC5AC, and surfactant protein B (SFTPB) proteins in apical washes of LAE and SAE cell cultures was performed by mass spectrometry (43, 44).
Statistical Analysis
Statistics were performed using R version 3.5.1 (R Foundation for Statistical Computing). Comparison between two groups was performed by Wilcoxon rank sum test and comparison between three or more groups was performed by Kruskal-Wallis test, followed by pairwise Wilcoxon rank sum test for post hoc analysis. A P value of less than 0.05 was considered significant.
Results
The Regional Distribution of Mucous Glycoproteins in Superficial Airway Epithelium
AB-PAS staining to quantitate airway regional variations in superficial epithelial mucous glycoproteins, which include MUC5B and MUC5AC, was performed in 10 lungs (Figure 1). Airway GMH, defined as an AB-PAS–stained volume density greater than 0.005 mm3/mm2 per airway superficial epithelial region (36), was identified in one or more airway regions in 5 of the 10 subjects. AB-PAS–stained volume densities in subjects with airway GMH were generally greater than those in subjects with histologically healthy airways, particularly in larger proximal airways (Figure 1B). Based on the exclusion criteria, the five subjects without GMH in any airway region were selected for the RNA ISH and immunohistochemistry study of mucin expression as representative of normal/healthy lungs.
Figure 1.
Regional distribution of mucus glycoproteins in superficial airway epithelia from 10 subjects with no prior lung disease history. (A) Hematoxylin and eosin and Alcian Blue and periodic acid–Schiff (AB-PAS) staining of the superficial epithelium of primary bronchi in (i and ii) a subject with histologically healthy airways versus (iii and iv) a subject with airway goblet cell metaplasia. (B) Quantification of AB-PAS–positive mucus glycoproteins in airway superficial epithelium of different airway regions. AB-PAS–stained volume densities in airway superficial epithelium were quantified (n = 10). Each circle represents AB-PAS–stained volume density for each airway region obtained from subjects with histologically healthy airways (open circles; n = 5; see Ai and Aii) and subjects with airway goblet cell metaplasia and/or hyperplasia (solid circles; n = 5; see Aiii and Aiv). Mean AB-PAS–stained volume densities in subjects with histologically healthy airways were compared with those in subjects with airway goblet cell metaplasia and/or hyperplasia by Wilcoxon rank sum test. Each circle in distal bronchi and bronchioles represent mean values of the AB-PAS–stained volume densities from multiple airways per subject. No proximal bronchiole was available in one of the five subjects with airway goblet cell metaplasia and/or hyperplasia. Histogram bars and error bars depict mean ± SD from the 10 subjects. *P < 0.05 and **P < 0.01. BM = basement membrane; H&E = hematoxylin and eosin; NS = not significant. Scale bars = 50 μm.
The Regional Distribution of MUC5B and MUC5AC in Normal/Healthy Human Airways
RNA ISH and immunohistochemistry revealed extensive MUC5B, but not MUC5AC, mRNA, and protein localization in SMG (Figure 2). Both RNA ISH and immunohistochemistry demonstrated significantly greater stained volume densities for MUC5B, and MUC5AC, in the superficial epithelium of larger cartilaginous airways, including the trachea and bronchi, compared with bronchioles (Figures 2 and 3; see Figure E6). Notably, robust MUC5B, but not MUC5AC, mRNA, and protein staining, were detected in the distal bronchioles. In the terminal bronchioles, neither MUC5B nor MUC5AC was detected.
Figure 2.
Regional distribution of MUC5B and MUC5AC mRNA and protein localization in normal/healthy human airways. Serial sections from five different regions of airways from one normal/healthy lung were stained by hematoxylin and eosin and Alcian Blue and periodic acid–Schiff, and probed for MUC5B and MUC5AC by RNA in situ hybridization and immunohistochemistry. Scale bars = 40 μm. For details on the methods of image acquisition, see Quantitation of mucous glycoproteins, MUC5AC, MUC5B, and CCSP in AB-PAS, immunohistochemistry and RNA ISH in the online supplement. AB-PAS = Alcian Blue and periodic acid–Schiff; H&E = hematoxylin and eosin; IHC = immunohistochemistry; ISH = in situ hybridization.
Figure 3.
Quantification of MUC5B and MUC5AC mRNAs and proteins in normal/healthy human airway superficial epithelia. (A) MUC5B (i) and MUC5AC (ii) mRNA–stained volume densities in airway superficial epithelium from normal/healthy lungs were quantified (n = 5). (B) MUC5B (i) and MUC5AC (ii) protein–stained volume densities by immunohistochemistry were also quantified (n = 5). Histogram bars and error bars represent mean ± SD. Symbols represent the five distinct subjects. For distal bronchi and bronchioles, more than one airway per region was examined per subject. BM = basement membrane; ISH = in situ hybridization.
Two approaches were used to verify the RNA ISH and immunohistochemistry data suggesting that MUC5B is the dominant mucin in bronchioles. First, the relative sensitivities of the RNA ISH MUC5B versus MUC5AC probes were tested by comparing RNA ISH with ddPCR signals in pellets derived from clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9–modified A549 cells expressing MUC5AC and MUC5B, MUC5AC only, or MUC5B only. Data from three independent experiments revealed that the MUC5B probe exhibited 2.1 ± 0.3-fold increased sensitivity compared with MUC5AC (see Figure E7). Adjusting the RNA ISH data by this factor did not alter the conclusion that MUC5B was the dominantly expressed mucin in bronchioles. Second, sections from large versus small airways were obtained from six lungs, and ddPCR of superficial epithelia from each region was performed to compare MUC5B with MUC5AC transcript copy number in each airway region (Figure 4A). Absolute quantification of MUC5B and MUC5AC transcripts revealed that MUC5B normalized to GAPDH (MUC5B/GAPDH) was not different from MUC5AC/GAPDH in the larger cartilaginous airways, including trachea and bronchi. In contrast, MUC5B/GAPDH was significantly greater than MUC5AC/GAPDH in bronchioles (Figure 4B). Based on the MUC5AC/MUC5B ratio (Figure 4C), MUC5B transcript copy number was 20-fold higher than MUC5AC in bronchioles, consistent with RNA ISH/immunohistochemistry data.
Figure 4.
Droplet digital PCR quantification of MUC5B and MUC5AC transcript copy numbers in freshly isolated human airway epithelium from subjects with no prior lung disease history. (A) Examples of dissected airway tissues including a trachea, bronchus, bronchiole, and peripheral lung parenchyma. (B) Absolute transcript copy numbers in different airway regions for MUC5B (circles) and MUC5AC (triangles). (C) MUC5AC/MUC5B ratios in each airway region. Measurements in B and C were performed by droplet digital PCR, with absolute MUC5B or MUC5AC transcript copy numbers normalized to GAPDH and shown as target/GAPDH ratios. Histogram bars and error bars represent mean ± SD. n = 6. Different symbol colors indicate results from six distinct individual subjects. **P < 0.01 by Wilcoxon rank sum test. NS = not significant.
What Cell Type Expresses MUC5B and MUC5AC mRNAs in Normal/Healthy Human Large and Small Airways?
Based on mouse airway data (10–14), CCSP+ secretory cells were identified as candidates for secretory mucin expression in normal/healthy human airways. Both RNA ISH and immunohistochemistry demonstrated widespread superficial epithelial localization of CCSP mRNA and protein from the trachea to the terminal bronchioles (Figures 5A–5D; see Figure E8). With respect to SMG, CCSP mRNAs were expressed in the ciliated ducts but not collecting ducts nor acini (Figures 5E and 5F). In contrast, MUC5B mRNAs were localized throughout all SMG epithelial structures. Quantification of CCSP mRNA–stained volume densities revealed robust expression in the segmental, distal bronchi, and bronchioles compared with trachea or primary bronchi (Figure 5G).
Figure 5.
Regional distribution of CCSP mRNA localization in normal/healthy human airways. Representative CCSP mRNA localization from one normal/healthy lung stained by RNA in situ hybridization (ISH) in (A) trachea, (B) primary bronchus, (C) segmental bronchus, and (D) distal lung parenchyma containing distal bronchi and bronchioles. (E) CCSP and MUC5B mRNA localization stained by RNA ISH in normal/healthy tracheal submucosal gland ducts. CCSP (red) mRNA signals stop in the middle of the submucosal gland ducts (arrows) with MUC5B (teal) mRNA signals being expressed throughout the submucosal gland ducts. (F) CCSP and MUC5B mRNA localization costained using RNA ISH in the normal/healthy tracheal superficial epithelium and submucosal glands acini. (G) Quantification of CCSP mRNA–stained volume densities in normal/healthy human airway superficial epithelium (n = 5). CCSP mRNA–stained volume densities by RNA ISH were quantified. Histogram bars and error bars represent mean ± SD. Symbols represent the five distinct subjects. For distal bronchi and bronchioles, more than one airway per region was examined per subject. BM = basement membrane; CCSP = club cell secretory protein; SE = superficial epithelium; SMG-A = submucosal glands acini; SMG-D = submucosal gland ducts; TB = terminal bronchiole.
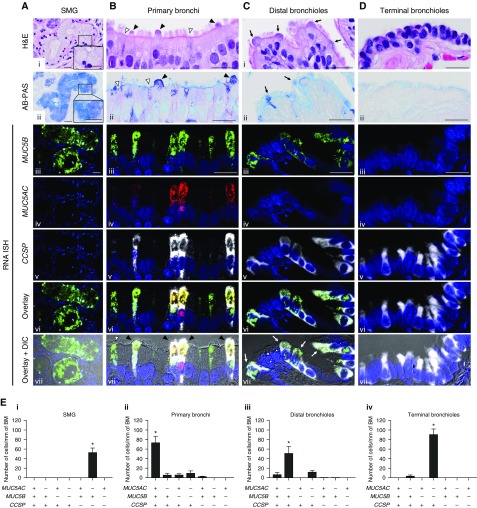
Quantitative colocalization studies were performed using fluorescent RNA ISH in five normal/healthy lungs (Figure 6). Typical goblet cells, characterized by AB-PAS–positive large secretory vesicles filling the cytoplasm, were rarely identified in superficial epithelia of these subjects (Figure 6Bii). In contrast, SMG consistently exhibited mucous cells with AB-PAS–definable large mucin granules (Figure 6Aii).
Figure 6.
MUC5B, MUC5AC, and CCSP mRNA coexpression is region-specific. (A–D) Colocalization of MUC5B and MUC5AC with CCSP mRNAs by RNA in situ hybridization (ISH) in four different regions of normal/healthy human airways. For cellular localization, MUC5B (green), MUC5AC (red), and CCSP (white) mRNAs were visualized by fluorescent RNA ISH. Single-color images were merged (Avi–Dvi, “overlay”) and the overlaid images superimposed on differential interference contrast (Avii–Dvii). In submucosal glands (A), mucus cells exhibited large mucin granules definable by Alcian Blue and periodic acid–Schiff (AB-PAS) staining (insets in Ai and Aii). In primary bronchial superficial epithelium (B), two nonciliated epithelial cell types were identified: 1) a nonciliated epithelial cell with an AB-PAS–stained apical bulge (Bi, Bii, and Bvii, black arrowheads) and 2) a nonciliated epithelial cell without the apical bulge (Bi, Bii, and Bvii, white arrowheads). In distal bronchioles (C), nonciliated epithelial cells with dome-shaped apical bulges (Ci and Cii, black arrows) corresponded to the CCSP+/MUC5B+/MUC5AC− cells (Cvii, white arrows). Nuclei were stained with DAPI (blue). Scale bars = 20 μm. (E) Quantification of cell types expressing MUC5B, MUC5AC, and/or CCSP mRNAs in different regions of normal/healthy human airways. Data are expressed as the number of each cell type per millimeter of basement membrane. Solid bars and error bars represent mean ± SD. n = 5. *P < 0.05 compared with every other cell type by pairwise Wilcoxon rank sum test for post hoc analysis, following significant Kruskal-Wallis test. BM = basement membrane; CCSP = club cell secretory protein; DIC = differential interference contrast; H&E = hematoxylin and eosin; SMG = submucosal glands.
Human airway cells expressing MUC5AC, MUC5B, CCSP mRNAs, or a combination of these markers were classified into the seven possible types (see Table E5). Four distinct, region-specific dominant cell types were identified among the seven possible types. First, CCSP−/MUC5B+/MUC5AC− cells were routinely found in SMG yet rarely, if at all, in superficial epithelia. Second, CCSP+/MUC5B+/MUC5AC+ cells were the dominant cell type in primary bronchial superficial epithelia. In this region, H&E and AB-PAS staining identified two nonciliated epithelial cell types: a nonciliated epithelial cell that had an AB-PAS–stained apical bulge protruding into the airway lumen; and a cell with apical cell membranes at the same height as those of adjacent ciliated cells without an apical bulge (Figures 6Bi and 6Bii). CCSP+/MUC5B+/MUC5AC+ cells exhibited morphologies similar to both cell types (Figure 6Bvii; see Figure E9). The number of CCSP+/MUC5B+/MUC5AC+ cells dramatically decreased in the bronchioles, and virtually disappeared in the terminal bronchioles, paralleling the decreased volume densities of MUC5AC mRNA and protein (Figure 3). Third, CCSP+/MUC5B+/MUC5AC− cells were the dominant cell type in the distal bronchiolar superficial epithelium. H&E staining identified nonciliated epithelial cells with dome-shaped apical bulges, which morphologically corresponded to the CCSP+/MUC5B+/MUC5AC− cells (Figures 6Ci and 6Cvii). Finally, although a small number of CCSP+/MUC5B+/MUC5AC− cells were detected, CCSP+/MUC5B−/MUC5AC− cells were the dominant cell type in the terminal bronchioles. Of note, CCSP+/MUC5B− cells in superficial epithelia of terminal bronchioles uniquely expressed SFTPB, which is known as a distal airway marker (see Figure E10) (45).
Primary Human SAE Cells Secrete MUC5B Protein In Vitro
Based on the reported distinct progenitor cell properties of large versus distal airway epithelial cells (40, 45, 46), we used an SAE versus LAE cell culture technique to test whether MUC5B protein was locally produced by small airway epithelia as predicted by in vivo RNA ISH and immunohistochemistry data. H&E staining revealed monolayer epithelia in SAE cell cultures versus stratified multilayers in LAE cells (Figures 7Ai and 7Aii), consistent with in vivo morphologic features of large versus small airway epithelia in human lungs (47, 48). As a distal airway marker (45), SFTPB was detected by quantitative PCR and mass spectrometry in SAE but not LAE cultured cells (Figures 7B and 7D). These findings indicate that our in vitro SAE cell culture model retained characteristics of distal airways distinguishable from large airways.
Figure 7.
MUC5B production in primary human small airway epithelial cell cultures. (A) Histologic images of large and small airway epithelial cell cultures. Air–liquid interface cultures of large airway epithelial (LAE) and small airway epithelial (SAE) cells were stained by hematoxylin and eosin (i and ii) and RNA in situ hybridization for MUC5B (iii and iv) and CCSP mRNAs (v and vi). MUC5B mRNAs (green) are localized in CCSP mRNA (red)–positive nonciliated cells in both LAE and SAE cells (v and vi, white arrows). Scale bars = 10 μm. (B) SFTPB transcript expression in LAE and SAE cells. SFTPB gene was specifically detected in SAE but not LAE cells by quantitative PCR. GAPDH was used as the reference gene. (C) Identification of MUC5B protein in apical washes of LAE and SAE cells. Immunoblots of apical washes were probed with antibody to MUC5B. Samples from both LAE and SAE cell cultures were run on the same gel. (D) Quantification of SFTPB protein in apical washes of LAE and SAE cells. SFTPB protein was identified by mass spectrometry (MS) using label-free quantification normalized to total precursor intensity. (E) Absolute concentrations of MUC5B protein in apical washes of LAE and SAE cells as determined by MS. (F) Absolute mucin concentrations for both MUC5B and MUC5AC in apical washes of SAE cells as determined by MS. B and C: n = 3, the order of three biologic replicates were the same in B and C. D and E: Histogram bars and error bars represent mean ± SD. n = 4, *P < 0.05 by Wilcoxon rank sum test. CCSP = club cell secretory protein; DIC = differential interference contrast; H&E = hematoxylin and eosin; ISH = in situ hybridization.
RNA ISH showed MUC5B mRNA localization in CCSP mRNA+ nonciliated secretory cells in both LAE and SAE cells (Figures 7Aiii–7Avi). Both Western blotting and mass spectrometry identified MUC5B protein in apical washes of SAE cells (Figures 7C and 7E), although less than secreted by LAE cells. In apical washes obtained from SAE cells, the concentration of MUC5B protein (6.04 ± 5.95 pmol/ml) was 9.2-fold higher than MUC5AC (0.66 ± 0.20 pmol/ml) (Figure 7F).
Region-Specific Superficial Epithelial Mucin Production in Normal/Healthy Human Lungs
To describe the airway regions contributing to total MUC5AC and MUC5B expression in the lung, the numbers, diameters, and total surface areas of airways as reported by Weibel and coworkers (37, 38) were used to calculate: total MUC5B, MUC5AC, or CCSP mRNA–stained volumes for the superficial epithelium of each airway region based on RNA ISH; and the percent of total MUC5B, MUC5AC, and CCSP mRNA–stained volumes for each airway region (Figure 8; see Table E4).
Figure 8.
Distinct regional distributions of MUC5AC, MUC5B, and CCSP mRNA localization in superficial epithelium of the normal/healthy lung. Data represent the calculated percent of total MUC5AC, MUC5B, or CCSP mRNA–stained volumes for each airway region. Total MUC5AC, MUC5B, or CCSP mRNA–stained volume for each airway region was calculated by multiplying the mean values of the RNA in situ hybridization volume densities for each airway region obtained from five normal/healthy lungs by predicted total surface area of corresponding airway regions. CCSP = club cell secretory protein.
MUC5B mRNA–stained volume in airway superficial epithelia increased from the proximal to the distal airways, reflecting the exponential increase in distal airway surface area. MUC5B mRNA–stained volume peaked in the distal bronchioles with a threefold higher value than observed in the cartilaginous large airways. In contrast, MUC5AC mRNA–stained volume peaked in the distal bronchi. Even though the combined surface area of proximal and distal bronchioles is more than 30-fold greater than distal bronchi, the percent of MUC5AC mRNA–stained volumes in bronchioles was less than distal bronchi. Importantly, both MUC5B and MUC5AC mRNA–stained volumes were negligible in the terminal bronchioles despite the fact that terminal bronchiolar surface area is the largest of the airway regions. For reference, CCSP mRNA–stained volume increased as a function of more distal regions and peaked in the terminal bronchioles.
Discussion
A comprehensive regional expression pattern of MUC5B and MUC5AC in normal/healthy human lungs has not been reported. Recent data from mice airways suggest that Muc5b is secreted by superficial epithelial club cells in both larger airways and bronchioles (9–12). Whether this paradigm pertains to normal/healthy human airways was the focus of this study.
It is difficult to obtain intact normal/healthy human lungs for studies of regional mucin expression. We adopted the criteria that a normal/healthy lung region had to be obtained from a lung donor: 1) without history of lung disease or smoking; 2) exposed to a mechanical ventilation for less than or equal to 7 days; and 3) did not exhibit GMH as defined by more than 0.005 mm3/mm2 AB-PAS–stained volume density in a superficial epithelial region, based on the mean value of mucin volume densities in healthy subject group in transbronchial biopsy study (36). Using these criteria, 5 of the 10 lungs from study subjects met the criteria for normal/healthy lungs. We speculate that the five subjects that exhibited airway GMH were more sensitive to the stresses of the mechanical ventilation, which has been shown to rapidly induce GMH in primary human cell culture models, animal models, and preterm infants (27–31). If indeed the excluded lungs were normal/healthy before mechanical ventilation, our data may document how rapidly and substantially large airway GMH can be induced by mechanical stresses (Figure 1B).
Our results demonstrate that MUC5B is extensively expressed in the superficial epithelium, in addition to SMG, of normal/healthy human airways. Importantly, these findings indicate that a major site for MUC5B production is the small airway superficial epithelia where SMG do not exist (Figure 8). These findings are consistent with the results of RNA-seq quantification of the normal human SAE transcriptome (49), and studies describing high expression of MUC5B versus MUC5AC in normal distal airway epithelium (21).
Our studies permitted a semiquantitative description of the relative expression of MUC5B in the SMG versus small airways of the normal/healthy human lung. In the large airways (e.g., from trachea to sixth generation bronchi) SMG occupy a volume of ∼0.10 mm3/mm2 airway surface area in nonsmokers (50), and the mucous cell percentage of total SMG volume is about 40% (51, 52). Based on these numbers and regional airway surface areas (37, 38) (see Table E6), the MUC5B mRNA–stained volume in distal bronchiolar superficial epithelia exceeded the total mucin volume of the SMG (see Figure E11). Although it is difficult to predict secretion rates based on stained volumes, the superficial epithelium of small airways may be considered a source of MUC5B production at least equal to SMG.
Interestingly, the SMG and superficial epithelium provide redundant sources for MUC5B in central airway locations. One unresolved issue is whether the superficial epithelium provides the basal MUC5B secretion in this region, as it likely does in mice (10, 11), with SMG providing intermittent secretion to acutely trap inhaled irritants that provoke cough responses. A second unresolved issue with regard to MUC5B biology in the large airways is whether superficial epithelial MUC5B differs from gland-derived MUC5B. Differences may occur because MUC5B seems to be secreted in large airways from different cell types. A CCSP-positive cell without definable granules secretes MUC5B in the superficial epithelium, whereas MUC5B is secreted in SMG from CCSP-negative cells with large mucin storage granules. MUC5B is known to exist as two glycoforms with different charges, a low and a high charge, and the low-charge MUC5B is elevated in the sputum from the subjects with asthma, cystic fibrosis, and chronic obstructive pulmonary disease (3, 53). Further studies are required to determine whether MUC5B glycan-based charge differs in MUC5B secreted by SMG versus the superficial epithelium.
Our results revealed that neither MUC5B nor MUC5AC is expressed in terminal bronchioles. This finding was also apparent in the group of five subjects with airway GMH (Figure 1B). Interestingly, this finding mimics the absence of Muc5b expression reported in terminal bronchioles of mice (10). Moreover, it is notable that a subtype of CCSP+ cells exists in the terminal bronchioles that do not express MUC5B but do express SFTPB (see Figure E10), consistent with previous RNA ISH findings (54). These data are consistent with reports that SFTPB and mucin gene expression are inversely regulated through NK2 homeobox1 at the transcription level (55–57). The finding that secretory mucins are not expressed in terminal bronchioles suggests that the physiology of terminal bronchioles requires a surfactant-rich, mucin-free zone to protect gas exchange functions in alveoli adjacent to terminal bronchioles. Because MUC5B overexpression in peripheral airways is reported in patients with IPF (21–23), it is possible that the loss of a surfactant-rich terminal bronchiolar mucin-free zone is associated with IPF pathogenesis.
In contrast to MUC5B, which is necessary to sustain mucociliary clearance (14), MUC5AC has been recognized as a “response mucin” with expression regulated by several inflammatory stimuli (9, 19, 29). The highly responsive nature of MUC5AC suggests that it provides critical innate immune functions during airway stresses. Our study revealed that MUC5AC-stained volume density peaked at the level of segmental bronchi. Maximal deposition of particles greater than 1 μm diameter per unit surface area occurs in this region (58), suggesting that MUC5AC is responding to the load of external stimuli deposited in this region. Importantly, it seemed that MUC5AC expression was superimposed on MUC5B/CCSP-expressing cells; and as MUC5AC expression increased, the cell morphology appeared more goblet cell–like.
The RNA ISH approach identified four distinct types of airway secretory cells based on the combination of MUC5AC, MUC5B, and CCSP transcript expression. Each cell-type predominantly exists in different airway regions and SMG versus superficial epithelium (Figure 6). Airway surface secretory epithelial cells have conventionally been divided into four distinct cell types based on morphology and ultrastructure: 1) mucous (goblet), 2) serous, 3) club (Clara), and 4) neuroendocrine cells (59). Mucous cells are mainly located in the tracheobronchial tree and rarely in bronchioles (60). Serous cells have been described in surface epithelium in adult human small bronchi and bronchioles, whereas club cells have been considered to be the predominant secretory cell type in the human bronchiole (61). Juxtaposing these data to our results, CCSP was shown to be expressed not only in typical club cells in distal airways but also nonciliated airway secretory cells in large airway superficial epithelia. The CCSP mRNA+ cells typically expressed MUC5B/MUC5AC mRNAs in large airway epithelia and MUC5B mRNAs in small airway epithelia. Thus, a subset of CCSP+ cells seems to define a mucin secretory cell type in superficial epithelia.
Consistent with our findings, single-cell transcriptome analysis of human airway epithelial club cells revealed that a subset of CCSP+ cells coexpressed MUC5B/MUC5AC, indicating possible lineage relationship between CCSP+ secretory cells and mucin-secreting cells in human airways (62). In contrast, MUC5B-producing cells in SMG may reflect a different lineage because CCSP expression was absent in SMG mucous cells. Morphologically, CCSP+/MUC5B+/MUC5AC− cells in distal airways resembled typical club cells, whereas the morphology of CCSP+/MUC5B+/MUC5AC+ cells in proximal larger airways was variable, reflecting club cells and cells that were termed “mucous cells,” “serous cells,” or “indeterminate cells” (63, 64). Our data suggest that the current nomenclature of airway secretory cells based on morphologic characteristics may need to be revisited.
Our in vivo finding that CCSP+ cells in small airways have the capacity to produce MUC5B was supported by in vitro LAE and SAE cell culture data. Interestingly, airway epithelial cells isolated from large and small airway regions were differentiated into phenotypes that maintained region-specific characteristics when cultured under identical conditions. This result recapitulates the previous findings that proximal and distal airway basal cells exhibited region-specific gene expression profiles and progenitor properties during lung regeneration in mice (46, 65).
There are limitations to our morphologic studies. First, the study group was small, particularly for the RNA ISH and immunohistochemistry studies, because of the strict inclusion criteria used to select normal/healthy lungs. Second, it is possible that degranulation of the stored mucin protein from superficial epithelial cells occurred during the tissue isolation, which might result in underestimation of AB-PAS- and mucin protein–stained volumes compared with those in vivo. However, MUC5B and MUC5AC localization patterns obtained from RNA ISH were consistent with carbohydrate/protein localization data, suggesting degranulation may not have occurred (Figure 3). Moreover, we tested whether expression of forkhead box A3 (FOXA3), a transcription factor typically expressed in goblet cells (66), might be helpful in addressing this question. Immunohistochemistry experiments revealed positive signals for FOXA3 protein in the nuclei of goblet cells in airway epithelium with GMH, whereas FOXA3 staining was absent in normal/healthy airway epithelium (see Figure E12). These data, therefore, suggest that the “normal/healthy” airway epithelium with less AB-PAS–stained area did not contain the degranulated goblet cells. Finally, the affinity of probes for RNA ISH differed for MUC5AC and MUC5B. Direct measurements of relative RNA ISH MUC5B versus MUC5AC probe affinities in gene-edited A549 cells, however, provided a useful correction factor. Collectively, the RNA ISH quantitation of MUC5AC and MUC5B, the absolute mucin transcript expression data by ddPCR in freshly isolated airway surface epithelium, and the cell culture data all agreed that MUC5B is the dominant expressed and secreted mucin in distal human superficial airway epithelia.
In conclusion, MUC5B is extensively expressed in human airway superficial epithelia in addition to SMG. The distal airway region is the major site for MUC5B production in the superficial epithelium of the human lung. MUC5AC is normally only produced by the superficial epithelia of the cartilaginous larger airways. Both MUC5B and MUC5AC are colocalized in CCSP+ cells in proximal superficial epithelium, whereas MUC5B is colocalized in CCSP+ cells of distal airway superficial epithelia. Our study suggests an important contribution to mucin secretion by the superficial epithelium of the distal airways that are the initial site of mucus plugging in mucoobstructive and possibly IPF diseases.
Supplementary Material
Acknowledgments
Acknowledgment
The authors appreciate University of North Carolina CF Center Tissue Procurement and Cell Culture Core for providing human lung tissues, Kimberlie A. Burns for histologic samples, Lisa C. Jones and Kristy A. Terrell for genotyping MUC5B promoter variant, and Matthew R. Markovetz for supporting the morphometric analysis.
Footnotes
Supported by grants from NIH NHLBI (R01 HL 136961, R01 HL 110906, R01 HL 103940, P01 HL 110873, P01 HL 108808, and UH2 HL 123645), NIH National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK 065988), and the Cystic Fibrosis Foundation (BOUCHE15R0 and SUBRAM1710) and by a fellowship from Cystic Fibrosis Research, Inc.
Author Contributions: S.H.R. provided human lung samples. K.O., T.K., and R.C.G. performed immunostainings and RNA in situ hybridization. C.M.D. analyzed histopathology. M.C. performed confocal microscopy imaging. K.O. and M.W. analyzed morphometry and quantified images. D.B.S. generated and provided gene-edited A549 cells. G.R. and M.K. performed mass spectrometry. G.C., A.L.-B., and C.E. provided technical knowledge. H.D. contributed to statistical analysis. H.M. and T.N. provided guidance and feedback to the overall work. W.K.O’N. and R.C.B. designed and supervised the project. K.O., W.K.O’N., and R.C.B. drafted and finalized the manuscript. All authors approved the final version of the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201804-0734OC on October 23, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kesimer M, Kirkham S, Pickles RJ, Henderson AG, Alexis NE, Demaria G, et al. Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol. 2009;296:L92–L100. doi: 10.1152/ajplung.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buisine MP, Devisme L, Copin MC, Durand-Réville M, Gosselin B, Aubert JP, et al. Developmental mucin gene expression in the human respiratory tract. Am J Respir Cell Mol Biol. 1999;20:209–218. doi: 10.1165/ajrcmb.20.2.3259. [DOI] [PubMed] [Google Scholar]
- 3.Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J. 2002;361:537–546. doi: 10.1042/0264-6021:3610537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kesimer M, Ehre C, Burns KA, Davis CW, Sheehan JK, Pickles RJ. Molecular organization of the mucins and glycocalyx underlying mucus transport over mucosal surfaces of the airways. Mucosal Immunol. 2013;6:379–392. doi: 10.1038/mi.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Audie JP, Janin A, Porchet N, Copin MC, Gosselin B, Aubert JP. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J Histochem Cytochem. 1993;41:1479–1485. doi: 10.1177/41.10.8245407. [DOI] [PubMed] [Google Scholar]
- 6.Groneberg DA, Eynott PR, Oates T, Lim S, Wu R, Carlstedt I, et al. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Respir Med. 2002;96:81–86. doi: 10.1053/rmed.2001.1221. [DOI] [PubMed] [Google Scholar]
- 7.Groneberg DA, Eynott PR, Lim S, Oates T, Wu R, Carlstedt I, et al. Expression of respiratory mucins in fatal status asthmaticus and mild asthma. Histopathology. 2002;40:367–373. doi: 10.1046/j.1365-2559.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- 8.Hovenberg HW, Davies JR, Carlstedt I. Different mucins are produced by the surface epithelium and the submucosa in human trachea: identification of MUC5AC as a major mucin from the goblet cells. Biochem J. 1996;318:319–324. doi: 10.1042/bj3180319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young HW, Williams OW, Chandra D, Bellinghausen LK, Pérez G, Suárez A, et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am J Respir Cell Mol Biol. 2007;37:273–290. doi: 10.1165/rcmb.2005-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Y, Ehre C, Abdullah LH, Sheehan JK, Roy M, Evans CM, et al. Munc13-2-/- baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol. 2008;586:1977–1992. doi: 10.1113/jphysiol.2007.149310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol. 2008;70:487–512. doi: 10.1146/annurev.physiol.70.113006.100638. [DOI] [PubMed] [Google Scholar]
- 12.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, et al. Mucin is produced by Clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defense. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson AG, Ehre C, Button B, Abdullah LH, Cai LH, Leigh MW, et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124:3047–3060. doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgel PR, Montani D, Danel C, Dusser DJ, Nadel JA. A morphometric study of mucins and small airway plugging in cystic fibrosis. Thorax. 2007;62:153–161. doi: 10.1136/thx.2006.062190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caramori G, Di Gregorio C, Carlstedt I, Casolari P, Guzzinati I, Adcock IM, et al. Mucin expression in peripheral airways of patients with chronic obstructive pulmonary disease. Histopathology. 2004;45:477–484. doi: 10.1111/j.1365-2559.2004.01952.x. [DOI] [PubMed] [Google Scholar]
- 19.Bonser LR, Zlock L, Finkbeiner W, Erle DJ. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J Clin Invest. 2016;126:2367–2371. doi: 10.1172/JCI84910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casalino-Matsuda SM, Monzon ME, Day AJ, Forteza RM. Hyaluronan fragments/CD44 mediate oxidative stress-induced MUC5B up-regulation in airway epithelium. Am J Respir Cell Mol Biol. 2009;40:277–285. doi: 10.1165/rcmb.2008-0073OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seibold MA, Smith RW, Urbanek C, Groshong SD, Cosgrove GP, Brown KK, et al. The idiopathic pulmonary fibrosis honeycomb cyst contains a mucociliary pseudostratified epithelium. PLoS One. 2013;8:e58658. doi: 10.1371/journal.pone.0058658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakano Y, Yang IV, Walts AD, Watson AM, Helling BA, Fletcher AA, et al. MUC5B promoter variant rs35705950 affects MUC5B expression in the distal airways in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193:464–466. doi: 10.1164/rccm.201509-1872LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conti C, Montero-Fernandez A, Borg E, Osadolor T, Viola P, De Lauretis A, et al. Mucins MUC5B and MUC5AC in distal airways and honeycomb spaces: comparison among idiopathic pulmonary fibrosis/usual interstitial pneumonia, fibrotic nonspecific interstitial pneumonitis, and control lungs. Am J Respir Crit Care Med. 2016;193:462–464. doi: 10.1164/rccm.201507-1322LE. [DOI] [PubMed] [Google Scholar]
- 24.Okuda K, Chen G, Kato T, Wolf M, Gilmore R, Burns K, et al. Regional expression of secretory mucins MUC5AC and MUC5B in normal human airways [abstract] Am J Respir Crit Care Med. 2018;197:A7636. [Google Scholar]
- 25.Okuda K, Chen G, Wolf M, Burns K, Chua M, Livraghi-Butrico A, et al. Localization of secretory mucins MUC5AC and MUC5B in normal human airways [abstract] Pediatr Pulmonol. 2017;52:S238. [Google Scholar]
- 26.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hislop AA, Haworth SG. Airway size and structure in the normal fetal and infant lung and the effect of premature delivery and artificial ventilation. Am Rev Respir Dis. 1989;140:1717–1726. doi: 10.1164/ajrccm/140.6.1717. [DOI] [PubMed] [Google Scholar]
- 28.Deputla N, Royse E, Kemp MW, Miura Y, Kallapur SG, Jobe AH, et al. Brief mechanical ventilation causes differential epithelial repair along the airways of fetal, preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2016;311:L412–L420. doi: 10.1152/ajplung.00181.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JA, Tschumperlin DJ. Chronic intermittent mechanical stress increases MUC5AC protein expression. Am J Respir Cell Mol Biol. 2009;41:459–466. doi: 10.1165/rcmb.2008-0195OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hillman NH, Moss TJ, Kallapur SG, Bachurski C, Pillow JJ, Polglase GR, et al. Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am J Respir Crit Care Med. 2007;176:575–581. doi: 10.1164/rccm.200701-051OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koeppen M, McNamee EN, Brodsky KS, Aherne CM, Faigle M, Downey GP, et al. Detrimental role of the airway mucin Muc5ac during ventilator-induced lung injury. Mucosal Immunol. 2013;6:762–775. doi: 10.1038/mi.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livraghi-Butrico A, Grubb BR, Wilkinson KJ, Volmer AS, Burns KA, Evans CM, et al. Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease. Mucosal Immunol. 2017;10:395–407. doi: 10.1038/mi.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim V, Oros M, Durra H, Kelsen S, Aksoy M, Cornwell WD, et al. Chronic bronchitis and current smoking are associated with more goblet cells in moderate to severe COPD and smokers without airflow obstruction. PLoS One. 2015;10:e0116108. doi: 10.1371/journal.pone.0116108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim V, Kelemen SE, Abuel-Haija M, Gaughan JP, Sharafkaneh A, Evans CM, et al. Small airway mucous metaplasia and inflammation in chronic obstructive pulmonary disease. COPD. 2008;5:329–338. doi: 10.1080/15412550802522445. [DOI] [PubMed] [Google Scholar]
- 36.Ordoñez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med. 2001;163:517–523. doi: 10.1164/ajrccm.163.2.2004039. [DOI] [PubMed] [Google Scholar]
- 37.Wiggs BR, Moreno R, Hogg JC, Hilliam C, Pare PD. A model of the mechanics of airway narrowing. J Appl Physiol (1985) 1990;69:849–860. doi: 10.1152/jappl.1990.69.3.849. [DOI] [PubMed] [Google Scholar]
- 38.Weibel ER. Morphometry of the human lung. Berlin: Springer; 1963. Geometric and dimensional airway models of conductive, transitory and respiratory zones of the human lung; pp. 136–142. [Google Scholar]
- 39.Stauber J, Shaikh N, Ordiz MI, Tarr PI, Manary MJ. Droplet digital PCR quantifies host inflammatory transcripts in feces reliably and reproducibly. Cell Immunol. 2016;303:43–49. doi: 10.1016/j.cellimm.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Tang XX, Vargas Buonfiglio LG, Comellas AP, Thornell IM, Ramachandran S, et al. Electrolyte transport properties in distal small airways from cystic fibrosis pigs with implications for host defense. Am J Physiol Lung Cell Mol Physiol. 2016;310:L670–L679. doi: 10.1152/ajplung.00422.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gentzsch M, Boyles SE, Cheluvaraju C, Chaudhry IG, Quinney NL, Cho C, et al. Pharmacological rescue of conditionally reprogrammed cystic fibrosis bronchial epithelial cells Am J Respir Cell Mol Biol 2017;56:568–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 43.Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med. 2017;377:911–922. doi: 10.1056/NEJMoa1701632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kesimer M, Cullen J, Cao R, Radicioni G, Mathews KG, Seiler G, et al. Excess secretion of gel-forming mucins and associated innate defense proteins with defective mucin un-packaging underpin gallbladder mucocele formation in dogs. PLoS One. 2015;10:e0138988. doi: 10.1371/journal.pone.0138988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang J, Zuo WL, Fukui T, Chao I, Gomi K, Lee B, et al. Smoking-dependent distal-to-proximal repatterning of the adult human small airway epithelium. Am J Respir Crit Care Med. 2017;196:340–352. doi: 10.1164/rccm.201608-1672OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells: current concepts and challenges. Proc Am Thorac Soc. 2008;5:772–777. doi: 10.1513/pats.200805-041HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hackett NR, Butler MW, Shaykhiev R, Salit J, Omberg L, Rodriguez-Flores JL, et al. RNA-Seq quantification of the human small airway epithelium transcriptome. BMC Genomics. 2012;13:82. doi: 10.1186/1471-2164-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whimster WF. Tracheobronchial submucous gland profiles in smokers and non-smokers. Appl Pathol. 1988;6:241–246. [PubMed] [Google Scholar]
- 51.Basbaum CB, Jany B, Finkbeiner WE. The serous cell. Annu Rev Physiol. 1990;52:97–113. doi: 10.1146/annurev.ph.52.030190.000525. [DOI] [PubMed] [Google Scholar]
- 52.Takizawa T, Thurlbeck WM. A comparative study of four methods of assessing the morphologic changes in chronic bronchitis. Am Rev Respir Dis. 1971;103:774–783. doi: 10.1164/arrd.1971.103.6.774. [DOI] [PubMed] [Google Scholar]
- 53.Welsh KG, Rousseau K, Fisher G, Bonser LR, Bradding P, Brightling CE, et al. MUC5AC and a glycosylated variant of MUC5B alter mucin composition in children with acute asthma. Chest. 2017;152:771–779. doi: 10.1016/j.chest.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phelps DS, Floros J. Localization of surfactant protein synthesis in human lung by in situ hybridization. Am Rev Respir Dis. 1988;137:939–942. doi: 10.1164/ajrccm/137.4.939. [DOI] [PubMed] [Google Scholar]
- 55.Attarian SJ, Leibel SL, Yang P, Alfano DN, Hackett BP, Cole FS, et al. Mutations in the thyroid transcription factor gene NKX2-1 result in decreased expression of SFTPB and SFTPC. Pediatr Res. 2018;84:419–425. doi: 10.1038/pr.2018.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo M, Tomoshige K, Meister M, Muley T, Fukazawa T, Tsuchiya T, et al. Gene signature driving invasive mucinous adenocarcinoma of the lung. EMBO Mol Med. 2017;9:462–481. doi: 10.15252/emmm.201606711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maeda Y, Chen G, Xu Y, Haitchi HM, Du L, Keiser AR, et al. Airway epithelial transcription factor NK2 homeobox 1 inhibits mucous cell metaplasia and Th2 inflammation. Am J Respir Crit Care Med. 2011;184:421–429. doi: 10.1164/rccm.201101-0106OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gerrity TR, Lee PS, Hass FJ, Marinelli A, Werner P, Lourenço RV. Calculated deposition of inhaled particles in the airway generations of normal subjects. J Appl Physiol. 1979;47:867–873. doi: 10.1152/jappl.1979.47.4.867. [DOI] [PubMed] [Google Scholar]
- 59.Jeffery PK, Li D. Airway mucosa: secretory cells, mucus and mucin genes. Eur Respir J. 1997;10:1655–1662. doi: 10.1183/09031936.97.10071655. [DOI] [PubMed] [Google Scholar]
- 60.Lumsden AB, McLean A, Lamb D. Goblet and Clara cells of human distal airways: evidence for smoking induced changes in their numbers. Thorax. 1984;39:844–849. doi: 10.1136/thx.39.11.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rogers AV, Dewar A, Corrin B, Jeffery PK. Identification of serous-like cells in the surface epithelium of human bronchioles. Eur Respir J. 1993;6:498–504. [PubMed] [Google Scholar]
- 62.Zuo WL, Shenoy SA, Li S, O’Beirne SL, Strulovici-Barel Y, Leopold PL, et al. Ontogeny and biology of human small airway epithelial club cells. Am J Respir Crit Care Med. 2018;198:1375–1388. doi: 10.1164/rccm.201710-2107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of Clara cells in normal human airway epithelium. Am J Respir Crit Care Med. 1999;159:1585–1591. doi: 10.1164/ajrccm.159.5.9806044. [DOI] [PubMed] [Google Scholar]
- 64.Jeffery PK, Gaillard D, Moret S. Human airway secretory cells during development and in mature airway epithelium. Eur Respir J. 1992;5:93–104. [PubMed] [Google Scholar]
- 65.Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y, et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517:616–620. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen G, Korfhagen TR, Karp CL, Impey S, Xu Y, Randell SH, et al. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med. 2014;189:301–313. doi: 10.1164/rccm.201306-1181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.