Abstract
The aim of this study was to determine the most appropriate culture combination and scalding temperature to provide standardization in the production of Mihaliç cheese. For this purpose, starter culture combinations consisting of Propionibacterium freudenreichii, Streptococcus thermophilus, Lactobacillus helveticus and Leuconostoc mesentroides subsp. cremoris were used. Two scalding temperatures (40 °C and 45 °C) were used for the Mihaliç cheese samples with same culture combinations. This study investigated the composition, acidity, proteolysis, lipolysis, microbial counts and sensory properties of Mihaliç cheese during 90 days of ripening. The lipolysis level was higher for cheese scalded at 40 °C. Eye formation, which is a characteristic feature of Mihaliç cheese, was seen in all cheese samples. The viability of the starters generally decreased significantly during ripening, and starter culture usage did not affect the characteristics of the cheeses adversely, which contrarily resulted in better sensory characteristics and increased ripening indexes in comparison to the control cheese. Especially the starter culture combination including P. freudenreichii, S. thermophilus and L. helveticus resulted in more desirable sensory characteristics.
Keywords: Mihaliç cheese, Propionic acid, Starter culture, Cheese ripening
Introduction
In Turkey, there are approximately 150 cheese varieties. Kashar, White cheese and Tulum cheese are the most popular ones with economic value. However, other traditional cheeses are getting to become popular (Bulut Solak and Akın 2013). Mihaliç cheese, which has been produced approximately for the last 250 years, is considered to be one of the oldest traditional cheeses in Turkey. It is also known as Maglıc, Mahlıç or Kelle cheese. It is a semi-hard cheese with a pale cream, yellow or straw yellow colour (Aday and Karagül Yüceer 2014). Mihaliç is a rather salty cheese with roundish holes of 3–4 mm diameter gradually decreasing from the centre to the surface (Özcan and Kurdal 2012). Propionic acid bacteria constitute the main flora of Mihaliç cheese and the cheese has a unique flavour and characteristic 3–4 mm eyes due to propionic acid fermentation. Propionic acid bacteria ferment lactate, which causes release of CO2, thus creating eye formation. Mihaliç cheese is mostly produced and consumed in the provinces of Bursa and Balıkesir in the Marmara Region of Turkey. Due to the production from raw milk and spontaneous fermentation without starter cultures, the native microflora of raw milk and natural contamination microorganisms are very important for specific properties of this cheese. In case of heat treatment applied to milk, starter cultures have to be used in cheese production for ensuring characteristic features. In cheese production, heat treatment reduces the diversity and level of raw milk microflora, and therefore, the biochemistry of ripening changes. Eventually, the desired flavour cannot be obtained. At this point, pasteurizing cheese milk, as well as using starter cultures, is a good alternative for obtaining quality. The use of commercial starters in cheese production decreases the probability of development of undesired microorganisms due to the indigenous flora of milk and ensures the production of a standardised product (Garcia et al. 2014). Starter bacteria that are encountered the most often are members of the genera Lactococcus, Lactobacillus, Leuconostoc and Enterococcus. Moreover, propionic acid bacteria are the characteristic microflora associated with Swiss-type cheeses. Mihaliç cheese undergoes propionic acid fermentation post-manufacture and the propionic and acetic acids that are produced contribute to the development of characteristic flavours of these cheeses (Beresford et al. 2001). Ripening has a major importance in cheese production. Cheeses have a specific pattern of proteolysis and are mainly characterised and classified using proteolytic data. Ripening index calculated using proteolytic data is used as an indicator of proteolysis in cheese (Hayaloğlu and Karabulut 2013). Cheese ripening is characterised by a series of microbiological and biochemical changes. Proteolysis is the most complex and maybe one of the most important primary biochemical events that occur during ripening of cheeses (McSweeney 2004). Proteolysis involves destabilisation of the casein matrix through the release of peptides and amino acids which have a direct effect on flavour and texture, where free amino acids are also substrates for a series of catabolic reactions that generate many important flavour compounds thiols, esters and others. Most of information available concerning the biochemical modifications that occur during ripening is related to proteolysis (Fox and McSweeney 1996; Grappin and Beuvier 1998). Moreover, lipolysis is one of the major events that contribute to flavour development with proteolysis in cheese. Hydrolysis of lipids is also used as a quality parameter for most types of matured/ripened cheeses. The level of lipolysis depends on the types of cheese (Hayaloğlu and Özer 2011). Extensive lipolysis is inevitable for the cheeses made from raw milk regarding indigenous lipase that is the main lipolytic agent in raw milk. However, lipolytic agents are also originated from rennet paste, starter, adjunct starter, non-starter bacteria and exogeneous lipase.
In Turkey, the manufacture of Mihaliç cheese has not reached an industrial level yet. So, a standard product cannot be obtained even in the same plant because of variable manufacturing conditions as well as milk quality. Additionally, manufacturers who cannot afford the cost of production distribute Mihaliç cheeses on the market although the cheese has not fully ripened. Thus, the objective of this study was to obtain a standard and healthy product. For this purpose, the effect of three different starter culture combinations on the composition, proteolytic, lipolytic, microbiologic and sensory characteristics of pasteurized Mihaliç cheeses were investigated. Besides, there is limited information in the literature regarding the effects of different scalding temperatures on the ripening parameters and microbiological properties of Mihaliç cheese. This study could also determine the effects of different scalding temperatures on the characteristics of Mihaliç cheese. This is necessary to suggest optimal starter culture combination and scalding temperature to produce industrial Mihaliç cheese while preserving the cheese’s traditional characteristics.
Materials and methods
Starter cultures
Commercial starter culture containing Streptococcus thermophilus (ST-B01) and Lactobacillus helveticus (LH-B02) were obtained from Chr. Hansen Holding A/S (Hoersholm, Denmark). Leuconostoc mesentroides subsp. cremoris NRRL B-3252 was obtained from ARS culture collection (Washington, USA). Propionibacterium freudenreichii (Propionici-LI061) was obtained from CSL Centro Sperimentale del Latte, Italy.
Mihaliç cheese production
The traditional method was used for Mihaliç cheese production. Seven different cheeses including a control cheese were produced. Cheese milk was inoculated with three different culture combinations, and two different scalding temperatures were applied. The culture combinations and scalding temperatures that were used for cheese manufacturing are given in Table 1. Raw cow milk was used during the manufacture of the control cheese, whereas the milk was pasteurized for other cheeses, food-grade calcium chloride was added to the milk on a level of 20 g/100 L after cooling. The milk was inoculated with one of the cultures that were tested and then left for approximately 30 min. Liquid rennet was added to cheese milk by an amount sufficient to coagulate the milk within 60 min. Following the coagulation, the coagulum was cut into cubes (approximately 1 cm3). The whey/curd mixture was then scalded by pouring hot water at 70 °C until the mixture’s temperature reached 40 °C (for HP40, StHP40, LeuSt40) or 80 °C hot water until the mixture’s temperature reached 45 °C (for HP45, StHP45, LeuSt45) within about 30 min. After resting for 15 min, the curds were transferred into a cotton cloth for whey drainage for 2 h. During whey drainage, the cheese cloth was pierced periodically with a needle to accelerate whey-off. After moulding and pressing for 15 h, the Mihaliç cheese blocks were left in an initial brine of 12% for 6 days at 24 ± 1 °C until eye formation occurred, followed by 10 days in 15% brine at 6 °C. Following portioning (~ 400 g) and vacuum packing in polyethylene foils, the cheeses were ripened at 4 ± 1 °C for 3 months. Each production process was carried out in two replicates, and the cheeses were analysed on the 1st, 15th, 30th, 60th and 90th days of ripening.
Table 1.
Experimental design of Mihaliç cheese production
| Cheese | Starter culture | Scalding temperature | ||||||
|---|---|---|---|---|---|---|---|---|
| ST-B01 (1%) | ST-B01 (0.5%) | LH-B02 (1%) | LH-B02 (0.5%) | NRRL B-3252 (0.5%) | Propionici (0.1%) | 40 °C | 45 °C | |
| HP40 | X | X | X | |||||
| HP45 | X | X | X | |||||
| StHP40 | X | X | X | X | ||||
| StHP45 | X | X | X | X | ||||
| LeuSt40 | X | X | X | |||||
| LeuSt45 | X | X | X | |||||
| Control | X | |||||||
Physicochemical analyses
The cheese samples were analysed for total solids (TS), fat and salt according to the method reported by Oysun (2001). Protein (total nitrogen × 6.38) was determined by the micro-Kjeldahl method. The pH values were measured by using a Hanna 210 pH meter. The ripening indexes regarding pH 4.6 soluble nitrogen (pH 4.6-SN) and nitrogen soluble in 12% trichloroacetic acid (TCA-SN) were estimated as percentage of total nitrogen (TN) to monitor the degree of proteolysis based on the method reported by Hayaloğlu et al. (2011). The acid degree value (ADV) was determined according to the method described by Renner (1993).
Microbiological analyses
For microbiological analyses, 10 g of cheese was added and homogenised in 90 mL of sterile Ringer’s solution and subjected to serial dilutions. Streptococcus thermophilus counts were determined on ST Agar as described by Dave and Shah (1996). Lactobacillus helveticus was enumerated on MRS-Agar (pH 5.4) according to the method used by Valence et al. (2000). Leuconostoc mesentroides subsp. cremoris was enumerated on MRS-Agar-containing vancomycin (30 µg/ml) (Güley 2008), and propionibacteria were enumerated on Yeast extract lactate agar (YELA) after anaerobic incubation at 30 °C for 7 days (Darılmaz 2010).
Sensory properties
For sensory properties, ten staff members of the Department of Dairy Technology evaluated the cheese samples by a scoring test (1–9 points). Panellists assigned scores in partitioned booths equipped with daylight to each cheese sample for colour, regular eye formation, texture, salinity and taste.
Statistical analyses
The effects of ripening and differences between the samples were tested by ANOVA, and significance was indicated by p < 0.05, using SPSS 15.0 (SPSS Inc., Chicago, USA). The significant differences were compared by using Duncan’s Multiple Range Test.
Result and discussion
Chemical composition
Compositions of experimental cheeses are given in Table 2. Regarding the total solid contents, there were no significant differences between the cheeses (p > 0.05). This may be attributed to the similar initial pH values of the cheese samples. This is because a significant decrease in pH level can increase the amount of dehydration from the casein matrix (Özcan and Kurdal 2012).
Table 2.
Gross composition of Mihaliç cheeses
| Samples | |||||||
|---|---|---|---|---|---|---|---|
| HP40 | HP45 | StHP40 | StHP45 | LeuSt40 | LeuSt45 | Control | |
| Total solid (%) | 56.40A | 56.71A | 57.64A | 58.69A | 56.87A | 58.61A | 58.50A |
| Fat (%) | 23.25C | 22.75BC | 21.50A | 22.50BC | 22.25AB | 23.00BC | 22.25AB |
| Protein (%) | 26.89AB | 27.16AB | 27.47AB | 28.16CD | 26.57A | 27.11AB | 26.73AB |
| Salt (%) | 6.73D | 5.61AB | 6.67D | 5.15AB | 5.85C | 5.09A | 5.68B |
A,B,C,DMeans in the same row with different superscripts among cheese samples significantly differ (p < 0.05)
The differences between the fat contents of cheeses were significant and ranged from 21.50 to 23.25%. The fat levels detected in our cheeses were lower than the Mihaliç cheeses in the previous studies by Bulut Solak and Akın (2013) and Aday and Karagül Yüceer (2014). The differences between the protein contents of the Mihaliç cheese samples were also significant (p < 0.05). The difference in the levels of protein may have been caused by different starter cultures, enzyme activities, moisture or titratable acidity (Bulut Solak and Akın 2013). Likewise, Özcan and Kurdal (2012) reported that the protein content of Mihaliç cheese samples changed significantly depending on the addition of enzymes and starter culture.
Differences between the salt contents of the cheese samples were significant (p < 0.05). HP40 had the highest salt content (6.73%). The salt contents of the samples in our study were generally lower than those reported by some previous studies on Mihaliç cheese (Anar and Şen 1991; Öner and Aloğlu 2004). This may be attributed to ripening in vacuum packages instead of ripening in brine. Similarly, Hayaloğlu et al. (2012) reported significantly lower salt contents in vacuum-packaged Mihaliç cheeses than brine-salted samples.
The Mihaliç cheeses scalded at different temperatures (40–45 °C) were generally grouped together in terms of their fat and protein contents except for the StHP cheese batches, while StHP45 had higher levels of fat and protein than StHP40 (p < 0.05). However, all cheeses scalded at 40 °C had significantly higher salt content. Similarly, Sheehan et al. (2007) and Yun et al. (1993) reported that the cheeses that were scalded at low temperatures had lower protein but significantly higher salt contents.
Acidity and physicochemical properties
The cheeses’ pH values, ripening index expressed by pH 4.6-SN (pH 4.6-SN, %TN) showing the level of primary proteolysis, ripening index expressed by 12% TCA-SN (12% TCA-SN, %TN) showing the depth of proteolysis and ADV values showing lipolysis rate are given in Table 3. Glycolysis of residual lactose and its constituent monosaccharides, which is one of the three primary biochemical events during the ripening process of cheeses has impact on the acidity of the cheeses (Anar and Şen 1991; Öner and Aloğlu 2004). Ripening had a significant effect on pH values except for the control cheese (p < 0.05). The lowest pH value was observed for StHP40 initially and for StHP45 at the end of ripening depending on the activity of the starter bacteria present in different culture combinations. A slight increase in pH at the end of ripening except the control may have been due to the formation of alkaline compounds. Similarly, Bulut Solak and Akın (2013) attributed the small increase in pH during ripening to limited production of alkaline compounds. While the pH values of the cheese samples were initially close to each other, the differences were statistically significant on further days (p < 0.05).
Table 3.
Acidity and physicochemical properties of Mihaliç cheeses during ripening
| Ripening days | Samples | ||||||
|---|---|---|---|---|---|---|---|
| HP40 | HP45 | StHP40 | StHP45 | LeuSt40 | LeuSt45 | Control | |
| pH | |||||||
| 1 | 5.18 ± 0.00cA | 5.15 ± 0.00cA | 5.00 ± 0.00eA | 5.02 ± 0.00dA | 5.25 ± 0.00cA | 5.06 ± 0.00cA | 5.20 ± 0.00aA |
| 15 | 5.08 ± 0.00bD | 4.81 ± 0.01aC | 4.70 ± 0.00cA | 4.74 ± 0.00cB | 5.21 ± 0.01bE | 4.75 ± 0.00aB | 5.20 ± 0.00aE |
| 30 | 5.09 ± 0.01bE | 4.80 ± 0.00aD | 4.68 ± 0.00bB | 4.57 ± 0.02bA | 5.20 ± 0.00bF | 4.76 ± 0.01aC | 5.18 ± 0.00aF |
| 60 | 4.85 ± 0.00aD | 4.80 ± 0.00aC | 4.65 ± 0.00aB | 4.48 ± 0.03aA | 4.99 ± 0.01aE | 4.98 ± 0.00bE | 5.19 ± 0.00aF |
| 90 | 5.20 ± 0.01dD | 5.00 ± 0.00bC | 4.81 ± 0.01 dB | 4.76 ± 0.00cA | 5.22 ± 0.00bE | 5.19 ± 0.01dD | 5.19 ± 0.00aD |
| pH 4.6-SN (%/TN) | |||||||
| 1 | 11.07 ± 0.16aC | 11.22 ± 0.23abC | 16.34 ± 0.39aD | 16.01 ± 0.18aD | 10.74 ± 0.00aBC | 10.17 ± 0.43aB | 10.74 ± 0.00dA |
| 15 | 10.98 ± 0.33aC | 10.50 ± 0.38aC | 17.21 ± 0.13bD | 16.84 ± 0.16bD | 10.74 ± 0.07aC | 9.92 ± 0.22aB | 7.83 ± 0.08aA |
| 30 | 11.73 ± 0.14bBC | 12.06 ± 0.38bC | 16.73 ± 0.48abD | 16.78 ± 0.01bD | 11.08 ± 0.11aB | 11.27 ± 0.54bB | 9.75 ± 0.12bA |
| 60 | 14.53 ± 0.16cC | 14.77 ± 0.13cC | 18.94 ± 0.13cD | 19.07 ± 0.28cD | 12.09 ± 0.27bB | 12.76 ± 0.30cBC | 10.49 ± 0.08cA |
| 90 | 15.21 ± 0.08dC | 15.27 ± 0.43dC | 19.78 ± 0.14dD | 19.72 ± 0.19dD | 12.86 ± 0.27cB | 12.89 ± 0.17cB | 10.88 ± 0.31dA |
| 12% TCA-SN (%TN) | |||||||
| 1 | 4.25 ± 0.21aB | 4.34 ± 0.22aBC | 4.76 ± 0.03aCD | 4.97 ± 0.23aD | 2.00 ± 0.08aA | 1.81 ± 0.28aA | 2.11 ± 0.01aA |
| 15 | 7.06 ± 0.06bD | 7.00 ± 0.12bD | 6.48 ± 0.05bC | 6.29 ± 0.01bC | 3.98 ± 0.00bB | 4.04 ± 0.18bB | 3.72 ± 0.06bA |
| 30 | 7.25 ± 0.02bcD | 7.50 ± 0.27bcD | 6.49 ± 0.08bC | 6.34 ± 0.05bC | 4.67 ± 0.04cB | 4.35 ± 0.01bcB | 3.81 ± 0.21bA |
| 60 | 7.38 ± 0.06cC | 7.74 ± 0.18cdD | 7.83 ± 0.21cD | 7.97 ± 0.19cD | 4.81 ± 0.06 dB | 4.65 ± 0.02cdB | 3.95 ± 0.06bcA |
| 90 | 7.81 ± 0.08dC | 8.11 ± 0.18dCD | 8.36 ± 0.01dD | 8.78 ± 0.35dE | 5.10 ± 0.02eB | 4.97 ± 0.16 dB | 4.09 ± 0.06cA |
| ADV (mg KOH/g-fat) | |||||||
| 1 | 2.37 ± 0.17aE | 1.84 ± 0.24aD | 1.29 ± 0.03aBC | 1.15 ± 0.20aABC | 0.90 ± 0.06aA | 1.07 ± 0.03bAB | 1.46 ± 0.00aC |
| 15 | 2.54 ± 0.03abF | 2.13 ± 0.08abE | 1.40 ± 0.04aD | 1.26 ± 0,00abC | 1.11 ± 0.04bB | 0.89 ± 0.04aA | 1.49 ± 0.00aD |
| 30 | 2.77 ± 0.11bE | 2.19 ± 0.04bD | 1.59 ± 0.01bC | 1.36 ± 0.01abB | 1.10 ± 0.05bA | 1.06 ± 0.05bA | 1.51 ± 0.00abC |
| 60 | 3.14 ± 0.04cF | 2.22 ± 0.05bE | 1.85 ± 0.11cD | 1.50 ± 0.05bcB | 1.19 ± 0.07bA | 1.16 ± 0.00cA | 1.64 ± 0.00bC |
| 90 | 3.34 ± 0.04cE | 2.47 ± 0.11bD | 2.29 ± 0.06dD | 1.73 ± 0.12cB | 1.38 ± 0.07cA | 1.33 ± 0.02dA | 2.06 ± 0.12cC |
a,b,c,dMeans in the same column with different superscripts significantly differ (p < 0.05)
A,B,C,DMeans in the same row with different superscripts among cheese samples significantly differ (p < 0.05)
The levels of pH 4.6-SN (%TN) increased constantly after 15th day of ripening (p < 0.05), while all cheese batches had their highest values at the end of the ripening period due to the increase in the pH 4.6-SN levels for all samples. Similar trends were also observed for Mihaliç cheese in a previous study (Öner and Aloğlu 2004). StHP40 had the highest initial pH 4.6-SN (%TN) value, followed by StHP45 with a level of 16.34 and 16.01%, respectively. At the end of ripening StHP40 had the highest ripening index value followed by StHP45, whereas the control cheese had the lowest values both initially and at the end of ripening (p < 0.05). These cheese batches had lower pH values that favour the action of chymosin on αs1-casein, resulting in rapid increases in soluble nitrogen. Lower pH may be more favourable for proteolytic activity of residual chymosin (Hayaloğlu et al. 2012; Sheehan et al. 2007). Besides, this may be related to the synergistic effect of the cultures on proteolytic potential. The degree of ripening in cheeses with the same starter cultures were generally similar although they were scalded at 40 °C or 45 °C except for LeuSt40–LeuSt45 on the 15th day of ripening. This may be attributed to the similar proteolytic activity of residual chymosin in cheese samples.
The levels of 12% TCA-SN increased significantly during ripening (p < 0.05), and the highest levels were determined at the end of ripening for all samples, so for the the degree of ripening. In a previous study, the level of total free amino acids in Mihaliç cheeses exhibited a similar trend with soluble nitrogen fractions including 12% TCA-SN (Hayaloğlu et al. 2012). The differences between the 12% TCA-SN (%TN) levels of our cheese samples were significant during ripening (p < 0.05). The highest degree of 12% TCA-SN (%TN) was determined in StHP45 initially and at the end of the ripening period, whereas the lowest values were determined for the control cheese at the end of ripening and for LeuSt45 initially. In previous studies a large increase in the amino acid concentration in cheeses that were inoculated with starter bacteria was reported. This is related to the intracellular enzymes released by bacterial lysis (Kawabata et al. 1997; Kiernan et al. 2000).
The ripening period significantly affected the ADV values of all cheese samples (p < 0.05). While the ADV of the HP and StHP cheese batches showed a continuous increase, the ADV of LeuSt samples showed fluctuations during ripening. Additionally, all cheese samples had their highest ADV values at the end of the ripening period. HP40–HP45 had significantly higher ADV levels at any stage of ripening followed by StHP40–StHP45 due to the culture combination used for their manufacturing, especially propionic acid bacteria. Propionic acid bacteria, especially P. freudenreichii, had 10–100 times higher lipolytic activity than lactic acid bacteria (Chamba and Irlinger 2004). Among the cheeses manufactured with the same culture but at different scalding temperatures, the samples scalded at 40 °C had higher ADV values than the batches scalded at 45 °C in their ripening periods when they were placed in different groups according to Duncan’s test. This may be due to the higher level of the curd-scalding process which inhibits the enzymes that come from the milk and the microorganisms. Therefore, lipolysis developed fairly slowly in the cheeses that were scalded at 45 °C.
Microbial composition
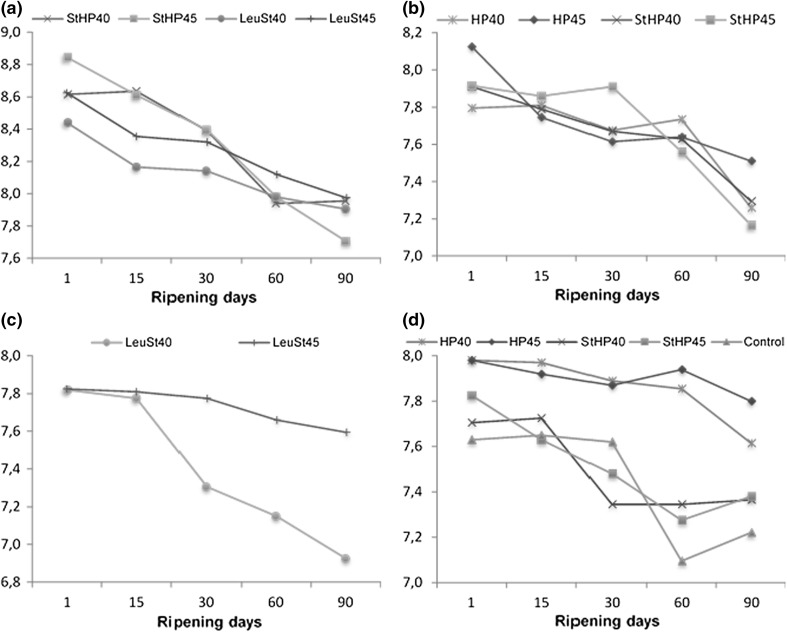
Changes in the populations of S. thermophilus, L. helveticus, Leu. mesentroides subsp. cremoris and P. freudenreichii during ripening are shown in Fig. 1. The viability values of all starters in the cheese samples were found significantly different from each other throughout the ripening period (p < 0.05). The counts of S. thermophilus decreased significantly as the ripening period progressed (p < 0.05). A significant decrease was reported for S. thermophilus in previous studies where it was used in addition to L. helveticus (Ong and Shah 2008) and those with adjunct Lactobacillus strains (Kocaoğlu-Vurma et al. 2008). Among the starters, the highest decline was noticed in the growth rate of S. thermophilus (8.64–7.89 log cfu/g). This decrease may be due to the unfavourable conditions in cheeses such as anaerobic atmosphere because of vacuum packaging, low water activity, low pH, low redox potential and high salt content (Ong and Shah 2008).
Fig. 1.
Changes in aS. thermophilus counts (log cfu/g); bL. helveticus counts (log cfu/g); cL. mesentroides subsp. cremoris counts (log cfu/g); dP. freudenreichii counts (log cfu/g) during ripening (n = 2)
Viable counts of L. helveticus decreased constantly for HP45 and StHP40, while fluctuations were observed in the counts of L. helveticus for HP40 and StHP45. Additionally, all had their lowest counts at the end of ripening (p < 0.05). Porcellato et al. (2013) reported that, microbial counts of Lactobacillus and Lactococcus in cheese made with added propionibacteria showed a large decrease because of high contents of propionic acid that have been correlated with the inhibition of other microorganisms.
A significant decrease was observed for L. mesentroides subsp. cremoris counts. It was reported in a previous study salt concentration did not seem to affect the growth of Leuconostoc strains up to a certain level. On the contrary, low pH seemed to exert a greater effect. Moreover, Liu et al. (1997) reported that low water activity in cheese reduce the activity of L. mesentroides subsp. cremoris.
Viable cell counts of P. freudenreichii showed a significant decrease during ripening. However, among the starters, the lowest decrease was noticed in P. freudenreichii counts. Bulut Solak and Akın (2013) reported a significant decrease in the counts of propionic acid bacteria during ripening for Mihaliç cheese due to the decline in water activity and the amount of nutrition elements, as well as acidity in the cheese Moreover, Sheehan et al. (2008), reported that increased cheese pH was significantly correlated with increased counts of propionic acid bacteria. Porcellato et al. (2013) observed that viable cell counts of propionic acid bacteria for different cheese samples were between 7.0 and 9.4 log cfu/g.
Regarding the scalding temperatures, StHP45 had significantly higher cell counts of S. thermophilus than StHP40. HP45 had higher cell counts of L. helveticus than HP40, StHP45 had higher cell counts of P. freudenreichii than StHP40, and the cell counts of P. freudenreichii for the HP batches were initially the same. So, it may be thought that scalding at 45 °C did not show an inhibition effect on starter bacteria. The viable cell counts of Leu. mesentroides subsp. cremoris were higher for LeuSt45 than LeuSt40 throughout ripening, and similar results were found for the same cheese batches regarding the S. thermophilus counts. Sheehan et al. (2007) showed that increasing scalding temperature from 47 to 53 °C had no significant effect on the mean viable cell counts of L. helveticus, but it significantly reduced the growth rate of S. thermophilus.
Sensory evaluation
The sensory evaluation scores of cheeses in terms of colour, regular eye formation, texture, salinity and taste during 90 days of ripening are given in Table 4. Higher scores were obtained at the end of the ripening period than the scores obtained initially for all attributes of the Mihaliç cheeses except for LeuSt40 regarding to salinity. The ripening period had a statistically significant effect (p < 0.05) on colour scores except for HP40–HP45. Among the samples, the highest colour score was in HP45 at the beginning, both in HP45 and LeuSt40 at the end of ripening, whereas the control sample received the lowest scores on these days. The differences between the colour scores of the samples were significant on the 1st and 60th days of ripening (p < 0.05).
Table 4.
Sensory evaluation of Mihaliç cheeses during ripening
| Ripening days | Samples | ||||||
|---|---|---|---|---|---|---|---|
| HP40 | HP45 | StHP40 | StHP45 | LeuSt40 | LeuSt45 | Control | |
| Colour | |||||||
| 1 | 6.17aAB | 7.38aC | 6.61aBC | 6.48aBC | 6.42aABC | 6.53BC | 5.43aA |
| 15 | 6.78aA | 7.31aA | 7.14abA | 7.53bcA | 6.87abA | 6.96abA | 7.27bA |
| 30 | 7.43aA | 7.57aA | 7.79cA | 7.29bA | 7.21bcA | 7.64bcA | 7.21bA |
| 60 | 7.40aB | 7.90aC | 8.40dC | 8.10cC | 7.00abAB | 8.10cC | 6.80bA |
| 90 | 7.41aA | 7.94aA | 7.56bcA | 7.63bcA | 7.94cA | 7.50bcA | 6.94bA |
| Regular eye formation | |||||||
| 1 | 6.09aB | 6.59aB | 5.33aA | 6.14aB | 6.11aB | 6.06aB | 4.42aA |
| 15 | 6.36aA | 7.67aB | 6.73bA | 7.32cB | 6.74aA | 7.46bB | 6.25bA |
| 30 | 7.14aA | 7.14aA | 7.29bA | 6.86bcA | 6.79aA | 7.21bA | 6.50bA |
| 60 | 7.50aC | 7.40aC | 7.20bBC | 6.50abAB | 7.50aC | 7.40bC | 6.20bA |
| 90 | 7.31aA | 7.19aA | 7.31bA | 7.13bcA | 7.72aA | 7.03bA | 5.44bA |
| Texture | |||||||
| 1 | 6.68abA | 7.14aA | 7.05aA | 6.82aA | 6.11aA | 6.31aA | 5.63aA |
| 15 | 6.96abA | 7.32aA | 7.07aA | 7.46aA | 7.08bcA | 6.92abA | 6.79aA |
| 30 | 6.29aA | 7.50aB | 7.71abB | 7.50aB | 6.57aA | 7.43bcB | 6.79aA |
| 60 | 7.50bA | 7.70aA | 7.50abA | 7.50aA | 7.40cA | 7.70cA | 6.80aA |
| 90 | 7.44bB | 7.88aB | 8.00bB | 7.75aB | 7.78cB | 7.63cB | 6.75aA |
| Salinity | |||||||
| 1 | 5.05aA | 6.36aA | 6.89aA | 6.30aA | 6.72aA | 5.69aA | 5.64aA |
| 15 | 6.08aA | 6.13aA | 7.35aBC | 7.77aC | 6.54aAB | 7.19bBC | 6.47aAB |
| 30 | 5.64aA | 7.29aC | 7.57aC | 7.39aC | 5.71aA | 7.57bC | 6.29aB |
| 60 | 6.10aA | 6.50aAB | 6.70aABC | 7.20aBC | 6.10aA | 7.50bC | 6.60aABC |
| 90 | 6.44aAB | 6.69aABC | 7.69aBC | 7.75aBC | 6.72aABC | 8.00bC | 5.75aA |
| Taste | |||||||
| 1 | 5.54aA | 6.33aA | 6.50aA | 6.56aA | 5.73aA | 5.79aA | 4.75aA |
| 15 | 6.36abAB | 7.04aBC | 6.65aAB | 7.63aC | 6.43aAB | 7.27bBC | 5.99cA |
| 30 | 6.36abA | 7.64aB | 7.75aB | 7.71aB | 6.14aA | 7.54bB | 5.75cA |
| 60 | 6.85bBC | 7.30aBC | 7.50aC | 7.30aBC | 6.60aB | 7.40bBC | 5.20bAB |
| 90 | 7.09bB | 7.25aBC | 8.09aC | 7.82aBC | 7.28aBC | 7.84bBC | 5.59bcA |
a,b,c,dMeans in the same column with different superscripts significantly differ (p < 0.05)
A,B,C,DMeans in the same row with different superscripts among cheese samples significantly differ (p < 0.05)
Characteristic eye formation produced by the action of propionibacteria has a critical importance because of consumer acceptability and visual appearance in Mihaliç cheeses (Özcan and Kurdal 2012). Leuconostoc creates an opening in cheese resulting from CO2 production, and when cell concentrations are between 5 × 106 and 5 × 107 cells/mL, very high numbers of cells in cheese trials provide an excess of opening (Hemme and Foucaud-Scheunemann 2004). HP45 had the highest regular eye formation score initially, while LeuSt40 had the highest score at the end of ripening. The control cheese had the lowest scores in these periods. These scores were generally consistent with the texture scores. It was reported in previous studies that an elastic, pliable texture is crucial in the formation of regular eyes (Lucey et al. 2003). A recent study found a similar trend related to texture attributes (Özcan and Kurdal 2012). Alvarado Gamboa et al. (2013) also reported that the positive changes in terms of the texture attributes of cheeses manufactured by lactic culture may be due to the extent of protein–protein interactions during the ripening process.
The salinity score of Mihaliç cheese was the highest in StHP40 initially, whereas it was the highest in LeuSt45 at the end of ripening. Generally, the chemically determined salt ratio is consistent with the salinity scores determined as a sensory attribute of Mihaliç cheeses. Among our cheeses, the ripening period had a significant effect in the scores of salinity only for LeuSt45. This may be attributed to vacuum packaging instead of packaging in brine due to the fact that more salt uptake occurs in cheeses stored in brine (Hayaloğlu et al. 2012). The highest taste scores were found in StHP45 initially. The panellists observed a nut-like, sweet flavour, due to either free fatty acids, peptides, amino acids or interactions between them, by the activity of the propionibacteria in StHP45 and StHP40. It was reported in previous studies that cheeses undergoing propionic acid fermentation have a characteristic nutty flavour (Beuvier et al. 1997; Thierry et al. 2004). It may be stated that the cheeses manufactured with the combination including S. thermophilus, L. helveticus and P. freudenreichii gained the highest acceptability from the panellists. All cheese samples with starter cultures were preferred more than the control cheese was. It was reported in a previous study that eye formation in Mihaliç cheese occurred from the addition of starter culture which also produced the desired colour, appearance, taste and aroma (Özcan and Kurdal 2012).
Conclusion
The use of different starter culture combinations in the manufacture of Mihaliç cheeses influenced some quality parameters of the cheeses. According to the results, using starter cultures in the manufacture of Mihaliç cheese provides contributions in terms of physical, chemical and sensory characteristics. The Mihaliç cheeses with starter culture combination had generally higher ripening indexes than control at any stage of ripening period. Proteolysis and lipolysis increased for all cheese samples throughout ripening. At the end of ripening all the cheeses had the highest ripening indexes and ADV values. The cheeses with P. freudenreichii had higher lipolysis levels than other cheeses owing to lipolytic characteristic of this starter bacteria. The starter cultures showed significant decrease during ripening although small fluctuations were observed. In terms of scalding temperature, 45 °C may be more favourable than 40 °C with regard to proteolysis, whereas 40 °C may be recommended for higher levels of lipolysis. In terms of sensory properties all cheese samples with starter cultures were generally preferred more than the control cheese was. According to the results, accelerated ripening with higher sensorial acceptability may be obtained by the starter culture addition to the pasteurized milk, especially by the starter culture combination including S. thermophilus, L. helveticus and P. freudenreichii. It may be concluded that this study provided a new approach for the production of Mihaliç cheese.
Acknowledgements
This study was supported financially by the Ege University Scientific Research Fund (2011-ZRF-011).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elif Özer, Phone: +90 232 311 44 69, Email: elif.ozer@ege.edu.tr.
Harun Kesenkaş, Phone: +90 232 311 16 39, Email: harun.kesenkas@ege.edu.tr.
References
- Aday S, Karagül Yüceer Y. Physicochemical and sensory properties of Mihalic cheese. Int J Food Prop. 2014;17:2207–2227. doi: 10.1080/10942912.2013.790904. [DOI] [Google Scholar]
- Alvarado Gamboa JG, Rojas Almaraz D, Ramon Canul LG, Ramirez Rivera EJ. Determination of the quality of cheese “Chihuahua” type: sensory and physicochemical approaches. Emir J Food Agric. 2013;25:409–417. doi: 10.9755/ejfa.v25i6.12496. [DOI] [Google Scholar]
- Anar S, Şen MKC. Determination of microbiological and chemical properties of Mihaliç cheese presented for consumption in Bursa. Uludağ Uni J Fac Vet Med. 1991;10:12–13. [Google Scholar]
- Beresford TP, Fitzsimons NA, Brennan NL, Cogan TM. Recent Advances in Cheese Microbiology. Int Dairy J. 2001;11:259–274. doi: 10.1016/S0958-6946(01)00056-5. [DOI] [Google Scholar]
- Beuvier E, Berthaud K, Cegarra S, Dasen A, Pochet S, Buchin S, Duboz G. Ripening and quality of Swiss-type cheese made from raw, pasteurized and microfiltered milk. Int Dairy J. 1997;7:311–323. doi: 10.1016/S0958-6946(97)00015-0. [DOI] [Google Scholar]
- Bulut Solak B, Akın N. Determination of some properties of traditional Mihaliç cheese made from raw and pasteurized cow’s milk during ripening period. Middle East J Sci Res. 2013;13:1180–1185. [Google Scholar]
- Chamba JF, Irlinger F. Secondary and adjunct cultures. In: Fox PF, Mc Sweeney PLH, Cogan TM, Guinee TP, editors. Cheese: chemistry, physics and microbiology. London: Elsevier; 2004. pp. 191–206. [Google Scholar]
- Darılmaz ÖD (2010) Determination of propionic acid bacterial species in traditional Turkish cheeses and searching some of their probiotic properties. Ph.D. Thesis. Gazi University Institute of Science and Technology, Ankara
- Dave I, Shah NP. Evaluation of media for selective enumeration of Streptococcus thermophilus, Lactobacillus delbrueckii ssp. bulgaricus, Lactobacillus acidophilus, and bifidobacteria. J Dairy Sci. 1996;1996:1529–1536. doi: 10.3168/jds.S0022-0302(96)76513-X. [DOI] [PubMed] [Google Scholar]
- Fox PF, McSweeney PLH. Proteolysis in cheese during ripening. Food Rev Int. 1996;12:457–509. doi: 10.1080/87559129609541091. [DOI] [Google Scholar]
- Garcia V, Rovira S, Boutoial KI, Ferrandini E, Morales MBL. Effect of starters and ripening time on the physicochemical, nitrogen fraction and texture profile of goat’s cheese coagulated with a vegetable coagulant. J Sci Food Agric. 2014;94:552–559. doi: 10.1002/jsfa.6292. [DOI] [PubMed] [Google Scholar]
- Grappin R, Beuvier E. Possible implications of milk pasteurization on the manufacture and sensory quality of ripened cheese. Int Dairy J. 1998;98:751–767. [Google Scholar]
- Güley Z (2008) Investigation of effects of some lactic acid bacteria isolated from naturally produced mouldy cheese on aflatoxin M1 and aflatoxin B1. Ph.D. Thesis. Ege University Institute of Science and Technology, Izmir
- Hayaloğlu AA, Karabulut I. Primary and secondary proteolysis in eleven Turkish cheese varieties. Int J Food Prop. 2013;16:1663–1675. doi: 10.1080/10942912.2011.604890. [DOI] [Google Scholar]
- Hayaloğlu AA, Özer B. Cheese Ripening. In: Hayaloğlu AA, Özer B, editors. Fundamentals of cheese science. Sidas Medya: İzmir; 2011. pp. 173–209. [Google Scholar]
- Hayaloğlu AA, Topçu A, Koca N. Cheese analyses. In: Hayaloğlu AA, Özer B, editors. Fundamentals of cheese science. Sidas Medya: İzmir; 2011. pp. 489–562. [Google Scholar]
- Hayaloğlu AA, Bansal N, McSweeney PLH. Influence of brine immersion and vacuum packaging on the chemistry, biochemistry, and microstructure of Mihaliç cheese made sing sheep’s milk during ripening. Dairy Sci Technol. 2012;92:671–689. doi: 10.1007/s13594-012-0083-4. [DOI] [Google Scholar]
- Hemme D, Foucaud-Scheunemann C. Leuconostoc, characteristics, use in dairy technology and prospects in functional foods. Int Dairy J. 2004;14:467–494. doi: 10.1016/j.idairyj.2003.10.005. [DOI] [Google Scholar]
- Kawabata S, Vassal L, Le Bars D, Cesselin B, Nardi M, Gripon JC, Chapot-Chartier MP. Phage-induced lysis of Lactococcus lactis during Saint-Paulin cheese ripening and its impact on proteolysis. Lait. 1997;77:229–239. doi: 10.1051/lait:1997216. [DOI] [Google Scholar]
- Kiernan RC, Beresford TP, O’Cuinn G, Jordan KN. Autolysis of lactobacilli during Cheddar cheese ripening. Ir J Agric Food Res. 2000;39:95–106. [Google Scholar]
- Kocaoğlu-Vurma NA, Harper WJ, Drake MA, Courtney MD. Microbiological, chemical, and sensory characteristics of Swiss cheese manufactured with adjunct Lactobacillus strains using a low cooking temperature. Am Dairy Sci Assoc. 2008;91:2947–2959. doi: 10.3168/jds.2007-0592. [DOI] [PubMed] [Google Scholar]
- Liu SQ, Asmundson RV, Holland R, Crow VL. Acetaldehyde metabolism by Leuconostoc mesentroides subsp. cremoris under stress conditions. Int Dairy J. 1997;7:175–183. doi: 10.1016/S0958-6946(96)00055-6. [DOI] [Google Scholar]
- Lucey A, Johnson ME, Horne DS. Invited review: perspectives on the basis of the rheology and texture properties of cheese. J Dairy Sci. 2003;86:2725–2743. doi: 10.3168/jds.S0022-0302(03)73869-7. [DOI] [PubMed] [Google Scholar]
- McSweeney PLH. Biochemistry of cheese ripening. Int Dairy J. 2004;57:127–144. doi: 10.1111/j.1471-0307.2004.00147.x. [DOI] [Google Scholar]
- Öner Z, Aloğlu H. Some characteristics of Mihaliç: a traditional Turkish cheese. Milchwissenschaft. 2004;59:628–631. [Google Scholar]
- Ong N, Shah NP. Influence of probiotic Lactobacillus acidophilus and L. helveticus on proteolysis, organic acid profiles, and ACE inhibitory activity of Cheddar cheese ripened at 4, 8 and 12°C. J Food Sci. 2008;73:111–120. doi: 10.1111/j.1750-3841.2008.00689.x. [DOI] [PubMed] [Google Scholar]
- Oysun G. Analysis methods for milk and dairy products. İzmir: Ege University Agricultural Faculty Press; 2001. p. 304. [Google Scholar]
- Özcan T, Kurdal E. The effect of using a starter culture, lipase and protease enyzmes on ripening of Mihaliç cheese. J Dairy Technol. 2012;65:585–593. doi: 10.1111/j.1471-0307.2012.00868.x. [DOI] [Google Scholar]
- Porcellato D, Ostlie HM, Brede ME, Martinovic A, Skeie SB. Dynamics of starter, adjunct non-starter lactic acid bacteria and propionic acid bacteria in low-fat and full-fat Dutch-type cheese. Int Dairy J. 2013;33:104–111. doi: 10.1016/j.idairyj.2013.01.007. [DOI] [Google Scholar]
- Renner E. Milchpraktikum Skriptum zu den übüngen. Giesen: Justus Liebig Universitat; 1993. [Google Scholar]
- Sheehan JJ, Oliveira JC, Kelly AL, Mc Sweeney PLH. Effect of cook temperature on primary proteolysis and predicted residual chymosin activity of a semi-hard cheese manufactured using thermophilic cultures. Int Dairy J. 2007;17:826–834. doi: 10.1016/j.idairyj.2006.08.012. [DOI] [Google Scholar]
- Sheehan JJ, Wilkinson MG, McSweeney PLH. Influence of processing and ripening parameters on starter, non-starter and propionic acid bacteria and on the ripening characteristics of semi-hard cheeses. Int Dairy J. 2008;18:905–917. doi: 10.1016/j.idairyj.2007.11.024. [DOI] [Google Scholar]
- Thierry A, Richoux R, Kerjean JR, Lortal S. A simple screening method for isovaleric acid production by Propionibacterium freudenreichii in Swiss Cheese. Int Dairy J. 2004;14:697–700. doi: 10.1016/j.idairyj.2004.01.001. [DOI] [Google Scholar]
- Valence F, Deutsch SM, Richoux R, Gagnaire V, Lortal S. Autolysis and related proteolysis in Swiss cheese for two Lactobacillus helveticus strains. J Dairy Res. 2000;2000:261–271. doi: 10.1017/S0022029900004118. [DOI] [PubMed] [Google Scholar]
- Yun JJ, Kiely J, Barbano DM, Kindstedt PS. Mozzarella cheese: impact of cooking temperature on chemical composition, proteolysis and functional properties. J Dairy Sci. 1993;76:3664–3673. doi: 10.3168/jds.S0022-0302(93)77708-5. [DOI] [Google Scholar]