Abstract
This study was conducted to investigate the effect of the collapse of the endogenous antioxidant enzymes, namely, catalase (CAT), glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) in post-mortem (PM) chicken thigh muscles on the extent of lipid and protein oxidation and the functionality of the muscle in terms of water-holding. To fulfil this objective, the samples were divided into two treatments: one group of muscles (n = 8) was subjected to delay cooling (DC) (at ~ 37 °C for 200 min PM) and then stored at 4 °C for 24 h. The second group (n = 8) was subjected to a normal cooling (NC): samples were immediately chilled at 4 °C for 24 h. DC samples presented a decrease in 16% of CAT, 25% GSH-Px and 20% SOD activity in relation to NC. Consistently, an increase of 36% of total carbonyl, 15% of Schiff bases and 27% of TBA-RS and 14% of tryptophan depletion was observed in DC samples, as compared to NC. The results suggested that DC challenged muscles to struggle against oxidative reactions, consuming endogenous antioxidant defenses and causing protein and lipid oxidation which in turn affect the quality and safety of chicken meat. These results emphasize the role of PM oxidative stress on chicken quality and safety. Antioxidant strategies like fast cooling may be combined with others (dietary antioxidants) to preserve chicken quality against oxidative stress.
Keywords: M. peroneus longus, Antioxidant enzymes, Protein oxidation, Carcass cooling, Lipid oxidation, Chicken quality
Introduction
In post-mortem (PM) muscle, the unbalance of the endogenous antioxidant defenses towards the generation of reactive oxygen species and other reactive compounds, promote oxidative stress in chicken meat. Oxidative stress in meat systems implies the oxidative damage to significant muscle components such as lipids and proteins (Estévez 2015). Among the assorted chemical changes induced by oxidative reactions in proteins, the loss of tryptophan residues and thiols and the accretion of carbonyl moieties and Schiff Bases, are commonly analyzed in raw and processed meat products (Estévez 2011; Carvalho et al. 2017; Utrera et al. 2014a; Rysman et al. 2016; Soladoye et al. 2015). Besides the impact of lipid and protein oxidation on meat quality traits such as texture, flavor and color, current studies have emphasized the that particular oxidation products may exert noxious effects on consumers upon intake and intestinal uptake, with these being involved in the pathogenesis of serious disorders (Estévez and Luna 2017).
In accordance to the aforementioned, it seems unavoidable to study the chemistry fundamentals of the oxidative reactions occurred in chicken meat from the farm to the fork and therefore, guarantee the utmost quality and safety. The incidence of oxidative reactions in PM muscle is intensely affected by the pH decline and denaturation of muscle proteins, as they may be responsible for the release of enzymes implicated in maintaining the redox homeostasis of the muscle cell. Muscle enzymes such as catalase (CAT), glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) are known for contributing to control pro-oxidant factors and protect lipid and proteins against oxidation in PM muscle (Liu et al. 2014). The loss of homeostasis during meat aging, which includes the collapse of the above mentioned endogenous antioxidant defenses, leads to an increased susceptibility of lipids and proteins to suffer oxidative degradation owing to the concurrence of pro-oxidants [i.e. Reactive Oxygen Species (ROS), iron, peroxides…] and susceptible muscle components. The severity of the oxidative stress suffered by PM muscle is of enormous interest as the damage at this stage is retained until food intake and make the whole system more susceptibility to further oxidative reactions.
The evolution of temperature and the rate and extent of pH decline exert considerable effects on the oxidative stability of muscle tissue and on various meat quality attributes and (Carvalho et al. 2017; Lesiów and Xiong 2013). The association between low pH value and high carcass temperature induces a fast glycolytic metabolism and an extensive denaturation of myofibril protein with a consequent loss of meat quality attributes and the onset of meat abnormalities such a Palid, Soft and Exudative (PSE) chicken meat (Carvalho et al. 2017; Wilhelm et al. 2010). After slaughter, in combination with denaturation and proteolysis, muscle proteins also undergo oxidative damage with subsequent impact on protein functionality, meat quality and safety (Estévez 2015; Estévez and Luna 2017).
Thus, the objective of this research was to investigate the effects of PM temperature fall on the ability of CAT, GSH-Px and SOD enzymes to protect muscle tissue against oxidative damage to lipids and proteins and the influence of such reactions on particular broiler meat quality traits such as water-holding capacity.
Materials and methods
Chemical compounds
All chemicals were of analytical grade and were purchased from Panreac (Panreac Quimica, S.A., Barcelona, Spain), Merck (Darmstadt, Germany), and Sigma Chemicals (Sigma-Aldrich, Steinheim, Germany). Water used was purified by passage through a Milli-Q system (Millipore Corp., Bedford, MA, USA). Chicken meat (peroneus longus muscle) was obtained from a local slaughterhouse.
Materials and experimental design
A total of 16 female Ross broilers (308 lineage, 34 days of age and 1.8 kg) were slaughtered and processed in a slaughterhouse in Cáceres (VerAvic, S.L.). Water was provided ad libitum while animals were deprived of feeds 10 h before slaughter. A commercial corn-soybean meal diet was used and the broilers were transported (155 km) to the slaughterhouse. The birds were stunned using CO2 facility and slaughtered according to standard industry practices, which consisted of hanging, bleeding, scalding, defeathering and evisceration. The carcasses were randomly allocated into two groups 5 min after slaughter (T ~ 38.5 °C). One group (n = 8) were placed in separate plastic bags (ziploc bags) and stored at ~ 37 °C in an oven (JP Selecta-2001244, Barcelona, Spain) for 200 min to generate delay cooling (DC). This procedure was replicated from that originally reported by Lesiów and Xiong (2013). The temperature changes were monitored full time using an internal thermometer as described below. After that, the carcasses were placed in separate plastic bags and stored at 4 °C for up to 24 h for further analysis. The other group of samples [normal cooling (NC); n = 8] was held by the hocks on shackles in the commercial cold room for approximately 60 min. After cooling, the carcasses (4 °C) were placed in separate plastic bags and stored at 4 °C for up to 24 h for further analysis. Thereafter, thigh muscles were dissected from carcasses and immediately subjected to color, pH and water holding capacity measurements as described below. The remaining samples were placed in plastic bags and kept in a − 80 °C freezer for determination of antioxidant enzymes (CAT, GSH-Px and SOD) and protein and lipid oxidation and other analyses.
Temperature and pH determination
Duplicate measurements of pH and temperature were applied to each carcass using a portable pH-meter (Crison pH25, Barcelona, Spain) and thermometer (Testo 735, Lenzkirch, Germany) by inserting electrodes into the peroneus longus M. as described by Carvalho et al. (2014).
Color determination
Color (L*, a*, b*) was measured using a Minolta chromameter CR-300 (Minolta Camera Corp., Meter Division, Ramsey, NJ) with illuminant D65 and 0° standard observer. Before each measurement, the chromameter was calibrated on the CIE color space system using a white tile. Five measurements were taken from each sample as previously described by Carvalho et al. (2014).
Water holding capacity (WHC)
Press method (WHC_p)
WHC was measured in the broiler thigh meat, following the description made by Hamm (1961) and Carvalho et al. (2017). After 24 h PM, samples were cut into cubes 1.0 ± 0.01 g. A total of 16 samples was analyzed in duplicate. Samples were allocated between two pieces of filter paper and then left under a 1 kg weight for 2 min. The samples were weighed and WHC was calculated using the subsequent equation: 100 − [(Wi − Wf/Wi) × 100], where Wi and Wf are the initial and final sample weight, respectively.
Centrifugation method (WHC_c)
A total of 16 samples were analyzed in duplicate as described by Honikel (1987), with minor modifications. Five grams of thigh meat was placed in a plastic bag (ziploc bags) and heated at 70 °C for 30 min in a water bath. Subsequently, samples were left for cooling at room temperature (21 °C) for 45 min. Later, samples were centrifuged at 500g for 15 min at 4 °C and the supernatant was discarded. WHC_c was calculated in accordance to the equation: 100 − [(Wi − Wf/Wi) × 100], where Wi and Wf are the initial and final sample weight, respectively.
Centrifugation method with salt addition (WHC_s)
Five grams of thigh meat was placed in falcon tubes and added 8 mL NaCl 0.6 M. Samples were then heated at 70 °C for 30 min in a water bath. At the end of the heating procedure, samples were left at room temperature (21 °C) for 45 min. WHC_s was determined by the centrifugation method previously described.
Extraction of antioxidant enzymes
Enzymes were extracted from thigh muscles following the instructions reported by Mahecha et al. (2011) with minor modifications. The procedure was performed twice in each sample. Five grams of each sample was mixed with 35 mL of ice-cold phosphate buffer (extraction solvent, pH 7.0, 50 mM; disodium phosphate heptahydrate (Na2HPO4·7H2O) and KH2PO4). Samples were homogenized by ultraturrax (12,000 rpm, ca. 45 s), then centrifuged (4500g, 40 min, 4 °C) and the supernatant was recovered by filtration using glass wool. The resulting extract was employed to analyze the activities of catalase (CAT), glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD).
Catalase (CAT)
CAT activity was assessed following the procedure described by Aebi (1974), with some modifications as follows. One hundred µL of the extract (kept in the ice-bath) was dispensed in a cuvette (1 cm path length), and 2.90 mL of H2O2 were added. Promptly, the absorbance was monitored at 240 nm during 180 s using a spectrophotometer (Shimadzu UV-1800, Japan). CAT activity was expressed in µmol min−1 g−1 (U/g). One unit (U) of CAT activity was defined as the amount of extract needed to decompose l µmol of H2O2 per min.
Glutathione peroxidase (GSH-Px)
GSH-Px activity was analyzed following a slightly modified version of the protocol reported by Mahecha et al. (2011). Six hundred µL of the muscle extract was mixed with 2.35 mL of 1.13 mM reduced glutathione, 0.57 mM EDTA, 1.13 mM NaN3 and 1.7 units glutathione reductase in 100 mL phosphate buffer (pH 7.0, 50 mM; disodium phosphate heptahydrate (Na2HPO4·7H2O) and KH2PO4 in MiliQ water). Twenty-six µL NADPH solution (17.3 mM), and 20 µL H2O2 solution (30%) were dispensed in cuvettes (1 cm path length). The absorbance was monitored at 340 nm during 600 s using a spectrophotometer (Shimadzu UV-1800). The extinction coefficient of 6.220 µL µmol−1 cm−1 for NADPH at 340 nm and 25 °C was used for the calculation. GSH-Px activity was expressed as µmol of oxidized NADPH µL−1 min−1 g−1 (U/g).
Superoxide dismutase (SOD)
SOD activity was measured by assessing the inhibition of pyrogallol autoxidation in a basic medium in accordance to the procedure described by Marklund and Marklund (1974). Fifty µL of pyrogallol (10 mM) were mixed with 2.9 mL of Tris–cacodylic buffer (50 mM with diethylenetriaminepentaacetic acid, DTPA, pH 8.2). The rate of pyrogallol autoxidation in presence of 50 µl of muscle extract was compared to a blank (with 50 µl of buffer), Increase of absorbance at 420 nm was measured during 360 s using a spectrophotometer (Shimadzu UV-1800). One unity was taken as the activity that inhibits the pyrogallol autoxidation by 50%.
Protein oxidation
Tryptophan fluorescence measurements
The methodology reported by Estévez et al. (2008) was followed to assess the concentration of tryptophan in broiler thigh muscles. Samples were diluted (1:10 w/v) using 8 M urea in 100 mM sodium phosphate buffer (pH 6.0) using an ultraturrax. Two mL of 6 M GuHCl in 20 mM sodium phosphate buffer (pH 6.5) was added to 0.5 mL of the muscle homogenate and vortexed. After subsequent dilutions (1:1000 v/v) samples were transferred to a 4 mL quartz cuvette with four flat walls (101-QS 10 × 10 mm, Analytics Hellma). The excitation wavelength was set at 283 nm while the emission spectrum for tryptophan fluorescence was recorded between 300 and 400 nm (Perkin-Elmer LS 55 Luminescence spectrometer, Beaconsfield, UK). Data were recorded at 500 nm per minute while the excitation and emission slit widths were established at 10 nm. Tryptophan concentration was calculated using a N-acetyl-l-tryptophan amide (NATA) standard curve ranging from 0.1 to 0.5 μM. Results were expressed as mg NATA equivalents per 100 g of sample.
Determination of total protein carbonyls
The total carbonyl content was analyzed following the dinitrophenylhydrazyl method (DNPH) method as reported by Ganhão et al. 2010) with minor modifications. One gram of thigh meat was homogenized 1:10 (w/v) in 20 mM sodium phosphate buffer containing 0.6 M NaCl (pH 6.5) using an ultraturrax. Two twin samples of 0.15 mL were taken from the homogenates and dispensed in 2 mL eppendorf tubes. Proteins were precipitated by cold 10% TCA (1 mL) and subsequently centrifuged at 4500g for 3 min. For protein concentration measurement, one sample was treated with 1 mL 2 N HCl while the sample aimed for carbonyl concentration measurement was treated with an equal volume of 0.2% (w/v) DNPH in 2 N HCl. Both samples were left to stand at room temperature for 1 h and stirred regularly (every 20 min). Proteins from samples were eventually precipitated by 10% TCA and washed twice with 1 mL ethanol:ethyl acetate (1:1, v/v). Proteins were then treated with 1.5 mL of 20 mM sodium phosphate buffer containing 6 M guanidine HCl (pH 6.5) and centrifuged at 2000g for 2 min. Protein was quantified by measuring absorbance at 280 nm using bovine serum albumin (BSA) as standard. A number of carbonyls was expressed as nmol of carbonyl per mg of protein using an absorption coefficient of 21.0 nM−1 cm−1 at 370 nm for protein hydrazones.
Analysis of Schiff bases
The method proposed by Estévez et al. (2008), with some modifications, was applied to analyze Schiff bases in broiler muscles. Sample homogenates were prepared following the procedure previously described for tryptophan analysis. After dilution (1:20 v/v), samples were transferred to a 4 mL quartz cuvette with four flat walls (101-QS 10 × 10 mm, Analytics Hellma). The excitation wavelength was set at 350 nm while the emission spectrum for tryptophan fluorescence was recorded between 400 and 500 nm (Perkin-Elmer LS 55 Luminescence spectrometer, Beaconsfield, UK). Data were recorded at 500 nm per minute while the excitation and emission slit widths were established at 10 nm. Results were expressed as units of fluorescence intensity emitted by Schiff bases structures at 450 nm. These values were corrected according to the protein concentration of each sample by applying a correction factor (Cf = Pt/Pp) where Pt is the total average of the amount of protein from all samples and Pp is the content of protein in each sample.
Determination of TBARS numbers
Lipid oxidation was assessed in thigh meat by means of thiobarbituric acid-reactive substances (TBA-RS) using the assay proposed by Ganhão et al. (2011). Thigh meat (2.5 g) was homogenized with 7.5 mL of 3.86% perchloric acid and 0.25 mL BHT (4.2%), using an ultraturrax. The homogenate was centrifuged at 4500g for 3 min and subsequently filtered through double filter paper. Two mL of the filtrate were mixed with 2 mL of 0.02 mol L−1 TBA in perchloric acid (3.86%) in test tubes (duplicate). The test tubes were first vortexed and then incubated in a water bath (90–100 °C) for 30 min. All tubes were centrifuged (4500g for 2 min) and the absorbance was measured at 532 nm using a spectrophotometer (Shimadzu UV-1800, Japan) against a blank containing 2 mL of 3.86% perchloric acid and 2 mL of TBA reagent. The results were plotted against a standard curve prepared with known concentrations of tetraethoxypropane (TEP) and expressed as mg malondialdehyde (MDA) per kg of thigh meat.
Statistical analysis
Statistical analyses were performed using SPSS software and two samples independent T test was used to determine the level of significance between two treatments: DC and NC. Principal Component Analysis (PCA) and Pearson correlations were calculated for testing correlations between variables. Significance level (p) was set at p < 0.05.
Results and discussion
Effect of cooling rate on biochemical parameters
The color, pH, WHC_p, WHC_c and WHC_s were assessed to evaluate the quality of chicken thigh meat (Table 1). Color (L*, a* and b*) and WHC_s did not significantly (p > 0.05) differ between groups. However, others parameters as pH, WHC_p and WHC_c were significantly (p < 0.05) affected by cooling rate. According to these findings, elevated PM temperatures (~ 37 °C) accelerated glycolysis depletion in the muscle. These findings were consistent with Carvalho et al. (2017), who reported that chicken pectoralis major muscles held at ~ 37 °C had increased rates of PM glycolysis, which was manifested as impaired WHC, higher pH and altered color. An increased rate of glycogen metabolism was also found by Lesiów and Xiong (2013) in pork longissimus muscle exposed to elevated temperatures of 35 °C for 7 h post-mortem. Furthermore, Carvalho et al. (2017) and Lesiów and Xiong (2013) found that ultimate pH had a significant effect on assorted traits linked to the ocurrence of PSE meat characteristics in broilers and pork, respectively. These studies emphasized that the early pH decline in PM muscle was found to be the most influential factor on the extent of exudation. In addition, data on the biochemical parameters (Table 1) show to what extent a near body temperature (~ 37 °C) during rigor development affected the WHC. The same was also observed by Liu et al. (2014). The delay cooling (DC) decreased WHC in broilers thigh meat compared to the normal cooling (NC) treatment. According to McKee and Sams (1998), an early development of rigor mortis in turkey meat (pectoralis major) combined with high carcass temperatures was responsible for the denaturation of sarcoplasmic proteins and a subsequent poor WHC. Liu et al. (2014) reported that a DC resulted in precipitation of phosphorylase and creatine kinase and hence reduction of WHC due to a combination of high temperature and low pH. The potential influence of the oxidative damage to proteins may have also played a role in the water functionality of broiler meat proteins, given the proven role of protein oxidation on protein functionality (Utrera and Estévez 2012). This will be discussed in due course.
Table 1.
Measurements of biochemical parameters (means ± standard deviations) in thigh chicken meat subjected to normal (NC) and delayed cooling (DC)
| NC | DC | p valueA | |
|---|---|---|---|
| L* | 55.22a ± 4.40 | 55.33a ± 3.20 | 0.958 |
| a* | 2.40a ± 0.93 | 2.23a ± 0.41 | 0.633 |
| b* | 0.10a ± 0.77 | 0.03a ± 1.04 | 0.879 |
| pH | 6.37a ± 0.15 | 6.26b ± 0.05 | 0.019 |
| WHC_p | 86.80a ± 1.15 | 84.82b ± 0.81 | 0.001 |
| WHC_c | 85.46a ± 0.73 | 84.11b ± 1.28 | 0.022 |
| WHC_s | 145.49a ± 4.92 | 144.55a ± 6.41 | 0.747 |
Color = L*, a*and b*; WHC_p = water holding capacity by press method; WHC_c = water holding capacity by centrifugation method and WHC_s = centrifugation method with salt addition
a–bMeans within each line with different superscripts are significantly different (p < 0.05)
AStatistical significance in Student’s T test
Activity of antioxidant enzymes
Endogenous antioxidant enzymes play a major role in the redox status of muscle tissue, and under physiological conditions, they counteract the pro-oxidant actions of ROS. SOD, CAT and GSH-Px are among the most active and efficient enzymatic systems against oxidative stress (Niu et al. 2017). The effect of cooling rate on the activity of the antioxidant enzymes in chicken thigh muscle was assessed (Table 2). SOD and CAT readily neutralize ROS, whereas GSH-Px regenerates oxidized glutathione, a peptide with antioxidant properties (Bai et al. 2016; Delles et al. 2014; Hoac et al. 2006; Mahecha et al. 2011). CAT, GSH-Px and SOD were found to occur in both DC and NC samples, yet, in DC samples, a decrease in activity of 16% (CAT), 25% (GSH-Px) and 20% (SOD) was observed in relation to that in NC samples. These results suggest that the cooling delay diminished the activity of the antioxidant enzymes. As far as I know, the information on the activity of antioxidant enzymes in poultry thigh muscles in the scientific literature is scarce. The effect of the delayed cooling could be explained by denaturation of the enzymes, by the waste antioxidant components during oxidative reactions occurred during the delayed cooling at 37 °C and also by the inactivation of these enzymes through the oxidation of their active sites. Therefore, the quantity and activity of antioxidant enzymes in the living muscles may not necessarily reflect their antioxidant potential as an inappropriate cooling rate after slaughter may severely lead to a loss of enzymatic activity in the PM muscle (Carvalho et al. 2017). These enzymes contribute to diverse mechanisms aimed to protect cell components against oxidative stress (Delles et al. 2014; Jiang et al. 2009; Niu et al. 2017). Hence, the depletion of antioxidant enzymes during delayed cooling may compromise the susceptibility of broiler thigh muscle towards oxidation which may eventually lead to elevated oxidation rates during successive storage and/or industrial processing (Niu et al. 2017). In good agreement with the present results, previously studies observed a decrease of GSH-Px in duck and chicken meat subjected to delayed cooling and that was revealed as increased lipid oxidation in such meats during succeeding storage (Hoac et al. 2006). Moreover, Niu et al. (2017) reported that weak endogenous antioxidant defenses cause increasing levels of TBA-RS and ROS in mitochondria which is manifested as oxidative damage and impaired quality traits in chicken breast meat.
Table 2.
Effect of normal (NC) and delayed cooling (DC) on activities of the antioxidants enzymes (means ± standard deviations) in thigh chicken meat
| NC | DC | p valueA | |
|---|---|---|---|
| CAT | 39.02 ± 3.31 | 32.78 ± 2.71 | 0.023 |
| GSH-Px | 0.52 ± 0.04 | 0.39 ± 0.04 | 0.003 |
| SOD | 69.38 ± 4.67 | 54.92 ± 3.42 | 0.009 |
CAT = catalase activity expressed as μmol min−1 g−1 (U/g); GSH-Px = glutathione peroxidase activity expressed as μmol of oxidized NADPH μL−1 min g−1 (U/g); SOD = superoxide dismutase activity expressed as U/g of sample
AStatistical significance in Student's t-test
Protein and lipid oxidation
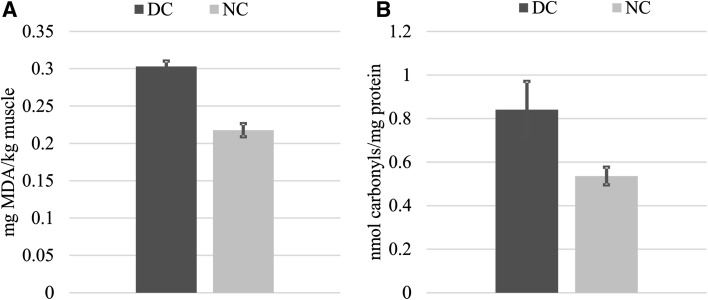
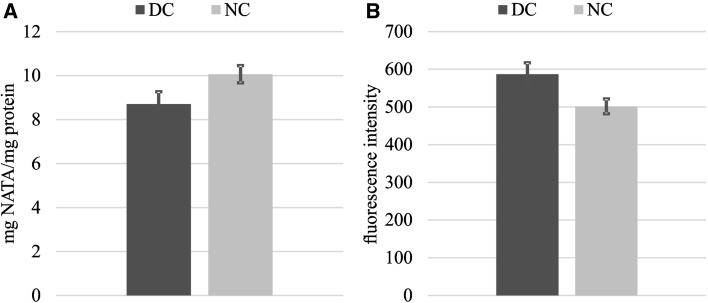
The susceptibility of thigh meat to lipid oxidation was assessed by TBA-RS levels (Fig. 1a) and higher TBA-RS numbers (~ 27%) were found in DC samples compared to the NC counterparts after 24 h of storage. A comparable outcome was obtained from protein oxidation measurements as assessed by the DNPH method (Fig. 2b). The formation of protein carbonyl was 36% higher in DC than in the NC samples. The concurrence of lipid and protein oxidation in meat systems suggests interactions via reciprocal transfer of reactive and non-reactive species between both biomolecules (Estévez 2015; Soladoye et al. 2015). The susceptibility of thigh meat from DC to suffer more intense oxidative reactions affected similarly to both, proteins and lipids (Fig. 1). Under pro-oxidant conditions, such as those occurred in PM muscle, lipids are oxidized earlier than proteins: traces of their oxidative damage are detected faster than protein oxidation products (Estévez 2011, 2015; Soladoye et al. 2015). Protein carbonyls are formed in as a result of the oxidative deamination of certain amino acids (lysine, proline and/or arginine) (Estévez 2011). The oxidation of lipids and the occurrence of protein carbonyls are certainly governed by different mechanisms (Estévez 2011; Soladoye et al. 2015). However, the peroxidation of polyunsaturated fatty acids as well as protein carbonylation, are initiated by radical species (i.e. superoxide and hydroxyl radicals). It is worth mentioning that the enzymes under study specifically catalyze the decomposition or stabilization of radical species. Therefore, the inactivation of CAT, SOD and GSH-Px during the delayed cooling process may have decisively contributed to the oxidation of lipids and proteins in DC samples. Enzymes such as SOD and CAT have been found to effectively counteract the harmful effect of ROS on proteins from diverse meats such as pork (Chen et al. 2010), beef (Utrera et al. 2014b) and poultry (Delles et al. 2014). Other measurements of the oxidative damage to proteins, such as the formation of Schiff basis and the depletion of tryptophan in chicken thigh meat, were consistent with the results presented above (Figs. 2a, b, 3a, b, respectively). The concentration of tryptophan was significantly low (~ 14%) in DC samples in relation to NC after 24 h PM. Conversely, 15% higher values were obtained for Schiff bases in DC samples compared to NC after 24 h PM as seen in Fig. 2b. Tryptophan is readily oxidized in the presence of ROS and hence, its depletion usually indicates oxidative damage to meat and poultry proteins (Estévez 2015). Given that tryptophan is implicated in assorted and relevant biological functions, the oxidation of this essential amino acid should be regarded as a remarkable nutritional loss (Estévez et al. 2008; Estévez 2011). The current results show that a delayed cooling leads to a more intense degradation of tryptophan in broiler thigh muscles, hence, affecting its nutritional value and quality.
Fig. 1.
Formation of oxidation products under normal cooling (NC) and delayed cooling (DC) in chicken thigh meat. a TBA-RS (mg MDA/kg muscle) and b protein carbonyls (nmol carbonyls/mg protein). Significant differences between means in Student’s T test are denoted on top of bars
Fig. 2.
Measurements of tryptophan (a) and Schiff bases (b) under normal cooling (NC) and delayed cooling (DC) chicken thigh meat. a tryptophan in mg NATA/g protein and b Schiff bases in fluorescence intensity. Significant differences between means in Student’s T test are denoted on top of bars
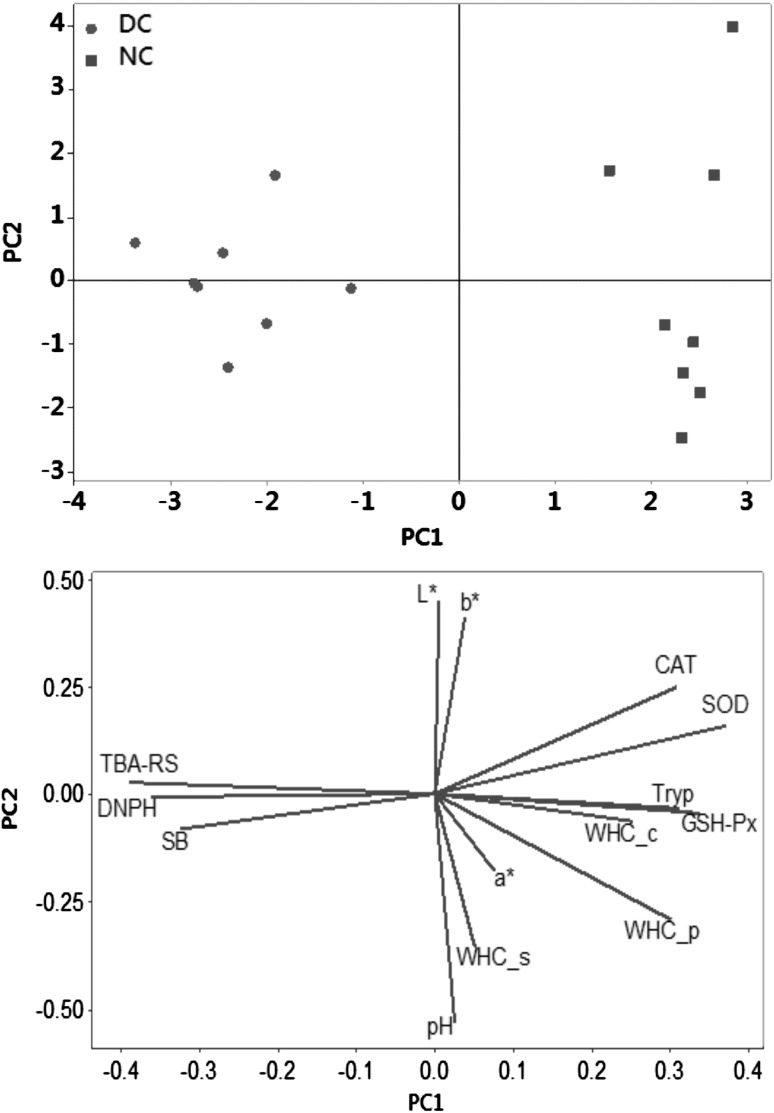
Fig. 3.
Overlay of similarity map of thigh meat samples and computed variables in principal component analysis (PCA). DC = delay cooling, NC = normal cooling, WHC_p = press method. WHC_c = centrifugation method. WHC_s = centrifugation method with salt addition, CAT = catalase (CAT), GSH-Px = glutathione peroxidase SOD = superoxide dismutase activity, DNPH = protein carbonyls TBA-RS = thiobarbituric acid-reactive substances, SB = Schiff base and Tryp = tryptophan
Schiff bases (Fig. 2b) are fluorescent products formed as a result of the reaction between carbonyls from both lipid and protein oxidation and secondary amines (Estévez 2011). In meat systems, amino groups from the side chains of alkaline amino acids such as lysine establish crosslinks with carbonyl moieties leading to Schiff base formation (Utrera et al. 2014a, b). Compared to NC samples, those subjected to a delayed cooling, were expected to have higher concentration of Schiff bases given the higher concentration in these samples of protein carbonyls and lipid-derived aldehydes (Fig. 1a). The formation of protein crosslinks in meat systems such as Schiff bases is thought to contribute to increasing meat consistency and toughening (Soladoye et al. 2015).
The negative impact of oxidative reactions on food quality and its nutritional value have been recurrently reported (Soladoye et al. 2015). Conversely, the impact of protein oxidation on nutritive and health aspects are otherwise disregarded though the intake of oxidized proteins is associated to in vivo oxidative stress and the onset and chronic diseases in experimental animals and humans (Estévez and Luna 2017). Hence, an early muscle cooling treatment can preserve the endogenous antioxidant defenses of broiler muscle tissues and thus contribute to diminishing lipid and protein oxidation in broiler thigh meat, enhancing, as a result, its nutritional value and safety.
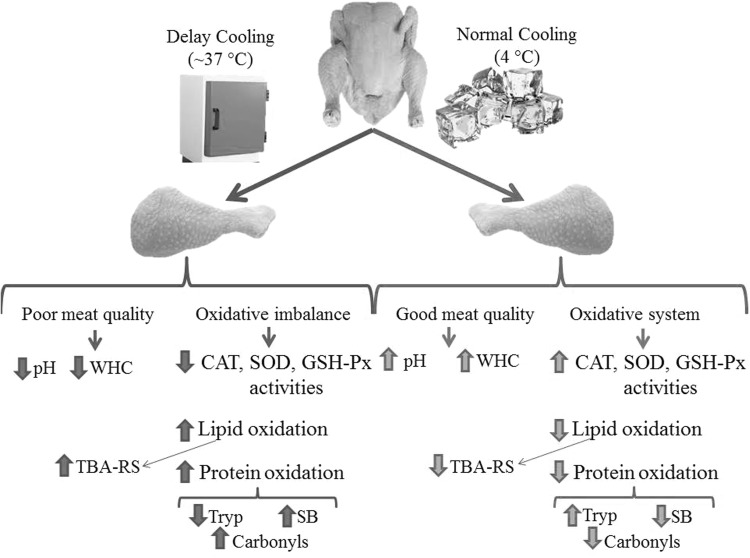
To establish interconnections between variables and corroborate some of the above explained hypothesis, a Principal Component Analysis was carried out (Fig. 3). As expected, the physico-chemical properties and oxidation markers measured in the present study enabled a clear discrimination between NC and DC broiler thigh meat in a PCA. PC1 and PC2 explained 50.4% and 16.6% of the total variance, whereas PC3 and PC4 explained 15.4% and 7.3% respectively. DC group was located in the negative axis of the PC#1 and defined by high rates of oxidative stress, proteolysis and poor ability to hold water. On the opposite side of the similarity map, NC group was located close to the antioxidant enzymes, normal pH values and WHC. Hence, significant and negative correlations were found between protein oxidation markers, such as DNPH, and WHC_p (r = 0.71) and WHC_c (r = 0.52), and TBA-RS with WHC_p (r = 0.72) and WHC_c (r = 0.59). Figure 4 summarizes the proposed mechanisms by which delay cooling may influence the oxidative damage to proteins and that, in turn, affect the water holding capacity of the chicken thigh meat.
Fig. 4.
Proposed mechanisms for the observed effects of cooling on the meat quality and oxidative systems in chicken thigh meat. WHC = water holding capacity, CAT = catalase, GSH-Px = glutathione peroxidase SOD = superoxide dismutase activity, TBA-RS = thiobarbituric acid-reactive substances, SB = Schiff base and tryp = tryptophan
Conclusion
Oxidative reactions are inserted into the center of the biochemical reactions having an influence on the altered quality of meat subjected to delayed PM cooling. Proteins from delay cooling of chicken thigh meat are more susceptible to oxidative stress due to lower pH, an impaired activity of endogenous antioxidant enzymes (CAT, GSH-Px and SOD), and other biochemical changes occurred in postmortem meat such as proteolysis. Though some relevant technological consequences of these reactions have already been identified here, other studies may clarify the extent to which the nutritional value and safety of such chicken meat is affected by a delayed cooling rate.
Funding
Funding was provided by Secretaría de Estado de Investigación, Desarrollo e Innovación (Grant No. AGL2017-84586-R).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Massami Shimokomaki (in memory) and Mario Estévez share the same author position.
References
- Aebi H (1974) Catalase. In: Methods of enzymatic analysis, pp 673–684. 10.1016/B978-0-12-091302-2.50032-3
- Bai WK, Zhang FJ, He TJ, Su PW, Ying XZ, Zhang LL, Wang T. Dietary probiotic Bacillus subtilis strain fmbj increases antioxidant capacity and oxidative stability of chicken breast meat during storage. PLoS ONE. 2016;11(12):1–17. doi: 10.1371/journal.pone.0167339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho RH, Soares AL, Honorato DCB, Guarnieri PD, Pedrão MR, Paião FG, Oba A, Ida EI, Shimokomaki M. The incidence of pale, soft, and exudative (PSE) turkey meat at a Brazilian commercial plant and the functional properties in its meat product. LWT Food Sci Technol. 2014;59(2):883–888. doi: 10.1016/j.lwt.2014.07.019. [DOI] [Google Scholar]
- Carvalho RH, Ida EI, Madruga MS, Martínez SL, Shimokomaki M, Estévez M. Underlying connections between the redox system imbalance, protein oxidation and impaired quality traits in pale, soft and exudative (PSE) poultry meat. Food Chem. 2017;215:129–137. doi: 10.1016/j.foodchem.2016.07.182. [DOI] [PubMed] [Google Scholar]
- Chen T, Zhou GH, Xu XL, Zhao GM, Li CB. Phospholipase A2 and antioxidant enzyme activities in normal and PSE pork. Meat Sci. 2010;84(1):143–146. doi: 10.1016/j.meatsci.2009.08.039. [DOI] [PubMed] [Google Scholar]
- Delles RM, Xiong YL, True AD, Ao T, Dawson KA. Dietary antioxidant supplementation enhances lipid and protein oxidative stability of chicken broiler meat through promotion of antioxidant enzyme activity. Poult Sci. 2014;93(6):1561–1570. doi: 10.3382/ps.2013-03682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez M. Protein carbonyls in meat systems: a review. Meat Sci. 2011;89(3):259–279. doi: 10.1016/j.meatsci.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Estévez M. Oxidative damage to poultry: from farm to fork. Poult Sci. 2015;94(6):1368–1378. doi: 10.3382/ps/pev094. [DOI] [PubMed] [Google Scholar]
- Estévez M, Luna C. Dietary protein oxidation: a silent threat to human health? Crit Rev Food Sci Nutr. 2017;57:3781–3793. doi: 10.1080/10408398.2016.1165182. [DOI] [PubMed] [Google Scholar]
- Estévez M, Kylli P, Puolanne E, Kivikari R, Heinonen M. Fluorescence spectroscopy as a novel approach for the assessment of myofibrillar protein oxidation in oil-in-water emulsions. Meat Sci. 2008;80(4):1290–1296. doi: 10.1016/j.meatsci.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Ganhão R, Morcuende D, Estévez M. Protein oxidation in emulsified cooked burger patties with added fruit extracts: influence on colour and texture deterioration during chill storage. Meat Sci. 2010;85(3):402–409. doi: 10.1016/j.meatsci.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Ganhão R, Esévez M, Morcuende D. Suitability of the TBA method for assessing lipid oxidation in a meat system with added phenolic-rich materials. Food Chem. 2011;126(2):772–778. doi: 10.1016/j.foodchem.2010.11.064. [DOI] [Google Scholar]
- Hamm R. Biochemistry of meat hydration. Adv Food Res. 1961;10:355–463. doi: 10.1016/S0065-2628(08)60141-X. [DOI] [PubMed] [Google Scholar]
- Hoac T, Daun C, Trafikowska U, Zackrisson J, Åkesson B. Influence of heat treatment on lipid oxidation and glutathione peroxidase activity in chicken and duck meat. Innov Food Sci Emerg Technol. 2006;7(1–2):88–93. doi: 10.1016/j.ifset.2005.10.001. [DOI] [Google Scholar]
- Honikel KO. How to measure the water-holding capacity of meat? Recommendation of standardized methods. In: Tarrant PV, Eikelenboom G, Monin G, editors. Evaluation and control of meat quality in pigs. Current topics in veterinary medicine and animal science. Dordrecht: Springer; 1987. pp. 129–142. [Google Scholar]
- Jiang Z, Lin Y, Zhou G, Luo L, Jiang S, Chen F. Effects of dietary selenomethionine supplementation on growth performance, meat quality and antioxidant property in yellow broilers. J Agric Food Chem. 2009;57(20):9769–9772. doi: 10.1021/jf902411c. [DOI] [PubMed] [Google Scholar]
- Lesiów T, Xiong YL. A simple, reliable and reproductive method to obtain experimental pale, soft and exudative (PSE) pork. Meat Sci. 2013;93(3):489–494. doi: 10.1016/j.meatsci.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Liu LL, He JH, Xie HB, Yang YS, Li JC, Zou Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult Sci. 2014;93(1):54–62. doi: 10.3382/ps.2013-03423. [DOI] [PubMed] [Google Scholar]
- Mahecha L, Nuernberg K, Nuernberg G, Martin J, Hubbermann EM, Knoeller S, Claeyse E, De Smet S, Dannenberger D. Antioxidant enzyme activities and antioxidant capacity in longissimus muscle from bulls fed diets rich in polyunsaturated fatty acids. Food Chem. 2011;127(2):379–386. doi: 10.1016/j.foodchem.2010.12.117. [DOI] [PubMed] [Google Scholar]
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem FEBS. 1974;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- McKee SR, Sams AR. Rigor mortis development at elevated temperatures induces pale exudative turkey meat characteristics. Poultry Sci. 1998;77:169–174. doi: 10.1093/ps/77.1.169. [DOI] [PubMed] [Google Scholar]
- Niu ZY, Min YN, Liu FZ. Dietary vitamin E improves meat quality and antioxidant capacity in broilers by upregulating the expression of antioxidant enzyme genes. J Appl Anim Res. 2017;2119:1–5. [Google Scholar]
- Rysman T, Utrera M, Morcuende D, Van Royen G, Van Weyenberg S, De Smet S, Estévez M. Apple phenolics as inhibitors of the carbonylation pathway during in vitro metal-catalyzed oxidation of myofibrillar proteins. Food Chem. 2016;211:784–790. doi: 10.1016/j.foodchem.2016.05.126. [DOI] [PubMed] [Google Scholar]
- Soladoye OP, Juárez ML, Aalhus JL, Shand P, Estévez M. Protein oxidation in processed meat: mechanisms and potential implications on human health. Compr Rev Food Sci Food Saf. 2015;14(2):106–122. doi: 10.1111/1541-4337.12127. [DOI] [PubMed] [Google Scholar]
- Utrera M, Estévez M. Oxidation of myofibrillar proteins and impaired functionality: underlying mechanisms of the carbonylation pathway. J Agricutural Food Chem. 2012;60:8002–8011. doi: 10.1021/jf302111j. [DOI] [PubMed] [Google Scholar]
- Utrera M, Morcuende D, Estévez M. Temperature of frozen storage affects the nature and consequences of protein oxidation in beef patties. Meat Sci. 2014;96(3):1250–1257. doi: 10.1016/j.meatsci.2013.10.032. [DOI] [PubMed] [Google Scholar]
- Utrera M, Parra V, Estévez M. Protein oxidation during frozen storage and subsequent processing of different beef muscles. Meat Sci. 2014;96(1):812–820. doi: 10.1016/j.meatsci.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Wilhelm AE, Maganhini MB, Hernández-Blazquez FJ, Ida EI, Shimokomaki M. Protease activity and the ultrastructure of broiler chicken PSE (pale, soft, exudative) meat. Food Chem. 2010;119(3):1201–1204. doi: 10.1016/j.foodchem.2009.08.034. [DOI] [Google Scholar]