Abstract
Chronic sympathetic nervous system overactivity is a hallmark of aging and obesity and contributes to the development of cardiovascular diseases including hypertension and heart failure. The cause of this chronic sympathoexcitation in aging and obesity is multifactorial and centrally mediated. In this mini-review, we have provided an overview of the key and emerging central mechanisms contributing to the pathogenesis of sympathoexcitation in obesity and healthy aging, specifically focusing on hypertension. A clear understanding of these mechanisms will pave way for targeting the sympathetic nervous system for the treatment of cardiovascular diseases in obesity and aging.
Keywords: Obesity, Aging, Hypertension, Sympathetic nerve activity, Inflammation, Leptin, Renin-angiotensin system, Oxidative stress, Cellular senescence
Introduction
It is now widely accepted that the risk of obesity markedly increases with aging. Currently, around 35% of adults aged 65 and older are obese with a body mass index of 30.0 or higher and it is projected to worsen where half of the elderly population will become obese by 2030 in the USA (Wang et al. 2007). On the other hand, obesity is associated with accelerated aging evident from increased susceptibility for age-related diseases and mortality in obese individuals (Perez et al. 2016). Obesity and aging share increased risk for cardiovascular diseases like hypertension, myocardial infarction, stroke, and heart failure. A systemic, low-grade pro-inflammatory state called “inflammaging” is believed to underlie many of the pathologies associated with aging and obesity. In addition, autonomic dysfunction, especially sympathetic nerve overactivity is increasingly recognized as a hallmark feature linking aging and obesity with increased cardiovascular risk (Fisher et al. 2009; Malpas 2010; Zucker et al. 2012).
Tonic sympathetic nerve discharge from the central nervous system plays a major role in the maintenance of resting vasomotor tone. In addition to blood pressure homeostasis through modulation of arterial baroreflex, the sympathetic nervous system (SNS) is also involved in the regulation of other physiological processes including metabolism and renal functions (Guyenet 2006). However, chronic increases in sympathetic nerve activity (SNA) have been documented to result in hypertension (Lambert et al. 2007), diastolic dysfunction (de Souza et al. 2013), increase in ventricular and aortic wall thickness (Dinenno et al. 2000), endothelial dysfunction (Hijmering et al. 2002), renal failure (Grassi et al. 2011), and metabolic dysfunction (Moreira et al. 2015), all of which increase the risk for cardiovascular events. Over the last few decades, the neuronal mechanisms that contribute to the sympathetic overactivity were studied in great detail only with limited success in the prevention of sympathetic overactivity under pathophysiological conditions. This is mainly attributed to the differential changes in sympathetic outflow to end-organs, i.e., the SNS activity is increased to the kidney in congestive heart failure, whereas it is not altered to the gut or liver (Kaye and Esler 2005). These region-specific changes in SNA are referred to as “sympathetic signature” which is seen in a variety of conditions including, but not limited to, aging and obesity (Osborn and Kuroki 2012; Subramanian and Mueller 2016). In this review, we have discussed the remarkable similarities in aging and obesity with respect to SNS dysregulation in cardiovascular diseases with a specific focus on hypertension. We have attempted to compare and contrast the mechanisms that contribute to the activation of SNS in both obesity and aging. It is also important to note that the elderly population is more vulnerable to the deleterious effects of obesity than young individuals suggesting that aging and obesity synergistically interact to exacerbate the adverse effects on the cardiovascular system. We have provided some insights into how cellular senescence, a basic mechanism of aging, could mediate the interaction between aging and obesity and potentially be a target for managing cardiovascular risk in aging and obesity.
Central regulation of sympathetic outflow
The sympathetic outflow originating from the central nervous system (CNS) involves an integration of multiple neural and hormonal inputs within the cardiovascular regions of the hypothalamus and brainstem. In particular, the paraventricular nucleus (PVN) of the hypothalamus and rostral ventrolateral medulla (RVLM) of the brainstem contains neurons that project directly to the intermediolateral cell column (IML) of the spinal cord, which innervates sympathetic preganglionic neurons and generates SNA to end organs via postganglionic neurons (Dampney 1994; Guyenet 2006). The activity of the neurons within the RVLM is modulated by excitatory or inhibitory neurotransmitters that are intrinsically produced or from inputs from other higher centers such as the PVN (Dampney 1994). In addition, the circumventricular organs like the subfornical organ (SFO) and area postrema (AP) that lack blood-brain barrier respond to circulatory molecules by transducing these signals to other regions of the brain and play an indirect role in modulating sympathetic outflow. Further, the neuronal population within the RVLM is not homogenous in nature with respect to generation of SNA to peripheral tissues. The topographical organization and neuroplasticity of sub-population of neurons within the RVLM have been attributed to differential responses in SNA to end organs contributing to the sympathetic signature in disease conditions (Ootsuka and Terui 1997, Subramanian and Mueller 2016). Several key and emerging central mechanisms by which overactivity of the SNS could contribute to end-organ damage in obesity and aging are discussed in the following sections.
Obesity and aging share outcomes on blood pressure and sympathetic nervous system activity
The risk for cardiovascular diseases dramatically increases in overweight or obese individuals compared to individuals with normal BMI. A recent longitudinal study carried out in almost 60,000 men and women demonstrate BMI as the strong predictor of future changes in blood pressure. A 5 kg/m2 higher BMI at age 30 was associated with a 2.12 mmHg increase in mean arterial pressure over 10 years (Van Hemelrijck et al. 2018), thus confirming several other previous reports on obesity-induced risk for hypertension (Rabkin et al. 1997; Huang et al. 1998; Forman et al. 2009; Juonala et al. 2011). This obesity-related increase in blood pressure roughly translates to a 12% increased risk for coronary heart disease (CHD) and 24% increased risk for stroke. A similar outcome is also observed in the aging population. The National Health and Nutrition Examination Survey (NHANES III) and longitudinal aging studies like Framingham Heart study (Franklin 1999) and the Baltimore Longitudinal study (AlGhatrif et al. 2013) have shown a continuous increase in systolic blood pressure and a gradual decrease in diastolic blood pressure with age (systolic hypertension). In the aging population of 65 years or older, cardiovascular diseases are the number one cause of all reported deaths and hypertension contributes at least in part to this phenomenon (Writing Group et al. 2016). Although an increase in BMI is positively associated with the increase in blood pressure in obesity and aging, it is important to note that not everyone with a higher BMI develops hypertension by clinical standards. Genetic factors and fat distribution pattern could explain the observed interindividual variability in blood pressure responses to weight gain. Studies have shown that visceral/retroperitoneal fat but not subcutaneous fat accumulation results in an unfavorable metabolic phenotype leading to hypertension (Koh et al. 2011; Elffers et al. 2017). On the other hand, individuals with normal BMI who exhibit ectopic fat deposition in tissues like the liver, heart, and muscle may also develop hypertension as part of the metabolic syndrome (Levelt et al. 2016). Since a traditional BMI measurement does not account for fat distribution patterns and ectopic fat deposition, BMI alone may not be a useful clinical tool for assessing the risk of developing hypertension.
Although numerous factors contribute to the pathogenesis of hypertension, sympathetic nerve overactivity appears to be a common central denominator in aging and obesity. Multiple lines of evidence, including direct nerve recordings of muscle sympathetic nerve activity (MSNA) in postganglionic nerve fibers, urinary noradrenaline excretion, norepinephrine (NE) spillover, and heart rate variability (HRV) suggest an overactive SNA in aging and obesity (Pfeifer et al. 1983; Smith and Minson 2012). Some studies have also reported a decreased sensitivity of arterial baroreceptor reflex in aged (Ebert et al. 1992) and obese (Indumathy et al. 2015) humans, which augments the risk for cardiovascular diseases. Also, the SNA responses to different regions or vascular beds are heterogeneous in nature. In obesity, sympathetic outflow is increased to the muscle and kidney (Fig. 1), while SNA to the heart and mesentery, i.e., splanchnic circulation remained unaltered (Vaz et al. 1997; Grassi et al. 2000; Esler et al. 2006; Lambert et al. 2007). Regional differences in SNA was also observed in healthy aging where MSNA was increased similar to obesity, but the changes in splanchnic, renal, and cardiac SNA followed a dissimilar trend compared to obesity (Jones et al. 1997; Kaye and Esler 2005). However, it should be noted that this sympathetic signature is specific to obesity-related hypertension and aging and it varies with other models of hypertension (Ang II-salt) and heart failure (Osborn and Kuroki 2012). Most of the SNA studies in animals were restricted to direct nerve recordings in acute, anesthetized preparations. However, emerging technology has made it possible to perform chronic SNA recordings in conscious animals which will undoubtedly advance our understanding on the role of region-specific SNA in disease conditions (Stocker and Muntzel 2013). The translatability of selective ablation of sympathetic nerves to a specific region or vascular bed is evident from the ongoing clinical trials on renal denervation in humans for essential hypertension, although the results are conflicting (Bhatt et al. 2014; de Beus et al. 2017; Warchol-Celinska et al. 2018). Nonetheless, such studies have proven that targeted sympathetic nerve ablation is clinically feasible and provide hope for extending such techniques to other disease conditions like aging and obesity-related hypertension.
Fig. 1.
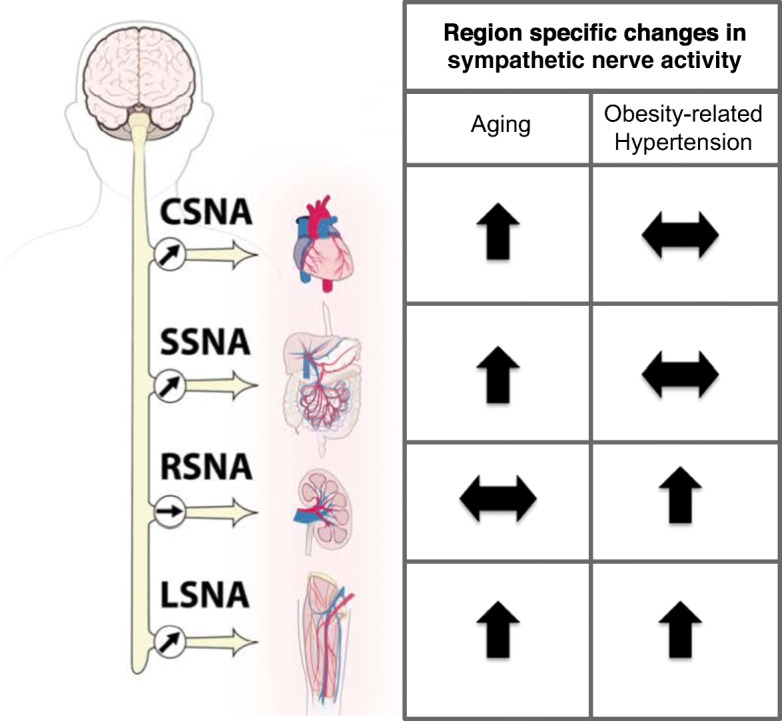
A comparison of regional patterns of sympathetic nerve activity in healthy aging and obesity-related hypertension in humans. The sympathetic outflow to the skeletal muscle vasculature (LSNA) is activated in both aging and obesity-related hypertension. Cardiac (CSNA) and splanchnic SNA (SSNA) are augmented in aging, whereas they are unaltered in obesity-related hypertension. On the other hand, renal SNA (RSNA) does not change with age, but it is elevated in obesity-related hypertension.
A strong association exists between SNA and feeding which could explain the underlying basis for SNS overdrive with fat accumulation in obesity and aging. SNA is inversely related to feeding, wherein SNA is activated with overfeeding or spontaneous food intake (Fagius and Berne 1994; Welle 1995) and decreased with fasting (Griggio et al. 1992; Young and Landsberg 1997). This suggests that SNA activation might be a homeostatic mechanism to prevent excess fat storage by stimulating lipolysis in adipose tissue in obesity. Elevations in circulating levels of leptin, insulin, and free fatty acids act as adiposity signals which cross BBB and through receptor-mediated mechanisms drive SNS outflow to metabolic tissues to stimulate β-adrenergic thermogenesis. Paradoxically, this protective mechanism of tonic sympathetic activation to stimulate thermogenesis pose adverse effects on cardiovascular system including, but not limited to, increases in arterial pressure, reduced peripheral blood flow, and hypertrophy of larger arteries. In addition, although physiologically appropriate at the initial stages, chronic SNS activation causes desensitization of β-adrenergic signaling in the adipose tissue which counterproductively affects both basal and acute energy intake-induced thermogenesis in the adipose tissue. This results in a vicious cycle of increased susceptibility for accruing fat mass amidst heightened SNS activity (Seals and Bell 2004; Perez et al. 2016).
Emerging evidence shows that aging exacerbates the deleterious effects of obesity on the central nervous system and in the cerebral vasculature by promoting neuroinflammation (Bailey-Downs et al. 2013; Tucsek et al. 2014a, b; Tarantini et al. 2018; Valcarcel-Ares et al. 2018). Tuscek et al. showed that obesity in aging promotes blood-brain barrier disruption, microglia activation and impairs long-term potentiation responses in the hippocampus, all of which contributes to cognitive decline in mice. In another study, the same group has demonstrated that aging exacerbates obesity-induced oxidative stress, microvascular rarefaction, and impairment in cerebral blood flow responses in the hippocampus. Based on these studies, we can expect that lean and obese elderly will exhibit dissimilar sympathetic phenotypes in lean versus obese patients with essential hypertension as reported previously (Esler et al. 2018). Understanding the mechanism by which obesity enhances the cardiovascular risk in the elderly is important so that we could reverse the deleterious effects of obesity as shown by others (Csipo et al. 2018). Nevertheless, physiological healthy aging and pathological obesity share remarkable similarities in terms of SNS activity and its effect on cardiovascular outcomes. Based on these evidences, it is plausible to consider obesity as an accelerated aging phenomenon.
Common mediators of sympathoexcitation
Leptin
Leptin is a circulating adipokine secreted in direct proportion to adipose tissue mass. After its release from the adipocytes, leptin enters the circulation and crosses the BBB to bind to its receptors in the hypothalamic and brainstem regions that are important for the control of metabolic and cardiovascular functions. The primary role of leptin is to reduce appetite and increase energy expenditure by the activation of sympathetic outflow to several vascular beds (Mark 2013; Hall et al. 2015). However, in obesity, the metabolic action of leptin, i.e., to suppress appetite is impaired whereas its cardiovascular actions, i.e., to stimulate SNA remain unaltered (Kuo et al. 2001; Correia et al. 2002; Engeli and Sharma 2002). This mechanism is referred to as “selective leptin resistance.” In this context, leptin has been suggested to play an important role in causing hypertension by overactivation of the SNS in obese animals.
The central actions of leptin have been implicated in increased sympathetic outflow and development of obesity-related hypertension in animals (Marsh et al. 2003; Rahmouni and Morgan 2007; Mark et al. 2009; Barnes and McDougal 2014). Intracerebroventricular (ICV) administration of leptin has been shown to increase mean arterial pressure and renal SNA in rabbits, with even higher increases under obese conditions (Prior et al. 2010). Also, overexpression of leptin results in hypertension in mice without affecting adipose tissue mass, indicating that leptin can mediate hypertension independent of obesity (Aizawa-Abe et al. 2000). Leptin receptors are distributed in various nuclei, including the arcuate nucleus, PVN, dorsomedial hypothalamus, ventromedial, and lateral hypothalamus. Among these nuclei, leptin receptors in the arcuate nucleus, specifically in the proopiomelanocortin (POMC)-expressing neurons, appear to mediate the sympathoexcitatory actions of leptin as conditional knockdown of leptin receptors in this subpopulation of neurons confers protection from leptin-mediated hypertension (do Carmo et al. 2011). However, the findings in animals were not translated well in humans. Although humans respond to leptin administration with enhanced MSNA, this occurred without changes in blood pressure and heart rate (Shek et al. 1998; Machleidt et al. 2013). Conclusive evidence that leptin increases SNA and cause hypertension in obese humans is still lacking. An in-depth analysis on the cellular and molecular mechanisms by which leptin causes hypertension in obesity and how these results should be interpreted in humans are discussed elsewhere (Kotsis et al. 2010; Head et al. 2014; do Carmo et al. 2016).
Leptin resistance documented with obesity-related hypertension is also observed in aging. Reduced leptin receptor expression and altered signal transduction pathways have been suggested to mediate leptin resistance in the elderly (Balasko et al. 2014). Weight gain or increase in fat mass is associated with leptin resistance in aged animals. The question remains whether aging alone leads to selective leptin resistance independent of obesity or fat distribution pattern. To answer this, Barzilai and colleagues compared leptin sensitivity in lifelong calorie-restricted aged mice (lean and metabolically similar to young mice) to ad libitum–fed aged mice. Although aged mice had the fat mass comparable to young mice, the ability of leptin to regulate appetite and peripheral metabolism was still impaired suggesting aging independently reduces leptin sensitivity (Gabriely et al. 2002). However, the role of leptin resistance in age-related sympathoexcitation has not been investigated in detail.
Renin-angiotensin system
Obesity-related hypertension in animals is associated with increased activity of the renin-angiotensin system (RAS) in the central sympathoregulatory nuclei, including PVN, OVLT, and RVLM (de Kloet et al. 2014). The major effector protein of angiotensinergic signaling, angiotensin II (Ang-II) binds to its receptors angiotensin II receptor type 1 (AT1R) in the brain and stimulates sympathetic outflow. Deletion of AT1a receptors in the PVN of high-fat-fed mice abrogated the development of hypertension despite obesity in these animals (de Kloet et al. 2013). In obesity-prone rats fed with a high-fat diet, the AT1R blockade in the RVLM inhibited SNA through antioxidant mechanisms (Konno et al. 2012). Further, central Ang-II appears to be a critical downstream mediator of leptin-induced neuroinflammation in obesity-related hypertension (discussed in the next section). Even though Ang-II has been shown to be involved in mediating obesity-related hypertension through a central pathway, the exact mechanisms are largely unknown and need to be investigated.
Brain RAS has also been implicated in the pathogenesis of age-related increase in arterial pressure. In aging, an autonomic imbalance characterized by reduced parasympathetic activity is created as a result of an age-related decline in Ang (1–7) generated by angiotensin-converting enzyme 2 (ACE2) by metabolizing Ang-II (Diz et al. 2008). In contrast, there is overactivity of the SNS influenced by circulating or brain Ang-II along with leptin. Although age-related changes in the expression of Ang-II-AT1R axis has been implicated in arterial aging (Yoon et al. 2016), there is a paucity of studies investigating the effects of Ang-II or RAS system in the RVLM or PVN with aging.
Neuroinflammation/oxidative stress
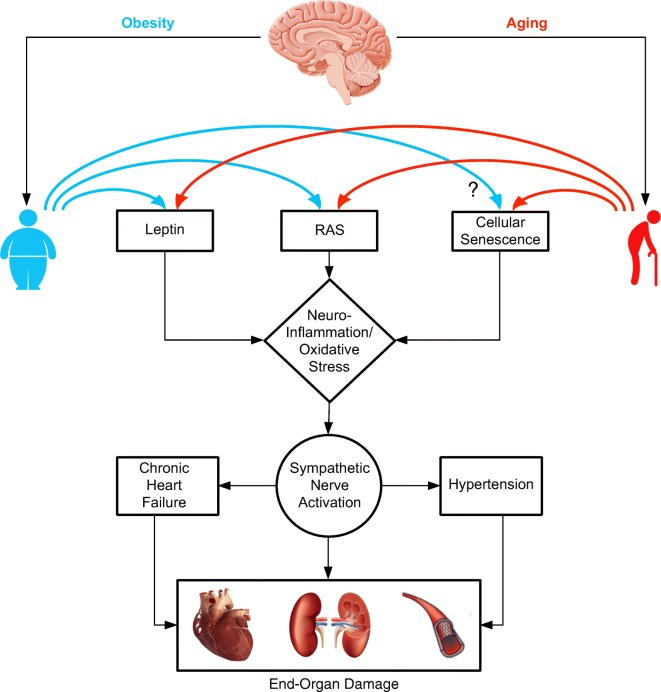
Both aging and obesity are associated with chronic low-grade systemic inflammation characterized by higher circulating pro-inflammatory cytokines secreted from adipose tissue and increased infiltration of macrophages in peripheral tissues (Perez et al. 2016). These peripheral pro-inflammatory signals are transmitted to the brain via the circumventricular organs of the hypothalamus and brainstem and in regions of BBB leakage where it activates microglia (Thaler et al. 2012) and astrocytes (Hsuchou et al. 2009; Buckman et al. 2013) promoting neuroinflammation. Cai and colleagues have demonstrated that blockade of the nuclear factor κB (NF-κB) pathway, a key transcription factor involved in initiating pro-inflammatory cytokine expression, in the mediobasal hypothalamus lowers arterial blood pressure in obese mice (Purkayastha et al. 2011). In the same study, the authors show that acute activation of NF-κB and its upstream activator IκB kinase-β in the mediobasal hypothalamus increases blood pressure by increasing sympathetic outflow suggesting neuroinflammation independent of obesity can also promote hypertension (Purkayastha et al. 2011). Central RAS has been implicated in mediating obesity and leptin-induced neuroinflammation in the regions regulating SNA. Diet-induced obesity increases the number of Iba1+ microglia and expression of AT1a receptors in the SFO and selective deletion of AT1a receptors in the PVN at least partially reversed glial fibrillary acidic protein (GFAP) immunoreactivity in the PVN of high-fat-fed mice (de Kloet et al. 2014). Further, obesity-related RAS activation and neuroinflammation have been demonstrated to increase the sensitivity to subsequent Ang-II-elicited hypertensive responses (Xue et al. 2016). Gene expression changes associated with neuroinflammation in the RVLM have also been documented in aged rats (Balivada et al. 2017); however, their mechanistic role in age-related SNS dysregulation has not been investigated (Fig. 2).
Fig. 2.
Possible central mechanisms linking aging and obesity to end-organ damage. RAS, renin angiotensin system
In addition to neuroinflammation, oxidative stress characterized by increased production of reactive oxygen species also contributes to the pathogenesis of neurogenic hypertension. It is believed that inflammation and oxidative stress go hand in hand where they mutually amplify each other in a disease setting. HF feeding has been associated with increased reactive oxygen species (ROS) production in the hypothalamus. On the other hand, ICV infusion of tempol or NADPH oxidase inhibitor decreased renal SNA and arterial pressure much greater in obese animals compared to lean animals (Nagae et al. 2009). The same mechanism holds true for hindbrain as well, where microinjection of tempol in the RVLM produced a greater depressor effect in obese prone versus obese resistant animals (Kishi et al. 2011). A recent study by Zucker and colleagues showed that selective deletion of nuclear factor erythroid 2-related factor (Nrf2), a master transcriptional regulator of anti-oxidant genes, in the RVLM increases mean arterial pressure, urinary norepinephrine and baseline renal sympathetic nerve activity in normal mice suggesting that Nrf2 is a critical modulator of redox status in the sympathetic neurons in the RVLM and contributes to sympathoregulation. (Gao et al. 2017). Adaptive activation of the Nrf2-ARE signaling pathway has also been demonstrated to confer protection against obesity-induced oxidative stress and inflammation. In addition to obesity, decreased Nrf2 signaling and ARE transcriptional activity has also been implicated in the pathogenesis of several age-related diseases, suggesting that Nrf2 signaling plays a pivotal role in cellular resilience during stress conditions in aging and obesity. In fact, a recent study by Tarantini et al., demonstrated that Nrf2 deficiency mimicks the aging phenotype by exacerbating obesity-induced cerebromicrovascular dysfunction, impairment in synaptic function, and neuroinflammation (Ungvari et al. 2011; Tarantini et al. 2018). These studies suggest that Nrf2 dysfunction in key brainstem regions may be a potential mechanism for age and obesity-related neuroinflammation leading to SNS overactivity and should be addressed in future studies.
Cellular senescence—a unifying theme linking obesity and aging
Cellular senescence is an anticancer mechanism that results in irreversible growth arrest in proliferating cells in response to several stimuli including, but not limited to, DNA damage, oxidative stress, mitochondrial dysfunction, oncogene activation, and telomere erosion (van Deursen 2014; Chinta et al. 2015; Palmer et al. 2015). In addition to growth arrest, senescent cells acquire senescence-associated secretory phenotype (SASP) and secrete pro-inflammatory cytokines, chemokines, growth factors, and proteases. This senescence associated secretome could be a potential contributor to neuroinflammation in aging and obesity. Through SASP, senescent cells can also affect the structure and function of neighboring cells and alter the tissue microenvironment in a paracrine manner. In addition to SASP, senescent cells are also characterized by an increase in cell and nuclear size, expression of cyclin-dependent kinase inhibitors (p16Ink4a, p21Cip1, p15, p19), senescence-associated β-galactosidase activity (SA-β-gal), reduced nuclear levels of laminB1, which is a nuclear envelope protein, reduced levels of high-mobility group box 2 (HMGB2), and senescence-associated heterochromatin foci (SAHF) (Rodier and Campisi 2011).
Senescent cells accumulate with age in all kinds of tissues, including the brain, and have been implicated in the pathogenesis of age-related diseases like cancer, osteoarthritis, and neurodegenerative diseases (Tan et al. 2014). This association is supported by murine studies showing delayed onset of age-related diseases when senescent cells were eliminated (Baker et al. 2011). In addition to diseases related to aging, cellular senescence is gaining significant attention in recent years as a target for aging-independent conditions like obesity and hypertension (Westhoff et al. 2008; Schafer et al. 2016). In fact, several stressors known to induce senescence in aging have also been implicated in obesity-induced senescence. A summary of the signaling pathways involved in senescence induction during aging and obesity is provided in Fig. 3. Recent studies demonstrate the accumulation of senescent immune cells like macrophages (Schafer et al. 2016) and T-cells (Shirakawa et al. 2016) in the visceral adipose tissue of high-fat diet–fed mice contributing to inflammation and insulin resistance in obesity. Also, in DOCA salt-treated rats, hypertension was associated with increased p16Ink4a expression in the kidneys and hearts (Westhoff et al. 2008). Similar results were also observed in humans, where kidney biopsies from patients with hypertensive nephrosclerosis showed increased p16 expression, suggesting the possibility of cellular senescence as a target for the treatment of hypertension (Westhoff et al. 2008).
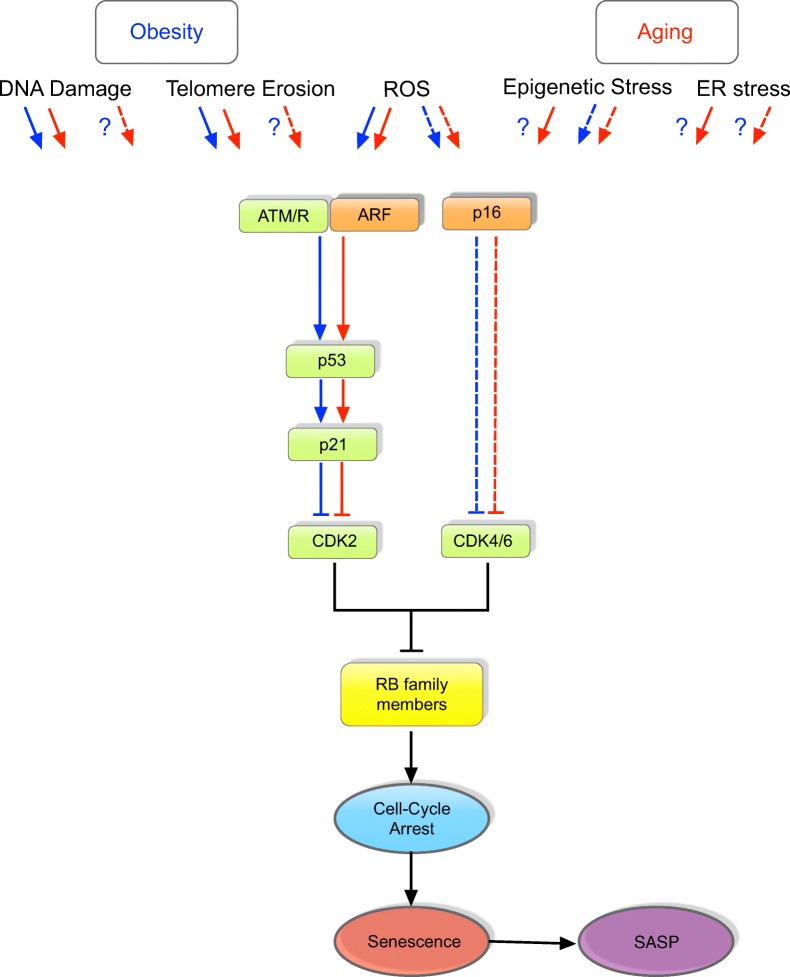
Fig. 3.
Signaling pathways regulating senescence in aging and obesity. Several stimuli can trigger the initiation of senescence program in aging and obesity. Based on available literature, pathways known to induce senescence in obesity and aging are denoted by blue and red arrows, respectively. Pathways that are unknown or unexplored are indicated using a question mark (e.g., activation of p16 in obesity-induced DNA damage has not been studied yet). Signaling pathways most commonly activated by known stressors include p53/p21 (indicated by straight lines) or p16 (indicated by dotted lines) or both. Stressors like reactive oxygen species (ROS) and telomere shortening are known to activate the DNA damage response (DDR) signaling, in which ATM/R and ARF stabilizes p53 and activates cyclin-dependent kinase (Cdk) inhibitor, p21. Activated p21 inhibits Cdk2 resulting in cell cycle arrest. Activation of p16 can also inhibit cell proliferation through the inhibition of Cdk4/6 complexes. Both p16 and p53/p21 pathways converge to prevent inactivation of retinoblastoma (Rb) family proteins to result in permanent growth arrest. Irrespective of the stressor, senescent cells acquire senescence-associated secretory phenotype (SASP) to secrete pro-inflammatory cytokines, chemokines, and proteases. It is important to note that much of what we know about the signaling pathway-inducing senescence are based on studies in peripheral tissues while the central pathways leading to senescence in the brain are largely unknown. We believe that senescent cells and SASP in the brain could potentially contribute to neuroinflammation and in turn, increases in SNS activity observed in obesity and aging
While several studies link cellular senescence in peripheral tissues with age-related diseases and obesity, studies on the existence of senescent cells in the brain and its involvement in neurodegenerative diseases are just emerging. Neurons are post-mitotic and do not undergo senescence, but replication competent glial cells are prone to senesce with age. Glial cells play a supporting role in neuronal function through regulation of neurotransmission (glutamate-glutamine cycle), myelination, synaptogenesis, secretion of neurotrophic factors that promote neuronal survival, immune modulation, and maintenance of BBB. Hence, senescence in glial cells, in addition to affecting its own structural and functional characteristics, may also impede neuronal function. In fact, senescent astrocytes have been implicated in the pathogenesis of Parkinson’s disease (Chinta et al. 2018) and Alzheimer’s disease (Bhat et al. 2012). Unpublished data from our lab provides evidence for accumulation of senescent cells in the aging brainstem which could potentially contribute to neuroinflammation and age-related sympathoexcitation. However, it is still not known whether there is induction of senescence in the brain during obesity, and if so, what kind of cells undergo senescence and what role does it play in obesity-related sympathetic overactivity.
Conclusions
Chronic elevations in SNA appear to be a common mechanism in obesity and aging and contribute to the development of a plethora of cardiovascular diseases including hypertension. However, it should be noted that the sympathetic outflow is not homogenous across target tissues and that the sympathetic signature differs with obesity and aging. These differences may have clinical significance as selective ablation of sympathetic nerves is gaining significant momentum in recent years. It is also important to distinguish the effects of aging per se versus obesity in aging on the observed perturbations in SNA. Although not discussed, consideration should also be given to additional factors influenced by obesity like insulin, adiponectin, non-esterified fatty acids (NEFA), and baroreflex sensitivity in mediating sympathoexcitation. Lastly, it is important to address glial cell–specific mechanisms and understand how changes in glia-neuron crosstalk contribute to sympathetic nerve overactivity in aging and obesity. In this context, cellular senescence in glial cells is emerging as an important area for investigation in modulating SNA, not only in aging and obesity but also in other models of neurogenic hypertension and heart failure.
Funding information
This work was supported by RAC fund to MS from the Center for Veterinary Health Sciences, Oklahoma State University.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000;105(9):1243–1252. doi: 10.1172/JCI8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62(5):934–941. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, Tucsek Z, Toth P, Sosnowska D, Gautam T, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci. 2013;68(7):780–792. doi: 10.1093/gerona/gls238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasko M, Soos S, Szekely M, Petervari E. Leptin and aging: review and questions with particular emphasis on its role in the central regulation of energy balance. J Chem Neuroanat. 2014;61-62:248–255. doi: 10.1016/j.jchemneu.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Balivada S, Ganta CK, Zhang Y, Pawar HN, Ortiz RJ, Becker KG, Khan AM, Kenney MJ. Microarray analysis of aging-associated immune system alterations in the rostral ventrolateral medulla of F344 rats. Physiol Genomics. 2017;49(8):400–415. doi: 10.1152/physiolgenomics.00131.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes MJ, McDougal DH. Leptin into the rostral ventral lateral medulla (RVLM) augments renal sympathetic nerve activity and blood pressure. Front Neurosci. 2014;8:232. doi: 10.3389/fnins.2014.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, Johnson FB, Trojanowski JQ, Sell C, Torres C. Astrocyte senescence as a component of Alzheimer’s disease. PLoS One. 2012;7(9):e45069. doi: 10.1371/journal.pone.0045069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL, Investigators SH. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- Buckman LB, Thompson MM, Moreno HN, Ellacott KL. Regional astrogliosis in the mouse hypothalamus in response to obesity. J Comp Neurol. 2013;521(6):1322–1333. doi: 10.1002/cne.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Woods G, Rane A, Demaria M, Campisi J, Andersen JK. Cellular senescence and the aging brain. Exp Gerontol. 2015;68:3–7. doi: 10.1016/j.exger.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Woods G, Demaria M, Rane A, Zou Y, McQuade A, Rajagopalan S, Limbad C, Madden DT, Campisi J, Andersen JK. Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson’s disease. Cell Rep. 2018;22(4):930–940. doi: 10.1016/j.celrep.2017.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia ML, Haynes WG, Rahmouni K, Morgan DA, Sivitz WI, Mark AL. The concept of selective leptin resistance: evidence from agouti yellow obese mice. Diabetes. 2002;51(2):439–442. doi: 10.2337/diabetes.51.2.439. [DOI] [PubMed] [Google Scholar]
- Csipo T, Fulop GA, Lipecz A, Tarantini S, Kiss T, Balasubramanian P, Csiszar A, Ungvari Z, Yabluchanskiy A (2018) Short-term weight loss reverses obesity-induced microvascular endothelial dysfunction. Geroscience [DOI] [PMC free article] [PubMed]
- Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74(2):323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- de Beus E, de Jager RL, Beeftink MM, Sanders MF, Spiering W, Vonken EJ, Voskuil M, Bots ML, Blankestijn PJ, S. s. group Salt intake and blood pressure response to percutaneous renal denervation in resistant hypertension. J Clin Hypertens (Greenwich) 2017;19(11):1125–1133. doi: 10.1111/jch.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, Seeley RJ, Herman JP, Woods SC, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J Neurosci. 2013;33(11):4825–4833. doi: 10.1523/JNEUROSCI.3806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet AD, Pioquinto DJ, Nguyen D, Wang L, Smith JA, Hiller H, Sumners C. Obesity induces neuroinflammation mediated by altered expression of the renin-angiotensin system in mouse forebrain nuclei. Physiol Behav. 2014;136:31–38. doi: 10.1016/j.physbeh.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza SB, Rocha JA, Cuoco MA, Guerra GM, Ferreira-Filho JC, Borile S, Krieger EM, Bortolotto LA, Consolim-Colombo FM. High muscle sympathetic nerve activity is associated with left ventricular dysfunction in treated hypertensive patients. Am J Hypertens. 2013;26(7):912–917. doi: 10.1093/ajh/hpt032. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR, Tanaka H. Age-associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. Am J Physiol Heart Circ Physiol. 2000;278(4):H1205–H1210. doi: 10.1152/ajpheart.2000.278.4.H1205. [DOI] [PubMed] [Google Scholar]
- Diz DI, Varagic J, Groban L. Aging and the brain renin-angiotensin system: relevance to age-related decline in cardiac function. Futur Cardiol. 2008;4(3):237–245. doi: 10.2217/14796678.4.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011;57(5):918–926. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo JM, da Silva AA, Wang Z, Fang T, Aberdein N, de Lara Rodriguez CE, Hall JE. Obesity-induced hypertension: brain signaling pathways. Curr Hypertens Rep. 2016;18(7):58. doi: 10.1007/s11906-016-0658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ. Effects of aging on baroreflex regulation of sympathetic activity in humans. Am J Phys. 1992;263(3 Pt 2):H798–H803. doi: 10.1152/ajpheart.1992.263.3.H798. [DOI] [PubMed] [Google Scholar]
- Elffers TW, de Mutsert R, Lamb HJ, de Roos A, Willems van Dijk K, Rosendaal FR, Jukema JW, Trompet S. Body fat distribution, in particular visceral fat, is associated with cardiometabolic risk factors in obese women. PLoS One. 2017;12(9):e0185403. doi: 10.1371/journal.pone.0185403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeli S, Sharma AM. Emerging concepts in the pathophysiology and treatment of obesity-associated hypertension. Curr Opin Cardiol. 2002;17(4):355–359. doi: 10.1097/00001573-200207000-00006. [DOI] [PubMed] [Google Scholar]
- Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48(5):787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- Esler M, Lambert G, Schlaich M, Dixon J, Sari CI, Lambert E. Obesity paradox in hypertension: is this because sympathetic activation in obesity-hypertension takes a benign form? Hypertension. 2018;71(1):22–33. doi: 10.1161/HYPERTENSIONAHA.117.09790. [DOI] [PubMed] [Google Scholar]
- Fagius J, Berne C. Increase in muscle nerve sympathetic activity in humans after food intake. Clin Sci (Lond) 1994;86(2):159–167. doi: 10.1042/cs0860159. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Young CN, Fadel PJ. Central sympathetic overactivity: maladies and mechanisms. Auton Neurosci. 2009;148(1–2):5–15. doi: 10.1016/j.autneu.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302(4):401–411. doi: 10.1001/jama.2009.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin SS. Ageing and hypertension: the assessment of blood pressure indices in predicting coronary heart disease. J Hypertens Suppl. 1999;17(5):S29–S36. [PubMed] [Google Scholar]
- Gabriely I, Ma XH, Yang XM, Rossetti L, Barzilai N. Leptin resistance during aging is independent of fat mass. Diabetes. 2002;51(4):1016–1021. doi: 10.2337/diabetes.51.4.1016. [DOI] [PubMed] [Google Scholar]
- Gao L, Zimmerman MC, Biswal S, Zucker IH. Selective Nrf2 gene deletion in the rostral ventrolateral medulla evokes hypertension and sympathoexcitation in mice. Hypertension. 2017;69(6):1198–1206. doi: 10.1161/HYPERTENSIONAHA.117.09123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Dell'Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension. 2000;36(4):538–542. doi: 10.1161/01.hyp.36.4.538. [DOI] [PubMed] [Google Scholar]
- Grassi G, Quarti-Trevano F, Seravalle G, Arenare F, Volpe M, Furiani S, Dell'Oro R, Mancia G. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension. 2011;57(4):846–851. doi: 10.1161/HYPERTENSIONAHA.110.164780. [DOI] [PubMed] [Google Scholar]
- Griggio MA, Richard D, Leblanc J. Effects of fasting and food restriction on sympathetic activity in brown adipose tissue in mice. J Comp Physiol B. 1992;162(7):602–606. doi: 10.1007/BF00296640. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7(5):335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991–1006. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head GA, Lim K, Barzel B, Burke SL, Davern PJ. Central nervous system dysfunction in obesity-induced hypertension. Curr Hypertens Rep. 2014;16(9):466. doi: 10.1007/s11906-014-0466-4. [DOI] [PubMed] [Google Scholar]
- Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol. 2002;39(4):683–688. doi: 10.1016/s0735-1097(01)01786-7. [DOI] [PubMed] [Google Scholar]
- Hsuchou H, He Y, Kastin AJ, Tu H, Markadakis EN, Rogers RC, Fossier PB, Pan W. Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain. 2009;132(Pt 4):889–902. doi: 10.1093/brain/awp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Willett WC, Manson JE, Rosner B, Stampfer MJ, Speizer FE, Colditz GA. Body weight, weight change, and risk for hypertension in women. Ann Intern Med. 1998;128(2):81–88. doi: 10.7326/0003-4819-128-2-199801150-00001. [DOI] [PubMed] [Google Scholar]
- Indumathy J, Pal GK, Pal P, Ananthanarayanan PH, Parija SC, Balachander J, Dutta TK. Decreased baroreflex sensitivity is linked to sympathovagal imbalance, body fat mass and altered cardiometabolic profile in pre-obesity and obesity. Metabolism. 2015;64(12):1704–1714. doi: 10.1016/j.metabol.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Jones PP, Davy KP, Alexander S, Seals DR. Age-related increase in muscle sympathetic nerve activity is associated with abdominal adiposity. Am J Phys. 1997;272(6 Pt 1):E976–E980. doi: 10.1152/ajpendo.1997.272.6.E976. [DOI] [PubMed] [Google Scholar]
- Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Sun C, Cheung M, Viikari JS, Dwyer T, Raitakari OT. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365(20):1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- Kaye D, Esler M. Sympathetic neuronal regulation of the heart in aging and heart failure. Cardiovasc Res. 2005;66(2):256–264. doi: 10.1016/j.cardiores.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Kishi T, Hirooka Y, Ogawa K, Konno S, Sunagawa K. Calorie restriction inhibits sympathetic nerve activity via anti-oxidant effect in the rostral ventrolateral medulla of obesity-induced hypertensive rats. Clin Exp Hypertens. 2011;33(4):240–245. doi: 10.3109/10641963.2011.583969. [DOI] [PubMed] [Google Scholar]
- Koh H, Hayashi T, Sato KK, Harita N, Maeda I, Nishizawa Y, Endo G, Fujimoto WY, Boyko EJ, Hikita Y. Visceral adiposity, not abdominal subcutaneous fat area, is associated with high blood pressure in Japanese men: the Ohtori study. Hypertens Res. 2011;34(5):565–572. doi: 10.1038/hr.2010.271. [DOI] [PubMed] [Google Scholar]
- Konno S, Hirooka Y, Kishi T, Sunagawa K. Sympathoinhibitory effects of telmisartan through the reduction of oxidative stress in the rostral ventrolateral medulla of obesity-induced hypertensive rats. J Hypertens. 2012;30(10):1992–1999. doi: 10.1097/HJH.0b013e328357fa98. [DOI] [PubMed] [Google Scholar]
- Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertens Res. 2010;33(5):386–393. doi: 10.1038/hr.2010.9. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Jones OB, Hall JE. Inhibition of NO synthesis enhances chronic cardiovascular and renal actions of leptin. Hypertension. 2001;37(2 Pt 2):670–676. doi: 10.1161/01.hyp.37.2.670. [DOI] [PubMed] [Google Scholar]
- Lambert E, Straznicky N, Schlaich M, Esler M, Dawood T, Hotchkin E, Lambert G. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension. 2007;50(5):862–868. doi: 10.1161/HYPERTENSIONAHA.107.094649. [DOI] [PubMed] [Google Scholar]
- Levelt E, Pavlides M, Banerjee R, Mahmod M, Kelly C, Sellwood J, Ariga R, Thomas S, Francis J, Rodgers C, Clarke W, Sabharwal N, Antoniades C, Schneider J, Robson M, Clarke K, Karamitsos T, Rider O, Neubauer S. Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J Am Coll Cardiol. 2016;68(1):53–63. doi: 10.1016/j.jacc.2016.03.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machleidt F, Simon P, Krapalis AF, Hallschmid M, Lehnert H, Sayk F. Experimental hyperleptinemia acutely increases vasoconstrictory sympathetic nerve activity in healthy humans. J Clin Endocrinol Metab. 2013;98(3):E491–E496. doi: 10.1210/jc.2012-3009. [DOI] [PubMed] [Google Scholar]
- Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90(2):513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- Mark AL. Selective leptin resistance revisited. Am J Physiol Regul Integr Comp Physiol. 2013;305(6):R566–R581. doi: 10.1152/ajpregu.00180.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension. 2009;53(2):375–380. doi: 10.1161/HYPERTENSIONAHA.108.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AJ, Fontes MA, Killinger S, Pawlak DB, Polson JW, Dampney RA. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension. 2003;42(4):488–493. doi: 10.1161/01.HYP.0000090097.22678.0A. [DOI] [PubMed] [Google Scholar]
- Moreira MC, Pinto IS, Mourão AA, Fajemiroye JO, Colombari E, Reis Â, Freiria-Oliveira AH, Ferreira-Neto ML, Pedrino GR. Does the sympathetic nervous system contribute to the pathophysiology of metabolic syndrome? Front Physiol. 2015;6:234. doi: 10.3389/fphys.2015.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation. 2009;119(7):978–986. doi: 10.1161/CIRCULATIONAHA.108.824730. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Terui N. Functionally different neurons are organized topographically in the rostral ventrolateral medulla of rabbits. J Auton Nerv Syst. 1997;67(1–2):67–78. doi: 10.1016/s0165-1838(97)00094-5. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Kuroki MT. Sympathetic signatures of cardiovascular disease: a blueprint for development of targeted sympathetic ablation therapies. Hypertension. 2012;59(3):545–547. doi: 10.1161/HYPERTENSIONAHA.111.182899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes. 2015;64(7):2289–2298. doi: 10.2337/db14-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez LM, Pareja-Galeano H, Sanchis-Gomar F, Emanuele E, Lucia A, Galvez BG. ‘Adipaging’: ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. J Physiol. 2016;594(12):3187–3207. doi: 10.1113/JP271691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer MA, Weinberg CR, Cook D, Best JD, Reenan A, Halter JB. Differential changes of autonomic nervous system function with age in man. Am J Med. 1983;75(2):249–258. doi: 10.1016/0002-9343(83)91201-9. [DOI] [PubMed] [Google Scholar]
- Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010;55(4):862–868. doi: 10.1161/HYPERTENSIONAHA.109.141119. [DOI] [PubMed] [Google Scholar]
- Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-beta and NF-kappaB. Nat Med. 2011;17(7):883–887. doi: 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin SW, Chen Y, Leiter L, Liu L, Reeder BA. Risk factor correlates of body mass index. Canadian Heart Health Surveys Research Group. CMAJ. 1997;157(Suppl 1):S26–S31. [PubMed] [Google Scholar]
- Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49(3):647–652. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192(4):547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MJ, White TA, Evans G, Tonne JM, Verzosa GC, Stout MB, Mazula DL, Palmer AK, Baker DJ, Jensen MD, Torbenson MS, Miller JD, Ikeda Y, Tchkonia T, van Deursen JM, Kirkland JL, LeBrasseur NK. Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes. 2016;65(6):1606–1615. doi: 10.2337/db15-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Bell C. Chronic sympathetic activation: consequence and cause of age-associated obesity? Diabetes. 2004;53(2):276–284. doi: 10.2337/diabetes.53.2.276. [DOI] [PubMed] [Google Scholar]
- Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31(1 Pt 2):409–414. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- Shirakawa K, Yan X, Shinmura K, Endo J, Kataoka M, Katsumata Y, Yamamoto T, Anzai A, Isobe S, Yoshida N, Itoh H, Manabe I, Sekai M, Hamazaki Y, Fukuda K, Minato N, Sano M. Obesity accelerates T cell senescence in murine visceral adipose tissue. J Clin Invest. 2016;126(12):4626–4639. doi: 10.1172/JCI88606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MM, Minson CT. Obesity and adipokines: effects on sympathetic overactivity. J Physiol. 2012;590(8):1787–1801. doi: 10.1113/jphysiol.2011.221036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Muntzel MS. Recording sympathetic nerve activity chronically in rats: surgery techniques, assessment of nerve activity, and quantification. Am J Physiol Heart Circ Physiol. 2013;305(10):H1407–H1416. doi: 10.1152/ajpheart.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian M, Mueller PJ. Altered differential control of sympathetic outflow following sedentary conditions: role of subregional neuroplasticity in the RVLM. Front Physiol. 2016;7:290. doi: 10.3389/fphys.2016.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan FC, Hutchison ER, Eitan E, Mattson MP. Are there roles for brain cell senescence in aging and neurodegenerative disorders? Biogerontology. 2014;15(6):643–660. doi: 10.1007/s10522-014-9532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares MN, Yabluchanskiy A, Tucsek Z, Hertelendy P, Kiss T, Gautam T, Zhang XA, Sonntag WE, de Cabo R, Farkas E, Elliott MH, Kinter MT, Deak F, Ungvari Z, Csiszar A. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood-brain barrier disruption, neuroinflammation, amyloidogenic gene expression, and cognitive decline in mice, mimicking the aging phenotype. J Gerontol A Biol Sci Med Sci. 2018;73(7):853–863. doi: 10.1093/gerona/glx177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122(1):153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Sosnowska D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2014;69(10):1212–1226. doi: 10.1093/gerona/glt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69(11):1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Bailey-Downs L, Gautam T, Jimenez R, Losonczy G, Zhang C, Ballabh P, Recchia FA, Wilkerson DC, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300(4):H1133–H1140. doi: 10.1152/ajpheart.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcarcel-Ares MN, Tucsek Z, Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, Galvan V, Ballabh P, Richardson A, Freeman WM, Wren JD, Deak F, Ungvari Z, Csiszar A (2018) Obesity in aging exacerbates Neuroinflammation, dysregulating synaptic function-related genes and altering eicosanoid synthesis in the mouse hippocampus: potential role in impaired synaptic plasticity and cognitive decline. J Gerontol A Biol Sci Med Sci [DOI] [PMC free article] [PubMed]
- van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509(7501):439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hemelrijck M, Ulmer H, Nagel G, Peter RS, Fritz J, Myte R, van Guelpen B, Foger B, Concin H, Haggstrom C, Stattin P, Stocks T. Longitudinal study of body mass index, dyslipidemia, hyperglycemia, and hypertension in 60,000 men and women in Sweden and Austria. PLoS One. 2018;13(6):e0197830. doi: 10.1371/journal.pone.0197830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96(10):3423–3429. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- Wang YC, Colditz GA, Kuntz KM. Forecasting the obesity epidemic in the aging U.S. population. Obesity (Silver Spring) 2007;15(11):2855–2865. doi: 10.1038/oby.2007.339. [DOI] [PubMed] [Google Scholar]
- Warchol-Celinska E, Prejbisz A, Kadziela J, Florczak E, Januszewicz M, Michalowska I, Dobrowolski P, Kabat M, Sliwinski P, Klisiewicz A, Topor-Madry R, Narkiewicz K, Somers VK, Sobotka PA, Witkowski A, Januszewicz A. Renal denervation in resistant hypertension and obstructive sleep apnea: randomized proof-of-concept phase II trial. Hypertension. 2018;72(2):381–390. doi: 10.1161/HYPERTENSIONAHA.118.11180. [DOI] [PubMed] [Google Scholar]
- Welle S. Sympathetic nervous system response to intake. Am J Clin Nutr. 1995;62(5 Suppl):1118S–1122S. doi: 10.1093/ajcn/62.5.1118S. [DOI] [PubMed] [Google Scholar]
- Westhoff JH, Hilgers KF, Steinbach MP, Hartner A, Klanke B, Amann K, Melk A. Hypertension induces somatic cellular senescence in rats and humans by induction of cell cycle inhibitor p16INK4a. Hypertension. 2008;52(1):123–129. doi: 10.1161/HYPERTENSIONAHA.107.099432. [DOI] [PubMed] [Google Scholar]
- Writing Group, M. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, C. American Heart Association Statistics and S. Stroke Statistics Heart disease and stroke statistics-2016 Update: a report from the American Heart Association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Xue B, Thunhorst RL, Yu Y, Guo F, Beltz TG, Felder RB, Johnson AK. Central renin-angiotensin system activation and inflammation induced by high-fat diet sensitize angiotensin II-elicited hypertension. Hypertension. 2016;67(1):163–170. doi: 10.1161/HYPERTENSIONAHA.115.06263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HE, Kim EN, Kim MY, Lim JH, Jang IA, Ban TH, Shin SJ, Park CW, Chang YS, Choi BS. Age-associated changes in the vascular renin-angiotensin system in mice. Oxidative Med Cell Longev. 2016;2016:6731093. doi: 10.1155/2016/6731093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JB, Landsberg L. Suppression of sympathetic nervous system during fasting. Obes Res. 1997;5(6):646–649. doi: 10.1002/j.1550-8528.1997.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Zucker IH, Patel KP, Schultz HD. Neurohumoral stimulation. Heart Fail Clin. 2012;8(1):87–99. doi: 10.1016/j.hfc.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]