Abstract
Egypt has successfully eliminated malaria during 2010–2013, however, between May to mid-June 2014, an outbreak was reported in Al-Adwa village, Aswan Governorate indicating that malaria may be potentially re-emerging in the country. The aim of this work was to reevaluate malaria in Al-Adwa and surrounding villages, 1 year after malaria cases have been reported through entomological and human screening in collaboration with the Ministry of Health and Population. Four trips were conducted during the period from March 2015 to March 2016. Mosquitoes were collected, sorted and identified. Anopheles species were processed for detection of Plasmodium by polymerase chain reaction (PCR), and engorged abdomens in blood-fed mosquitoes were analyzed for host preference using multiplex PCR. Thick and thin blood films were prepared from all apparently healthy children (n = 188) attending El-Sheikh Mostafa preparatory school. Results showed that Anopheles genus existed only in East Al-Adwa village. A total of 38 Anopheles mosquitoes were collected and identified as Anopheles multicolor 70% (A. multicolor), A. sergenti 20% and A. pharoensis 10%. The latter showed 100% human blood preference compared to A. sergenti (20%) and An. multicolor (0%). All female Anopheles were 100% negative for Plasmodium DNA, and all blood films showed no detectable parasite. The absence of Plasmodium in the area under study does not rule out the risk of future infections as the vector is still present together with the imported cases and carriers. Regular screening for the presence of Plasmodium in the area is absolutely paramount for early warning.
Keywords: Anopheles mosquito, Malaria, Egypt, Host preference, PCR
Introduction
Malaria is a mosquito-borne serious illness caused by genus Plasmodium. The majority of the world’s population live in areas at risk of malaria transmission. Since the year 2000, substantial progress has been made in fighting malaria worldwide; however, in 2018, the World Health Organization (WHO) has requested an urgent action to get the global fight against malaria back on track because after an unprecedented period of success in malaria control between 2000 and 2015, progress has stalled with 216 million reported malaria cases in 2016 compared to 211 million cases in 2015 (WHO 2018).
Malaria is transmitted by the bite of female Anopheline mosquitoes of which 30 species are vectors of major importance (Blandin and Levashina 2004). Twelve Anopheles species are currently present in Egypt but only five are known to be malaria vectors: Anopheles pharoensis (A. pharoensis), the most important all over Egypt, especially in the Delta, A. sergenti, the primary vector in the Oases of the Western Desert, A. multicolor in Al-Fayoum, A. stephensi in the Red Sea Coast and A. superpictus in Sinai (Wassim 2014).
In 1943, a major malaria epidemic occurred in Egypt associated with the spread of A. arabiensis from Sudan along the Nile Valley (Malcolm et al. 2009). Between 1982 and 1991, Plasmodium vivax (P. vivax) and P. falciparum were reported in 7 governorates: Port Said, Suez, Sharkia, Menofia, Beni Suef, Fayoum, and Aswan. In 1989, Egypt was listed among the malaria endemic countries. The disease control efforts were shared between the Malaria Control Program and general health services (WHO 1990) until Egypt has achieved a steady decline in malaria transmission. Very few sporadic cases of malaria were then diagnosed, especially from Al-Fayoum governorate, between 1992 and 2001, which was categorized as a high-risk area for malignant malaria (Morsy et al. 1995). Between October 2003 to July 2004, Zaher et al. (2007) reported 16 malaria cases in Almaza Military Fever Hospital, nine of which were imported pilgrims suffering from P. falciparum and 7 cases acquired P. vivax locally. During 2010–2013, Egypt successfully eliminated malaria but remained in the stage of prevention of re-introduction of malaria (WHO 2015). Unfortunately, between late May to mid-June 2014, the Egyptian Ministry of Health (MoHP) reported the presence of 23 cases of P. vivax and one case of P. falciparum in East Al-Adwa village, Aswan governorate (Kenawy 2015). Aswan governorate is along the southern part of the Nile River and it is believed that the P. vivax malaria came from Sudanese migrants. The MoHP and local government have engaged in an intensive malaria control program in the affected villages.
The risk of localized outbreaks of malaria cases in Egypt due to infection of local anopheline mosquitoes by imported cases does exist (Saleh et al. 2016). Several factors contribute to this risk including the continuous movement of people between Aswan governorate and Sudan and the influx of large populations from Africa and Asia to Cairo and other cities for educational and religious purposes (Hassan et al. 2003). In Aswan governorate, Edfu villages have reported settlement of some illegal borders crossing Sudanese individuals who came to work and teach locals gold mining. Such activity would result in the formation of more mosquito breeding places (Kandeel et al. 2016). People coming from endemic areas may act as carriers for the parasite and this, together with the presence of anopheline vectors, may be the cause of the past and future outbreaks. Moreover, with global warming and the continuous increase in temperature, it is possible that A. gambiae expands its range of distribution to Egypt.
Dahesh and Mostafa (2015) took the initiative to reevaluate malaria in Al-Fayoum and reported 9 positive cases among 2044 examined stained thick films, 3 of which were P. falciparum and the rest P. vivax. All positive cases were males, imported from Sudan, and most of them were merchants having trade activities in Sudan. The aim of this study was to reevaluate malaria in Al-Adwa and surrounding villages, 1 year after malaria cases have been reported to ensure the absence of Plasmodium parasite in both vectors and human blood.
Materials and methods
Study area
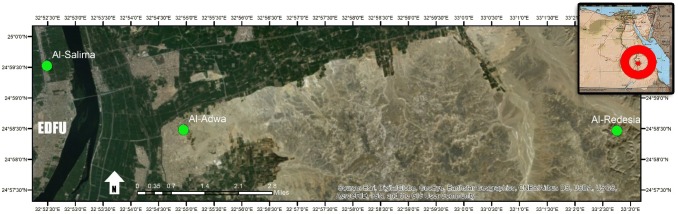
Screening was conducted in Al-Adwa (El-Sheikh Mostafa) (24°58.484 N, 32 54.946E), Al-Salima (24°59.526 N, 32°52.486E) and Al-Redesia (24°58.470 N, 32°54.929E) villages, Edfu district, Aswan governorate, during the period between March 2015 and March 2016, where a previous malaria outbreak has been recorded (Fig. 1)
Fig. 1.
Map showing the 3 study sites (Al-Adwa, Al-Salima and Al-Redesia) in which malaria screening took place between March 2015 and March 2016
Mosquito sampling
Four field trips of one-week duration each were carried out in March, May, October 2015 and March 2016 to collect mosquitoes. Outdoor trapping was conducted for 5 consecutive nights at selected field sites using CDC light traps (Hock 2012). Traps were set in the evening around 17:00 and collected the following morning around 08:00 for approximately 12 h. trap period. Pyrethrum spray catch was conducted for indoor mosquito collection from houses near known breeding sites after obtaining approval of the occupants (WHO 1992). GPS readings (Garmin Oregon 550t handheld Global Positioning System) and a text description of each collection site were recorded to identify the sites when returning for follow-up and resampling.
Morphological identification and processing of mosquitoes
Anopheline mosquitoes were identified using the morphological keys of Gilles and De Meillon (1968). The head and thorax of all female Anopheles vectors were separated from the abdomen to test for Plasmodium, while abdomens of blood fed mosquitoes were used for blood meal analysis.
Detection of Plasmodium by PCR
DNA was extracted from mosquitoes using QIAamp DNA mini kit (Qiagen, Valencia, CA). A screening reaction for the presence of Plasmodium genus with conserved primers pair forward primer, Plasmo 1, and a reverse primer, Plasmo 2, were selected to amplify a 157- to 165-bp segment of the four plasmodial 18S genes and TaqMan probe was labeled with 5′FAM and 3′TAMRA as the reporter and quencher, respectively according to Rougemont et al. (2004). Plasmodium positive DNA, prepared from previous samples, was used as a positive control for PCR runs.
Identification of blood meal origin
Extracted DNA from engorged abdomens was used in a multiplex PCR targeting Cytochrome b using primers established by Kent and Norris (2005) for five mammal species: cow, human, pig, goat, and dog. Band size (bp) specific for each mammal was identified and visualized after electrophoresis on ethidium bromide–stained 2% agarose gels. Specific positive control was used for PCR run confirmation. All multiplex PCR runs included positive and negative controls.
Informed consent
An informed consent was obtained from all legal guardians of children who participated in the study.
Human screening
In March 2016, a representative sample including all school children (n = 188) from both sexes attending El-Sheikh Mostafa preparatory school whose ages ranged from 12 to 14 years old, living in East Al-Adwa (El-Sheikh Mostafa) village were screened for the presence of Plasmodium parasites by thin and thick blood films. Blood films were prepared and read by an expert parasitologist. Also, a sheet was formulated to collect relevant data as regards age, sex, residence, past history of malaria and antimalarial treatment.
Ethical clearance
The protocol for this study was reviewed and approved by the Research Committee of Medical Parasitology Department, Research Ethics Committee at Faculty of Medicine, Ain Shams University and the Egyptian MoHP. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Results and discussion
A total of 3789 adult mosquitoes were collected in all four trips. Among the collected mosquitoes, Culex pipiens mosquitoes were the most abundant (80.0%), followed by Culex antennatus (8.9%), Aedes caspius (5.5%), Culiseta longiareolata (2.2%), Culex univitatus (1.5%), Phlebotomus papatasi (1.2%), while anopheline mosquitoes were detected in very low densities (1.0%). Anopheles mosquitoes were detected only in March 2015 and 2016 which corresponds to the appropriate temperature (17.5 -18.1 °C) and humidity (37%). This correlates with the finding of Ahmadi et al. (2015) who reported a high prevalence of Anopheles during November– March. The absence of Anopheles in May, October 2015 could be due to the higher temperature (≥ 35 °C). Also, Anopheles were found only in East Al-Adwa village, although the collection methodology was the same for all sites. This was probably because of the presence of under-ground water at the quarry area in this specific village, a suitable breeding place, as Anopheles prefer clean and unpolluted waters.
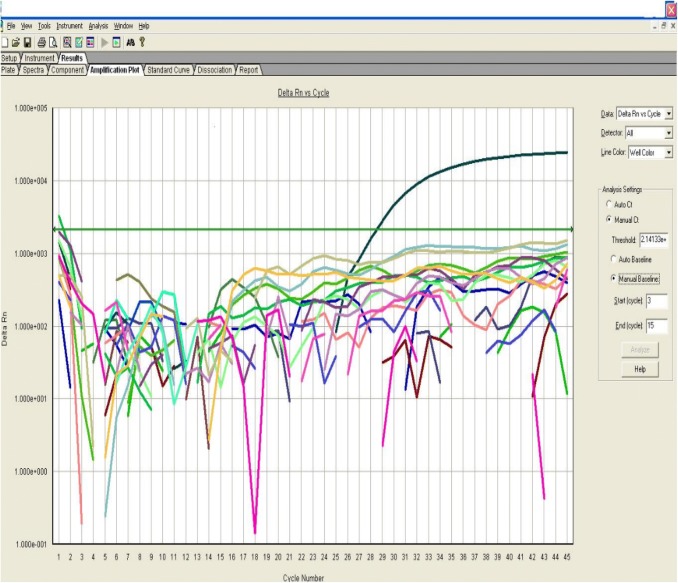
In the current work, three Anopheles species were identified; A. multicolor (n = 28, 22 fed and 2 unfed, 4 males) A. sergenti (n = 7, 6 fed and 1 unfed) and A. pharoensis (n = 3, 2 fed and 1 unfed). The MoHP declared that 20% of anopheline species recorded during Aswan malaria outbreak were A. sergenti (Kandeel et al. 2016). This coincides with the present study as A. sergenti accounted 18.4% from the collected anophelines, and agrees with a previous study conducted by El-Said et al. (1986) who incriminated A. sergenti as the responsible vector of malaria in Egypt in the Nile Delta. Kenawy (1988) confirmed that A. sergenti was responsible for P. falciparum transmission in Al- Fayoum, while A. pharoensis for P. vivax transmission. El-Bahnasawy et al. (2011) recorded A. multicolor, A. sergenti and A. algeriensis in Toshka project confirming A. sergenti as a malaria-vector and A. multicolor as a suspected vector. El-Said et al. (1986) documented that A. sergenti is more efficient than A. pharoensis in transmitting malaria as they found a higher sporozoite rate (0.85%) in A. sergenti than in A. pharoensis (0.36%) collected from Al-Fayoum. Such efficiency may be attributed to its relatively longer adult life. All Anopheles female mosquitoes tested for the presence of Plasmodium DNA were negative as shown in Fig. 2.
Fig. 2.
Amplification plot curve of the real-time PCR assay with positive control amplification detected at cycle 27 and no detection of PCR products for all tested samples
Identification of mosquito host preference is useful in understanding the disease transmission profile in an area. The current work showed that all tested A. pharoensis and one A. sergenti exhibited anthropophilic behavior, while the rest of A. sergenti preferred cow blood, and 18 of the A. multicolor fed on cow and 4 fed on dog blood (Figs. 3 and 4). This confirmed work that was done by Kenawy et al. (1990) who reported that A. sergenti and A. multicolor, collected from 3 Western desert oases, fed primarily on large domestic hosts. Also, A. pharoensis collected from Aswan governorate were 100% anthroponotic while A. sergenti and A. multicolor were zoophilic (48.1% and 68.75% respectively) (Morsy et al. 1995). The initial attraction emanating from cattle, goats, and sheep kept inside or around human houses may influence the feeding behavior of mosquitoes. A possible change in behavior may increase their risk of having the man as a regular source of the blood meal by zoophilic species transmitting other mosquito-borne diseases in animals and human (Oyewole et al. 2007). Therefore, the human preference of A. sergenti deviating from its normal zoophagic habit may be due to, either, accidental feeding on the human being or a shifting pattern of host preference from animals to human. Once the human preference is detected for a mosquito, the next step would be the need to examine the same mosquito for the probable occurrence of Plasmodium. Simultaneous detection of host and parasite determines the vectorial capacity of an Anopheles mosquito (Mohanty et al. 2007).
Fig. 3.

Stained agarose gel electrophoresis (2%) of PCR assay for blood meal identification. Lane A DNA marker (100 bp), lanes 1–9 Anopheles abdomen samples, lanes 1,2,7 human blood, lanes 4,5 cow and lane 9 dog, lanes N1, N2: Negative controls showing no PCR product
Fig. 4.

Stained agarose gel electrophoresis (2%) of PCR assay for blood meal identification. Lane A DNA marker (100 bp), lanes 17–19, 21–26 Anopheles abdomen samples, All lanes show cow blood preference except lane 26 dog blood, lanes N1, N2 negative control showing no PCR product
Several factors, which are the highly zoophilic preference of A. multicolor and A. sergenti, the efficient measures undertaken by MoHP in terms of screening contacts, diagnosis and treatment of cases, dissemination of information to medical and paramedical staff on malaria status in affected areas and enhancement of malaria awareness among the risk population contributed significantly to the limited malaria outbreak that occurred in Aswan governorate (Kandeel et al. 2016).
As regards human screening, examining a thick and thin stained blood film is the gold standard test for the detection of a very low level of parasitemia in blood. The preference of this technique over the malaria rapid antigen test is the false negative results documented by the latter (Koita et al. 2012). All examined blood films showed no detectable Plasmodium parasites (100% negative). Data collected showed that one child had a previous history of malaria infection as well as the history of antimalarial medication, and four children had residence adjacent to the site of the previous outbreak.
The notion that malaria may be potentially re-emerging in the country raises the flag for continuous monitoring and evaluation. Although no Plasmodium parasites were detected either in Anopheles mosquitoes or human blood films, yet, the risk of subsequent malaria outbreaks still exists due to the presence of local anopheline mosquitoes which can be infected by imported cases. Also, the continuous monitoring of insecticide resistance status of Anopheles mosquitoes in the area is crucial for integrated vector control strategies.
Author contributions
DMM research idea conceiving and manuscript revision, HMH Link with Ministry of Health and manuscript linguistic revision, BMREG Results confirmation and manuscript writing, HST Lab work and manuscript writing, MAH Sample collection permission and Manuscript editing, RAAM Field and lab work.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Ahmadi MS, Vatandoost H, Zar M, Turki H, Alizadeh A. Topographical distribution of anopheline mosquitoes in an area under elimination programme in the south of Iran. Malar J. 2015;14:262. doi: 10.1186/s12936-015-0771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandin SA, Levashina EA. Mosquito immune responses against malaria parasites. Curr Opin Immunol. 2004;16:16–20. doi: 10.1016/j.coi.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Dahesh SMA, Mostafa HI. Reevaluation of malaria parasites in El-Fayoum Governorate, Egypt using rapid diagnostic tests (Rdts) J Egypt Soc Parasitol. 2015;45(3):617–628. doi: 10.12816/0017929. [DOI] [PubMed] [Google Scholar]
- El-Bahnasawy MM, Saleh NM, Khalil MF, Morsy TA. The impact of three anopheline mosquito species in Toshka on the introduction of chloroquine resistant P. falciparum to Egypt. J Egypt Soc Parasitol. 2011;41(3):573–592. [PubMed] [Google Scholar]
- El-Said S, Beier JC, Kenawy MA, Morsy ZS, Merdan AI. Anopheles population dynamics in two malaria endemic villages in Faiyum Governorate, Egypt. J Am Mosq Control Assoc. 1986;2:158–163. [PubMed] [Google Scholar]
- Gilles MT, De Meillon B. The anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region) 2. Johannesburg: The South African Institute for Medical Research; 1968. [Google Scholar]
- Hassan AN, Kenawy MA, Kamal H, Abdel Sattar AA, Sowilem MM. GIS-based prediction of malaria risk in Egypt. East Mediterr Health J. 2003;9(4):548–558. [PubMed] [Google Scholar]
- Hock JW (2012) CDC miniature light trap. Model 512. (Gainesville, Florida)
- Kandeel A, Haggag AA, Abo El Fetouh M, Naiel M, Refaey SA, Hassan AH, Ramzy RMR. Control of malaria outbreak due to Plasmodium vivax in Aswan Governorate, Egypt. East Mediterr Health J. 2016;22(4):274–279. doi: 10.26719/2016.22.4.274. [DOI] [PubMed] [Google Scholar]
- Kenawy MA. Anopheline mosquitoes (Diptera: Culicidae) as malaria carriers in A.R. Egypt “History and Present status”. J Egypt Public Health Assoc. 1988;63:67–85. [PubMed] [Google Scholar]
- Kenawy MA. Review of Anopheles mosquitoes and malaria in ancient and modern Egypt. J M R. 2015;5(4):1–8. [Google Scholar]
- Kenawy MA, Beier JC, Asiago CM, EL Said S. Factors affecting the human feeding behavior of anopheline mosquitoes in Egyptian Oases. J Am Mosq Control Assoc. 1990;6(3):451–464. [PubMed] [Google Scholar]
- Kent RJ, Norris DE. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am J Trop Med Hyg. 2005;73:336–342. doi: 10.4269/ajtmh.2005.73.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koita OA, Doumbo OK, Ouattara A, Tall LK, Konaré A, Diakité M, Diallo M, Sagara I, Masinde GL, Doumbo SN, Dolo A, Tounkara A, Traoré I, Krogstad DJ. False-negative rapid diagnostic tests for malaria and deletion of the histidine-rich repeat region of the hrp2 gene. Am J Trop Med Hyg. 2012;86(2):194–198. doi: 10.4269/ajtmh.2012.10-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm CA, El Sayed B, Babiker A, Girod R, Fontenille D, Knols BGJ, Nugud AH, Benedict MQ. Field site selection: getting it right first time around. Malar J. 2009;8(Suppl 2):S9. doi: 10.1186/1475-2875-8-S2-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A, Mohanty A, kar P, Mishra K, Singh DV, Mohapatra N, Kar SK, Dash AP, Hazra RK. Multiplex PCR assay for the detection of Anopheles fluviatilis Species complex, human host preference and Plasmodium falciparum sporozoite presence, using a unique mosquito processing method. Am J Trop Med Hyg. 2007;76(5):837–843. doi: 10.4269/ajtmh.2007.76.837. [DOI] [PubMed] [Google Scholar]
- Morsy TA, El Kadry AA, Salama MMI, Sabry AA, El Sharkawy IMA. Studies on bionomics and vector competence of adult anopheline mosquitoes in El Faiyum Governorate, Egypt. J Egypt Soc Parasitol. 1995;25:213–244. [PubMed] [Google Scholar]
- Oyewole IO, Awolola TS, Ibidabo CA, Oduola AO, Okwa OO, Obansa JA. Behavior and population dynamics of major anopheline vectors in a malaria endemic area in southern Nigeria. J Vector Borne Dis. 2007;44:56–64. [PubMed] [Google Scholar]
- Rougemont M, Van Saanen M, Sahli R, Hinrikson HP, Bille J, Jaton K. Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J Clin Microbiol. 2004;42:5636–5643. doi: 10.1128/JCM.42.12.5636-5643.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MA, Adam SM, Ibrahim AMA, Tosson AM. Malaria: a general minireview with reference to Egypt. J Egypt Soc Parasitol (JESP) 2016;46(1):35–48. doi: 10.12816/0026148. [DOI] [PubMed] [Google Scholar]
- Wassim NM. Secondary structure and sequence of its2-rDNA of the Egyptian malaria vector Anopheles pharoensis (theobald) J Egypt Soc Parasitol (JESP) 2014;44(1):197–204. doi: 10.12816/0006459. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1990) Consultation on epidemiological malaria information at national and regional levels. http://applications.emro.who.int/docs/who_em_mal_217_e_en.pdf. Accessed 10 Oct 2018
- World Health Organization . Entomological field techniques for malaria control: Part 1. Learner’s guide. Geneva: World Health Organization; 1992. [Google Scholar]
- World Health Organization (2015) World Malaria Report 2015. http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/. Accessed 10 Oct 2018
- World Health Organization (2018). http://www.who.int/docs/default-source/documents/key-messages.pdf?sfvrsn=73faaf09_0. Accessed 9 Oct 2018
- Zaher T, Ahmadi M, Ibrahim A, El-Bahnasawy M, Gouda H, et al. Malaria in Egypt, Saudi Arabia and Yemen: a clinical pilot study. J Egypt Soc Parasitol. 2007;37(3):969–976. [PubMed] [Google Scholar]