Abstract
Echinostoma revolutum is known as a significant intestinal trematode in various species of animals and humans. It presents complexities in terms of both the morphological and molecular biological data. This is the first study of the application of Cytochrome B gene (CYTB) as a target for studying the phylogeny and designing species-specific primer of E. revolutum. Adult trematodes were harvested from experimentally infected hamsters at 18 days of post-infection. Each worm was identified based on their morphological appearance. The novel CYTB primers were designed from other Echinostoma species to initially amplify CYTB region in E. revolutum. All sequence data of E. revolutum in five provinces of Central Thailand were used as the target for designing the species-specific primer for E. revolutum. The results revealed that CYTB gene can separate E. revolutum into two sister groups by geographical distribution, comprising the eastern and western area groups. Moreover, it also separates E. revolutum from other Echinostoma species, including two sibling species; E. caproni and E. paraensei. In addition, we developed the high performance species-specific primer of E. revolutum. It can detect DNA from a single egg, as well as cercaria, metacercaria and adult stages of this trematode with no cross-reactions to other trematodes and their hosts. Therefore, this research is a positive initial step for the future study of E. revolutum CYTB. The future studies based on this gene should be continued with all species in revolutum complex to overcome the problems of systemic classification that arise in this complex group.
Electronic supplementary material
The online version of this article (10.1007/s12639-018-1057-0) contains supplementary material, which is available to authorized users.
Keywords: Revolutum complex, Cytochrome B, Species-specific primer, Phylogenetic relationship
Introduction
The intestinal trematode, Echinostoma revolutum (Froelich, 1802), is the predominant species belonging to the species complex of the revolutum group. This complex group is characterized by thirty-seven collar spines around their collar head (Fried and Graczyk 2004; Toledo et al. 2009). This complex group has been recognized as a significant intestinal trematode in medical and veterinary terms for decades (Huffman and Fried 1990; Chai 2009; Chai et al. 2011; Sohn et al. 2011; Toledo and Esteban 2016). This trematode is a food-borne zoonosis by which people may be infected by eating freshwater snails that have an infective stage known as the metacercarial stage (Chai et al. 2011; Fried et al. 2004; Toledo et al. 2014; Nagataki et al. 2015). E. revolutum is a cosmopolitan species (Toledo and Esteban 2016), which has been reported in Australia, Europe and Asia (Morgan and Blair 1998; Georgieva et al. 2014). However, most human cases have occurred in various areas in Asia (Toledo and Esteban 2016) such as Cambodia (Sohn et al. 2011), China, Indonesia, Russia, Taiwan and Thailand (Chai 2009). The symptoms of abdominal pain, diarrhea, fatigue and weight loss have commonly occurred in Echinostomiasis patients (Fried et al. 2004; Toledo and Esteban 2016). These symptoms may be more severe than those produced by other intestinal trematodes due to the many sharp collared spines that can seriously injure the intestines of patients (Toledo and Esteban 2016).
Nowadays, epidemic studies of E. revolutum are difficult due to the large number of morphological similarities in the revolutum group (Noikong and Wongsawad 2014) and lacked of accuracy that is associated with the identification tools. This is especially true for the eggs, cercaria and metacercaria; as they are very small and soft-bodied with fewer morphological features than adults. As a result, they are virtually impossible to identify at a species level. These problems can lead to misidentification and can have a negative impact on the accuracy of prevalence value. In order to overcome these systematic problems, the applications of molecular biological data have dramatically increased in the investigation of taxonomic and systematic studies of the Echinostoma species, especially E. revolutum.
The molecular biological data studies continued to discriminate E. revolutum from other Echinostoma species for a decade, and most of them were focused on two important mitochondrial genes; NADH dehydrogenase subunit 1 (ND1) and Cytochrome c oxidase subunit 1 (CO1) (Morgan and Blair 1998; Detwiler et al. 2010; Georgieva et al. 2014; Noikong and Wongsawad 2014; Nagataki et al. 2015). However, some conflicting sequences of E. revolutum and other sibling species have occurred in several continents. Some of these were also clustered in the same clade in several other studies (Kostadinova et al. 2003; Detwiler et al. 2010; Saijuntha et al. 2011; Georgieva et al. 2013; Nagataki et al. 2015). As a consequence of some of the problems associated with these genes, there have been no reports on the molecular identification tools or a species-specific primer for E. revolutum detection in their intermediate hosts until now. To solve this problem, new DNA regions should be investigated and applied in future E. revolutum studies.
In this study, a new candidate gene for E. revolutum identification based on the Cytochrome B gene (CYTB) was investigated. This gene is one of the most highly conserved mitochondrial genes in terms of divergence levels (Simon et al. 1994; Blasco-Costa et al. 2016; Valadas et al. 2016; Huyse et al. 2017). It contains species-specific information and has been used in phylogenetic investigations in a number of studies (Parson et al. 2000; Valadas et al. 2016; Huyse et al. 2017). Various reports have been known to use CYTB sequences for species identification in several kinds of organisms including parasites (Hsieh et al. 2001; Hüttner et al. 2008; Hanelt et al. 2015; Xu et al. 2015). Moreover, it is one of the most suitable genes and has been recommended for use as a marker gene for the molecular studies of trematodes (Blasco-Costa et al. 2016). As has been previously mentioned, CYTB gene has a potential feature which could be used to construct the species-specific primers for species identification and can also be used in phylogenetic studies.
This is the first study of E. revolutum CYTB gene. The sequences of this gene in E. revolutum have never been reported in any nucleotide database. The novel Echinostoma primers were generated for use in phylogenetic studies. Moreover, the novel species-specific primers for E. revolutum identification were generated for detection of the larval stages of E. revolutum. They are capable of detecting E. revolutum at the early stages of infections, such as with the eggs, cercariae and metacercariae with high specificity and sensitivity and are easier to apply than the morphological characterization method.
Materials and methods
Animal ethics
All experimental procedures involving animals were conducted in accordance with the National Research Council of Thailand and approved by the Committee for Biological Experimentation on Animals from the Department of Biology in the Faculty of Science at Srinakharinwirot University, Thailand.
Specimen collection
The freshwater snails were collected from freshwater resources in five provinces of the Chao-Phraya basin of Thailand, including Chainat, Nakhon Pathom, Lopburi, Saraburi and Suphanburi provinces, respectively. All snails were hand-picked following the stratified random sampling methods (Freedman et al. 2007). For the specimens of E. revolutum, the echinostome cercariae and metacercariae were isolated from freshwater snails using the crushing method (Hung et al. 2015) and kept at − 20 °C. Some living metacercariae were also fed to the experimental hamsters. After 18 days of experimental infection, the experimental hamsters were euthanized according to AVMA guidelines for the euthanasia of animals (Leary et al. 2013) and the adult trematodes of E. revolutum were collected from their intestines. For other species of trematodes, three Echinostoma species (E. cinetorchis, E. ilocanum and E. hortense) and one Echinochasmus species (Echinochasmus japonicus) were obtained from the Department of Parasitology and Tropical Medicine, Seoul National University College of Medicine.
Morphological characterization
The twenty-five adult worms were cleaned and any debris or adhering mucus was removed from their surface using PBS. Then, they were fixed with 4% formalin under coverslip pressure for at least 10 days. After that, the fixed-specimens were rehydrated with distilled water, stained with hematoxylin and left overnight. The stained specimens were dehydrated with the ethanol series for 45 min in each concentration (10%, 20%, 30%, 50% and 70%) and destained in acid alcohol (1% conc. HCl in 70% ethanol). Next, they were washed with 70% ethanol to remove any acid before being dehydrated in the ethanol series of 80%, 95%, 100% (absolute ethanol) and butanol, respectively. Finally, the specimens were washed in xylene to clear the samples, and they were mounted with Permount™ and measured under a high magnification light microscope.
The ultrastructure of the tegumental surface of E. revolutum was investigated by scanning electron microscope (SEM). The adult worms were completely cleaned of all debris and adhering mucus on their surface with PBS and pre-fixed with 2.5% glutaraldehyde at 4 °C for seven days. The fixed-specimens were washed with PBS for 15 min three times and post-fixed with osmium tetroxide for 6 h to increase the electron scattering rate. After metal coating, they were washed with PBS followed by rehydration with an ethanol series (10%, 20%, 30%, 50%, 70%, 80%, 95% and absolute), dried with a critical point drying machine (CPD machine), coated with gold particles and examined by a scanning electron microscope (JEOL, JSM-6610LV) with an accelerating voltage of 15 kV.
DNA extraction, DNA amplification and phylogenetic analysis
The genomic DNA of E. revolutum, other trematodes and host specimens were extracted using the DNeasy® blood and tissue kit (Qiagen®) according to the manufacture’s protocol. The novel Echinostoma primers were manually designed from the conserved regions in the CYTB sequences of Echinostoma and other related species, including Echinostoma hortense (KR062182.1, NC_028010.1), Echinostoma caproni (AP017706.1), Echinostoma paraensei (KT008005.1) and Echinochasmus japonicus (KP844722.1, NC_030518.1) (Table 1). The E. revolutum CYTB gene was amplified with the gradient-PCR method using this primer set which was comprised of the following; EchCYTB-F (5′-TGC TGA TTC ATG TTT GAG GTT TGG-3′) and EchCYTB-R (5′-AAG GGT ACT CTG GAT GAC AAG C-3′). The gradient-PCR condition started with pre-denaturation at 94 °C for 5 min followed by a thirty-five cycle of DNA amplification reaction (30 s for denaturation at 94 °C, 50 s for primer annealing from 51 to 61.6 °C, and for 1 min at 72 °C for primer extension). Then, the final extension was performed at 72 °C for 7 min. The PCR products were purified and sequenced directly by 1st BASE DNA Sequencing Services (Axil Scientific Pty. Ltd). The sequences of E. revolutum were aligned with CYTB sequences that were acquired from the GenBank database (Table 1). The sequences were aligned using the ClustalW program and constructed by Maximum-Likelihood (ML) trees with the Kimura-2-parameter model using MEGA7® program. For this method, an initial tree was first built using Maximum Parsimony and its branch lengths are adjusted to maximize the likelihood of the data set for that tree topology under the Kimura-2-parameter model with nodal support and an estimated 10,000 bootstrap re-sampling.
Table 1.
The sequences of the Cytochrome B gene, acquired from the GenBank database
| Trematode species | GenBank accession number |
|---|---|
| Echinostoma hortense | KR062182.1 |
| Echinostoma hortense | NC_028010.1 |
| Echinostoma caproni | AP017706.1 |
| Echinostoma paraensei | KT008005.1 |
| Echinochasmus japonicus | KP844722.1 |
| Echinochasmus japonicus | NC_030518.1 |
| Fasciola hepatica | AP017707.1 |
Species-specific primer designs
The DNA sequences were aligned and manually designed the species-specific primers for E. revolutum (ER primer set). To confirm the specificity of primers in primer design process, the species-specific primers were performed using an in silico specificity test in an in silico program (Primer-BLAST program, https://www.ncbi.nlm.nih.gov/tools/primer-blast/) and the results revealed no cross-reactions with the taxon of Trematoda, Gastropoda and all their definitive hosts (data not shown). The ER primer set consisted of two primers; ER1 (5′-ACC ACA TCA CCA TAT CCC GC-3′) and ER2 (5′-GGG CAG CCA CAG TTC TTA CT-3′) and the expected length of a PCR product was 235 base pairs.
PCR optimization and primer validation
The primer validation phase was divided into three parts consisting of gradient-PCR, a specificity test and a sensitivity test. First, to regulate the optimal annealing temperature of ER primer set, gradient-PCR amplifications were performed in 25 μl, the reactions containing 1.6 mM of MgCl2, 0.28 µM of each primer, 50 µM of each dNTP, 0.2 unit of Taq polymerase (Vivantis), 1 × of PCR buffer and 1 μl of 8.5 ng/µl of genomic DNA. The gradient-PCR was performed for optimizing the annealing temperature of PCR reactions. This condition started with pre-denaturation at 94 °C for 5 min followed by 35 cycles of thirty-second denaturation at 94 °C, 50 s for primer annealing from 49.9 to 61.6 °C, and 1 min at 72 °C for primer extension. Lastly, a final extension was performed at 72 °C for 7 min.
The specificity test of primers was conducted on related Echinostoma species (E. cinetorchis, E. ilocanum and E. hortense), E. japonicus and other trematodes which have frequently co-infected the same species of intermediate snail hosts, namely Thapariella anastomusa, Haplorchis taichui and Stellantchasmus falcatus, respectively. Moreover, the specificity of primers was tested on the intermediate and definitive hosts of E. revolutum, comprised of Lymnaea auricularia, Filopaludina sumatrensis polygramma, F. martensi martensi, Indoplanorbis exustus, Bithynia siamensis, Clea helena, Phodopus roborovskii (hamsters), Gallus gallus (domestic chicken) and Anas platyrhynchos, (domestic duck). The PCR reaction was performed in 25 μl under the same conditions as the gradient-PCR, but the annealing temperature was fixed at an optimal annealing temperature. The reaction contained 1.6 mM of MgCl2, 0.28 µM of each primer, 150 µM of each dNTP, 0.2 unit of Taq polymerase (Vivantis), 1 × of PCR buffer (Vivantis) and 1 μl of 8.5 ng/µl genomic DNA. Regarding the sensitivity of these primers, the PCR reaction was performed under the same conditions as the specificity test, E. revolutum DNA was started at 8.5 ng/µl and diluted with the ten-fold dilution method to identify the minimum detectable concentration.
Results
Morphological characterization
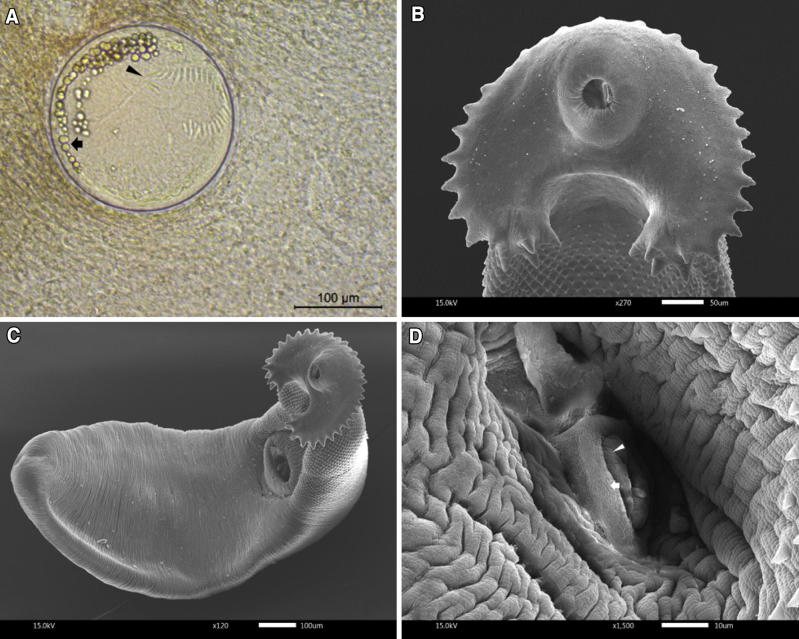
The metacercarial stage of E. revolutum was folded within a spherically-shaped cyst. The cyst has a bilayer transparent wall (Fig. 1a). Morphological characterization of the adult stage of E. revolutum was identified using the taxonomic key (Yamaguti 1958). It has a well-developed collar head at the anterior part of body. The collar head presented two alternating rows of thirty-seven collar spines that were arranged in the same way as in the metacercarial stage (Fig. 1b). The body was elongated and flattened, ranging from 5.4 to 6.5 mm in length and from 0.9 to 1.2 mm in width (n = 25) (Fig. 1c). The huge ventral ridge was clearly visible and located on the anterior of the muscular acetabulum found on one-third of its body. The anterior part of the tegumental surface had numerous tiny spines directed toward the posterior part of body. The subterminal oral sucker was smaller than the acetabulum and connected to an extremely short prepharynx. The muscular pharynx was clearly visible. The bifurcated intestine was elongated and ended near the posterior terminal. The round ovary was located posterior to acetabulum and connected to the moderate loop of the uterus. The uterus was filled with numerous eggs. It had two tandem testes located at the posterior of the ovary. The cirrus was well-developed and located in the cirrus sac near the acetabulum (Fig. 1d).
Fig. 1.
The morphological appearance of E. revolutum.a The metacercarial stage show five clearly visible corner spines (arrowhead) and large excretory granules (arrow). b The collar head of E. revolutum presents two alternating rows, consisting of thirty-seven collar spines. c Adult stage of E. revolutum, which was obtained from the experimental hamster 18 days after infection. d The well-developed cirrus (arrowhead) located in the cirrus sac (arrow) near the acetabulum
Cytochrome B amplification and phylogenetic analysis
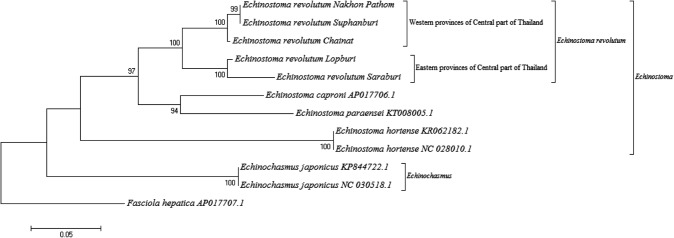
The gradient-PCR results revealed that the optimum annealing temperature (Ta) of EchCYTB primer was 59.4 °C and the PCR product was recorded as 837 base pairs (supplementary figure 1). In terms of the phylogenetic tree, the ML tree of CYTB sequences showed two separating clades of Echinostoma and Echinochasmus species. All E. revolutum results from five provinces in Central Thailand were clustered together in E. revolutum clade, which were distinct from all other Echinostoma genus (Fig. 2). Moreover, E. revolutum lineage was divided into two sister groups by different specimen geographies consisting of the Eastern and Western provinces of Central Thailand.
Fig. 2.
The maximum likelihood (ML) trees of E. revolutum and other related species. The maximum likelihood (ML) trees constructed from the sequences of E. revolutum in this study and other related species in the GenBank database. The nodal support was estimated using 10,000 bootstrap re-sampling
PCR optimization and primer validations
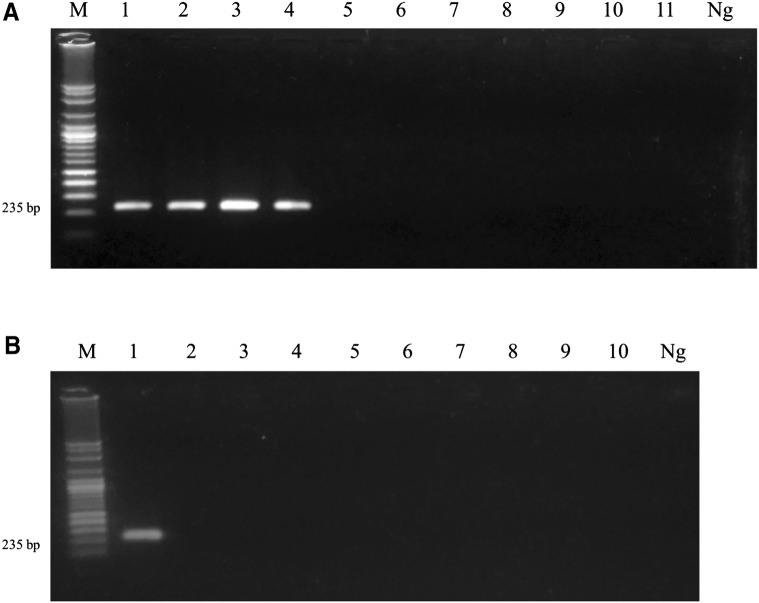
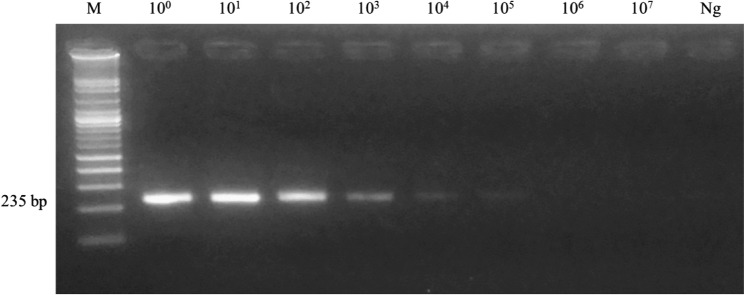
After validation of the optimal annealing temperature in ER primer set, the results indicated that the best annealing temperature was 60 °C and the PCR product was 235 base pairs (supplementary figure 2). The specificity test of primers was performed at all stages of E. revolutum and other related species and their hosts. The results found that the primers can amplify CYTB gene in all stages of E. revolutum, including the egg, cercaria, metacercaria and adult stage with no cross-reactions to other trematodes, including E. cinetorchis, E. ilocanum, E. hortense, Echinochasmus japonicus, T. anastomusa, H. taichui and S. falcatus, respectively (Fig. 3a). Moreover, these primers did not have any cross-reactions with freshwater snails (important intermediate hosts) and definitive hosts, such as L. auricularia, F. sumatrensis, F. martensi, I. exustus, B. siamensis, C. helena, P. roborovskii, G. gallus and A. platyrhynchos (Fig. 3b). With regard to sensitivity, the PCR results demonstrated that primers can detect E. revolutum with an extremely high degree of sensitivity. The minimum DNA concentration of E. revolutum which can be amplified was 0.85 pg/µl (Fig. 4).
Fig. 3.
Specificity tests for the ER primer set. a The specificity test for each stage: (1) egg; (2) cercaria; (3) metacercaria; (4) adult; and other trematodes consisting of (5) E. cinetorchis; (6) E. ilocanum; (7) E. hortense; (8) Echinochasmus japonicus; (9) Thapariella anastomusa; (10) Haplorchis taichui; and (11) Stellantchasmus falcatus, respectively. b The specificity test for E. revolutum hosts include the following: (1) DNA of E. revolutum; (2) Lymnaea auricularia; (3) Filopaludina sumatrensis; (4) F. martensi; (5) Indoplanorbis exustus; (6) Bithynia siamensis; (7) Clea helena; (8) Phodopus roborovskii; (9) Gallus gallus; and (10) Anas platyrhynchos, respectively. The HPLC water was used as negative control in all tests
Fig. 4.
PCR results of the sensitivity testing of the ER primer set. The initial Echinostoma revolutum DNA concentration was 8.5 ng and then diluted using the ten-fold dilution method. The minimum DNA concentration of E. revolutum which can be amplified was 0.85 pg/µl
Discussion
The DNA-sequence data from the mitochondrial genes were used with increasing frequency in order to estimate phylogenetic relationships among Echinostoma species. Even most previous Echinostoma studies were focused on ND1 and CO1. However, substantial genetic variations on ND1 and CO1 genes of Echinostoma were reported on various continents (Kostadinova et al. 2003; Detwiler et al. 2010; Saijuntha et al. 2011; Georgieva et al. 2013; Nagataki et al. 2015). This problem resulted in the conflicting sequences that have occurred in several reports, in which they tried to separate some species in revolutum complex to each species clade. In Europe, the isolates of E. revolutum formed two separating clades with strongly supported clusters and E. revolutum (AY168933.1) was clustered to E. friedi (AY168937.1) clade based on ND1 sequences (Kostadinova et al. 2003). In North America, many accession numbers of Echinostoma species obtained from the NCBI database are in conflict among originally report and other re-clustered clade reports. First, a tissue sample from E. revolutum (AY222132.1/AY222246.1) was clustered in E. trivolvis clade and two Echinostoma species; E. robustum (U58102) and E. friedi (AY168937) were found within the same monophyletic clade based on ND1 marker (Detwiler et al. 2010). In Asia, Noikong and colleagues reported that the infective stage of echinostome were classified as an E. revolutum-like group and belonged to several species base on ND1 sequences (Noikong et al. 2014). According to these reports, the molecular confirmation based on ND1 and CO1 markers are insufficient to classify the species of Echinostoma and the taxonomic status of this group remains unclear. Therefore, new DNA regions must be investigated to solve the taxonomical problems in this complex species. In this study, the new primers were designed for Echinostoma CYTB amplification. This gene has been used as a target for separating the sibling species in many types of parasites, such as parasitic protozoa (Kato et al. 2016), cestode (Hüttner et al. 2008, Xu et al. 2015) and nematode (Hanelt et al. 2015). With regard to trematodes, this is the first study on the applications of the E. revolutum CYTB gene. Eleven sibling species of E. revolutum have been validated in the revolutum species complex consisting of E. cinetorchis (Chai 2009), E. echinatum (Chai et al. 2011), E. caproni (Toledo et al. 2009; Chai et al. 2011), E. deserticum (Kechemir et al. 2002), E. friedi, E. jurini, E. luisreyi, E. miyagawai, E. paraensei, E. parvocirrus and E. trivolvis (Toledo et al. 2009). Unfortunately, there are only four sequences of data of the Echinostoma CYTB gene including E. caproni, E. paraensei and two sequences of E. hortense in the database. The Maximum-likelihood tree analysis was developed for all of E. revolutum specimens that were examined in this study. From our results, the CYTB region is capable of separating each Echinostoma species to their own clade. The phylogenetic tree demonstrated that all E. revolutum specimens were clustered in the same clade, which were distinctly separated from two cryptic species; E. caproni and E. paraensei. which are the members of the revolutum group. This result confirmed that CYTB gene has the informative sites that could be helpful in terms of phylogenetic studies on other sibling Echinostoma species. Moreover, this gene can be used to estimate the geographical distribution of the specimens. The results revealed that E. revolutum is divided into two sister groups in two distinct areas of Central, Thailand; the east part (Lopburi and Saraburi provinces) and the west part (Chainat, Nakhon Pathom and Suphanburi provinces) with a monophyletic clade. Consequently, CYTB can be applied as a molecular marker for phylogenetic studies and geographical studies about the substantial genetic variations of the revolutum complex species in each region in future studies.
The presence of E. revolutum is well-known in northern and northeastern Thailand (Chantima et al. 2013; Saijuntha et al. 2013; Noikong and Wongsawad 2014; Nagataki et al. 2015). Notably, the microscopic examination has been the most effective method of investigating the cercariae and metacercariae in intermediate hosts. However, it is virtually impossible to identify them at a species level, especially in the case of multiple infections in definitive hosts. To overcome this problem, this study used the CYTB gene as a target for detection of E. revolutum. The species-specific primer of E. revolutum obtained from this study can detected a single egg in the specimen with no cross-reaction to other related trematode species or their intermediate and definitive hosts. Therefore, the successful development of a high-performance species-specific primer is helpful for discriminating between E. revolutum eggs and other trematode eggs that are similar in color and morphological appearance, such as Echinostoma eggs, Fasciola eggs, Fasciolopsis eggs and Gastrodiscoides eggs (Qureshi et al. 2016; Sah et al. 2018). The findings of this study can be applied for use in the development of detection tools for fecal examination in Echinostomiasis patients due to the fact that it would take considerably less time than the conventional method. Moreover, it can applied for use in species identification tools in the study of the larval stages of E. revolutum in the snail intermediate hosts, especially at the cercarial stage which is extremely important in terms of the epidemiological study, because only one snail with a horde of cercariae can rapidly infect more than hundreds of secondary intermediate hosts and highly increase the risk opportunities for humans and other definitive hosts in the infection area. Consequently, the species-specific primer obtained from this study can be helpful in the related studies of trematodes, including medical and veterinary research because of the shorter time required and the higher degree of specificity of results obtained when compared with the conventional method.
Conclusion
This is the first study that revealed the applications of the Cytochrome B gene in E. revolutum. It can separate Echinostoma into each species clade and has the potential to be used as a molecular marker gene of the Echinostoma species. The species-specific primer of E. revolutum obtained from this study proved useful in the detection of and discrimination between E. revolutum and other related species. It can be applied to epidemic studies and would be helpful for disease control strategies, for example, in constructing E. revolutum risk maps, and as a part of local health education initiatives. Moreover, our study confirmed that the E. revolutum that remains in Central Thailand has two genetic structures that can be divided into two sister groups depending on their specimen geographies. However, there are still some cryptic species in the revolutum complex that have not yet been reported on in terms of molecular genotyping. Thus, a comprehensive investigation of the genetic variations and the relationships between the populations in this complex group based on the CYTB gene should continue to employ the use of larger sample sizes covering all species in the complex to increase the validity of the sequence data. This may enable the researcher to overcome systematic problems of revolutum complex species in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We greatly acknowledge Srinakharinwirot University, Thailand for providing funding and relevant facilities (Grant No. 239/2560). Finally, we would like to thank Mr. Simon McIver and Mr. Russell Kirk Hollis for their assistance in editing this manuscript.
Author’s contribution
AS and CT: Conception and design of study. CJY and AS: Support specimens. AS: Acquisition of data. AS, TT and CT: Analysis and/or interpretation of data. AS and CT: Drafting the manuscript. AS, CT and CJY: Revising the manuscript critically for important intellectual content. AS, TT, CT and CJY: Approval of the version of the manuscript to be published.
Compliance with ethical standards
Conflict of interest
We declare that all authors have no conflict of interest.
References
- Blasco-Costa I, Cutmore SC, Miller TL, Nolan MJ. Molecular approaches to trematode systematics: ‘best practice’ and implications for future study. Syst Parasitol. 2016;93(3):295–306. doi: 10.1007/s11230-016-9631-2. [DOI] [PubMed] [Google Scholar]
- Chai JY. Echinostomes in humans. In: Fried B, Toledo R, editors. The biology of echinostomes. Berlin: Springer; 2009. pp. 147–183. [Google Scholar]
- Chai JY, Sohn WM, Na BK, Van De N. Echinostoma revolutum: metacercariae in Filopaludina snails from Nam Dinh province, Vietnam, and adults from experimental hamsters. Korean J Parasitol. 2011;49(4):449–455. doi: 10.3347/kjp.2011.49.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantima K, Chai JY, Wongsawad C. Echinostoma revolutum: freshwater snails as the second intermediate hosts in Chiang Mai, Thailand. Korean J Parasitol. 2013;51(2):183–189. doi: 10.3347/kjp.2013.51.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler JT, Bos DH, Minchella DJ. Revealing the secret lives of cryptic species: examining the phylogenetic relationships of echinostome parasites in North America. Mol Phylogenet Evol. 2010;55(2):611–620. doi: 10.1016/j.ympev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Freedman D, Pisani R, Purves R. Statistics (International Student Edition) 4. New York: WW Norton & Company; 2007. [Google Scholar]
- Fried B, Graczyk TK. Recent advances in the biology of Echinostoma species in the “revolutum” group. Adv Parasitol. 2004;58:139–195. doi: 10.1016/S0065-308X(04)58003-X. [DOI] [PubMed] [Google Scholar]
- Fried B, Graczyk TK, Tamang L. Food-borne intestinal trematodiases in humans. Parasitol Res. 2004;93(2):159–170. doi: 10.1007/s00436-004-1112-x. [DOI] [PubMed] [Google Scholar]
- Georgieva S, Selbach C, Faltýnková A, Soldánová M, Sures B, Skírnisson K, Kostadinova A. New cryptic species of the ‘revolutum’ group of Echinostoma (Digenea: Echinostomatidae) revealed by molecular and morphological data. Parasit Vectors. 2013;6(1):64. doi: 10.1186/1756-3305-6-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieva S, Faltýnková A, Brown R, Blasco-Costa I, Soldánová M, Sitko J, Scholz T, Kostadinova A. Echinostoma ‘revolutum’ (Digenea: Echinostomatidae) species complex revisited: species delimitation based on novel molecular and morphological data gathered in Europe. Parasit Vectors. 2014;7(1):520. doi: 10.1186/s13071-014-0520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanelt B, Schmidt-Rhaesa A, Bolek MG. Cryptic species of hairworm parasites revealed by molecular data and crowdsourcing of specimen collections. Mol Phylogenet Evol. 2015;82:211–218. doi: 10.1016/j.ympev.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Hsieh HM, Chiang HL, Tsai LC, Lai SY, Huang NE, Linacre A, Lee JCI. Cytochrome b gene for species identification of the conservation animals. Forensic Sci Int. 2001;122(1):7–18. doi: 10.1016/S0379-0738(01)00403-0. [DOI] [PubMed] [Google Scholar]
- Huffman JE, Fried B. Echinostoma and echinostomiasis. Adv Parasitol. 1990;29:215–269. doi: 10.1016/S0065-308X(08)60107-4. [DOI] [PubMed] [Google Scholar]
- Hung NM, Anh NTL, Van PT, Thanh BN, Van HN, Van HH. Current status of fish-borne zoonotic trematode infections in Gia Vien district, Ninh Binh province, Vietnam. Parasit Vectors. 2015;8(1):21. doi: 10.1186/s13071-015-0643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttner M, Nakao M, Wassermann T, Siefert L, Boomker JD, Dinkel A, Sako Y, Mackenstedt U, Romig T, Ito A. Genetic characterization and phylogenetic position of Echinococcus felidis Ortlepp, 1937 (Cestoda: Taeniidae) from the African lion. Int J Parasitol. 2008;38(7):861–868. doi: 10.1016/j.ijpara.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Huyse T, Oeyen M, Larmuseau MH, Volckaert FA. Co-phylogeographic study of the flatworm Gyrodactylus gondae and its goby host Pomatoschistus minutus. Parasitol Int. 2017;66(2):119–125. doi: 10.1016/j.parint.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Kato H, Gomez EA, Martini-Robles L, Muzzio J, Velez L, Calvopiña M, Daniel RA, Mimori T, Uezato H, Hashiguchi Y. Geographic distribution of Leishmania species in Ecuador based on the cytochrome b gene sequence analysis. PLoS Negl Trop Dis. 2016;10(7):e0004844. doi: 10.1371/journal.pntd.0004844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kechemir N, Jourdane J, Mas-Coma S. Life cycle of a new African echinostome species reproducing by parthenogenesis. J Nat Hist. 2002;36(15):1777–1784. doi: 10.1080/00222930110062633. [DOI] [Google Scholar]
- Kostadinova A, Herniou E, Barrett J, Littlewood DTJ. Phylogenetic relationships of Echinostoma Rudolphi, 1809 (Digenea: Echinostomatidae) and related genera re-assessed via DNA and morphological analyses. Syst Parasitol. 2003;54(3):159–176. doi: 10.1023/A:1022681123340. [DOI] [PubMed] [Google Scholar]
- Leary SL, Underwood W, Anthony R, Gwaltney-Brant S, Poison A, Meyer R. AVMA guidelines for the euthanasia of animals. 2013. Schaumburg: American Veterinary Medical Association Schaumburg; 2013. [Google Scholar]
- Morgan J, Blair D. Mitochondrial ND1 gene sequences used to identify echinostome isolates from Australia and New Zealand. Int J Parasitol. 1998;28(3):493–502. doi: 10.1016/S0020-7519(97)00204-X. [DOI] [PubMed] [Google Scholar]
- Nagataki M, Tantrawatpan C, Agatsuma T, Sugiura T, Duenngai K, Sithithaworn P, Andrews RH, Petney TN, Saijuntha W. Mitochondrial DNA sequences of 37 collar-spined echinostomes (Digenea: Echinostomatidae) in Thailand and Lao PDR reveals presence of two species: Echinostoma revolutum and E. miyagawai. Infect Genet Evol. 2015;35:56–62. doi: 10.1016/j.meegid.2015.07.022. [DOI] [PubMed] [Google Scholar]
- Noikong W, Wongsawad C. Epidemiology and molecular genotyping of echinostome metacercariae in Filopaludina snails in Lamphun province, Thailand. Asian Pac J Trop Med. 2014;7(1):26–29. doi: 10.1016/S1995-7645(13)60186-8. [DOI] [PubMed] [Google Scholar]
- Noikong W, Wongsawad C, Chai JY, Saenphet S, Trudgett A. Molecular analysis of echinostome metacercariae from their second intermediate host found in a localised geographic region reveals genetic heterogeneity and possible cryptic speciation. PLoS Negl Trop Dis. 2014;8:e2778. doi: 10.1371/journal.pntd.0002778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson W, Pegoraro K, Niederstätter H, Föger M, Steinlechner M. Species identification by means of the cytochrome b gene. Int J Legal Med. 2000;114(1–2):23–28. doi: 10.1007/s004140000134. [DOI] [PubMed] [Google Scholar]
- Qureshi AW, Tanveer A, Mas-Coma S. Epidemiological analysis of human fascioliasis in northeastern Punjab, Pakistan. Acta Trop. 2016;156:157–164. doi: 10.1016/j.actatropica.2015.12.023. [DOI] [PubMed] [Google Scholar]
- Sah R, Khadka S, Hamal R, Poudyal S. Human echinostomiasis: a case report. BMC Res Notes. 2018;11(1):17. doi: 10.1186/s13104-018-3133-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijuntha W, Sithithaworn P, Duenngai K, Kiatsopit N, Andrews RH, Petney TN. Genetic variation and relationships of four species of medically important echinostomes (Trematoda: Echinostomatidae) in South-East Asia. Infect Genet Evol. 2011;11(2):375–381. doi: 10.1016/j.meegid.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Saijuntha W, Duenngai K, Tantrawatpan C. Zoonotic echinostome infections in free-grazing ducks in Thailand. Korean J Parasitol. 2013;51(6):663–667. doi: 10.3347/kjp.2013.51.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am. 1994;87(6):651–701. doi: 10.1093/aesa/87.6.651. [DOI] [Google Scholar]
- Sohn WM, Chai JY, Yong TS, Eom KS, Yoon CH, Sinuon M, Socheat D, Lee SH. Echinostoma revolutum infection in children, Pursat province, Cambodia. Emerg Infect Dis. 2011;17(1):117–119. doi: 10.3201/eid1701.100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo R, Esteban J. An update on human echinostomiasis. Trans R Soc Trop Med Hyg. 2016;110(1):37–45. doi: 10.1093/trstmh/trv099. [DOI] [PubMed] [Google Scholar]
- Toledo R, Esteban JG, Fried B. Recent advances in the biology of echinostomes. Adv Parasitol. 2009;69:147–204. doi: 10.1016/S0065-308X(09)69003-5. [DOI] [PubMed] [Google Scholar]
- Toledo R, Muñoz Antoli C, Esteban JG. Intestinal trematode infections. In: Toledo R, Muñoz-Antoli C, Esteban JG, editors. Digenetic trematodes. Berlin: Springer; 2014. pp. 201–240. [Google Scholar]
- Valadas SY, da Silva JI, Lopes EG, Keid LB, Zwarg T, de Oliveira AS, Sanches TC, Joppert AM, Pena HF, Oliveira TM. Diversity of Sarcocystis spp. shed by opossums in Brazil inferred with phylogenetic analysis of DNA coding ITS1, cytochrome B, and surface antigens. Exp Parasitol. 2016;164:71–78. doi: 10.1016/j.exppara.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Xu W, Morris U, Aydin-Schmidt B, Msellem MI, Shakely D, Petzold M, Björkman A, Mårtensson A. SYBR green real-time PCR-RFLP assay targeting the plasmodium cytochrome b gene: a highly sensitive molecular tool for malaria parasite detection and species determination. PLoS ONE. 2015;10(3):e0120210. doi: 10.1371/journal.pone.0120210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguti S. Systema helminthum. The digenetic trematodes of vertebrates. New York: Interscience; 1958. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.