Abstract
Cold pressing technology is a new technology using during the apple juice processing, which involved peeling and deseeding of apples at low temperature. The phenolics of apple juice, apple vinegar and apple pomace generated by cold pressing and traditional process were investigated. The results showed that the total phenols and flavanols of cold pressing apple juice were lower than those of traditional process. The total phenols content of peel pomace extract was significantly higher than that of the pulp pomace by almost tenfold, which showed that the peels and seeds were valuable sources of phenolic compounds. The total phenols of apple vinegars were significantly different. The predominant compounds in apple products were phloridzin and chlorogenic acid, while the apple pomaces based on cold pressing technology had significantly high content of phenolic compounds, indicating that the cold pressing technology could facilitated the use of apple pomace for bioactive compounds.
Keywords: Apple, Phenolic compound, Cold pressing, Apple production
Introduction
Apple is one of the most commonly consumed fruits worldwide, and it is beneficial to human health (Antal et al. 2015; Markowski et al. 2015) thanks to the sugar, sugar alcohol, organic acid, amino acid and phenolic compounds (Maragò et al. 2016; Pires et al. 2018; Zielinski et al. 2014). Apple polyphenols have been reported to have various physiological functions including in vivo and clinical antiallergic activity (Heinmaa et al. 2016), in vivo anti-caries activity, and in vitro and in vivo inhibitory activity against some enzymes and receptors (Yanagida et al. 2000). In addition, apple and apple production are important source of bioavailable polyphenols, which contribute to the antioxidant activity and anti-proliferation activity (Silva et al. 2015).
Apple polyphenols are widely common secondary metabolites of plants and fruits, play an important part in the sensory characteristics of fruit and fruit wine, particularly the color (Le Deun et al. 2015), aroma, bitterness, astringency and mouth feel (Alberti et al. 2017; Laaksonen et al. 2017; Ye et al. 2014). They are generally recognized as the main determinants of the biological activities of apples. Apple is one of the best source of antioxidant and phenolic compounds, including quercetin, catechin, phlorizdin and chlorogenic acid (Pires et al. 2018; Raudone et al. 2016). The leading polyphenols in apple juice are chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, catechin, epicatechin, procyanidines (B1, B2, trimer C1), rutin and phloridzin (Guo et al. 2013; Verdu et al. 2013). Herrera Alvarez et al. (2017) have informed that vinegars usually have the higher content of polyphenols, including phloridzin, chlorogenic acid, caffeic acid, gallic acid, catechin and so on. It is worth mentioning that apple pomace has long been recognized as a valuable material for nutritional, pharmacological and cosmetic purposes because of its dietary fiber and polyphenols (Leyva-Corral et al. 2016; Sudha et al. 2016). Apple pomace contains over 60 different phenolic compounds (Zhang et al. 2016), which means the apple pomace has a broad application prospect.
Furthermore, different processing technology has a great effect on apple production (Markowski et al. 2017). Cold pressing or cold extraction is a new technology during the apple juice concentrates processing, which has a considerable impact on product quality. It uses cold crushing equipment to remove peel, stalk and seeds in the initial stage, and then breaks the apples at low temperature without preheating. Compared with the traditional process, cold pressing is an optimized technology by prevention of heat and oxidative damages on antioxidant components. The cold pressing not only can reduce the activity of pulp polyphenol oxidase, peroxidase and the pesticide residues in apple juice, but also make the oxidative browning due to the enzymatic browning lower, and thus remain the original color of apple puree. In addition, the cold pressing technology can greatly simplify the process of the subsequent use of pomace.
Apple industry is one of the most competitive industries in northwest of China, and lots of apples are processed into juices or juice concentrates, ciders, vinegars and dried products. Apple juice and concentrates are the most important and traditional processed products; vinegar is one of the most popular beverages in China. Meanwhile, large quantities of apple pomace are accumulated each year in course of apple juice, cider and vinegar production as agro-industrial by-products. The objective of this study was to investigate the polyphenolic profile of apple juices, ciders and pomace under the cold pressing technology and traditional process.
Materials and methods
Chemicals
The following chemicals were supplied from the indicated sources: catechin, epicatechin, epicatechin gallate, epigallocatechin gallate, quercetin, rutin, caffeic acid, ferulic acid, chlorogenic acid, p-coumaric acid, vanillic acid, gallic acid, gentisic acid, salicylic acid, resveratrol, phlorizin (all purchased from Sigma, USA). Acetonitrile (Spectrum Corporation, USA); Saccharomyces cerevisiae (Angel Yeast Co., Ltd.), acetic acid bacteria powder (Shanghai stuffed 1.01 No. acetic acid bacteria); Methanol (HPLC grade); acetic acid, ethyl acetate, Folin reagent, Na2CO3 (Tianjin Tianli).
Preparation of apple juice samples
The Fuji apples were harvested in the Experimental Orchard of the Northwest University of Agriculture and Forestry (Liquan, China).The scale for each was 1000 kg; the apple juices were divided into cold pressing apple juice and traditional process apple juice.
Traditional process apple juice Apples were washed by internal spray after handpick steps. Then apples were transported to a hammer mill (Kean, Wu Xi, China) equipped with a screen of 9 mm diameter mounted in the bottom of the mill. These purees were crushed through the continuous belt filter-press (Kean, Wu Xi, China), and thus the juices were separated from the pulp. The dosage of 200 mg/L ascorbic acid was used for oxidation inhibition. The juice was heated to 90 °C in a steam-heated tube heat exchanger and cooling down. A total of three samples of juice were collected at the end of tube with intervals of 10 min between each sample.
Cold pressing apple juice Apples were washed by internal spray after handpick steps. Apples were then transported to cold crushing equipment equipped with cooler of ice water. The apples were peeled off, deseeded at low temperature, and the apple pulp was separated. The pulps were crushed through the continuous belt filter-press (Kean, Wu Xi, China). The dosage of 200 mg/L ascorbic acid was used for oxidation inhibition. The juice was heated to 90 °C in a steam-heated tube heat exchanger and cooling down. A total of 3 samples of juice were collected at the end of tube with intervals of 10 min between each sample.
All fruit juices were frozen at − 20 °C until use. In all cases, samples were centrifuged at 10,000g at 10 °C during 10 min in a Sigma 3K 30 centrifuge (Osterode, Germany) to remove any solid residue. Supernatants were filtered before analysis with 0.45 μm mesh filter.
Preparation of apple pomace
In this experiment, the apple pomace was provided by cold pressing technology. Based on the cold pressing technology, the flesh was the edible portion of the apple without the peel and seed; the pomace (peel and seed) were the parts of the apple removed by the apple parer through cold pressing process. The pulp and pomace were dried at 60 °C for 48 h until it reached a constant weight. Dry peel was reduced to a 40 mesh particle size, and then was frozen at − 40 °C.
Dry pulp and pomace samples (5 g) were ground in 10 mL of 70% methanol containing 2% formic acid, separately. Another 10 mL of extraction solution was used to wash the mortar and pestle and Combined with the first homogenate. Then the mixture was transferred into an Eppendorf tube. After being shaken at 30 °C for 30 min in a thermo mixer at 1000 rpm, the combined homogenate was centrifuged at 10,000g for 10 min. The supernatant was filtered through a 0.45 μm syringe filter prior to analysis.
Apple cider vinegar
Seven kinds of apple cider vinegar were chosen in this experiment. Fresh apple cider vinegar (V1) and concentrated apple cider vinegar (V2) were made in our laboratory, and samples were made from the same quality of raw material apple juice. And other common vinegars were selected from the local market, which were Rauch apple cider vinegar (V3), Sempio apple vinegar (V4), Dongkang apple cider vinegar beverage (V5), Health Road apple cider vinegar beverage (V6) and Guoran apple vinegar drink (V7).
Total phenolic content (TPC), total flavanol content (TFA) and total flavonoids content (TFO) of apple products
Total phenolics were determined using the Folin–Ciocalteu assay (Ganesan et al. 2008; Iora et al. 2015). The reaction mixture was prepared by mixing 100 μL extract with 7 mL water and 1.5 mL of 20% Na2CO3 in 10 mL tubes. The mixture was allowed to stand for 1 min at room temperature followed by the addition of 0.5 mL of Folin–Ciocalteu’s phenol reagent. After 60 min incubation at room temperature in the dark the absorbance was measured at 765 nm and the results were expressed in gallic acid equivalents (GAE).
The amount of total flavonoids was determined according to a previously described protocol (Kim et al. 2003). Absorbances were measured at 510 nm. Results were expressed as catechin equivalents (mg/L of CTE). Catechin standard solutions were prepared at a concentration ranging from 6.25 to 300 mg/L.
The amount of total flavanol was estimated using the slightly modified p-dimethylaminocinnamaldehyde (DMACA) method (Li et al. 1996; Xu et al. 2010). Absorbances were measured at 640 nm. Results were expressed as catechin equivalents (mg/L of CTE). Catechin standard solutions were prepared at a concentration ranging from 6.25 to 200 mg/L.
Free radical-scavenging ability (DPPH)
The ability for apple products to scavenge DPPH free radicals was determined. Scavenging activity was based on the slightly modified method (Li et al. 2009; Maragò et al. 2016). Briefly, 0.1 mL of extract was added to 3.9 mL of a 6 × 10−5 M solution of DPPH in methanol. A control sample containing the same volume of solvent in place of extract was used to measure the maximum DPPH absorbance. After the reaction was allowed to take place in the dark for 20 min, the absorbance at 515 nm was recorded to determine the concentration of remaining DPPH. The percentage inhibition of initial concentration of DPPH radical was calculated as: % inhibition = [(ADPPH − Awine)/ADPPH] × 100. Results were expressed as Trolox equivalent antioxidant capacity. Trolox standard solutions were prepared at a concentration ranging from 0 to 1200 M/L.
HPLC analysis of phenolic fractions
All analyses were conducted using high performance liquid chromatography. The analyses were carried out using a Waters XBridgeTM Shield RP18 (4.6 * 250 mm, 3.5 µm). The column temperature was 30 °C. Gradient elution system was applied, using a mobile phase comprised of (A) 98% (v/v) acetonitrile containing 2% (v/v) glacial acetic acid, and (B) 2% (v/v) glacial acetic acid.
The elution gradient was from 5 to 15% A for 60 min, 15% A for 5 min, from 15 to 20% for 1 min, 20% A for 7 min, from 20 to 30% for 1 min, 30% A for 6 min, from 30 to 40% for 1 min, 40% A for 12 min, and from 40 to 5% A for 2 min, with a flow rate at 0.8 mL/min. The injection volume was 20 μL, and the detection wavelength was 280 nm and 320 nm.
Statistical analysis
All data were reported as mean ± standard deviation of three replicates. Statistical analyses were performed by DPS v 7.05 for Windows. Principal component analysis (PCA) (software SPSS v19.0) was applied to separate the products according to phenolic composition and antioxidant activity.
Results and discussion
TPC, TFO and DPPH of apple juice
The comparisons of concentrations of total phenolic, total flavonoid and DPPH scavenging activity present in traditional process and cold pressing apple juices are reported in Table 1. Significant differences were observed between traditional process apple juice and cold pressing apple juice. The results presented in Table 1 indicated that the TPC of the traditional process apple juice was 307.3 ± 16.7 mg/L GAE, and the value of the cold pressing apple juice was 190.5 ± 21.2 mg/L GAE. It’s similar to the values measured by Alberti et al. (2016), which the TPC of Fuji apple juices varied from 171 to 279 mg/L GAE with the apples in three ripening stage. The total flavonoid content (TFO) of traditional process apple juice (70.0 ± 9.1 mg/L CTE) was higher than the cold pressing apple juice (51.7 ± 3.6 mg/L CTE). The values were in accordance with the levels found by Herrera Alvarez et al. (2017), who assessed apple juices produced in South America, North America and Europe.
Table 1.
The content of TPC, TFO and DPPH of the apple juice
| Apple juice | TPC (mg/L GAE) | TFO (mg/L CTE) | DPPH (mM/L Trolox) |
|---|---|---|---|
| Traditional process apple juice | 307.3 ± 16.7 | 70.0 ± 9.1 | 369.4 ± 22.8 |
| Cold pressing apple juice | 190.5 ± 21.2 | 51.7 ± 3.6 | 248.8 ± 20.5 |
Free radical scavenging activity of apple extracts was assessed by the most valid and easy-to use DPPH assay (Prior et al. 2005). As shown in Table 1, the result of traditional process apple juice was 369.4 ± 22.8 mM/L Trolox, and cold pressing apple juice had 248.8 ± 20.5 mM/L Trolox. The phenolic compounds present in apple juice crude extracts showed strong DPPH scavenging activity.
HPLC analysis of phenolic fractions
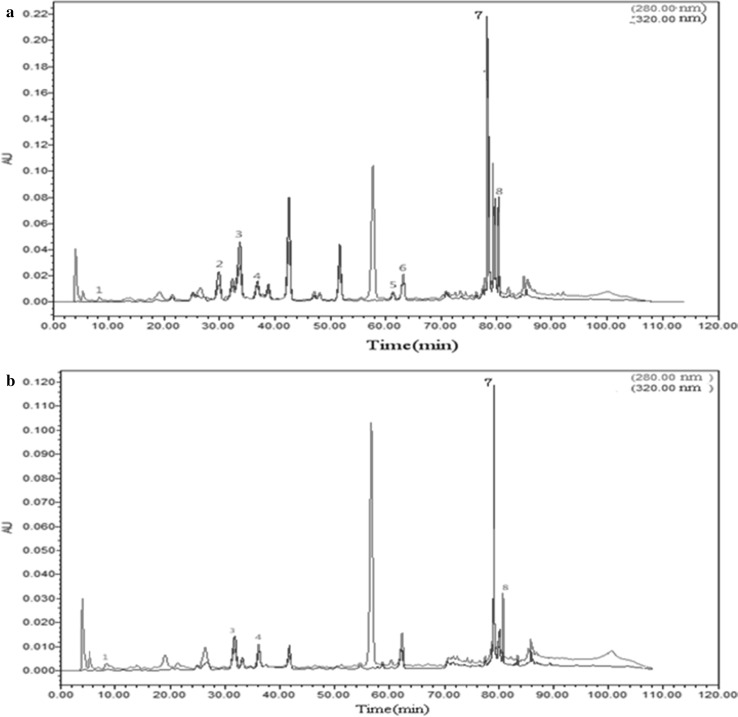
The polyphenol profile of apple juices were analyzed by HPLC, and the results were presented in Fig. 1. The experimental results show a stable baseline with low SNR. At different detection wavelengths, the detection peaks of substances were diverse. Labeled substances can be detected at both wavelengths. However, some peaks were displayed under 280 nm, but not in 320 nm. As can be seen from Fig. 1a, the essential phenolics that can be identified and quantified in apple juices were gallic acid (0.08 mg/L), vanillic acid (0.09 mg/L), chlorogenic acid (0.46 mg/L), caffeic acid (0.21 mg/L), p-coumaric acid (0.08 mg/L), ferulic acid (0.26 mg/L), rutin (4.54 mg/L) and phlorizin (0.86 mg/L), and these findings are generally in accordance with literature reports (Tian et al. 2018; Venkatachalam et al. 2018) showing significant variations in composition. Vanillic acid, p-coumaric acid and ferulic acid that have been reported to exist in trace quantities were not found in the analysed cold pressing apple juice (Fig. 1b), and five kinds of individual phenolic compounds in cold pressing fruit juice were gallic acid (0.06 mg/L), chlorogenic acid (0.43 mg/L), caffeic acid (0.11 mg/L), rutin (2.23 mg/L) and phlorizin(0.34 mg/L) (quantified as a whole). The results also indicated that chlorogenic acid (7%), rutin (69%) and phlorizin (13%) were the major phenolic compounds present in apple juice and that these three compounds represent at least 88% of total individual phenolics. These results are in agreement with Karaman et al. (2010) and Verdu et al. (2013) reported.
Fig. 1.
The polyphenol chromatogram of apple juice and apple cider vinegar. Notea Traditional process apple juice for individual phenolics, b cold pressing apple juice. The peaks are (1) gallic acid, (2) vanillic acid, (3) chlorogenic acid, (4) caffeic acid, (5) p-coumaric acid, (6) ferulic acid, (7) rutin and (8) phlorizin identified
As can be observed, concentrations of rutin, chlorogenic acid and phlorizin were higher than other phenolic compounds in the apple juices. The contents of individual phenolic compounds in traditional process apple juice were higher than those in cold pressing apple juice, which indicated that apple peels and seeds were rich in phenolic compounds. Even though the extracts of traditional process apple juice were more active than those obtained from the cold pressing apple juice, the latter process to treatment apple pomace was an optimized process which prevented heat, oxidative damages such as oxidative browning on antioxidant components due to the too much polyphenols.
TPC, TFA and DPPH of apple pomace and cider vinegars
The antioxidant activity of apple pomace and cider vinegars could be predicted from the contents of gallic acid, catechin, vanillic acid, chlorogenic acid, caffeic acid, epicatechin gallate and phlorizin. Polyphenols from apple pomace were considered highly valuable compounds which might be used as functional food ingredients (Schieber et al. 2003), and the apple pomace could also be the material to make apple cider vinegars. It was believed that the apple pomace shows high potential for cider production. The contents of total phenolic, total flavanol and DPPH of the apple pomace and apple cider vinegars are shown in the Table 2.
Table 2.
The comparison of the total phenols, total flavanols and DPPH scavenging activity in seven kinds of apple cider vinegar and pomace by cold pressing
| Samples | TPC (mg/L GAE) | TFA (mg/L CTE) | DPPH (μM/L Trolox) |
|---|---|---|---|
| Peel pomace | 2228.49 ± 66.78 | 170.33 ± 5.34 | 4203.59 ± 0.00 |
| Pulp pomace | 208.75 ± 9.28 | 40.91 ± 0.53 | 3830.72 ± 5.33 |
| V1 | 274.08 ± 35.48 | 3.360 ± 0.07 | 112.445 ± 35.59 |
| V2 | 284.73 ± 6.37 | 4.049 ± 0.08 | 624.345 ± 1.34 |
| V3 | 281.25 ± 3.33 | 0.893 ± 0.00 | 539.595 ± 3.99 |
| V4 | 33.197 ± 5.02 | 1.915 ± 0.31 | 73.495 ± 5.32 |
| V5 | 38.193 ± 3.94 | 1.693 ± 0.40 | 113.990 ± 9.32 |
| V6 | 57.363 ± 6.68 | 1.693 ± 0.69 | 67.845 ± 10.66 |
| V7 | 56.803 ± 2.55 | 0.893 ± 0.00 | 161.07 ± 14.65 |
Values represent means of triplicate determination ± SD
V1, fresh apple cider vinegar; V2, concentrated apple cider vinegar; V3, Rauch apple cider vinegar; V4, Sempio apple vinegar; V5, Dongkang apple cider vinegar beverage; V6, Health Road apple cider vinegar beverage; V7, Guoran apple vinegar drink
Both apple peels and pulp showed high phenolic content at 2228.49 ± 66.78 and 208.75 ± 9.28 mg/L GAE, respectively. The total phenolic content of peels extract was significantly higher than the pulp, almost tenfold. This is consistent with the findings of a previous study demonstrating that peels extracts exhibit higher TPC values than those obtained by pulps extraction (González-Laredo et al. 2007). The TFA of pulp pomace was 40.91 ± 0.53 mg/L CTE, and the content of the peels was fourfold higher than the pulp, which was 170.33 ± 5.34 mg/L CTE. The phenolic compounds present in apple peel and pulp crude extracts showed strong DPPH scavenging activity. The result of apple peel pomace was 4203.59 ± 0.00 μM/L Trolox, and apple pulp pomace had 3830.71 ± 5.33 μM/L Trolox.
Apple cider vinegars produced by different techniques had significantly different contents of individual phenolics and total polyphenols. The content of total phenols and flavanols showing considerable correlations with the antioxidant capacity of vinegar was measured, the higher total phenolic content, the better its antioxidant activity. In Table 2, it can be observed that the average antioxidant values of these vinegars were more than fivefold higher than vinegar beverages, and these phenols were a key nutritional factor in vinegar.
It can be seen that the total phenol values of seven apple vinegars varied greatly (33.19–284.73 mg/L). Total phenolic content of apple cider vinegar was maintained at between about 270 and 290 mg/L, while the total phenolic content of apple cider vinegar beverages only maintained at between 30 and 60 mg/L, which was lower than that Du (2009) reported (68.63 mg/L).
As for the total flavanols and DPPH scavenging activity, the data showed that concentrated apple cider vinegar (4.69 mg/L CTE and 1248.69 μM/L Trolox) had the most values, maybe due to the concentrated process that made the vinegar had a higher content of phenols. Flavanol substance, with a variety of roles delaying aging, antibacterial, deodorant, was a good antioxidant and improved the nutritional value of apple vinegar. In addition, a considerable difference between the apple cider vinegar and vinegar beverages was observed. The total flavanol content of apple cider vinegar was maintained at between about 3 and 5 mg/L CTE, and that of apple vinegar beverage only maintained at between 0.5 and 2 mg/L CTE.
DPPH value for apple cider vinegar content was maintained at between 100 and 700 μM/L Trolox, and the antioxidant ability of apple cider vinegar beverages only lasted between 50 and 100 μM/L Trolox. The DPPH scavenging activity of concentrated apple vinegar samples was significantly higher than that of the other varieties. That indicated that the concentrate apple vinegar had strong ability of free radical scavenging and the brewing apple cider vinegar had much higher antioxidant ability than apple vinegar beverages, with a large gap.
To sum up, the TPC, TFA and DPPH were found to be higher in apple pomace extracts. These are important selection criterion when considering the potential biological activity for apple pomace to as material to make cider vinegar. This characteristics of the apple pomace from cold pressing technology make it feasible to use the apple pomace as the raw material to obtain cider vinegar.
Major polyphenols of apple peal, pulp and cider
Massias et al. (2015) and Herrera Alvarez et al. (2017) pointed out that apple can provide a lot of polyphenols for the human body to improve the body’s condition. Table 3 shows the contents of major polyphenols of peel, pulp and seven kinds of apple cider vinegar and beverages. Gallic acid, catechin, epicatechin gallate, chlorogenic acids, phloridzin and rutin were identified in both peel and pulp extracts. Phloridzin was always the main dihydrochalcone present in the apple pomaces, with contents ranging between 12 and 60 mg/L. Vanillic acid was the second one, with the content range between 56 and 59 mg/L. Maximum content of vanillic acid was 40.42 mg/L. Four hydroxycinnamic acids were identified in the apple pomace: chlorogenic acid, caffeic acid, p-coumaric acid and ferulic acid, as described by others (Sánchez-Rabaneda et al. 2004). Epicatechin gallate and catechin contents varied 13–22 mg/L and 35–38 mg/L, separately. Compared with previous results on apple pomace, the sample results reported here exhibited higher level of polyphenolic compounds (Cetkovic et al. 2008). It was indicated that the individual phenolic compounds content of apple peels were higher than that of pulp. The concentrations of phlorizin in apple peels were remarkably higher than those found in apple pulp. The pomace which was collected through cold pressing technology could be more easily used, the peels and seeds contained much more phenols were a valuable source of phenolic compounds and the pulps could provide dietary fiber.
Table 3.
Major polyphenols (mg/L) of apple peels, pulps, cider vinegar and beverages
| Samples | Gallic acid (mg/L) | Vanillic acid (mg/L) | Caffeic acid (mg/L) | Chlorogenic acid (mg/L) | p-Coumaric acid (mg/L) | Ferulic acid (mg/L) | Catechin (mg/L) | Epicatechin gallate (mg/L) | Phlorizin (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| Peels | 40.42 ± 1.45 | 58.70 ± 3.05 | 0.21 ± 0.03 | 7.05 ± 0.18 | – | – | 37.67 ± 1.07 | 12.85 ± 0.55 | 60.28 ± 0.15 |
| Pulps | 29.57 ± 5.19 | 56.02 ± 3.33 | – | 5.29 ± 1.71 | – | – | 35.40 ± 9.32 | 21.68 ± 0.70 | 12.81 ± 0.94 |
| V1 | 0.35 ± 0.02 | 0.06 ± 0.04 | 3.03 ± 0.02 | 6.56 ± 0.43 | 0.33 ± 0.28 | 0.24 ± 0.07 | – | 0.77 ± 0.34 | 1.76 ± 0.14 |
| V2 | – | – | – | 17.63 ± 1.04 | 0.09 ± 0.00 | 0.03 ± 0.00 | – | – | – |
| V3 | 16.72 ± 0.18 | 3.44 ± 0.07 | 1.07 ± 0.05 | 26.42 ± 0.62 | 0.21 ± 0.01 | 0.18 ± 0.00 | 37.55 ± 0.61 | – | 10.10 ± 0.13 |
| V4 | 11.55 ± 0.21 | – | – | 1.26 ± 0.01 | 0.02 ± 0.00 | 0.24 ± 0.01 | 2.05 ± 0.02 | – | – |
| V5 | 9.92 ± 0.16 | – | 0.04 ± 0.00 | 0.83 ± 0.02 | – | – | – | – | – |
| V6 | 9.54 ± 0.50 | 0.08 ± 0.01 | 0.04 ± 0.00 | 1.24 ± 0.02 | – | 0.04 ± 0.00 | – | – | – |
| V7 | 7.70 ± 0.06 | – | 0.17 ± 0.00 | 2.79 ± 0.01 | 0.18 ± 0.00 | 0.06 ± 0.00 | – | – | 0.92 ± 0.01 |
Values represent means of triplicate determination ± SD
(V1, fresh apple cider vinegar; V2, concentrated apple cider vinegar; V3, Rauch apple cider vinegar; V4, Sempio apple vinegar; V5, Dongkang apple cider vinegar beverage; V6, Health Road apple cider vinegar beverage; V7, Guoran apple vinegar drink; –, not detected
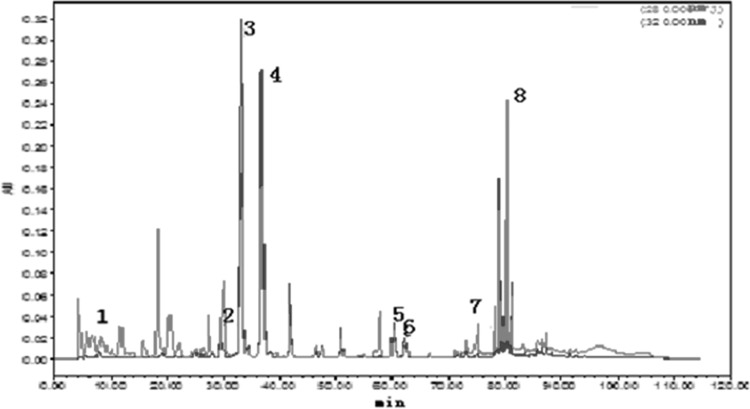
As presented in Fig. 2, eight kinds of polyphenols can be quantified in apple cider vinegar under test conditions, including chlorogenic acid, caffeic acid, phlorizin, vanilla acid, gallic acid, coumaric acid and ferulic acid. This result was two more than the six phenols determined by Budak et al. (2011). Chlorogenic acid, caffeic acid, and phlorizin had the highest levels of the concentrations of up to 6.56 mg/L, 3.03 mg/L and 1.76 mg/L, while vanilla acid with only 0.06 mg/L which was the lowest concentrations, gallic acid (0.35 mg/L), coumaric acid (0.33 mg/L) and ferulic acid (0.24 mg/L)with a relatively low concentration. The total concentration of the quantitative polyphenols in apple cider vinegar was up to 13.09 mg/L (Table 3), and the ratios of them were: gallic acid: vanilla acid: chlorogenic acid: caffeic acid: coumaric acid: ferulic acid: epigallocatechin gallate: phlorizin = 5:1:111:51:6:4:13:30.
Fig. 2.
Fresh apple cider vinegar sample. The peaks are (1) gallic acid, (2) vanillic acid, (3) chlorogenic acid, (4) caffeic acid, (5) p-coumaric acid, (6) ferulic acid, (7) rutin and (8) phlorizin identified
The major polyphenols in different vinegars is shown in Table 2, while the V1’s chromatograms is presented in Fig. 2. The phenols of peels and pulps showed in Tables 1 and 2 indicated that apple peels had higher polyphenols than the pulps, just like most other fruits (Singh et al. 2016). The individual antioxidant contents of the tested vinegars showed significant changes depending on the apple cider vinegar variety, especially only three kinds of individual phenols can be detected in concentrated apple cider vinegar due to the production process. Chlorogenic acid is the leading phenolic constituent of apple cider vinegar, the decreasing order of apple cider vinegars as follows (Table 3): Rauch apple cider vinegar > concentrated apple cider vinegar > fresh apple cider vinegar > Guoran apple vinegar drink > Sempio apple vinegar > Health Road apple cider vinegar beverage > Dongkang apple vinegar beverage.
Principal component analysis (PCA) on the combined data
In order to better discriminate the cultivars under investigation, principal component analysis (PCA) was performed on the combined data set of total polyphenols, total flavanols plus DPPH scavenging activity. The two principal components (PCs) accounted for 99.35% of the total variance, with PC1 (90.16%) explaining 10 times as much as PC2 (9.19%). By the analysis, it is indicated that PC1 and PC2 contain a lot of the samples’ information. From the PCA result, fresh apple cider vinegar (V1) were clearly separated with other products, apple peels were also separated with pulps. Apple peels had the highest score on the positive side of first axe, whereas fresh apple cider vinegar had the highest score on the second axe. According to the eigenvectors all the apple products were highly positively associated with PC1. Health Road apple vinegar drink (V6) and apple juice, Dongkang apple vinegar (V5) and Guoran apple vinegar drink (V7), apple peels and Rauch apple cider vinegar (V3) were positively associated with PC2, whereas concentrated apple cider vinegar (V2), Sempio apple vinegar (V4), and apple pulp were negatively associated with PC2.
Conclusion
The cold pressing technology involved cold crushing equipment to remove peels, stalk and seeds in the initial stage and then crush the apples at low temperature without preheating. Compared with the traditional process, cold pressing decreased not only the pesticide residues in apple juice, but also the oxidative browning due to the lower temperature. Nevertheless, the result obtained had highlighted the concentration of individual phenols in cold pressing apple juice lower than in traditional process apple, it still retained in the range of previous research data. Meanwhile the apple peels obtained by cold pressing could be an effective method to avoid discarding the valuable by-products of apple processing industry. The apple pomaces obtained by cold pressing technology possess high levels of antioxidant and bioactive compounds, and it could be a better material to make apple cider vinegar. The apple cider vinegars as one of China popular products, also contained favorable phenolic compounds. These results suggested that apple products and by-products based on cold pressing technology had health-promoting qualities and could be a competitive product in the commercial market.
Acknowledgements
The research was supported by the Xi’an Science and Technology Bureau (2017050NC/NY006) and Science and Technology Department of Shaanxi Province (2017NY-149).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guorong Du and Yanyun Zhu have contributed equally to this work and should be considered co-first authors.
References
- Alberti A, Machado dos Santos TP, Ferreira Zielinski AA, Eleutério dos Santos CM, Braga CM, Demiate IM, Nogueira A. Impact on chemical profile in apple juice and cider made from unripe, ripe and senescent dessert varieties. LWT Food Sci Technol. 2016;65:436–443. doi: 10.1016/j.lwt.2015.08.045. [DOI] [Google Scholar]
- Alberti A, Zielinski AAF, Couto M, Judacewski P, Mafra LI, Nogueira A. Distribution of phenolic compounds and antioxidant capacity in apples tissues during ripening. J Food Sci Technol. 2017;54(6):1511–1518. doi: 10.1007/s13197-017-2582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal T, Kerekes B, Sikolya L, Tarek M. Quality and drying characteristics of apple cubes subjected to combined drying (FD pre-drying and HAD finish-drying) J Food Process Preserv. 2015;39(6):994–1005. doi: 10.1111/jfpp.12313. [DOI] [Google Scholar]
- Budak NH, Kumbul Doguc D, Savas CM, Seydim AC, Kok Tas T, Ciris MI, Guzel-Seydim ZB. Effects of apple cider vinegars produced with different techniques on blood lipids in high-cholesterol-fed rats. J Agric Food Chem. 2011;59(12):6638–6644. doi: 10.1021/jf104912h. [DOI] [PubMed] [Google Scholar]
- Cetkovic G, Canadanovic-Brunet J, Djilas S, Savatovic S, Mandic A, Tumbas V. Assessment of polyphenolic content and in vitro antiradical characteristics of apple pomace. Food Chem. 2008;109(2):340–347. doi: 10.1016/j.foodchem.2007.12.046. [DOI] [PubMed] [Google Scholar]
- Du GR (2009) Study on the total antioxidant capacity and bioactive compounds of kiwi, persimmon and apple fruits. Northwest A&F University, pp 3–73
- Ganesan P, Kumar CS, Bhaskar N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour Technol. 2008;99(8):2717–2723. doi: 10.1016/j.biortech.2007.07.005. [DOI] [PubMed] [Google Scholar]
- González-Laredo RF, Reyes-Navarrete MG, Lerma AMPY, Rosales-Castro M, Morales-Castro J, Gallegos-Infante JA, Rocha-Guzmán NE. Antioxidant evaluation and chemoprotection of phenolic extracts from apple seeds. Grasas Aceites. 2007;58(1):5–9. doi: 10.3989/gya.2007.v58.i1.1. [DOI] [Google Scholar]
- Guo J, Yue T, Yuan Y, Wang Y. Chemometric classification of apple juices according to variety and geographical origin based on polyphenolic profiles. J Agric Food Chem. 2013;61(28):6949–6963. doi: 10.1021/jf4011774. [DOI] [PubMed] [Google Scholar]
- Heinmaa L, Moor U, Põldma P, Raudsepp P, Kidmose U, Scalzo RL. Content of health-beneficial compounds and sensory properties of organic apple juice as affected by processing technology. LWT Food Sci Technol. 2016;85:372–379. doi: 10.1016/j.lwt.2016.11.044. [DOI] [Google Scholar]
- Herrera Alvarez LV, Zielinski AAF, Alberti A, Nogueira A. Monitoring of the phenolic compounds and in vitro antioxidant activity of apple beverages according to geographical origin and their type: a chemometric study. LWT Food Sci Technol. 2017;84:385–393. doi: 10.1016/j.lwt.2017.05.078. [DOI] [Google Scholar]
- Iora SRF, Maciel GM, Zielinski AAF, da Silva MV, Pontes PVDA, Haminiuk CWI, Granato D. Evaluation of the bioactive compounds and the antioxidant capacity of grape pomace. Int J Food Sci Technol. 2015;50(1):62–69. doi: 10.1111/ijfs.12583. [DOI] [Google Scholar]
- Karaman Ş, Tütem E, Sözgen Başkan K, Apak R. Comparison of total antioxidant capacity and phenolic composition of some apple juices with combined HPLC–CUPRAC assay. Food Chem. 2010;120(4):1201–1209. doi: 10.1016/j.foodchem.2009.11.065. [DOI] [Google Scholar]
- Kim D-O, Chun OK, Kim YJ, Moon H-Y, Lee CY. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J Agric Food Chem. 2003;51(22):6509–6515. doi: 10.1021/jf0343074. [DOI] [PubMed] [Google Scholar]
- Laaksonen O, Kuldjarv R, Paalme T, Virkki M, Yang B. Impact of apple cultivar, ripening stage, fermentation type and yeast strain on phenolic composition of apple ciders. Food Chem. 2017;233:29–37. doi: 10.1016/j.foodchem.2017.04.067. [DOI] [PubMed] [Google Scholar]
- Le Deun E, Van der Werf R, Le Bail G, Le Quere JM, Guyot S. HPLC–DAD–MS profiling of polyphenols responsible for the yellow-orange color in apple juices of different French cider apple varieties. J Agric Food Chem. 2015;63(35):7675–7684. doi: 10.1021/acs.jafc.5b00988. [DOI] [PubMed] [Google Scholar]
- Leyva-Corral J, Quintero-Ramos A, Camacho-Dávila A, Zazueta-Morales JDJ, Aguilar-Palazuelos E, Ruiz-Gutiérrez MG, Ruiz-Anchondo TDJ. Polyphenolic compound stability and antioxidant capacity of apple pomace in an extruded cereal. LWT Food Sci Technol. 2016;65:228–236. doi: 10.1016/j.lwt.2015.07.073. [DOI] [Google Scholar]
- Li YG, Tanner G, Larkin P. The DMACA–HCl protocol and the threshold proanthocyanidin content for bloat safety in forage legumes. J Sci Food Agric. 1996;70(1):89–101. doi: 10.1002/(SICI)1097-0010(199601)70:1<89::AID-JSFA470>3.0.CO;2-N. [DOI] [Google Scholar]
- Li H, Wang X, Li Y, Li P, Wang H. Polyphenolic compounds and antioxidant properties of selected China wines. Food Chem. 2009;112(2):454–460. doi: 10.1016/j.foodchem.2008.05.111. [DOI] [Google Scholar]
- Maragò E, Michelozzi M, Calamai L, Camangi F, Sebastiani L. Antioxidant properties, sensory characteristics and volatile compounds profile of apple juices from ancient Tuscany (Italy) apple varieties. Eur J Hortic Sci. 2016;81(5):255–263. doi: 10.17660/eJHS.2016/81.5.4. [DOI] [Google Scholar]
- Markowski J, Baron A, Le Quéré J-M, Płocharski W. Composition of clear and cloudy juices from French and Polish apples in relation to processing technology. LWT Food Sci Technol. 2015;62(1):813–820. doi: 10.1016/j.lwt.2014.11.048. [DOI] [Google Scholar]
- Markowski J, Celejewska K, Rosłonek A, Kosmala M. Impact of different thermal preservation technologies on the quality of apple-based smoothies. LWT Food Sci Technol. 2017;85:470–473. doi: 10.1016/j.lwt.2017.01.004. [DOI] [Google Scholar]
- Massias A, Boisard S, Baccaunaud M, Leal Calderon F, Subra-Paternault P. Recovery of phenolics from apple peels using CO2+ ethanol extraction: kinetics and antioxidant activity of extracts. J Supercrit Fluid. 2015;98:172–182. doi: 10.1016/j.supflu.2014.12.007. [DOI] [Google Scholar]
- Pires T, Dias MI, Barros L, Alves MJ, Oliveira M, Santos-Buelga C, Ferreira I. Antioxidant and antimicrobial properties of dried Portuguese apple variety (Malus domestica Borkh. cv Bravo de Esmolfe) Food Chem. 2018;240:701–706. doi: 10.1016/j.foodchem.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53(10):4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Raudone L, Raudonis R, Liaudanskas M, Viskelis J, Pukalskas A, Janulis V. Phenolic profiles and contribution of individual compounds to antioxidant activity of apple powders. J Food Sci. 2016;81(5):C1055–C1061. doi: 10.1111/1750-3841.13277. [DOI] [PubMed] [Google Scholar]
- Sánchez-Rabaneda F, Jáuregui O, Lamuela-Raventós RM, Viladomat F, Bastida J, Codina C. Qualitative analysis of phenolic compounds in apple pomace using liquid chromatography coupled to mass spectrometry in tandem mode. Rapid Commun Mass Spectrom. 2004;18(5):553–563. doi: 10.1002/rcm.1370. [DOI] [PubMed] [Google Scholar]
- Schieber A, Hilt P, Streker P, Endreß H-U, Rentschler C, Carle R. A new process for the combined recovery of pectin and phenolic compounds from apple pomace. Innov Food Sci Emerg. 2003;4(1):99–107. doi: 10.1016/S1466-8564(02)00087-5. [DOI] [Google Scholar]
- Silva LCA, Almeida PS, Rodrigues S, Fernandes FAN. Inactivation of polyphenoloxidase and peroxidase in apple cubes and in apple juice subjected to high intensity power ultrasound processing. J Food Process Preserv. 2015;39(6):2081–2087. doi: 10.1111/jfpp.12451. [DOI] [Google Scholar]
- Singh JP, Kaur A, Shevkani K, Singh N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J Food Sci Technol. 2016;53(11):4056–4066. doi: 10.1007/s13197-016-2412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudha ML, Dharmesh SM, Pynam H, Bhimangouder SV, Eipson SW, Somasundaram R, Nanjarajurs SM. Antioxidant and cyto/DNA protective properties of apple pomace enriched bakery products. J Food Sci Technol. 2016;53(4):1909–1918. doi: 10.1007/s13197-015-2151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Gou X, Niu P, Sun L, Guo Y. Multivariate data analysis of the physicochemical and phenolic properties of not from concentrate apple juices to explore the alternative cultivars in juice production. Food Anal Methods. 2018;11(6):1735–1747. doi: 10.1007/s12161-018-1169-2. [DOI] [Google Scholar]
- Venkatachalam K, Techakanon C, Thitithanakul S. Impact of the ripening stage of wax apples on chemical profiles of juice and cider. ACS Omega. 2018;3(6):6710–6718. doi: 10.1021/acsomega.8b00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdu CF, Gatto J, Freuze I, Richomme P, Laurens F, Guilet D. Comparison of two methods, UHPLC–UV and UHPLC–MS/MS, for the quantification of polyphenols in cider apple juices. Molecules. 2013;18(9):10213–10227. doi: 10.3390/molecules180910213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Zhang Y, Cao L, Lu J. Phenolic compounds and antioxidant properties of different grape cultivars grown in China. Food Chem. 2010;119(4):1557–1565. doi: 10.1016/j.foodchem.2009.09.042. [DOI] [Google Scholar]
- Yanagida A, Kanda T, Tanabe M, Matsudaira F, Oliveira Cordeiro JG. Inhibitory effects of apple polyphenols and related compounds on cariogenic factors of mutans streptococci. J Agric Food Chem. 2000;48(11):5666. doi: 10.1021/jf000363i. [DOI] [PubMed] [Google Scholar]
- Ye M, Yue T, Yuan Y. Evolution of polyphenols and organic acids during the fermentation of apple cider. J Sci Food Agric. 2014;94(14):2951–2957. doi: 10.1002/jsfa.6639. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wei X, Miao Z, Hassan H, Song Y, Fan M. Screening for antioxidant and antibacterial activities of phenolics from Golden Delicious apple pomace. Chem Cent J. 2016;10:47. doi: 10.1186/s13065-016-0195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski AAF, Braga CM, Demiate IM, Beltrame FL, Nogueira A, Wosiacki G. Development and optimization of a HPLC-RI method for the determination of major sugars in apple juice and evaluation of the effect of the ripening stage. Food Sci Technol. 2014;34(1):38–43. doi: 10.1590/S0101-20612014005000003. [DOI] [Google Scholar]