Abstract
Cardiovascular disease, rare in premenopausal women, increases sharply at menopause and is typically accompanied by chronic inflammation. Previous work in our laboratory demonstrated that replacing senescent ovaries in post-reproductive mice with young, actively cycling ovaries restored many health benefits, including decreased cardiomyopathy and restoration of immune function. Our objective here was to determine if depletion of germ cells from young transplanted ovaries would alter the ovarian-dependent extension of life and health span. Sixty-day-old germ cell-depleted and germ cell-containing ovaries were transplanted to post-reproductive, 17-month-old mice. Mean life span for female CBA/J mice is approximately 644 days. Mice that received germ cell-containing ovaries lived 798 days (maximum = 815 days). Mice that received germ cell-depleted ovaries lived 880 days (maximum = 1046 days), 29% further past the time of surgery than mice that received germ cell-containing ovaries. The severity of inflammation was reduced in all mice that received young ovaries, whether germ cell-containing or germ cell-depleted. Aging-associated inflammatory cytokine changes were reversed in post-reproductive mice by 4 months of new-ovary exposure. In summary, germ cell depletion enhanced the longevity-extending effects of the young, transplanted ovaries and, as with germ cell-containing ovaries, decreased the severity of inflammation, but did so independent of germ cells. Based on these observations, we propose that gonadal somatic cells are programed to preserve the somatic health of the organism with the intent of facilitating future germline transmission. As reproductive potential decreases or is lost, the incentive to preserve the somatic health of the organism is lost as well.
Keywords: Ovarian, Menopause, Inflammation, Life span, Germ cell, Aging
Introduction
The influence of reproduction on health and life span is often thought of as being restricted only to the period of reproductive competency, particularly in women. However, reproductive status influences health throughout all phases of the chronological life span. Evidence over the past decade indicates that an individual’s reproductive status is associated with an increased risk of developing chronic health conditions (National Institutes of Health-Eunice Kennedy Shriver National Institute of Child Health and Human Development, Research Priorities Bulletin 2016). One study documented an association between shorter life spans and reproductive failure for a cohort of men (Eisenberg et al. 2014). The association is even more striking in women. Insulin resistance and bone loss increase at menopause and almost two-thirds of Americans with Alzheimer’s disease are women (Kulaksizoglu et al. 2013; Johnell and Kanis, 2006; Rosario et al. 2011). Cardiovascular disease is rare in premenopausal women, but increases sharply at menopause and in young women with premature ovarian failure (Thom et al. 2006; Shuster et al. 2010; Jacobsen et al. 1999).
Premature cardiovascular disease and an increased risk of atherosclerotic events are typically accompanied by chronic inflammation (Mason and Libby 2014). Persistent inflammation is also a traditional finding in the majority of epidemiologic studies of commonly observed pathophysiologic alterations in patients with chronic kidney disease (Carrero and Stenvinkel 2009). Age-related chronic inflammation, characterized by a mild elevation of inflammatory components in a process known as “inflammaging” has been associated with most age-related diseases, including cardiovascular disease (Franceschi et al. 2000; Frasca and Blomberg 2016). Inflammaging is predominantly triggered by metabolic surplus. Oxidation of excess, circulating lipoproteins activates cellular stress pathways, which initiate and then sustain a continuous, non-resolving inflammatory response (Gregor and Hotamisligil 2011). Circulating lipids and inflammatory mediators interact with each other at multiple levels, thereby aggravating the development of disease. Cholesterol and modified lipids can directly activate inflammatory pathways. Pro-inflammatory cytokines can also directly affect lipid metabolism (van Diepen et al. 2013).
The beneficial effects of dietary restriction on glucose and lipid metabolism in intact female rodents are distinct from the effects in ovariectomized rodents, supporting a central role for the ovaries in female metabolic health (Monteiro et al. 2014; Sterin et al. 1989). In addition, naturally menopausal women possess a health advantage over surgically menopausal women, suggesting that even senescent ovaries provide a health advantage, independent of active germ cells (Yoshida et al. 2011). A classic view of the initiating cause of menopause is the exhaustion of ovarian germ cells. Inconsistent with this view is the observation that, in primitive species, removal of the gonadal germ cells in young invertebrates improves health and longevity (Hsin and Kenyon 1999; Flatt et al. 2008). However, these effects are dependent on the retention of the somatic cells of the gonad. In female mice, dietary restriction inactivates germ cells and extends health span (Nelson et al. 1985; Selesniemi et al. 2008). Both of these observations bring into question the commonly held views that (1) ovarian hormones produced by actively cycling, ovarian germ cells are essential for the maintenance of female health and (2) that the function of gonadal somatic cells is solely to support germ cell maturation. For species to persist, they must pass on their germline to the next generation. We propose that gonadal somatic cells are programed to preserve the somatic health of the organism with the intent of facilitating future germline transmission. As reproductive potential decreases or is lost, such as at menopause in women, the incentive to preserve the somatic health of the organism is lost as well.
Well-defined changes in ovarian signaling mark the end of the traditional reproductive life span. Ovarian transplantation is an efficient experimental method to separate the influence of the reproductive life span or reproductive aging from chronological aging per se. Previous work in our laboratory demonstrated that replacement of the senescent ovaries in post-reproductive female mice with young, actively cycling ovaries can restore many health benefits, including an increase in life span and an improvement in immune function. However, the factors responsible for this ovary-dependent enhancement of health remain unknown. We originally hypothesized that this phenomenon was driven by germ cell-stimulated ovarian hormone production from the new ovaries. The well-established supportive role for ovarian hormones in many aspects of female health implicates the loss of hormone production from actively cycling germ cells, as the principal cause of increased disease risks at menopause. While the value of ovarian hormones in female health is unquestionable, efforts to replace the hormonal milieu of actively cycling ovaries in peri- and post-menopausal women have struggled to reliably restore the health benefits enjoyed by young women with young ovaries.
The observation that replacement of the senescent ovaries of post-reproductive female mice with young ovaries increased health span is robust (Cargill et al. 2003; Mason et al. 2009, 2011, 2015). However, the question remained; what was the role of ovarian germ cells in this ovarian-dependent extension of health? Our objective here was to determine if removal of germ cell influence would alter the ovarian-dependent extension of life and health span. In the current study, we chemically depleted ovarian germ cells in pre-pubertal ovaries and then transplanted young ovaries either with or without germ cells to post-reproductive mice. Germ cell depletion did not diminish the anti-inflammatory benefits and extended the longevity benefits past that of transplanted young, germ cell-containing ovaries.
Material and methods
Mice
The CBA/J strain (used in the current study) and the DBA strain of mice are unique in that they prematurely lose their ovarian follicles, becoming reproductively senescent by 10–12 months of age (Thung et al. 1956; Jones and Krohn 1961; Faddy et al. 1987). A reduction of ovarian follicles in the human is associated with the onset of menopause. For this reason, CBA/J mice may serve as an appropriate experimental model to study age-related changes in human reproduction (Gosden et al. 1978; Barnett et al. 2006).
Twenty-one-day and 8-month-old CBA/J strain female mice were obtained from Jackson Laboratory (Bar Harbor, ME). In addition, 14-month-old female CBA/J mice were obtained from the National Institute on Aging rodent colony. All mice were housed in a standard laboratory animal environment (fresh filtered air, 15 changes/h; temperature, 21 ± 2 °C; humidity, 50 ± 20%; and light-dark cycle, 12:12 h). The mice were housed individually in ventilated cages (Green Line IVC Sealsafe Plus, Tecniplast, West Chester, PA, USA) on corn cob bedding (7097 Corncob, Harlan Teklad, Bartonville, IL, USA) changed once a week, with added enrichment (nestlets and multiple paper tubes), in a specific-pathogen-free colony where pathology on sentinel mice was done quarterly and pathological results showed no breach in this status. The mice were housed individually to prevent complications during healing of surgical wounds and to decrease potential influence of the Whitten effect (synchronous estrus in females). The mice received deionized water and a certified laboratory diet ad libitum (2018 Teklad Global 18% Protein Rodent Diet, Harlan Teklad, Bartonville, IL, USA).
Anesthetics were used during surgery (see Surgical Procedures) and analgesia was provided for 48-h post-operatively, longer if deemed necessary. Animals with acute, but not severe weight loss were treated with subcutaneous fluids and moistened food. Animals with acute, but not severe urine staining or rectal/vaginal prolapse were manually cleaned and treated with Desitin®. Mice were monitored at least twice daily and weights were recorded monthly, more frequently when concerns arose. Aged, moribund mice found with overt clinical signs (catatonia) were euthanized. Mice were euthanized by cervical dislocation. Immediately after cervical dislocation, a thoracotomy was performed followed by rapid exsanguination via cardiocentesis. The heart and arterial tree were then removed.
Mice were maintained in an American Association for Accreditation of Laboratory Animal Care (AAALAC)-approved facility in accordance with the National Institutes of Health animal-use guidelines. Animal care and use protocols were developed under the National Research Council guidelines found in the Guide for the Care and Use of Laboratory Animals. This project was approved by the Utah State University Institutional Animal Care and Use Committee (IACUC-2277).
Experimental design
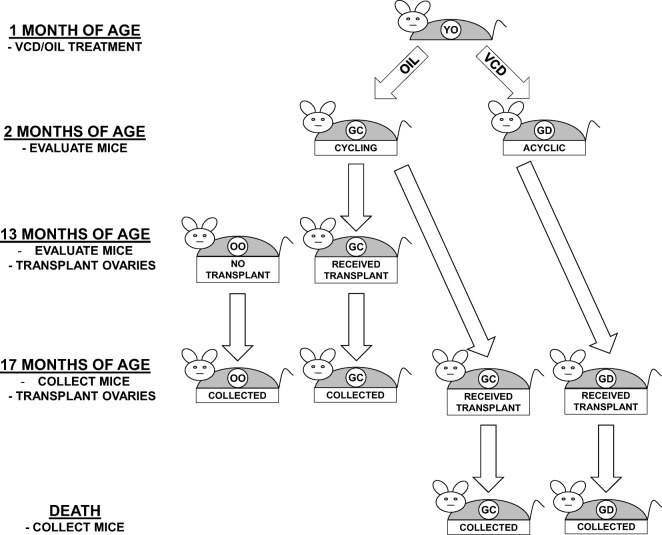
Animals were randomly assigned to control or transplant groups as follows (Fig. 1):
Fig. 1.
Experimental design. One-month-old mice were treated for 15 days with VCD or oil-only. Ovaries were collected from 2-month-old oil-only mice for transplantation to 13-month-old, acyclic recipients. Ovaries were also collected from 2-month-old VCD and oil-only mice for transplantation to 17-month-old, acyclic recipients. Thirteen-month-old controls and transplant recipients were collected at 17 months of age. Seventeen-month-old VCD and oil-only transplant recipients were collected at death
Experimental groups
Thirteen-month-old control mice (no transplant—no-Tx@13mo)
Reproductively senescent (acyclic) control mice were evaluated for cycle status at 13 months of age, kept their old ovaries (OO) and were collected at 17 months of age.
Thirteen-month-old transplant recipient mice (GC-Tx@13mo)
Reproductively senescent (acyclic) control mice were evaluated for cycle status at 13 months of age, at which time their senescent endogenous ovaries were removed and replaced with a pair of actively cycling, germ cell-containing donor ovaries (GC) from a 2-month-old mouse. These transplant recipient mice were reproductively cycling and were collected at 17 months of age.
Seventeen-month-old transplant recipient mice (GC-Tx@17mo)
Reproductively senescent (acyclic) control mice were evaluated for cycle status at 17 months of age, at which time their senescent endogenous ovaries were removed and replaced with pair of actively cycling, GC ovaries from a 2-month-old mouse. These mice were evaluated at the time of natural death/end of life euthanasia.
Seventeen-month-old germ cell-depleted transplant recipient mice (GD-Tx@17mo)
Reproductively senescent (acyclic) control mice were evaluated at 17 months of age, at which time their senescent endogenous ovaries were removed and replaced with a pair of GD ovaries from a 2-month-old mouse. These mice were evaluated at the time of natural death/end of life euthanasia.
Germ cell depletion
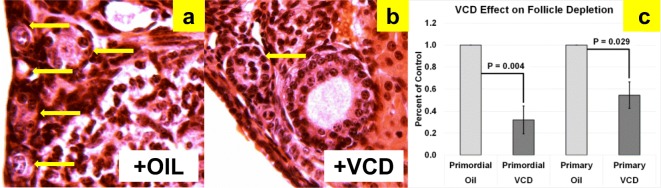
Intact donor animals at 28 days of age received daily intraperitoneal injections of 160 mg/kg 4-vinylcyclohexene diepoxide (VCD; Sigma-Aldrich, St. Louis, MO) in sesame oil or injections of sesame oil only for 15 days. At 43 days of age, VCD and vehicle treatments were stopped. In our lab, treatment of 28-day-old female CBA/J mice for 15 days with VCD results in reduced ovarian weights (1.7 mg in oil-only vs. 0.9 mg in VCD-treated, P = 0.030) and depleted primordial (P = 0.004) and primary (P = 0.029) ovarian follicles at 43 days of age, compared with controls (Fig. 2). Cessation of reproductive cyclicity (persistent vaginal cornification) was documented for all VCD-treated mice prior to 60 days of age. Because VCD-treatment eliminates primordial and primary follicles, any existing secondary or later stage follicles will be exhausted and not replaced.
Fig. 2.
VCD depletion of small follicles in CBA/J mice. H&E-stained sections showing (a) abundant small follicles in oil-treated (n = 6) mice, (b) reduced numbers of small follicles in VCD-treated (n = 4) mice, and (c) and already displayed significant differences in both primordial and primary follicle numbers by 45 days of age. Arrows indicate primordial and primary follicles
Age at manipulation
Rodents do not undergo menopause, but instead, have an estropause-like decrease in reproductive function. Female mice of the CBA /J strain become reproductively competent between 45 and 60 days of age. Initiation of germ cell depletion at 28 days of age was chosen to avoid major up-regulation of the reproductive system at the onset of puberty and to eliminate other influences the female gonad might have in addition to direct effects of gonadal hormones. These influences may include positive or negative feedback mechanisms, or system-wide “imprinting” influences the intact ovary may normally provide upon reproductive maturation. Reproductive decline in CBA/J mice usually begins with irregular cycles at 8–10 months of age. At 11 months of age, many CBA/J mice have become reproductively incompetent (Cargill et al. 2003). All 13- and 17-month-old recipients and all germ cell-depleted donor mice used in these experiments displayed a complete lack of reproductive cycling, as determined by vaginal cytology.
Surgical procedures
Thirteen-month and 17-month-old animals underwent a bilateral ovariectomy and subsequent ovarian transplantation and received a pair of 2-month-old ovaries from a donor mouse of the same strain. Bilateral ovarian transplantation surgeries were performed as previously described (Cargill et al. 1999; Mason et al. 2018). Briefly, the ovaries were exposed by paralumbar incision under anesthesia (50–100 mg/kg ketamine, 10–15 mg/kg xylazine, and 2–3 mg/kg acepromazine, intraperitoneal) and removed by incising the ovarian bursa opposite the ovarian hilum. The ovary was gently removed from the ovarian bursa and excised by clamping the ovarian hilum to prevent bleeding. Excised ovaries were placed in cold saline prior to transfer/replacement. After transfer/replacement, the ovarian bursa was closed with one to three sutures of 10–0 Ethilon monofilament (Ethicon, Inc.). The abdominal wall was sutured with 6–0 Vicryl (Ethicon, Inc.), and the skin was closed with 9 mm wound clips (MikRon Precision, Inc.).
Data on vaginal cytology were collected for at least 10 consecutive days pre- and post-operatively to ensure (1) cessation of cycling and (2) success of the ovarian transplantation procedure in mice that received germ cell-containing ovary transplants. Daily vaginal cytology was re-initiated beginning 10–14 days post-operatively. One estrous cycle was defined as the period from the day nucleated epithelial cells first appeared (i.e., proestrus) to the day preceding the next appearance of nucleated epithelial cells in the vaginal smear, provided there was a period of leukocytic presence (i.e., diestrus) in between. Estrus was determined by the presence of large, squamous epithelial cells, with or without nuclei. Success of the ovarian transplantation procedure in mice that received germ cell-depleted ovary transplants was determined by an increase in the androgen:estrogen ratio, which increases significantly after germ cell depletion in mice (Rivera et al. 2009). No immunosuppressive techniques were employed and no evidence of graft-versus-host disease was detected post-transplantation or at death.
Exclusion criteria
Mice that displayed cytological evidence of cyclic gonadal input prior to surgery at 13 or 17 months of age were excluded from these experiments. 4-vinylcyclohexene diepoxide-treated donor mice that displayed cytological evidence of gonadal input at 2 months of age were also excluded from these experiments. Cyclic gonadal input was defined as cyclic changes on vaginal cytology, presumably due to cyclic influence of ovarian hormones. No cyclic gonadal input was defined as the lack of cyclic changes on vaginal cytology. Germ cell-containing transplant recipients that failed to display evidence of cyclic gonadal input post-operatively based on vaginal cytology or germ cell-depleted transplant recipients that failed to display evidence of change in the androgen:estrogen ratio post-operatively were also excluded from analysis. Mice that fit these criteria were the only mice used for analysis throughout this study.
Determination of life span
The life span for individual mice was determined by recording the age of spontaneous death or euthanasia. Aged, moribund mice found with overt clinical signs (catatonia) were euthanized. Criteria for euthanasia specific for aged mice were determined in coordination with the attending veterinarian and included, but were not limited to mice found in poor condition with or without crusting around the perineum and diarrhea, urine staining, persistent vaginal prolapse, chronic vulva/rectal swelling, kyphosis, respiratory distress, anorexia, poor coat condition and lack of grooming, moribund mentation, hind-limb weakness/paresis, wounds not healing, limited mobility, neoplastic growth, and unusual weight loss (or gain). Average weight loss in aged, female CBA/J mice, from peak weight to death is approximately 12% per month (Mason et al. 2010). An increased rate of weight loss, but not total weight loss was the most critical factor for determining a moribund state. Unexpected deaths were uncommon, but included neoplastic growths (most commonly mammary), decubitus ulcers (extremely old animals) and uncontrolled cataleptic seizures (normally between 11 and 13 months of age).
Determination of inflammatory pathology at death
Pathological and histological analysis was conducted by Dr. Yuji Ikeno at the Barshop Institute for Longevity and Aging Studies. Mice in all groups were submitted for necropsy at death/euthanasia. After mice were necropsied for gross pathological lesions, the following organs and tissues were excised and preserved in 10% buffered formalin: brain, pituitary gland, heart, lung, trachea, thymus, aorta, esophagus, stomach, small intestine, colon, pancreas, spleen, kidneys, urinary bladder, reproductive system (ovaries, oviduct, uterus, cervix and vagina), thyroid gland, adrenal glands, parathyroid glands, psoas muscle, tibiofemoral joint, sternum, and vertebrae. Other tissues with gross lesions, including liver were also excised. Liver tissue without gross lesions was frozen for further analysis. The fixed tissues were processed conventionally, embedded in paraffin, sectioned at 5 μm and stained with hematoxylin-eosin. Although autolysis of varying severity can occur, it normally does not prevent the histopathological evaluation of lesions.
Diagnosis of each histopathological change was made with histological classifications in aging mice previously described (Bronson and Lipman 1991; Ikeno et al. 2005). A list of pathological lesions was constructed for each mouse that included both neoplastic and non-neoplastic diseases. Based on these histopathological data, the probable cause of death in each mouse was assessed.
Cytokine analysis
Circulating cytokine analysis was conducted in mice that received new ovaries at 13 months of age and were collected at 17 months of age, along with age-matched controls. Cytokine analysis was conducted by Dr. Björn Schumacher, Chair for Genome Stability in Ageing and Disease, CECAD Research Center, University of Cologne. Circulating factors in serum were analyzed using a G-Series Mouse Cytokine Antibody Array (GS4000), which is a combination of five, non-overlapping arrays to measure the relative expression levels of 200 mouse cytokines as per the manufacturer’s protocol. Briefly, the assay slides were dried, blocked, and incubated with sample; washed and incubated with biotinylated antibody cocktail; washed and incubated with IRDye 800CW Streptavidin Antibody; and washed and imaged using an LI-COR Odyssey CLx. Data was extracted using the manufacturer’s GAL file and the raw numerical data extracted from the array scan was analyzed with the GSM-CAA-4000 data analysis software specific for the Mouse Cytokine Array GS4000.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7.01 (GraphPad Software, Inc. La Jolla, CA). A D’Agostino-Pearson omnibus test was used to determine normality. Data were analyzed with two-factor ANOVA and a Tukey-Kramer post-hoc test was used to determine difference between groups. Individual treatments were further analyzed by paired Student’s t test, two-tailed, unequal distribution of variance assumed. Test results were considered significant for P values P < 0.05.
Results
Longevity
Mean life span for female CBA/J mice is approximately 644 days (Yuan et al. 2009). In the current experiments, 2-month-old germ cell-depleted and germ cell-containing ovaries were transplanted to post-reproductive mice at 17 months of age (517 days of age). Mice that received GC ovaries lived 798 days of age (maximum life span of 815 days). Mice that received GD ovaries lived 880 days (maximum life span of 1046 days). Mice that received new ovaries at 13 months were collected at 17 months, and therefore were not included in the longevity analysis.
Current transplantation protocols were the same as in previous experiments, as evidenced by the < 1% difference in results between previous intact ovary transplants at 18 months and the current intact ovary transplants at 17 months. In previous experiments, mice subjected to sham surgeries were necessarily selected to live at least until the time of surgery (11 months of age). In these previous experiments, sham mice lived 728 days of age and post-reproductive mice that received new, transplanted ovaries from young, 2-month-old mice lived mice lived 793 days of age (Mason et al. 2009). Mice that had received young ovaries at 18 months of age were no different in mean age at death (within 2%; J.B. Mason, unpublished observations) from mice that received ovaries at 11 months (Figs. 3 and 4).
Fig. 3.
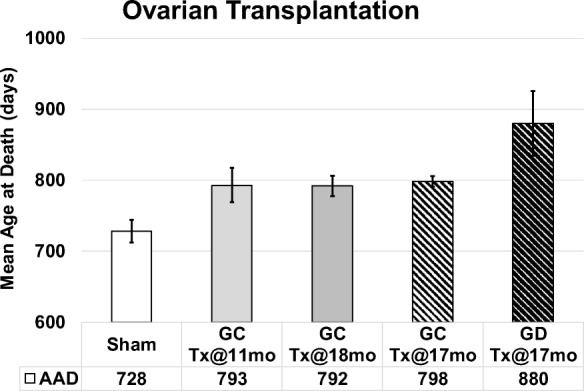
Influence of young ovaries on life span in post-reproductive recipients. Young ovaries transplanted (Tx) to 11 month-old mice (GC-Tx@11mo, n = 30) extended life span by 13% past the time of surgery, compared with sham operated mice (Sham, n = 34). Young ovaries Tx at 17mo (GC-Tx@17, n = 5) or 18mo (GC-Tx@18, n = 6) were no different from 11mo Tx. Depleting the germ cells from young ovaries prior to Tx (GD-Tx@17mo, n = 5) more than doubled (29%) the life span extension of GC ovaries. AAD, age at death. Patterned bars represent the current longevity experiments. * P < 0.05, ** P < 0.1. Error bars are SE. (Mason et al. 2009)
Fig. 4.
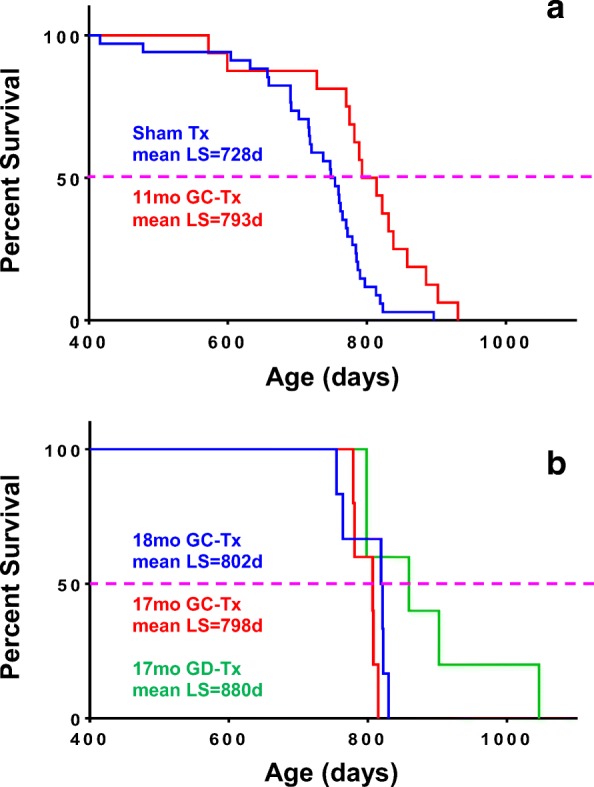
Influence of young ovaries on survival. At 750 days of age, 50% of sham mice had died. At this same age, 81% of mice transplanted with young ovaries at 11 months of age were still alive and 100% of mice transplanted at 17 and 18 months of age were still alive. Maximum life span, but not mean life span was influenced by the number of mice per group. a Mice that underwent sham surgery (mean LS = 728 days) and mice that received 60-day ovaries at 11 months of age (mean LS = 793 days). b Mice that received intact 60-day ovaries at 17 and 18 months of age (mean LS = 798 days and 802 days, respectively) and mice that received germ cell-depleted 60-day ovaries at 17 months of age (mean LS = 880 days)
Inflammatory pathology
Mice that received young ovaries at 13 months and control mice were collected at 17 months of age (after 4 months of exposure to new ovaries in transplant recipients). Mice with 4 months of exposure to GC ovaries displayed decreased severity of inflammation and decreased glomerulonephritis, compared with age-matched controls (Fig. 5). Among mice that received young ovaries at 17 months and that were collected at death, germ cell depletion of the transplanted young ovaries had no influence in severity of inflammation or glomerulonephritis (Fig. 6).
Fig. 5.
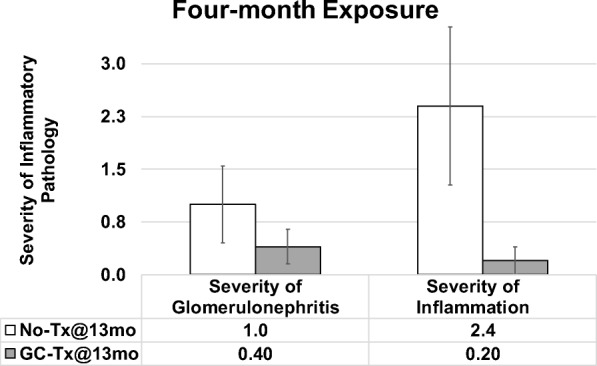
Influence of young ovaries on glomerulonephritis and inflammation in post-reproductive recipients. Young ovaries transplanted (Tx) to 13 month-old mice (GC-Tx@13mo) decreased the severity of glomerulonephritis and inflammation at 4 months post-transplantation (17 months of age), compared with mice that did not receive new ovaries (No-Tx@13mo). Error bars are SE
Fig. 6.
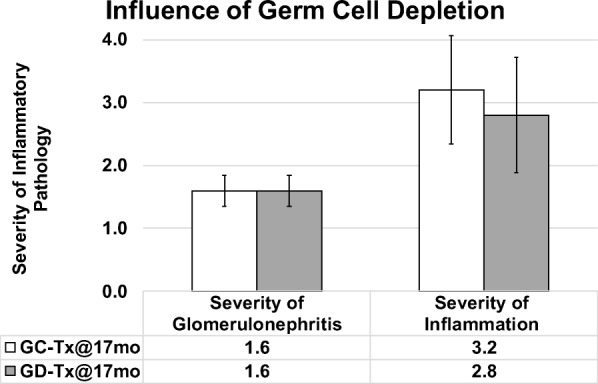
Influence of germ cell depletion of young ovaries on glomerulonephritis and inflammation in post-reproductive recipients. Germ cell depletion of young ovaries prior to transplantation to 17 month-old mice (GD-Tx@17mo) had little to no influence of the severity of inflammation or glomerulonephritis at death, compared with mice that received germ cell-containing new ovaries at 17 months of age (GC-Tx@17mo). Error bars are SE
Inflammatory cytokines
Over a 4-month period, beginning shortly after the time of reproductive senescence at 13 months of age, until the time of collection at 17 months of age, aging/ovarian failure lead to a decrease in circulating inflammation-associated cytokines, both pro- and anti-inflammatory. These decreases in cytokine levels were restored in mice that received transplanted young ovaries at the start of the 4-month period (Fig. 7).
Fig. 7.
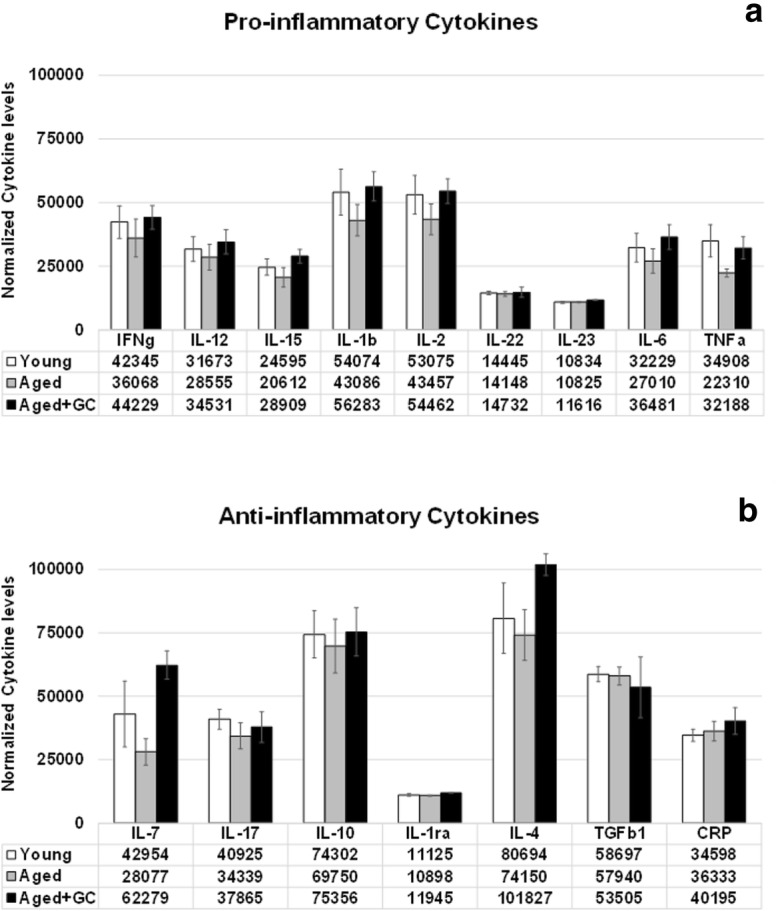
Inflammatory cytokines. Both pro- and anti-inflammatory cytokines decreased from the time of reproductive senescence at 13 months to 17 months of age. These decreases were reversed by exposure to new ovaries in mice that received new ovaries at 13 months of age (GC-Tx@17mo). Values are 1/1000 of actual values. * P < 0.05. Error bars are SE
Discussion
Longevity
In our model, the observed extension of life span was ovary-dependent, but ovarian germ cell-independent. Intact young ovaries provided young ovarian tissue, which extended life span. Germ cell-depleted young ovaries also provided young ovarian tissue, but no germ cells, which extended life span even further. Ovarian hormones and the feedback effects of these hormones produced by germ cell-directed follicular development in actively cycling ovaries likely have a positive influence on health. Hormone replacement therapy, when initiated early during peri-menopause can have a positive effect on menopausal health. However, it appears that the health benefits of germ cell-driven, cyclic ovarian hormones and the longevity benefits of ovarian somatic cell signaling may be distinct mechanisms.
Transplanted intact and germ cell-depleted ovaries both provided young ovarian somatic cells to the recipient. However, germ cell-containing, actively cycling ovaries supported developing follicles, which continually recruited these somatic cells to support follicle maturation during the period when the ovary was cycling. These waves of follicular development constantly recruit new oocytes and somatic cells from the resting ovarian reserve. Over time, as the ovarian germ cells become depleted, the somatic cells recruited by these maturing germ cells likely become depleted as well. In the germ cell-depleted ovaries, the somatic cells are not recruited to developing follicles, but may instead be available to support the somatic health of the organism and would be expected to persist much longer than somatic cells in the intact, cycling ovaries.
The GH/IGF-1 signaling axis is well known to influence longevity, particularly when reduced prepubertally (Podlutsky et al. 2017). Post-reproductive mice in the current study had experienced a full reproductive life span and exposure to normal levels of GH/IGF-1 signaling prior to initiation of longevity-extending treatments. This may suggest that GH/IGF-1 signaling was not the dominant factor in the observed life span extension in the current study. However, the effects of IGF-1 are dependent on the tissue, gender, and the age of the animal (Ashpole et al. 2017). Circulating IGF-1 levels were reduced in our transplant recipients with extended longevity (unpublished observations). Reduced ovarian IGF-1 signaling can lead to increased Foxo signaling levels, a common factor in extension of longevity in primitive species and with dietary restriction. Interestingly, the beneficial effects of dietary restriction on glucose and triglyceride metabolism in intact female rodents do not appear in ovariectomized rodents, supporting a central role for the ovary in female metabolic health (Casalino et al. 1994). The observation that naturally menopausal women with reproductively senescent ovaries possess a health advantage over surgically menopausal women suggests that something about the ovary provides a health advantage, independent of active germ cells.
In primitive species, the extension of longevity due to germ cell depletion is dependent on the presence/retention of gonadal somatic cells (Arantes-Oliveira et al. 2002; Flatt et al. 2008). Complete gonad removal in female mice shortens the life span (Mason et al. 2009). In our model, germ cell depletion may have provided an extended period of exposure to naïve ovarian somatic cells (cells not recruited to support reproduction), compared with mice that received intact ovaries.
Inflammation
Chronic inflammation is a common component of many aging-associated pathological conditions. Changes that positively influence inflammation are likely to positively influence many other aging pathologies. In previous work, transplantation with young ovaries positively influenced immune and renal function, reduced cardiomyopathy, decreased sarcopenia, decreased unintentional age-associated weight loss, improved cognitive behavior and sensory function, and decreased arthritis in recipient mice (Peterson et al. 2017; Mason et al. 2011; Peterson et al. 2016; Mason et al. 2010; Parkinson et al. 2017; Mason et al. 2015). In the current study, young ovaries transplanted to old mice produced a major reduction in the severity of inflammation, suggesting a significant ovarian influence on immune function. Surprisingly, these effects were not diminished in mice that received germ cell-depleted ovaries. This points toward an ovarian dependent, but germ cell-independent, positive influence on aging-associated chronic inflammation.
Among mice analyzed over a 4-month period, beginning at the time of reproductive senescence, inflammation-associated cytokines decreased with aging/reproductive failure, but were restored/maintained by 4 months of exposure to new, intact ovaries. T cell function was also improved in these mice and in 13-month-old mice with in situ germ cell-depleted ovaries (Peterson et al. 2017; Habermehl TL, Parkinson KC, Mason JB (2018) Germ cell depletion influenced neuromuscular, sensory, renal and metabolic function in post reproductive female mice. (submitted)). Interleukin 7 (IL-7), which has a positive influence on immune function was decreased by 35% with aging. Transplantation of new ovaries reversed this change to a 45% increase in circulating IL-7. Both IL-6 and IL-10 have been reported to increase during aging in rodent models (Longo and Finch 2003; Panda et al. 2009). However, IL-10 knock-out mice showed increased inflammation, increased frailty and increased mortality (Deepa et al. 2017). Elevation of both IL-6 and IL-10 is associated with decreased production of reactive oxygen species (ROS) in the aging rat brain (Xie et al. 2003; Sparkman and Johnson 2008). In contrast to much of the previous research on immune cytokines in aging, in our model, both IL-6 and IL-10 decreased with aging (− 16% and − 6%, respectively) and were restored by ovarian transplantation at or above levels found in pre-transplant mice (+ 13% and + 1%, respectively). Aging is highly heterogeneous and it is possible that because we began treatments in aged animals, these selected animals may have been healthier than the general population of CBA/J female mice and may have not displayed the classical hallmarks of immune senescence (Rais et al. 2017). In addition, the TGFβ1 decrease in recipients (9%), which did not reach statistical significance may have provided an additional vascular protective effect (Ungvari et al. 2017).
Two major hallmarks of the menopausal transition include chronic inflammation and dyslipidemia. Reduced lipid catabolism at menopause can lead to oxidation of excess circulating lipids and systemic inflammation. Exogenous estrogens can reduce the serum levels of several markers for inflammation in post-menopausal women (Stork et al. 2002). However, germ cell-depleted ovaries do not provide cyclic estrogens. Based on pilot data suggesting an increase in lipid metabolism with new ovaries (unpublished observations, Dr. Mason), germ cell-depleted ovaries may provide a non-estrogenic metabolic correction of lipid profiles.
Conclusions
In summary, depletion of the germ cells prior to transplantation of young ovaries to post-reproductive females did not compromise the anti-inflammatory benefits of the young ovaries. Depletion of the germ cells prior to transplantation also did not compromise the life span-extending effect of the young ovaries, but instead extended life span even further than transplantation with intact young ovaries. Estrogens and progestins have a well-established role in maintaining/improving female health. Mounting evidence suggests that estrogen and progesterone have disparate, sometimes opposing effects on inflammation, immunity, and autoimmunity (Hughes and Clark, 2007). The observations that chronic estrogen replacement therapy may exacerbate chronic neuroinflammation and exacerbate disease in lupus erythematosus suggest using caution when considering the use of hormone replacement therapy to treat age- or menopause-associated diseases, many of which are associated with inflammatory processes (Marriott et al. 2002; Hughes et al. 2009). A weakness of the current work is the small number of animals included in each group, which often precluded results from reaching statistical significance, even though there were often large percentage differences between groups. Results from previous experiments involving transplantation of young, intact ovaries are all within 1–2% of the results for mean age at death for the current group. This strongly suggests that these longevity results are reliable and repeatable. In addition, the immune data, even with the small number of animals included in this analysis provides a strong indication of trends toward a major ovarian influence on immune function.
The current results suggest the presence of a germ cell-independent positive ovarian influence on health. We propose that gonadal somatic cells are programed to preserve the somatic health of the organism with the intent of facilitating future germline transmission. As reproductive potential decreases or is lost, the incentive to preserve the somatic health of the organism is lost as well (Fig. 8). Results seen with germ cell-depletion suggest that more than one mechanism of influence may exist in young ovaries (germ cell-dependent and germ cell-independent) toward the preservation of health in young females and the restoration of health in post-reproductive transplant recipients. Future work will include identification of a potentially evolutionarily conserved, germ cell-independent molecular mechanism that contributes to the ovarian tissue-dependent extension of health and life span.
Fig. 8.
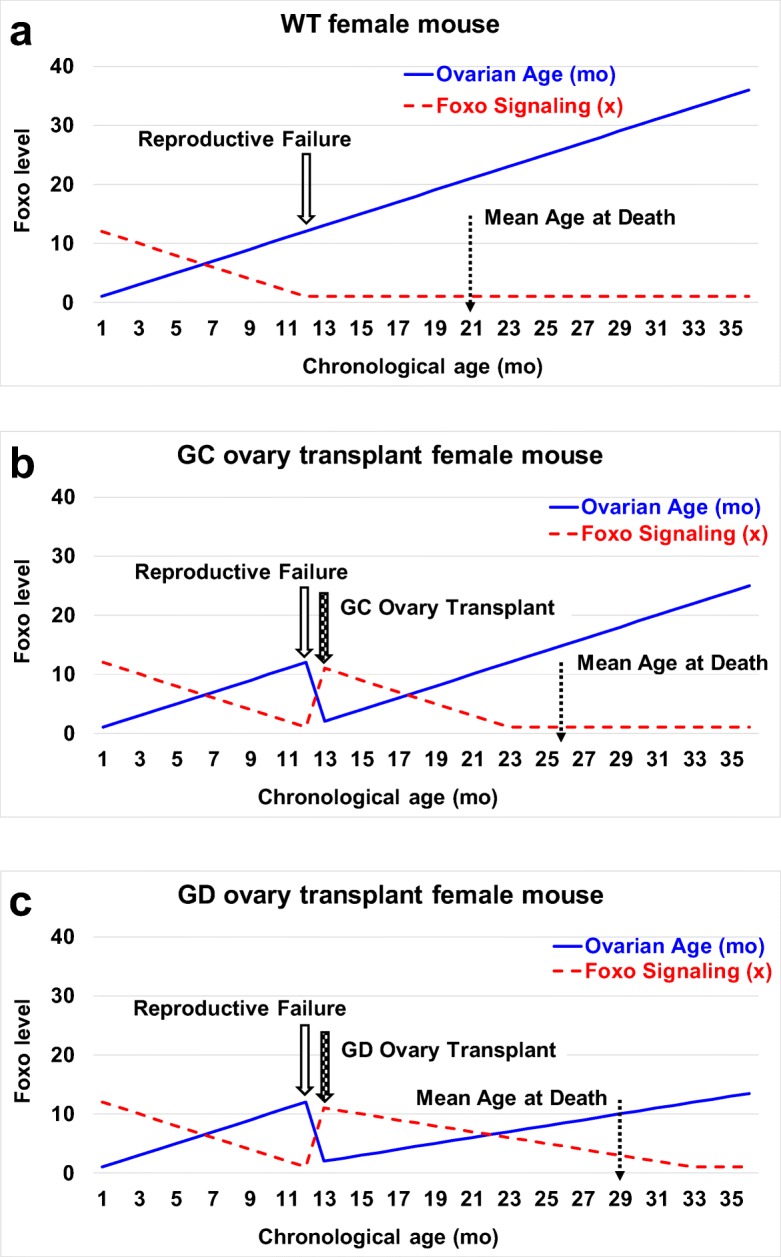
Theory of longevity extension in post-reproductive recipients. a In wild-type mice, reproductive cycling continuously recruits both ovarian germ and somatic cells. At reproductive senescence, both germ and somatic cells are lost, along with the female health advantage. We hypothesize that Foxo signaling from ovarian somatic cells contributes to the female health advantage. In mammals, Foxo suppresses the de novo methyltransferase Dnmt3b and reduces the age-associated erosion of methylation patterns and epigenetic reprogramming. Foxo signaling is also linked to gender-specific longevity in centenarians. Ovarian Foxo signaling is significantly reduced at menopause due to the loss of Foxo-producing ovarian tissue. b Young, germ cell-containing ovaries (GC) transplanted to 13 month-old mice extended life span by supplying new ovarian somatic cells and resetting the Foxo clock. c Deleting the germ cells from young ovaries (GD) prior to transplantation prevented reproductive cycling and the continuous recruitment of ovarian somatic cells. This extended the influence of the transplanted somatic cells and further extended the Foxo clock
Acknowledgments
The authors thank Dr. Aaron Olsen and Ms. Lisa DeSoi for help with the mice, Dr. Erik Eide and Mr. Mahdi Nazokkarmaher for help with VCD injections, and Ms. Sumira Phatak for guidance with magnetic resonance imaging. The authors also thank Dr. Shelley Cargill and Dr. Chris Pearl for critical review of the manuscript. Research reported in this publication was supported by Utah State University, School of Veterinary Medicine, Department of Animal, Dairy and Veterinary Sciences Research Initiation Funds, the Cluster of Excellence for Aging Research (CECAD) at the University of Cologne and by a generous gift of aged CBA/J female mice from Nancy Nadon at the National Institute on Aging.
Author contributions
Conceived and designed the experiments: TH, JM. Performed the experiments: TH, KP, GH, YI, JE, BS, JM. Analyzed the data: TH, GH, YI, JE, BS, JM. Contributed reagents/materials/analysis tools: YI, BS, JM. Wrote the paper: TH, KP, JM.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295(5554):502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges E, LUngvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. GeroScience. 2017;39(2):129–145. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett KR, Schilling C, Greenfeld CR, Tomic D, Flaw JA. Ovarian follicle development and transgenic mouse models. Hum Reprod Update. 2006;12(5):537–555. doi: 10.1093/humupd/dml022. [DOI] [PubMed] [Google Scholar]
- Bronson RT, Lipman RD. Reduction in rate of occurrence of age-related lesions in dietary restricted laboratory mice. Growth Dev Aging. 1991;55(3):169–184. [PubMed] [Google Scholar]
- Cargill SL, Medrano JF, Anderson GB. Infertility in a line of mice with the high growth mutation is due to luteal insufficiency resulting from disruption at the hypothalamic-pituitary axis. Biol Reprod. 1999;61(1):283–287. doi: 10.1095/biolreprod61.1.283. [DOI] [PubMed] [Google Scholar]
- Cargill SL, Carey JR, Muller HG, Anderson GB. Age of ovary determines remaining life expectancy in old ovariectomized mice. Aging Cell. 2003;2(3):185–190. doi: 10.1046/j.1474-9728.2003.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrero J, Stenvinkel P. Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: a hypothesis proposal. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S49–S55. doi: 10.2215/CJN.02720409. [DOI] [PubMed] [Google Scholar]
- Casalino SM, Linares JA, Goldraij A. Different effect of a restricted diet on isolated uteri of ovariectomized and non-ovariectomized rats. Influence of indomethacin and prostaglandins. Prostaglandins Leukot Essent Fat Acids. 1994;51(1):41–45. doi: 10.1016/0952-3278(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Bhaskaran S, Espinoza S, Brooks SV, McArdle A, Jackson MJ, Van Remmen H, Richardson A. A new mouse model of frailty: the Cu/Zn superoxide dismutase knockout mouse. GeroScience. 2017;39(2):187–198. doi: 10.1007/s11357-017-9975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Li SF, Behr B, Cullen MR, Galusha D, Lamb DJ, Lipshultz LI. Semen quality, infertility and mortality in the USA. Hum Reprod. 2014;29(7):1567–1574. doi: 10.1093/humrep/deu106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faddy MJ, Telfer E, Gosden RG. The kinetics of pre-antral follicle development in ovaries of CBA/Ca mice during the first 14 weeks of life. Cell Tissue Kinet. 1987;20:551–560. doi: 10.1111/j.1365-2184.1987.tb01364.x. [DOI] [PubMed] [Google Scholar]
- Flatt T, Min K, D’Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, Jones D, Tatar M. Drosophila germ-line modulation of insulin signaling and lifespan. Proc Natl Acad Sci U S A. 2008;105:6368–6373. doi: 10.1073/pnas.0709128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology. 2016;17(1):7–19. doi: 10.1007/s10522-015-9578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosden RG, Jones EC, Jacks F. Pituitary-ovarian relationships during post-reproductive phase of inbred mice. Exp Gerontol. 1978;13:159–166. doi: 10.1016/0531-5565(78)90008-6. [DOI] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Hsin H, Kenyon C (1999) Signals from the reproductive system regulate the lifespan of C-elegans. Nature 399:362–366. 10.1038/20694 [DOI] [PubMed]
- Hughes GC, Clark EA. Regulation of dendritic cells by female sex steroids: relevance to immunity and autoimmunity. Autoimmunity. 2007;40:470–481. doi: 10.1080/08916930701464764. [DOI] [PubMed] [Google Scholar]
- Hughes GC, Martin D, Zhang K, Hudkins HL, Alpers CL, Clark EA, Elkon KB. Decrease in glomerulonephritis and Th1-associated autoantibody production after progesterone treatment in NZB/NZW mice. Arthritis Rheum. 2009;60:1775–1784. doi: 10.1002/art.24548. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Hubbard GB, Lee S, Richardson A, Strong R, Diaz V, Nelson JF. Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci. 2005;60(12):1510–1517. doi: 10.1093/gerona/60.12.1510. [DOI] [PubMed] [Google Scholar]
- Jacobsen BK, Knutsen SF, Fraser GE. Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist health study. J Clin Epidemiol. 1999;52(4):303–307. doi: 10.1016/s0895-4356(98)00170-x. [DOI] [PubMed] [Google Scholar]
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- Jones EC, Krohn PL. Relationships between age, numbers of oocytes and fertility in virgin and multiparious mice. J Endocrinol. 1961;21:469–495. doi: 10.1677/joe.0.0210469. [DOI] [PubMed] [Google Scholar]
- Kulaksizoglu M, Ipekci SH, Kebapcilar L, Kebapcilar AG, Korkmaz H, Akyurek F, Baldane S, Gonen MS. Risk factors for diabetes mellitus in women with primary ovarian insufficiency. Biol Trace Elem Res. 2013;154(3):313–320. doi: 10.1007/s12011-013-9738-0. [DOI] [PubMed] [Google Scholar]
- Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299(5611):1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- Marriott LK, Hauss-Wegrzyniak B, Benton RS, Vraniak PD, Wenk GL. Long-term estrogen therapy worsens the behavioral and neuropathological consequences of chronic brain inflammation. Behav Neurosci. 2002;116(5):902–911. doi: 10.1037//0735-7044.116.5.902. [DOI] [PubMed] [Google Scholar]
- Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J. 2014;36:482–489. doi: 10.1093/eurheartj/ehu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JB, Cargill SL, Anderson GB, Carey JR. Transplantation of young ovaries to old mice increased life span in transplant recipients. J Gerontol A Biol Sci Med Sci. 2009;64(12):1207–1211. doi: 10.1093/gerona/glp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JB, Cargill SL, Anderson GB, Carey JR. Ovarian status influenced the rate of body-weight change but not the total amount of body-weight gained or lost in female CBA/J mice. Exp Gerontol. 2010;45:435–441. doi: 10.1016/j.exger.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JB, Cargill SL, Griffey SM, Reader JR, Anderson GB, Carey JR. Transplantation of young ovaries restored cardioprotective influence in post-reproductive-aged mice. Aging Cell. 2011;10:448–456. doi: 10.1111/j.1474-9726.2011.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JB, Terry BC, Merchant SS, Mason HM, Nazokkarmaher M. Manipulation of ovarian function significantly influenced trabecular and cortical bone volume, architecture and density in mice at death. PLoS One. 2015;10(12):e0145821. doi: 10.1371/journal.pone.0145821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JB, Parkinson KC, Habermehl TL (2018) Orthotopic ovarian transplantation procedures to investigate the life- and health-span influence of ovarian senescence in female mice. J Vis Exp (132):e56638. 10.3791/56638 [DOI] [PMC free article] [PubMed]
- Monteiro R, Teixeira D, Calhau C. Estrogen signaling in metabolic inflammation. Mediat Inflamm. 2014;615917:1–20. doi: 10.1155/2014/615917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Fertility and Infertility Branch. New research priorities. 'Fertility status as a marker of overall health'. 2016. "Support studies that investigate fertility status as a marker of overall health for both men and women"
- Nelson JF, Gosden RG, Felicio LS. Effect of dietary restriction on estrous cyclicity and follicular reserves in aging C57/BL6 mice. Biol Reprod. 1985;32(3):515–522. doi: 10.1095/biolreprod32.3.515. [DOI] [PubMed] [Google Scholar]
- Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR, Lord JM, Shaw AC. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 2009;30(7):325–333. doi: 10.1016/j.it.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson KC, Peterson RL, Mason JB. Cognitive behavior and sensory function were significantly influenced by restoration of active ovarian function in post-reproductive mice. Exp Gerontol. 2017;92:28–33. doi: 10.1016/j.exger.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Peterson RL, Parkinson KC, Mason JB (2016) Manipulation of ovarian function significantly influenced sarcopenia in post-reproductive-age mice. J Transplant. 10.1155/2016/4570842 [DOI] [PMC free article] [PubMed]
- Peterson RL, Parkinson KC, Mason JB (2017) Immune and renal function, which are critical for reproductive success suffer substantial declines in aged females, but are significantly restored by re-establishment of active ovarian function in post-reproductive females. Reprod Fertil Dev. 10.1071/RD16333 [DOI] [PubMed]
- Podlutsky A, Valcarcel-Ares MN, Yancey K, Podlutskaya V, Nagykaldi E, Gautam T, Miller RL, Sonntag WE, Csiszár A, Ungvari Z. The GH/IGF-1 axis in a critical period early in life determines cellular DNA repair capacity by altering transcriptional regulation of DNA repair-related genes: implications for the developmental origins of cancer. GeroScience. 2017;39:147–160. doi: 10.1007/s11357-017-9966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rais M, Wilson RM, Urbanski HF, Messaoudi I. Androgen supplementation improves some but not all aspects of immune senescence in aged male macaques. GeroScience. 2017;39:373–384. doi: 10.1007/s11357-017-9979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera Z, Christian PJ, Marion SL, Brooks HL, Hoyer PB. Steroidogenic capacity of residual ovarian tissue in 4-vinylcyclohexene diepoxide-treated mice. Biol. Reprod. 2009;80:328–336 [DOI] [PMC free article] [PubMed]
- Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ (2011) Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol Aging 32(4):604–613. 10.1016/j.neurobiolaging.2009.04.008 [DOI] [PMC free article] [PubMed]
- Selesniemi K, Lee HJ, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell. 2008;7(5):622–629. doi: 10.1111/j.1474-9726.2008.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: Long-term health consequences. Maturitas. 2010;65(2):161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15(4–6):323–330. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterin AB, Linares JA, Goldraij A. Effect of dietary restriction on triglyceride levels in the uterus isolated from pregnant rats. Influences of prostaglandins and indomethacin. Prostaglandins Leukot Essent Fatty Acids. 1989;38(2):129–135. doi: 10.1016/0952-3278(89)90097-5. [DOI] [PubMed] [Google Scholar]
- Stork S, Vonschacky C, Angerer P. The effect of 17 beta-estradiol on endothelial and inflammatory markers in post-menopausal women: a randomized and controlled trial. Atherosclerosis. 2002;165:301–307. doi: 10.1016/s0021-9150(02)00242-3. [DOI] [PubMed] [Google Scholar]
- Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2006 update—a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):E85–E151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- Thung PJ, Boot LM, Muhlbock O. Senile changes in the oestrous cycle and in ovarian structure in some inbred strains of mice. Acta Endocrinol. 1956;23:8–32. doi: 10.1530/acta.0.0230008. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Fülöp GÁ, Kiss T, Csiszár A. Connective tissue growth factor (CTGF) in age-related vascular pathologies. GeroScience. 2017;39:491–498. doi: 10.1007/s11357-017-9995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Diepen JA, Berbée JF, Havekes LM, Rensen PC. Interactions between inflammation and lipid metabolism: relevance for efficacy of anti-inflammatory drugs in the treatment of atherosclerosis. Atherosclerosis. 2013;228(2):306–315. doi: 10.1016/j.atherosclerosis.2013.02.028. [DOI] [PubMed] [Google Scholar]
- Xie Z, Morgan TE, Rozovsky I, Finch CE. Aging and glial responses to lipopolysaccharide in vitro: greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Exp Neurol. 2003;182(1):135–141. doi: 10.1016/s0014-4886(03)00057-8. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Takahashi K, Yamatani H, Takata K, Kurachi H. Impact of surgical menopause on lipid and bone metabolism. Climacteric. 2011;14:445–452. doi: 10.3109/13697137.2011.562994. [DOI] [PubMed] [Google Scholar]
- Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, Rosen CJ, Sundberg JP, Harrison DE, Churchill GA, Paigen B. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8(3):277–287. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]