Abstract
Phenolic compounds are considered the main bioactive components in okra fruits. In order to well understand the accumulation pattern of phenolic compounds in okra fruits during maturation, and to obtain okra fruits with high level of health-beneficial phenolic compounds, physicochemical properties, phenolic profiles, antioxidant capacities, and inhibitory effects on digestive enzymes of okra fruits at different maturation stages were investigated. Noticeable variations in physicochemical properties and phenolic profiles of okra were observed at different maturation stages. Phenolic compounds, including quercetin-3-O-gentiobioside, quercetin-3-O-glucoside (isoquercitrin), rutin, quercetin derivative, protocatechuic acid, and catechin derivative, were determined to be the major compounds in okra fruits, while quercetin-3-O-gentiobioside was the most abundant phenolic compound. Considering the accumulation patterns of fruit size, firmness, and total flavonoid content of okra fruits, the optimal harvest time of okra fruits with relatively high level of health-beneficial phenolic compounds was determined. Furthermore, okra fruits at different maturation stages exerted remarkable antioxidant capacities and inhibitory effects on the pancreatic lipase, α-glucosidase, and α-amylase. The Pearson’s correlation showed that quercetin-3-O-gentiobioside was one of the major contributors to the antioxidant capacities and inhibitory effects on digestive enzymes. Results are beneficial for understanding of the accumulation pattern of phenolic compounds in okra fruits during maturation, and can aid in the targeting of specific maturation stages with an optimal phenolic profile for the production of health-beneficial products.
Keywords: Okra fruit, Phenolic compounds, HPLC analysis, Antioxidant capacity, Enzyme inhibition
Introduction
The fruit of okra (Abelmoschus esculentus), known as lady’s finger and gumbo, is an important vegetable crop (Xia et al. 2015). It is an annual plant native to Africa, and has been grown in different countries around the world, mainly in tropical, sub-tropical, and warm temperate regions (Xia et al. 2015). In recent years, okra has also been widely cultivated in the North and South China (Jiang et al. 2017). Okra fruits have a wide range of medicinal value, and have been used to control various diseases and disorders (Sabitha et al. 2011). The health-promoting effects of okra fruit are owing to its various biological activities. Pharmacological studies have demonstrated that okra fruit possesses various bioactivities, such as antioxidant, anti-diabetic, anti-hyperlipidemic, and anti-hyperglycemic effects (Graham et al. 2017; Lu et al. 2016; Sabitha et al. 2011; Zhang et al. 2018). Generally, phenolic compounds (Jiang et al. 2017; Liao et al. 2012; Lu et al. 2016; Xia et al. 2015; Zeng et al. 2015) are considered the main bioactive components in okra fruit, which are responsible for its various bioactivities. The content of phenolic compounds and their bioactivities of okra fruits are influenced by cultivars, growing conditions, and fruit sizes (Olivera et al. 2012; Petropoulos et al. 2018). Nevertheless, studies on the changes of phenolic compounds, and their antioxidant capacities and inhibitory effects on digestive enzymes (pancreatic lipase, α-glucosidase, and α-amylase) of okra fruits at different maturation stages have seldom been investigated.
The ideal fruit harvesting time is an important factor affecting fruit quality (Petropoulos et al. 2018). Generally, the physicochemical properties, such as fruit weight, fruit sizes, firmness, free sugars, and organic acids are considered as the primary indicators for harvest (Olivera et al. 2012; Petropoulos et al. 2018). Especially, the fruit size has been used as a harvest index for okra harvest. Nowadays, with the improvement in quality of life, consumers are attaching increasing importance to health (Ma et al. 2017). Therefore, besides the physicochemical properties are necessary for okra fruit harvest, the changes of bioactive components and bioactivities of okra fruits during maturation are also important. Whether the accumulation pattern of health-beneficial phenolic compounds in okra fruits during development and maturation is consistent with that of fruit size remains unknown. Therefore, in order to well understand the accumulation pattern of phenolic compounds in okra fruits during maturation, and to obtain okra fruits with high level of health-beneficial phenolic compounds, physicochemical properties, phenolic profiles, antioxidant capacities, and inhibitory effects on digestive enzymes (pancreatic lipase, α-glucosidase, and α-amylase) of okra fruits at different maturation stages were systematically investigated.
Materials and methods
Samples and chemicals
“Wufu” (cultivar) okra fruits (A. esculentus) were harvested from 100 of marked plants (similar in vigor, size, and anthesis) at a commercial orchard (30°46′ 18.50″N, 104°02′ 20. 02″ E) located in Chengdu, Sichuan province, China. After 4 days post-anthesis (DPA), the fruits were harvested every day for six times during the period from 19th July to 24th July, 2017 (Fig. 1). At each time, forty of fruits were harvested and weighted, and then delivered to the laboratory. The whole okra fruits were washed with distilled water, and divided into two parts. The first part of fruits was used to measure the physicochemical properties. And the second part of fruits was frozen and freezing dried. Subsequently, the samples were ground to pass through a 60 mesh sieve, and stored at − 20 °C for further analysis.
Fig. 1.

Morphological characteristic of “Wufu” okra fruits at different maturation stages. DPA days post-anthesis
Protocatechuic acid, caffeic acid, catechin, quercetin-3-O-gentiobioside, quercetin, rutin, quercetin-3-O-glucoside (isoquercitrin), gallic acid, 6-hydroxy-2,5,7,8-tetramethyl chroman-2-carboxylic acid (Trolox), 2,2-diphenyl-1-picrylhydrazyl (DPPH), pancreatic lipase, α-glucosidase, α-amylase, starch, p-nitrophenyl-α-D-glucopyranoside (pNPG), p-nitrophenyl acetate, and Folin-Ciocalteu reagent were purchased from Sigma-Aldrich (St. Louis, Mo, U.S.A). All other reagents and chemicals used were of analytical grade.
Determination of length, diameter, and firmness
Twenty of okra fruits were immediately measured for length and major diameter by using a digital vernier caliper with a sensibility of 0.01 mm. The firmness of okra fruits was measured by a penetration test using a TA.XT-plus Texture Analyser (Stable Micro System, United Kingdom) equipped with a 5-kg load cell. The penetrometer was fitted with the p/5 (5 mm diameter) needle probe. The probe was driven into the flesh okra fruit with a trigger force of 8.0 g at a pre-test speed of 5 mm/s, a test speed of 1.0 mm/s, and a post-test speed 10 mm/s. Firmness was measured five times at the equator of each okra fruit.
Extraction of phenolic compounds
Phenolic compounds of okra fruits were extracted according to a previously reported method with minor modifications (Yang et al. 2015). Briefly, 30 mL of 70% acidified methanol (0.1% HCl, v/v) was added into 1.0 g of each sample. Then, the mixture was extracted twice with ultrasound (50 kHz, 480 W) for 60 min at room temperature. After centrifugation (4000 × g, 10 min), the supernatants were combined, and concentrated to dryness using a rotary evaporator at 45 °C. Finally, the dried residue was reconstituted in 70% of methanol, and stored at − 20 °C in dark for the further determination of total flavonoids, individual phenolic compounds, antioxidant capacities, and inhibitory effects on digestive enzymes. The extract was filtered through a 0.22 µm organic membrane prior to analysis using high performance liquid chromatography (HPLC).
Determination of total flavonoid content
The total flavonoid content (TFC) of okra fruit extract was estimated according to a previously reported method with minor modifications (Lin et al. 2018). Briefly, 100 µL of each okra extract or rutin standard solution was added into 30 µL of 5% sodium nitrite solution (w/v). After 6 min, 30 µL of 10% aluminum nitrate solution (w/v) was added. Subsequently, 400 µL of 4% sodium hydroxide (w/v) was added, and incubated at room temperature for 25 min. Finally, the absorbance of the mixture was measured at 510 nm, and the TFC was expressed as milligram of rutin equivalents per gram of okra fruits dry weight (mg RE/g DW).
HPLC analysis of individual phenolic compounds
Phenolic compounds of okra fruit extract were evaluated by an Agilent 1260 series LC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a diode-array detector (DAD). Chromatographic separations were conducted at 25 °C on a ZORBAX Eclipase XDB-C18 column (250 mm × 4.6 mm, 5 µm). The chromatographic separation was achieved by gradient elution with 0.5% (v/v) acetic acid solution (A) and acetonitrile (B) according to a previous study with minor modifications (Ma et al. 2017). Briefly, samples were eluted as follows: 0 min, 5% B; 5 min, 5% B; 50 min, 5–20% B, 70 min, 20–70% B; 72 min, 70–5% B; 72–77 min, 5% B. The flow rate was 0.8 mL/min and the injection volume was 20 µL for all samples. Detection was made at 280 nm, 320 nm, and 360 nm, respectively. Identification of phenolic compounds was carried out by comparing retention times and absorption spectra of commercial standards, and spiking of external reference standards, as well as comparing the information of the same type of samples previously reported (Arapitsas 2008). The identified compounds were quantified by external calibration method using calibration curves. Briefly, the contents of protocatechuic acid (y = 28.455x + 0.2127, R2 = 0.999), catechin (y = 16.756x − 9.765, R2= 0.999), quercetin (y = 80.507x + 9.5089, R2 = 0.999), quercetin-3-O-gentiobioside (y = 43.945x − 310.13, R2 = 0.998), rutin (y = 30.337x − 6.2411, R2 = 0.998), and isoquercitrin (y = 56.55x + 68.103, R2 = 0.999) were measured by external calibration method using calibration curves. The contents of catechin derivative and quercetin derivative were expressed as catechin equivalents and quercetin equivalents, respectively. The content of each phenolic compound was expressed as microgram per gram dry weight (µg/g DW).
Determination of antioxidant capacity of okra fruit extract
The Folin–Ciocalteu reagent assay (FCR)
The FCR assay, according to (Lin et al. 2018), measures sample reducing capacity, which does not reflect the total phenolic content; therefore, it is advised to use the FCR method as a reducing capacity assay (Górnas´ et al. 2015). Briefly, 250 µL of each okra fruit extract or gallic acid standard solution was added into 1.25 mL of Folin–Ciocalteu reagent. Then the mixture was incubated at room temperature for 3 min in dark, and 1.25 mL of sodium carbonate solution (20%, w/v) was added. After incubated at room temperature for 30 min in dark, the absorbance was measured at 765 nm using a Varioskan flash multimode reader (Thermo Fisher, Waltham, Mass., USA). The FCR reducing capacity was express as milligram of gallic acid equivalents per gram of okra fruits dry weight (mg GAE/g DW).
DPPH radical scavenging capacity
The DPPH radical scavenging antioxidant capacity of okra fruit extract was determined according to a previously reported method with minor modifications (Lin et al. 2018). Briefly, 25 µL of each okra fruit extract or methanol as negative control was mixed with 200 µL of DPPH solution (0.35 mM) in a 96-well microplate. Then the mixture was shaken, and incubated at 37 °C for 30 min in dark. The absorbance of the mixture was measured at 517 nm with a blank contain only DPPH solution and methanol. Trolox was used as the standard, and the DPPH radical scavenging capacity was expressed as µmol of Trolox equivalents per gram of okra fruits dry weight (µmol TE/g DW). The IC50 value of DPPH radical scavenging capacity was expressed as mg of okra fruits dry weight/mL (mg DW/mL).
Reducing power
The reducing power of okra fruit extract was determined according to a previously reported method with minor modifications (Lin et al. 2018). Briefly, an aliquot of 100 µL okra fruit extract or Trolox solution was mixed with 100 µL of potassium ferricyanide (1%, w/v) in PBS (0.2 M, pH 6.8). Then the mixture was incubated at 50 °C for 20 min, and followed by the addition of 100 µL of trichloroacetic acid (10%, w/v). After centrifugation (3000 × g, 10 min), 100 µL of supernatant was mixed with 100 µL of distilled water and 20 µL of ferric chloride (0.1%, w/v). Finally, the absorbance of the mixture was measured at 700 nm after 30 min incubation. The reducing power was expressed as µmol of Trolox equivalents per gram of dry weight (µmol TE/g DW).
Inhibitory effects of okra fruit extract on pancreatic lipase, α-glucosidase, and α-amylase
In vitro pancreatic lipase inhibition assay
The in vitro pancreatic lipase inhibition assay was conducted according to a previously reported method with minor modifications (Tan et al. 2017). In brief, the p-nitrophenyl acetate (10 mM, dissolved in DMSO) stock solution was diluted with distilled water to reach a final concentration of 2 mM. 100 µL of each okra extract at different concentrations was mixed with 200 µL of Tris buffer (50 mM, pH 7.4) and 100 µL of pancreatic lipase solution (5 mg/mL, dissolved in 50 mM, pH 7.4 Tris buffer). After incubated at 37 °C for 10 min, 100 µL of p-nitrophenyl acetate solution (2 mM) was added, and then incubated at 37 °C for 15 min. A portion of 200 µL of reaction mixture was taken and added into a 96-well microplate, and the absorbance was measured at 410 nm. The commercial capsule of orlistat was used as a positive control. Results were expressed as inhibition (%) of pancreatic lipase activity according to the following equation below. Pancreatic lipase inhibitory effect was measured at five different concentrations, and a logarithmic regression curve was established to calculate IC50 values (mg of okra fruit dry weight/mL).
where Asample is the absorbance of the mixture of okra fruit extract, Tris buffer, pancreatic lipase solution, and p-nitrophenyl acetate solution; Ablank is the absorbance of the mixture of okra fruit extract, Tris buffer, Tris buffer (instead of pancreatic lipase solution), and p-nitrophenyl acetate solution; Acontrol is the absorbance of the mixture of 70% methanol (instead of okra fruit extract), Tris buffer, pancreatic lipase solution, and p-nitrophenyl acetate solution.
In vitro α-glucosidase inhibition assay
The in vitro α-glucosidase inhibitory activity was conducted according to the previously described method with slight modifications (Tan et al. 2017). In brief, 100 µL of okra fruit extract at different concentrations was mixed with 100 µL of α-glucosidase (0.5 U/mL, dissolved in 0.1 M, pH 6.8 phosphate buffer), and incubated at 37 °C for 15 min. Then, 25 µL of 4 mM p-nitrophenyl-α-D-glucopyranoside (pNPG) solution (dissolved in 0.1 M, pH 6.8 phosphate buffer) was added to initiate the reaction at 37 °C for 20 min. The absorbance of the mixture was measured at 405 nm. Acarbose standard was used as a positive control. Results were expressed as inhibition (%) of α-glucosidase activity according to the following equation below. α-Glucosidase inhibitory effect was measured at five different concentrations, and a logarithmic regression curve was established to calculate IC50 values (mg of okra fruit dry weight/mL).
where Asample is the absorbance of the mixture of okra fruit extract, α-glucosidase solution, and pNPG solution; Ablack is the absorbance of the mixture of okra fruit extract, buffer, and α-glucosidase solution; Acontrol is the absorbance of the mixture of α-glucosidase solution, buffer, and pNPG solution.
In vitro α-amylase inhibition assay
The in vitro α-amylase inhibition assay was carried out by a previously described method with minor modifications (Tan et al. 2017). Briefly, 100 µL of okra fruit extract was mixed with 100 µL of α-amylase solution (50 U/mL, dissolved in 0.1 M, pH 6.8 phosphate buffer), and incubated at 37 °C for 30 min with continuous shaking. Then 200 µL of soluble starch (0.5%, w/v) was added into the mixture, and incubated at 37 °C for 10 min. Subsequently, 1.6 mL of 3, 5-dinitrosalicylic acid (DNS) reagent was added into the mixture, and incubated at a boiling water bath for 5 min. Finally, the absorbance of the mixture was measured at 540 nm. Acarbose standard was used as a positive control. Results were expressed as inhibition (%) of α-amylase activity according to the following equation below. α-Amylase inhibitory activity was measured at five different concentrations, and a logarithmic regression curve was established to calculate IC50 values (mg of okra fruit dry weight/mL).
where Asample is the absorbance of the mixture of okra fruit extract, α-amylase solution, soluble starch, and DNS reagent; Acontrol is the absorbance of the mixture of okra fruit extract, buffer (instead of α-amylase), soluble starch, and DNS reagent; Atest is the absorbance of the mixture of 70% methanol (instead of okra fruit extract), α-amylase solution, soluble starch, and DNS reagent; Ablank is the absorbance of the mixture of 70% methanol (instead of okra fruit extract), soluble starch, buffer (instead of α-amylase), and DNS reagent.
Statistical analysis
All experiments were conducted in triplicate, and data were expressed in means ± standard deviations (n ≥ 3). Statistical analysis was performed using SPSS 21.0 software, and the differences among mean values were tested by one-way ANOVA, taking a level of p < 0.05 as significant to Duncan’s multiple range test. Pearson’s correlation coefficients were determined by Origin 2017 software.
Results and discussion
Weight, size, and firmness of okra fruits at different maturation stages
The fresh weight of per fruit, length, major diameter, and firmness are considered the primary indicators for okra fruit harvest (Olivera et al. 2012). The changes of physicochemical properties of okra fruits at different maturation stages are shown in Table 1. The fresh weight, length, and major diameter increased significantly (p < 0.05) during maturation, and reached the maximum values at 9 DPA, respectively. The okra fruit grown rapidly during the stages from 5 to 8 DPA, and the growth rate of weight of per fruit was more than 5 g/day. In addition, the length/major diameter increased rapidly at the first three stages, and then reached fairly stable values during the next stages. The firmness of okra fruits increased significantly during maturation, and reached the fairly stable values at the stages from 8 to 9 DPA. The differences in firmness of okra fruit harvested at 8 DPA and 9 DPA were not statistically significant. As shown in Table 1, the lengths of okra fruits increased from 9.72 ± 0.55 to 17.08 ± 0.64 cm during maturation stages from 4 DPA to 9 DPA. Generally, the length of okra fruit has been considered the most important harvest index for okra fruits (Olivera et al. 2012; Petropoulos et al. 2018). Both small and big sizes of fruits are harvested and consumed (Petropoulos et al. 2018). Therefore, considering both firmness and length of okra fruits during maturation, the harvest time of “Wufu” okra fruits could be the stages from 4 DPA to 9 DPA.
Table 1.
Fruit weight, length, diameter, firmness, total flavonoid content (TFC), Folin–Ciocalteu reagent assay (FCR), DPPH radical scavenging capacities, and reducing power of okra fruits at different maturation stages
| Stages | Fruit weight (g) | Length (cm) | Diameter (cm) | Length/diameter | Firmness (kg/cm2) | TFC (mg RE/g DW) | FCR (mg GAE/g DW) | DPPH (µmol TE/g DW) | Reducing power (µmol TE/g DW) |
|---|---|---|---|---|---|---|---|---|---|
| 4 DPA | 8.90 ± 0.38f | 9.72 ± 0.55f | 1.32 ± 0.09e | 6.70 ± 0.45c | 7.42 ± 1.61c | 1.88 ± 0.03e | 5.19 ± 0.14d | 25.77 ± 0.64d | 42.37 ± 0.55e |
| 5 DPA | 10.66 ± 0.36e | 10.50 ± 0.47e | 1.45 ± 0.07e | 7.28 ± 0.31b | 7.99 ± 1.85bc | 2.72 ± 0.04bc | 7.84 ± 0.17c | 31.70 ± 0.91c | 61.26 ± 0.56d |
| 6 DPA | 15.72 ± 0.69d | 12.78 ± 0.43d | 1.63 ± 0.10d | 7.72 ± 0.31a | 8.16 ± 1.18bc | 2.95 ± 0.06a | 9.43 ± 0.14a | 36.32 ± 1.05a | 73.53 ± 0.60a |
| 7 DPA | 21.35 ± 0.97c | 14.05 ± 0.65c | 1.83 ± 0.07c | 7.61 ± 0.41ab | 8.24 ± 1.17bc | 2.86 ± 0.04ab | 8.51 ± 0.13b | 35.74 ± 0.12a | 70.25 ± 1.48b |
| 8 DPA | 27.33 ± 1.61b | 15.64 ± 0.25b | 2.02 ± 0.12b | 7.73 ± 0.26a | 8.75 ± 0.92ab | 2.64 ± 0.08 cd | 8.34 ± 0.09b | 33.76 ± 0.74b | 70.20 ± 1.83b |
| 9 DPA | 30.79 ± 1.01a | 17.08 ± 0.64a | 2.21 ± 0.06a | 7.80 ± 0.37a | 9.41 ± 0.78a | 2.54 ± 0.08d | 8.15 ± 0.21bc | 32.64 ± 0.89bc | 64.29 ± 0.71c |
Each value represents the mean ± standard deviation. Different letters in the same column indicate significant differences at p < 0.05
Phenolic profiles of okra fruits at different maturation stages
Phenolic compounds are considered the main bioactive components in okra fruits (Liao et al. 2012; Xia et al. 2015). Nevertheless, the accumulation pattern of phenolic compounds in “Wufu” okra fruit has seldom been investigated. Table 1 summarized the changes of TFC in okra fruits at different maturation stages. The TFC of okra fruits changed significantly during fruit maturation. Briefly, the TFC of okra fruits increased from 1.88 ± 0.03 to 2.95 ± 0.06 mg RE/g DW at the stages from 4 DPA to 6 DPA, and then decreased slowly from 2.86 ± 0.04 to 2.54 ± 0.08 mg RE/g DW at the stages from 7 to 9 DPA, respectively. The possible reason for this observation could be due to the stoppage or slow rate of new biosynthesis of phenolic compounds during fruit maturation (a dilution effect as fruits increase in size) (Anand and Aradhya 2005). Considering the accumulation patterns of TFC, fruit size, and firmness of okra fruits during maturation, the optimal harvest time of “Wufu” okra fruits with higher level of health-beneficial phenolics could be the stages from 6 to 9 DPA.
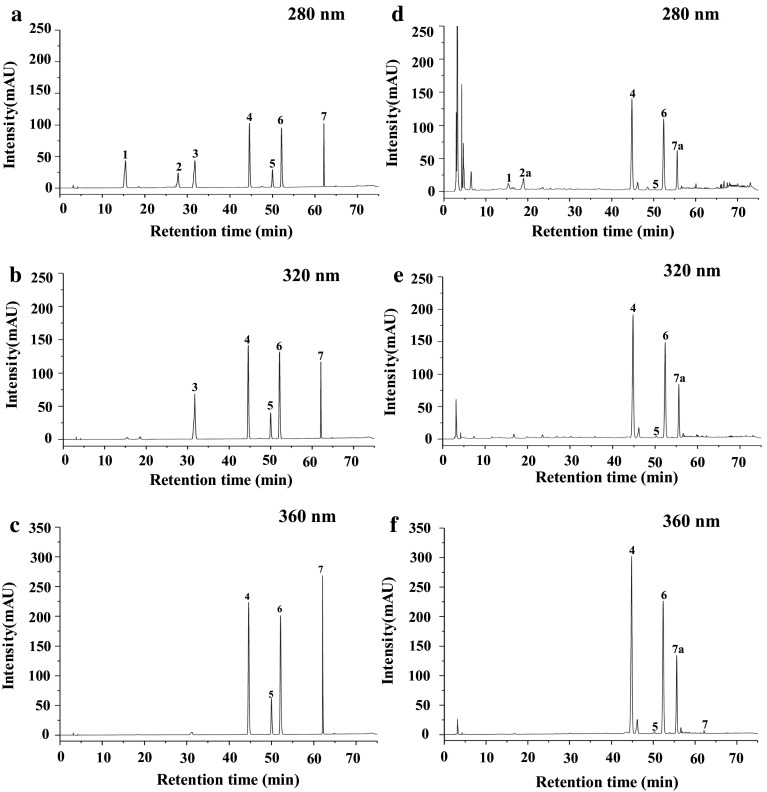
Furthermore, in order to well understand the change patterns of individual phenolic compounds in okra fruits during maturation, the HPLC–DAD analysis was performed. Previous studies have shown that quercetin, isoquercitrin, rutin, quercetin-3-O-gentiobioside, hydroxycinnamic derivatives, and catechin derivatives have been found in okra seeds and skins (Arapitsas 2008; Lin et al. 2014; Xia et al. 2015). Therefore, a total of 7 phenolic compounds, including quercetin, isoquercitrin, rutin, quercetin-3-O-gentiobioside, catechin, caffeic acid, and protocatechuic acid, were selected and investigated in okra fruit extract. Figure 2 showed the chromatograms of mixed standards and representative phenolic profiles of “Wufu” okra fruit. As shown in Fig. 2, five phenolic compounds, including protocatechuic acid, quercetin-3-O-gentiobioside, quercetin, rutin, and isoquercitrin, were identified based on their HPLC retention time and UV spectral information. Moreover, one catechin derivative and one quercetin derivative were also determined based on their UV spectral information. Briefly, the catechin derivative had the same characteristic UV spectrum of catechin (UV λmax, 225 nm and 280 nm). The quercetin derivative also had the same characteristic UV spectrum of quercetin (UV λmax, 255 nm and 365 nm). The changes of these phenolic compounds in okra fruits during maturation were presented in Table 2.
Fig. 2.
HPLC chromatograms of mixed standards (a–c) and representative phenolic profiles of okra fruits (d–f). 1 protocatechuic acid; 2 catechin; 3 caffeic acid; 4 quercetin-3-O-gentiobioside; 5 rutin; 6 isoquercitrin; 7 quercetin; 2a catechin derivative; 7a quercetin derivative
Table 2.
Contents (µg/g DW) of individual phenolic compounds in okra fruits at different maturation stages
| Peak | Phenolic compounds (µg/g DW) | Stages | |||||
|---|---|---|---|---|---|---|---|
| 4 DPA | 5 DPA | 6 DPA | 7 DPA | 8 DPA | 9 DPA | ||
| 1 | Protocatechuic acid | 107.67 ± 0.37b | 114.77 ± 0.35a | 97.36 ± 0.44c | 89.74 ± 0.28d | 83.13 ± 0.33e | 64.13 ± 0.34f |
| 2 | Catechin | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 2a | Catechin derivative | 157.64 ± 0.34e | 277.85 ± 0.40b | 284.92 ± 0.44a | 226.00 ± 0.42c | 226.22 ± 0.43c | 216.63 ± 0.30d |
| 3 | Caffeic acid | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 4 | Quercetin-3-O-gentiobioside | 625.09 ± 0.44f | 1028.27 ± 0.37e | 1359.04 ± 0.36a | 1178.42 ± 0.45b | 1167.71 ± 0.45c | 1044.40 ± 0.23d |
| 5 | Rutin | 23.19 ± 0.25f | 38.40 ± 0.37e | 52.86 ± 0.33a | 44.81 ± 0.39b | 43.66 ± 0.33c | 40.01 ± 0.38d |
| 6 | Isoquercitrin | 647.05 ± 0.33e | 905.45 ± 0.46a | 812.35 ± 0.37b | 806.55 ± 0.42c | 721.72 ± 0.62e | 551.23 ± 0.42f |
| 7 | Quercetin | N.Q. | N.Q. | N.Q. | N.Q. | N.Q. | N.Q. |
| 7a | Quercetin derivative | 181.45 ± 0.59e | 278.84 ± 0.42a | 255.03 ± 0.35b | 252.46 ± 0.53c | 226.33 ± 0.15d | 176.59 ± 0.37f |
| Total | 1743.55 | 2646.37 | 2864.79 | 2600.23 | 2471.00 | 2093.76 | |
N.D.: the compound can not be detected; N.Q.: the amount is not available for quantification
Each value represents the mean ± standard deviation. Content of catechin derivative was expressed as catechin equivalents, and content of quercetin derivative was expressed as quercetin equivalents
Significant (p < 0.05) differences are shown by data bearing different letters (a–f)
The peaks were the same as in Fig. 2
As shown in Table 2, one phenolic acid, protocatechuic acid, was detected. The contents of protocatechuic acid in okra fruits increased from 107.67 ± 0.37 to 114.77 ± 0.35 µg/g DW at the stages from 4 DPA to 5 DPA, and then decreased significantly from 114.77 ± 0.35 to 64.13 ± 0.34 µg/g DW at the stages from 5 to 9 DPA. One catechin derivative was also detected. Its contents increased from 157.64 ± 0.34 to 284.92 ± 0.44 µg/g DW at the stages from 4 to 6 DPA, and then decreased slowly from 284.92 ± 0.44 to 216.63 ± 0.30 µg/g DW at the stages from 6 to 9 DPA. In addition, five flavonols, including quercetin-3-O-gentiobioside, isoquercitrin, rutin, quercetin, and quercetin derivative, were detected in okra fruits. As shown in Table 2 and Fig. 2, the quercetin-3-O-gentiobioside was not only the most abundant individual flavonols, but also the most abundant individual phenolic compounds in okra fruits, which is in accordance with previous studies (Lin et al. 2014; Xia et al. 2015). The contents of quercetin-3-O-gentiobioside increased significantly (p < 0.05) from 625.09 ± 0.44 to 1359.04 ± 0.36 µg/g DW at the stages from 4 to 6 DPA, and then decreased slightly from 1359.04 ± 0.36 to 1044.40 ± 0.23 µg/g DW at the stage from 6 to 9 DPA. In addition, the contents of another abundant flavonol, isoquercitrin, increased from 647.05 ± 0.33 to 905.45 ± 0.46 µg/g DW at the stages from 4 DPA to 5 DPA, and then decreased from 905.45 ± 0.46 to 551.23 ± 0.42 µg/g DW at the stage from 5 to 9 DPA. The significant decrease in contents of isoquercitrin at the stages from 5 to 6 DPA might be attributed to the biosynthesis of quercetin-3-O-gentiobioside using isoquercitrin as a substrate (Cho et al. 2016), which results a significant increase in contents of quercetin-3-O-gentiobioside at the stages from 5 to 6 DPA. The accumulation pattern of rutin was similar with that of quercetin-3-O-gentiobioside during okra maturation. Besides, the contents of quercetin derivative increased from 181.45 ± 0.59 to 278.84 ± 0.42 µg/g DW at the stages from 4 to 5 DPA, and then decreased slowly from 255.03 ± 0.35 to 176.59 ± 0.37 µg/g DW at the stage from 6 DPA to 9 DPA.
Antioxidant capacities of okra fruits at different maturation stages
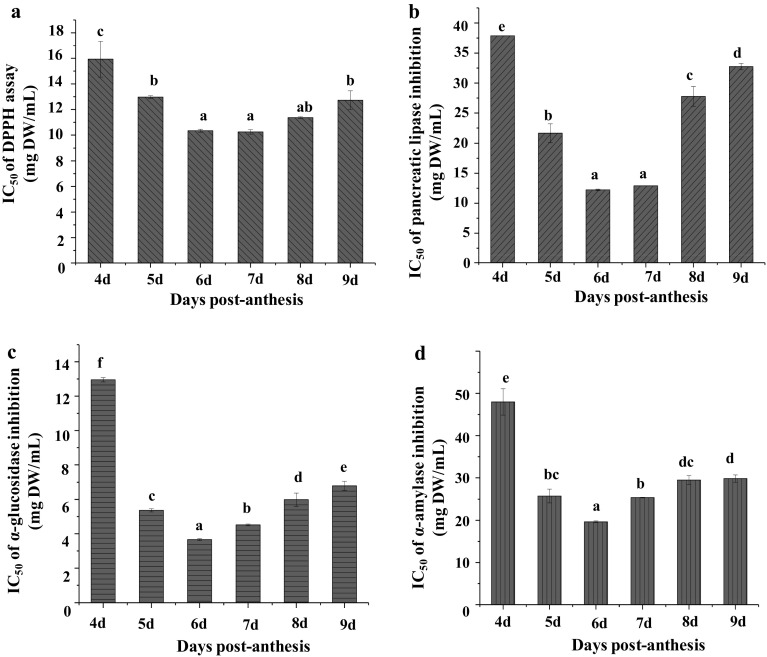
The contribution of okra fruits to health improvement has been partially attributed to their antioxidant capacities (Xia et al. 2015). Table 2 summarized the antioxidant capacities of okra fruits at different maturation stages, and the IC50 values of DPPH radical scavenging capacity was shown in Fig. 3a. The antioxidant capacities of okra fruits changed significantly during fruit maturation. Briefly, the DPPH radical scavenging capacities increased from 25.77 ± 0.64 to 36.32 ± 1.05 µmol TE/g DW at the stages from 4 to 6 DPA, and then decreased slightly from 35.74 ± 1.05 to 32.64 ± 0.89 µmol TE/g DW at the stages from 7 to 9 DPA. The DPPH radical scavenging capacities of “Wufu” okra fruit were similar with previous studies (Liao et al. 2012). The FCR assay was also used to assess the reducing capacity of the samples (Górnas´ et al. 2015). The FCR values increased from 5.19 ± 0.14 to 9.43 ± 0.14 mg GAE/g DW at the stages from 4 DPA to 6 DPA, and then decreased slightly from 8.51 ± 0.13 to 8.15 ± 0.21 mg GAE/g DW at the stages from 7 to 9 DPA. Furthermore, the reducing power also increased from 42.37 ± 0.55 to 73.53 ± 0.60 µmol TE/g DW at the stages from 4 to 6 DPA, and then declined slightly from 70.25 ± 1.48 to 64.29 ± 0.71 µmol TE/g DW at the stages from 7 to 9 DPA. The antioxidant capacities of okra fruits among FCR, DPPH and reducing power assays showed a similar change pattern during fruit maturation. The change pattern of antioxidant capacities was also similar with the accumulation pattern of TFC as abovementioned. Moreover, the IC50 values of DPPH assay decreased from 15.94 ± 1.41 to 10.25 ± 0.17 mg DW/mL, and then increased from 10.25 ± 0.17 to 12.73 ± 0.73 mg DW/mL during maturation (Fig. 3a), which is in accordance with the antioxidant capacities. The low IC50 values confirmed that okra fruits possessed high antioxidant capacity, which suggested that okra fruits could be potential resources of antioxidants for the production of health-beneficial products.
Fig. 3.
DPPH radical scavenging capacity (a), and inhibitory activities on pancreatic lipase (b), α-glucosidase (c), and α-amylase (d) of okra fruits at different maturation stages Significant (p < 0.05) differences are shown by data bearing different letters (a–f)
Inhibitory effects on digestive enzymes of okra fruits at different maturation stages
Pancreatic lipase is a key enzyme involved in triglyceride digestion. The inhibition of pancreatic lipase is a key approach to the control of hyperlipidaemia and obesity (Zhang et al. 2015). Figure 3b showed that the inhibitory activities of okra fruit extract on pancreatic lipase changed significantly at different maturation stages. Briefly, the IC50 values significantly decreased from 37.88 ± 0.02 to 12.23 ± 0.11 mg DW/mL at the stages from 4 DAP to 6 DPA, and then significantly increased from 12.23 ± 0.11 to 32.74 ± 0.52 mg DW/mL at stages from 6 to 9 DPA. The change pattern of inhibitory effects on pancreatic lipase was also similar with the accumulation pattern of TFC. Furthermore, compared with the commercial orlistat drug (IC50 = 6.34 mg/mL), the inhibitory effects on pancreatic lipase of okra fruit with the highest level of flavonols also presented moderate IC50 value (12.23 ± 0.11 mg DW/mL). Therefore, okra fruits could be explored as functional food ingredients for the prevention of hyperlipidaemia and obesity.
α-Glucosidase and α-amylase are key enzymes responsible for the breakdown of oligosaccharides and disaccharides into monosaccharides suitable for absorption. Therefore, the inhibition of α-glucosidase and α-amylase is one of the main strategies to counteract metabolic alterations related to hyperglycaemia and type 2 diabetes (Nowicka et al. 2016). Previous studies have shown that the okra extract exhibits strongly inhibitory effect on α-glucosidase and α-amylase (Cahyana et al. 2017; Karim et al. 2014). However, the changes of inhibitory effects on α-glucosidase and α-amylase of okra fruit at different maturation stages have seldom been investigated. Figure 3c, d showed that the IC50 values of inhibitory effects on α-glucosidase and α-amylase varied from 3.66 ± 0.05 to 12.95 ± 0.12 mg DW/mL, and from 19.64 ± 0.21 to 47.97 ± 3.13 mg DW/mL, respectively. The change patterns of inhibitory effects on α-glucosidase and α-amylase of okra fruit during maturation were also similar with the accumulation pattern of TFC. Furthermore, compared with the acarbose standard (IC50 = 4.63 mg/mL), the okra fruit with the highest level of flavonols exerted remarkable inhibitory effects on α-glucosidase (IC50 = 3.66 ± 0.05 mg DW/mL). Therefore, okra fruits could be potential anti-hyperglycemic agents.
Correlations between bioactivities and phenolic compounds
The correlations among phenolic compounds, antioxidant capacities, and inhibitory effects on digestive enzymes (pancreatic lipase, α-glucosidase, and α-amylase) were summarized in Table 3. The TFC (r ≥ 0.944) showed significantly (p < 0.01) positive correlations with antioxidant capacities measured by FCR, DPPH, and reducing power assays, respectively. The total flavonoids might be the main contributors toward the antioxidant capacities of okra fruits. Previous studies have also noticed good correlations between phenolic compounds and antioxidant capacities of okra fruits (Ahmed and Kumar 2016; Chao et al. 2014). Furthermore, highly positive correlations among quercetin-3-O-gentiobioside (r ≥ 0.979), rutin (r ≥ 0.974) and antioxidant capacities were measured, which suggested that quercetin-3-O-gentiobioside and rutin might play significant roles in antioxidant capacities of okra fruits. Similar studies have shown that quercetin-3-O-gentiobioside exhibits remarkable antioxidant activity in vitro (Xia et al. 2015; Fan et al. 2014). Moreover, significant correlations were also observed between phenolic compounds and inhibitory effects on digestive enzymes. Briefly, the strong correlations were observed between IC50 values of pancreatic lipase inhibition and TFC (r = − 0.882), which is in accordance with previous studies that phenolic compounds exerted strongly inhibitory activity on pancreatic lipase (Podsedek et al. 2014; Zhang et al. 2015). In addition, the IC50 values of pancreatic lipase inhibition were significantly (p < 0.05) correlated with quercetin-3-O-gentiobioside (r = − 0.813), quercetin derivative (r = − 0.827), and rutin (r = − 0.814), respectively. The IC50 values of pancreatic lipase inhibition were also correlated with isoquercitrin (r = − 0.745). Previous studies have shown that quercetin and its derivatives exert inhibition effect on the pancreatic lipase (Sergent et al. 2012; Sakulnarmrat and Konczak 2012). Therefore, quercetin-3-O-gentiobioside and isoquercitrin could be the major contributors toward the pancreatic lipase inhibition effects of okra fruits due to their high contents. There were also significant (p < 0.01) correlations among the IC50 values of α-glucosidase and α-amylase inhibition and TFC (r ≤ − 0.941). The IC50 values of α-glucosidase and α-amylase inhibition were significantly (p < 0.01) correlated with catechin derivative (r ≤ − 0.873), quercetin-3-O-gentiobioside (r ≤ − 0.946), and rutin (r ≤ − 0.934), respectively. As a matter of fact, proanthocyanidins from okra fruits, which are composed of (epi)gallocatechins and (epi)catechins, exhibit remarkable α-amylase and α-glucosidase inhibitory activities (Lu et al. 2016). Previous studies have also indicated that phenolic compounds, such as quercetin derivatives, are major contributors to the inhibitory activities on digestive enzymes of okra fruit (Karim et al. 2014; Zeng et al. 2015). Therefore, quercetin-3-O-gentiobioside and catechin derivative might play important roles in α-amylase and α-glucosidase inhibition effects of okra fruits due to their high contents. However, for further understanding of the structure–function relationships of phenolics from okra, the chemical structures of the catechin derivative and quercetin derivative are required to be elucidated, and individual phenolic compounds with well known chemical structure are also required for the investigation of bioactivities in future study.
Table 3.
Pearson’s correlation coefficients among phenolic compounds, antioxidant capacities, and inhibitory activities on digestive enzymes of okra fruits at different maturation stages
| TFC | PA | CD | QOG | RU | IS | QUD | FCR | DPPH | RP | PL | α-Glu | α-Amy | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TFC | 1 | ||||||||||||
| PA | − 0.171 | 1 | |||||||||||
| CD | 0.867* | 0.156 | 1 | ||||||||||
| QOG | 0.955** | − 0.301 | 0.797 | 1 | |||||||||
| RU | 0.947** | − 0.309 | 0.792 | 0.998** | 1 | ||||||||
| IS | 0.574 | 0.687 | 0.707 | 0.416 | 0.393 | 1 | |||||||
| QUD | 0.733 | 0.518 | 0.814* | 0.586 | 0.563 | 0.976** | 1 | ||||||
| FCR | 0.964** | − 0.367 | 0.810* | 0.988** | 0.987** | 0.368 | 0.553 | 1 | |||||
| DPPH | 0.960** | − 0.365 | 0.723 | 0.979** | 0.977** | 0.384 | 0.560 | 0.976** | 1 | ||||
| RP | 0.944** | − 0.413 | 0.726 | 0.982** | 0.974** | 0.344 | 0.529 | 0.982** | 0.985** | 1 | |||
| PL | − 0.882* | − 0.160 | − 0.726 | − 0.813* | − 0.814* | − 0.745 | − 0.827* | − 0.775 | − 0.834* | − 0.755 | 1 | ||
| α-Glu | − 0.947** | 0.233 | − 0.873** | − 0.950** | − 0.934** | − 0.493 | − 0.662 | − 0.958** | − 0.909* | − 0.949** | 0.725 | 1 | |
| α-Amy | − 0.941** | 0.202 | − 0.912** | − 0.946** | − 0.936** | − 0.491 | − 0.657 | − 0.958** | − 0.891* | − 0.926** | 0.726 | 0.992** | 1 |
TFC total flavonoid content, PA protocatechuic acid, CD catechin derivative, QOG quercetin-3-O-gentiobioside, RU rutin, IS isoquercitrin, QUD quercetin derivative, FCR Folin-Ciocalteu reagent assay, DPPH DPPH radical scavenging capacity, RP reducing power, PL pancreatic lipase inhibition activity, α-Glu α-glucosidase inhibition activity, α-Amy α-amylase inhibition activity; Correlation is significant at *p < 0.05, **p < 0.01 level (two-tailed)
Conclusion
In the present study, noticeable variations in okra fruit physicochemical properties and phenolic profiles were observed at different maturation stages. Considering the accumulation patterns of fruit size, firmness, and phenolic compounds in okra fruit during maturation, the optimum harvest time of okra fruits with relatively high level of health-beneficial phenolic compounds was determined. Results are beneficial for better understanding of the accumulation pattern of phenolic compounds, and can aid in the targeting of specific maturation stages with an optimal phenolic profile for the production of health-beneficial products. Furthermore, the okra fruits exerted remarkable antioxidant capacities and inhibitory effects on digestive enzymes, which suggested that okra fruits could be explored further as function food ingredients for industrial applications.
Acknowledgements
This work was supported by the Scientific Research Foundation of Sichuan Agricultural University (Grant number 03120321) and the Scientific Research Fund Project of Science and Technology Department of Sichuan Province (Grant numbers 2017NZ0039, 2018NZ0010, and 2018JY0149).
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dan-Dan Shen and Xu Li have contributed equally to this work.
Contributor Information
Wen Qin, Phone: +86 0835 2883219, Email: Qinwen@sicau.edu.cn.
Ding-Tao Wu, Phone: +86 0835 2883219, Email: DT_Wu@sicau.edu.cn.
References
- Ahmed BT, Kumar SA. Antioxidant and antidiabetic properties of Abelmoschus esculentus extract. Res J Biotechnol. 2016;3(11):31–43. [Google Scholar]
- Anand PK, Aradhya SM. Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food Chem. 2005;93(2):319–324. doi: 10.1016/j.foodchem.2004.09.029. [DOI] [Google Scholar]
- Arapitsas P. Identification and quantification of polyphenolic compounds from okra seeds and skins. Food Chem. 2008;110(4):1041–1045. doi: 10.1016/j.foodchem.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Cahyana AH, Kam N, Ellyn Study on the stability of antioxidant and anti α-glucosidase activities using soaking treatment in okra (Abelmoschus esculentus L.) mucilage extraction. Chem Int. 2017;3(3):201–211. [Google Scholar]
- Chao PY, Lin SY, Lin KH, Liu YF, Hsu JI, Yang CM, Lai JY. Antioxidant activity in extracts of 27 indigenous Taiwanese vegetables. Nutrients. 2014;6:2115–2130. doi: 10.3390/nu6052115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho AR, An DG, Lee Y, Ahn JH. Biotransformation of quercetin to quercetin 3-O-gentiobioside using engineered Escherichia coli. Appl Biol Chem. 2016;59(5):689–693. doi: 10.1007/s13765-016-0212-5. [DOI] [Google Scholar]
- Fan SJ, Zhang Y, Sun QH, Yu LJ, Li MX, Zheng B, Wu XM, Yang BC, Li YM, Huang C. Extract of okra lowers blood glucose and serum lipids in high-fat diet-induced obese C57BL/6 mice. J Nutr Biochem. 2014;25:702–709. doi: 10.1016/j.jnutbio.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Górnas´ P, Dwiecki K, Siger A, Tomaszewska-Gras J, Michalak M, Polewski K. Contribution of phenolic acids isolated from green and roasted boiled-type coffee brews to total coffee antioxidant capacity. Eur Food Res Technol. 2015;242:641–653. doi: 10.1007/s00217-015-2572-1. [DOI] [Google Scholar]
- Graham JO, Agbenorhevi JK, Kpodo FM. Total phenol content and antioxidant activity of okra seeds from different genotypes. Am J Food Nutr. 2017;5(3):90–94. doi: 10.12691/ajfn-5-3-2. [DOI] [Google Scholar]
- Jiang N, Liu C, Li D, Zhang Z, Liu C, Wang D, Niu L, Zhang M. Evaluation of freeze drying combined with microwave vacuum drying for functional okra snacks: antioxidant properties, sensory quality, and energy consumption. LWT Food Sci Technol. 2017;82:216–226. doi: 10.1016/j.lwt.2017.04.015. [DOI] [Google Scholar]
- Karim MR, Islam MS, Sarkar SM, Murugan AC, Makky EA, Rashid SS, Yusoff MM. Anti-amylolytic activity of fresh and cooked okra (Hibiscus esculentus L.) pod extract. Biocatal Agric Biotechnol. 2014;3:373–377. doi: 10.1016/j.bcab.2014.07.006. [DOI] [Google Scholar]
- Liao H, Dong W, Shi X, Liu H, Yuan K. Analysis and comparison of the active components and antioxidant activities of extracts from Abelmoschus esculentus L. Pharmacogn Mag. 2012;8(30):156–161. doi: 10.4103/0973-1296.96570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Lu MF, Liao HB, Li YX, Han W, Yuan K. Content determination of the flavonoids in the different parts and different species of Abelmoschus esculentus L. by reversed phase-high performance liquid chromatograph and colorimetric method. Pharmacogn Mag. 2014;10(39):278–284. doi: 10.4103/0973-1296.137368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Guo H, Gong JDB, Lu M, Lu MY, Wang L, Zhang Q, Qin W, Wu DT. Phenolic profiles, β-glucan contents, and antioxidant capacities of colored Qingke (Tibetan hulless barley) cultivars. J Cereal Sci. 2018;81:69–75. doi: 10.1016/j.jcs.2018.04.001. [DOI] [Google Scholar]
- Lu Y, Demleitner MF, Song L, Rychlik M, Huang D. Oligomeric proanthocyanidins are the active compounds in Abelmoschus esculentus Moench for its α-amylase and α-glucosidase inhibition activity. J Funct Food. 2016;20:463–471. doi: 10.1016/j.jff.2015.10.037. [DOI] [Google Scholar]
- Ma T, Sun X, Zhao J, You Y, Lei Y, Gao G, Zhan J. Nutrient compositions and antioxidant capacity of kiwifruit (Actinidia) and their relationship with flesh color and commercial value. Food Chem. 2017;218:294–304. doi: 10.1016/j.foodchem.2016.09.081. [DOI] [PubMed] [Google Scholar]
- Nowicka P, Wojdyło A, Samoticha J. Evaluation of phytochemicals, antioxidant capacity, and antidiabetic activity of novel smoothies from selected Prunus fruits. J Funct Food. 2016;25:397–407. doi: 10.1016/j.jff.2016.06.024. [DOI] [Google Scholar]
- Olivera DF, Mugridge A, Chaves AR, Mascheroni RH, Viña SZ. Quality attributes of okra (Abelmoschus esculentus L. Moench) pods as affected by cultivar and fruit size. J Food Res. 2012;1(4):224–235. doi: 10.5539/jfr.v1n4p224. [DOI] [Google Scholar]
- Petropoulos S, Fernandes A, Barros L, Ferreira ICFR. Chemical composition, nutritional value and antioxidant properties of Mediterranean okra genotypes in relation to harvest stage. Food Chem. 2018;242:466–474. doi: 10.1016/j.foodchem.2017.09.082. [DOI] [PubMed] [Google Scholar]
- Podsedek A, Majewska I, Redzynia M, Sosnowska D, Koziolkiewicz M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J Agric Food Chem. 2014;62:4610–4617. doi: 10.1021/jf5008264. [DOI] [PubMed] [Google Scholar]
- Sabitha V, Ramachandran S, Naveen KR, Panneerselvam K. Antidiabetic and antihyperlipidemic potential of Abelmoschus esculentus (L.) Moench. in streptozotocin-induced diabetic rats. J Pharm Bioall Sci. 2011;3(3):397–402. doi: 10.4103/0975-7406.84447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakulnarmrat K, Konczak I. Composition of native Australian herbs polyphenolic-rich fractions and in vitro inhibitory activities against key enzymes relevant to metabolic syndrome. Food Chem. 2012;134(2):1011–1019. doi: 10.1016/j.foodchem.2012.02.217. [DOI] [PubMed] [Google Scholar]
- Sergent T, Vanderstraeten J, Winand J, Beguin P, Schneider YJ. Phenolic compounds and plant extracts as potential natural anti-obesity substances. Food Chem. 2012;135(1):68–73. doi: 10.1016/j.foodchem.2012.04.074. [DOI] [Google Scholar]
- Tan Y, Chang SKC, Zhang Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017;214:259–268. doi: 10.1016/j.foodchem.2016.06.100. [DOI] [PubMed] [Google Scholar]
- Xia F, Zhong Y, Li M, Chang Q, Liao Y, Liu X, Pan R. Antioxidant and anti-fatigue constituents of okra. Nutrients. 2015;7(10):8846–8858. doi: 10.3390/nu7105435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Shen Q, Li LQ, Huang YQ, Cheung HY. Phytochemical profiles, antioxidant activities of functional herb Abrus cantoniensis and Abrus mollis. Food Chem. 2015;177:304–312. doi: 10.1016/j.foodchem.2015.01.054. [DOI] [PubMed] [Google Scholar]
- Zeng H, Liu Q, Yu J, Wang M, Chen M, Wang R, He X, Gao M, Chen X. Separation of α-amylase inhibitors from Abelmoschus esculentus (L). Moench by on-line two-dimensional high-speed counter-current chromatography target-guided by ultrafiltration-HPLC. J Sep Sci. 2015;38:3897–3904. doi: 10.1002/jssc.201500824. [DOI] [PubMed] [Google Scholar]
- Zhang B, Deng Z, Ramdath DD, Tang Y, Chen PX, Liu R, Liu Q, Tsao R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chem. 2015;172:862–872. doi: 10.1016/j.foodchem.2014.09.144. [DOI] [PubMed] [Google Scholar]
- Zhang T, Xiang J, Zheng G, Yan R, Min X. Preliminary characterization and anti-hyperglycemic activity of a pectic polysaccharide from okra (Abelmoschus esculentus (L.) Moench) J Funct Food. 2018;41:19–24. doi: 10.1016/j.jff.2017.12.028. [DOI] [Google Scholar]