Abstract
This study evaluated the total phenolic content (TPC) and the antioxidant activity (AA) of cow’s milk enriched with phenolic compounds extracted from jaboticaba peel, either by adding jaboticaba crude extract or a jaboticaba-loaded nanoemulsion. Three nanoemulsions with 5, 10 and 15% of jaboticaba extract were prepared. Average particle diameter (166.7–181.7 nm), polydispersity index (0.138–0.156) and zeta potential (ranging from − 35.30 to − 38.60 mV) were measured for the three different colloidal systems. The nanoemulsion with 15% of jaboticaba extract (J15-NE) was chosen for milk enrichment. J15-NE showed an encapsulation efficiency of 85.6% and remained stable for 60 days at 8 °C. Transmission electron microscopy of J15-NE displayed nanoparticles with a well-defined spherical shape. Reference milk, milk enriched with jaboticaba extract and milk enriched with J15-NE were characterised by a TPC of 93, 171 and 161 µg/ml GAE (gallic acid equivalent), respectively, and an AA of 0.04, 0.17 and 0.14 µg/ml TEAC (trolox equivalent antioxidant capacity), respectively. Thus, this study showed that nanoemulsion with jaboticaba peel extract could be exploited as an ingredient to enrich the properties of milk.
Keywords: Plinia peruviana, Phenolic compounds, Antioxidant activity, Cow’s milk, Nanoemulsion, High-pressure homogenisation
Introduction
Interest is increasing in the incorporation of bioactive compounds in foods owing to their potential effect on human health and well-being (Hasler 1998; Pang et al. 2012). In particular, phenolic compounds (PCs) are considered of high value because of their antioxidant activity (AA), along with other beneficial health effects (Servili et al. 2009; Yilmaz 2006).
Phenolic compounds are secondary metabolites of the plant kingdom and are synthesized by plants during normal development and in response to physical injury, infection or other stress conditions (Beckman 2000). They constitute a group of about 8000 molecules, characterised by the presence of an aromatic ring with one or more hydroxyl substituents, and they range from simple phenolic molecules to highly polymerised compounds (Bravo 1998).
Phenolic compounds are widespread in the human diet (Manach et al. 2005). Main dietary sources of polyphenols are fruits, beverages (e.g., tea, coffee), and vegetables. The average daily intake of PCs is estimated to be around 1 g/day (Scalbert and Williamson 2000), but this value can vary considerably, depending on the dietary habits of different populations. For example, recent studies show an average consumption of 460, 820, 863 and 1193 mg/day for Brazilian, Spanish, Finnish and French populations, respectively (Corrêa et al. 2015; Ovaskainen et al. 2008; Pérez-Jiménez et al. 2011; Tresserra-Rimbau et al. 2013).
Epidemiological studies suggest that the long-term consumption of diets rich in these compounds can offer protection against oxidative stress recognised as the main role in human aging and in the development of chronic and degenerative diseases, such as cancer, arthritis, and autoimmune disorders, as well as cardiovascular and neurodegenerative diseases (Pandey and Rizvi 2009; Ness and Powles 1997; Steinmetz and Potter 1996). This has driven efforts to design specific nutritional supplements and include these compounds to enrich foods with functional and health properties.
In food, polyphenols may contribute to bitterness, astringency, colour, flavour, odour and oxidative stability (Pandey and Rizvi 2009). The manufacture of food products enriched with phenolic compounds, either extracted or recovered from natural sources, such as fruits, vegetables and herbs, requires proper design and optimisation to comply with aspects related to both quality and sensory properties of the products, as well as bioavailability and health benefits.
Milk and dairy products are characterised by a high nutritional value based on the presence of essential macronutrients which, along with minor compounds (e.g. calcium, vitamins, immunoglobulins and peptides), contribute to their health-giving properties. Some studies have shown that milk contains a limited amount of phenolic compounds, most of them derived from feed (Hoikkala et al. 2007; King et al. 1998; Kuhnen et al. 2014; Mustonen et al. 2009), although a fraction may be the product of amino acid catabolism by bacteria and contamination with sanitising agents (O’Connell and Fox 2001). In addition, polyphenols of bovine origin can be formed by the action of gut bacterial flora on plant PCs, and secondary metabolites, such as equol, can be found in the milk of dairy cows (Mustonen et al. 2009). Thus, any variation of the phenolic profile of milk could mainly depend on feed formulation.
Some studies have been carried out to investigate the intrinsic antioxidant activity (AA) and phenolic content of milk derivatives (e.g., cheese) in the absence of food supplements (Han et al. 2011; Hilario et al. 2010). In this case, phenolic compounds are present in low concentrations and seem to have limited AA. Most recently, the potential of improving the content of bioactive compounds in milk and dairy products has been investigated, and to this aim, two main strategies have been differently applied: (1) enrichment of the diet with feed fortified with specific phenolic compounds or with ingredients rich in these compounds (Branciari et al. 2014; Santos et al. 2014) and (2) post-milking addition (Rashidinejad et al. 2014; Servili et al. 2011).
Tropical fruits have been attracting the interest of researchers for the diversity and quantity of compounds they contain (Denardin et al. 2015; Rufino et al. 2010). Among these, jaboticaba (Plinia peruviana), a Brazilian native fruit, has been recognised as a promising source of phenolic compounds since the fresh whole fruit has a total phenolic content of about 440 mg GAE/100 g, much higher than many other fruits (Rufino et al. 2010; Fu et al. 2011). Jaboticaba fruit contains a large variety of PCs, such as cyanidin-3-O-glucoside, delphinidin-3-O-glucoside, ellagic acid, gallic acid, rutin, quercetin, quercitrin, isoquercitrin, quercimeritrin, myricitrin, kaempferol, and tannins (Bailão et al. 2015; Borges et al. 2014). In addition to their antioxidant power, these compounds give jaboticaba anti-inflammatory, antibacterial, antifungal, antiproliferative, antimutagenic, hypoglycemic and hypolipidemic bioactive properties (Borges et al. 2014; Leite-Legatti et al. 2012). During extraction of jaboticaba juice, pomace (ca. 40%), a waste containing mainly skins and seeds, is produced. This waste product, much richer in phenolics and anthocyanins than the whole fruit (ca. 2.5 and 3.5 times, respectively), could constitute a cheap source of such molecules for use in various sectors, including the food and pharmaceutical industries (Gurak et al. 2014).
Phenolic compounds are rather unstable molecules, but their inclusion in milk and milk derivatives, rich in protein and calcium, could contribute to their protection and at the same time enrich the nutritional and health value of the products. However, phenolic compounds and tannins could bind onto various sites of proteins and also crosslink separate molecules affecting both physical and sensory properties of the food system. Furthermore, some phenolic compounds are characterised by low water solubility and poor bioavailability (Rashidinejad et al. 2014). To enhance the gastrointestinal absorption of phenolic compounds, improve their bioavailability and prevent their degradation caused by environmental stress agents, different colloidal systems have been developed (Joung et al. 2016), and emulsion-based delivery systems have been investigated to overcome these problems (Huang et al. 2010; McClements and Decker 2000). Nanoemulsions are kinetically stable submicron-sized emulsions wherein droplets of oil or water are finely dispersed in the opposite phase with the aid of an adequate emulsifier that stabilizes the colloidal dispersion (Sutradhar and Amin 2013). Nanoemulsions can offer several advantages compared to conventional emulsions, such as improving physical stability, absorption penetration, and bioavailability (Gupta et al. 2016).
Therefore, this study aimed to evaluate the total phenolic content and the antioxidant activity of cow’s milk enriched with phenolic compounds extracted from jaboticaba peel, either by adding jaboticaba crude extract, or by adding a jaboticaba-loaded nanoemulsion. Colourimetry was also performed to evaluate any differences between the reference milk and the two differently enriched milks.
Materials and methods
Materials
Jaboticaba fruits, collected in October 2015, were obtained from an orchard cultured in Guaxupé County (Minas Gerais, Brazil). The voucher specimen (FLOR 55902) was preserved at the FLOR herbarium (Department of Botany, Federal University of Santa Catarina, Florianopolis, Brazil). These fruits were produced by native jaboticaba trees located in the remaining Atlantic Forest (Mata Atlântica) territories and were not subjected to any kind of agrochemicals for the control of disease and pests. Peels were manually separated from pulp and seeds and stored at − 20 °C until use. All chemicals of analytical grade used in this study were purchased from Sigma-Aldrich (São Paulo, Brazil) and Vetec (São Paulo, Brazil). Miglyol 812 N was kindly donated by PIC Química (São Paulo, Brazil).
Methods
Extraction of phenolic compounds
Jaboticaba peels were first freeze dried in a freeze-drying system (FreeZone 6, Labconco, Missouri, US) at − 52 °C and at a pressure of 0.021 mbar for 24 h. After lyophilisation, dried peels were finely and uniformly milled (Cadence MDR301, Santa Catarina, Brazil). Phenolic compounds were extracted from powder using a high-pressure method (125 mPa, 3 h) in a 50% (v/v) ethanol solution acidified with hydrochloric acid at pH 3.6. This pH was chosen for extraction because most polyphenols are more stable against oxidation under acidic conditions (Janeiro and Oliveira-Brett 2004). Ethanol was removed by means of a rotary evaporator at 50 °C in dark conditions; then, the aqueous extract was filtered with paper with pore size = 14 μm (80 g/m2, Unifil, Niederlenz, Switzerland).
Characterisation of jaboticaba extract
Determination of total phenolic compounds
Total phenolic compounds (TPC) were determined according to the Folin–Ciocalteu method (Singleton and Rossi 1965). In this procedure, 1 ml diluted (1:10, v/v, in water) Folin reagent and 800 µl sodium carbonate aqueous solution (7.5%, w/v) were added to 200 µl of properly diluted extract. The mixture was kept protected from light at room temperature for 30 min before recording the absorbance at 760 nm (Kuhnen et al. 2014). An external standard curve for gallic acid (Sigma, 5–160 µg/ml, r2 = 0.999) was constructed for the quantification. Results are expressed as gallic acid equivalents (GAE) in mg/ml and are the average of three replicates.
Determination of total anthocyanins
Total anthocyanins (TA) were determined using the pH differential method (Wrolstad et al. 2005). An aliquot of jaboticaba extract was diluted in 0.025 M chloride buffer (pH 1) at a dilution factor ratio of 1:150, and another aliquot of the same extract was diluted in 0.4 M acetate buffer (pH 4.5) at the same ratio. The two solutions were separately mixed and incubated for 30 min at room temperature under dark conditions. Then, the absorbance of both solutions was measured at 510 nm and 700 nm. Cyanidin-3-glucoside (c3g), with a molar attenuation coefficient (ε) of 26900 and molar weight (MW) of 449.2, was used as the standard.
Total anthocyanins content in mg/l was calculated as
| 1 |
where D is the dilution factor, and A is the absorbance calculated as
| 2 |
Results are expressed as mg cyanidin-3-glucoside (c3g) for ml and are the average of three replicates.
Evaluation of in vitro antioxidant activity
The evaluation of jaboticaba crude extract antioxidant activity (AA) was performed using the DPPH method (Sharma and Bhat 2009) based on the capture of the free radical DPPH (1,1-diphenyl-2-picrylidrazyl) by antioxidants. A 0.00316% DPPH solution (w/v) in 80% methanol was used. 2600 μl of DPPH solution and 400 μl of properly diluted extract were placed in a glass cuvette, gently shaken and incubated for 5 min at room temperature protected from light. The control assay was prepared according to the procedure described above, but adding 400 μl of 80% methanol instead of the extract. The decrease of absorbance was recorded at 530 nm, and the free radical scavenging activity was calculated as
| 3 |
where CA is the control assay absorbance, and SA is the sample absorbance.
Results are expressed in TEAC (Trolox Equivalent Antioxidant Capacity).
Preparation of jaboticaba nanoemulsions
An important part of this study involved the production of stable nanoemulsions containing as much encapsulated jaboticaba extract as possible so that milk could be enriched using smaller amounts of nanoemulsion. To accomplish this, different colloidal systems were prepared and added with 5, 10 and 15% of jaboticaba crude extract. Nanoemulsions were obtained by high-pressure (HP) homogenisation and were made of 1% polysorbate 80 (Tween 80, Vetec), 5% medium chain triglycerides (MCT, Miglyol 812 N) and 94% aqueous phase, according to the procedure previously described by Mazzarino et al. (2017). The oil phase (MCT) was previously mixed with Tween 80, the aqueous phase was added, and then the mixture was homogenised for 15 min by magnetic stirring. Next, the pre-emulsion was transferred to a high-pressure homogeniser (Homolab, FBF Italy, Parma, Italy) and homogenised at 500 bar for 3 cycles. First, a reference nanoemulsion, consisting of Tween 80, TCM and ultrapure water (ref-NE), was prepared. Then, by using the same procedure, three jaboticaba-loaded nanoemulsions were prepared and added to the aqueous phase with different concentrations of jaboticaba peel extract: 5% (J5-NE), 10% (J10-NE), and 15% (J15-NE) (w/v). Extract quantities higher than 15% were added and tested, but the present experimental conditions did not allow us to obtain homogeneous pre-emulsions owing to the formation of a precipitate. Therefore, we did not transfer such extracts to high-pressure homogenisation.
Characteristics of jaboticaba nanoemulsions
Particle size and zeta potential
Mean particle size, polydispersity index (PDI) and zeta potential were determined by dynamic light scattering (DLS) and laser-doppler anemometry (LDA), respectively, using a Zetasizer Nano ZS90 (Malvern Instruments, Worcestershire, UK). The measurements were performed at 25 °C after appropriate dilution (1:30) in ultrapure water. The particle size analyses were performed at a fixed angle of 90°. For the measurements of zeta potential, samples were placed in electrophoretic cells, followed by applying a potential of ± 150 mV. Each datum reported is the average of at least three measurements.
As the goal of this study was to enrich milk with a stable colloidal system, without excessively diluting the product, the experiments of milk enrichment were carried out only with the most concentrated nanoemulsion (J15-NE).
pH
Nanoemulsion pH was measured using a pH-meter SP1800 equipped with an electrode SC09 (Sensoglass, São Paulo, SP, Brazil).
Encapsulation efficiency
The encapsulation efficiency (%) of nanoemulsions containing 15% of jaboticaba extract was determined using the ultrafiltration/centrifugation technique. Nanoemulsions were placed in Amicon Centrifugal Filter Devices with Ultracel-100 membrane (100 kDa, Millipore Corp., Billerica, MA) and centrifuged (2000g, 30 min) to separate the free compounds in the supernatant from the compounds loaded in the colloidal dispersion. Encapsulation efficiency was calculated as the difference between the total phenolic content of the suspensions of nanoparticles and the TPC present in the supernatant. TPC contents were determined spectrophotometrically using the Folin–Ciocalteu assay (Singleton and Rossi 1965).
Morphological evaluation
The morphology of J15-NE was evaluated by transmission electron microscopy (TEM) using Jeol JEM-1011 equipment (Jeol, Tokyo, Japan). Droplets of nanoparticle suspensions were deposited on carbon-coated grids and stained with 5% uranyl acetate.
Nanoemulsion colloidal stability
J15-NE stability was evaluated by monitoring particle size, polydispersity index, ζ-potential and pH for 60 days at 8 °C and at 20 °C. The samples were stored in amber glass bottles under dark conditions. Tests were performed at 7, 14, 21, 30, and 60 days after preparation.
Milk enrichment
Commercial fresh pasteurised milk was differently enriched, either by adding the jaboticaba crude extract (10.5% dry matter), or by adding the J15-NE. The jaboticaba extract was previously diluted with ultrapure water to a concentration of 15%. The diluted extract, or J15-NE, was added to milk in the same quantity (10%). Milk was then thoroughly mixed for 15 min with a magnetic stirrer at 4 °C and kept at this temperature until analysis. All analyses were carried out within a maximum of 6 h from the preparation of the enriched milks.
Milk characteristics
Colour measurement
The colour analysis of the milk samples was performed using a Chroma Meter CR-400 (Konica Minolta, Tokyo, Japan) adjusted to operate with a D65 illuminator and 10º observation angle and previously calibrated. The CIELab scale was used to determine the values of the parameters L*, a* and b*, where L* represents the luminosity ranging from black (0) to white (100), a* represents the variation of the color from red (+) to green (−), and b* represents the variation of the color from yellow (+) to blue (−). For each milk sample, the results were expressed as the mean of three measurements.
Total phenolic compounds and in vitro antioxidant activity
Milk samples were first deproteinized according to the following procedure (Kuhnen et al. 2014). Briefly, a volume of 15 ml methanol was added to 5 ml of milk in a test tube with a screw cap. After shaking for 30 s, the mixture was incubated for 40 min at room temperature and centrifuged (15 min, 4000×g); then the supernatant was removed for analysis. Reference milk (no addition) and differently enriched milks were analysed for total phenolic compounds (TPC) and antioxidant activity (DPPH method), as described above, and the correlation between TPC and AA was determined.
Statistical analysis
All analyses were performed in triplicate, and the data are presented as mean ± standard deviation (SD). Significant differences were evaluated by analysis of variance (ANOVA) using SPSS Statistics, v 20, software (IBM, New York, USA).
Results and discussion
Characteristics of jaboticaba peel extract
The yield of the jaboticaba aqueous extract (%) was 10.54 ± 0.02. The values of pH, total phenolic compounds (TPC), total anthocyanins (TA) and antioxidant activity (AA) are shown in Table 1. Similar values were reported by other authors (Gurak et al. 2014; Leite-Legatti et al. 2012; Silva et al. 2010; Souza et al. 2017).
Table 1.
pH, total phenolic compounds (TPC), total anthocyanins (TA) and antioxidant activity (AA) of the Jaboticaba aqueous extract
| pH | 3.33 ± 0.01 |
| TPC (mg GAE/ml) | 18.51 ± 0.52 |
| TA (mg c3g/ml) | 2.10 ± 0.02 |
| AA (µg TEAC/ml) | 36.75 ± 0.70 |
Jaboticaba whole fruit was shown to contain a greater amount of total phenolic compounds compared to different blueberry species (Dai et al. 2009; Reynertson 2007). Among the different classes of polyphenols, flavonoids and, in particular, anthocyanins represent the major compounds in jaboticaba fruit (Reynertson et al. 2006). A study carried out by Abe et al. (2012) showed that the major contribution to the total phenolic content of the whole fruit came from jaboticaba peel.
These results confirm the high total phenolic and anthocyanin content of the jaboticaba fruit and its high antioxidant power, in turn validating this fruit and, more particularly, its skin as an excellent source of these bioactive compounds. This also highlights the importance of their use in the pharmaceutical industry (Shahidi 2009) and, more recently, in food additives (Ayala-Zavala et al. 2010).
Characterisation of jaboticaba nanoemulsions
The nanoemulsions containing both different jaboticaba extract and reference were characterised for their colloidal properties, and in Table 2, the results of zeta average diameter, polydispersity index and zeta potential are reported. The addition of the jaboticaba extract affected the average diameter of the nanoparticles such that it decreased with increasing concentration of the extract. This result could be attributed to a surfactant effect of some bioactives and phenolics present in the extract that could locate at the oil/water interface during emulsification, thus affecting interfacial tension (Richards et al. 2002; Frankel et al. 1996). Some studies have highlighted this ability of plant phenolics, such as that found in olive and olive leaves, to play a major role in the stabilisation of food colloids owing to their amphiphilic nature (Di Mattia et al. 2009, 2011; Polychniatou and Tzia 2016).
Table 2.
Average particle diameter (Z-mean diameter), polydispersity index (PDI) and zeta potential of the nanoemulsions, without jaboticaba extract (reference, ref-NE) and with different concentrations of extract: 5% (J5-NE), 10% (J10-NE), and 15% (J15-NE)
| Sample | Z-mean diameter (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|
| Ref-NE | 186.6 ± 0.75a | 0.149 ± 0.022a | − 17.70 ± 0.40a |
| J5-NE | 181.7 ± 1.85b | 0.156 ± 0.08a | − 35.30 ± 1.48b |
| J10-NE | 175.6 ± 1.11c | 0.172 ± 0.08a | − 40.03 ± 2.06c |
| J15-NE | 166.7 ± 0.41d | 0.138 ± 0.027a | − 38.60 ± 0.26c |
a,b,c,dValues with different letters in the same column differ significantly (p < 0.05)
With regard to PDI, no statistically significant differences between the different preparations were determined. A PDI value lower than 0.2 means that the particles in the nanoemulsion are monodisperse (Izquierdo et al. 2005). A lower, i.e., negative, zeta potential value was observed in the ref-NE and could be attributed to the stabilisation of droplets of triglycerides by non-ionic surfactant (Mazzarino et al. 2017). The addition of the fruit extract in the nanoemulsion caused a decrease of zeta potential (Table 2). This result could confirm the effect of minor amphyphilic compounds of fruit extracts on the colloidal properties of nanoemulsions. The increased negative charge of the jaboticaba-enriched nanoemulsions could also contribute to their physical stabilisation by increased charge repulsion (Patel and Agrawal 2011).
Nanoemulsions made with 15% jaboticaba showed a pH of 3.53, a TPC equal to ca. 2.8 mg GAE/ml , and an encapsulation efficiency of 85.6%. In previous studies focused on the preparation of nanoemulsions containing at most 10% of jaboticaba peel extract, Mazzarino et al. (2017) found that an encapsulation efficiency of more than 90% was achieved.
Despite the lower encapsulation efficiency, J15-NE was selected for milk enrichment as it contains a higher quantity of encapsulated phenolic compounds by volume. Figure 1 shows TEM images of J15-NE at two different magnifications. Micrographs highlight the spherical shape of the nanoparticles and their nanoscale size on the same order as that determined by scattering dynamic light. At higher magnification, it is also possible to clearly distinguish the structure of the nanoparticles, the surfactant interface of which surrounds the lipid phase containing the jaboticaba extract.
Fig. 1.
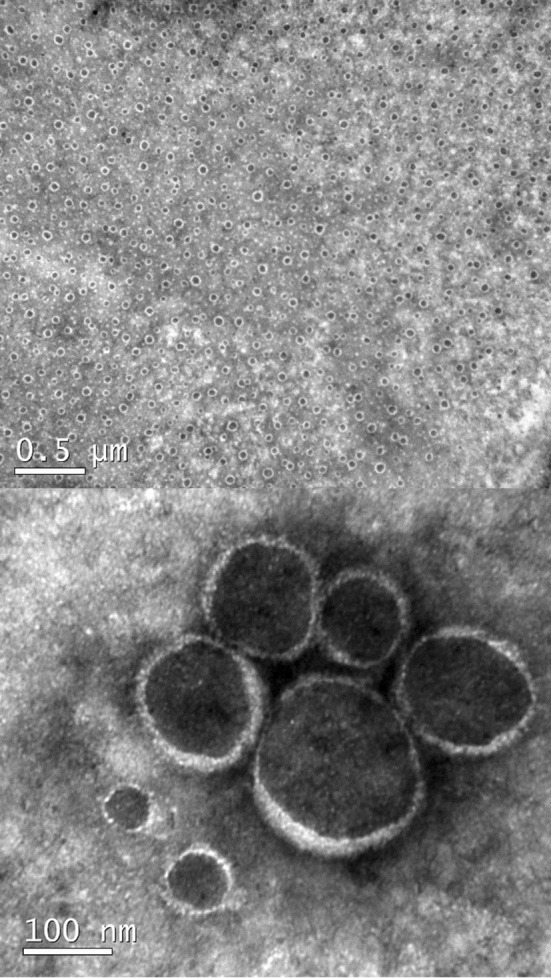
TEM micrographs of J15-NE at two different magnifications
The physicochemical stability of the J15-NE system was evaluated over storage time at two different temperatures, and the change in colloidal properties is summarised in Table 3. After 60 days of storage at 8 °C, a slight, but significant, increase of nanoparticles size was observed, along with an insignificant increase of the polydispersity index that remained below 0.2. No visual phase separation was observed. Neither pH nor zeta potential changed over the selected storage time, and, overall, these results confirm the relative colloidal stability of J15-NE at 8 °C. On the contrary, at 20 °C, J15-NE showed a significant change in the physicochemical properties of the nanoemulsion after 21 days of storage, and for longer storage time, a loss of monodispersity occurred.
Table 3.
J15-NE physicochemical stability test at 8 °C and 20 °C
| J15-NE | Z-mean d. (nm) | PdI | Zeta potential (mV) | pH | ||||
|---|---|---|---|---|---|---|---|---|
| 8 °C | 20 °C | 8 °C | 20 °C | 8 °C | 20 °C | 8 °C | 20 °C | |
| T0 | 167.5 ± 3.81a | 166.7 ± 0.42a | 0.173 ± 0.011a | 0.168 ± 0.027a | − 38.4 ± 1.16a | − 38.6 ± 0.26a | 3.5 ± 0.015 | 3.5 ± 0.006 |
| T7 | 181.7 ± 1.50b | 187.9 ± 5.69c | 0.179 ± 0.007a | 0.200 ± 0.003a | − 37.2 ± 1.08a | − 38.5 ± 0.25a | 3.6 ± 0.010 | 3.5 ± 0.015 |
| T14 | 183.1 ± 1.42b | 226.1 ± 3.47d | 0.180 ± 0.005a | 0.248 ± 0.003b | − 38.3 ± 0.81a | − 38.5 ± 0.40a | 3.6 ± 0.020 | 3.4 ± 0.015 |
| T21 | 184.1 ± 1.63b | 322.4 ± 1.78e | 0.181 ± 0.003a | 0.365 ± 0.040c | − 38.4 ± 0.21a | − 34.5 ± 1.00b | 3.7 ± 0.010 | 3.4 ± 0.006 |
| T30 | 184.5 ± 3.43b | 716.6 ± 90.23f | 0.182 ± 0.009a | 0.744 ± 0.241d | − 38.3 ± 1.76a | − 42.4 ± 0.90c | 3.7 ± 0.006 | 3.3 ± 0.015 |
| T60 | 188.9 ± 0.14c | – | 0.199 ± 0.011a | – | − 37.7 ± 0.25a | – | 3.6 ± 0.010 | – |
a,b,cValues with different letters in the same column differ significantly (p < 0.01)
Milk characteristics
Reference milk (milk-R), milk enriched with jaboticaba diluted extract (milk-E) and milk enriched with J15-NE (milk-N) were characterised by a total phenolic content of 93, 171 and 161 µg GAE/ml, respectively, and an antioxidant activity of 0.04, 0.17 and 0.14 TEAC, respectively (Table 4). These results show that enrichment arising from extract and nanoemulsions increased both phenolic content and in vitro antioxidant activity of the reference milk. Several studies have been performed with the aim of evaluating the characteristics of milk and milk-based products enriched with phenolic compounds, or their derivatives (Branciari et al. 2014; Han et al. 2011; Rashidinejad et al. 2014; Santos et al. 2014; Servili et al. 2011). Phenolic compounds and phenolic derivatives found in milk can be modified, either by supplementing the diet of the lactating animal or by directly adding phenolic compounds to milk. Post-milking enrichment has been carried out in different ways, e.g., by adding single phenolic compounds, plant extracts, microencapsulated phenolic compounds or phenolic compounds incorporated in nanoliposomes. The use of encapsulated phenolic compounds in the form of nanoemulsions or liposomes presents the merits of protecting the compounds from degradation and improving their bioavailability (Huang et al. 2010). In the present study, the small, but significant, differences in total phenolic content and antioxidant activity between milk-E and milk-N can be explained by the encapsulation of phenolic compounds inside the droplets of nanoemulsion, thereby avoiding contact with the reagent. This result was also observed by Mazzarino et al. (2017). The antioxidant activity of the milk increases proportionally with the content of phenolic compounds with a significant correlation (r = 0.997).
Table 4.
TPC and AA of the milk samples in the presence and absence of Jaboticaba peel as nanoemulsion or crude extract
| Sample | TPC (µg GAE/ml) | AA (µg TEAC/ml) |
|---|---|---|
| Reference milk | 93 ± 2.42a | 0.04 ± 0.00052a |
| Milk enriched with the crude extract | 171 ± 1.90b | 0.17 ± 0.00002b |
| Milk enriched with the J15-NE | 161 ± 1.68c | 0.14 ± 0.00482c |
a,b,cValues with different letters in the same column differ significantly (p < 0.01)
Table 5 shows the mean values of the main colorimetric parameters, as well as Yellow Index (YI) and a*/b* chromatic index of the reference and enriched milk samples. In general, both milk-E and milk-N presented lower L* (lightness), higher a* (redness), lower b* (yellowness), and lower YI (yellow index) when compared to the reference milk based on the addition of the jaboticaba fruit peel extract, which is characterised by an intense and dark purple colour. The mode of adding extract to milk affected the colourimetric and chromatic properties since milk-N, compared to Milk-E, had a stronger degree of lightness, lower redness, as well as a stronger degree of yellowness and YI. These differences result from the different colour of J15-NE compared to the crude extract, and, in particular, the chromatic changes of a solution undergoing emulsification. Colourimetric and visual properties of a colloidal system are affected by various factors, such as particle size, concentration, refractive index and spatial distribution of the droplets, as well as the presence of any coloured component (McClements 2002). Thus, the use of nanoemulsions containing jaboticaba extract, instead of crude extract, resulted in less change in the chromatic properties of the milk, while significantly improving the content of bioactives and their in vitro antioxidant activity. In Brazil, the addition of 10% of jaboticaba extract in milk could be sold as a milk-based product because it does not comply with the legislative requirements regarding colour. Therefore, a milk-based product enriched with phenolic compounds would comply with nutritional characteristics as set forth by Brazilian legislation. However, future studies are needed to optimise milk enrichment and evaluate additional quality and nutritional properties of milk enriched with jaboticaba-loaded nanoemulsion, including digestibility and bioavailability of the added bioactive compounds.
Table 5.
Colour analysis of the milk samples
| Sample | L* | a* | b* | YI | a*/b* |
|---|---|---|---|---|---|
| Reference milk | 80.43 ± 0.323a | − 3.03 ± 0.023a | 7.70 ± 0.072a | 13.73 ± 0.048a | − 0.39 ± 0.001a |
| Milk enriched with crude extract | 62.70 ± 0.156b | 4.17 ± 0.061b | 0.16 ± 0.032b | 0.33 ± 0.012b | 26.16 ± 4.980b |
| Milk enriched with J15-NE | 66.69 ± 0.299c | 2.23 ± 0.032c | 1.76 ± 0.031c | 3.75 ± 0.056c | 1.26 ± 0.029c |
a,b,cValues with different letters in the same column differ significantly (p < 0.01)
Conclusion
This study confirmed the feasibility of obtaining nanoemulsions enriched with jaboticaba fruit peel extract at a relatively high extract concentration (15%) to be exploited as an ingredient to improve the antioxidant properties of milk. Milk enriched in this way resulted in more phenolic compounds and more antioxidant activity compared to reference milk, while, at the same time, limiting chromatic changes to milk with the addition of crude jaboticaba peel extract.
Acknowledgements
This study was funded by CNPq (National Council on Scientific and Technological Development; Brasília, Brazil) through Project No. 402539/2013-3, Edital 29/2013.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abe LT, Lajolo FM, Genovese MI. Potential dietary sources of ellagic acid and other antioxidants among fruits consumed in Brazil: Jabuticaba (Myrciaria jaboticaba (Vell.) Berg) J Sci Food Agric. 2012;92:1679–1687. doi: 10.1002/jsfa.5531. [DOI] [PubMed] [Google Scholar]
- Ayala-Zavala J, Rosas-Domınguez C, Vega-Vega V, Gonzalez-Aguilar G. Antioxidant enrichment and antimicrobial protection of fresh-cut fruits using their own byproducts: looking for integral exploitation. J Food Sci. 2010;75:R175–R181. doi: 10.1111/j.1750-3841.2010.01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailão EF, Devilla IA, da Conceicao EC, Borges LL. Bioactive compounds found in Brazilian cerrado fruits. Int J Mol Sci. 2015;16:23760–23783. doi: 10.3390/ijms161023760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman CH. Phenolic-storing cells: keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol Mol Plant Path. 2000;57:101–110. doi: 10.1006/pmpp.2000.0287. [DOI] [Google Scholar]
- Borges LL, Conceição EC, Silveira D. Active compounds and medicinal properties of Myrciaria genus. Food Chem. 2014;153:224–233. doi: 10.1016/j.foodchem.2013.12.064. [DOI] [PubMed] [Google Scholar]
- Branciari R, Ranucci D, Trabalza-Marinucci M, Codini M, Orru M, Ortenzi R, Forte C, Ceccarini MR, Valiani A. Evaluation of the antioxidant properties and oxidative stability of Pecorino cheese made from the raw milk of ewes fed Rosmarinus officinalis L. leaves. Int J Food Sci Technol. 2014;50:558–565. doi: 10.1111/ijfs.12712. [DOI] [Google Scholar]
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Corrêa VG, Tureck C, Locateli G, Peralta RM, Koehnlein EA. Estimate of consumption of phenolic compounds by Brazilian population. Rev Nutr. 2015;28:185–196. doi: 10.1590/1415-52732015000200007. [DOI] [Google Scholar]
- Dai J, Gupte A, Gates L, Mumper RJ. A comprehensive study of anthocyanin-containing extracts from selected blackberry cultivars: extraction methods, stability, anticancer properties and mechanisms. Food Chem Toxicol. 2009;47:837–847. doi: 10.1016/j.fct.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Denardin CC, Hirsch GE, da Rocha RF, Vizzotto M, Henriques AT, Moreira JCF, Guma FTCR, Emanuelli T. Antioxidant capacity and bioactive compounds of four Brazilian native fruits. J Food Drug Anal. 2015;23:387–398. doi: 10.1016/j.jfda.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mattia CD, Sacchetti G, Mastrocola D, Pittia P. Effect of phenolic antioxidants on the dispersion state and chemical stability of olive oil O/W emulsions. Food Res Int. 2009;42:1163–1170. doi: 10.1016/j.foodres.2009.05.017. [DOI] [Google Scholar]
- Di Mattia CD, Sacchetti G, Pittia P. Interfacial behavior and antioxidant efficiency of olive phenolic compounds in O/W olive oil emulsions as affected by surface active agent type. Food Biophys. 2011;6:295–302. doi: 10.1007/s11483-010-9195-7. [DOI] [Google Scholar]
- Frankel EN, Huang S-W, Prior E, Aeschbach R. Evaluation of antioxidant activity of rosemary extracts, carnosol and carnosic acid in bulk vegetable oils and fish oil and their emulsions. J Sci Food Agric. 1996;72:201–208. doi: 10.1002/(SICI)1097-0010(199610)72:2<201::AID-JSFA632>3.0.CO;2-Q. [DOI] [Google Scholar]
- Fu L, Xu BT, Xu XR, Gan RY, Zhang Y, Xia EQ, Li HB. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011;129:345–350. doi: 10.1016/j.foodchem.2011.04.079. [DOI] [PubMed] [Google Scholar]
- Gupta A, Eral HB, Hatton TA, Doyle PS. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12:2826–2841. doi: 10.1039/C5SM02958A. [DOI] [PubMed] [Google Scholar]
- Gurak PD, De Bona GS, Tessaro IC, Marczak LDF. Jaboticaba pomace powder obtained as a co-product of juice extraction: a comparative study of powder obtained from peel and whole fruit. Food Res Int. 2014;62:786–792. doi: 10.1016/j.foodres.2014.04.042. [DOI] [Google Scholar]
- Han J, Britten M, St-Gelais D, Champagne CP, Fustier P, Salmieri S, Lacroix M. Polyphenolic compounds as functional ingredients in cheese. Food Chem. 2011;124:1589–1594. doi: 10.1016/j.foodchem.2010.08.021. [DOI] [Google Scholar]
- Hasler CM. Functional foods: their role in disease prevention and health promotion. Food Technol. 1998;52:57–62. [Google Scholar]
- Hilario MC, Puga CD, Ocana AN, Romo FPG. Antioxidant activity, bioactive polyphenols in Mexican goats’ milk cheeses on summer grazing. J Dairy Res. 2010;77:20–26. doi: 10.1017/S0022029909990161. [DOI] [PubMed] [Google Scholar]
- Hoikkala A, Mustonen E, Saastamoinen I, Jokela T, Taponen J, Saloniemi H, Wähälä K. High levels of equol in organic skimmed Finnish cow milk. Mol Nutr Food Res. 2007;51:782–786. doi: 10.1002/mnfr.200600222. [DOI] [PubMed] [Google Scholar]
- Huang Q, Yu H, Ru Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J Food Sci. 2010;75:R50–R57. doi: 10.1111/j.1750-3841.2009.01457.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo P, Feng J, Esquena J, Tadros TF, Dederen JC, Garcia MJ, Azemar N, Solans C. The influence of surfactant mixing ratio on nano-emulsion formation by the pit method. J Colloid Interface Sci. 2005;285:388–394. doi: 10.1016/j.jcis.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Janeiro P, Oliveira-Brett AM. Catechin electrochemical oxidation mechanisms. Anal Chim Acta. 2004;518:109–115. doi: 10.1016/j.aca.2004.05.038. [DOI] [Google Scholar]
- Joung HJ, Choi MJ, Kim JT, Park SH, Park HJ, Shin GH. Development of food-grade curcumin nanoemulsion and its potential application to food beverage system: antioxidant property and in vitro digestion. J Food Sci. 2016;81:N745–N753. doi: 10.1111/1750-3841.13224. [DOI] [PubMed] [Google Scholar]
- King RA, Mano MM, Head RJ. Assessment of isoflavonoid concentrations in Australian bovine milk samples. J Dairy Res. 1998;65:479–489. doi: 10.1017/S0022029998002891. [DOI] [PubMed] [Google Scholar]
- Kuhnen S, Moacyr JR, Mayer JK, Navarro BB, Trevisan R, Honorato LA, Maraschin M, Pinheiro Machado Filho LC. Phenolic content and ferric reducing-antioxidant power of cow’s milk produced in different pasture-based production systems in southern Brazil. J Sci Food Agric. 2014;94:3110–3117. doi: 10.1002/jsfa.6654. [DOI] [PubMed] [Google Scholar]
- Leite-Legatti AV, Batista AG, Dragano NRV, Marques AC, Malta LG, Riccio MF, Eberlin MN, Machado ART, Carvalho-Silva LB, Ruiz ALTG, Carvalho JE, Pastore GM, Júnior MRM. Jaboticaba peel: antioxidant compounds, antiproliferative and antimutagenic activities. Food Res Int. 2012;49:596–603. doi: 10.1016/j.foodres.2012.07.044. [DOI] [Google Scholar]
- Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- Mazzarino L, da Silva PH, Lorenzen Voytena AP, Dias Trevisan AC, Ribeiro-Do-Valle RM, Maraschin M. Jaboticaba (Plinia peruviana) extract nanoemulsions: development, stability, and in vitro antioxidant activity. Drug Dev Ind Pharm. 2017;29:1–9. doi: 10.1080/03639045.2017.1405976. [DOI] [PubMed] [Google Scholar]
- McClements DJ. Colloidal basis of emulsion color. Curr Opin Colloid Interface Sci. 2002;7:451–455. doi: 10.1016/S1359-0294(02)00075-4. [DOI] [Google Scholar]
- McClements DJ, Decker EA. Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems. J Food Sci. 2000;8:1270–1282. doi: 10.1111/j.1365-2621.2000.tb10596.x. [DOI] [Google Scholar]
- Mustonen EA, Tuori M, Saastamoinen I, Taponen J, Wähälä K, Saloniemi H, Vanhatalo A. Equol in milk of dairy cows is derived from forage legumes such as red clover. Br J Nutr. 2009;102:1552–1556. doi: 10.1017/S0007114509990857. [DOI] [PubMed] [Google Scholar]
- Ness AR, Powles JW. Fruit and vegetables, and cardiovascular disease: a review. Int J Epidemiol. 1997;26:1–13. doi: 10.1093/ije/26.1.1. [DOI] [PubMed] [Google Scholar]
- O’Connell JE, Fox PF. Significance and applications of phenolic compounds in the production and quality of milk and dairy products: a review. Int Dairy J. 2001;11:103–120. doi: 10.1016/S0958-6946(01)00033-4. [DOI] [Google Scholar]
- Ovaskainen ML, Törrönen R, Koponen JM, Sinkko H, Hellström J, Reinivuo H, Mattila P. Dietary intake and major food sources of polyphenols in Finnish adults. J Nutr. 2008;138:562–566. doi: 10.1093/jn/138.3.562. [DOI] [PubMed] [Google Scholar]
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang G, Xie J, Chen Q, Hu J. How functional foods play critical roles in human health. Food Sci Hum Wellness. 2012;1:26–60. doi: 10.1016/j.fshw.2012.10.001. [DOI] [Google Scholar]
- Patel VR, Agrawal YK. Nanosuspension: an approach to enhance solubility of drugs. J Adv Pharm Technol Res. 2011;2:81–87. doi: 10.4103/2231-4040.79799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Jiménez J, Fezeu L, Touvier M, Arnault N, Manach C, Hercberg S, Galan P, Scalbert A. Dietary intake of 337 polyphenols in French adults. Am J Clin Nutr. 2011;93:1220–1228. doi: 10.3945/ajcn.110.007096. [DOI] [PubMed] [Google Scholar]
- Polychniatou V, Tzia C. Study of the emulsifying ability of olive oil endogenous compounds in co-surfactant free olive oil w/o nanoemulsions with food grade non-ionic surfactants. Food Bioprocess Technol. 2016;9:882–891. doi: 10.1007/s11947-015-1668-8. [DOI] [Google Scholar]
- Rashidinejad A, Birch EJ, Sun-Waterhouse D, Everett DW. Delivery of green tea catechin and epigallocatechin gallate in liposomes incorporated into low-fat hard cheese. Food Chem. 2014;156:176–183. doi: 10.1016/j.foodchem.2014.01.115. [DOI] [PubMed] [Google Scholar]
- Reynertson KA (2007) Phytochemical analysis of bioactive constituents from edible Myrtaceae fruits. Thesis-Graduate Faculty in Biology—City University of New York
- Reynertson KA, Wallace AM, Adachi S, Gil RR, Yang H, Basile MJ, D’Armiento J, Weinstein IB, Kennelly EJ. Bioactive depsides and anthocyanins from jaboticaba (Myrciaria cauliflora) J Nat Prod. 2006;69:1228–1230. doi: 10.1021/np0600999. [DOI] [PubMed] [Google Scholar]
- Richards MP, Chaiyasit W, McClements DJ, Decker EA. Ability of surfactant micelles to alter the partitioning of phenolic antioxidants in oil-in-water emulsions. J Agric Food Chem. 2002;50:1254–1259. doi: 10.1021/jf011324p. [DOI] [PubMed] [Google Scholar]
- Rufino MSR, Alves RE, de Brito ES, Pérez-Jiménez J, Saura-Calixto F, Mancini-Filho J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010;121:996–1002. doi: 10.1016/j.foodchem.2010.01.037. [DOI] [Google Scholar]
- Santos NW, Santos GTD, Silva-Kazama DC, Grande PA, De Marchi FE, Jobim CC, Petit HV. Production, composition and antioxidants in milk of dairy cows fed diets containing soybean oil and grape residue silage. Livest Sci. 2014;159:37–45. doi: 10.1016/j.livsci.2013.11.015. [DOI] [Google Scholar]
- Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- Servili M, Esposto S, Fabiani R, Urbani S, Taticchi A, Mariucci F, Selvaggini R, Montedoro GF. Phenolic compounds in olive oil: antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology. 2009;17:76–84. doi: 10.1007/s10787-008-8014-y. [DOI] [PubMed] [Google Scholar]
- Servili M, Rizzello CG, Taticchi A, Esposto S, Urbani S, Mazzacane F, Di Maio I, Gobbetti M, Di Cagno R. Functional milk beverage fortified with phenolic compounds extracted from olive vegetation water, and fermented with functional lactic acid bacteria. Int J Food Microbiol. 2011;147:45–52. doi: 10.1016/j.ijfoodmicro.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Shahidi F. Nutraceuticals and functional foods: whole versus processed foods. Trends Food Sci Technol. 2009;20:376–387. doi: 10.1016/j.tifs.2008.08.004. [DOI] [Google Scholar]
- Sharma OP, Bhat TK. DPPH antioxidant assay revisited. Food Chem. 2009;113:1202–1205. doi: 10.1016/j.foodchem.2008.08.008. [DOI] [Google Scholar]
- Silva GJF, Figueiredo RW, Moura SM. Formulação e estabilidade de corantes de antocianinas extraídas das casas de jabuticaba (Myrciaria ssp.) Alim Nutr Araraquara. 2010;21:429–436. [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Souza ACP, Gurak PD, Marczak LDF. Maltodextrin, pectin and soy protein isolate as carrier agents in the encapsulation of anthocyanins-rich extract from jaboticaba pomace. Food Bioprod Process. 2017;102:186–194. doi: 10.1016/j.fbp.2016.12.012. [DOI] [Google Scholar]
- Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc. 1996;96:1027–1039. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- Sutradhar KB, Amin L. Nanoemulsions: increasing possibilities in drug delivery. Eur J Nanomed. 2013;5:97–110. doi: 10.1515/ejnm-2013-0001. [DOI] [Google Scholar]
- Tresserra-Rimbau A, Medina-Remón A, PérezJiménez J, Martínez-González MA, Covas MI, Corella D, Salas-Salvado J, Gómez-Gracia E, Lapetra J, Arós F, Fiol M, Ros E, Serra-Majem L, Pintó X, Muñoz MA, Saez GT, Ruiz-Gutiérrez V, Warnberg J, Estruch R, Lamuela-Raventós RM. Dietary intake and major food sources of polyphenols in a Spanish population at high cardiovascular risk: the PREDIMED study. Nutr Metab Cardiovasc Dis. 2013;23:953–959. doi: 10.1016/j.numecd.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Wrolstad RE, Durst RW, Lee J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci Technol. 2005;16:423–428. doi: 10.1016/j.tifs.2005.03.019. [DOI] [Google Scholar]
- Yilmaz Y. Novel uses of catechins in foods. Trends Food Sci Technol. 2006;17:64–71. doi: 10.1016/j.tifs.2005.10.005. [DOI] [Google Scholar]


