Abstract
Plant defensins are mainly known for their antifungal activity. However, limited information is available regarding their function in abiotic stresses. In this study, a defensin gene, Ca-AFP, from Cicer arietinum, commonly known as chickpea, was cloned and transformed in Arabidopsis thaliana for its functional characterization under simulated water-deficit conditions. Under simulated water-deficit conditions (mannitol and polyethylene glycol-6000 induced), the transgenic A. thaliana plants had higher accumulation of the Ca-AFP transcript compared to that under non-stress condition and showed higher germination rate, root length, and biomass than the wild-type (WT) plants. To get further insights into the role of Ca-AFP in conferring tolerance to water-deficit stress, we determined various physiological parameters and found significant reduction in the transpiration rate and stomatal conductance whereas the net photosynthesis and water use efficiency was increased in the transgenic plants compared to that in the WT plants under water deficit conditions. The transgenic plants showed enhanced superoxide dismutase, ascorbate peroxidase, and catalase activities, had higher proline, chlorophyll, and relative water content, and exhibited reduced ion leakage and malondialdehyde content under water-deficit conditions. Overall, our results indicate that overexpression of Ca-AFP could be an efficient approach for conferring tolerance to water-deficit stress in plants.
Keywords: Arabidopsis thaliana, Ca-AFP, defensin, water-deficit stress, mannitol, polyethylene glycol, transgenic
Introduction
Drought tolerance is the ability of plants to resist water-deficit conditions with low tissue water potential (Ingram and Bartels, 1996). A significant alteration in the expression of genes related to various physiological, regulatory, and molecular functions, which could be upregulated or downregulated, is reported in plants under stress conditions (Ingram and Bartels, 1996; Blum, 1998; Trethowan et al., 2001; Ramanjulu and Bartels, 2002; Bartels and Sunkar, 2005). Under water-deficit conditions in soil, plants adopt mechanisms either to escape or resist the stress (Levitt, 1980; Price et al., 2002). Traits like reduced epidermal (stomatal and cuticular) conductance, radiation absorption, and evaporative surface together with improved root architecture are some of the response of plants exposed to such conditions (Price et al., 2002).
The role of several plant proteins has been studied in transgenic Arabidopsis thaliana plants under osmotic stress; these include AtFP3 (Zhang et al., 2016), VvMBF1 (Yan et al., 2014), AtRabG3e (Mazel et al., 2004), EcGBF3 (Ramegowda et al., 2017), CarNAC4 (Yu et al., 2016), and GmDhn8 (Maitra and Cushman, 1998). Plant defensins are known for their important roles in biotic stress, especially against fungal pathogens. For example, Pisum sativum defensin 1 (Psd1) is involved in the response against Neurospora crassa (Lobo et al., 2007), Medicago truncatula defensin (MtDef4) is effective against Fusarium graminearum (Sagaram et al., 2013), and Nicotiana alata defensin (NaD1) has growth inhibitory activity against Fusarium oxysporum (Lay et al., 2012). Plant defensins have also been reported to play an important role during abiotic stresses. The soybean defensin gene (Dhn8) was demonstrated to be induced by drought (Maitra and Cushman, 1998) and Nicotiana defensins, NeThio1 and NeThio2, were induced by NaCl-induced salinity stress (Komori et al., 1997). The pepper defensin gene (CADEF1) was shown to be induced by drought and salinity stresses in Capsicum annuum (Do et al., 2004). The expression of a plant defensin, AhPDF1.1, from zinc-hyperaccumulating Arabidopsis halleri, under the control of 35S promoter conferred Zn tolerance in transgenic A. thaliana plants (Mirouze et al., 2006).
Plant defensins are small, basic, cysteine-rich peptides, found ubiquitously in the plant kingdom (Osborn et al., 1995; Broekaert et al., 1997; Shewry and Lucas, 1997; Osborn and Broekaert, 1999; Thomma et al., 2002) that exhibit three-dimensional folding pattern stabilized by eight cysteine residues linked by four disulfide bridges (Broekaert et al., 1995; Almeida et al., 2002). More than 300 defensin-like genes have been identified in the model plant A. thaliana till date (Silverstein et al., 2005). These have been isolated from seeds (Broekaert et al., 1995; Thomma et al., 2003), roots (Sharma and Lönneborg, 1996), leaves (Segura et al., 1998; Do et al., 2004), and pods (Chiang and Hadwiger, 1991). We identified a defensin gene, Ca-AFP, along with several other drought-responsive differentially expressed genes, in our transcriptome sequencing of chickpea (Cicer arietinum) root samples exposed to water-deficit conditions (Table 1). The expression of these genes was validated through quantitative real time PCR (qRT-PCR) and Ca-AFP was observed to be upregulated under water-deficit conditions. In the present study, we investigated, for the first time, the role of chickpea defensin gene under water-deficit conditions by overexpressing it in A. thaliana. Our results show that Ca-AFP confers tolerance to water-deficit stress in transgenic A. thaliana plants and could, therefore, be used for generating drought-tolerant commercially important plants.
Table 1.
Chickpea drought responsive genes identified through transcriptome analysis.
| S.N. | LOC ID | log2(fold_change) | GeneID | Chromosome | Description of gene | Type_of_gene |
|---|---|---|---|---|---|---|
| 1 | LOC101512255 | 2.1274 | 101512255 | Ca1 | geraniol 8-hydroxylase-like | protein-coding |
| 2 | LOC101512255 | –2.95378 | 101512255 | Ca1 | geraniol 8-hydroxylase-like | protein-coding |
| 3 | LOC101508810 | –1.51518 | 101508810 | Ca7 | uncharacterized LOC101508810 | protein-coding |
| 4 | LOC101508810 | 5.78519 | 101508810 | Ca7 | uncharacterized LOC101508810 | protein-coding |
| 5 | LOC101503379 | –2.22106 | 101503379 | Ca3 | RNA-binding protein 38-like | protein-coding |
| 6 | LOC101491913 | –3.3973 | 101491913 | Ca1 | 14 kDa proline-rich protein DC2.15-like | protein-coding |
| 7 | LOC101509326 | 1.54692 | 101509326 | Ca6 | proline-rich receptor-like protein kinase PERK2-like | protein-coding |
| 8 | LOC101513365 | –2.59857 | 101513365 | Ca4 | GATA transcription factor 8-like | protein-coding |
| 9 | LOC101495554 | –3.78102 | 101495554 | Ca4 | gibberellin-regulated protein 1-like | protein-coding |
| 10 | LOC101512021 | 5.91122 | 101512021 | Ca1 | defensin-like protein-like | protein-coding |
| 11 | LOC101490679 | –2.0441 | 101490679 | Ca8 | abscisic acid 8′-hydroxylase 1-like | protein-coding |
| 12 | LOC101505927 | –2.22819 | 101505927 | Ca7 | abscisic acid 8′-hydroxylase 1-like | protein-coding |
| 13 | LOC101496585 | –3.16129 | 101496585 | Ca7 | 36.4 kDa proline-rich protein-like | protein-coding |
| 14 | LOC101490393 | –2.8389 | 101490393 | Ca4 | 3-oxo-Delta(4,5)-steroid 5-beta-reductase-like | protein-coding |
| 15 | LOC101501606 | –2.73243 | 101501606 | Ca5 | acidic mammalian chitinase-like | protein-coding |
| 16 | LOC101509704 | –2.09366 | 101509704 | Ca3 | actin-3-like | protein-coding |
| 17 | LOC101494386 | –3.46903 | 101494386 | Ca6 | aspartic proteinase PCS1-like | protein-coding |
| 18 | LOC101509714 | –2.92187 | 101509714 | Ca4 | basic 7S globulin 2-like | protein-coding |
| 19 | LOC101495910 | –1.75766 | 101495910 | Ca1 | basic 7S globulin-like | protein-coding |
| 20 | LOC101509822 | –2.33358 | 101509822 | Ca4 | basic 7S globulin-like | protein-coding |
| 21 | LOC101510034 | –2.25005 | 101510034 | Ca4 | basic 7S globulin-like | protein-coding |
| 22 | LOC101513089 | –2.2806 | 101513089 | Ca1 | beta-fructofuranosidase, cell wall isozyme-like | protein-coding |
| 23 | LOC101513528 | –1.56005 | 101513528 | Ca1 | beta-glucosidase 12-like | protein-coding |
| 24 | LOC101489749 | 2.08897 | 101489749 | Ca5 | bis(5′-adenosyl)-triphosphatase-like | protein-coding |
| 25 | LOC101496665 | –3.26285 | 101496665 | Ca5 | brassinosteroid-regulated protein BRU1-like | protein-coding |
| 26 | LOC101494236 | –1.90685 | 101494236 | Ca8 | calcium-transporting ATPase 2, plasma membrane-type-like | protein-coding |
| 27 | LOC101501496 | –2.06753 | 101501496 | Ca4 | calmodulin-like protein 11-like | protein-coding |
| 28 | LOC101499686 | 1.72218 | 101499686 | Ca5 | cellulose synthase-like protein G1-like | protein-coding |
| 29 | LOC101496722 | 1.69591 | 101496722 | Ca8 | cinnamoyl-CoA reductase 1-like | protein-coding |
| 30 | LOC101509871 | –1.87905 | 101509871 | Ca7 | COBRA-like protein 7-like | protein-coding |
| 31 | LOC101495399 | –1.56719 | 101495399 | Ca2 | cysteine proteinase inhibitor-like | protein-coding |
| 32 | LOC101513755 | –3.30691 | 101513755 | Ca8 | cytochrome P450 71D8-like | protein-coding |
| 33 | LOC101496712 | 1.73721 | 101496712 | Ca2 | cytochrome P450 83B1-like | protein-coding |
| 34 | LOC101491312 | –1.7198 | 101491312 | Ca8 | cytochrome P450 84A1-like | protein-coding |
| 35 | XLOC_031917 | –2.29121 | 101497136 | Ca7 | diacylglycerol kinase zeta-like | protein-coding |
| 36 | LOC101491646 | –2.43042 | 101491646 | Ca3 | disease resistance response protein 206-like | protein-coding |
| 37 | LOC101504947 | –2.0409 | 101504947 | Ca6 | disease resistance response protein 206-like | protein-coding |
| 38 | LOC101511741 | –1.58078 | 101511741 | Ca3 | DNA ligase 1-like | protein-coding |
| 39 | LOC101490396 | 2.426 | 101490396 | Ca5 | eugenol synthase 1-like | protein-coding |
| 40 | LOC101514490 | –1.99149 | 101514490 | Ca6 | expansin-like A2-like | protein-coding |
| 41 | LOC101489892 | 1.96486 | 101489892 | Ca8 | expansin-like B1-like | protein-coding |
| 42 | LOC101507544 | –3.92787 | 101507544 | Ca7 | expansin-like B1-like | protein-coding |
| 43 | LOC101490554 | –1.55537 | 101490554 | Ca2 | fasciclin-like arabinogalactan protein 2-like | protein-coding |
| 44 | LOC101500481 | 6.17303 | 101500481 | Ca7 | FBD-associated F-box protein At4g10400-like | protein-coding |
| 45 | LOC101505686 | 1.61881 | 101505686 | Ca5 | gamma-glutamyltranspeptidase 2-like | protein-coding |
| 46 | LOC101513567 | –1.89271 | 101513567 | Ca3 | GDSL esterase/lipase CPRD49-like | protein-coding |
| 47 | LOC101514168 | –2.91426 | 101514168 | Ca6 | geraniol 8-hydroxylase-like | protein-coding |
| 48 | LOC101514489 | –3.41582 | 101514489 | Ca6 | geraniol 8-hydroxylase-like | protein-coding |
| 49 | LOC101489131 | –1.52061 | 101489131 | Ca1 | glucan endo-1,3-beta-glucosidase-like | protein-coding |
| 50 | LOC101503489 | –1.76305 | 101503489 | Ca3 | hevein-like preproprotein-like | protein-coding |
| 51 | LOC101504416 | –2.14451 | 101504416 | Ca7 | histone H3.3-like | protein-coding |
| 52 | LOC101508417 | –2.1537 | 101508417 | Ca8 | isoflavone reductase-like protein-like | protein-coding |
| 53 | LOC101498105 | –3.17152 | 101498105 | Ca7 | L-ascorbate oxidase homolog | protein-coding |
| 54 | LOC101510707 | –2.02723 | 101510707 | Ca4 | L-ascorbate oxidase homolog | protein-coding |
| 55 | LOC101511136 | –2.28784 | 101511136 | Ca5 | L-ascorbate oxidase-like | protein-coding |
| 56 | LOC101495962 | –1.7916 | 101495962 | Ca3 | L-gulono-1,4-lactone dehydrogenase-like | protein-coding |
| 57 | LOC101514307 | –2.74197 | 101514307 | Ca7 | lysine-rich arabinogalactan protein 18-like | protein-coding |
| 58 | LOC101509335 | –3.2507 | 101509335 | Ca7 | major latex allergen Hev b 5-like | protein-coding |
| 59 | LOC101500484 | –1.95148 | 101500484 | Ca2 | mitochondrial uncoupling protein 5-like | protein-coding |
| 60 | LOC101503951 | –1.54123 | 101503951 | Ca4 | MLP-like protein 34-like | protein-coding |
| 61 | LOC101500008 | 5.23079 | 101500008 | Ca5 | NAD(P)H-dependent 6’-deoxychalcone synthase-like | protein-coding |
| 62 | LOC101503208 | 2.51526 | 101503208 | Ca5 | NADH-ubiquinone oxidoreductase chain 5-like | protein-coding |
| 63 | LOC101510843 | –2.29995 | 101510843 | Ca7 | NADP-dependent glyceraldehyde-3-phosphate dehydrogenase-like | protein-coding |
| 64 | LOC101491385 | 2.751 | 101491385 | Ca6 | non-cyanogenic beta-glucosidase-like | protein-coding |
| 65 | LOC101503860 | 1.7591 | 101503860 | Ca5 | non-specific lipid-transfer protein-like | protein-coding |
| 66 | LOC101511984 | –2.31268 | 101511984 | Ca4 | patatin-2-Kuras 3-like | protein-coding |
| 67 | LOC101504619 | –3.57814 | 101504619 | Ca6 | polygalacturonase inhibitor 2-like | protein-coding |
| 68 | LOC101506971 | 1.52448 | 101506971 | Ca5 | probable glutathione S-transferase parA-like | protein-coding |
| 69 | LOC101492282 | 1.8497 | 101492282 | Ca3 | probable non-specific lipid-transfer protein AKCS9-like | protein-coding |
| 70 | LOC101506095 | –3.64235 | 101506095 | Ca5 | probable pectinesterase/pectinesterase inhibitor 25-like | protein-coding |
| 71 | LOC101489717 | –2.13157 | 101489717 | Ca3 | probable pectinesterase/pectinesterase inhibitor 7-like | protein-coding |
| 72 | LOC101515495 | 1.62498 | 101515495 | Ca7 | probable peptide/nitrate transporter At3g54450-like | protein-coding |
| 73 | LOC101503329 | –2.01431 | 101503329 | Ca1 | probable polygalacturonase-like | protein-coding |
| 74 | LOC101489781 | –3.64932 | 101489781 | Ca7 | probable xyloglucan endotransglucosylase/hydrolase protein 23-like | protein-coding |
| 75 | LOC101493216 | –2.41189 | 101493216 | Ca5 | probable xyloglucan glycosyltransferase 12-like | protein-coding |
| 76 | LOC101498412 | 1.50025 | 101498412 | Ca5 | protein EARLY FLOWERING 4-like | protein-coding |
| 77 | LOC101492463 | –1.94625 | 101492463 | Ca6 | putative lipid-transfer protein DIR1-like | protein-coding |
| 78 | LOC101501102 | –3.05622 | 101501102 | Ca7 | putative nuclease HARBI1-like | protein-coding |
| 79 | LOC101491061 | –1.9557 | 101491061 | Ca6 | receptor-like protein kinase HERK 1-like | protein-coding |
| 80 | LOC101508898 | –3.48617 | 101508898 | Ca1 | reticuline oxidase-like protein-like | protein-coding |
| 81 | LOC101490106 | 4.69002 | 101490106 | Ca1 | ribulose-phosphate 3-epimerase, cytoplasmic isoform-like | protein-coding |
| 82 | LOC101488811 | 2.10436 | 101488811 | Ca1 | serine carboxypeptidase-like 45-like | protein-coding |
| 83 | LOC101493761 | 2.52261 | 101493761 | Ca6 | serine hydroxymethyltransferase 1-like | protein-coding |
| 84 | LOC101513097 | –3.47589 | 101513097 | Ca7 | snakin-2-like | protein-coding |
| 85 | LOC101503877 | 2.73941 | 101503877 | Ca6 | UDP-glucose flavonoid 3-O-glucosyltransferase 7-like | protein-coding |
| 86 | LOC101494020 | 1.55845 | 101494020 | Ca2 | uncharacterized LOC101494020 | protein-coding |
| 87 | LOC101494177 | –2.98733 | 101494177 | Ca5 | uncharacterized LOC101494177 | protein-coding |
| 88 | LOC101494198 | 1.6535 | 101494198 | Ca6 | uncharacterized LOC101494198 | pseudo |
| 89 | LOC101494609 | –1.55765 | 101494609 | Ca6 | uncharacterized LOC101494609 | pseudo |
| 90 | LOC101495255 | 4.69856 | 101495255 | Ca6 | uncharacterized LOC101495255 | protein-coding |
| 91 | LOC101496044 | 7.03616 | 101496044 | Ca7 | uncharacterized LOC101496044 | protein-coding |
| 92 | LOC101499217 | –4.2123 | 101499217 | Ca7 | uncharacterized LOC101499217 | protein-coding |
| 93 | LOC101500065 | –1.89539 | 101500065 | Ca8 | uncharacterized LOC101500065 | protein-coding |
| 94 | LOC101501038 | –2.60722 | 101501038 | Ca3 | uncharacterized LOC101501038 | protein-coding |
| 95 | LOC101508795 | –1.99777 | 101508795 | Ca6 | uncharacterized LOC101508795 | protein-coding |
| 96 | LOC101510669 | 2.23982 | 101510669 | Ca8 | uncharacterized LOC101510669 | protein-coding |
| 97 | LOC101511932 | –2.82384 | 101511932 | Ca2 | uncharacterized LOC101511932 | protein-coding |
| 98 | LOC101512423 | –3.25588 | 101512423 | Ca5 | uncharacterized LOC101512423 | protein-coding |
| 99 | LOC101515113 | 1.63132 | 101515113 | Ca4 | uncharacterized LOC101515113 | protein-coding |
| 100 | LOC101515418 | –1.84621 | 101515418 | Ca4 | uncharacterized LOC101515418 | protein-coding |
| 101 | LOC101489665 | –4.03999 | 101489665 | Ca1 | xyloglucan endotransglucosylase/hydrolase protein 9-like | protein-coding |
Materials and Methods
Plant Materials and RNA Isolation
Seeds of chickpea (Cicer arientinum L.) genotypes BG362 (drought tolerant) and P1003 (drought sensitive) were aseptically grown in Hoagland’s medium in a culture room at 24 ± 2°C under 16-h light: 8-h dark cycle, with a light intensity of ∼200 μmol m-2 s-1. Seven-day-old plants were subjected to polyethylene glycol (PEG 6000; SD Fine Chemicals Limited, India)-simulated osmotic stress for 4 days. The roots of PEG-treated and control samples were harvested and crushed in liquid N2. Total RNA was isolated using RNA Spectrum Plant Total RNA Kit (Sigma-Aldrich, United States).
Arabidopsis thaliana (Col-0 ecotype) seeds were surface-sterilized with Tween-20 for 5 min, and then with 70% ethanol for 5 min followed by 4% sodium hypochlorite (NaOCl; Sigma-Aldrich, United States) for 7 min, and were subsequently washed with autoclaved MilliQ water for 4–5 times. The sterilized seeds were stratified at 4°C for 3 days, sown in 300 g sterile soilrite filled in 10 cm × 10 cm (height × width) plastic pots, and kept at 22°C, under 75% relative atmospheric humidity and 16-h light:8-h dark cycle, with a light intensity of ∼200 μmol m-2 s-1 (Philips, Amsterdam, Netherlands).
Validation, Isolation, and Sequence Analysis of Ca-AFP Gene
For validation of the expression of Ca-AFP, cDNA was prepared from stressed chickpea root and leaf samples from BG-362 and P-1003 genotypes and used for qRT-PCR analysis. cDNA was synthesized from 1 μg of total RNA using Verso cDNA synthesis kit (Thermo Fisher Scientific, United States). For qRT-PCR analysis, 10 μL reaction mixture contained 1 μL of cDNA, 1 μL each of forward and reverse primers (from 5 pmol stock), 5 μL SYBR green (Agilent Technologies, CA, United States), and 2 μL nuclease free water. The reaction was carried out in Stratagene Mx3000P (Agilent Technologies, CA, United States) using the following thermal cycling conditions: initial denaturation at 95°C for 2 min, followed by 40 cycles at 95°C for 15 s, 60°C for 20 s, and 72°C for 30 s. All the reactions were performed in triplicate. The fold-change in the expression of transcripts was calculated using the standard 2–ΔΔCT method (Livak and Schmittgen, 2001). The expression patterns of the transcripts were plotted in Microsoft Excel 2007.
The full length cDNA of Ca-AFP was isolated using RT-PCR from chickpea roots. The primer pair utilized for PCR amplification was as follows: forward primer: 5′-ATCAACAAATATATCAACCACACCA-3′ and reverse primer: 5′-TAATAATGAATATTTATTGTTGTTGTATATATG-3′. The Ca-AFP sequence was BLASTed using the NCBI online tool1. Multiple sequence alignment with other defensin proteins from Cajanus cajan, M. truncatula, Medicago sativa, Phaseolus vulgaris, Tephrosia villosa, Vigna radiata, and Vigna angularis was performed using Clustal Omega online tool2.
Preparation of the Transformation Construct and Generation of Transgenic Plants
For overexpression of Ca-AFP in A. thaliana, the full-length cDNA was cloned under the control of CaMV35S promoter in pBI121 vector. For this, the full-length Ca-AFP gene was PCR-amplified from the cDNA prepared from the roots of chickpea BG-362 with gene-specific primers described above using PrimeSTAR GXL DNA Polymerase (Takara, Japan). The cDNA was cloned in an intermediate vector, pBluescript SK+ (Addgene Cambridge, MA, United States) by creating ends. The cloned cDNA was then excised from this vector by digestion with BamHI and Eco53kI and sub-cloned at the site for these restriction endonucleases in the binary vector, pBI121, in sense orientation. The resulting construct (35S:Ca-AFP) was introduced into Agrobacterium tumefaciens strain GV3101 using electroporation (Hercules, CA, United States). The transformation of Arabidopsis was done by Agrobacterium-mediated floral dip method (Clough and Bent, 1998). The seeds were harvested from the infiltrated plants and positive plants were selected on ½ Murashige and Skoog’s (MS) medium supplemented with 50 mg L-1 kanamycin (Sigma-Aldrich, United States). The kanamycin-resistant plants were transferred to soil after 8 days of germination and were grown in a growth chamber. Arabidopsis plants transformed with empty pBI121 vector (EV control) were also generated.
PCR and qRT-PCR Analysis of Transgenic A. thaliana Plants
Genomic DNA (gDNA) was isolated from transgenic Arabidopsis lines using DNeasy mini prep kit (Qiagen, Germany). DNA quantification was done using NanoDrop spectrophotometer (Eppendorf, Germany). For PCR, the reaction mixture contained 100 ng gDNA, 2 μL 10× Taq buffer, 1 μL of 10 pmol each of forward and reverse gene-specific primers, 1 μL of 10 mM dNTP mixture (Genei Laboratories Pvt. Ltd., India), and 0.4 μL of Taq DNA polymerase (3 U/μL) (Genei Laboratories Pvt. Ltd., India), and the volume was made up to 20 μL using autoclaved MilliQ water. In the positive control reaction, 30 ng of the recombinant pBI121 plasmid was taken in place of gDNA. For negative control reaction, 100 ng gDNA of wild-type untransformed (WT) and EV transformed plants was taken. The PCR was performed under following conditions: initial denaturation at 95°C for 10 min followed by 30 cycles at 95°C for 45 s, 60°C for 30 s, and 72°C for 40 s, and a final extension at 72°C for 7 min. The PCR product was visualized by electrophoresis on a 1.2% agarose gel.
The expression of Ca-AFP in the transgenic plants was assessed using qRT-PCR. Total RNA was extracted from 100 mg leaves of the WT and transgenic lines using Spectrum Plant Total RNA Kit (Sigma Life Science, United States), according to manufacturer’s instructions. The quality and quantity of RNA samples were analyzed by agarose gel electrophoresis and NanoDrop spectrophotometer. The qRT-PCR was done taking three biological replicates using the above-mentioned primers and reaction conditions. Actin2 of A. thaliana (AtActin2; GenBank accession number: U41998) was used as an internal control for normalization and was amplified using the primers: 5′-AGTAAGGTCACGTCCAGCAAGG-3′ (forward) and 5′-GCACCCTGTTCTTCTTACCGAG-3′ (reverse). The expression levels of genes (ETHYLENE RESPONSE FACTOR1 and VEGETATIVE STORAGE PROTEIN 1) related to hormones like ethylene and jasmonic acid was also assayed through qRT-PCR. The sequences of primers designed for A. thaliana AtERF1 (GenBank accession number: AT3G23240) were 5′-ACGTTCTCAACCGCCTACAG-3′ (forward) and 5′-CGGACTCGCTCTCTGGTG-3′ (reverse) and those designed for AtVSP1 (GenBank accession number: AT5G24780) were 5′-TTTTACGCCAAAGGACTTGC-3′ (forward) and 5′-AATCCCGAGTTCCAAGAGGT3-3′ (reverse).
Assessment of Water-Deficit Stress Tolerance of Transgenic A. thaliana Plants
The water-deficit stress tolerance of Ca-AFP overexpressing transgenic A. thaliana plants was analyzed using three homozygous lines (#1, #6, and #9). For this, the surface sterilized seeds of transgenic and WT A. thaliana were stratified at 4°C for 3 days and then inoculated in ½MS medium supplemented with mannitol (0, 100, 200, 250, and 300 mM) or PEG (0, 1.5, 3, 4.5, and 6%) and their germination rate was determined after 3, 6, and 9 days. Similarly, for root length and biomass measurement, 4-day-old seedlings of transgenic lines and WT were placed on ½MS medium containing different concentrations of mannitol (Sigma-Aldrich, United States) and PEG and the measurements were made after 10 days.
For assessment of the performance of transgenic lines and WT plants under simulated physiological drought conditions they were grown on soilrite in well-watered pots for 20 days. These plants were then subjected to water-deficit conditions by withholding water for the next 15 days. The control plants were watered regularly. The survival rate of plants was recorded. Thereafter, the plants were irrigated again for 5 days and their recovery was monitored. The leaf samples from plants exposed to well-watered and water-deficit conditions were harvested for different enzymatic assays. The total soluble protein was extracted from the leaf samples in bicarbonate buffer (Himedia, India) and quantified using the Bradford assay (Bradford, 1976) using Bradford reagent (Hercules, CA, United States). The previously described methods were used for the estimation of proline (Bates et al., 1973), superoxide dismutase (SOD) (Beauchamp and Fridovich, 1971), catalase (CAT) (Aebi, 1974), ascorbate peroxidase (APX) (Nakano and Asada, 1981), malondialdehyde (MDA), NADPH oxidase (NADPHox) (Cakmak and Marschner, 1988), peroxidase (POX) (Hemeda and Klein, 1990), and chlorophyll (Arnon, 1949). The accumulation of superoxide anion radical (O2-) and hydrogen peroxide (H2O2) in Arabidopsis plants transformed with Ca-AFP and empty vector (EV) and in WT plants was determined using nitrobluetetrazollium (NBT) and 3,3’-Diaminobenzidine (DAB) staining, respectively. The staining intensity of NBT and DAB in leaves was determined by densitometry using ImageJ software (ver. 1.46 for Windows 8) (Schneider et al., 2012). To measure the water loss, 3-week-old plants were exposed to 15 days of water-deficit condition as described above. The leaves from these plants were harvested and weighed immediately. These were placed on a dry filter paper at 25°C under an RH of 50–60% and weighed at designated time intervals (1, 2, and 3 h) after detachment. The water loss was calculated based on the initial fresh weight of plants. The experiment was conducted thrice for each transgenic and WT plant. To determine the electrolytic leakage (EL), leaves were rinsed with deionized water (dH2O) and immersed in 10 mL dH20; they were then kept on a gyratory shaker at 100 rpm at room temperature. After 2 h, the conductivity (C1) of the samples was measured. Subsequently, the samples were boiled for 10 min and cooled to room temperature. The conductivities (C2) of the samples were measured again. The C1/C2 ratio was calculated to evaluate the relative electrolytic leakage from leaves samples (Liu et al., 2006).
The relative water content (RWC) was determined using rosette leaves of transgenic and WT plants exposed to water-deficit stress for different periods (3, 6, 9, 12, and 15 days). The detached leaves with intact petioles were immediately weighed for determining the leaf fresh weight (LFW) and then kept in falcon tubes containing 10 mL of dH20. These leaves were kept for 5 h and allowed to imbibe water and were then weighed to obtain the leaf turgid weight (LTW). The leaves were then dried at 70°C for 5 h after wrapping in filter paper and weighed to obtain the leaf dry weight (LDW). The RWC was calculated using the formula: RWC = (LFW–LDW)/(LTW–LDW). The physiological parameters like transpiration rate, stomatal conductance, photosynthetic rate, and water use efficiency (WUE) were measured using Li-Cor 6800 gas exchange portable photosynthesis system (Li-Cor, Lincoln, NB, United States). The measurements were taken around 10:00 a.m. using three leaves from each plant. The stomatal conductance was used to determine the degree of stomatal opening and closing that defines the plant water status (Pei et al., 1997). For evaluation of stomatal size, epidermis of leaves of transgenic and WT plants were peeled and imaged under Leica DM 2500 microscope (Wetzlar, Germany) and the size measurements were made using the LAS V4.2 software (Lawson et al., 1998).
Statistical Analysis
Statistical analysis was performed using the SPSS software (SPSS 16.0). Analysis of variance (ANOVA) was used to compare the significant differences (p < 0.05) based on Duncan Multiple Range Test (DMRT) in triplicate (n = 3).
Results
Validation, Characterization, and Cloning of the Ca-AFP Gene
The Ca-AFP gene was identified to be overexpressed in chickpea plants exposed to water-deficit stress (Table 1). The validation of higher expression of Ca-AFP in the drought tolerant chickpea genotype was done using qRT-PCR analysis, the results of which showed its 4-fold upregulation in the roots and leaves of BG362 as compared to that in the drought sensitive (P-1003) genotype (Supplementary Figure S1). The Ca-AFP gene (LOC101512021) is located on chickpea chromosome number 1 (Ca1)3 and has a length of 542 bp and is predicted to encode a protein of 74 amino acids with a calculated MW of 8.3 kDa. The multiple sequence alignment of defensin proteins from different pulse crops showed that Ca-AFP shares 90.41, 87.67, 89.19, 89.19, 87.84, 85.14, and 81.94% similarity with the proteins from C. cajan (XP_020240649.1), M. sativa (AAT66096.1), T. villosa (AAX86993.1), Vigna radiata (XP_014492172.1), P. vulgaris (AIN35082.1), V. angularis (XP_017424299.1), and M. truncatula (AAT66097.1), respectively. All these proteins have eight common cysteine residues involved in four disulfide bridges (Figure 1A). The phylogenetic analysis revealed the identity of chickpea defensin with that of other pulses (Figure 1B). For overexpression of Ca-AFP in A. thaliana, the full-length cDNA was cloned into the plant expression vector pBI121 downstream of CaMV35S constitutive promoter (Supplementary Figure S2).
FIGURE 1.
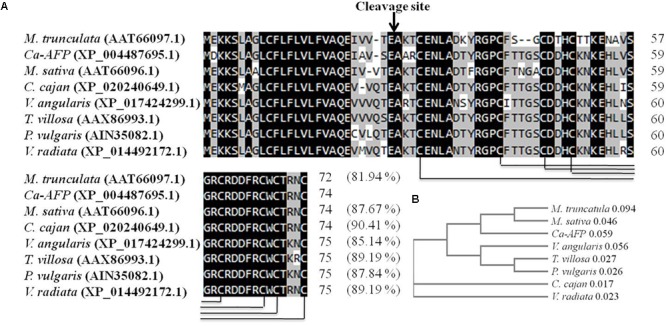
Comparison of Ca-AFP with other legume defensins (A) Multiple sequence alignment of Ca-AFP amino acid sequence with other legume defensin proteins using Clustal Omega software. (B) Phylogenetic analysis of Ca-AFP and other legume defensins. The defensin proteins whose sequences were used in this analysis were from Cajanus cajan (XP_020240649.1), Medicago sativa (AAT66096.1), Tephrosia villosa (AAX86993.1), Vigna radiata (XP_014492172.1), Phaseolus vulgaris (AIN35082.1), Vigna angularis (XP_017424299.1), and Medicago truncatula (AAT66097.1).
PCR Screening and Expression of Ca-AFP in A. thaliana
A total of nine independent kanamycin resistant transgenic lines of Arabidopsis (T1 generation) were generated by floral dip method (Supplementary Figure S3A). Out of the nine lines, three homozygous transgenic lines (named as #1, #6, and #9) generated in the T3 generation were selected for further analysis (Supplementary Figure S3B). The PCR results showed the amplification of a 542-bp fragment of Ca-AFP confirming the integration of the transgene in the genome of all the nine A. thaliana lines (Supplementary Figure S3C). The expression of Ca-AFP in the transgenic A. thaliana leaves was assessed in the nine transgenic lines under control and water-deficit condition using qRT-PCR. The relative expression of Ca-AFP was found to be increased by 6.8-, 5.0-, 6.2-, 5.5-, 6.5-, 7.6-, 5.6-, 5.3-, and 7.0-folds in transgenic Arabidopsis lines #1, #2, #3, #4, #5, #6, #7, #8, and #9 under the water-deficit conditions compared to its expression in the respective plants under well-watered conditions (Supplementary Figure S3D).
Response of Transgenic Plants to Mannitol and PEG Simulated Stress
The germination of seeds was normal under the control conditions in the WT as well as in the transgenic lines. However, the germination rate decreased in the WT compared to that in the transgenic lines under stress conditions. On 9th day of the experiment, the average germination rate of transgenic seedlings was 84, 69, 52, and 48% in comparison to 54, 24, 12, and 15% for WT in the presence of 100, 200, 250, and 300 mM mannitol, respectively (Figure 2A). The germination rates of the transgenic lines were significantly higher compared to that of WT under PEG simulated osmotic stress conditions. After 9th day, the average germination rates for the transgenic lines were 88, 66, 58, and 50% in comparison to 59, 20, 17, and 15% for WT in the presence of 1.5, 3, 4.5, and 6% PEG, respectively (Figure 2B).
FIGURE 2.
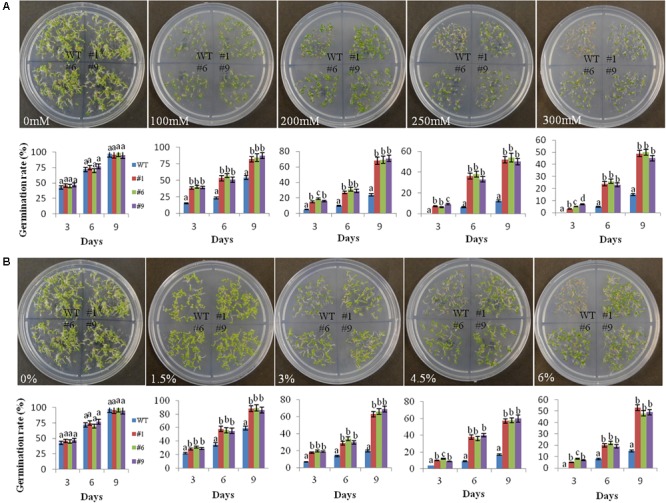
Germination rate of transgenic seeds under mannitol and polyethylene glycol (PEG) induced stress. Germination rates of seeds of the three transgenic lines (#1, #6, #9) and wild-type (WT) plants on MS medium supplemented with 100, 200, 250, and 300 mM mannitol (A) and on MS medium supplemented with 1.5, 3, 4.5, and 6% PEG-6000 (B) during a period from 3 to 9 days of stratification. At least 100 seeds were taken for each line in the plates and photographs were taken on the 9th day. The germination rates of seeds are shown graphically below each plate. Values are means ± SE (n = 3). Different letters above the bars indicate significant differences (p < 0.05) as analyzed by Duncan Multiple Range Test applied to different transgenic and WT lines.
Overall, the root growth was reduced in the presence of mannitol and PEG. However, in comparison to WT, transgenic lines showed significantly longer roots. The average root lengths of the transgenic lines were 4.8, 4.3, 3.8, and 2.9 cm compared to 4.2, 3.3, 2.2, and 1.3 cm for WT in the presence of 100, 200, 250, and 300 mM of mannitol, respectively (Figure 3A). Similarly, the average root lengths of the transgenic lines were 4.9, 4.18, 3.7, 2.74 cm compared to 4, 3.4, 2.6, and 1.2 cm for WT in the presence of 1.5, 3, 4.5, and 6% PEG, respectively (Figure 3B). The average biomass of the transgenic lines was 1.3-, 1.8-, 1.7-, and 2.8-times higher compared to that of the WT plants in the presence of 100, 200, 250, and 300 mM mannitol, respectively; it was 1.4-, 1.9-, 2.3-, and 2.8-times higher than that of the WT plants in the presence of 1.5, 3, 4.5, and 6% PEG, respectively (Figure 4A,B).
FIGURE 3.
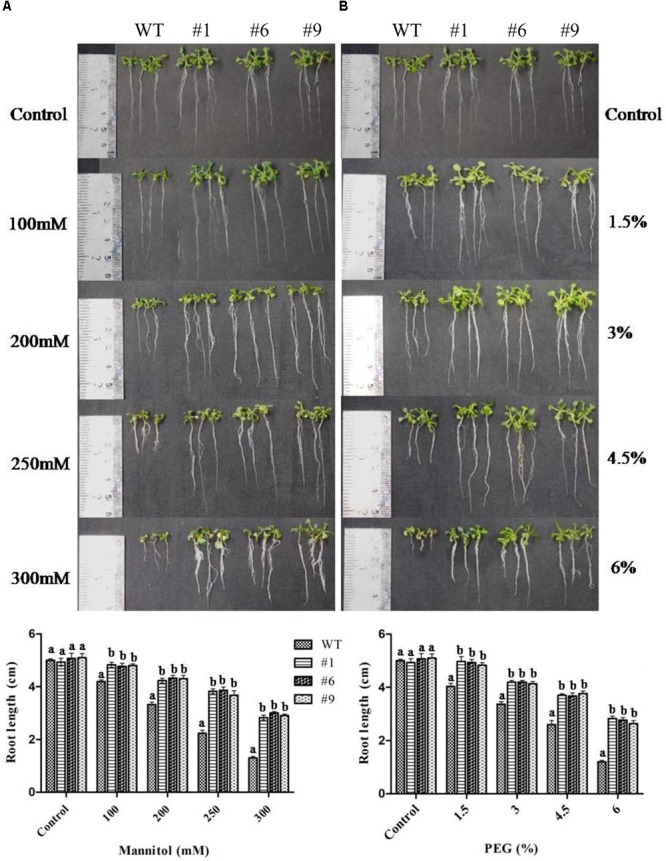
Root growth of transgenic plants under mannitol and polyethylene glycol (PEG) induced stress. Four-day-old seedlings of transgenic lines (#1, #6, #9) and wild type (WT) were grown on ½MS medium supplemented with 100, 200, 250, and 300 mM mannitol (A) or ½MS medium supplemented with 1.5, 3, 4.5, and 6% PEG (B). Photographs were taken after 10 days. Values are means ± SE (n = 3). Different letters above the bars indicate significant differences (p < 0.05) as analyzed by Duncan Multiple Range Test applied to different transgenic and WT lines.
FIGURE 4.
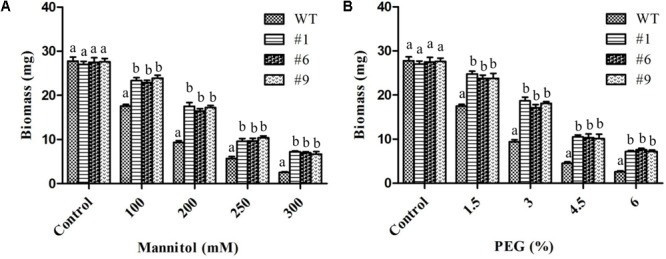
Biomass of transgenic plants under mannitol and polyethylene glycol (PEG) induced stress. In vitro grown transgenic plants were used for biomass measurements after 10 days of stress. Histograms showing biomass of plants exposed to 100, 200, 250, and 300 mM mannitol (A) or 1.5, 3, 4.5, and 6% PEG (B). Values are means ± SE (n = 3). Different letters above the bars indicate significant differences (p < 0.05) as analyzed by Duncan Multiple Range Test applied to different transgenic and WT lines.
Tolerance of Transgenic Plants to Water-Deficit Conditions
To assess the tolerance of Ca-AFP overexpressing transgenic A. thaliana plants to water-deficit stress, they were grown in soilrite for 3 weeks in a growth chamber and were then exposed to water-deficit stress by withholding of water for the next 15 days (Figure 5A). Under well-watered conditions, the growth of the transgenic and WT plants was identical. However, under water-deficit condition only 17% of the WT plants survived whereas about 84% survival rate was recorded for the transgenic plants (Figure 5B). After 5 days of recovery, most of the transgenic plants recovered but very low recovery was observed for the WT plants. We also measured the biomass and root length in all the nine lines of transgenic Arabidopsis plants, and EV transformed and WT plants exposed to 15 days of water-deficit conditions. Under well-watered conditions, transgenic plants and control plants showed almost similar biomass. However, biomass and root length were upto 2- and 1.8-fold, respectively, in the transgenic plants compared to that in the WT and EV transformed plants under water-deficit conditions (Supplementary Figure S4). The biomass of EV transformed and WT plants was almost the same under water-deficit conditions. Moreover, transgenic, WT, and EV transformed plants were evaluated for tolerance to water-deficit stress imposed for 15 days at pre-bolting stage and subsequent recovery for 5 days. In this case, the WT and EV transformed plants died whereas more than 85% transgenic plants survived (Supplementary Figure S5). The water loss and antioxidant parameters were measured after 15 days of imposition of water-deficit condition whereas RWC was measured after 3, 6, 9, 12, and 15 days. The values of average RWC for the transgenic plants were 82, 75, 72, 67, and 62% whereas the same for WT were 71, 63, 60, 54, and 45% after 3, 6, 9, 12, and 15 days of water-deficit treatment. The average RWC of the transgenic plants was 62% whereas it was 42% for the WT plants (Figure 5C). The average EL in the transgenic lines was 24.35% compared to 37% in the WT plants (Figure 6A) whereas the average water loss after 1, 2, and 3 h was 20, 39, and 52% in the transgenic plants compared to 22.5, 43, and 60% in the WT plants under water-deficit condition (Figure 6B). The contents of proline and chlorophyll were found to be increased in the transgenic plants. In the transgenic lines #1, #6, and #9, the proline content was determined to be 1.55-, 1.5-, and 1.5-fold higher, respectively, than the content in the WT plants under the water-deficit condition (Figure 6C). The chlorophyll content was found to be 1.7-, 1.7-, and 1.65-fold higher in the transgenic lines #1, #6, and #9, respectively, compared to that in the WT plants under water-deficit conditions (Figure 6D).
FIGURE 5.
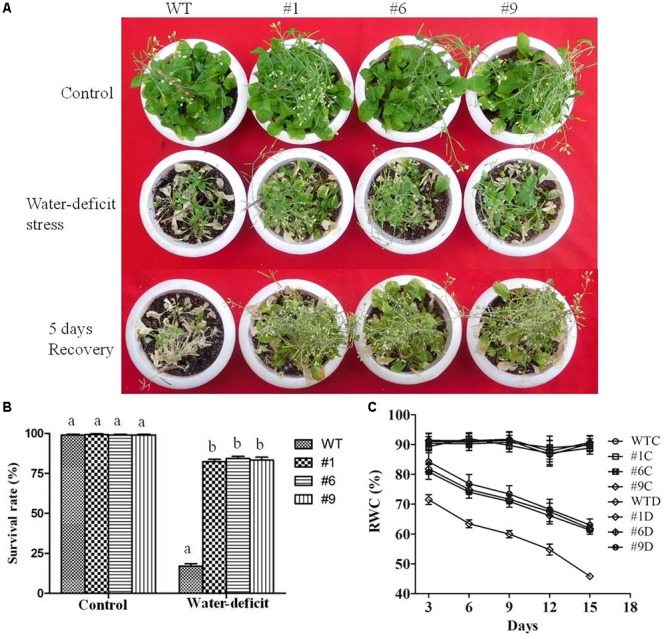
Water-deficit stress tolerance of Arabidopsis thaliana plants overexpressing Ca-AFP. (A) Transgenic A. thaliana (#1, #6, #9) plants under control and water-deficit (15 days) conditions. (B) Survival rates of the transgenic plants under water-deficit conditions. (C) Relative water content (RWC) of the transgenic plants. Values are means ± SE (n = 3). Different letters above the bars indicate significant differences (p < 0.05) as analyzed by Duncan Multiple Range Test applied to the transgenic and wild type (WT) lines.
FIGURE 6.
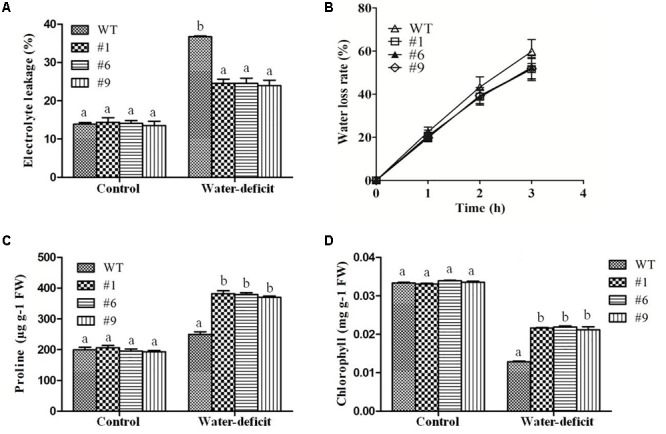
Physiological changes in transgenic plants under water-deficit condition. Electrolytic leakage (EL) (A), rate of water loss (B), proline content (C), and chlorophyll content (D) in transgenic (#1, #6, #9) lines and wild-type (WT) plants. Values are means ± SE (n = 3). Different letters above the bars indicate significant differences (p < 0.05) as analyzed by Duncan Multiple Range Test applied to the transgenic and WT lines.
The results of assays for SOD, APX, CAT, NADPHox, POX, and MDA did not show any significant differences between the transgenic lines and WT plants under normal conditions whereas under water-deficit condition, the average SOD, APX, and CAT activities were 1.3-, 1.50-, and 2.40-fold higher in the transgenic plants compared to that in the WT plants, respectively (Figure 7A–C). The MDA content was lower in the transgenic plants compared to that in the WT plants under water-deficit stress (Figure 7D). The NADPHox activity was found to be significantly decreased in transgenic plants compared to that in WT plants whereas the activity of apoplastic POX was found to be significantly increased in transgenic plants compared to that in WT plants under water-deficit condition (Figure 7E,F). A comparative assessment of the accumulation of two major ROS, O2- and H2O2, in the different lines was done by histochemical staining. Under non-stressed control condition no staining was observed in any of the lines. However, after 15 days of imposition of water-deficit stress, the WT and EV transformed plants showed dark blue (for NBT) and deep brown (for DAB) staining whereas the staining was much less in the Ca-AFP transformed lines, suggesting lower accumulation of these ROS in the Ca-AFP transformed lines (Supplementary Figures S6A,B). A comparative analysis of the staining intensities is presented in Supplementary Figures S6C,D. The fact that the EV transformed plants behaved similar to the WT plants with respect to their accumulation of ROS as well as biomass and root length under control and water-deficit stress conditions suggests that the better performance of the Ca-AFP transformed lines vis-à-vis the WT and EV plants was related to the expression of Ca-AFP in these lines.
FIGURE 7.
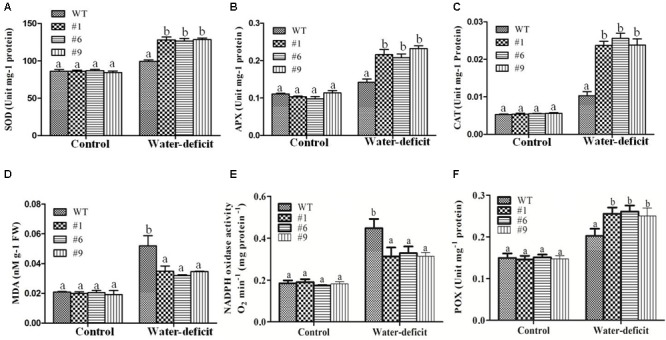
Changes in activities of superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), malondialdehyde (MDA), NADPH oxidase (NADPHox), and apoplastic peroxidise (POX) content under water-deficit condition. Activities of SOD (A), APX (B), CAT(C), MDA (D), NADPHox (E) and POX (F) content in the wild-type (WT) and transgenic A. thaliana (#1, #6, and #9) lines under control and water-deficit conditions. Values are means ± SE (n = 3). Different letters above the bars indicate significant differences (p < 0.05) as analyzed by Duncan Multiple Range Test applied to the different transgenic and WT lines.
In the WT plants, the MDA content was found to be 1.53-fold higher than in the transgenic plants. The transpiration rate and stomatal conductance were measured under well-watered and water-deficit stress conditions in the WT and transgenic plants. The stomatal closure, which enhances the water-deficit tolerance, was observed in the transgenic plants. The transpiration rate and stomatal conductance were found to be higher in the WT compared to those in the transgenic plants under water-deficit stress (Figure 8A,B). The average transpiration rate and stomatal conductance in the transgenic plants were 1.2 mol H2O m-2 s-1 and 150 mmol H2O m-2 s-1 compared to 1.64 mol H2O m-2 s-1 and 176 mmol H2O m-2 s-1 in the WT plants, respectively. The photosynthetic rate was found to be normal in the WT and transgenic plants under well-watered conditions whereas it decreased in both under the water-deficit conditions. The photosynthetic rate was 2.7-fold and WUE was 3-fold higher in the transgenic plants than in the WT plants under water-deficit conditions (Figure 8C,D). The size of guard cells (width and length) in the leaf epidermis of WT plants was significantly larger compared to that in the transgenic lines (Supplementary Figure S7). The average guard cell width to length dimension was 14.7 by 25.5 μm for the WT, in contrast to 12.8 by 21.5 μm, 13.2 by 22.5 μm, and 12.3 by 22 μm for transgenic lines (#1, #6, #9). Therefore, Ca-AFP expression decreased the stomatal size in transgenic Arabidopsis lines resulting in the reduction of water loss rates and transpiration efficiency that probably conferred tolerance to water-deficit stress.
FIGURE 8.
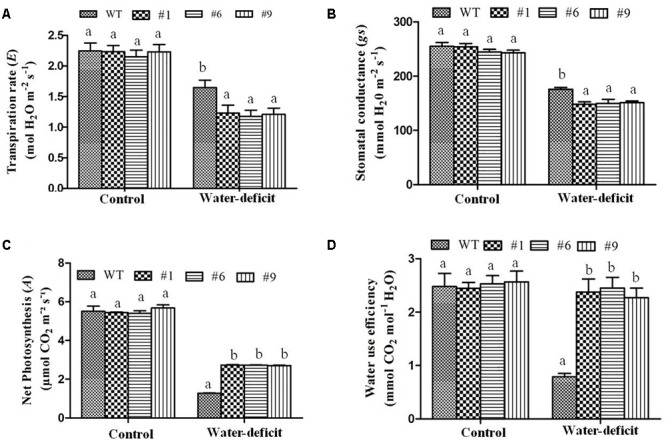
Physiological parameters of transgenic plants under water-deficit condition. (A) Transpiration rate, (B) stomatal conductance, (C) photosynthetic rate, and (D) water use efficiency in the wild-type (WT) and transgenic A. thaliana (#1, #6, and #9) lines under control and water-deficit conditions. Values are means ± SE (n = 3). Different letters above the bars indicate significant differences (p < 0.05) as analyzed by Duncan Multiple Range Test applied to different transgenic and WT lines.
Methyl jasmonate plays an important role in plant defense and in various developmental pathways including seed germination, root growth, flowering, fruit ripening, and senescence. ABA plays highly diverse roles in plants and it has been well known to promote leaf senescence. Therefore, we analyzed the effects of ABA and methyl jasmonate on germination rate and leaf senescence of transgenic and WT plants. Leaf disks of WT plants were damaged after 16 h of incubation in 5 μM ABA and 5 μM MeJA containing medium under dark condition (Supplementary Figure S8A). However, leaf disks of transgenic plants were less deteriorated compared to those of WT. Surface sterilized seeds of transgenic and WT were inoculated on ½ MS medium containing 5 μM ABA or 5 μM MeJA and then the germination rate was measured after 4 days of inoculation. The average germination rate of WT and transgenic seeds were 79 and 78% on ½MS medium (control). The average germination rates of transgenic lines were 53 and 58% in 5 μM ABA and 5 μM MeJA, respectively (Supplementary Figures S8B,D), whereas germination rates of WT seeds were 21 and 20% in 5 μM ABA and 5 μM MeJA, respectively. The root length of germinated seedlings of transgenic lines was higher than that of WT under ABA and MeJA treatments (Supplementary Figure S8C). The expression levels of both the hormone-related genes, AtERF1 and AtVSP1, were elevated under water-deficit condition in transgenic lines as well as in WT compared to the levels in well-watered plants. It was observed that in transgenic and WT plants, the expression of AtERF1 was upregulated 6- and 2.5-fold, respectively, under water-deficit condition. Similarly, the expression of AtVSP1 was upregulated 8-fold in transgenic lines and 3-fold in WT plants (Supplementary Figures S9A,B).
Discussion
Chickpea is one of the most important pulse crops cultivated worldwide and forms a rich source of dietary protein. Studies over the past decade have revealed a huge amount of information related to chickpea genome (Jain et al., 2013; Varshney et al., 2013; Gupta et al., 2016) and transcriptome analyses of this plant under stress conditions have been reported (Hiremath et al., 2011; Agarwal et al., 2012; Jhanwar et al., 2012; Singh et al., 2013; Pradhan et al., 2014; Garg et al., 2016; Iquebal et al., 2017). Herein, we report the cloning and characterization of Ca-AFP and its Agrobacterium-mediated genetic transformation in A. thaliana. The Ca-AFP gene encodes an 8.3-kDa protein containing 78 amino acids. Plant defensins display a conserved tertiary structure that is made up of a triple-stranded antiparallel β-sheet and one α-helix, stabilized into a compact shape by the disulfide bridges. These disulfide bridges develop a cysteine-stabilized α-helix β-sheet motif (CSα/β) (Kobayashi et al., 1991; Zhu et al., 2005). Apart from CSα/β motif, two additional conserved motifs, named α-core, surrounding the loop linking the first β-strand to the α-helix, and the γ-core containing the hairpin loop that connect β-strands 2 and 3 (Lβ2β3) were also found in the defensin structure (Yount and Yeaman, 2004; Yount et al., 2007). Ca-AFP has a typical signal peptide similar to all the other reported plant defensins (Meyer et al., 1996). Moreover, Ca-AFP is significantly identical to XP_020240649.1, AAT66096.1, AAX86993.1, XP_014492172.1, AIN35082.1, XP_017424299.1, and AAT66097.1, because of which these proteins lie in the same subgroup in the phylogenetic analysis (Figure 1A,B). Triticum aestivum defensin 1 (TAD1) gene showed tolerance to cold stress in T. aestivum (Koike et al., 2002). Under the control of the CAMV35S promoter, A. halleri defensin gene conferred Zn tolerance in A. thaliana plants (Mirouze et al., 2006). Although defensins are mainly known to be upregulated in response to biotic stress, their induction under abiotic stress, such as drought, has been observed (Govind et al., 2009). We used the CaMV35S promoter for over-expression of Ca-AFP in A. thaliana for improving water-stress tolerance. The qRT-PCR analysis of the generated transgenic lines showed an upregulation of the Ca-AFP gene under water-deficit condition (Supplementary Figure S3D). To characterize the mechanisms involved, mannitol and PEG 6000 were used to simulate water deficit conditions. Three homozygous (T3) transgenic lines of A. thaliana were used for the stress tolerance assay. These transgenic Arabidopsis plants showed higher germination rate (Figure 2) under mannitol- and PEG-induced stress. The process of maintaining water relations under osmotic stress is termed as osmotic adjustment. The accumulation of solutes under water deficit conditions results in a decrease in the osmotic potential of the cell, which pulls water molecules inside the cells and helps in maintaining turgor. For artificial induction of osmotic stress, PEG (Rauf et al., 2007; Khakwani et al., 2011) and mannitol (Bohnert et al., 1999) are used widely and these solutes decrease the osmotic potential. Previous studies have shown that transgenic Arabidopsis plants overexpressing stress related proteins exhibit higher germination rate, root length, and biomass under mannitol and NaCl stress (Huang et al., 2015; Xu et al., 2015; Yu et al., 2016). In the present study, the root length in the transgenic lines was about 2-times more than in the WT in the presence of 300 mM mannitol and 6% PEG (Figure 3). Similarly, the transgenic lines also showed higher biomass compared to the WT plants under the induced stress conditions.
In earlier studies, transgenic Arabidopsis plants overexpressing stress related proteins showed higher survival rates compared to the WT plants under water deficit conditions (Yan et al., 2014; Huang et al., 2015; Yao et al., 2017). It was reported that the overexpression of JcPR-10a, an antifungal protein of Jatropha curcas caused reduction in the transpiration efficiency and increased WUE during biotic and abiotic stress in transgenic Nicotiana tabacum (Agarwal et al., 2016). It was also reported that a C. annuum gene, CaPMEI1, which encodes an antifungal pectin methylesterase inhibitor protein, enhanced disease resistance, as well as drought and oxidative stress tolerance in transgenic Arabidopsis plants. The CaPMEI1 overexpressing Arabidopsis showed reduced transpiration and enhanced root elongation (An et al., 2008). In another study, an antimicrobial protein gene, CaAMP1, was found to be strongly induced in pepper leaves exposed to ABA, NaCl, drought, or low temperature. The overexpression of CaAMP1 conferred enhanced tolerance to high salinity and drought in transgenic Arabidopsis plants. These transgenic plants were also highly tolerant to osmotic stress caused by higher concentrations of mannitol. The transgenic Arabidopsis plants overexpressing CaAMP1 had stimulated growth of roots and decreased transpiration rate and enhanced stomatal closure that prevented water loss resulting in the protection of the plants from various environmental stresses (Lee and Hwang, 2009). The constitutive overexpression of a rose expansin gene in A. thaliana plants resulted in drought tolerance with decrease in water loss rates and higher RWC in the transgenic plants (Lu et al., 2013). A significant reduction in RWC of transgenic A. thaliana and WT plants was observed under drought stress (Brini et al., 2007) with RWC being upto 82% in the transgenic plants compared to 55% in the WT plants under drought conditions (Butt et al., 2017). Similarly, increased RWC was observed in the transgenic plants compared to that in the WT plants in the present study (Figure 5C). Furthermore, EL and MDA content were found to be normal in both the transgenic and WT whereas under water-deficit condition higher values were obtained in the WT plants compared to that in the transgenic plants (Figure 6A, 7D). Malondialdehyde is usually used as a marker for lipid peroxidation (Dong et al., 2003). After exposure of plants to drought/oxidative stress, the higher content of ROS generated in plants can be evaluated in terms of the accumulation of MDA (Tu et al., 2016). The rate of water loss was reported to be higher in the WT than in the transgenic A. thaliana plants expressing different stress-related proteins under drought stress (Brini et al., 2007; Yan et al., 2014). We also found higher rate of water loss in the WT plants compared to that in the transgenic plants (Figure 6B). The reduced rate of water loss in the transgenic plants resulted in water-stress tolerance.
The accumulation of osmoprotectants in plants has also been reported under stress conditions. Osmotic potential is a typical indicator of the osmotic adjustment ability under varying physiological conditions. It has been used as an efficient index to assess genotypes for tolerance against osmotic stress (Zhu, 2002). Proline is an important amino acid whose accumulation in plants indicates stress tolerance and it adjusts the intracellular osmoticum under drought conditions. It scavengers free radicals (Ashraf and Foolad, 2007), and is involved in the overexpression of genes responsible for drought tolerance (Sharma et al., 2011) and in protecting plant cells from damage (Kishor et al., 1995). We observed the accumulation of higher amounts of proline in transgenic plants under water-deficit condition (Figure 6C). Similarly, the chlorophyll content was found to be higher in the transgenic plants compared to that in the WT plants under water-deficit conditions (Figure 6D). The chlorophyll content correlated with higher net photosynthesis and confirmed that the transgenic plants could maintain better photosynthesis under drought stress (Figure 8C). The C. annuum defensin 1 gene was reported to be expressed in leaves under drought, salinity, and wound stress. The expression of this gene was not found under normal conditions (Do et al., 2004) and it was observed that plant defensins are involved in the adaptation of plants to environmental stresses.
The production of reactive oxygen species (ROS), and hence oxidative stress, most often plays a role in defensin-mediated cell death, as has been reported for many plant defensins, including RsAFP2 (Aerts et al., 2007), HsAFP1 (Aerts et al., 2011), DmAMP1 (Aerts et al., 2006), and NaD1 (van der Weerden et al., 2008). Plants can regulate the levels of ROS through antioxidant enzymes, such as SOD, APX, CAT and POX, which scavenge the ROS molecules to impart enhanced resistance to drought (Hameed et al., 2012; Cai et al., 2015; Xu et al., 2015; Kumar et al., 2016; Yao et al., 2017). The NADPHox catalyzes the production of superoxides, an important ROS in cells. These enzymes are conserved in plants, fungi and animals and are actively involved in ROS production (Torres and Dangl, 2005). In present study, the activity of NADPHox was found to be lower in transgenic lines under water-deficit conditions. However, no significant changes were seen under well-watered conditions. It reflects that the ROS production was lower in transgenic lines compared to that in WT plants correlating with the results of ROS assay in transgenic lines under water-deficit conditions. The accumulation of ROS is observed under abiotic stress conditions, like drought, that results in oxidative damage and cell death in plants. To reduce the excessive ROS accumulation, plants have developed a complex set of antioxidant strategies to eliminate their damaging effects and maintain redox homeostasis (Gill and Tuteja, 2010). Plants can regulate the ROS levels through modulation of ROS scavenging enzymes, such as SOD, APX, CAT, and MDA. SOD is the front-line enzyme in protection against ROS attack because it rapidly scavenges superoxide, one of the first ROS to be produced, dismutating it to oxygen and H2O2 (Bowler et al., 1992). The major enzymatic cellular scavengers of H2O2 are CAT and APX (Noctor and Foyer, 1998). APX, an enzyme located in every cellular compartment that produces ROS, might function as a fine regulator of steady-state levels of intracellular ROS, possibly for signaling purposes, whereas CAT located exclusively in the peroxisomes, might function as a bulk remover of excess ROS produced under stress conditions (Mittler, 2002). Our results showed that the SOD, APX, CAT, and POX activities were significantly higher in the Ca-AFP overexpressing transgenic plants under water-deficit condition (Figure 7A–C,F). Our study with the transgenic plants overexpressing defensin shows that better ROS homeostasis is involved in the tolerance of these plants to simulated drought stress.
The regulation of stomata plays an important role in the exchange of gases between plants and atmosphere. The stomatal opening is responsible for 90% water loss from plants through transpiration (Wang et al., 2009). The reduction in stomatal conductance results in a decrease in net photosynthetic and transpiration rates with progressive water stress. It leads to increased WUE because transpiration is repressed more than photosynthesis (Xu et al., 2010). We observed that the transpiration rate and stomatal conductance were normal under well-watered conditions whereas under water-deficit conditions, the rates were significantly decreased in the transgenic plants (Figure 8A,B). The stomatal behavior could also be regulated by H2O2 for optimizing the WUE. The tolerance to drought was positively correlated with the increasing WUE in rice plants (Huang et al., 2009). Our results showed that the WUE was significantly increased in the transgenic plants under water-deficit conditions (Figure 8D). In a previous study, TAD1 was robustly and rapidly induced when wheat plants were exposed to cold stress whereas its expression was not induced by the plant hormones, such as ABA, salicylic acid, and methyl jasmonate (Brown et al., 2003; Do et al., 2004), that normally drive the expression of defensins. Its strong induction during exposure to cold implies that this plant defensin is involved in the adaptation of wheat to the cold. Production of plant defensins is also induced in response to environmental stress, such as drought (Maitra and Cushman, 1998), and signaling molecules, including methyl jasmonate, ethylene, and salicylic acid (Hanks et al., 2005). A hypothesized model for the role of defensin gene during water-deficit condition is proposed. A common regulatory defense mechanism explains the involvement of some antimicrobial peptides (AMPs). These peptides are generally induced against biotic stressors, especially defensin is induced by fungal pathogens. In addition to biotic stress, many of the abiotic stressors, for example, drought, wounding, cold, and salinity may induce the synthesis of defensins (Lay and Anderson, 2005). Ethylene Response Factor1 (ERF1) is known as an upstream component in both jasmonate (JA) and ethylene (ET) signaling and is involved in pathogen resistance. It was also reported that ERF1 was highly induced by high salinity and drought stress in A. thaliana (Cheng et al., 2013). ERF1 plays a positive role in salt, drought, and heat stress tolerance by stress-specific gene regulation, which integrates JA, ET, and abscisic acid signals. In response to various stress signals ERF1 binds to different cis elements (DRE element or GCC box). The induction of ERF1 required ET and JA signaling under abiotic stress and it was negatively regulated by ABA (Cheng et al., 2013). JA signaling enhances the activities of antioxidant enzymes, such as SOD, POD, CAT, and APX, as well as the tolerance to salinity stress in wheat (Qiu et al., 2014). ET causes closing of stomata in Arabidopsis through ethylene-induced H2O2 synthesis (Desikan et al., 2006). MeJA-mediated stomatal closure has been linked to cytoplasmic alkalinization in guard cells, production of ROS and NO, and activation of K+ efflux channels (Evans, 2003) and slows the activities of anion channels (Suhita et al., 2004; Munemasa et al., 2007). These modulations are similar to those of ABA, suggesting an overlapping use of signaling components for closing of stomata. Constitutive expression of ERF1 activates the transcription of downstream effector genes, such as Plant Defensin1.2 (PDF1.2), to promote the ET response (Solano et al., 1998). JA and ET often induce the up-regulation of genes involved in plant defense, such as PDF1.2, VSP2, LOX2, and chitinases (Boter et al., 2004). Drought stress induces hypersensitive response mediated necrotic lesion in Arabidopsis plants (Tang et al., 2005). In Arabidopsis, the constitutive expression of pepper CaHIR1 was reported to increase the resistance to P. syringae and H. parasitica, and the sensitivity to Botrytis cinerea, and it also conferred hypersensitivity to drought and salt stresses (Jung et al., 2008). Based on our results, we propose a model for the functional regulation of Ca-AFP under water-deficit conditions caused by water deficiency, mannitol, and PEG. The imposition of drought/osmotic stress causes hypersensitive response and production of stress related hormones like jasmonic acid, abscisic acid, and ethylene in plants (Haa et al., 2014). JA and ET trigger ERFs, which induce drought responsive genes (like Ca-AFP). These genes modulate the antioxidant activities, causing physiological changes and accumulation of osmoprotectants, resulting in water stress tolerance (Figure 9). AtERF1 was reported to be highly induced under high salt and drought stress conditions and overexpression of AtERF1 in A. thaliana resulted in enhanced tolerance to drought and salinity stress (Cheng et al., 2013). Involvement of JA trigger is indicated from the accumulation of transcripts of the jasmonic acid-marker gene, AtVSP1, after 15 days of drought stress in A. thalaina plants (Cho et al., 2013). In present study, we observed that the expression of AtERF1 and AtVSP1 was upregulated in transgenic A. thaliana plants under water-deficit stress conditions (Supplementary Figures S9A,B).
FIGURE 9.
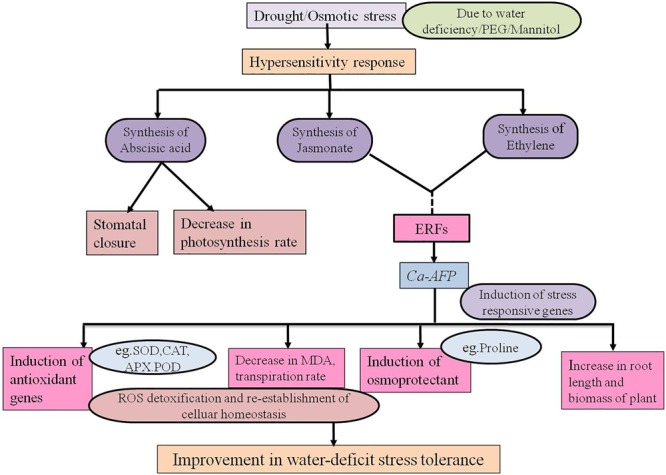
Proposed model for the regulatory network of the Ca-AFP response under water-deficit condition.
In conclusion, the overexpression of chickpea defensin gene in A. thaliana confers water stress tolerance. However, an understanding of the signaling pathways involved in the induction of Ca-AFP under water-deficit condition would need further investigation. Our results indicate that Ca-AFP can be a potential candidate gene for the development of drought tolerance trait in economically important crop plants.
Author Contributions
MK (first author), PY, and SN conceived, designed and conducted the experiments. MK (last author) and MY analyzed the data and results. MK (first author), MY, and MK (last author) wrote the manuscript. MK (last author) monitored the experiments and critically commented on the manuscript. All the authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The study was financially supported under the project titled “Characterization of gene(s) responsible for tyloses formation in chickpea during Fusarium oxysporum infection” from Science & Engineering Research Board (SERB), New Delhi, India. The manuscript communication number assigned to this manuscript by the Dean, R&D, Integral University, Lucknow, is IU/R&D/2018-MCN000377.
Footnotes
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2019.00290/full#supplementary-material
References
- Aebi H. (1974). “Catalase,” in Methods of Enzymatic Analysis Vol. 2 ed. Bergmeyer H. U. (New York, NY: Academic Press Inc.) 673–685. 10.1016/B978-0-12-091302-2.50032-3 [DOI] [Google Scholar]
- Aerts A. M., Bammens L., Govaert G., Carmona-Gutierrez D., Madeo F., Cammue B. P., et al. (2011). The antifungal plant defensin HsAFP1 from Heuchera sanguinea induces apoptosis in Candida albicans. Front. Microbiol. 2:47. 10.3389/fmicb.2011.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerts A. M., Francois I. E., Bammens L., Cammue B. P., Smets B., Winderickx J., et al. (2006). Level of M(IP)2C sphingolipid affects plant defensin sensitivity, oxidative stress resistance and chronological life-span in yeast. FEBS Lett. 580 1903–1907. 10.1016/j.febslet.2006.02.061 [DOI] [PubMed] [Google Scholar]
- Aerts A. M., Francois I. E., Meert E. M., Li Q. T., Cammue B. P., Thevissen K. (2007). The antifungal activity of RsAFP2, a plant defensin from Raphanus sativus, involves the induction of reactive oxygen species in Candida albicans. J. Mol. Microbiol. Biotechnol. 13 243–247. 10.1159/000104753 [DOI] [PubMed] [Google Scholar]
- Agarwal G., Jhanwar S., Priya P. (2012). Comparative analysis of Kabuli chickpea transcriptome with desi and wild chickpea provides a rich resource for development of functional markers. PLoS One 7:e52443. 10.1371/journal.pone.0052443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal P., Dabi M., More P., Patel K., Jana K., Agarwal P. K. (2016). Improved shoot regeneration, salinity tolerance and reduced fungal susceptibility in transgenic tobacco constitutively expressing PR-10a gene. Front. Plant Sci. 7:217. 10.3389/fpls.2016.00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M. S., Cabral K. M. S., Kurtenbach E., Almeida F. C. L., Valente A. P. (2002). Solution structure of Pisum sativum defensin 1 by high resolution NMR: plant defensins, identical backbone with different mechanisms of action. J. Mol. Biol. 315 749–757. 10.1006/jmbi.2001.5252 [DOI] [PubMed] [Google Scholar]
- An S. H., Sohn K. H., Choi H. W., Hwang I. S., Lee S. C., Hwang B. K. (2008). Pepper pectin methylesterase inhibitor protein CaPMEI1 is required for antifungal activity, basal disease resistance and abiotic stress tolerance. Planta 228 61–78. 10.1007/s00425-008-0719-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. (1949). Copper enzyme polyphenoloxides in isolated chloroplast in Beta vulgaris. Plant Physiol. 24 1–15. 10.1104/pp.24.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M., Foolad M. R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 59 206–216. 10.1016/j.envexpbot.2005.12.006 [DOI] [Google Scholar]
- Bartels D., Sunkar R. (2005). Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 24 23–58. 10.1080/07352680590910410 [DOI] [Google Scholar]
- Bates L., Waldren R. P., Teare I. D. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39 205–207. 20688380 [Google Scholar]
- Beauchamp C., Fridovich I. (1971). Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. Rev. 44 276–287. 10.1016/0003-2697(71)90370-8 [DOI] [PubMed] [Google Scholar]
- Blum A. (1998). Plant Breeding for Stress Environment. Florida: CRC Press Inc. 208. [Google Scholar]
- Bohnert H. J., Su H., Shen B. (1999). “Molecular mechanisms of salinity tolerance,” in Molecular Responses to Cold, Drought, Heat, and Salt Stress in Higher Plants eds Shinozaki K., Yamaguchi-Shinozaki K. (Austin, TX: Landes RG Company; ) 29–62. [Google Scholar]
- Boter M., Ruiz-Rivero O., Abdeen A., Prat S. (2004). Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes Dev. 18 1577–1591. 10.1101/gad.297704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C., Van Montagu M., Inzé D. (1992). Superoxide dismutases and stress tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43 83–116. 10.1146/annurev.pp.43.060192.000503 [DOI] [Google Scholar]
- Bradford M. M. (1976). Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 248–254. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Brini F., Hanin M., Mezghani I., Berkowitz G. A., Masmoudi K. (2007). Overexpression of wheat Na+ /H+ antiporter TNHX1 and H+ -pyrophosphatase TVP1 improve salt- and drought-stress tolerance in Arabidopsis thaliana plants. J. Exp. Bot. 58 301–308. 10.1093/jxb/erl251 [DOI] [PubMed] [Google Scholar]
- Broekaert W. F., Cammue B., DeBolle M., Thevissen K., DeSamblanx G., Osborn R. (1997). Antimicrobial peptides from plants. Crit. Rev. Plant Sci. 16 297–323. 10.1080/07352689709701952 [DOI] [Google Scholar]
- Broekaert W. F., Terras F. R. G., Cammue B. P. A., Osborn R. W. (1995). Plant defensins: novel antimicrobial peptides as components of the host defense system. Plant Physiol. 108 1353–1358. 10.1104/pp.108.4.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. L., Kazan K., McGrath K. C., Maclean D. J., Manners J. M. (2003). A role for the GCC-box in jasmonate-mediated activation of the PDF1.2 gene of Arabidopsis. Plant Physiol. 32 1020–1032. 10.1104/pp.102.017814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt H. I., Yang Z., Chen E., Zhao G., Gong Q., Yang Z., et al. (2017). Functional characterization of cotton GaMYB62L, a novel R2R3 TF in transgenic Arabidopsis. PLoS One 12:e0170578. 10.1371/journal.pone.0170578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W., Liu W., Wang W.-S., Fu Z.-W., Han T.-T., Lu Y.-T. (2015). Overexpression of rat neurons nitric oxide synthase in rice enhances drought and salt tolerance. PLoS One 10:e0131599. 10.1371/journal.pone.0131599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak I., Marschner H. (1988). Zinc-dependent changes in electron spin resonance signals, NADPH oxidase and plasma membrane permeability in cotton roots. Physiol. Plant. 73 182–186. 10.1111/j.1399-3054.1988.tb09214.x [DOI] [Google Scholar]
- Cheng M. C., Liao P. M., Kuo W. W., Lin T. P. (2013). The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol. 162 1566–1582. 10.1104/pp.113.221911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C. C., Hadwiger L. A. (1991). The Fusarium solani-induced expression of a pea gene family encoding high cysteine content proteins. Mol. Plant Microbe Interact. 4 324–331. 10.1094/MPMI-4-324 [DOI] [PubMed] [Google Scholar]
- Cho S. M., Kang B. R., Kim Y. C. (2013). Transcriptome analysis of induced systemic drought tolerance elicited by Pseudomonas chlororaphis O6 in Arabidopsis thaliana. Plant Pathol. J. 29 209–220. 10.5423/PPJ.SI.07.2012.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S. J., Bent A. F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. 10.1046/j.1365-313x.1998.00343.x [DOI] [PubMed] [Google Scholar]
- Desikan R., Last K., Harrett-Williams R., Tagliavia C., Harter K., Hooley R., et al. (2006). Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J. 47 907–916. 10.1111/j.1365-313x.2006.02842 [DOI] [PubMed] [Google Scholar]
- Do H. M., Lee S. C., Jung H. W., Sohn K. H., Hwang B. K. (2004). Differential expression and in situ localization of a pepper defensin (CADEF1) gene in response to pathogen infection, abiotic elicitors and environmental stresses in Capsicum annuum. Plant Sci. 166 1297–1305. 10.1016/j.plantsci.2004.01.008 [DOI] [Google Scholar]
- Dong J., Chen C., Chen Z. (2003). Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol. Biol. 51 21–37. 10.1023/A:1020780022549 [DOI] [PubMed] [Google Scholar]
- Evans N. H. (2003). Modulation of guard cell plasma membrane potassium currents by methyl jasmonate. Plant Physiol. 131 8–11. 10.1104/pp.014266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R., Shankar R., Thakkar B., Kudapa H., Krishnamurthy L., Mantri N., et al. (2016). Transcriptome analyses reveal genotype and developmental stage-specific molecular responses to drought and salinity stresses in chickpea. Sci. Rep. 6:19228. 10.1038/srep19228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. S., Tuteja N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48 909–930. 10.1016/j.plaphy.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Govind G., Harshavardhan V. T., Patricia J. K., Dhanalakshmi R., Senthil Kumar M., Sreenivasulu N., et al. (2009). Identification and functional validation of a unique set of drought induced genes preferentially expressed in response to gradual water stress in peanut. Mol. Genet. Genomics 281 591–605. 10.1007/s00438-009-0432-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Nawaz K., Parween S., Roy R., Sahu K., Pole A. K., et al. (2016). Draft genome sequence of Cicer reticulatum L., the wild progenitor of chickpea provides a resource for agronomic trait improvement. DNA Res. 24 1–10. 10.1093/dnares/dsw042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haa C. V., Leyva-Gonzálezc M. A., Osakabed Y., Trana U. T., Nishiyamaa R., Watanabea Y., et al. (2014). Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. U.S.A. 111 851–856. 10.1073/pnas.1322135111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed A., Goher M., Iqbal N. (2012). Drought induced programmed cell death and associated changes in antioxidants, proteases, and lipid peroxidation in wheat leaves. Biol. Plant. 57 370–374. 10.1007/s10535-012-0286-9 [DOI] [Google Scholar]
- Hanks J. N., Snyder A. K., Graham M. A., Shah R. K., Blaylock L. A., Harrison M. J., et al. (2005). Defensin gene family in Medicago truncatula: structure, expression and induction by signal molecules. Plant Mol. Biol. 58 385–399. 10.1007/s11103-005-5567-7 [DOI] [PubMed] [Google Scholar]
- Hemeda H. M., Klein B. P. (1990). Effect of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J. Food Sci. 55 184–185. 10.1111/j.1365-2621.1990.tb06048.x [DOI] [Google Scholar]
- Hiremath P. J., Farmer A., Cannon S. B., Woodward J., Kudapa H., Tuteja R., et al. (2011). Large-scale transcriptome analysis in chickpea (Cicer arietinum L.), an orphan legume crop of the semi-arid tropics of Asia and Africa. Plant Biotechnol. J. 9 922–931. 10.1111/j.1467-7652.2011.00625.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q., Wang Y., Li B., Chang J., Chen M., Li K., et al. (2015). TaNAC29, a NAC transcription factor from wheat, enhances salt and drought tolerance in transgenic Arabidopsis. BMC Plant Biol. 15:268. 10.1186/s12870-015-0644-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X. Y., Chao D. Y., Gao J. P., Zhu M. Z., Shi M., Xuan H. (2009). A previously unknown xinc finger protein, DST, regulates drought and salt. Gene Dev. 23 1709–1713. 10.1101/gad.1812409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram J., Bartels D. (1996). The molecular basis of dehydration tolerance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47 377–403. 10.1146/annurev.arplant.47.1.377 [DOI] [PubMed] [Google Scholar]
- Iquebal M. A., Soren K. R., Gangwar P., Shanmugavadivel P. S., Aravind K., Singla D., et al. (2017). Discovery of putative herbicide resistance genes and its regulatory network in chickpea using transcriptome sequencing. Front. Plant Sci. 8:958. 10.3389/fpls.2017.00958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M., Misra G., Patel R. K., Priya P., Jhanwar S., Khan A. W., et al. (2013). A draft genome sequence of the pulse crop chickpea Cicer arietinum L. Plant J. 74 715–729. 10.1111/tpj.12173 [DOI] [PubMed] [Google Scholar]
- Jhanwar S., Priya P., Garg R., Parida S. K., Tyagi A. K., Jain M. (2012). Transcriptome sequencing of wild chickpea as a rich resource for marker development. Plant Biotechnol. J. 10 690–702. 10.1111/j.1467-7652.2012.00712.x [DOI] [PubMed] [Google Scholar]
- Jung H. W., Lim C. W., Lee S. C., Choi H. W., Hwang C. H., Hwang B. K. (2008). Distinct roles of the pepper hypersensitive induced reaction protein gene CaHIR1 in disease and osmotic stress, as determined by comparative transcriptome and proteome analyses. Planta 227 409–425. 10.1007/s00425-007-0628-6 [DOI] [PubMed] [Google Scholar]
- Khakwani A. A., Denett M. D., Munir M. (2011). Early growth response of six wheat varieties under artificial osmotic stress condition. Pak. J. Agric. Sci. 48 121–126. [Google Scholar]
- Kishor P. B. K., Hong Z., Miao G. H., Hu C. A. A., Verma D. P. S. (1995). Over-expression of [delta]-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol. 108 1387–1394. 10.1104/pp.108.4.1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Sato A., Takashima H., Tamaoki H., Nishimura S., Kyogoku Y., et al. (1991). A new α-helical motif in membrane active peptides. Neurochem. Int. 18 525–534. 10.1016/0197-0186(91)90151-3 [DOI] [PubMed] [Google Scholar]
- Koike M., Okamoto T., Tsuda S., Imai R. (2002). A novel plant defensin-like gene of winter wheat is specifically induced during cold acclimation. Biochem. Biophys. Res. Commun. 298 46–53. 10.1016/S0006-291X(02)02391-4 [DOI] [PubMed] [Google Scholar]
- Komori T., Yamada S., Imaseki H. (1997). A cDNA clone for γ-thionin from Nicotiana paniculata (accession no. AB005250; PGR97–132). Plant Physiol. 115 314. [Google Scholar]
- Kumar M., Mishra S., Dixit V. K., Kumar M., Agrawal L., Chauhan P. S., et al. (2016). Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea. Plant Signal. Behav. 11:e1071004. 10.1080/15592324.2015.1071004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T., James W., Weyers J. (1998). A surrogate measure of stomatal aperture. J. Exp. Bot. 49 1397–1403. 10.1093/jxb/49.325.1397 [DOI] [Google Scholar]
- Lay F. T., Anderson M. A. (2005). Defensins–components ofthe innate immune system in plants. Curr. Protein Peptide Sci. 6 85–101. 10.2174/1389203053027575 [DOI] [PubMed] [Google Scholar]
- Lay F. T., Mills G. D., Poon I. K. H. (2012). Dimerization of plant defensin NaD1 enhances its antifungal activity. J. Biol. Chem. 287 19961–19972. 10.1074/jbc.M111.331009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Hwang B. K. (2009). Functional roles of the pepper antimicrobial protein gene, CaAMP1, in abscisic acid signaling, and salt and drought tolerance in Arabidopsis. Planta 229 383–391. 10.1007/s00425-008-0837-7 [DOI] [PubMed] [Google Scholar]
- Levitt J. (1980). Responses of Plants to Environmental Stresses: Water, Radiation, Salt and Other Stresses Vol. 2 New York, NY: Academic Press; 93–128 [Google Scholar]
- Liu J. H., Nada K., Honda C., Kitashiba H., Wen X. P., Pang X. M., et al. (2006). Polyamine biosynthesis of apple callus under salt stress: importance of the arginine decarboxylase pathway in stress response. J. Exp. Bot. 57 2589–2599. 10.1093/jxb/erl018 [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real- time quantitation PCR and the 2 (-Delta Delta C(T)) method. Methods 25 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lobo D. S., Pereira I. B., Fragel-Madeira L. (2007). Antifungal Pisum sativum defensin 1 interacts with Neurospora crassa cyclin F related to the cell cycle. Biochemistry 30 987–996. 10.1021/bi061441j [DOI] [PubMed] [Google Scholar]
- Lu P., Kang M., Jiang X., Dai F., Gao J., Zhang C. (2013). RhEXPA4, a rose expansin gene, modulates leaf growth and confers drought and salt tolerance to Arabidopsis. Planta 237 1547–1559. 10.1007/s00425-013-1867-3 [DOI] [PubMed] [Google Scholar]
- Maitra N., Cushman J. C. (1998). Characterization of a drought induced soybean cDNA encoding a plant defensin. Plant Physiol. 118 1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel A., Leshem Y., Tiwari B. S., Levine A. (2004). Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7 (AtRabG3e). Plant Physiol. 134 118–128. 10.1104/pp.103.025379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B., Houlne G., Pozueta-Romero J., Schantz M., Schantz R. (1996). Fruit-specific expression of a defensin-type gene family in bell pepper. Plant Physiol. 112 615–622. 10.1104/pp.112.2.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirouze M., Sels J., Richard O., Czernic P., Loubet S., Jacquier A., et al. (2006). A putative novel role for plant defensins: a defensin from the zinc hyper-accumulating plant, Arabidopsis halleri, confers zinc tolerance. Plant J. 47 329–342. 10.1111/j.1365-313X.2006.02788.x [DOI] [PubMed] [Google Scholar]
- Mittler R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7 405–410. 10.1016/S1360-1385(02)02312-9 [DOI] [PubMed] [Google Scholar]
- Munemasa S., Oda K., Watanabe-Sugimoto M., Nakamura Y., Shimoishi Y., Murata Y. (2007). The coronatine-insensitive1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiol. 143 1398–1407. 10.1104/pp.106.091298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y., Asada K. (1981). Hydrogen peroxide scavenged by Ascorbate- specific peroxidase in spinach chloroplast. Plant Cell Physiol. 22 867–880. [Google Scholar]
- Noctor G., Foyer C. H. (1998). Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49 249–279. 10.1146/annurev.arplant.49.1.249 [DOI] [PubMed] [Google Scholar]
- Osborn R. W., Broekaert W. F. (1999). “Antifungal proteins,” in Seed Proteins eds Shewry P. R., Casey R. (Dordrecht: Kluwer Academic Publishers; ) 727–751. 10.1007/978-94-011-4431-5_31 [DOI] [Google Scholar]
- Osborn R. W., De Samblanx G. W., Thevissen K., Goderis I., Torrekens S., Van Leuven F., et al. (1995). Isolation and characterisation of plant defensins from seeds of Asteraceae, Fabaceae, Hippocastanaceae and Saxifragaceae. FEBS Lett. 368 257–262. 10.1016/0014-5793(95)00666-W [DOI] [PubMed] [Google Scholar]
- Pei Z. M., Kuchitsu K., Ward J. M., Schwarz M., Schroeder J. I. (1997). Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9 409–423. 10.1105/tpc.9.3.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan S., Bandhiwal N., Shah N., Kant C., Gaur R., Bhatia S. (2014). Global transcriptome analysis of developing chickpea (Cicer arietinum L.) seeds. Front. Plant Sci. 5:698. 10.3389/fpls.2014.00698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A. H., Cairns J. E., Horton P., Jones H. G., Griffiths H. (2002). Linking drought-resistance mechanisms to drought avoidance in upland rice using a QTL approach: progress and new opportunities to integrate stomatal and mesophyll responses. J. Exp. Bot. 53 989–1004. 10.1093/jexbot/53.371.989 [DOI] [PubMed] [Google Scholar]
- Qiu Z., Guo J., Zhu A., Zhang L., Zhang M. (2014). Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol. Environ. Saf. 104 202–208. 10.1016/j.ecoenv.2014.03.014 [DOI] [PubMed] [Google Scholar]
- Ramanjulu S., Bartels D. (2002). Drought- and desiccation-induced modulation of gene expression in plants. Plant Cell Environ. 25 141–151. 10.1046/j.0016-8025.2001.00764.x [DOI] [PubMed] [Google Scholar]
- Ramegowda V., Gill U. S., Sivalingam P. N., Gupta A. (2017). GBF3 transcription factor imparts drought tolerance in Arabidopsis thaliana. Sci. Rep. 7:9148. 10.1038/s41598-017-09542-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauf M., Munir M., Hassan M. U., Ahmad M., Afzal M. (2007). Performance of wheat genotypes under osmotic stress at germination and early seedling growth stage. Afr. J. Biotechnol. 6 971–975. [Google Scholar]
- Sagaram U. S., El-Mounadi K., Buchko G. W., Berg H. R., Kaur J., Pandurangi R. S., et al. (2013). Structural and functional studies of a phosphatidic acid-binding antifungal plant defensin MtDef4: identification of an RGFRRR motif governing fungal cell entry. PLoS One 8:e82485. 10.1371/journal.pone.0082485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S., Eliceiri K. W. (2012). NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9 671–675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura A., Moreno M., Molina A., Garcia-Olmedo F. (1998). Novel defensin subfamily from spinach (Spinacia oleracea). FEBS Lett. 435 159–162. 10.1016/S0014-5793(98)01060-6 [DOI] [PubMed] [Google Scholar]
- Sharma P., Lönneborg A. (1996). Isolation and characterization of a cDNA encoding a plant defensin-like protein from roots of Norway spruce. Plant Mol. Biol. 31 707–712. 10.1007/BF00042244 [DOI] [PubMed] [Google Scholar]
- Sharma S., Villamor J. G., Verslues P. E. (2011). Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol. 157 292–304. 10.1104/pp.111.183210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewry P. R., Lucas J. A. (1997). Insecticidal and antifungic proteins of the latex from Manihot glaziovii Muell. Arg. Adv. Bot. Res. 26 135–192. [Google Scholar]
- Silverstein K. A., Graham M. A., Paape T. D., VandenBosch K. A. (2005). Genome organization of more than 300 defensin-like genes in Arabidopsis. Plant Physiol. 138 600–610. 10.1104/pp.105.060079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V. K., Garg R., Jain M. (2013). A global view of transcriptome dynamics during flower development in chickpea by deep sequencing. Plant Biotechnol. J. 11 691–701. 10.1111/pbi.12059 [DOI] [PubMed] [Google Scholar]
- Solano R., Stepanova A., Chao Q., Ecker J. R. (1998). Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE FACTOR1. Genes Dev. 12 3703–3714. 10.1101/gad.12.23.3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhita D., Raghavendra A. S., Kwak J. M., Vavasseur A. (2004). Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol. 134 1536–1545. 10.1104/pp.103.032250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Christiansen K. M., Innes R. W. (2005). Regulation of plant disease resistance, stress responses, cell death, and ethylene signaling in Arabidopsis by the EDR1 protein kinase. Plant Physiol. 138 1018–1026. 10.1104/pp.105.060400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma B. P., Cammue B. P., Thevissen K. (2002). Plant defensins. Planta 216 193–202. 10.1007/s00425-002-0902-6 [DOI] [PubMed] [Google Scholar]
- Thomma B. P., Cammue B. P., Thevissen K. (2003). Mode of action of plant defensins suggests therapeutic potential. Curr. Drug Targets Infect. Dis. 3 1–8. 10.2174/1568005033342000 [DOI] [PubMed] [Google Scholar]
- Torres M. A., Dangl J. L. (2005). Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 8 397–403. 10.1016/j.pbi.2005.05.014 [DOI] [PubMed] [Google Scholar]
- Trethowan R. M., Crossa J., Van G. M., Rajaram S. (2001). Relationships among bread wheat international yield testing locations in dry areas. Crop Sci. 41 1461–1469. 10.2135/cropsci2001.4151461x [DOI] [Google Scholar]
- Tu M., Wang X., Huang L., Guo R., Zhang H., Cai J., et al. (2016). Expression of a grape bZIP transcription factor, VqbZIP39, in transgenic Arabidopsis thaliana confers tolerance of multiple abiotic stresses. Plant Cell Tiss. Org. Cult. 125 537–551. 10.1007/s11240-016-0969-6 [DOI] [Google Scholar]
- van der Weerden N. L., Lay F. T., Anderson M. A. (2008). The plant defensin, NaD1, enters the cytoplasm of Fusarium oxysporum hyphae. J. Biol. Chem. 283 14445–14452. 10.1074/jbc.M709867200 [DOI] [PubMed] [Google Scholar]
- Varshney R. K., Song C., Saxena R. K., Azam S., Yu S., Sharpe A. G., et al. (2013). Draft genome sequence of chickpea (Cicer arietinum L.). Nat. Biotechnol. 31 240–246. 10.1038/nbt.2491 [DOI] [PubMed] [Google Scholar]
- Wang J., Griffiths R., Ying J., McCourt P., Huang Y. (2009). Development of drought-tolerant (Brassica napus L.) through genetic modulation of ABA-mediated stomatal responses. Crop Sci. 49 1539–1554. 10.2135/cropsci2008.09.0568 [DOI] [Google Scholar]
- Xu J., Xing X. J., Tian Y. S., Peng R. H., Xue Y., Zhao W., et al. (2015). Transgenic Arabidopsis plants expressing tomato glutathione S-transferase showed enhanced resistance to salt and drought stress. PLoS One 10:e0136960. 10.1371/journal.pone.0136960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Zhou G., Shimizu H. (2010). Plant responses to drought and rewatering. Plant Signal. Behav. 5 649–654. 10.4161/psb.5.6.11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q., Hou H. M., Singer S. D., Yan X. X., Guo R. R., Wang X. P. (2014). The grape VvMBF1 gene improves drought stress tolerance in transgenic Arabidopsis thaliana. Plant Cell Tiss. Org. Cult. 118 571–582. [Google Scholar]
- Yao P. F., Li C. L., Zhao X. R., Li M. F., Zhao H. X., Guo J. Y., et al. (2017). Overexpression of a tartary buckwheat gene, FtbHLH3, enhances drought/oxidative stress tolerance in transgenic Arabidopsis. Front. Plant Sci. 8:625. 10.3389/fpls.2017.00625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount N. Y., Andrés M. T., Fierro J. F., Yeaman M. R. (2007). The gammacore motif correlates with antimicrobial activity in cysteine-containing kaliocin-1 originating from transferrins. Biochim. Biophys. Acta 1768 2862–2872. 10.1016/j.bbamem.2007.07.024 [DOI] [PubMed] [Google Scholar]
- Yount N. Y., Yeaman M. R. (2004). Multidimensional signatures in antimicrobial peptides. Proc. Natl. Acad. Sci. U.S.A. 101 7363–7368. 10.1073/pnas.0401567101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Liu Y., Wang S., Tao Y., Wang Z., Shu Y., et al. (2016). CarNAC4, a NAC-type chickpea transcription factor conferring enhanced drought and salt stress tolerances in Arabidopsis. Plant Cell Rep. 35 613–627. 10.1007/s00299-015-1907-5 [DOI] [PubMed] [Google Scholar]
- Zhang A., Liu D., Hua C., Yan A., Liu B., Wu M., et al. (2016). The Arabidopsis gene zinc finger protein 3(ZFP3) is involved in salt stress and osmotic stress response. PLoS One 11:e0168367. 10.1371/journal.pone.0168367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. K. (2002). Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 53 247–273. 10.1146/annurev.arplant.53.091401.143329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., Gao B., Tytgat J. (2005). Phylogenetic distribution, functional epitopes and evolution of the CSab superfamily. Cell. Mol. Life Sci. 62 2257–2269. 10.1007/s00018-005-5200-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


