Abstract
The incidence of neurodegenerative disorders and cognitive impairment is increasing. Rising prevalence of age-related medical conditions is associated with a dramatic economic burden; therefore, developing strategies to manage these health concerns is of great public health interest. Nutritionally based interventions have shown promise in treatment of these age-associated conditions. Astaxanthin is a carotenoid with reputed neuroprotective properties in the context of disease and injury, while emerging evidence suggests that astaxanthin may also have additional biological activities relating to neurogenesis and synaptic plasticity. Here, we investigate the potential for astaxanthin to modulate cognitive function and neural plasticity in young and aged mice. We show that feeding astaxanthin to aged mice for 1 month improves performance on several hippocampal-dependent cognitive tasks and increases long-term potentiation. However, we did not observe an alteration in neurogenesis, nor did we observe a change in microglial-associated IBA1 immunostaining. This demonstrates the potential for astaxanthin to modulate neural plasticity and cognitive function in aging.
Keywords: Aging, Cognitive decline, Inflammation, Nutraceutical, Neuroplasticity
Introduction
Aging is an intricate process that encompasses multiple physiological changes, eventually leading to reduced organ homeostasis and resilience. Aging is a prominent predisposing factor for many neurodegenerative disorders, but even normal aging in the absence of pathology can be associated with mild cognitive impairment in a subset of the elderly. The clinical presentation of cognitive dysfunction is highly variable, but is typically described as impaired memory, attention, or loss of executive function (Arvanitakis et al. 2016). The population of people over 65 years of age is increasing and this demographic shift is expected to lead to increased incidence of neurodegenerative disease. Additionally, the rising prevalence of age-related medical issues is presumed to be accompanied by a dramatic economic burden; therefore, developing strategies to manage the deleterious consequences of aging is becoming a significant issue in public health (Johnson et al. 2013; Velkoff 2010).
One promising strategy in the intervention or prevention of the insidious effects of aging is the use of nutritionally based factors. Dietary foods, extracts, and natural compounds have been successful in generally promoting health and preserving cognitive function (Bickford et al. 2017; Bickford et al. 2015; Grimmig et al. 2017b; Miller et al. 2017). Carotenoids have been investigated for their impact on human health; one carotenoid that has gained significant attention recently is the xanthophyll astaxanthin (AXT). AXT has been investigated for a potential therapeutic role in various clinical conditions, notably cardiovascular disease, metabolic syndrome, athletic performance, and cancer (Guerin et al. 2003; Kidd 2011; Yamashita 2013). Interestingly, even though these conditions are diverse, they do share common features of oxidative stress and inflammation, both of which are known to increase with age and contribute to neural dysfunction. AXT has been used therapeutically in models of neurodegenerative disease and neuronal injury. For example, the application of AXT was found to mitigate cytotoxicity from amyloid β fragments in vitro, by interrupting the apoptotic cascade and calcium dysregulation (Chang et al. 2010; Lobos et al. 2016; Wang et al. 2010). AXT attenuated neurotoxicity in an in vivo model of Parkinson’s disease (Grimmig et al. 2018; Grimmig et al. 2017). AXT-treated animals were also protected from the hippocampal damage in an amygdala kindling model of epilepsy (Lu et al. 2015), from aluminum chloride toxicity (Al-Amin et al. 2016a), and in traumatic brain injury (Ji et al. 2017). These observations establish that AXT has a neuroprotective effect against pathological conditions. However, emergent evidence from recent work suggests that AXT may also have the capacity to increase neurogenesis and neural plasticity and may therefore play a role in modulating cognitive function, recently reviewed here: (Galasso et al. 2018; Grimmig et al. 2017a). Yook et al. 2016 demonstrate that AXT treatment can increase stem cell proliferation in the hippocampus and this was associated with improved spatial memory in adult mice (Yook et al. 2016). It has also been shown that consumption of AXT may increase brain-derived neurotrophic factor (BDNF) (Wibrand et al. 2013; Wu et al. 2014), an important growth factor for neural development, growth, and function (Binder and Scharfman 2004).
A few studies have reported that AXT can improve aspects of cognition, although this is most frequently attributed to a neuroprotective mechanisms in animal and cellular models of neurodegeneration and neural injury (Ji et al. 2017; Wibrand et al. 2013; Xu et al. 2015). Given that symptoms of cognitive dysfunction are also seen in some elderly individuals during normal aging, it is possible that AXT treatment may be effective in rescuing the age-related cognitive decline in non-pathological conditions. However, relatively few studies have assessed the effect of AXT on cognitive function in normal, healthy mice, while the impact of AXT on the aged CNS has been largely ignored. Here, we investigate the potential for AXT to modulate cognitive function and synaptic plasticity under physiological conditions in both young and aged mice.
Materials and methods
All procedures were conducted according to the National Institute of Health Guide and Use of Laboratory Animals and the University of South Florida IACUC. Male C57BL/6J were used in these studies at both 3 and 18 months of age. Animals were housed in groups of 4 and maintained in controlled conditions of 21 °C and a 12-h light/dark cycle.
Dietary treatment
Mice were treated with a natural astaxanthin supplement sold as Bioastin®, generously donated to the lab by Cyanotech Corporation. This material was incorporated into a Harlan Teklad rodent diet to achieve a dose of 30 mg/kg body weight. Because Bioastin® is a product that contains a relatively high volume of inactive ingredients such as cellulose and gelatin, we included an additional dietary condition where mice were fed equal concentrations of the Bioastin® product without astaxanthin as a vehicle control. However, we have previously reported that the vehicle condition does not have an effect different from standard chow, and it is unlikely that the inactive ingredients should effect the endpoints in this study. These compounds were delivered to the mice by an experimenter 3× per week in order to ensure the freshness of the food and test compounds. Diets were available ad libitum for 1 month prior to behavioral analysis. In order to confirm that the mice were consuming the enriched diets, research staff regularly recorded the food intake per cage and body weight of the mice and did not observe any significant differences in weight gain over the course of the experiment due to the dietary treatments.
Behavioral testing
Novel object/novel place
This test is a useful tool to evaluate spatial memory and recognition in rodents. Mice were allowed to explore a moderately lit arena (40 cm × 40 cm) for 15 min 24 h prior to training in order to habituate the animals to the test environment. Mice were placed back in the arena the next day and allowed to explore two identical objects placed equidistantly from the center as a 5-min familiarization trial, before being placed into a holding container for a 10-min intertrial interval. This was repeated two more times for a total of 3 training trials. Mice were returned to the home cage for 1 h then returned to the test chamber. The testing phase included one familiar object in the same location from the training session and one novel object that was placed in opposite corners of the chamber. Animal behavior was recorded using ANY-maze software (Stoelting) and test videos were evaluated for time interacting with either the familiar or novel object. Data from the videos was scored by an experimenter blind to treatment conditions.
Fear conditioning
Hippocampal function and memory formation were further assessed by training the mice to associate a 90-dB tone (CS-conditioned stimulus) presented for 30 s and co-terminating with a 0.5-mA foot shock (US-unconditioned stimulus). Twenty-four hours after training, mice were put back in the training chamber without exposure to the tone and shock, and freezing behavior was monitored for 3 min. Freezing was operationally defined by no activity for two consecutive seconds. Freezing behavior was also monitored in an altered context paradigm. Briefly, mice were placed to a novel environment and allowed to explore for 3 min before being exposed to the same 90-dB CS for the remaining 3 min of the test. Animal behavior was recorded and monitored by ANY-maze software (Stoelting). Freezing behavior in response to the conditioned stimulus was evaluated and presented as a percentage of the time freezing relative to the beginning phase of the test without the tone.
Electrophysiology
Mice assigned to electrophysiology experiments were rapidly euthanized immediately before 400 μm sections were prepared by vibratome. Hippocampi were carefully dissected out of the sections and allowed to recover in the recording chamber for an hour before experimentation. Field recordings (fEPSPs) were generated by stimulating in the CA3 Schaffer collaterals, and collected from the stratum radiatum of the CA1 with a glass microelectrode filled with artificial cerebrospinal fluid and impedance between 1 and 5 mΩ. After placement of electrodes and stable responses were observed from each slice, the input/output relationship was established by 30 repeated stimulations from 0 to 15 mV, steadily increasing stepwise by 0.5 mV. The following experiments were conducted using the stimulus determined to evoke a fEPSP of approximately 50% of the max response. Paired-pulse facilitation (PPF) was used to evaluate the presynaptic, short-term responses by delivering two stimulations every 20 ms, where each subsequent pair of stimuli was separated by an additional 20 ms (up to 300 ms); long-term potentiation (LTP) was evoked using a theta-burst protocol, and waveforms were evaluated for the change in slope of the fEPSPs.
Immunohistochemistry
Brains were fixed with 4% paraformaldehyde for 24 h then incubated in a 30% sucrose solution for 3 days to protect the tissue from cryo-damage during sectioning. Forty-micrometer-thick sagittal sections were prepared on a cryostat. Every sixth section was selected for all IHC procedures, this periodicity allows consistent sampling of the structure, although extra sections medial and lateral of the hippocampus were also included to ensure thorough investigation of the entire region. Free-floating sections were blocked in 10% serum before being incubated in primary antibody (KI 67, Jackson Chemicals 1:500; Doublecortin, Santa Cruz 1:200; IBA 1, WAKO Chemicals 1:2500). Primary antibodies were diluted in PBS containing serum and Triton X-100 overnight at 4 °C while the sections were incubated in biotinylated secondary antibody for 60 min at room temperature. Avidin-biotin complex (Vector Labs) was used to increase substrate formation from the precipitation reactions which were developed with diaminobenzidine (Sigma-Aldrich).
Quantification
Estimated population was determined using the optical fractionator probe and unbiased stereological methods (Microbrightfield, Cholchester VT). The hippocampus was sampled with a counting frame and grid size of 150 × 150 μm in order to count all of the positive cells within the structure of interest because aged animals have substantially reduced neurogenesis. IBA 1 was assessed using a digital image analysis program, NearCYTE (nearcyte.org), as previously described (Grimmig et al. 2017; Nash et al. 2015). Briefly, we were able to quantify the area of positive IBA 1 staining by creating high-resolution images with the AxioScan microscope (× 20 objective). Using NearCYTE software, regions of interest were applied to the images, and then compared to a threshold of intensity set by an experimenter blind to treatment conditions. This comparison generates a ratio of the number of pixels that match the user’s defined criteria within the region compared to those that fall below the threshold. We have previously determined that data collected with NearCYTE and expressed as a percent positive area reliably and accurately corroborates the data collected with unbiased stereology and presented as an estimated population of positive cells within a region (Nash et al. 2015).
Data analysis
The data is presented graphically as the group mean and standard error of the mean. Statistical analysis was performed using GraphPad Prism software. Two-way ANOVA was conducted, while the Bonferroni multiple comparisons post hoc tests (unless otherwise specified) were used to further compare differences between groups. Cued and context fear-conditioning data were analyzed using SPSS statistics 23 software for a repeated measure ANOVA.
Results
AXT modulates synaptic plasticity
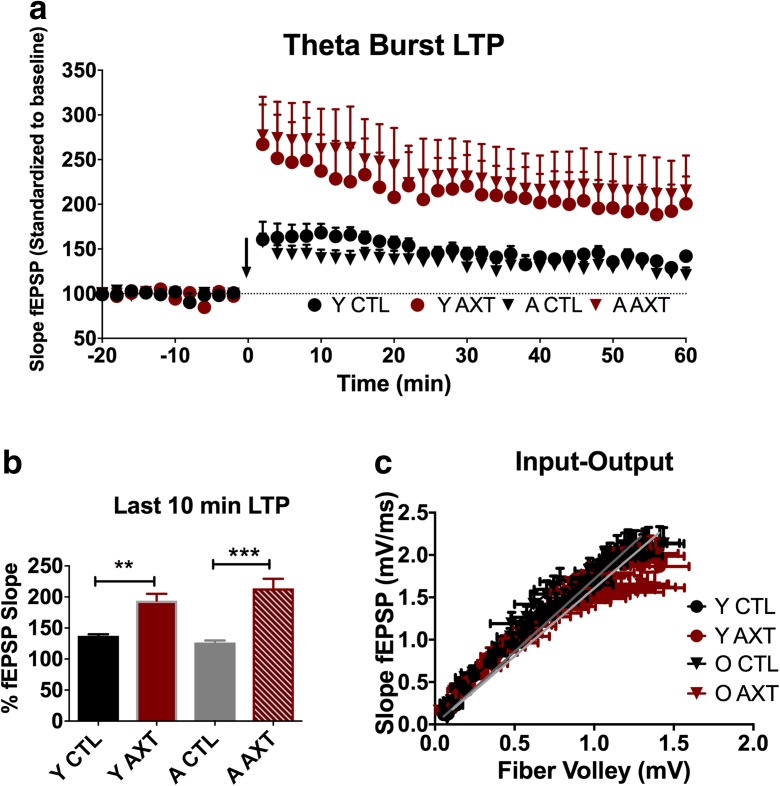
Recent research has generated substantial evidence to establish AXT as a neuroprotective agent, while results from the most current studies are just beginning to come together to suggest the potential for AXT to have a role in promoting or maintaining neural plasticity to modulate cognitive function in healthy animals. Here, we show for the first time that AXT supplementation can modulate synaptic plasticity in both young and aged mice. Animals that consumed a diet enriched with AXT displayed enhanced early-phase LTP induced using a theta-burst stimulation protocol. Both young and aged mice showed increased slopes of the fEPSPs after theta-burst stimulation (Fig. 1a and b). We determined that AXT supplementation alone does not significantly alter synaptic transmission through the calculation of input/output curves comparing all experimental groups (Fig. 1c), nor was any alteration detected for paired-pulse facilitation, suggesting unaffected presynaptic function (data not shown).
Fig. 1.
One month of dietary supplementation of AXT was able to enhance early-phase LTP at the CA1-CA3 synapse in the hippocampus. Both age groups that consumed an AXT-enriched diet demonstrate an increased slope of the fEPSPs after theta-burst stimulation compared to their age-matched controls. Aged animals treated with AXT (A AXT) show enhanced LTP compared to the aged control mice (A CTL). Young ATX-supplemented animals (Y AXT) also show enhanced LTP compared to the young mice on the CTL diet (Y CTL) (a), and this trend remains significantly different during the last 10 min of the experiment (b). One-way ANOVA; F 24.72; **p < 0.01, ***p < 0.001. The observed trends in enhanced early-phase LTP were not due to direct dietary-induced alterations of synaptic transmission, indicated by similar relationships between stimulus intensity and synaptic response (c) or by short-term plasticity mediated at the presynaptic site (data not shown)
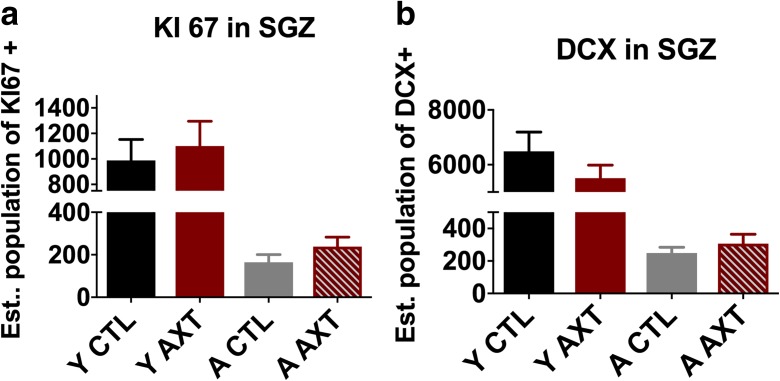
AXT modulation of synaptic plasticity is not explained by increased neurogenesis
Yook et al. 2016 demonstrated that AXT treatment was associated with increased immunolabeling of BrdU within the subgranular layer of the hippocampus in young mice (Yook et al. 2016). This suggests that AXT supplementation may have the capacity to stimulate the proliferation of neural progenitor cells (NPCs), and ultimately neurogenesis in the dentate gyrus. Given this preliminary result, the impact of AXT supplementation on neural growth in both young and aged mice was evaluated. This investigation used unbiased stereological quantification of immunohistochemical labeling of KI 67 (a marker of dividing cells). As expected, we observed an age-related decrease in KI 67-positive cells in the subgranular zone, as it is known that stem cell proliferation slows dramatically with age. We did not observe a significant increase in NPCs of diet-treated animals even though we did observe a slight trend towards increased cell numbers (Fig. 2a). The population of neural progenitors in the dentate gyrus is of interest, in part because of their capacity to give rise to neurons. Stem cell proliferation can support the growth of new neurons that can be recruited into and fortify existing circuits in the brain. Given the recently reported findings that AXT treatment can stimulate NPC proliferation and upregulate BDNF, a molecule important for the structural growth and development of neurons, we also evaluated the number of newly differentiated neurons within the subgranular zone of the hippocampus. As expected, we measured a significant reduction in young neurons labeled with doublecortin (DCX) in the aged animals. However, the marginal increase in KI 67 was not correlated with a rescue in DCX in the AXT-treated aged animals (Fig. 2b). We have determined that AXT supplementation also does not significantly increase the differentiation to neurons, and that neurogenesis does not explain the increased LTP.
Fig. 2.
One month of dietary supplementation of AXT does not significantly alter neurogenesis in the dentate gyrus. AXT treatment does not significantly stimulate the proliferation of neural progenitor cells in the subgranular zone of the hippocampus (a) or increase the differentiation to neuronal lineage in the same region (b). Two-way ANOVA n.s.
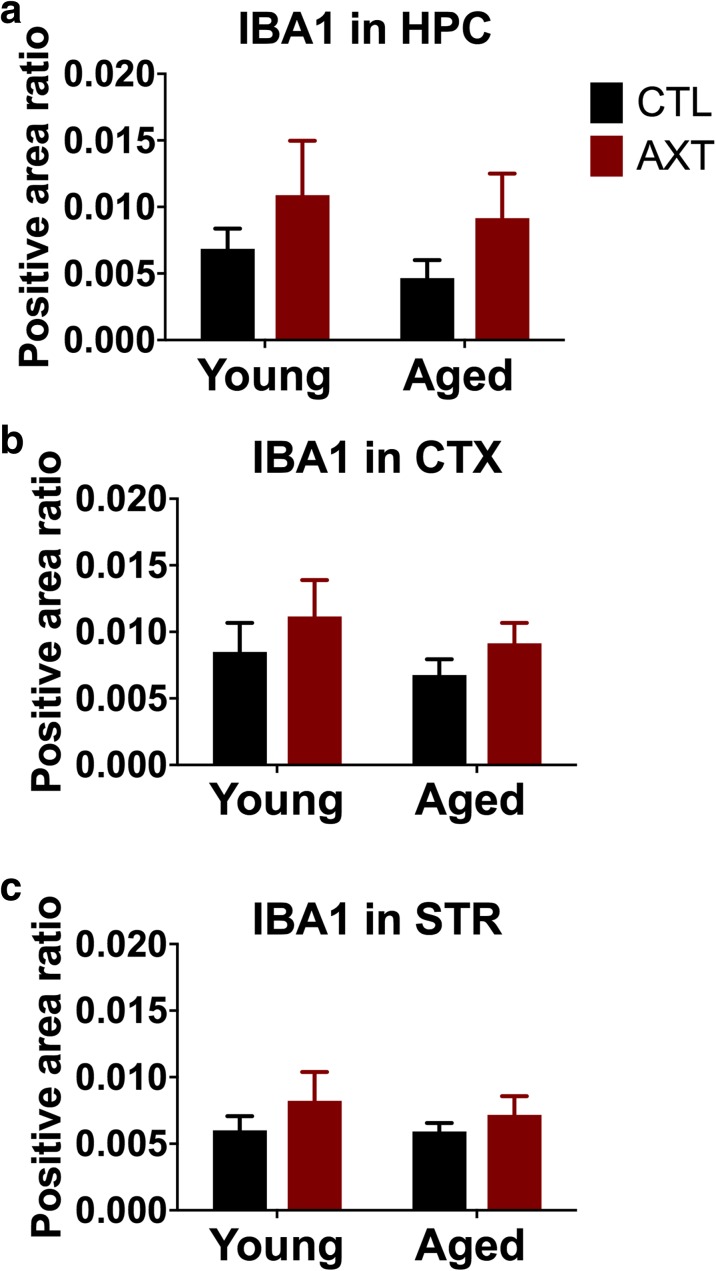
AXT does not significantly alter microglial function
Multiple studies report that AXT treatment can alter the proinflammatory profile both in vivo and in vitro, suggesting that AXT may be directly involved in modulating microglial activity (Al-Amin et al. 2016b; Balietti et al. 2016; Kim et al. 2016; Kim et al. 2010; Park et al. 2013; Zhang et al. 2014). This mechanism of action may underlie the observed neuroprotection in models using CNS insults, given the role of microglial activation in neurodegenerative disease and injury. Microglia are also involved in synaptic pruning in early postnatal periods, and are therefore heavily involved in establishing the neural circuitry in the developing brain, as well as sculpt and refine these connections later on (Paolicelli and Gross 2011; Schafer et al. 2012). Given the relationship between microglial function and neural integrity, microglia morphology and activation were evaluated in this model by assessing alterations in IBA1 expression levels in the brain. All microglia express IBA 1 regardless of activation state, although perturbations that activate microglia are known to correlate with increased expression of IBA 1; therefore, the relationship between IBA 1 expression levels across experimental conditions can elucidate a dietary effect on microglial activity. Digital image analysis of IBA 1 staining indicates that AXT treatment does not significantly alter the expression of IBA 1 in the hippocampus cortex or striatum (Fig. 3a–c). However, there does appear to be general trends for AXT to marginally increase IBA 1 levels in both age groups.
Fig. 3.
Consuming a diet enriched with AXT does not significantly alter the expression of IBA 1 in the hippocampus (a), cortex (b), or striatum (c). Two-way ANOVA n.s.
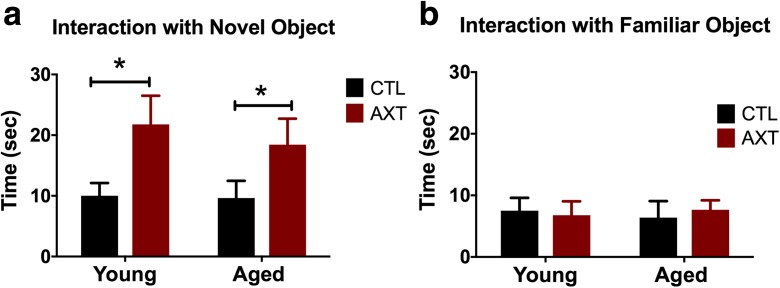
AXT modulates cognitive function
AXT supplementation improves performance in cognitive behavioral tasks. One month of consuming a diet enriched with AXT improved recognition of the novel object in the novel object recognition task. This task exploits a rodent’s innate curiosity and propensity to explore new stimuli in the environment; exploration time will decrease with repeated exposure to an object as animals become more familiar with that object. Test videos were hand scored by an observer blind to experimental conditions and evaluated for time interacting with each object. We observed that animals that consumed the AXT-enriched diet show increased preference for the novel object during the testing phase, as they spent more time interacting with the novel object than the familiar one presented during the training phase (Fig. 4a and b, respectively). This preference for novelty is evident in both the young and aged mice, indicating that AXT supplementation improved short-term recognition memory regardless of age. These trends did not seem to be influenced by locomotor activity around the chamber during the test (data not shown).
Fig. 4.
One month of consuming a diet supplemented with AXT increased time of interaction with the novel object (a). One-way ANOVA, p < 0.001; F 4.436. Two-tailed T tests, *p < 0.0.5. The exploration of the familiar object was similar across all experimental conditions (b). One-way ANOVA n.s. This dietary effect on increased recognition of the novel object is apparent in both young and aged animals, although not due to alterations in locomotor activity around the test chamber (data not shown)
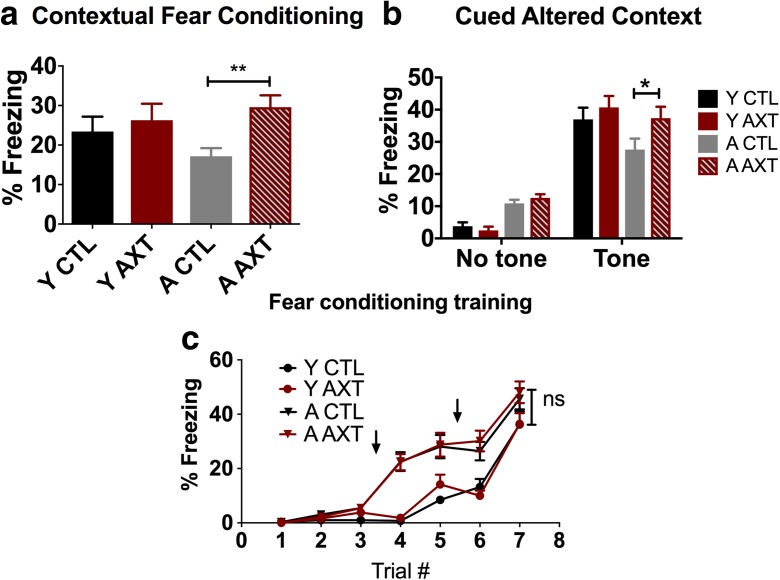
AXT supplementation led to a behavioral improvement in 24 h contextual fear conditioning, a test that indicates hippocampal function and associative memory. Freezing behavior was monitored during the course of this training event and was comparable across treatment conditions by the end of the training (Fig. 5a). Twenty-four hours later, the mice were re-exposed to the training chamber without the tone, and freezing behavior was evaluated as a gauge of associating the environment with the aversive training stimuli. Aged animals that were treated with AXT displayed more freezing behavior to the training environment, indicating that they had a better memory of the aversive training events compared to their age-matched controls. This AXT-mediated improvement for contextual memory was only observed in the aged animals; the young AXT-treated mice demonstrated similar freezing behavior to the context compared those who consumed the standard NIH diet. Interestingly, we also observed a dietary effect in the cued context paradigm. For cued context, animals were placed in novel environment and allowed to explore before being exposed to the training tone representing two distinct 3 min phases of the test. An increase of freezing behavior to the tone indicates an association of the tone and foot shock retained from the training event; the formation of the fear memory is a process also largely mediated by the amygdala (Duvarci and Pare 2014). We observed an overall dietary effect of AXT supplementation increasing the freezing response to the tone, evident in both age groups. However, in agreement with the contextual fear conditioning, the effect was only significant in the aged animals, reiterating that AXT rescues an age-related deficit in fear associative learning but does not augment this cognitive process in the young (Fig. 5b). Aged mice show a heightened freezing response after the first presentation of the tone during fear-conditioning training. This age-related discrepancy normalizes by the end of training as seen during the minute 7 of training in which experimental groups show comparable freezing behavior and there is no significant impact of AXT on acquisition in either age group (Fig. 5c).
Fig. 5.
One month of dietary supplementation of AXT improves the memory retention of aged mice in the 24-h fear-conditioning paradigm, indicated by increased freezing behavior in response to the environment where they previously experienced aversive events (a). Two-way ANOVA, dietary effect, *p < 0.05; F 1, 89. Two-tailed T test, **p < 0.01. Both young and aged mice treated with AXT show an improved performance in the altered context paradigm, demonstrating an increase in freezing behavior to the presentation of the conditioned stimulus compared to the freezing behavior in the first phase of the test before exposure to the tone associated with the foot shock. However, the effect is only significant in the aged animals (b). Repeated measure ANOVA, *p < 0.05. Aged animals show more freezing behavior compared to the young during training. However, by the end of the training, mice show a comparable freezing response and there is no discernable influence of AXT on the learning curve (c). One-way ANOVA of the percent freezing during the last minute of training indicates no significant interaction
Discussion
AXT has steadily gained attention for a potential therapeutic role in neurodegenerative disease. There are numerous reports to establish that AXT treatment is effective at protecting neurons from various CNS insults in models of specific neurodegenerative disorders and neural injury (Al-Amin et al. 2016a; Grimmig et al. 2017; Ji et al. 2017; Zhang et al. 2014), while there are relatively few studies investigating the benefit of AXT in the healthy or aged CNS (Balietti et al. 2016; Park et al. 2013). Here, we investigate the capacity of AXT to modulate cognitive function in young and aged mice. We show for the first time that AXT supplementation enhances synaptic plasticity at the CA1-CA3 synapses of the hippocampus in both young and aged animals. This observation is noteworthy because we expected to only see a rescue of synaptic function in the aged animals, as aging is associated with deteriorating synaptic plasticity. In contrast, young animals do not suffer from impaired synaptic plasticity, yet AXT supplementation was able to improve LTP compared to their age-matched counterparts who consumed the control diet. This suggests that AXT may have a direct mechanism of action in facilitating LTP, in addition to the anti-inflammatory and antioxidant properties that have been the overwhelming focus of studies investigating the therapeutic potential of this molecule in various diseases. It has been documented in that AXT will cross the blood-brain barrier and accumulates in the brain (Choi et al. 2011; Manabe et al. 2018) and that there are no age-related differences in AXT blood levels (Grimmig et al. 2018). Thus, it is possible that the effect we are observing is a direct effect of AXT in the brain. We did not observe that the presence of AXT modulates synaptic transmission on its own, evidenced by no differences in relationships of stimulus intensity (input) to synaptic response (output) across experimental conditions. We also did not observe significant differences in presynaptic activity (PPF). These findings suggest that AXT supplementation is exerting its action on facilitating long-term potentiation in the post synaptic signaling cascades that promote calcium influx and lead to increased insertion of AMPA receptors into the post synaptic membrane. To date, it is unclear if AXT is directly acting on downstream signaling cascades that regulate calcium signaling or AMPA receptor modulation, but there are numerous studies that report changes in signaling pathways that are known to play a role in early-phase LTP (Kim et al. 2010; Wang et al. 2010; Xu et al. 2015). AXT has been shown to increase levels of BDNF (Gite et al. 2018; Ji et al. 2017), and AKT/GSK3ß/CREB signaling (Qiao et al. 2017). These reports suggest potential mechanisms that could contribute to a post synaptic mechanisms being altered by the presence of AXT that is responsible for increasing LTP in our treated animals. As there are many potential mechanisms, future studies could thoroughly examine the post synaptic mechanisms that underlie these observations of enhanced LTP.
It is interesting to note that an AXT-mediated effect of increasing neurogenesis was not observed in this study, as hypothesized. It has been shown that AXT treatment can stimulate cellular division of neural progenitor cells in the dentate gyrus, and that consumption of AXT is associated with increased levels of BDNF. Therefore, it was hypothesized that a diet enriched with AXT would promote the proliferation of neural progenitors and be associated with increased differentiation of doublecortin-positive neurons in this region. However, our stereological estimates revealed that while AXT may slightly increase the number of KI 67-positive progenitor cells in the hippocampus, it did not lead to notable increases in the number of new neurons in the same regions. Our electrophysiological data confirms that, in fact, dietary treatment of AXT is likely not functionally increasing the number of new neurons or active synaptic connections in the hippocampus, as the input/output curves of diet treated and control conditions are similar in both age groups suggesting that there are comparable number of active synapses in the field that contribute to the fEPSP. This observation is notable because previous reports regarding possible mechanisms of action of AXT in studies that assess neuroprotection and neurodegeneration all come together to suggest likely neurogenic, structural changes, even at the level of the synapse as a viable mechanism for AXT to preserve neural integrity and cognitive function (Wibrand et al. 2013; Wu et al. 2014; Yook et al. 2016). However, our data does not support a structural change as the basis for improved performance on the cognitive tasks, as evidenced by the KI 67 and doublecortin staining combined with the input/output relationship assessed by electrophysiology.
Many studies establish anti-inflammatory properties of AXT (Al-Amin et al. 2016b; Balietti et al. 2016; Kim et al. 2016; Kim et al. 2010; Xu et al. 2015; Zhou et al. 2015). Given the role of neuroinflammation in neurodegeneration, and the role for microglia in maintaining synaptic connections, we assessed the impact of AXT on gross microglial morphology by measuring immunoreactivity to IBA 1. IBA 1 is a protein expressed on the cellular surface of all microglia. While this marker will label all microglia, these cells are known to upregulate this protein when they respond to various insults. Therefore, the relative expression of IBA 1 can indicate differences in microglial activity across the diets and ages of animals used in our study. We did not detect significant age-related or diet-related changes in gross microglial morphology. Aging is known to increase microglial activation and is associated with an upregulation of IBA 1 and can relate to gross morphological changes that typically correlate with microglial function (Norden and Godbout 2013), and so this was an unexpected result. However, based on our digital image analysis of immunoreactivity to IBA 1, AXT does not significantly alter the expression of IBA 1 in the hippocampus, cortex, or striatum. It is possible that our animals were very healthy and as yet did not display age-related alterations of microglial activity. However, it is also possible that AXT is modulating microglial function in subtle ways that do not influence overall morphology that would be detected in the image analysis performed in this study. Future studies could look at older mice and could focus on precisely characterizing the impact of AXT on microglial physiology and function.
Our data demonstrates that mice treated with AXT show improved performance in hippocampal-dependent behavioral tasks. We observed an improvement in spatial and recognition memory in the novel object/novel place recognition tests. Furthermore, we observed similar trends in both young and aged mice, suggesting that AXT treatment both rescued the deficit for exploring the novel object in the aged mice, but also improved recognition in the young mice even above and beyond the young counterparts. As stated previously, the capacity for AXT to enhance learning and memory in the young animals is a novel finding that suggests a direct mechanism of action in neural plasticity.
We also observed a behavioral improvement in fear conditioning. As expected, aged animals that consumed the standard rodent diet demonstrate impaired recall of the fear-associated memory of the aversive events they encountered during training. This impairment is evident in the aged mice on the control diet displaying less freezing behavior in the 24-h contextual fear-conditioning test than the young control mice, who typically do not suffer from any learning or memory impairment. In contrast, the AXT-enriched diet seemed to rescue the age-related memory deficit. Aged animals that were fed the AXT-supplemented diet show better retention of the fear-related memory foot shock and freeze more during exposure to training context. The association of the aversive events with the training context is a process mediated by the hippocampus and thus indicates that AXT treatment is associated with improved hippocampal function in aged animals.
We also observed a behavioral improvement in the altered context paradigm, in which the trained animals are exposed to a novel environment and presented with the same training tone that was presented preceding the foot shock during training. In this paradigm, all sensory cues except the training tone are altered, so that the only stimuli the animals are likely to associate with the fear-conditioning training are the specific tone. In these experiments, mice from all experimental conditions show minimal freezing to the altered context before the presentation of the tone. These results were expected, as there should have been no negative associations formed to any of the stimuli presented in the novel context. Interestingly, there is a main effect for the AXT supplementation to increase freezing behavior to the conditioned stimulus in both young and aged mice. However, the effect is only significant in the aged animals, reflecting the AXT-mediated rescue of an age-associated cognitive deficit, similar to the trends observed in fear conditioning. The formation of this fear-associated memory is a process that is mediated by the amygdala and may be indicative of another cognitive domain affected by AXT supplementation.
Thus, our study reveals that AXT treatment may have a direct role in modulating synaptic plasticity in the hippocampus. Here, we show that enhanced synaptic plasticity is a mechanism of action of AXT that underlies some of the improved performance on hippocampal-dependent tasks. Therefore, our data support the premise that AXT has the potential to restore cognitive function in an aged animals.
Abbreviations
- AXT
Astaxanthin
- BDNF
Brain-derived neurotrophic factor
- LTP
Long-term potentiation
- fEPSP
Field excitatory post synaptic potentials
- PPF
Paired-pulse facilitation
- DCX
Doublecortin
- NPC
Neural progenitor cell
- IBA1
Ionized calcium-binding adaptor protein
Funding
Funding was provided from the NIA R01 AG044919 (PCB) and the Veterans Administration I01 BX003421 (PCB).
Disclaimer
The contents of this presentation do not represent the views of the Department of Veterans Affairs or the United States Government.
Conflict of interest
PCB is the co-Founder of Natura Therapeutics, Inc., and has served on the scientific advisory board for Nutrex Hawaii.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Amin MM, et al. Astaxanthin ameliorates aluminum chloride-induced spatial memory impairment and neuronal oxidative stress in mice. Eur J Pharmacol. 2016;777:60–69. doi: 10.1016/j.ejphar.2016.02.062. [DOI] [PubMed] [Google Scholar]
- Al-Amin MM, Sultana R, Sultana S, Rahman MM, Reza HM. Astaxanthin ameliorates prenatal LPS-exposed behavioral deficits and oxidative stress in adult offspring. BMC Neurosci. 2016;17:11. doi: 10.1186/s12868-016-0245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z, Fleischman DA, Arfanakis K, Leurgans SE, Barnes LL, Bennett DA. Association of white matter hyperintensities and gray matter volume with cognition in older individuals without cognitive impairment. Brain Struct Funct. 2016;221:2135–2146. doi: 10.1007/s00429-015-1034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balietti M, Giannubilo SR, Giorgetti B, Solazzi M, Turi A, Casoli T, Ciavattini A, Fattorettia P. The effect of astaxanthin on the aging rat brain: gender-related differences in modulating inflammation. J Sci Food Agric. 2016;96:615–618. doi: 10.1002/jsfa.7131. [DOI] [PubMed] [Google Scholar]
- Bickford PC, Kaneko Y, Grimmig B, Pappas C, Small B, Sanberg CD, Sanberg PR, Tan J, Douglas Shytle R. Nutraceutical intervention reverses the negative effects of blood from aged rats on stem cells. Age (Dordr) 2015;37:103. doi: 10.1007/s11357-015-9840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford PC, Flowers A, Grimmig B. Aging leads to altered microglial function that reduces brain resiliency increasing vulnerability to neurodegenerative diseases. Exp Gerontol. 2017;94:4–8. doi: 10.1016/j.exger.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors (Chur, Switzerland) 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Chen CY, Chiou JY, Peng RY, Peng CH. Astaxanthine secured apoptotic death of PC12 cells induced by beta-amyloid peptide 25-35: its molecular action targets. J Med Food. 2010;13:548–556. doi: 10.1089/jmf.2009.1291. [DOI] [PubMed] [Google Scholar]
- Choi HD, Kang HE, Yang SH, Lee MG, Shin WG. Pharmacokinetics and first-pass metabolism of astaxanthin in rats. Br J Nutr. 2011;105:220–227. doi: 10.1017/S0007114510003454. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasso C, Orefice I, Pellone P, Cirino P, Miele R, Ianora A, Brunet C, Sansone C. On the neuroprotective role of astaxanthin: new perspectives? Marine drugs. 2018;16:247. doi: 10.3390/md16080247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gite S, Ross RP, Kirke D, Guihéneuf F, Aussant J, Stengel DB, Dinan TG, Cryan JF, Stanton C (2018) Nutraceuticals to promote neuronal plasticity in response to corticosterone-induced stress in human neuroblastoma cells. Nutr Neurosci:1–18. 10.1080/1028415X.2017.1418728 [DOI] [PubMed]
- Grimmig BA, Daly L, Hudson C, Nash K, Bickford PC. Astaxanthin attenuates neurotoxicity in a mouse model of PD. Funct Foods Health Dis. 2017;7:562–567. doi: 10.31989/ffhd.v7i8.352. [DOI] [Google Scholar]
- Grimmig B, Kim S-H, Nash K, Bickford PC, Douglas Shytle R. Neuroprotective mechanisms of astaxanthin: a potential therapeutic role in preserving cognitive function in age and neurodegeneration. GeroScience. 2017;39:19–32. doi: 10.1007/s11357-017-9958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmig B, Kim SH, Nash K, Bickford PC, Douglas Shytle R. Neuroprotective mechanisms of astaxanthin: a potential therapeutic role in preserving cognitive function in age and neurodegeneration. Geroscience. 2017;39:19–32. doi: 10.1007/s11357-017-9958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmig B, Daly L, Subbarayan M, Hudson C, Williamson R, Nash K, Bickford PC. Astaxanthin is neuroprotective in an aged mouse model of Parkinson’s disease. Oncotarget. 2018;9:10388–10401. doi: 10.18632/oncotarget.23737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin M, Huntley ME, Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. 2003;21:210–216. doi: 10.1016/S0167-7799(03)00078-7. [DOI] [PubMed] [Google Scholar]
- Ji X, Peng D, Zhang Y, Zhang J, Wang Y, Gao Y, Lu N, Tang P. Astaxanthin improves cognitive performance in mice following mild traumatic brain injury. Brain Res. 2017;1659:88–95. doi: 10.1016/j.brainres.2016.12.031. [DOI] [PubMed] [Google Scholar]
- Johnson EJ, et al. Relationship between serum and brain carotenoids, alpha-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia Centenarian Study. J Aging Res. 2013;2013:951786. doi: 10.1155/2013/951786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern Med Rev. 2011;16:355–364. [PubMed] [Google Scholar]
- Kim YH, Koh HK, Kim DS. Down-regulation of IL-6 production by astaxanthin via ERK-, MSK-, and NF-kappaB-mediated signals in activated microglia. Int Immunopharmacol. 2010;10:1560–1572. doi: 10.1016/j.intimp.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Kim B, Farruggia C, Ku CS, Pham TX, Yang Y, Bae M, Wegner CJ, Farrell NJ, Harness E, Park YK, Koo SI, Lee JY. Astaxanthin inhibits inflammation and fibrosis in the liver and adipose tissue of mouse models of diet-induced obesity and nonalcoholic steatohepatitis. J Nutr Biochem. 2016;43:27–35. doi: 10.1016/j.jnutbio.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Lobos P, Bruna B, Cordova A, Barattini P, Galáz JL, Adasme T, Hidalgo C, Muñoz P, Paula-Lima A. Astaxanthin protects primary hippocampal neurons against noxious effects of Aβ-oligomers. Neural Plasticity. 2016;2016:13. doi: 10.1155/2016/3456783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Xie T, He XX, Mao ZF, Jia LJ, Wang WP, Zhen JL, Liu LM. Astaxanthin rescues neuron loss and attenuates oxidative stress induced by amygdala kindling in adult rat hippocampus. Neurosci Lett. 2015;597:49–53. doi: 10.1016/j.neulet.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Manabe Y, Komatsu T, Seki S, Sugawara T. Dietary astaxanthin can accumulate in the brain of rats. Biosci Biotechnol Biochem. 2018;82:1433–1436. doi: 10.1080/09168451.2018.1459467. [DOI] [PubMed] [Google Scholar]
- Miller MG, Thangthaeng N, Poulose SM, Shukitt-Hale B. Role of fruits, nuts, and vegetables in maintaining cognitive health. Exp Gerontol. 2017;94:24–28. doi: 10.1016/j.exger.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Nash KR, Moran P, Finneran DJ, Hudson C, Robinson J, Morgan D, Bickford PC. Fractalkine over expression suppresses alpha-synuclein-mediated neurodegeneration. Mol Ther. 2015;23:17–23. doi: 10.1038/mt.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Godbout JP. Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. 2013;39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Gross CT. Microglia in development: linking brain wiring to brain environment. Neuron Glia Biol. 2011;7:77–83. doi: 10.1017/S1740925X12000105. [DOI] [PubMed] [Google Scholar]
- Park JS, Mathison BD, Hayek MG, Zhang J, Reinhart GA, Chew BP. Astaxanthin modulates age-associated mitochondrial dysfunction in healthy dogs. J Anim Sci. 2013;91:268–275. doi: 10.2527/jas.2012-5341. [DOI] [PubMed] [Google Scholar]
- Qiao J, Rong L, Wang Z, Zhang M. Involvement of Akt/GSK3beta/CREB signaling pathway on chronic omethoate induced depressive-like behavior and improvement effects of combined lithium chloride and astaxanthin treatment. Neurosci Lett. 2017;649:55–61. doi: 10.1016/j.neulet.2017.03.048. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velkoff GVaV (2010) The next four decades The older population in the United States: 2010 to 2050 Current population reports
- Wang HQ, Sun XB, Xu YX, Zhao H, Zhu QY, Zhu CQ. Astaxanthin upregulates heme oxygenase-1 expression through ERK1/2 pathway and its protective effect against beta-amyloid-induced cytotoxicity in SH-SY5Y cells. Brain Res. 2010;1360:159–167. doi: 10.1016/j.brainres.2010.08.100. [DOI] [PubMed] [Google Scholar]
- Wibrand K, Berge K, Messaoudi M, Duffaud A, Panja D, Bramham CR, Burri L. Enhanced cognitive function and antidepressant-like effects after krill oil supplementation in rats. Lipids Health Dis. 2013;12:6. doi: 10.1186/1476-511X-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Wang X, Xiang Q, Meng X, Peng Y, du N, Liu Z, Sun Q, Wang C, Liu X. Astaxanthin alleviates brain aging in rats by attenuating oxidative stress and increasing BDNF levels. Food Funct. 2014;5:158–166. doi: 10.1039/C3FO60400D. [DOI] [PubMed] [Google Scholar]
- Xu L, Zhu J, Yin W, Ding X. Astaxanthin improves cognitive deficits from oxidative stress, nitric oxide synthase and inflammation through upregulation of PI3K/Akt in diabetes rat. Int J Clin Exp Pathol. 2015;8:6083–6094. [PMC free article] [PubMed] [Google Scholar]
- Yamashita E. Astaxanthin as a medical food. Funct Foods Health Dis. 2013;3:254–258. doi: 10.31989/ffhd.v3i7.49. [DOI] [Google Scholar]
- Yook JS, Okamoto M, Rakwal R, Shibato J, Lee MC, Matsui T, Chang H, Cho JY, Soya H. Astaxanthin supplementation enhances adult hippocampal neurogenesis and spatial memory in mice. Mol Nutr Food Res. 2016;60:589–599. doi: 10.1002/mnfr.201500634. [DOI] [PubMed] [Google Scholar]
- Zhang XS, Zhang X, Zhou ML, Zhou XM, Li N, Li W, Cong ZX, Sun Q, Zhuang Z, Wang CX, Shi JX. Amelioration of oxidative stress and protection against early brain injury by astaxanthin after experimental subarachnoid hemorrhage. J Neurosurg. 2014;121:42–54. doi: 10.3171/2014.2.JNS13730. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhang F, Hu X, Chen J, Wen X, Sun Y, Liu Y, Tang R, Zheng K, Song Y. Inhibition of inflammation by astaxanthin alleviates cognition deficits in diabetic mice. Physiol Behav. 2015;151:412–420. doi: 10.1016/j.physbeh.2015.08.015. [DOI] [PubMed] [Google Scholar]