Abstract
Rationale: Approximately 2.8 billion people are exposed daily to household air pollution from polluting cookstoves. The effects of prenatal household air pollution on lung development are unknown.
Objectives: To prospectively examine associations between prenatal household air pollution and infant lung function and pneumonia in rural Ghana.
Methods: Prenatal household air pollution exposure was indexed by serial maternal carbon monoxide personal exposure measurements. Using linear regression, we examined associations between average prenatal carbon monoxide and infant lung function at age 30 days, first in the entire cohort (n = 384) and then stratified by sex. Quasi-Poisson generalized additive models explored associations between infant lung function and pneumonia.
Measurements and Main Results: Multivariable linear regression models showed that average prenatal carbon monoxide exposure was associated with reduced time to peak tidal expiratory flow to expiratory time (β = −0.004; P = 0.01), increased respiratory rate (β = 0.28; P = 0.01), and increased minute ventilation (β = 7.21; P = 0.05), considered separately, per 1 ppm increase in average prenatal carbon monoxide. Sex-stratified analyses suggested that girls were particularly vulnerable (time to peak tidal expiratory flow to expiratory time: β = −0.003, P = 0.05; respiratory rate: β = 0.36, P = 0.01; minute ventilation: β = 11.25, P = 0.01; passive respiratory compliance normalized for body weight: β = 0.005, P = 0.01). Increased respiratory rate at age 30 days was associated with increased risk for physician-assessed pneumonia (relative risk, 1.02; 95% confidence interval, 1.00–1.04) and severe pneumonia (relative risk, 1.04; 95% confidence interval, 1.00–1.08) in the first year of life.
Conclusions: Increased prenatal household air pollution exposure is associated with impaired infant lung function. Altered infant lung function may increase risk for pneumonia in the first year of life. These findings have implications for future respiratory health.
Clinical trial registered with www.clinicaltrials.gov (NCT 01335490).
Keywords: household air pollution, lung development, lung function, pneumonia, sex-specific effects
At a Glance Commentary
Scientific Knowledge on the Subject
Household air pollution exposure likely begins to impact lung development prenatally, increasing risk for future disease. Fetal lung development occurs across gestation with sex-specific trajectories; therefore, the impact of prenatal household air pollution on lung function may differ based on fetal sex.
What This Study Adds to the Field
This is the first study to measure maternal household air pollution exposure serially across gestation, perform infant lung function, and assess incident pneumonia in the first year of life. These data demonstrate that increased prenatal household air pollution exposure alters infant lung function, especially in girls. Exploratory analyses suggest that altered lung function may increase risk for pneumonia. Understanding the influence of prenatal household air pollution on lung development could identify at-risk populations and direct future public health interventions.
Approximately 2.8 billion people, or 40% of the world’s households, cook with solid fuels (1) resulting in approximately 3.5 million deaths every year (2, 3). In sub-Saharan Africa, more than 75% of households are reliant on solid fuels (4). These fuels are burned on combustion-inefficient traditional cook stoves, releasing several known toxicants including carbon monoxide (CO). Exposures are termed household air pollution (HAP) because they occur indoors or near the house. HAP exposure results in substantial respiratory morbidity and mortality across the life course, including nearly half a million deaths in children under 5 because of acute lower respiratory infections (ALRI), specifically pneumonia (PNA) (2, 5, 6). All household members are exposed and women, who tend to be the primary cooks, experience the highest exposures. Women continue to cook while pregnant, thus exposure to HAP begins in utero.
The developmental origins of health and disease theory posits that many disease processes begin in the fetal period (7). Altered prenatal lung development impairs lung function and lung growth and increases risk for respiratory disease in childhood and adulthood (8). Low lung function in infancy and early childhood has been associated with subsequent wheeze, airway hyperresponsiveness, and asthma and, importantly for HAP exposure, may increase risk for PNA. Reduced infant lung function has been associated with adult lung function including FEV1 (9), an important determinant of adult chronic respiratory diseases, such as chronic obstructive pulmonary disease (10), and mortality (11).
Genetic factors account for a relatively small proportion of risk for reduced lung function, highlighting the need to consider the effect of prenatal environmental exposures on lung development (12). To our knowledge, only one study has investigated associations between prenatal maternal air pollution and infant lung function. That study demonstrated that, similar to prenatal tobacco exposure, infants born to mothers with increased particulate matter exposure during pregnancy have higher respiratory rates (RRs) and minute ventilation (MV) and altered breathing flows (13–15). Prenatal air pollution has also been linked to impaired lung function in childhood (16, 17). No study has examined associations between prenatal HAP exposure and infant lung function and PNA risk. Understanding the impact of prenatal HAP exposure on infant lung function, and the subsequent impact of infant lung function on PNA risk, has implications for respiratory health across the life course and may help identify at-risk children and develop preventive efforts.
Recent studies have highlighted the importance of sex-specific effects of prenatal air pollution exposure on childhood respiratory outcomes (18–20). Lung development begins as early as 4 weeks gestation and male and female fetuses display differences in lung development trajectories, suggesting that sex-differential responses to prenatal toxicants may occur (21). Supporting this, surfactant production and branching morphogenesis occur later in male fetuses and sex-specific differences in the developing lung transcriptome have been identified (22). Identification of sex-specific effects may help elucidate underlying mechanistic pathways.
We examined the impact of prenatal HAP exposure, as measured by personal CO monitoring, on infant lung function at age 30 days in a prospective birth cohort in rural Ghana. Because of the clinical importance of HAP-associated PNA, we explored associations between infant lung function and PNA in the first year of life. First, we used multivariable linear regression models to estimate associations between average prenatal CO exposure and infant lung function. Next, we performed sex-stratified analyses. Finally, we fit quasi-Poisson generalized additive models to estimate associations between infant lung function variables and PNA incidence. We hypothesized that children born to mothers with higher prenatal CO exposure would have impaired lung function relative to those born to mothers with lower prenatal CO exposure and that impaired lung function would increase risk for PNA in the first year of life.
Methods
Study Participants
Subjects were from GRAPHS (Ghana Randomized Air Pollution and Health Study), a cluster-randomized cookstove intervention trial in rural Ghana that has been described elsewhere (23). Briefly, between August 2013 and March 2016, a total of 1,414 nonsmoking, pregnant women were recruited from communities in the Kintampo North Municipality and Kintampo South District of Ghana. Gestational age at enrollment was established by ultrasound (24). All households were enrolled before 24 weeks gestation. The analyses in this manuscript include 404 GRAPHS mothers who were in the third trimester of pregnancy between October 2014 and August 2015 and agreed to have their infant participate in a lung function visit. Procedures were approved by human studies and institutional ethics committees at Kintampo Health Research Centre, the Icahn School of Medicine at Mount Sinai, and the Columbia University Mailman School of Public Health, and written consent was obtained from all mothers.
Prenatal CO Exposures
Pregnant mothers underwent four prenatal 72-hour personal CO monitoring sessions (Lascar EL-CO-USB Data Logger). Participants were asked to wear the personal monitor except while sleeping or bathing, during which times they were told to keep the monitor nearby and off the floor. The CO monitor was protected from rain and dust by a custom plastic case and tea bag filter paper and was affixed within the breathing zone of the pregnant women.
The Lascar monitors were set to record readings in parts per million every 10 seconds. The Lascar monitors were exposed to certified span gas (50 ppm CO in zero air) at the Kintampo Health Research Centre laboratory every 6 weeks to quantify response and adjust field values. Additional quality assurance/quality control checks on the functioning of the CO monitors were made based on run time and visual inspection of each deployment following GRAPHS protocols. Data used in this analysis passed all three quality assurance/quality control checks. Over the course of the trial we collected 4,923 sessions of prenatal CO monitoring data. Of these, 388 (7.9%) did not run a full 48 hours and were excluded. An additional 516 (10.5%) were excluded because of not passing quality assurance/quality control checks for validity.
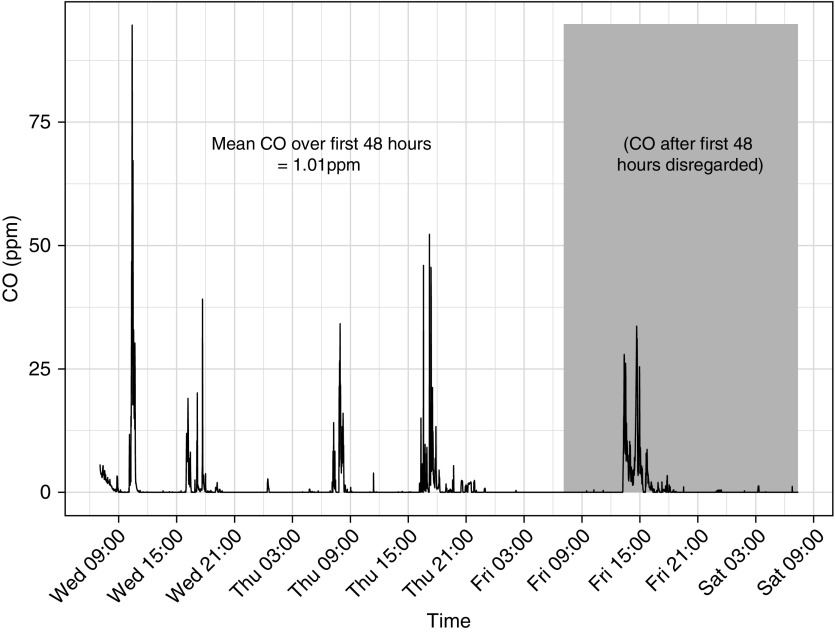
For this analysis, CO exposure at each prenatal time point was based on the first 48 hours of each of the 72-hour deployments. We excluded the last day to avoid cases where battery issues or field pick up schedules could have resulted in missing a cooking event on the last day. Forty-eight-hour data completeness for the GRAPHS cohort was high (93.6%). A representative plot of CO exposure over the exposure assessment period is shown in Figure 1.
Figure 1.
Representative plot of a maternal prenatal personal exposure assessment. This figure demonstrates personal carbon monoxide (CO) exposures (in parts per million) plotted against time, for a 72-hour exposure assessment period. The Lascar EL-CO-USB Data Logger measured CO levels every 10 seconds and was worn in the participant’s breathing zone. Peaks correspond to reported cooking periods; CO exposures outside of these periods were largely zero. Data for this study used the first 48 hours, to avoid cases where battery issues or field pick up schedules could have resulted in missing a cooking event on the last day. For this session, the 48-hour average CO exposure is 1.01 ppm (range, 0–94.65 ppm).
Pulmonary Function Testing
Infant pulmonary function testing was performed at the Kintampo Municipal Hospital in 30 day-old healthy-appearing infants without acute respiratory symptoms. Research assistants measured child length to the nearest 0.1 cm and weight to the nearest 0.1 kg. Pediatricians performed lung function testing with the Whistler LFMI (Nuenen), which displays real-time flow–volume plots to facilitate testing. All tests were over-read by the lead pulmonologist. Infants were studied unsedated, supine, and breathing through a sterile, single-use facemask following a standardized operating procedure and acceptance criteria (25). As in other studies (14, 26), at least 50 flow–volume loops with inspiratory and expiratory volumes reproducible to 15% determined the ratio of the time to peak tidal expiratory flow to expiratory time (Tptef:Te), Vt (ml), RR (breaths/min), and MV (ml/min). The single occlusion technique was used to obtain passive respiratory system compliance per kilogram (Crs/kg, ml/cm H2O/kg); acceptance criteria comprised a stable end-expiratory baseline and plateau pressure for more than 100 milliseconds that varied by less than or equal to 0.125 cm H2O. We confirmed the flow–volume curve by visual inspection.
Physician-assessed PNA and Severe PNA
Trained fieldworkers visited mother-infant pairs in the home each week for the infant’s first year of life to assess for respiratory symptoms, following the World Health Organization Integrated Management of Childhood Illness guidelines (27). Any child with respiratory symptoms or who was otherwise unwell was referred to the Kintampo Municipal Hospital for evaluation by a trained study physician. GRAPHS provided transportation to the hospital and assistance with follow-up care as needed. Study physicians were trained in the Integrated Management of Childhood Illness guidelines. The primary PNA outcome was physician-assessed PNA, defined as cough or difficulty breathing and tachypnea (60 or 50 breaths/min in children aged 0–2 mo or 2–12 mo, respectively); physician-assessed severe PNA (SPNA), defined as PNA with the presence of chest-indrawing, stridor, oxygen saturation less than 90%, or any general danger sign (including convulsions, lethargy or unconsciousness, vomiting, or inability to drink or breastfeed) was also determined.
Covariates
Maternal ethnicity, age at enrollment, education, and secondhand smoke exposure, defined as a smoking household member, were obtained through questionnaires. Questionnaires also determined wealth index, which was used to specify the wealth of households compared with others within the study. At the end of each exposure assessment, mothers were asked if they had performed activities that may have resulted in other sources of CO exposure, including burning of mosquito coils, selling wares by the roadside, kerosene lamp use, and charcoal burning. Birth weight within 24 hours of birth was measured to the nearest gram using the Tanita BD 585 digital baby scale and gestational age at delivery determined using the previously established ultrasound dates. Child sex was recorded at delivery. Child length to the nearest 0.1 cm and weight to the 0.1 kg were recorded at lung function test. Date of lung function test was used to determine child age at lung function test. Infants underwent three 72-hour personal CO monitoring sessions over the first year of life, following the maternal prenatal CO protocol detailed previously, to determine average postnatal CO exposure.
Statistical Analysis
First, we used univariate and multivariable linear regression models to determine associations between average prenatal CO exposure and infant lung function measures, including Tptef:Te, Vt, RR, MV, and Crs/kg. In addition to standard pulmonary function adjustment variables of child sex, length, weight, and age at lung function, we adjusted for other potential confounders/covariates including maternal education, ethnicity, and household wealth index (model 1). We then additionally adjusted for birth weight and gestational age at delivery to understand the effect of prenatal CO on infant lung function independent of these variables (model 2).
Sensitivity analyses were performed to examine the impact of secondhand tobacco smoke exposure and other sources of CO exposure on these results. Specifically, we used univariate regression to examine associations between secondhand tobacco smoke exposure and prenatal average CO and ANOVA to examine associations between reported mosquito coil use, kerosene lamp use, charcoal burning, and selling wares by the roadside, and prenatal average CO. We then additionally adjusted for each of these covariates. We also explored the impact of weight gain during the first month of life. Effect modification by child sex was then examined in stratified analyses and by fitting interaction terms.
To explore the relationship between infant lung function at age 30 days and PNA over the first year of life, we fit univariate and multivariable quasi-Poisson generalized additive models. Specifically, we analyzed associations with the infant lung function variables that were found to be significantly associated with prenatal HAP exposure in all children. The outcome variable was the number of physician-assessed PNA or SPNA episodes, considered separately, per child over the study period. Multivariable models were adjusted for child sex and length, weight and age at time of lung function test, month of delivery, child postnatal CO exposure, mother’s education and ethnicity, and wealth index. As above, we additionally included birth weight and gestational age at delivery and secondhand smoke and other sources of exposure, such as reported mosquito coil use, kerosene lamp use, charcoal burning, or selling wares by the roadside. Person-time in child-weeks of completed fieldworker follow-up was included as an offset in all models.
Main effects were considered statistically significant if the P value was less than 0.05. In subgroup analyses, interaction terms were considered suggestive of an interaction if the P value was less than 0.10. Statistical analyses were implemented in R version 3.3.3. Quasi-Poisson generalized additive models were fit using the R package “mgcv.”
Results
Of the 404 children recruited for the lung function visit, 384 (95%) provided acceptable lung function tests. Table 1 summarizes participant characteristics. Half of children included in the analyses were boys (n = 191; 50%). Most mothers had no (46%) or only primary school (25%) education. Asset index indicated a good distribution of relative wealth in the selected population. Median prenatal average CO exposure was 1.1 ppm (interquartile range, 0.7–1.9). There were no statistically significant differences in wealth index, maternal education, ethnicity, tobacco smoke exposure, or prenatal CO exposures between boys and girls. Boys’ birth weight was higher, and they were longer and heavier at the time of lung function testing as compared with girls. Boys’ Vt and MV were larger than girls. Lung passive compliance (Crs) was similar between sexes; however, after adjusting for weight, boys Crs/kg was smaller than the girls.
Table 1.
Participant Characteristics
| Categorical Variables | All Children (N = 384) |
Boys (n = 191) |
Girls (n = 193) |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Wealth index | ||||||
| 1 (very poor) | 77 | 20.1 | 36 | 18.9 | 41 | 21.2 |
| 2 | 85 | 22.1 | 35 | 18.3 | 50 | 25.9 |
| 3 | 64 | 16.7 | 35 | 18.3 | 29 | 15.0 |
| 4 | 65 | 16.9 | 37 | 19.4 | 28 | 14.5 |
| 5 (least poor) | 93 | 24.2 | 48 | 25.1 | 45 | 23.4 |
| Maternal education | ||||||
| None | 178 | 45.6 | 86 | 45.0 | 92 | 47.6 |
| 1–6 yr (primary school) | 100 | 24.7 | 52 | 27.2 | 48 | 24.9 |
| >6 yr (secondary school) | 106 | 26.3 | 53 | 27.8 | 53 | 27.5 |
| Ethnicity | ||||||
| Akan | 65 | 16.9 | 31 | 16.3 | 34 | 17.6 |
| Dagarti | 86 | 22.4 | 41 | 21.5 | 45 | 23.3 |
| Gonja | 55 | 14.3 | 27 | 14.1 | 28 | 14.5 |
| Konkonba | 58 | 15.1 | 27 | 14.1 | 31 | 16.1 |
| Other (Sisala, Mo, Fulani, Bimoba, Ga, Banda) | 120 | 31.2 | 65 | 34.0 | 55 | 28.5 |
| Secondhand smoke exposure | ||||||
| Yes | 50 | 13.0 | 23 | 12.0 | 27 | 14.0 |
| Other reported exposures | ||||||
| Kerosene lamp | 3 | 0.8 | 1 | 0.5 | 2 | 1.0 |
| Charcoal | 33 | 8.6 | 16 | 8.4 | 17 | 8.8 |
| Mosquito coil | 22 | 5.7 | 10 | 5.2 | 12 | 6.2 |
| Selling wares by roadside | 28 | 7.3 | 13 | 6.8 | 15 | 7.8 |
| Continuous Variables | All Children (N = 384) |
Boys (n = 191) |
Girls (n = 193) |
|||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |
| Average prenatal CO level, ppm | 1.1 | 0.7–1.9 | 1.1 | 0.6–2.1 | 1.1 | 0.7–1.8 |
| Child birth weight, kg | 3.00 | 2.72–3.21 | 3.01 | 2.76–3.28 | 2.97 | 2.62–3.17 |
| Child gestational age at delivery, wk | 39.7 | 39.0–40.7 | 39.8 | 39.1–40.6 | 39.7 | 38.9–40.9 |
| Child age at lung function, d | 30 | 27–33 | 30 | 27–33 | 29 | 27–33 |
| Child length at lung function, cm | 54.6 | 52.5–63.0 | 55.8 | 53.0–64.9 | 54.1 | 51.9–62.0 |
| Child weight at lung function, kg | 4.0 | 3.7–4.3 | 4.1 | 3.8–4.5 | 3.9 | 3.5––4.2 |
| Lung function outcomes | ||||||
| Tptef:Te | 0.56 | 0.42–0.69 | 0.57 | 0.45–0.69 | 0.55 | 0.40–0.69 |
| RR, breaths/min | 55.8 | 47.1–64.8 | 56.4 | 46.9–65.6 | 55.5 | 47.4–63.9 |
| Vt, ml | 32.9 | 28.7–37.0 | 33.6 | 30.2–38.6 | 31.5 | 27.3–35.8 |
| MV, ml/min | 1,810 | 1,560–2,130 | 1,880 | 1,600–2,220 | 1,740 | 1,520–2,000 |
| Crs/kg, ml/cm H2O/kg | 0.80 | 0.68–0.93 | 0.79 | 0.68–0.90 | 0.81 | 0.68–0.98 |
Definition of abbreviations: CO = carbon monoxide; Crs/kg = passive respiratory system compliance per kilogram; IQR = interquartile range; MV = minute ventilation; RR = respiratory rate; Tptef:Te = ratio of the time to peak tidal expiratory flow to expiratory time.
Table E1 in the online supplement demonstrates the characteristics of the GRAPHS cohort and those included in the study. Notably, in this sample of healthy, nonwheezing children selected for infant lung function testing, birth weight and maternal education were significantly different from that of the entire GRAPHS cohort.
Effect of Average Prenatal CO on Infant Lung Function
We identified an exposure-response relationship between average prenatal CO and infant lung function. Specifically, increased average prenatal CO was associated with reduced Tptef:Te (β = −0.004; SE = 0.002; P = 0.01), increased RR (0.28; SE = 0.11; P = 0.02), and increased MV (β = 7.26; SE = 3.76; P = 0.05), considered separately, per 1 ppm increase in average prenatal CO following adjustment for child sex; length, weight, and age at lung function; maternal education; ethnicity; and wealth index (Table 2, model 1). Additional adjustment for child’s birth weight and gestational age at delivery did not significantly change the results (Table 2, model 2).
Table 2.
Estimated Effects of Average Prenatal CO on Infant Lung Function: Linear Regression
| Lung Function Outcomes | Univariate Model |
Model 1* |
Model 2† |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | P Value | β | SE | P Value | β | SE | P Value | |
| Tptef:Te | −0.004 | 0.001 | 0.01 | −0.004 | 0.002 | 0.01 | −0.004 | 0.002 | 0.01 |
| RR, breaths/min | 0.28 | 0.11 | 0.01 | 0.28 | 0.11 | 0.02 | 0.28 | 0.11 | 0.01 |
| Vt, ml | −0.02 | 0.06 | 0.77 | 0.001 | 0.05 | 0.97 | 0.001 | 0.05 | 0.97 |
| MV, ml/min | 6.68 | 3.94 | 0.09 | 7.26 | 3.76 | 0.05 | 7.21 | 3.77 | 0.05 |
| Crs/kg, ml/cm H2O/kg | 0.001 | 0.001 | 0.34 | 0.001 | 0.001 | 0.52 | 0.001 | 0.001 | 0.53 |
Definition of abbreviations: CO = carbon monoxide; Crs/kg = passive respiratory system compliance per kilogram; MV = minute ventilation; RR = respiratory rate; Tptef:Te = ratio of the time to peak tidal expiratory flow to expiratory time.
Models adjusted for child sex, length, weight, and age at examination, and maternal education, ethnicity, and wealth index, per 1 ppm increase in average prenatal CO.
Models additionally adjusted for child birth weight and gestational age at delivery.
Sensitivity Models
Univariate models did not demonstrate an association between reported household member smoking and prenatal average CO exposure (β = −0.79; SE = 0.87; P = 0.37). Models additionally adjusted for tobacco smoke exposure did not substantively change the reported findings (Table 3). Participant report of mosquito coil use, selling wares by roadside, kerosene lamp use, and charcoal burning was not associated with prenatal average CO exposure (P = 0.62 by ANOVA). Additional adjustment for these other sources of CO exposure did not change the reported findings. Additional adjustment for weight gain in the first month of life instead of birth weight similarly did not change results.
Table 3.
Sensitivity Models of Effects of Average Prenatal CO on Infant Lung Function: Linear Regression
| Lung Function Outcomes | Tobacco Smoke* |
Other Environmental Exposures† |
Weight Gain‡ |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | P Value | β | SE | P Value | β | SE | P Value | |
| Tptef:Te | −0.004 | 0.002 | 0.01 | −0.004 | 0.002 | 0.01 | −0.004 | 0.002 | 0.01 |
| RR, breaths/min | 0.27 | 0.11 | 0.02 | 0.26 | 0.11 | 0.03 | 0.28 | 0.11 | 0.02 |
| Vt, ml | 0.003 | 0.05 | 0.96 | −0.008 | 0.05 | 0.88 | 0.001 | 0.05 | 0.98 |
| MV, ml/min | 7.10 | 3.77 | 0.06 | 7.09 | 3.78 | 0.06 | 7.21 | 3.77 | 0.05 |
| Crs/kg, ml/cm H2O/kg | 0.001 | 0.001 | 0.50 | 0.001 | 0.001 | 0.50 | 0.001 | 0.001 | 0.52 |
For definition of abbreviations, see Table 2.
Model 2, adjusting for child sex, length, weight, and age at examination; maternal education, ethnicity, and wealth index; birth weight and gestational age at delivery; additionally, adjusted for tobacco smoke exposure, per 1 ppm increase in average prenatal CO.
Model 2 additionally adjusted for environmental exposures (including burning mosquito coils, selling wares by roadside, kerosene lamp use, and charcoal burning).
Model 2, adjusting for weight gain in first month of life instead of birth weight.
Sex-Specific Effect of Average Prenatal CO on Infant Lung Function
Table 4 presents the stratified analyses for the association between average prenatal CO and infant lung function using the fully adjusted model (model 2). Among girls, increased prenatal CO was associated with reduced Tptef:Te (β = −0.003; SE = 0.001; P = 0.05; P for interaction [P-int] = 0.86), increased RR (β = 0.36; SE = 0.15; P = 0.01; P-int = 0.49), increased MV (β = 11.25; SE = 4.88; P = 0.01; P-int = 0.29), and increased Crs/kg (β = 0.005; SE = 0.002; P = 0.01; P-int < 0.01). Among boys, increased prenatal CO was associated with decreased Crs/kg (β = −0.004; P = 0.02).
Table 4.
Sex-Specific Effects of Average Prenatal CO Exposure on Infant Lung Function: Linear Regression
| Lung Function Outcomes | Adjusted Model* |
Boys |
Girls |
P Value for Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | P Value | β | SE | P Value | β | SE | P Value | ||
| Tptef:Te | −0.004 | 0.002 | 0.01 | −0.001 | 0.001 | 0.33 | −0.003 | 0.001 | 0.05 | 0.86 |
| RR, breaths/min | 0.28 | 0.11 | 0.01 | 0.18 | 0.17 | 0.29 | 0.36 | 0.15 | 0.01 | 0.49 |
| Vt, ml | 0.001 | 0.05 | 0.97 | −0.04 | 0.08 | 0.53 | 0.06 | 0.07 | 0.42 | 0.23 |
| MV, ml/min | 7.21 | 3.77 | 0.05 | 3.43 | 6.03 | 0.57 | 11.25 | 4.88 | 0.01 | 0.29 |
| Crs/kg, ml/cm H2O/kg | 0.001 | 0.001 | 0.53 | −0.004 | 0.002 | 0.02 | 0.005 | 0.002 | 0.01 | <0.01 |
For definition of abbreviations, see Table 2.
Model adjusted for child sex, length, weight, and age at examination; maternal education, ethnicity, wealth index; and child birth weight and gestational age at delivery.
Effect of Infant Lung Function on PNA Risk
Fieldworkers made a median of 51.3 (interquartile range, 48.0–51.9) weekly visits over the children’s first year of life. Fifty-two (14%) children had one episode, 14 (4%) had two episodes, and three (1%) had three episodes of physician-assessed PNA. Eighteen children received a diagnosis of physician-assessed SPNA.
Table 5 presents the associations between infant lung function at age 30 days, specifically Tptef:Te and RR, and physician-assessed PNA and SPNA, considered separately. Multivariable models suggested that increased RR at infant age 30 days was associated with an increased risk of physician-assessed PNA (relative risk, 1.02; 95% confidence interval [CI], 1.00–1.04; P = 0.03) and physician-assessed SPNA (relative risk, 1.04; 95% CI, 1.00–1.08; P = 0.04) in the first year of life, per 1 breath/min increase in RR following adjustment for child sex, length, weight, and age at the time of lung function test, month of delivery, and postnatal CO exposure; maternal education, ethnicity, and wealth index; and child-weeks of fieldworker follow-up (Table 5, model 1). Additional adjustment for birth weight and gestational age at delivery (Table 5, model 2) or secondhand smoke and other sources of exposure (Table 5, model 3) did not substantively change these findings. Increase in Tptef:Te at infant age 30 days was suggestive of a decreased risk for physician-assessed PNA in the first year of life; however, this finding did not reach statistical significance (relative risk, 0.31; 95% CI, 0.07–1.43; P = 0.13). Because of the small number of PNA and SPNA cases, sex-stratified analyses could not be performed.
Table 5.
Relative Risk for Physician-assessed PNA and SPNA Associated with Infant Lung Function
| Lung Function Variable | Outcome | Univariate Model |
Model 1* |
Model 2† |
Model 3‡ |
||||
|---|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | ||
| Tptef:Te | PNA | 0.54 (0.13–2.33) | 0.41 | 0.31 (0.07–1.43) | 0.13 | 0.35 (0.07–1.68) | 0.18 | 0.32 (0.07–1.51) | 0.15 |
| SPNA | 0.43 (0.02–9.46) | 0.59 | 0.72 (0.03–17.6) | 0.84 | 0.66 (0.03–16.5) | 0.80 | 0.66 (0.03–17.3) | 0.80 | |
| Respiratory rate | PNA | 1.02 (1.00–1.04) | 0.04 | 1.02 (1.00–1.04) | 0.03 | 1.02 (1.00–1.04) | 0.03 | 1.02 (1.00–1.04) | 0.03 |
| SPNA | 1.03 (1.00–1.07) | 0.07 | 1.04 (1.00–1.08) | 0.04 | 1.04 (1.00–1.08) | 0.04 | 1.04 (1.00–1.07) | 0.04 | |
Definition of abbreviations: CI = confidence interval; CO = carbon monoxide; PNA = pneumonia; RR = relative risk; SPNA = severe pneumonia; Tptef:Te = ratio of the time to peak tidal expiratory flow to expiratory time.
RR values are given per 1 unit change of lung function parameter.
Model adjusted for child sex and length, weight, and age at time of lung function test, month of delivery, and postnatal CO exposure; maternal education, ethnicity, and wealth index; and child-weeks of completed fieldworker follow-up.
Model 1 additionally adjusted for birth weight and gestational age at delivery.
Model 1 additionally adjusted for secondhand smoke and other sources of exposure (including burning mosquito coils, selling wares by roadside, kerosene lamp use, and charcoal burning).
Discussion
This is the first prospective study to serially measure prenatal HAP exposure as indexed by personal maternal CO measurements and demonstrate associations between prenatal CO and infant lung function, including identification of sex-specific effects. Furthermore, we begin to explore the effect of altered infant lung function on PNA risk, a major cause of HAP-associated childhood morbidity and mortality. Although HAP research is increasing, only one prior randomized controlled cookstove intervention trial exclusively recruited pregnant women (28) and no prior study has performed infant lung function. Our data suggest that children, especially girls, born to mothers with higher HAP CO exposures during pregnancy were at increased risk for impaired lung function measured 1 month after birth. Altered infant lung function may increase risk for PNA in the first year of life, even after controlling for postnatal exposures. Collectively, these results suggest that prenatal HAP exposure alters lung development and may begin to program future respiratory disease starting in utero.
Our findings that increased prenatal HAP exposure is associated with reduced Tptef:Te and altered respiratory compliance are consistent with the prenatal tobacco literature (15) and animal models of prenatal particulate matter exposure (29). Alterations in expiratory flow and compliance may reflect developmental changes in airway growth and caliber. Reduced Tptef:Te has been shown to precede and predict important childhood pulmonary outcomes, including wheeze and lung function (14, 26, 30, 31). Furthermore, lung function in infancy is inversely related to, and may account for an estimated 16% of the variance in, maximally attainable FEV1 (9), a determinant of chronic respiratory disease in adulthood, such as chronic obstructive pulmonary disease (10), and mortality (11). Therefore, changes in infant lung function may set the course for future respiratory health.
Animal data suggest that prenatal air pollution exposure may “prime” the developing lung for postnatal injury secondary to continued exposure (32). We demonstrate that prenatal CO is associated with increased RR and MV, in concert with prior ambient air pollution study (33). Postnatally, a child’s air pollution dose is approximately proportional to RR and MV (34). Thus, these findings suggest that the respiratory mechanics of children born to mothers with higher HAP exposure during pregnancy may result in children having a higher postnatal HAP dose than similarly exposed peers with lower prenatal HAP exposures. In this way, altered prenatal lung development may influence postnatal HAP dose, impairing postnatal lung development and increasing risk for future respiratory disease.
Our data suggest that girls are more susceptible to the effects of prenatal HAP. Sex-specific differences in lung maturation and trajectory have been described, with girls having earlier alveolar maturation and surfactant production and being born with smaller lungs (35). Although the sex-specific effect of HAP has not been previously studied, response to prenatal tobacco exposure differs in boys versus girls (36). Furthermore, adolescent girls exposed to tobacco smoke have reduced lung function growth and increased risk of wheeze, as compared with boys (37). Animal models of other prenatal environmental exposures and experimentally induced intrauterine growth restriction also find that girls may be differentially affected (38, 39). Mechanisms driving these differences have not been fully delineated; however, the lung developmental transcriptome does demonstrate sex-specific differences (22). The finding that female fetuses may be more vulnerable to prenatal HAP is especially important given that females are the predominant cooks in most LMIC communities and thus are differentially exposed across the life course.
Experimental in utero tobacco smoke data suggest that cigarette smoke stimulates dysanaptic airway growth, decreases the numbers of saccules and septal crests, and increases collagen and decreases elastin airway deposition (40–42). HAP has not been studied; however, animal models of ambient air pollution similarly found that pups exposed to in utero particulate matter less than or equal to 2.5 μm in aerodynamic diameter had decreased surface-to-volume ratios and altered elastic properties with corresponding impairments in lung function (32). Altered epithelial-to-mesenchymal transition, thickened alveoli septum, and destroyed alveoli and interstitial proliferation have also been demonstrated (29). Together, these histologic changes are consistent with a phenotype of reduced expiratory flow and altered lung compliance.
ALRI secondary to HAP results in an estimated 455,000 deaths annually in children younger than 5 (43). Our data begin to explore associations between infant lung function at 1 month of age and PNA risk over the first year of life and suggest that infants born with impaired lung function may be at increased risk for future PNA. Prior studies support an association between impaired lung function, wheeze, asthma, and ALRI risk, in particular PNA, in children less than 3 years of age (44, 45). These studies, however, have been unable to disentangle whether the impaired lung function precedes (46), or is a result of (47), ALRI. Our study adds to this literature suggesting that impaired prenatal lung development, potentially via alterations in airway structure and function secondary to environmental exposures, such as HAP, may increase risk for future PNA (48). Additional studies are needed to corroborate this finding. Because of the sample size we are unable to perform formal mediation analyses. Future work should explore whether associations between prenatal HAP exposure and infant ALRI are mediated by impaired infant lung function.
Personal exposure assessment strategies in HAP research are limited by cost and participant fatigue. Prior research suggests that the predictive ability of short-term personal exposures to estimate long-term exposures is improved with repeated sampling (49). The GRAPHS exposure strategy thus used serial, personal CO exposure measurements across pregnancy and Year 1 of life to estimate average HAP prenatal and postnatal exposure. Observed personal 48-hour CO measures provide unbiased estimates of CO exposure during the observed interval; however, exposures vary over time, which may attenuate regression coefficients (50). Future studies would be strengthened by the development of a biomarker of exposure.
We note several strengths of our study. We prospectively assessed personal prenatal HAP exposure as measured by CO for up to 72 hours at multiple time points across pregnancy in a rural, low-resource setting, an approach that minimizes exposure misclassification. Our well-characterized cohort with ultrasound-confirmed gestational age at enrollment allowed us to control for several confounders and covariates, including birth weight and gestational age, suggesting these results are independent of the effect of HAP on global fetal growth. We measured lung function at 30 days of life, thereby isolating the prenatal period. Our cohort included nonsmoking mother-infant dyads from rural, lower socioeconomic communities most affected by HAP. Finally, our physician-assessed PNA case ascertainment included active fieldworker surveillance with weekly household visits, fieldworkers and physicians trained in the validated World Health Organization Integrated Management of Childhood Illness–PNA assessment tool, and support for participant travel and follow-up care needs.
We also acknowledge limitations. Although we adjusted for many potential confounders, we did not have data on diet or covarying environmental exposures. We measured personal HAP exposure at multiple time points; however, because of cost and logistics of performing a study in rural Ghana, we were unable to perform continuous measurements over pregnancy. Future studies with more extensive prenatal HAP exposure assessments should explore the effect of timing of exposure on lung development. HAP is not the only source of CO exposure; however, we note that on visual inspection exposures were largely zero except during cooking periods when they rose to higher levels. Furthermore, sensitivity analyses additionally adjusting for other sources of CO, including tobacco smoke exposure, did not substantively change results. Although measuring lung function in infancy is a strength, the gold standard for assessing lung function is spirometry. As we continue to follow this cohort, it is important to prospectively assess respiratory symptoms, ALRI, lung function, and contemporaneous personal HAP exposure, to better understand the contribution of prenatal and postnatal HAP exposure on lung development and child respiratory health (51). Although our PNA case ascertainment uses validated methodologies used in other HAP studies in rural, resource-poor communities, access to radiologic methodologies, such as chest radiograph or lung ultrasound, would have strengthened our PNA diagnoses (52, 53). Finally, although we focus on a high-risk population, our results may not be generalizable to other HAP-exposed communities that often have even higher exposures; a stronger effect may be seen in mothers with higher exposures.
In summary, we demonstrate that prenatal HAP exposure is associated with impaired infant lung function, especially in girls, and that altered lung function at age 30 days may increase risk for PNA in the first year of life. Given previously established associations between early life lung function and respiratory morbidity across the life course, these findings support the urgent need for reductions in exposure to HAP, including during the prenatal period.
Supplementary Material
Footnotes
GRAPHS (Ghana Randomized Air Pollution and Health Study) was funded by the NIH (R01 ES019547). Infant lung function phenotyping was funded by a Thrasher Research Fund Early Career Award (A.G.L.) and a CHEST Foundation Grant in Women’s Lung Health (A.G.L.). Ghana Health Service facilities in the Kintampo North Municipality and Kintampo South Districts provided facilities for GRAPHS and infant lung function measurements. During preparation of this manuscript, A.G.L. was supported by NIH grant K23 HL135349 and B.J.W. was supported by NIH grant K23 ES021471. Additional support was provided by National Institute of Environmental Health Sciences (ES009089) and the Global Alliance for Clean Cookstoves. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. NIH or Department of Health and Human Services.
Author Contributions: A.G.L., D.J., and K.P.A. conceived of the study and oversaw implementation. A.G.L. led data analysis and manuscript writing. P.L.K., S.C., and D.J. led exposure assessment teams. A.Q. and K.B. led analysis of carbon monoxide exposure data. B.J.W. oversaw obstetric outcomes and gestational age ultrasound assessment. S.K., R.D., A.K.Y., K.A.A.-N., and J.O.-M. oversaw daily field operations. S.K. and A.K.Y. performed all infant lung function testing. All authors assisted with data interpretation, manuscript preparation, and final manuscript review.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201804-0694OC on September 26, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.WHO Household air pollution and health.Fact sheetGeneva, Switzerland: 2014 [Google Scholar]
- 2.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assad NA, Balmes J, Mehta S, Cheema U, Sood A. Chronic obstructive pulmonary disease secondary to household air pollution. Semin Respir Crit Care Med. 2015;36:408–421. doi: 10.1055/s-0035-1554846. [DOI] [PubMed] [Google Scholar]
- 4.Bonjour S, Adair-Rohani H, Wolf J, Bruce NG, Mehta S, Prüss-Ustün A, et al. Solid fuel use for household cooking: country and regional estimates for 1980-2010. Environ Health Perspect. 2013;121:784–790. doi: 10.1289/ehp.1205987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dherani M, Pope D, Mascarenhas M, Smith KR, Weber M, Bruce N. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ. 2008;86:390–398C. doi: 10.2471/BLT.07.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. for the Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry CE, Billheimer D, Jenkins IC, Lu ZJ, Stern DA, Gerald LB, et al. A distinct low lung function trajectory from childhood to the fourth decade of life. Am J Respir Crit Care Med. 2016;194:607–612. doi: 10.1164/rccm.201604-0753OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–764. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 11.Vasquez MM, Zhou M, Hu C, Martinez FD, Guerra S. Low lung function in young adult life is associated with early mortality. Am J Respir Crit Care Med. 2017;195:1399–1401. doi: 10.1164/rccm.201608-1561LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soler Artigas M, Loth DW, Wain LV, Gharib SA, Obeidat M, Tang W, et al. Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet. 2011;43:1082–1090. doi: 10.1038/ng.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korten I, Ramsey K, Latzin P. Air pollution during pregnancy and lung development in the child. Paediatr Respir Rev. 2017;21:38–46. doi: 10.1016/j.prrv.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 14.McEvoy CT, Schilling D, Clay N, Jackson K, Go MD, Spitale P, et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA. 2014;311:2074–2082. doi: 10.1001/jama.2014.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEvoy CT, Spindel ER. Pulmonary effects of maternal smoking on the fetus and child: effects on lung development, respiratory morbidities, and life long lung health. Paediatr Respir Rev. 2017;21:27–33. doi: 10.1016/j.prrv.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jedrychowski WA, Perera FP, Maugeri U, Mroz E, Klimaszewska-Rembiasz M, Flak E, et al. Effect of prenatal exposure to fine particulate matter on ventilatory lung function of preschool children of non-smoking mothers. Paediatr Perinat Epidemiol. 2010;24:492–501. doi: 10.1111/j.1365-3016.2010.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee AG, Le Grand B, Hsu HL, Chiu YM, Brennan KJ, Bose S, et al. Prenatal fine particulate exposure associated with reduced childhood lung function and nasal epithelia GSTP1 hypermethylation: Sex-specific effects. Respir Res. 2018;19:76. doi: 10.1186/s12931-018-0774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu HH, Chiu YH, Coull BA, Kloog I, Schwartz J, Lee A, et al. Prenatal particulate air pollution and asthma onset in urban children. identifying sensitive windows and sex differences. Am J Respir Crit Care Med. 2015;192:1052–1059. doi: 10.1164/rccm.201504-0658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee A, Leon Hsu HH, Mathilda Chiu YH, Bose S, Rosa MJ, Kloog I, et al. Prenatal fine particulate exposure and early childhood asthma: effect of maternal stress and fetal sex. J Allergy Clin Immunol. 2018;141:1880–1886. doi: 10.1016/j.jaci.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bose S, Chiu YM, Hsu HL, Di Q, Rosa MJ, Lee A, et al. Prenatal nitrate exposure and childhood asthma. influence of maternal prenatal stress and fetal sex. Am J Respir Crit Care Med. 2017;196:1396–1403. doi: 10.1164/rccm.201702-0421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax. 1999;54:1119–1138. doi: 10.1136/thx.54.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kho AT, Chhabra D, Sharma S, Qiu W, Carey VJ, Gaedigk R, et al. Age, sexual dimorphism, and disease associations in the developing human fetal lung transcriptome. Am J Respir Cell Mol Biol. 2016;54:814–821. doi: 10.1165/rcmb.2015-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack DW, Asante KP, Wylie BJ, Chillrud SN, Whyatt RM, Ae-Ngibise KA, et al. Ghana Randomized Air Pollution and Health Study (GRAPHS): study protocol for a randomized controlled trial. Trials. 2015;16:420. doi: 10.1186/s13063-015-0930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boamah EA, Asante K, Ae-Ngibise K, Kinney PL, Jack DW, Manu G, et al. Gestational age assessment in the Ghana Randomized Air Pollution and Health Study (GRAPHS): Ultrasound Capacity Building, Fetal Biometry Protocol Development, And Ongoing Quality Control. JMIR Res Protoc. 2014;3:e77. doi: 10.2196/resprot.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bates JH, Schmalisch G, Filbrun D, Stocks J for the European Respiratory Society/American Thoracic Society. Tidal breath analysis for infant pulmonary function testing. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. Eur Respir J. 2000;16:1180–1192. doi: 10.1034/j.1399-3003.2000.16f26.x. [DOI] [PubMed] [Google Scholar]
- 26.Håland G, Carlsen KC, Sandvik L, Devulapalli CS, Munthe-Kaas MC, Pettersen M, et al. ORAACLE. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355:1682–1689. doi: 10.1056/NEJMoa052885. [DOI] [PubMed] [Google Scholar]
- 27.WHO. Integrated management of childhood illness: chart booklet. 2014 Mar [accessed 2019 Feb 22]. Available from: https://apps.who.int/iris/bitstream/handle/10665/104772/9789241506823_Chartbook_eng.pdf?sequence=16.
- 28.Alexander DA, Northcross A, Karrison T, Morhasson-Bello O, Wilson N, Atalabi OM, et al. Pregnancy outcomes and ethanol cook stove intervention: a randomized-controlled trial in Ibadan, Nigeria. Environ Int. 2018;111:152–163. doi: 10.1016/j.envint.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Tang W, Du L, Sun W, Yu Z, He F, Chen J, et al. Maternal exposure to fine particulate air pollution induces epithelial-to-mesenchymal transition resulting in postnatal pulmonary dysfunction mediated by transforming growth factor-β/Smad3 signaling. Toxicol Lett. 2017;267:11–20. doi: 10.1016/j.toxlet.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Hoo AF, Henschen M, Dezateux C, Costeloe K, Stocks J. Respiratory function among preterm infants whose mothers smoked during pregnancy. Am J Respir Crit Care Med. 1998;158:700–705. doi: 10.1164/ajrccm.158.3.9711057. [DOI] [PubMed] [Google Scholar]
- 31.Martinez FD, Morgan WJ, Wright AL, Holberg C, Taussig LM, for the Group Health Medical Associates. Initial airway function is a risk factor for recurrent wheezing respiratory illnesses during the first three years of life. Am Rev Respir Dis. 1991;143:312–316. doi: 10.1164/ajrccm/143.2.312. [DOI] [PubMed] [Google Scholar]
- 32.Mauad T, Rivero DH, de Oliveira RC, Lichtenfels AJ, Guimarães ET, de Andre PA, et al. Chronic exposure to ambient levels of urban particles affects mouse lung development. Am J Respir Crit Care Med. 2008;178:721–728. doi: 10.1164/rccm.200803-436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Latzin P, Röösli M, Huss A, Kuehni CE, Frey U. Air pollution during pregnancy and lung function in newborns: a birth cohort study. Eur Respir J. 2009;33:594–603. doi: 10.1183/09031936.00084008. [DOI] [PubMed] [Google Scholar]
- 34.Dong J, Zhang S, Xia L, Yu Y, Hu S, Sun J, et al. Physical activity, a critical exposure factor of environmental pollution in children and adolescents health risk assessment. Int J Environ Res Public Health. 2018;15:E176. doi: 10.3390/ijerph15020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carey MA, Card JW, Voltz JW, Arbes SJ, Jr, Germolec DR, Korach KS, et al. It’s all about sex: gender, lung development and lung disease. Trends Endocrinol Metab. 2007;18:308–313. doi: 10.1016/j.tem.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Naeem E, Tian J, Lombardi V, Kwong K, Akbari O, et al. Sex-specific perinatal nicotine-induced asthma in rat offspring. Am J Respir Cell Mol Biol. 2013;48:53–62. doi: 10.1165/rcmb.2011-0344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gold DR, Wang X, Wypij D, Speizer FE, Ware JH, Dockery DW. Effects of cigarette smoking on lung function in adolescent boys and girls. N Engl J Med. 1996;335:931–937. doi: 10.1056/NEJM199609263351304. [DOI] [PubMed] [Google Scholar]
- 38.Joss-Moore L, Carroll T, Yang Y, Fitzhugh M, Metcalfe D, Oman J, et al. Intrauterine growth restriction transiently delays alveolar formation and disrupts retinoic acid receptor expression in the lung of female rat pups. Pediatr Res. 2013;73:612–620. doi: 10.1038/pr.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zazara DE, Perani CV, Solano ME, Arck PC. Prenatal stress challenge impairs fetal lung development and asthma severity sex-specifically in mice. J Reprod Immunol. 2018;125:100–105. doi: 10.1016/j.jri.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Collins MH, Moessinger AC, Kleinerman J, Bassi J, Rosso P, Collins AM, et al. Fetal lung hypoplasia associated with maternal smoking: a morphometric analysis. Pediatr Res. 1985;19:408–412. doi: 10.1203/00006450-198519040-00018. [DOI] [PubMed] [Google Scholar]
- 41.Sekhon HS, Keller JA, Proskocil BJ, Martin EL, Spindel ER. Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung. Association with alpha7 nicotinic acetylcholine receptors. Am J Respir Cell Mol Biol. 2002;26:31–41. doi: 10.1165/ajrcmb.26.1.4170. [DOI] [PubMed] [Google Scholar]
- 42.Wongtrakool C, Roser-Page S, Rivera HN, Roman J. Nicotine alters lung branching morphogenesis through the alpha7 nicotinic acetylcholine receptor. Am J Physiol Lung Cell Mol Physiol. 2007;293:L611–L618. doi: 10.1152/ajplung.00038.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith KR, Bruce N, Balakrishnan K, Adair-Rohani H, Balmes J, Chafe Z, et al. HAP CRA Risk Expert Group. Millions dead: how do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu Rev Public Health. 2014;35:185–206. doi: 10.1146/annurev-publhealth-032013-182356. [DOI] [PubMed] [Google Scholar]
- 44.Castro-Rodríguez JA, Holberg CJ, Wright AL, Halonen M, Taussig LM, Morgan WJ, et al. Association of radiologically ascertained pneumonia before age 3 yr with asthmalike symptoms and pulmonary function during childhood: a prospective study. Am J Respir Crit Care Med. 1999;159:1891–1897. doi: 10.1164/ajrccm.159.6.9811035. [DOI] [PubMed] [Google Scholar]
- 45.Chan JY, Stern DA, Guerra S, Wright AL, Morgan WJ, Martinez FD. Pneumonia in childhood and impaired lung function in adults: a longitudinal study. Pediatrics. 2015;135:607–616. doi: 10.1542/peds.2014-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. N Engl J Med. 1988;319:1112–1117. doi: 10.1056/NEJM198810273191702. [DOI] [PubMed] [Google Scholar]
- 47.Castleman WL, Sorkness RL, Lemanske RF, Grasee G, Suyemoto MM. Neonatal viral bronchiolitis and pneumonia induces bronchiolar hypoplasia and alveolar dysplasia in rats. Lab Invest. 1988;59:387–396. [PubMed] [Google Scholar]
- 48.Young S, O’Keeffe PT, Arnott J, Landau LI. Lung function, airway responsiveness, and respiratory symptoms before and after bronchiolitis. Arch Dis Child. 1995;72:16–24. doi: 10.1136/adc.72.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCracken JP, Schwartz J, Bruce N, Mittleman M, Ryan LM, Smith KR. Combining individual- and group-level exposure information: child carbon monoxide in the Guatemala woodstove randomized control trial. Epidemiology. 2009;20:127–136. doi: 10.1097/EDE.0b013e31818ef327. [DOI] [PubMed] [Google Scholar]
- 50.Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108:419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grigg J. Particulate matter exposure in children: relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:564–569. doi: 10.1513/pats.200905-026RM. [DOI] [PubMed] [Google Scholar]
- 52.Mortimer K, Ndamala CB, Naunje AW, Malava J, Katundu C, Weston W, et al. A cleaner burning biomass-fuelled cookstove intervention to prevent pneumonia in children under 5 years old in rural Malawi (the Cooking and Pneumonia Study): a cluster randomised controlled trial. Lancet. 2017;389:167–175. doi: 10.1016/S0140-6736(16)32507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith KR, McCracken JP, Weber MW, Hubbard A, Jenny A, Thompson LM, et al. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet. 2011;378:1717–1726. doi: 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.