Abstract
Echium oil is rich in omega-3, however, is unstable. The objective of this work was the co-encapsulation of echium oil and sinapic acid (SA) by emulsification using Arabic gum as emulsifier/carrier, followed by spray or freeze-drying. Eight treatments (S0, S200, S600 and S1000: particles spray dried with different concentrations of SA; L0, L200, L600 and L1000: particles freeze dried with different concentrations of SA) were analyzed in relation to microscopy, water activity (Aw), hygroscopicity, moisture, solubility, particle size, X-ray diffraction, thermogravimetry and accelerated oxidation. Particles of rounded shape and undefined form were obtained by spray and freeze-drying, besides ideal physicochemical properties for application (values from 0.091 to 0.365, 3.22 to 4.89%, 57 to 68% and 2.32 to 12.42 µm for Aw, moisture, solubility and particle size, respectively). All treatments protected the oil against oxidation, obtaining induction time of 5.31 h for oil and from 7.88 to 12.94 h for treatments. The better protection to oil was obtained with it emulsified and freeze-dried (L600); the encapsulation increased oxidative stability of the oil, besides facilitating its application over the fact the material is in powder form.
Keywords: Omega-3 fatty acids, Oxidation, Microencapsulation, Phenolic compound, Arabic gum
Introduction
Omega-3 fatty acids (ω-3) are compounds widely used in food, pharmaceuticals and cosmetics products due to its beneficial effects on health (Kralovec et al. 2012). The oil extracted from the seed of echium (Echium plantagineum L.) contains from 9 to 16% of stearidonic fatty acid and it is being considered an alternative in the substitution of fish oil since it presents a proportion of ω-3 to ω-6 fatty acids that is not found in any other oil (1.8:1 of omega-3 to omega-6) (Berti et al. 2007). However, this oil is very susceptible to oxidation, and as any oil, it does not solubilize in aqueous media, factors that hamper its storage and application into foods. The microencapsulation technique and the addition of an antioxidant compound are strategies that would facilitate applications of these compounds.
The phenolic compound sinapic acid (SA) has been of great interest in the food industry due to its beneficial effects on human health, acting as an antioxidant agent (Comunian et al. 2017a). In the case of the encapsulation technique, the protection of the material encapsulated is the main objective, providing the controlled release of the active material, besides facilitating the storage and application (Favaro-Trindade et al. 2008). There are many encapsulation techniques, such as complex coacervation (Comunian et al. 2016a), spray drying (Vincenzetti et al. 2018), microfluidic devices (Comunian et al. 2017b), emulsions (Ho et al. 2017) and ionic gelation (Moura et al. 2018). These authors used different biopolymers as wall materials/carriers such as combination of gelatin-arabic gum, whey proteins, alginate, soy and pea proteins, sodium caseinate and pectin. The method of emulsification followed by spray drying and freeze-drying techniques consists in preparing a mixture of two immiscible liquids (aqueous phase and oil phase), subjected to atomization at high temperature (in the range of 100–180 °C) (spray drying) or to freezing followed by sublimation under vacuum conditions and low temperature (lyophilization). In addition, they are fast techniques and the most used by the food industries, being spray drying an inexpensive and continuous method (Davis and Walker 2018).
In this context, the objective of this study was to co-encapsulate echium oil and SA by emulsification using Arabic gum as encapsulating agent/emulsifier, followed by spray and freeze-drying, resulting in innovative vehicles that will bring greater oxidative stability to the oil and facilitates its application.
Materials and methods
Materials
Echium seed oil was used as the source of omega-3 (Echium plantagineum L.) (De Wit Speciality Oils, Netherlands). Its fatty acid composition is: palmitic acid (C16:0) (6.8%), stearic acid (C18:0) (3.4%), oleic acid (C18:1) (14.9%), linoleic acid (C18:2) (15.3%), alpha linolenic acid (C18:3) (31.3%), gamma linoleic acid (C18:3) (11.2%) and stearidonic acid (C18:4) (13.6%). Sinapic acid and Arabic gum were obtained from Sigma Chemical Co. (St. Louis/MO, United States) and Nexira (São Paulo/SP, Brazil), respectively.
Study of emulsions
Preparation of emulsions
For the preparation of single emulsion oil in water (O/W), echium oil was used as oil phase and solution of Arabic gum 30% (w/w) as aqueous phase and emulsifier. Two formulations were prepared, varying the ratio of oil phase to aqueous phase in 1:2 and 1:3, in volume. Concentrations/ratios were determined after preliminary tests.
Study of stability and droplet size
Emulsions were visually observed in a measuring cylinder at room temperature and also by optical microscopy for 7 days. In addition, the emulsions were analyzed according to the instability index and droplet size with the aid of the equipment of dispersion and stability analyzer (LumiSizer, Berlin/Germany) at 4000 rpm and 25 °C. The instability index was calculated by the software (SepView 6.0, LUM, Berlin, Germany). According to Hoffmann and Schrader (2015), this index was quantified by the clarification at a given separation time, divided by the maximum clarification, and it is a value in the range of 0–1 (0 = very stable and 1 = very unstable).
Incorporation of SA
For choosing the best incorporation method of SA, the process was done in two ways, according to Comunian et al. (2016b). The first method of incorporation of SA was with its addition (200 ppm in relation to the oil) after preparation of single emulsion oil in water (O/W), which was kept under slow magnetic stirring for 12 h at 15 °C. The second method of incorporation of SA was with its addition in the aqueous solution of Arabic gum 30% (w/w), kept under magnetic stirring for 1 min before preparation of the emulsion.
After choosing the best incorporation method of the phenolic compound, three SA concentrations were used (200, 600 and 1000 ppm in relation to the oil).
Drying
The emulsions were frozen in a freezer (− 18 °C) and freeze-dried (Terroni, São Carlos, Brazil) for 24 h, with a pressure of 1–0.1 kPa, a condenser temperature of − 20 °C and a final temperature of 30 °C. Samples were also dried by atomization, using a spray dryer model LM MSD 1.0 (Labmaq, Ribeirão Preto, Brazil), with heated air flow of 2.50 m/s, inlet air temperature of 150 °C, feed flow rate of 0.6 L/h, nozzle diameter of 1.2 mm, parameters determined after numerous preliminary tests. Thus, eight treatments were obtained (Table 1).
Table 1.
Values of water activity (Aw), moisture, solubility, hygroscopicity, yield of the drying process, average particle size and oxidation index for each treatment
| Treatments | Aw | Moisture (%) | Solubility (%) | Hygroscopicity (g of water/100 g of sample) | Yield of the drying process (%) | Average particle size (µm) | Oxidation index (h) |
|---|---|---|---|---|---|---|---|
| S0 | 0.274 ± 0.06b | 4.44 ± 0.50abc | 64.27 ± 1.75ab | 1.32 ± 0.09a | 60.82 ± 3.89bc | 5.14 ± 2.81a | 8.14 ± 0.03cd |
| S200 | 0.365 ± 0.11a | 4.58 ± 0.09abc | 68.53 ± 2.70a | 1.58 ± 0.21a | 55.52 ± 7.73c | 2.41 ± 0.53a | 7.88 ± 0.51d |
| S600 | 0.281 ± 0.03ab | 4.65 ± 0.60ab | 62.64 ± 0.98ab | 1.38 ± 0.09a | 52.07 ± 1.63c | 3.28 ± 0.98a | 8.83 ± 0.15cd |
| S1000 | 0.268 ± 0.03b | 3.94 ± 0.20cd | 60.73 ± 2.97ab | 1.55 ± 0.56a | 49.04 ± 3.96c | 2.32 ± 1.57a | 9.53 ± 0.71bcd |
| L0 | 0.091 ± 0.01d | 3.22 ± 0.36e | 60.76 ± 3.79ab | 2.38 ± 0,72a | 79.09 ± 2.68a | – | 10.49 ± 0.21abc |
| L200 | 0.146 ± 0.01cd | 3.50 ± 0.07de | 56.42 ± 2.27b | 1.85 ± 0.06a | 82.93 ± 6.18a | – | 11.71 ± 0.74ab |
| L600 | 0.180 ± 0.01c | 4.19 ± 0.30bc | 59.08 ± 1.96b | 1.87 ± 0.08a | 83.27 ± 2.09a | – | 12.94 ± 1.03a |
| L1000 | 0.201 ± 0.01bc | 4.89 ± 0.39a | 57.86 ± 3.00b | 2.31 ± 0.19a | 77.98 ± 4.11ab | – | 11.55 ± 1.00ab |
| Pure oil | – | – | – | – | – | – | 5.31 ± 0.02e |
Equal letters in the same column do not differ at 5% of probability by Tukey test
S0, S200, S600 and S1000: particles obtained by spray drying with 0, 200, 600 and 1000 ppm of sinapic acid in relation to the oil
L0, L200, L600 and L1000: particles obtained by freeze drying with 0, 200, 600 and 1000 ppm of sinapic acid in relation to the oil
Characterization of particles
Morphological characterization by scanning electron microscopy (SEM)
The characterization of the microparticles was performed by scanning electron microscopy using the microscope Tabletop Microscope Hitachi (Tokyo, Japan) TM 3000.
Particle size and particle size distribution of samples obtained by spray drying
To obtain the particle size and particle size distribution of the atomized treatments, particle analyzer by laser diffraction SALD-201V, Shimadzu (Kyoto, Japan) was used. Ethanol was used as sedimentation medium and the average particle size was expressed as D [4,3] by volume distribution.
Aw and hygroscopicity
Aw was determined using AQUALAB equipment (Decagon Devices, Pullman, WA). For the determination of hygroscopicity, 0.25 g of samples were stored for one week in a desiccator containing a saturated solution of NaCl [relative humidity (RH) = 75%]. The hygroscopicity was determined by the mass of water absorbed per 100 g of sample after 7 days of storage, according to the methodology used by Cai and Corke (2000) with some modifications.
Moisture and solubility
The determination of moisture was performed in the moisture analyzer model MB 35, Ohaus (Ohio, USA). Water solubility was determined by the gravimetric method according to Eastman and Moore (1984) and Cano-Chauca et al. (2005), adding 0.40 g of sample into a flask containing 40.0 mL of distilled water. It was homogenized at 100 rpm for 30 min, at room temperature, and the solution was centrifuged with an Eppendorf Centrifuge, Model 5430 R (Hamburg, Germany) at 1400 g for 5 min. An aliquot of 20.0 mL of the supernatant was transferred to a porcelain cup with known mass and kept in an oven at 105 °C until complete evaporation of the water.
Thermogravimetric analysis (TGA)
The TGA curves were obtained using a Thermogravimetric Analyzer, model STA 449 F3 (Netzsh of Brazil, São Paulo, Brazil). Samples of 10 mg were weighed and put in a platinum sample holder. The furnace atmosphere was saturated with super-pure nitrogen with a flow rate of 50 mL/min. The analysis temperature range was from 25 to 800 °C.
X-ray diffraction
The structure of Arabic gum and particles were analyzed by X-ray diffraction (diffractometer Siemens 5100) according to Xiao et al. (2015). The measurements were obtained by 2θ scanning between 5° and 55° and a scanning rate of 6 °C/min, a tension of 40 kV and current of 30 mA.
Accelerated oxidation by Rancimat
Accelerated oxidation tests were carried out with the Rancimat equipment (model 873, Metrohm, Switzerland). The samples were subjected to heating under a purified air flow rate of 20 L/h at 90 °C. The induction time of the sample, measured in hours, was used as oxidative stability index (OSI). Four g of pure oil and 1.5 g of dried microparticle were used.
Statistical analysis
All experiments were performed in triplicate, with the exception of Rancimat, in duplicate. The data were statistically analyzed using SAS statistical software (Statistic Analysis System), version 9.3, by ANOVA and Tukey test, at 5% of probability.
Results and discussion
Study of emulsions
To select the most stable emulsion, before application of SA, two formulations were tested, varying the ratio of aqueous and oil phase. The two emulsions were stable visually for 7 days, besides not presenting morphological difference by optical microscopy (micrographs not presented). The great stability of the two formulations can be explained due to the emulsifying function of Arabic gum added in the aqueous phase. Emulsions were also evaluated regarding the instability index, using the conditions which estimate the period of 48 days of storage (corresponding to 30 min of analysis). The formulation related to the ratio of oil to aqueous phase 1:3 was the most stable, with the lowest instability index (0.101) (Fig. 1a).
Fig. 1.
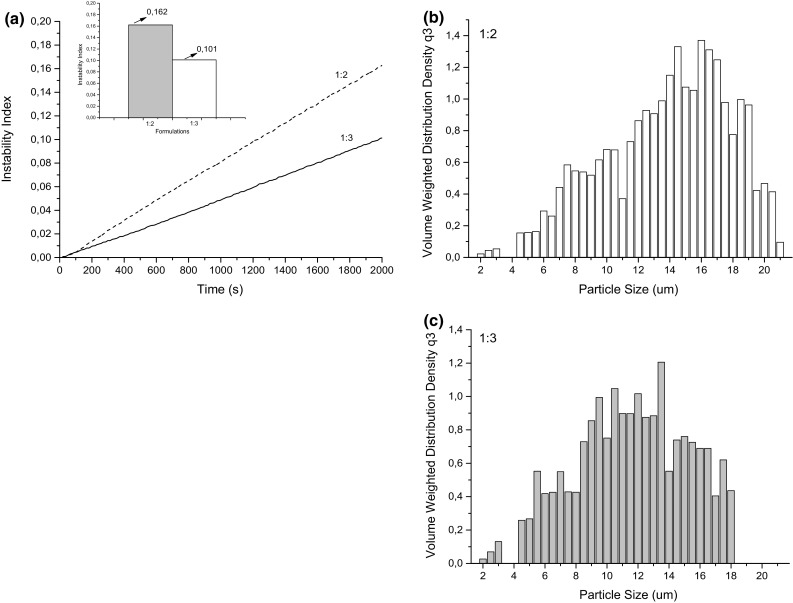
a Instability index of each emulsion according to the analyzed time and general instability index of the emulsion; b droplet size distribution of the emulsions in the ratio of 1:2 and c 1:3 of oil phase: aqueous phase
It is possible to verify that there was a difference between the emulsions from 200 s of analysis (Fig. 1a), time which the best behavior for the emulsion with the proportion of 1:3 of oil phase: aqueous phase was already noticed. The values of average size were 14.31 and 10.03 µm for emulsions with the proportion of 1:2 and 1:3 of oil phase: aqueous phase and a unimodal distribution of droplet size were obtained for both formulations (Fig. 1b).
According to the above, the emulsion related to the ratio of 1:3 of oil phase: aqueous phase was chosen for the addition of SA and to continue the research.
Definition of SA incorporation way
To define the best incorporation of SA, it was added in two different ways according to Comunian et al. (2016b) and subjected to spray drying and freeze drying, resulting in four treatments: (1) S1 (magnetic stirring for 1 min and spray dried), (2) S12 (magnetic stirring for 12 h and spray dried), (3) L1 (magnetic stirring for 1 min and freeze-dried) and (4) L12 (magnetic stirring for 12 h and freeze-dried). For the treatments stirred for 12 h, SA was added in the prepared O/W emulsion. However, for the treatments in which SA was stirred for 1 min, it was added in the aqueous solution of Arabic gum before preparation of the emulsion.
The treatments were analyzed in relation to accelerated oxidation by Rancimat, obtaining induction time values (or oxidative stability index) (OSI) of 7.51 ± 0.01, 6.91 ± 0.06, 11.71 ± 0.74 and 11.02 ± 1.15 h, respectively.
The periods of 1 min and 12 h were based on the procedure used by Comunian et al. (2016b), in which the authors added SA to the microcapsule obtained by complex coacervation. In this case, SA acted as antioxidant and crosslinker. According to these authors, the crosslinking reaction of SA occurred mainly with the polysaccharide. For this reason, SA was incorporated in the formulations of this research in order to, besides acting as an antioxidant, to verify its crosslinking function. However, this function was effective only when applied in microparticles dried by freeze-drying, as it will be observed through the results and discussion. Furthermore, its antioxidant function was significant for both drying methods when compared to the oil not encapsulated.
Moreover, it can be said that there was no significant difference in the incorporation of SA when stirring continuously for 1 min and for 12 h and when subjected to the same drying technique; different SA incorporations did not influence its function as an antioxidant. Thus, the continuation of the research was done with the incorporation of SA in the microparticles kept under stirring for only 1 min.
Characterization of microparticles
Morphology by SEM
The morphology of the microparticles was evaluated by SEM and are presented in Fig. 2. Regarding microparticles obtained by spray drying, it was not possible to observe difference between the ones produced with different concentrations of SA. In general, the microparticles showed a broad particle size distribution (which corroborate to Sect. 3.3.4), rounded shape and surface with formation of concavities. This formation of concavities may be explained due to shrinkage of the particles due to evaporation of water during drying in the atomizer (Comunian et al. 2011). Botrel et al. (2014) studied the optimization of drying of fish oil by spray drying using inulin as the wall material and obtained morphology similar to that obtained for particles containing echium oil.
Fig. 2.
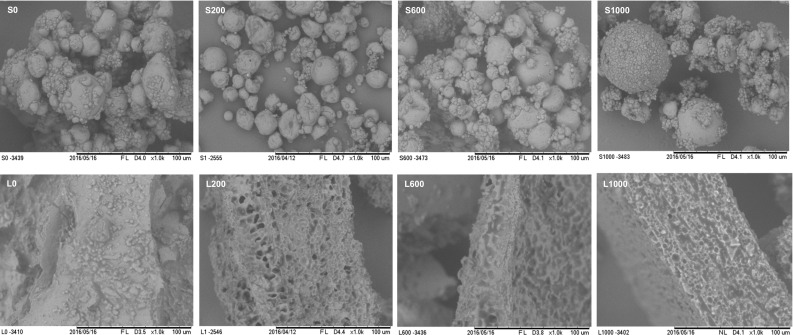
Micrographs obtained by scanning electron microscopy of dried treatments by spray dryer: S0, S200, S600 and S1000 (with 0, 200, 600 and 1000 ppm of sinapic acid, respectively); and treatments dried by freeze dryer: L0, L200, L600 and L1000 (with 0, 200, 600 and 1000 ppm of sinapic acid, respectively)
Regarding freeze-dried treatments, it was also not observed difference in the morphology of these treatments according to the way of SA addition, since non-defined shape particles were obtained. The rounded shape of particles obtained by spray drying has the advantage of facilitating the application and the flow of this material, properties that are not possible with the material obtained by lyophilization. On the other hand, the drying by lyophilization does not use high temperatures as the atomization process, which decreases the chance of degradation of oil by oxidation.
Aw, moisture, water solubility and hygroscopicity
The values of Aw, moisture, hygroscopicity, and solubility of each treatment are shown in Table 1.
Aw values ranged from 0.091 to 0.365 and were considered low and ideal for storage of material since it ensured microbiological stability. There were significant differences between the dried treatments by different methods; lyophilized particles presented lower Aw values than atomized ones. This can be explained due to the rapid drying process in the atomizer; it is related to a certain amount of unbounded water still remaining in the sample. Comunian et al. (2016a) also co-encapsulated echium oil with phenolic compounds, however by complex coacervation technique using gelatin and Arabic gum as wall materials and obtained Aw values larger than the values obtained for particles of this work, being within the range from 0.36 to 0.51. This difference can be attributed to different methods of encapsulation and the use of gelatin, besides Arabic gum.
Moisture and hygroscopicity values the range of 3.22–4.89% and 1.32–2.38 g water/100 g sample were obtained, respectively. These values were considered low and facilitate storage and handling. The moisture values were similar for treatments atomized and freeze-dried. Anwar and Kunz (2011) studied the influence of spray drying and freeze drying of fish oil microcapsules and obtained moisture values from 2.23 to 3.01% and 2.7 to 3.23% for treatments dried by atomization and freeze-drying, respectively, which were lower than those obtained for the echium oil particles. In this case, this difference can be attributed to the combinations of materials (SSPS—soybean soluble polysaccharide, maltodextrin, hydroxypropyl-beta-cyclodextrin, and OSA-starch) and the different methods of encapsulation. Comunian et al. (2016a) evaluated the moisture of echium oil microcapsules with phenolic compounds obtained by complex coacervation using gelatin and Arabic gum as wall materials, and obtained moisture values from 6.9 to 9.4%, values nearly twice than obtained for the atomized and freeze-dried echium oil particles. Botrel et al. (2014) reported moisture values of 1.89% for fish oil particles dried by spray dryer using inulin as the carrier. The low values of moisture are important to ensure the stability of atomized products, because, according to Rosenberg et al. (1990), the increased moisture of the particles can cause the merging of them, reducing the retention of encapsulated material.
According to Nadeau and Puiggali (1995), the hygroscopicity can be defined as the measurement of food ability to retain moisture from the environment and it is important for the storage powders. The hygroscopicity values obtained for the echium oil particles were from 5 to 7 times smaller than observed by Botrel et al. (2014) (from 9.1 to 11.9 g/100 g of sample). This large difference can be explained due to different wall materials and parameters used during drying.
In relation to the solubility, values within the range 57–68% were obtained. This high water solubility is a positive factor in the case of echium oil particles since it indicates that the presence of Arabic gum provides dispersibility of the oil in water, facilitating its use in foods. Botrel et al. (2014) obtained solubility values within the range 71.9–84.2%, greater values than those obtained for the echium oil particles, probably due to the different materials used as carrier agents.
Regarding the incorporation of SA, it is important to emphasize that its application did not influence on the Aw, moisture, solubility, and hygroscopicity of echium oil particles. Even presenting statistical difference, as shown in Table 1, this difference can be considered minimal.
Particle size and particle size distribution for particles atomized
The average particle size values and particle size distribution of each treatment dried by atomization are shown in Table 1 and Fig. 3. Values of particle size, even with a broad particle size distribution, were within the range from 2.3 to 5.14 µm and presented no significant difference between the treatments; different SA concentrations did not affect the average particle size.
Fig. 3.
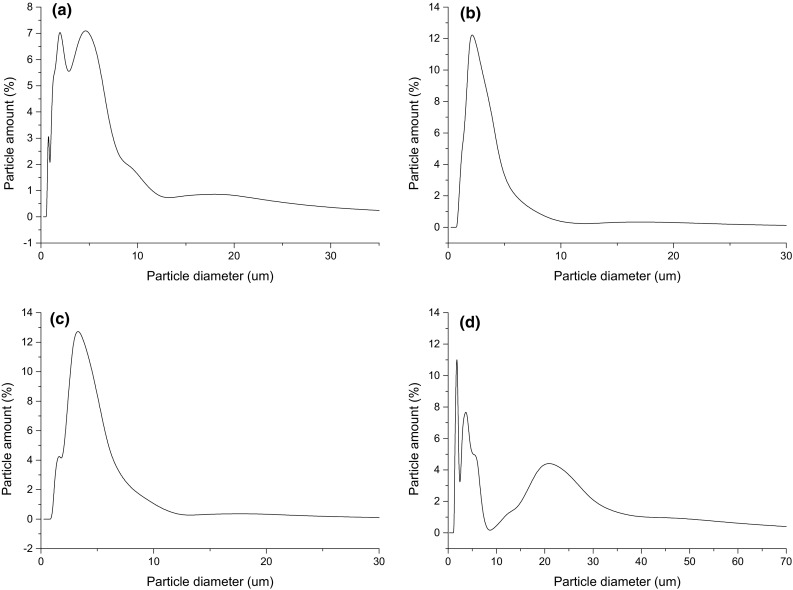
Particle size distribution of the atomized treatments: a S0 (without the addition of sinapic acid); b S200 (200 ppm of sinapic acid); c S600 (600 ppm of sinapic acid) and d S1000 (1000 ppm of sinapic acid)
The treatments with 200 and 600 ppm of SA showed particle size distribution with unimodal behavior, while treatments without SA and 1000 ppm of it showed multimodal behavior with three populations; the particles were not homogenous, result from the agglomerating of them. According to Comunian et al. (2011), it can be considered a positive factor since smaller particles occupy the space among the larger, reducing the total volume.
Values of particle sizes from 9.6 to 13.7 µm were obtained by Botrel et al. (2014) when fish oil was atomized using inulin as wall material. Polavarapu et al. (2011) obtained particle sizes from 0.41 to 0.43 µm when studied the physicochemical characteristics of fish oil encapsulated by atomization using pectin from beet sugar as a carrier. This wide range can be explained due to the different compositions of treatments and process parameters used.
Thermogravimetric analysis (TGA)
Thermogravimetric analysis is being widely used to evaluate the thermal stability and the weight loss of different materials according to a wide range of temperature. The echium oil particles submitted to the same type of drying showed no difference in thermogravimetric behavior; different SA concentrations did not influence on weight loss during the temperature range analyzed, mainly up to the temperature of 250 °C, an important factor since the high temperatures during food processing do not exceed 150–160 °C.
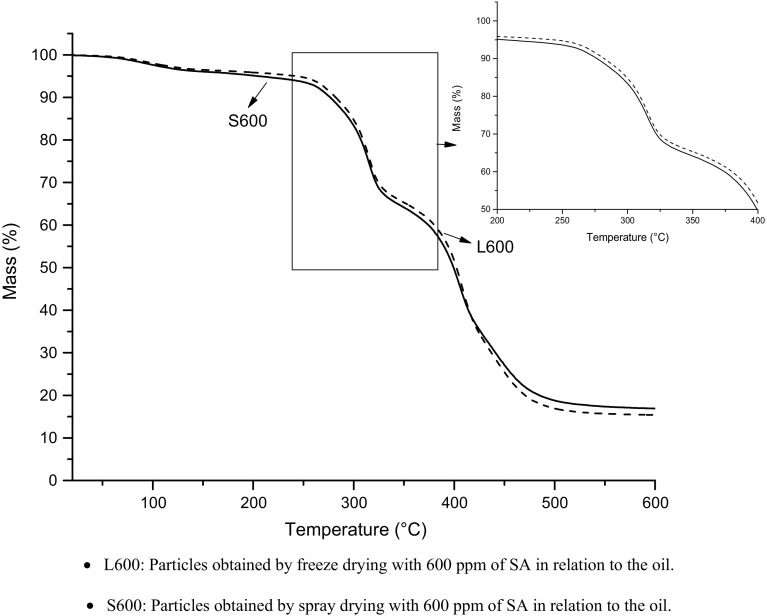
In relation to different drying techniques, particles obtained by spray drying exhibited behavior slightly weaker than particles obtained by freeze-drying (Fig. 4); this can be explained due to the conditions used during these drying processes: high temperature and exposure to oxygen in the drying by spray dryer and low temperature and vacuum in the drying by lyophilization. Moreover, the crosslinking reaction of SA and Arabic gum was observed by Comunian et al. (2016b, 2017a) when it was added in microcapsules obtained by complex coacervation using gelatin-Arabic gum and gelatin-cashew gum combinations as wall materials. According to this affirmation, the function of SA as crosslinker can be also observed in the freeze-dried emulsions, since these particles were more thermally stable than particles obtained by spray-drying. This result corroborates with those obtained by accelerated oxidation by Rancimat (Sect. 3.3.6), which shows that particles obtained by freeze-drying showed longer induction time than particles obtained by spray drying, i.e., greater oxidative protection to the encapsulated oil, which can also be attributed to SA crosslinker function, besides the process parameters.
Fig. 4.
TG curves for treatments S600 and L600 (treatments with 600 ppm of sinapic acid, submitted to spray drying and freeze drying, respectively)
The treatments showed three weight loss steps (Fig. 4). The first step is observed due to the loss of water in the range of 25–100 °C. The other two steps are observed within the range of 250–400 °C and 400–500 °C. According to Xiao et al. (2015), these last two steps of weight loss can be attributed to the decomposition of materials, in this case, Arabic gum and echium oil. Moreover, the fact that the particles were stable up to the temperature of 250 °C indicates that the method of emulsification followed by spray drying or freeze drying was feasible for oil protection.
X-ray diffraction
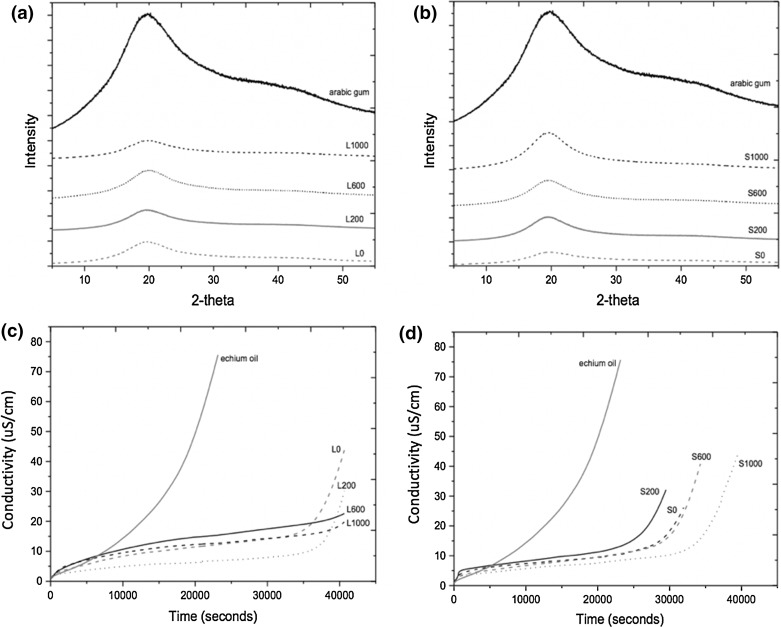
The x-ray diffraction analyzes are used in studies of encapsulation in order to check the state of the material (crystalline or amorphous). The diffractograms obtained for the echium oil and SA particles produced by spray drying or lyophilization, showed broad peaks, indicating that this material has an amorphous phase. This behavior was expected since the material used as carrier agent is Arabic gum (Fig. 5a, b).
Fig. 5.
X-ray diffraction of particles obtained by a lyophilization with 0, 200, 600 and 1000 ppm of sinapic acid in relation to the oil and b spray drying with 0, 200, 600 and 1000 ppm of sinapic acid in relation to the oil and behavior of accelerated oxidation of pure oil and particles obtained by: c lyophilization with 0, 200, 600 and 1000 ppm of sinapic acid in relation to the oil and d spray drying with 0, 200, 600 and 1000 ppm of sinapic acid in relation to the oil
Different characteristics were obtained by Cerimedo et al. (2014) when they encapsulated fish oil in emulsion using trehalose and sodium caseinate as wall materials followed by freeze drying. In this case, the samples showed crystalline characteristics due to the presence of trehalose. According to Cerimedo et al. (2014), the protection function of the matrix which surrounds the encapsulated material is lost when in the crystalline state due to the release of encapsulated material. In other words, the amorphous character of echium oil particle is a positive factor in the protection of this oil.
Accelerated oxidation by Rancimat
The analysis of accelerated oxidation by Rancimat was performed in order to verify the oxidation of the oil encapsulated by emulsification followed by different drying techniques, besides the different concentrations of SA (Table 1 and Fig. 5c, d).
The spray dried treatments showed oxidation index values within the range 7.8–9.5 h, while the pure oil oxidized in 5.3 h. So, the encapsulation and drying by atomization increased the oxidative stability of oil in 3 h. The freeze-dried treatments showed OSI values between 10.4 and 12.9 h. Therefore, the encapsulation by emulsion followed by lyophilization promoted an oxidative stability twice higher in the oil when compared to the free one. Thus, the freeze-drying process was more effective than spray drying. This can be explained due to high exposure to oxygen at the time of nebulization in the spray dryer, which does not happen with the freeze-dried particle. Furthermore, due to the smaller particle size obtained by atomization, they have higher exposure area, resulting in degradation of the oil. It is also important to note that the high temperature used in the spray dryer is crucial to start the oxidation process, mainly to compounds which are sensitive to heat, such as echium oil. In addition, according to Anwar and Kunz (2011), during the spray drying process, there is the breakdown of the large emulsion droplets, which results in a higher remaining oil on the surface of particles, and consequently, the oil is exposed to oxygen and therefore subject to oxidation.
It is also important to highlight the protection promoted by the crosslinking reaction of SA in lyophilized particles. Besides the peculiarities of this drying technique, this phenolic compound promoted the formation of a more resistant matrix, obtaining greater protection to the echium oil (Treatments L200, L600, and L1000). Gallardo et al. (2013) studied linseed oil microencapsulation by spray drying using Arabic gum, maltodextrin, methylcellulose and whey protein isolate as carriers and evaluated the accelerated oxidation of these particles, resulting in OSI values from 3.8 to 9.5 h, while the pure linseed oil showed a value of 2.04 h. Encapsulated echium oil showed values of oxidation index of 12.9 h, in other words, the encapsulation by emulsion followed by lyophilization was more effective than spray drying technique used by Gallardo et al. (2013).
Comunian et al. (2016a, b) studied the microencapsulation of echium oil by complex coacervation using gelatin-Arabic gum and gelatin-cashew gum as wall materials, respectively, and obtained oxidation index values within the range 9.97–18.77 h and 8.7–26.5 h, respectively, values higher than those obtained for the echium oil particles. This difference can be explained due to the higher concentration of SA used in coacervated capsules and obtainment of a more rigid capsule since the wall is formed of two polymers linked by ionic interaction and not only Arabic gum. However, even so, the values obtained in this work are satisfactory since the encapsulated oil was much more stable than free one.
Conclusion
The co-encapsulation of echium oil and SA by emulsification followed by spray or freeze drying were suitable techniques for the oxidative protection of the oil, since all treatments promoted protection of it; the treatment in which 600 ppm of SA was added and the particle dried by lyophilization (L600) increased oxidative stability of the oil in 2.5 times in relation to the free one. Furthermore, the morphology and values of hygroscopicity, moisture and solubility were optimum for the application, storing and handling of the powder.
Acknowledgements
The authors thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the scholarship for T. A. Comunian (Process 2013/25862-5) and De Wit Speciality Oils for the echium oil donation.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anwar SH, Kunz B. The influence of drying methods on the stabilization of fish oil microcapsules: comparison of spray granulation, spray drying, and freeze drying. J Food Eng. 2011;105:367–378. doi: 10.1016/j.jfoodeng.2011.02.047. [DOI] [Google Scholar]
- Berti M, Johnson BL, Dash S, Fischer S, Wilckens RH. Echium: a source of stearidonic acid adapted to the northern great plains in the US. Issues in new crops and new uses. Alexandria: ASHS Press; 2007. [Google Scholar]
- Botrel DA, Borges SV, Fernandes RVB, Carmo EL. Optimization of fish oil spray drying using a protein: inulin system. Dry Technol. 2014;32(3):279–290. doi: 10.1080/07373937.2013.823621. [DOI] [Google Scholar]
- Cai YZ, Corke H. Production and properties of spray-dried Amaranthus betacyanin pigments. J Food Sci. 2000;65(6):1248–1252. doi: 10.1111/j.1365-2621.2000.tb10273.x. [DOI] [Google Scholar]
- Cano-Chauca M, Stringheta PC, Ramos AM, Cal-Vidal J. Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov Food Sci Emerg Technol. 2005;5(4):420–428. doi: 10.1016/j.ifset.2005.05.003. [DOI] [Google Scholar]
- Cerimedo MSA, Candal RJ, Herrera ML. Physical properties and oxidative status of concentrated-from-fish oils microencapsulated in trehalose/sodium caseinate matrix. Food Bioprocess Technol. 2014;7:3536–3547. doi: 10.1007/s11947-014-1367-x. [DOI] [Google Scholar]
- Comunian TA, Monterrey-Quintero ES, Thomazini M, Balieiro JCC, Piccone P, Pittia P, Favaro-Trindade CS. Assessment of production efficiency, physicochemical properties and storage stability of spray-dried chlorophyllide, a natural food colourant, using gum Arabic, maltodextrin and soy protein isolate-based carrier systems. Int J Food Sci Technol. 2011;46:1259–1265. doi: 10.1111/j.1365-2621.2011.02617.x. [DOI] [Google Scholar]
- Comunian TA, Boillon MRG, Thomazini M, Nogueira MS, Castro IA, Favaro-Trindade CS. Protection of echium oil by microencapsulation with phenolic compounds. Food Res Int. 2016;88:114–121. doi: 10.1016/j.foodres.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Comunian TA, Gomez-Estaca J, Ferro-Furtado R, Conceição GJA, Moraes ICF, de Castro IA, Favaro-Trindade CS. Effect of different polysaccharides and crosslinkers on echium oil microcapsules. Carbohydr Polym. 2016;150:319–329. doi: 10.1016/j.carbpol.2016.05.044. [DOI] [PubMed] [Google Scholar]
- Comunian TA, Chaves IE, Thomazini M, Moraes ICF, Ferro-Furtado R, Castro IA, Favaro-Trindade CS. Development of functional yogurt containing free and encapsulated echium oil, phytosterol and sinapic acid. Food Chem. 2017;237:948–956. doi: 10.1016/j.foodchem.2017.06.071. [DOI] [PubMed] [Google Scholar]
- Comunian TA, Ravanfar R, Castro IA, Dando R, Favaro-Trindade CS, Abbaspourrad A. Improving oxidative stability of echium oil emulsions fabricated by microfluidics: effect of ionic gelation and phenolic compounds. Food Chem. 2017;233:125–134. doi: 10.1016/j.foodchem.2017.04.085. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker G. Recent strategies in spray drying for the enhanced bioavailability of poorly water-soluble drugs. J. Control Release. 2018;269:110–127. doi: 10.1016/j.jconrel.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Eastman JE, Moore CO (1984) Cold water soluble granular starch for gelled food composition. U.S. Patent 4465702
- Favaro-Trindade CS, Pinho SC, Rocha GA. Microencapsulation of food ingredients. Braz J Food Technol. 2008;11(2):103–112. [Google Scholar]
- Gallardo G, Guida L, Martinez V, Lopez MC, Bernhardt D, Blasco R, Pedroza-Islas R, Hermida LG. Microencapsulation of linseed oil by spray drying for functional food application. Food Res Int. 2013;52:473–482. doi: 10.1016/j.foodres.2013.01.020. [DOI] [Google Scholar]
- Ho KKHY, Schroën K, Martin-González FS, Berton-Carabin CC. Physicochemical stability of lycopene-loaded emulsions stabilized by plant or dairy proteins. Food Struct. 2017;12:34–42. doi: 10.1016/j.foostr.2016.12.001. [DOI] [Google Scholar]
- Hoffmann W, Schrader K. Dispersion analysis of spreadable processed cheese with low content of emulsifying salts by photocentrifugation. Int J Food Sci Technol. 2015;50:950–957. doi: 10.1111/ijfs.12725. [DOI] [Google Scholar]
- Kralovec JA, Zhang S, Zhang W, Barrow CJ. A review of the progress in enzymatic concentration and microencapsulation of omega-3 rich oil from fish and microbial sources. Food Chem. 2012;131:639–644. doi: 10.1016/j.foodchem.2011.08.085. [DOI] [Google Scholar]
- Moura SCSR, Berling CL, Germer SPM, Alvim ID, Hubinger MD. Encapsulating anthocyanins from Hibiscus sabdariffa L. calyces by ionic gelation: pigment stability during storage of microparticles. Food Chem. 2018;241:317–327. doi: 10.1016/j.foodchem.2017.08.095. [DOI] [PubMed] [Google Scholar]
- Nadeau JP, Puiggali JR. Séchage: des processus physiques aux procédés industriels. 1. Paris Cedex: Technique et Documentation-Lavoisier; 1995. [Google Scholar]
- Polavarapu S, Oliver CM, Ajlouni S, Augustin MA. Physicochemical characterisation and oxidative stability of fish oil and fish oil-extra virgin olive oil microencapsulated by sugar beet pectin. Food Chem. 2011;127:1694–1705. doi: 10.1016/j.foodchem.2011.02.044. [DOI] [Google Scholar]
- Rosenberg M, Kopelman IJ, Talmon Y. Factors affecting retention in spray-drying microencapsulation of volatile materials. J Agric Food Chem. 1990;36:1288–1294. doi: 10.1021/jf00095a030. [DOI] [Google Scholar]
- Vincenzetti S, Cecchi T, Perinelli DR, Pucciarelli S, Polzonetti V, Bonacucina G, Ariani A, Parrocchia L, Spera DM, Ferretti E, Vallesi P, Polidori P. Effects of freeze-drying and spray-drying on donkey milk volatile compounds and whey proteins stability. LWT Food Sci Technol. 2018;88:189–195. doi: 10.1016/j.lwt.2017.10.019. [DOI] [Google Scholar]
- Xiao Z, Li W, Zhu G. Effect of wall materials and core oil on the formation and properties of styralyl acetate microcapsules prepared by complex coacervation. Colloid Polym Sci. 2015;293:1339–1348. doi: 10.1007/s00396-015-3515-x. [DOI] [Google Scholar]