Abstract
The present work was conducted to evaluate the quality of milk (fresh/acidic/neutralized) on the physico-chemical, textural and fatty acid profile of khoa prepared from buffalo milk and stored in poly-alu-poly laminates for 30 °C/7 days and 5 °C/21 days, respectively. The degree of deterioration of common quality parameters was rapid during storage at 30 °C as compared to storage at 5 °C. Khoa stored at 30 °C showed greater variation in various physico-chemical and textural parameters as compared to khoa stored at 5 °C. Acidity, ash, tyrosine value, furosine, HMF, FFA, peroxide value, TBA value, butyric acid and stearic acid showed an increasing trend whereas, decrease in pH and oleic acid was observed as storage period progressed. Noticeable changes were observed in textural attributes of khoa during storage. However, the SDS–PAGE pattern of caseins from different types of khoa showed almost negligible deviation during storage.
Keywords: Khoa, Neutralization, Tyrosine, Furosine, SDS–PAGE
Introduction
Khoa is a very famous heat desiccated milk product which serves as base material for several Indian delicacies such as burfi, peda, kalakand, gulabjamun etc. (Amruthakala 2012; Choudhary et al. 2016; 2017a, b). It is prepared by continuous heating, stirring and scraping in an open pan until a semi solid consistency is observed. Londhe et al. (2012) reported that almost 7% of total milk share in India is used for khoa preparation. During storage, milk based sweets undergo various physical, biochemical and microbiological changes which makes them unacceptable for consumption. Storage conditions (time and temperature) along with packing material affect the nutritional quality and physico-chemical attributes of khoa at ambient and high temperature storage. Considerable deterioration in quality of khoa was observed on storage under high temperature as compared to storage under low temperatures (Acharya and Agrawal 2010). Khoa is a fat and protein rich product with quite high water activity which makes it more prone to proteolytic and oxidative changes adversely affect its storage stability. India is a tropical country and due to its comparatively higher atmospheric temperature, development of acidity is one of the most common reasons of milk getting sour easily (Choudhary et al. 2018). Such milk is not suitable for thermal processing (Choudhary et al. 2017a, b). Therefore, milk handlers intentionally add neutralizer to such milk. In India, surplus milk is converted to various traditional milk products primarily as a means of preservation (Londhe et al. 2012). As marketing of khoa is primarily carried out by the unorganized sector wherein lack of strict quality parameters is prescribed to khoa, hence adulteration is a common norm to gain easy profit (Amruthakala 2012). Most of the published reports on khoa have focused either on standardization of manufacturing techniques, preservation or improvement in keeping quality of khoa during storage since it is a topmost consumed dairy product in India (Acharya and Agrawal 2010). Rajorhia et al. (1990) reported the effect of buffalo milk quality on compositional and physico-chemical parameters of khoa, however, relevant information was lacking on the effect of acidic and neutralized milk on physico-chemical parameters of resulting khoa during storage. Therefore, study was designed to generate pertinent information on the changes in physico-chemical parameters of resulting khoa during storage at 30 °C and 5 °C.
Materials and methods
Materials
Sodium bicarbonate, Coomassie brilliant blue, sodium methoxide, Folin–Ciocalteau, mercaptoethanol, HCl, 5-hydroxy methyl furfural, oxalic acid, dichloromethane and were procured from Sigma Aldrich, St. Louis, Missouri, USA. Furosine was purchased from polypeptide laboratory, Strasbourg, France. Water, methanol, O-phosphoric acid of HPLC grade, cyclohexanone were purchased from Thermo Fisher Scientific India Pvt. Ltd., Delhi, India.
Preparation of khoa
Khoa was prepared according to the method of De (2004). Fresh buffalo milk was obtained from Experimental dairy of the ICAR-National Dairy Research Institute, Karnal. For acidic milk, fresh milk was allowed to develop acidity up to 0.18% LA in an incubator (Narang Scientific Works Pvt. Limited, Delhi, India) by checking acidity hourly. Keeping aside the representative of acidic milk sample for khoa preparation, developed acidity was neutralized with calculated amount of sodium bicarbonate at required rate to adjust the acidity to 0.14% LA (~ acidity of fresh milk). The fresh, acidic and neutralized buffalo milk samples were used to prepare khoa. Polythene-aluminium foil-polythene laminates of 100 µm thickness was used for packaging of khoa at two different storage conditions, i.e. 30 °C/7 days and 5 °C/21 days. Packaging material was treated with UV light for using laminar air flow before being used. All the trials were carried out in triplicates.
Compositional analysis
Fat, lactose, ash, total solids, pH and acidity of khoa was determined by the methods as described in Indian Standard (IS: SP: 18 part XI 1981). Moisture and protein content of khoa was determined by Indian Standard (IS: 16072 2012 and AOAC 1970), respectively. The gross composition of three types of khoa prepared from buffalo is presented in Table 1.
Table 1.
Gross composition of fresh, acidic and neutralized buffalo milk khoa.
(Choudhary et al. 2016)
| Parameters | Fat (%) | Protein (%) | Lactose (%) | Moisture (%) | Ash (%) | Total solids (%) | pH | Acidity (% LA) |
|---|---|---|---|---|---|---|---|---|
| Sample | ||||||||
| FBMK | 35.13 ± 1.06A | 17.56 ± 0.17A | 20.59 ± 0.78B | 23.94 ± 0.41B | 2.74 ± 0.025B | 76.06 ± 0.41B | 6.48 ± 0.003B | 0.57 ± 0.003B |
| ABMK | 35.50 ± 1.53A | 17.70 ± 0.14A | 17.66 ± 1.02A | 22.43 ± 0.55A | 2.64 ± 0.034A | 77.57 ± 0.55C | 6.38 ± 0.005A | 0.62 ± 0.002C |
| NBMK | 35.41 ± 1.35A | 17.76 ± 0.29A | 17.03 ± 1.32A | 25.44 ± 0.70C | 2.85 ± 0.040C | 74.56 ± 0.70A | 6.68 ± 0.005C | 0.55 ± 0.005A |
Data are presented as mean ± SEM (n = 3)
FBMK fresh buffalo milk Khoa, ABMK acidic buffalo milk Khoa, NBMK neutralized buffalo milk Khoa
A−CMeans within column with different upper case superscript are significantly different (p < 0.05) from each other
Physico-chemical analysis during storage
Acidity, pH and ash
pH, acidity and ash of khoa was determined by the methods described in Indian Standard (IS: SP: 18 part XI 1981).
Tyrosine value
Tyrosine content was evaluated in the khoa filtrate using the enzyme trypsin according to the method of Lowry et al. (1951), followed by Choudhary et al. (2016). The absorbance of the developed colour was read at 750 nm in spectrophotometer. Standard curve of tyrosine concentration was drawn for a range from 10 to 130 µg tyrosine per mL and results were represented as µg/g of khoa.
SDS–PAGE
SDS–PAGE was performed according to the method described by Simon (2004) on 12.5% gel concentration. Casein samples were prepared from khoa samples by following the method of Gupta and Ganguli (1965). 20 µl of sample was injected in the wells of the gel.
Hydroxy methyl furfural (HMF)
HMF content in khoa was evaluated according to the spectrophotometric method of Keeney and Bassette (1958) with slight modification as described by Choudhary et al. (2017b). Optical density was measured at 443 nm and a standard curve was prepared. Standard curve of HMF was drawn for a range from 0.5 to 50 µM/L and results were represented as micromoles HMF.
Furosine
Furosine in khoa was evaluated by the method of Choudhary et al. (2017b). Standard curve of furosine was drawn from a range of 0.5–200 mg furosine/L and the final results were represented as mg furosine/100 g of protein.
Peroxide value
Peroxide value in khoa was evaluated by the method as described in IS: 3508 (1966). Final results were represented as milliequivalent of oxygen per kg of fat.
FFA value
Free fatty acid of the khoa was evaluated by the method as described in IS: 3508 (1966). The FFA content was represented as percent oleic acid.
TBA value
The extent of secondary oxidation of fat in Khoa was measured in terms of TBA value according to the method of Choudhary et al. (2016). TBA value was represented as absorbance at 532 nm.
Fatty acid profile
Fatty acid profile of khoa was determined by the method of DeMan (1961) as modified by Choudhary et al. (2016) in packed glass column (10% DEGS, 1.85 m length and 3.10 mm inner diameter) using GC-NETEL equipped with flame ionization detector and temperature control modules.
Texture profile analysis
Texture profile analysis (TPA) was conducted as per the method of Choudhary et al. (2017a) using a texture analyzer TAXT21 (Stable Micro Systems, Godalming, Surrey, UK) fitted with a 25 kg load cell and was calibrated with 5 kg standard dead weight prior to use. A minimum of three replicates per sample was run.
Statistical analysis
Means and standard error mean (SEM) were calculated using Microsoft excel (2007, Microsoft Corp., Redmond, WA). Significant difference between values was verified by one way or two way analysis of variance and comparison between means was made by critical difference value (Snedecor and Cochran 1994).
Results and discussion
Physico-chemical analysis of khoa during storage
The average fat, protein, lactose, total solids, moisture and ash content of fresh, acidic and neutralized buffalo milk khoa are presented in Table 1. Fresh, acidic and neutralized buffalo milk khoa sample did not show significant difference (p > 0.05) in fat and protein content (Table 1), whereas lactose content of fresh buffalo milk khoa samples differed significantly (p < 0.05) from their respective khoa samples from acidic and neutralized milk. This might be due to conversion of some lactose into lactic acid during development of acidity in milk prior to acidic and neutralized milk khoa preparation. Moisture and ash content of fresh, acidic and neutralized khoa samples differed significantly (p < 0.05) indicating higher moisture and ash content in neutralized samples which might be due to the presence of neutralizer, which increased the moisture retention and ash content in khoa samples from neutralized milk while lower moisture content in khoa samples from acidic milk could be explained by the fact that decrease in pH resulted in lower moisture and yield (Sachdeva and Singh 1988).
During storage of khoa samples at 30 °C/7 days (Fig. 1a-i) and 5 °C/21 days (Fig. 1b-i) a significant decrease (p < 0.05) in pH and an increase (p < 0.05) in acidity (Fig. 1a, b-ii) was observed. These differences in acidity and pH might be due to microbial growth in samples during storage as the nutritive value and water activity (0.96) of khoa is very high which is favorable to microbial growth. Similar observations were reported by Acharya and Agarwal (2010) in khoa packed in low density polythene (LDPE) and stored at 25 °C for 30 days. Gawande et al. (2015) and Arora et al. (2007) also reported similar observations in kalakand and burfi (khoa based sweetmeats). During storage, ash content of khoa (Fig. 1a-iii, B-iii) increased significantly (p < 0.05). This might be due to the release of moisture during storage. Moisture is significantly affected by storage temperature and period. Therefore with increase in storage period, moisture decreased and ash increased since moisture plays an important role in determination of ash content. Gaikwad and Hembade (2013) also reported an increase (p < 0.05) in ash content of Ujaini basundi (a heat desiccated milk product) with corresponding decrease in moisture content during storage. Results correlate well with those of Rajorhia et al. (1990) who also reported high pH and ash in neutralized buffalo milk khoa and higher acidity in acidic buffalo milk khoa.
Fig. 1.
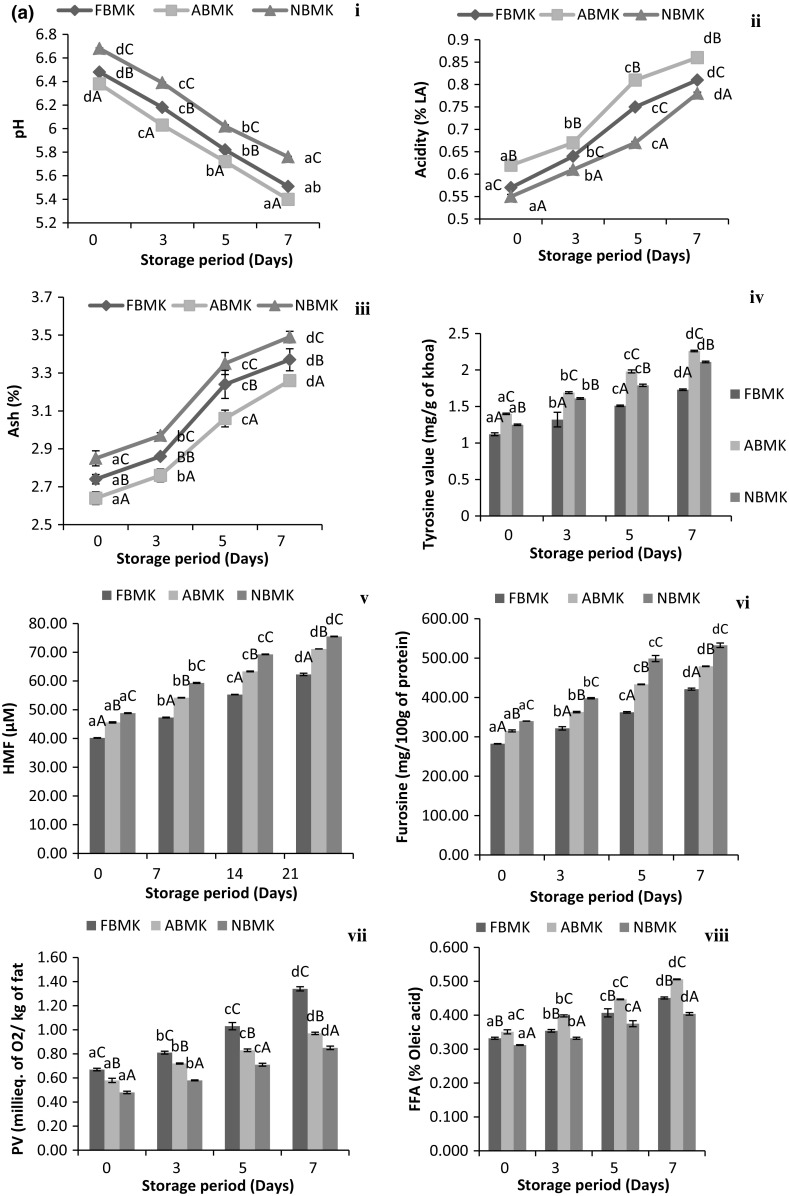
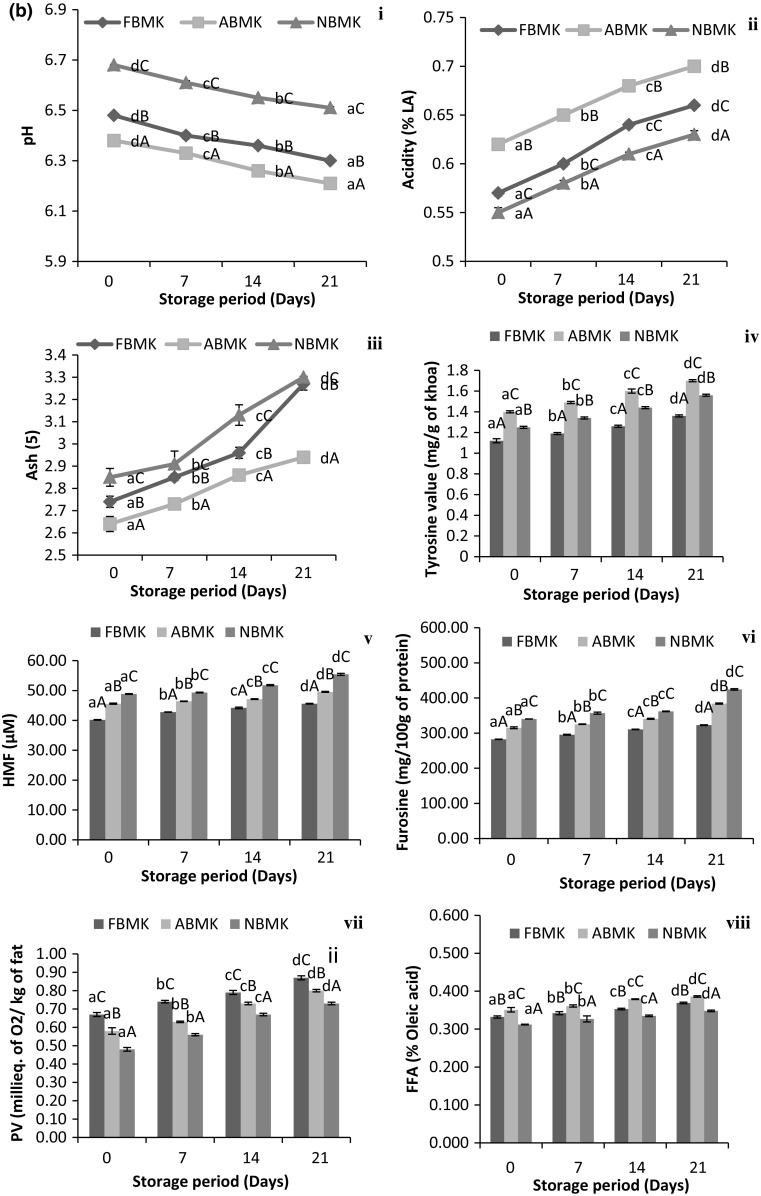
Physicochemical properties of khoa stored at a 30 °C/7 days. b 5 °C/21 days. (i) pH. (ii) Acidity. (iii) Ash. (iv) Tyrosine value. (v) HMF. (vi) Furosine. (vii) Peroxide value. (viii) FFA. Data are presented as mean ± SEM (n = 3). a–cDifferent lowercase letters denote significant difference (p < 0.05) between groups (No. of days (0, 7th, 14th and 21st) of storage at refrigerated temperature (5 °C/21 days). Error bars show the variations of three determinations in terms of standard error of mean. A–CDifferent uppercase letters denote significant difference (p < 0.05) across subgroups [(FBMK, ABMK and NBMK)]. FBMK fresh buffalo milk Khoa, ABMK acidic buffalo milk Khoa, NBMK neutralized buffalo milk Khoa
HMF and Furosine are the browning indicators in milk and milk products and exhibited significant increase (p < 0.05) in all the khoa samples and during storage (Fig. 1a, b-v, vi). However, the extent of increase was more pronounced (p < 0.05) during storage at 30 °C (Fig. 1a-v, vi) as compared to 5 °C (Fig. 1b-v, vi). HMF and furosine were significantly lower (p < 0.05) in fresh milk khoa followed by acidic and neutralized milk khoa. Natural souring of milk at 30 °C enhanced the fermentation of lactose by lactic acid bacteria converting lactose into glucose, galactose and lactic acid. Glucose and galactose, being monosaccharides are more prone to maillard browning (Choudhary et al. 2017b). This could be the reason for higher HMF and furosine in acidic milk khoa, whereas, presence of neutralizers in milk resulted in shifting of pH towards alkaline values which generally favored maillard reaction (Choudhary et al. 2017b). Hence, the added effect of presence of monosaccharides (glucose and galactose) and neutralizers in milk resulted in higher concentration of HMF and furosine in khoa samples prepared from neutralized milk. Our findings were supported by Guerra-Hernández et al. (2002) who also reported an increase (p < 0.05) in HMF and furosine content in powdered and liquid infant formula during storage. They observed that the extent of increase was higher when stored at 55 °C than at 20 °C for 90 days. However, the furosine content in infant formula (701 mg/100 g of protein) was comparatively higher (p < 0.05) than khoa samples (in the present study) due to the intense maillard reaction occurring in these low moisture foods. Khoa has comparatively high water activity (0.96) which probably resulted in lower furosine content as compared to powdered infant formula (aw 0.2) (Gurtler and Beuchat 2007).
Peroxide value (PV) is a measure of primary oxidative deterioration of milk fat whereas Free Fatty Acids (FFA) in khoa is an indicator of the lipolytic activity due to the presence of microorganisms. The PV and FFA content of three types of khoa differed significantly (p < 0.05) (Fig. 1a-vii, viii). It was evident from the results that neutralized samples showed lower (p < 0.05) peroxide value than acidic and fresh samples. Added neutralizer in milk resulted in intense maillard reaction in khoa. Maillard reaction is a type of non-enzymatic browning reaction between reducing sugars and amino acids, forming various chemical complexes/compounds known as maillard reaction products (MRPs). These MRPs are known to impart antioxidant activity in food products (Maillard et al. 2007). Intense maillard reaction in neutralized samples resulted in production of MRPs such as dicarbonyls which probably prevented the formation of peroxides during oxidation (Choudhary et al. 2016).
During storage at 30 °C/7 days (Fig. 1a-vii) as well as refrigerated temperature storage at 5 °C/21 days (Fig. 1b-vii) significant increase (p < 0.05) in peroxide value was observed in all the khoa samples, however, the extent of oxidation was more pronounced at 30 °C as compared to 5 °C. Similarly, the rate of autoxidation was rapid in the lal peda samples (a khoa based sweet) stored at higher temperature (37 °C) as compared to samples stored at 4 °C (Jha et al. 2014). Similar trend in peroxide value was also observed by Acharya and Agarwal (2010) for khoa samples stored at 25 °C in polythene material and Kumar et al. (1975) for khoa samples stored in parchment paper for 5-10 days at 37 °C.
FFA was significantly higher (p < 0.05) in khoa samples prepared from acidic milk (Fig. 1a-viii). This could be due to higher microbial count in acidic milk (due to induced acidity) used for the preparation of khoa. In case of neutralized milk; acidic milk was neutralized using sodium bicarbonate which formed sodium salts of fatty acids. FFA has a tendency to form suitable salts with bases which serve as the basis for determination of FFA content. These particular salts were highly water soluble and were not extracted with organic solvents during solvent extraction process. This probably resulted in lower titration value of alkali (0.1 N NaOH) used during titration.
Significant increase (p < 0.05) was observed in FFA content of khoa stored at 30 °C (Fig. 1a-viii) and 5 °C (Fig. 1b-viii). Continuous stirring and scrapping of milk during khoa preparation, resulted in rupturing of fat cells eventually releasing free fat in the final product. This free fat in addition to the inherent moisture in khoa initiated the lipolysis, which is very much affected by storage temperature. Therefore, the extent of lipolysis was higher at room temperature storage (30 °C) as compared to refrigerated storage at 5 °C. Indigenous milk lipase is inactivated by heat treatment during the manufacture of khoa. It is mainly microbial and fungal lipase which causes an increase of FFA in dairy products during storage (Hanus et al. 2008). Hydrolysis of milk fat was catalyzed by lipase resulting in formation of glycosides and FFA. This led to an increase in FFA during storage. Similar trend of increase in FFA was observed by Kumar et al. (2010) in khoa during storage at different temperature and Jha et al. (2014) in lal peda (a Khoa based sweet) stored at 37 °C and 4 °C.
The extent of secondary oxidation in fat rich dairy products is characterized by TBA value. Non-significant differences (p > 0.05) was observed in the TBA values of all three types of khoa samples (Table 2a, b). During storage of khoa samples at 30 °C (Table 2a), non-significant difference (p > 0.05) was observed in TBA value up to the 3rd day of storage and from 5th day onwards all the khoa samples exhibited significant increase (p < 0.05) in TBA values. During refrigerated storage (Table 2b) non-significant increase (p > 0.05) was observed in all the three types of buffalo milk khoa samples up to the 14th day of storage. However, on the 21st day a noteworthy increase (p < 0.05) was observed in TBA vale of stored khoa samples. Kumar et al. (2010) reported a similar trend in khoa samples packed and stored at 32 °C and 11 °C/180 days under vacuum. Khan et al. (2008) observed a similar trend in groundnut burfi stored at ambient temperature (15–34 °C) for 6 months in polypropylene pouches, Jha et al. (2014) also reported similar observations for lal peda (a khoa based delicacy) stored at 37 °C/9 days and 4 °C/31 days in paper board boxes.
Table 2.
Changes in TBA value of khoa stored at (A) 30 °C/7 days and, (B) 5 °C/21 days
| Periods of analysis | TBA value (O. D.) | |||
|---|---|---|---|---|
| 0 day | 3rd day | 5th day | 7th day | |
| (i) (A) Sample | ||||
| FBMK | 0.007 ± 0.0005aA | 0.011 ± 0.0005aA | 0.016 ± 0.0006bA | 0.021 ± 0.0003bA |
| ABMK | 0.006 ± 0.0005aA | 0.010 ± 0.0003aA | 0.019 ± 0.0003bA | 0.023 ± 0.0005bA |
| NBMK | 0.006 ± 0.0003aA | 0.011 ± 0.0007aA | 0.018 ± 0.0003bA | 0.024 ± 0.0003bA |
| Periods of analysis | TBA value (O. D) | |||
|---|---|---|---|---|
| 0 day | 7th day | 14th day | 21st day | |
| (ii) (B) Sample | ||||
| FBMK | 0.003 ± 0.0005aA | 0.007 ± 0.0005aA | 0.013 ± 0.0006aA | 0.019 ± 0.0004bA |
| ABMK | 0.006 ± 0.0005aA | 0.009 ± 0.0003aA | 0.015 ± 0.0007aA | 0.021 ± 0.0006bA |
| NBMK | 0.005 ± 0.0003aA | 0.007 ± 0.0007aA | 0.016 ± 0.0003aA | 0.020 ± 0.0003bA |
Data are presented as mean ± SEM (n = 3)
FBMK fresh buffalo milk Khoa, ABMK acidic buffalo milk Khoa, NBMK neutralized buffalo milk Khoa
A−CMeans within column with different upper case superscript are significantly different (p < 0.05) from each other
a-bMeans within row with different lower case superscript are significantly different (p < 0.05)
Proteolytic changes during storage
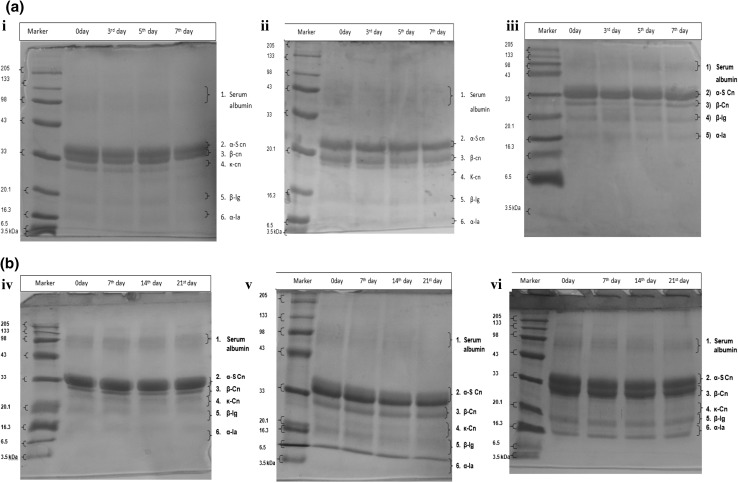
The extent of proteolysis in khoa was measured by SDS–PAGE. The SDS–PAGE gels (Fig. 2) revealed that casein (CN) isolated from the three different types of khoa samples stored at 30 °C and 5 °C, fractionated into casein and whey protein fractions. Casein protein was majorly fractioned as αs-casein (αs-CN), β-casein (β-CN) and κ-casein (κ-CN) while whey protein fractions i.e. serum proteins, beta lactoglobulin (β-lg) and alpha-lactalbumin (α-la) might have appeared in the SDS–PAGE gels from the heat induced interaction between CN and whey proteins in milk during preparation of khoa.
Fig. 2.
SDS–PAGE of fresh, acidic and neutralized buffalo milk khoa during storage at a 30 °C/7 days, b 5 °C/21 days. Where, (i) FBMK stored at 30 °C/7 days. (ii) ABMK stored at 30 °C/7 days. (iii) NBMK stored at 30 °C/7 days. (iv) FBMK stored at 5 °C/21 days. (v) ABMK stored at 5 °C/21 days. (vii) NBMK stored at 5 °C/21 days. FBMK fresh buffalo milk Khoa, ABMK acidic buffalo milk Khoa, NBMK neutralized buffalo milk Khoa
No evident degradation was observed in the SDS–PAGE gels (performed for khoa samples stored at 30 °C/7 days) for serum albumin, α- and β-casein, β-lg and α-la fractions of fresh buffalo milk khoa casein. However, in κ- casein no visible degradation was observed up to 3rd day of storage (Fig. 2a-i) and from 5th day onwards an additional faint band appeared below the band of κ- casein up to 7th day indicating degradation of κ-casein, which might be due to proteolysis in samples during storage at 30 °C/7 days. Similarly, no evident degradation was observed in the SDS–PAGE gels of serum albumin, α, β, κ-casein, β-lg and α-la fractions during storage of acidic (Fig. 2a-ii) and neutralized buffalo milk khoa (Fig. 2a-iii) up to 7th day of storage. Similarly, under refrigerated storage (5 °C/21 days) no visible degradation was observed for isolated protein fractions from respective khoa samples at different time intervals (Fig. 2b-iv, v, vi). The results observed were in accordance with Tossavainen and Kallioinen (2007) and Oh et al. (2014) who reported that the extent of proteolysis (SDS–PAGE patterns) increased at higher temperature of storage and were almost constant under refrigerated storage for UHT milk and cheese. It was evident from SDS–PAGE of khoa that the extent of proteolysis in khoa was not as extreme as in case of cheese which could be observed even at room temperature (30 °C/7 days) or refrigerated storage (5 °C/21 days).
Hence, Tyrosine value was determined in khoa samples during storage as level of tyrosine could be an effective indicator for the extent of proteolysis in milk proteins due to the microbial enzymes present in khoa (Choudhary et al. 2016). Due to high nutritive value and water activity of khoa, it is conducive to the growth of bacteria. Khoa prepared from fresh, acidic and neutralized buffalo milk differed significantly (p < 0.05) in tyrosine value. Tyrosine value was observed to be significantly higher (p < 0.05) in acidic milk khoa (Fig. 1a-iv, B-iv), which might be due to the use of sour milk for preparation of aforesaid khoa. During natural souring of milk, lactose is broken down to monosaccharides i.e. glucose and galactose, doubling the concentration of these reducing sugars in milk and making acidic milk more vulnerable to proteolysis (Tossavainen and Kallioinen 2007).
Significant increase (p < 0.05) was observed in the tyrosine value of khoa stored at 30 °C/7 days (Fig. 1a-iv) and 5 °C/21 days (Fig. 1b-iv) which might be due to microbial growth in samples during storage. Proteolysis in terms of tyrosine value was more pronounced during storage at room temperature (30 °C) as compared to refrigerated storage owing to higher microbial activity at former storage conditions (Choudhary et al. 2016). Our results correlated well with those of Tossavainen and Kallioinen (2007) who reported that the development of α-amino nitrogen/total nitrogen reflected the total proteolytic activity in milk during storage. This activity may originate from milk itself (indigenous proteases) or from microbial contamination of milk. They also reported an increase of proteolysis in lactose hydrolysed UHT milk as compared to lactose unhydrolysed UHT milk during storage at 22 °C, 30 °C and 45 °C/4 weeks revealing higher proteolysis in UHT milk stored at higher temperature (45 °C).
Textural changes of khoa during storage
Textural attributes are one of the most important acceptance characteristics of khoa from consumer’s point of view. Changes in textural attributes during storage at 30 °C/7 days as well as 5 °C/21 days of khoa are presented in Fig. 3a, b, respectively. The textural attributes such as hardness, springiness, cohesiveness, chewiness and gumminess differed significantly (p < 0.05) in all three types of khoa indicating significantly (p < 0.05) higher values in acidic milk khoa. Similar observation was reported by Rajorhia et al. (1990) for acidic milk khoa samples. Comparatively lower moisture content of acidic milk khoa resulted in higher total solids and higher hardness (Table 1). Considering the texture, pH plays a significant role in moisture retention in khoa (Choudhary et al. 2017a). The hardness values increased significantly (p < 0.05) throughout the storage period (Fig. 3a-i, b-i) due to decrease in moisture and subsequent increase in total solids. This was in accordance with the findings of Gupta et al. (1990) who had reported that the increase in hardness of khoa correlated with the increase in total solids. Similar observations were reported by Londhe et al. (2012) and Jha et al. (2014) for peda (khoa based delicacy) during storage.
Fig. 3.
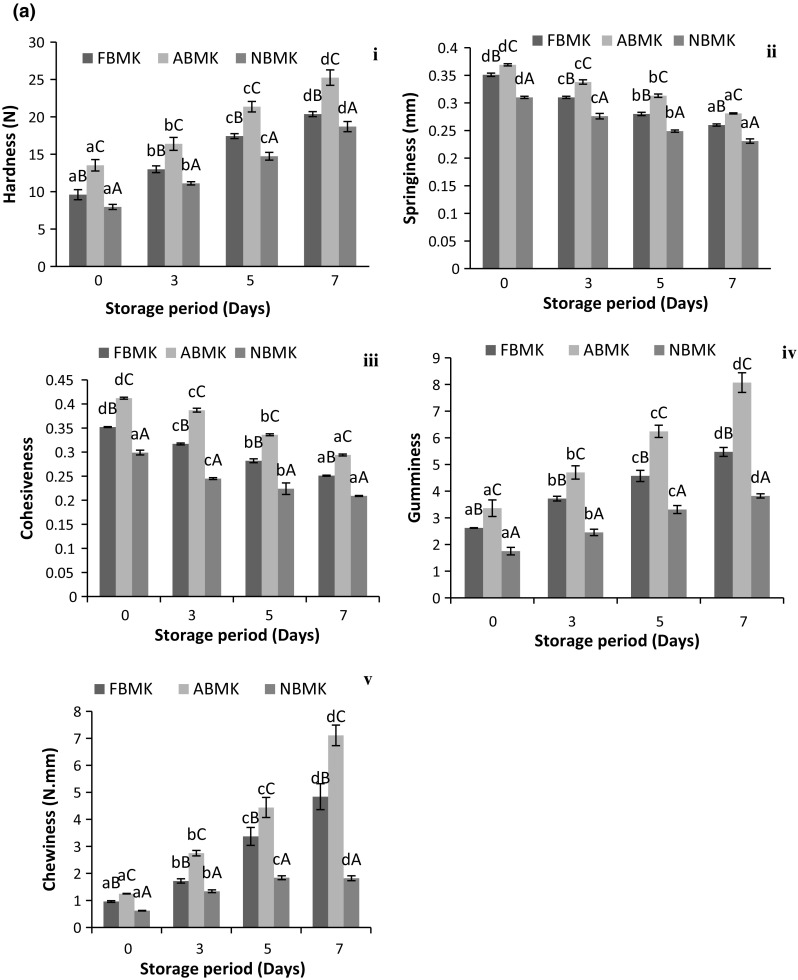
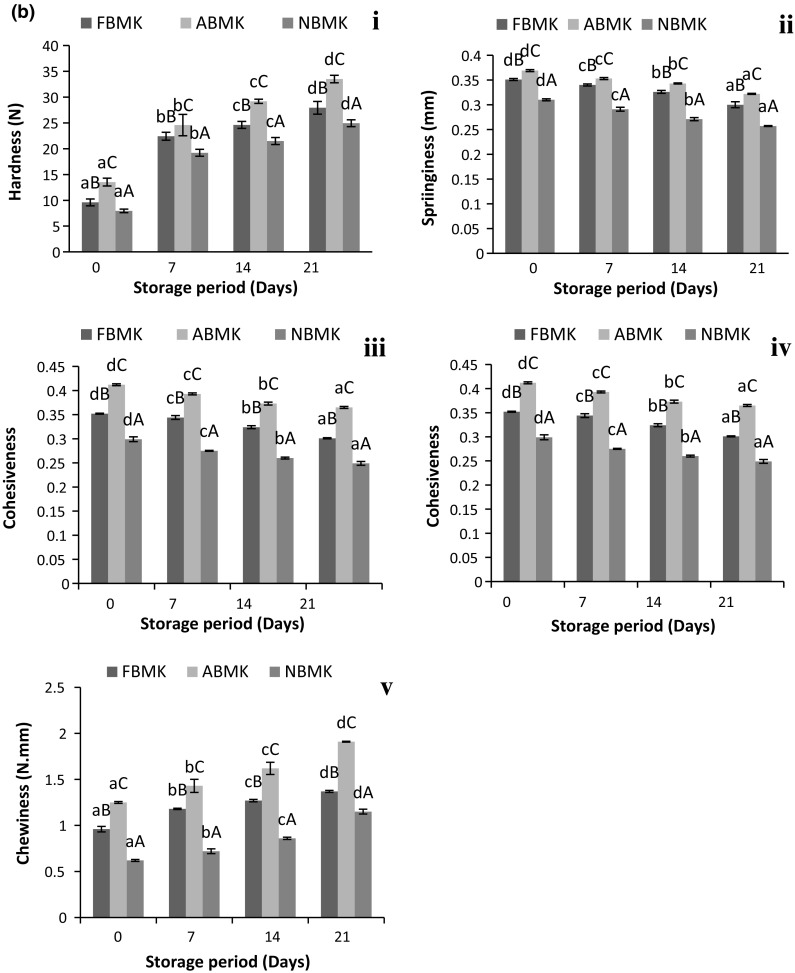
Changes in textural attributes of khoa stored at a 30 °C/7 days, b 5 °C/21 days, where, (i) Hardness. (ii) Springiness. (iii) Cohesiveness. (iv) Gumminess. (v) Chewiness. Data are presented as mean ± SEM (n = 3). a–cDifferent lowercase letters denote significant difference (p < 0.05) between groups (No. of days (0, 7th, 14th and 21st) of storage at refrigerated temperature (5 °C/21 days). Error bars show the variations of three determinations in terms of standard error of mean. A–CDifferent uppercase letters denote significant difference (p < 0.05) across subgroups ((FBMK, ABMK and NBMK)]. FBMK fresh buffalo milk Khoa, ABMK acidic buffalo milk Khoa, NBMK neutralized buffalo milk Khoa)
Springiness values of khoa manufactured from acidic milk was highest (p < 0.05) among all three types of khoa samples used and reduced significantly (p < 0.05) throughout the storage period at 30 °C and 5 °C (Fig. 3a-ii, 3b-ii) which was due to its well defined granular texture. Our results were in accordance with Rajorhia et al. (1990) who also reported highest springiness in buffalo milk khoa prepared from acidic milk. Lal peda, a khoa based delicacy also resulted in decrease in springiness during storage at 37 °C and 5 °C (Jha et al. 2014). Cohesiveness values were highest in acidic milk khoa, which might be due to the moisture difference in the respective khoa samples owing to the fact that moisture and cohesiveness of khoa shared a negative correlation (Fig. 3a-iii, b-iii). Our results were in accordance with earlier observations of Rajorhia et al. (1990), Adhikari et al. (1994) and Garg et al. (1989). Cohesiveness of khoa reduced consistently (p < 0.05) throughout the storage period due to loss of moisture in the samples. However, the extent of decrease was higher during storage at 30 °C owing to the greater moisture loss at ambient temperature conditions. Our findings were in consonance with those of Jha et al. (2014) who observed a decrease in cohesiveness of lal peda during storage at both 37 °C and 5 °C.
Gumminess and chewiness of khoa prepared using acidic milk was significantly higher (p < 0.05) than other khoa samples due to their comparatively higher total solids content (Fig. 3a-iv, v, 3b-iv, v). Similar observations were reported by Rajorhia et al. (1990). It was also observed that with an increase in total solids, gumminess and chewiness in khoa also increased (Gupta et al. 1990). Negative correlation has also been suggested between moisture and gumminess and chewiness of khoa (Adhikari et al. 1994). During storage of khoa significant increase (p < 0.05) was observed in both these parameters. More variation in gumminess and chewiness was observed for khoa stored at 30 °C (Fig. 3a-iv, v) as compared to khoa stored at 4 °C (Fig. 3b-iv, v). Results were in accordance with Londhe et al. (2012) and Jha et al. (2014) who reported an increase in gumminess and chewiness during storage of brown peda (30 °C) and in lal peda (37 °C and 5 °C), respectively. Deteriorative textural changes were rapid at 30 °C in comparison to 5 °C during storage. Gumminess and chewiness both being secondary parameters derived from hardness, cohesiveness and springiness therefore, slight variation in anyone of them directly affected gumminess and chewiness. Changes in textural parameters had deteriorative effect on the final product and the changes were more pronounced at room temperature storage as compared to refrigerated storage.
Fatty acid profile of khoa during storage
Fatty acid profile of fat extracted from khoa was determined in terms of major fatty acids present in milk fat i.e. butyric, palmitic, stearic and oleic acid (Fig. 4). Concentration of these particular fatty acids did not differ significantly (p > 0.05) in khoa prepared using fresh, acidic and neutralized buffalo milk except for butyric acid. Butyric acid is a unique fatty acid of milk fat. Its concentration in fat extracted from khoa prepared using acidic buffalo milk was significantly higher (p < 0.05) than fat extracted from khoa prepared from fresh and neutralized milk, which may be due to greater microbial count in acidic milk (induced acidity) used for the preparation of khoa. Butyric acid increased significantly (p < 0.05) in fresh, acidic and neutralized buffalo milk khoa fat samples up to 5th day of storage at 30 °C/7 days (Fig. 4a-i) however, from 5th day onwards butyric acid decreased significantly (p < 0.05). Non-significant difference (p > 0.05) was observed in butyric acid up to 14th day of storage in case of fresh and neutralized buffalo milk khoa fat samples during refrigerated conditions (Fig. 4b-i), however, significant increase (p < 0.05) was observed on 21st day of storage.
Fig. 4.
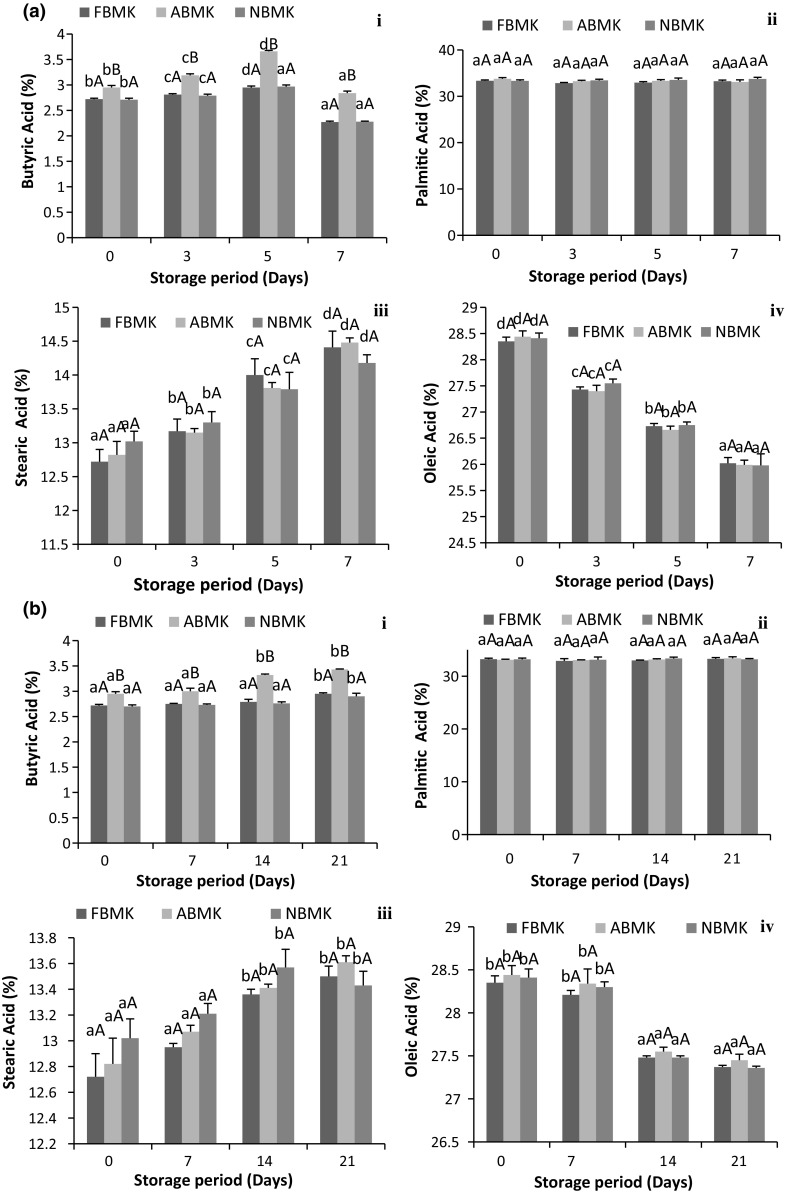
Fatty acid profile of buffalo milk khoa stored at a 30 °C/7 days, b 5 °C/21 days, where, (i) Butyric Acid. (ii) Palmitic Acid. (iii) Stearic Acid. (iv) Oleic Acid. Data are presented as mean ± SEM (n = 3). a–cDifferent lowercase letters denote significant difference (p < 0.05) between groups (No. of days (0, 3rd, 5th and 7th) of storage at room temperature (30 °C/7 days). Error bars show the variations of three determinations in terms of standard error of mean. A–CDifferent uppercase letters denote significant difference (p < 0.05) across subgroups [(FBMK, ABMK and NBMK)]. FBMK fresh buffalo milk Khoa, ABMK acidic buffalo milk Khoa, NBMK neutralized buffalo milk Khoa
During storage at 30 °C, the increased lipolytic activity may be connected to the increase in lipase activity. Increase in lipolytic activity during storage resulted in an increase in FFA concentration which contributed to the increase in butyric acid concentration. Butyric acid is soluble in ethanol and diethyl ether and was probably extracted in the fat portion during solvent extraction of fat and contributed to the increased butyric acid concentration.
Considering the fatty acid profile of milk, palmitic acid (16:0) is one of the most important and contributes approximately 30% to the total fatty acids from quantitative view point. Non-significant differences (p > 0.05) were observed in palmitic, stearic (saturated fatty acid) and oleic acid content of fat derived from khoa prepared from fresh, acidic and neutralized buffalo milk. Palmitic acid content exhibited non-significant increase (p > 0.05) throughout storage (Fig. 4a, b-ii). Similar results were observed by Omer Abdelaziz and Hamid (2013) in Sudanese white cheese stored in plastic cans at room temperature for 28 days. In case of stearic acid, significant increase (p < 0.05) was observed in all the khoa fat samples stored up to 7th day of storage at 30 °C (Fig. 4a-iii) while in case of khoa stored under refrigeration (Fig. 4b-iii) non-significant difference (p > 0.05) was observed in fresh, acidic and neutralized buffalo milk khoa fat samples up to 7th day of storage and from 7th day onwards significant increase (p < 0.05) was observed in stearic acid.
During storage at 30 °C, oleic acid (mono-unsaturated fatty acid) concentration decreased significantly (p < 0.05) up to 7th day of storage for khoa fat samples (Fig. 4a-iv). However, during refrigerated storage (5 °C/21 days) (Fig. 4b-iv) of fresh, acidic and neutralized buffalo milk khoa, the samples showed non-significant difference (p > 0.05) up to 7th day of storage however, on further storage all the khoa samples exhibited significant decrease (p < 0.05) up to 21st day of storage. Gulla and Waghray (2011) also reported significant increase of stearic and a decrease in oleic acid in oil blends of saturated, monounsaturated and polyunsaturated fatty acids stored in polyethylene terephthalate (PET) bottles at room temperature for 12 months. During storage, stearic acid content increased while that of oleic acid decreased due to oxidative cleavage of the fatty acids on storage (Gulla and Waghray 2011).
Conclusion
It is evident from the present investigation that the development of acidity and subsequent neutralization of milk significantly affected (p < 0.05) the physico-chemical properties of khoa during storage. Developed acidity and neutralization of milk resulted in deteriorative changes in khoa. These deteriorative changes were more pronounced at higher temperature of storage (30 °C) as compared to lower temperature (5 °C). However, TBA value, SDS–PAGE pattern and fatty acid profile of the three khoa variants (from fresh/acidic/neutralized milk) were not much affected except butyric acid content.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sonika Choudhary, Email: sonikachoudhary15@gmail.com.
Sumit Arora, Phone: 0184-2259156, Email: sumitak123@gmail.com.
Anuradha Kumari, Email: anu.ndri@gmail.com.
Vikrant Narwal, Email: vikrantnarwal7@gmail.com.
A. K. Singh, Email: aksndri@gmail.com
References
- Acharya PP, Agrawal J. Effect of packaging materials and modified atmosphere packaging on the shelf-life of khoa. Nepal J Sci Technol. 2010;11:87–94. doi: 10.3126/njst.v11i0.4129. [DOI] [Google Scholar]
- Adhikari AK, Mathur ON, Patil GR. Interrelationships among instron textural parameters, composition and microstructure of khoa and gulabjamun made from buffalo milk. J Food Sci Technol. 1994;31:279–284. [Google Scholar]
- Amruthakala AL. A survey of lipid composition of khoa samples in relation to possible adulteration. Int J Dairy Technol. 2012;65(3):444–450. doi: 10.1111/j.1471-0307.2012.00847.x. [DOI] [Google Scholar]
- AOAC . Official method of analysis of AOAC international. Washington: Association of Official Analytical Chemist; 1970. [Google Scholar]
- Arora S, Singh VP, Yarrakula S, Gawande H, Narendra K, Sharma V, Wadhwa BK, Tomer SK, Sharma GS. Textural and microstructural properties of burfi made with various sweeteners. J Texture Stud. 2007;38:684–697. doi: 10.1111/j.1745-4603.2007.00120.x. [DOI] [Google Scholar]
- Choudhary S, Arora S, Kumari A, Narwal V, Sharma V. Impact of developed acidity in milk and subsequent neutralization on changes in physico-chemical properties and oxidative stability of khoa. Indian J Dairy Sci. 2016;69(6):665–675. [Google Scholar]
- Choudhary S, Arora S, Kumari A, Narwal V, Sharma V. Effect of quality of milk on maillard reaction and protein oxidation during preparation of cow and buffalo milk khoa. J Food Sci Technol. 2017;54(9):2737–2745. doi: 10.1007/s13197-017-2710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary S, Arora S, Kumari A, Narwal V, Tomar SK, Singh AK. Effect of developed acidity and neutralization of milk on sensory, microstructural and textural changes in khoa prepared from cow and buffalo milk. J Food Sci Technol. 2017;54(2):349–358. doi: 10.1007/s13197-016-2468-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary S, Arora S, Kumari A, Narwal V, Sharma V. Effect of type and quality of milk on heat induced protein–protein interactions in khoa. J Food Sci Technol. 2018;55(10):4321–4329. doi: 10.1007/s13197-018-3380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S. Outlines of dairy technology. New Delhi: Oxford Publishing Company; 2004. [Google Scholar]
- DeMan JM. Physical properties of milk fat. I. Influence of chemical modification. J Dairy Res. 1961;28:81–86. doi: 10.1017/S0022029900010645. [DOI] [Google Scholar]
- Gaikwad SM, Hembade AS. Standardization and production of traditional Indian milk product Ujanibasundi from buffalo milk. Glob J Dairy Farm Milk Prod. 2013;1(1):032–036. [Google Scholar]
- Garg FC, Patel AA, Patil GR, Rajorhia GS, Gupta SK. Textural changes in khoa during holding. Indian J Dairy Sci. 1989;42:804–809. [Google Scholar]
- Gawande HM, Arora S, Sharma V, Wadhwa BK. Aspartame: safety and stability in kalakand. J Food Sci Technol. 2015;52(4):2373–2379. doi: 10.1007/s13197-013-1206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Hernandez E, Leon C, Corzo N, Garcıa-Villanova B, Romera JM. Chemical changes in powdered infant formulas during storage. Int J Dairy Technol. 2002;55:171–176. doi: 10.1046/j.1471-0307.2002.00049.x. [DOI] [Google Scholar]
- Gulla S, Waghray K. Effect of storage on physico-chemical characteristics and fatty acid composition of selected oil blends. J Life Sci. 2011;3:35–46. doi: 10.1080/09751270.2011.11885167. [DOI] [Google Scholar]
- Gupta SK, Ganguli NC. Sailic acid content of casein preparation from cow and buffalo milks. Milchwissenschaft. 1965;20:10. [Google Scholar]
- Gupta SK, Patil GR, Patel AA, Garg FC, Rajorhia GS. Instron texture profile parameters of khoa as influenced by composition. J Food Sci Technol. 1990;27:213–219. [Google Scholar]
- Gurtler JB, Beuchat LR. Survival of Enterobacter sakazakii in powdered infant formula as affected by composition, water activity and temperature. J Food Prot. 2007;70(7):1579–1586. doi: 10.4315/0362-028X-70.7.1579. [DOI] [PubMed] [Google Scholar]
- Hanus O, Vegricht J, Frelich J, Macek A, Bjelka M, Louda F, Janu L. Fatty acid composition of cow milk fat produced on low-input mountain farms. Czech J Anim Sci. 2008;53(1):17–30. doi: 10.17221/2717-CJAS. [DOI] [Google Scholar]
- IS 3508 . Methods for sampling and test of ghee (butter fat) New Delhi: Bureau of Indian Standards; 1966. [Google Scholar]
- IS: 16072 . Determination of moisture content in milk powder and similar products. New Delhi: Bureau of Indian standards; 2012. [Google Scholar]
- IS: SP (Part XI) (1981) Indian standard’s on dairy products. In: Handbook of food analysis XI dairy products. Bureau of Indian Standards, New Delhi
- Jha A, Kumar A, Jain P, Om H, Singh R, Bunkar DS. Physicochemical and sensory changes during the storage of lalpeda. J Food Sci Technol. 2014;51:1173–1178. doi: 10.1007/s13197-012-0613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney M, Bassette R. Detection of intermediate compounds in the early stages of browning reactions in milk products. J Dairy Sci. 1958;42:945–960. doi: 10.3168/jds.S0022-0302(59)90678-2. [DOI] [Google Scholar]
- Khan MA, Semwal AD, Sharma GK, Yadav DN, Srihari KA. Studies on the development and storage stability of groundnut (Arachis hypogea) burfi. J Food Qual. 2008;31(5):612–626. doi: 10.1111/j.1745-4557.2008.00224.x. [DOI] [Google Scholar]
- Kumar A, Rajorhia GS, Srinivasan MR. Effect of modern packaging materials on the keeping quality of khoa. J Food Sci Technol. 1975;12(4):172–177. [Google Scholar]
- Kumar M, Beniwal BS, Rai DC. Effect of antioxidant on shelf life of khoaunder refrigerated conditions. Egypt J Dairy Sci. 2010;38:211–218. [Google Scholar]
- Londhe G, Pal D, Raju PN. Effect of packaging techniques on shelf life of brown peda, a milk-based confection. LWT-Food Sci Technol. 2012;47:117–125. doi: 10.1016/j.lwt.2011.12.025. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RL. Protein measurement with the folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maillard MN, Billaud C, Chow YN, Ordonaud C, Nicholas J. Free radical scavenging of polyphenoloxidase activity and copper chelating properties of model maillard systems. LWT Food Sci Technol. 2007;40:1434–1444. doi: 10.1016/j.lwt.2006.09.007. [DOI] [Google Scholar]
- Oh NS, Lee HA, Myung JH, Joung JY, Lee JY, Shin YK, Baick SCJ. Effects of temperature and supplementation with skim milk powder on microbial and proteolytic properties during storage of cottage cheese. J Microbiol Biotechnol. 2014;24(6):795–802. doi: 10.4014/jmb.1402.02007. [DOI] [PubMed] [Google Scholar]
- Omer Abdelaziz EA, Hamid OIA. Characterization of free fatty acids contents of sudanese white cheese during storage. J Adv Sci Res. 2013;4(1):22–26. [Google Scholar]
- Rajorhia GS, Pal D, Garg FC, Patel RS. Effect of quality of milk on chemical, sensory and rheological properties of khoa. Indian J Dairy Sci. 1990;43:220–224. [Google Scholar]
- Sachdeva S, Singh S. Optimization of processing parameters in themanufacture of paneer. J Food Sci Technol. 1988;25:142–145. [Google Scholar]
- Simon R. Protein purification, applications and practical approach. Oxford: Oxford University Press; 2004. [Google Scholar]
- Snedecor GW, Cochran WG. Statistical methods. 8. Ames: Iowa State University Press; 1994. [Google Scholar]
- Tossavainen O, Kallioinen H. Proteolytic changes in lactose hydrolysed UHT milks during storage. Milchwissenschaft. 2007;62(4):410–414. [Google Scholar]