Abstract
Milk components have the ability to interact with functional compounds, such as polyphenols. This may result in altered biological activity of the compounds, and changes in the technological properties of dairy products. The objective of this study was to examine the effect of the addition of yerba mate (YM) on the physico-chemical and sensory properties of fresh cheese (FC) during storage (21 days). Different concentrations of YM were used for FC production: 0.0% (control), 0.5% (FC5), 1.0% (FC10) and 2.0% (FC20); and the bioactive compound concentration, antioxidant activity, color, texture, structure and sensory acceptance were evaluated. YM conferred antioxidant activity to FC; and affected the color, texture and structure. Storage time influenced all evaluated parameters except for color. FCs with 0.5 and 1.0% YM were scored as having higher sensory acceptability than FC with 2.0% YM. However, all supplemented FCs were well accepted by consumers (scores above 6). The YM could represent a source of bioactive compounds to improve the biological activity of dairy products.
Keywords: Bioactive compounds, Dairy products, Natural antioxidants, Texture
Introduction
Functional foods have attracted consumers who believe that there is a connection between disease prevention and food. When consumed as part of a usual diet; in addition to the basic nutritional composition provided by these foods, compounds such as bioactive compounds can contribute beneficial effects for health (Gul et al. 2016). These compounds, whether synthetic or natural in origin, can exhibit different functional properties such as antioxidant activity (Giroux et al. 2013; Han et al. 2011a). Natural antioxidants are generally safe for use in food products, whereas some synthetic alternatives can have harmful effects on health (Yıldırım et al. 2001; Zheng and Wang 2001).
Many plants produce phenolic compounds with antioxidant activity (Brewer 2011), which are the subject of considerable research interest. One such example is Ilex paraguariensis, a native plant of the subtropical and temperate regions of South America; such as Argentina, Brazil, Uruguay and Paraguay (Cardozo Junior and Morand 2016). It is known as yerba mate (YM), and commonly sold as a product of the dried and crushed leaves for consumption as a hot or cold infusion (chimarrão or tererê, respectively) prepared with water (Filip et al. 2000a). The highest per capita consumption of YM is observed in Uruguay (8–10 kg/capita/year); followed by Argentina (around 6.5 kg/capita/year) then the southern region of Brazil (3–5 kg/capita/year) (Cardozo Junior and Morand 2016).
The phenolic compounds of YM are mainly represented by chlorogenic acids, flavonoids and saponins (Mejía et al. 2010), that together with methylxanthines are responsible for the high antioxidant capacity and physiological effects of YM (Riachi and Maria 2017). In addition to these phytochemicals, YM contains nutrients (proteins and carbohydrates), minerals (potassium, magnesium, phosphorus, calcium, sulfur, manganese, zinc, iron and copper) and vitamins (primarily C, B1, B2 and B6) (Cardozo Junior and Morand 2016).
The pharmacological properties of YM have long been known, and utilized in popular medicine for the treatment of digestive problems, liver diseases, obesity and headache. The Food Code of Argentina and Latin American recognize YM as a stimulant, diuretic, antirheumatic, lipolytic, tonic and digestive and chronotropic agent (Filip et al. 2000b).
Food enrichment can involve the addition of nutrients such as proteins, minerals, vitamins and carbohydrates; or biologically active compounds such as phenolic compounds (Livney 2010). Addition of phenolic compounds to dairy products enables alteration of their rheological and structural properties (Han et al. 2011a; Vital et al. 2015). Proteins can interact with phenolic compounds through hydrophobic or hydrophilic interactions to form complexes, which may be soluble or insoluble (Bandyopadhyay et al. 2012). The formation of polyphenol-protein complexes may reduce or enhance the antioxidant activity of polyphenols, as well as potentially altering the properties of both (Bandyopadhyay et al. 2012).
Gel maintenance is achieved in cheese processing through the formation of interactions between amino acids of the milk proteins with surrounding solvent molecules (Lucey 2002). The addition of functional compounds to milk may alter these interacting properties, affecting the cheese-making process and the product characteristics such as flavor, color and odor (Han et al. 2011b). The objective of this study was to analyze the effects of the enrichment of fresh cheese with yerba mate on the physical and sensorial characteristics of the cheese.
Materials and methods
Materials and reagents
Yerba mate (Chimarrão 81 Premium, Guarapuava, Brazil), pasteurized whole milk (Lacto Bom, Toledo, Brazil) and chymosin enzyme (HA-LA®, CHR Hansen, Danmark) were procured from a local supplier. Folin-Ciocalteu reagent, gallic acid, 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,2-Diphenyl-1-picrylhydrazyl (DPPH), methyl alcohol, hydrochloric acid, trichloroacetic acid, phosphate buffer, potassium persulfate, sodium carbonate and commercial standards of caffeic acid, chlorogenic acid, catechin, high performance liquid chromatography (HPLC) grade methanol and acetic acid were purchased from Sigma Aldrich (São Paulo, Brazil). Potassium ferricyanide, aluminum chloride and ferric chloride were of analytical grade.
Preparation of extracts
Standardized samples of YM were prepared at a mesh size of 60. Methanol (100%) (9 mL) was added to YM (1 g), homogenized for 10 min and centrifuged at 3000 rpm for 10 min. The supernatant was recovered and diluted in Methanol (1:1500; v/v) to use for analyses of bioactive compounds and antioxidant activity.
To prepare the fresh cheese (FC) extract, methanol was added in FC (1:10; w/v) and the mixture homogenized for 10 min and centrifuged for 10 min at 3000 rpm. The supernatant was recovered for analyses of bioactive compounds and antioxidant activity.
Bioactive compounds
Total phenolic compounds
Total phenolic compounds (TPC) were determined according to the protocol of Singleton and Rossi (1965), with some modifications. Extract (125 µL) was mixed with an equivalent volume of Folin-Ciocalteu reagent (diluted 1:1 in deionized water) and 2.25 mL sodium carbonate (28 g/L). The mixture was incubated in the dark for 30 min, then the absorbance measured at 725 nm using a spectrophotometer (Evolution™ 300, Thermo Fisher Scientific, UK). A standard absorbance curve was prepared using 0–300 mg/L gallic acid, and results were obtained by interpolation into this curve and expressed as mg gallic acid equivalent (GAE)/g.
Antioxidant activity
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) assay
The antioxidant activity was determined by 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay according to the protocol of Re et al. (1999), with some modifications. The ABTS+ cation was formed by incubating ABTS (7 mM) with potassium persulfate (140 mM) for 16 h at room temperature in dark conditions. The ABTS activated radical was diluted with ethanol until an absorbance of 0.70 ± 0.02 was achieved, and 1960 µL of the resulting solution mixed 40 µL of with extract. The absorbance at 734 nm was measured after 6 min and the radical scavenging activity (%) was calculated using Eq. 1:
| 1 |
where = absorbance of the sample at 6 min, and = absorbance of the sample at time zero.
Ferric reducing antioxidant power
The ferric reducing antioxidant power (FRAP) was determined according to the published protocol of Zhu et al. (2002). Extract (250 µL) was added to 1.25 mL of 50 mM sodium phosphate buffer pH 7.0 and 1.25 mL of 1% potassium ferricyanide. The mixture was incubated at 50 °C for 20 min. Next, 1.25 mL of trichloroacetic acid (10%) was added and the mixture was centrifuged at 3000 rpm for 10 min. The supernatant (2.5 mL) was mixed with 500 µL of ferric chloride (0.1%) and the absorbance at 700 nm read immediately. Results were expressed as mg GAE/g, determined using the standard curve of gallic acid from 0 to 300 mg/L.
2,2-Diphenyl-1-picrylhydrazyl assay
The antioxidant activity was determined by 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay according to Li et al. (2009), with some modifications. Extract (150 μL) was mixed with DPPH solution (2.85 mL) (60 μM) for 10 s, was incubated for 30 min in dark conditions, and the absorbance measured at 515 nm. The antioxidant activity was calculated using Eq. 2:
| 2 |
where = absorbance of the samples at 30 min, and = absorbance of the sample at time zero.
The result was expressed as the minimum concentration of compound required to inhibit activity by 50% (IC50).
High performance liquid chromatography
New extraction of the phenolic compounds of YM was performed for HPLC analysis using methanol diluted in water (80:20; v/v) in a ratio of 1:10 (YM:methanol; w/v). The mixture was homogenized for 10 min and centrifuged for 10 min at 3000 rpm. The supernatant (3 mL) was diluted with 20% methanol and passed into a solid phase extraction cassette (Oasis HLB, Waters, Barueri, SP, Brazil) which had been previously conditioned with pure methanol and ultrapure water. After a water wash step, compounds were eluted with 80% methanol, and 10 μL of the eluent was injected into an HPLC system (Alliance Waters e2695, Waters corporation, Micromass Ltd, UK), equipped with a separation module, quaternary pump and photodiode detector. Separation was achieved using a reverse-phase C18 column (Shim-pack CLC-ODS, 250 mm × 4.6 mm × 5 μm) at room temperature. The mobile phase consisted of water in 2% acetic acid (solvent A) and methanol in 2% acetic acid (solvent B) in a gradient, as described by Deladino et al. (2013) in flow 0.9 mL/min. Detection of the compounds was performed between 255 and 330 nm. Phenolic compounds were identified and quantified by interpolation with standard curves prepared using commercial caffeine standards, caffeic acid, chlorogenic acid and catechin.
Fresh cheese production
Milk used for the production of fresh cheese had the following composition: 3.4% protein, 3.4% fat, 4.0% carbohydrate (lactose), 0.10% calcium and 0.05% sodium. The pH was 6.56. Four formulations of FC were trialed: without YM (control), and with 0.5%, 1.0% and 2.0% YM (FC5, FC10 and FC20, respectively). The percentages of YM were determined by previous trials, where it was found that milk enriched with more than 2% of YM did not form a gel (data not shown). For the FC control, milk was heated to 35 °C and calcium chloride (1 g/L milk) was added. Chymosin (0.06 g/40 L) was then added, the solution mixed for 15 s and rested for 60 min at 35 °C to allow coagulation. After coagulation, the cheese mass was cut into cubes of 0.5 cm3, the cubes rested for 30 min at 35 °C and then transferred to stainless steel containers where they remained for 60 min at 4 °C for whey expulsion. The FC was packed using a vacuum packer (Sulpack SVC 620, Brazil, operated for 15 s), so as not to alter the structure. The resulting packed FC was stored at 4 °C until the analyses at 1, 7, 14 and 21 days of storage. For the production of formulations which included YM, the milk was homogenized with YM for 15 min prior to the heating step (35 °C). The procedures were the same as for the control formulation from thereon.
Physical and sensory analysis of fresh cheese
The color of FC was evaluated according to the Commission Internationale de l’Eclairage (CIELab) color scale. The parameters of L* (100, white; 0, black), a* (+, red; −, green) and b* (+, yellow; −, blue) were measured with a colorimeter (Chroma Meter CR-400, Minolta, USA) and the illuminant D65 was used as a reference with a 10° observer.
Texture profile analysis (TPA) was performed on whole FC (with a diameter of 7.0 cm and height of 1.2 cm) using a Brookfield Texture Analyzer CT-III. An acrylic circular probe was used (with a diameter of 38.1 mm and height of 20 mm) with a trigger force of 10 g, speed of 1 mm/s and compression distance of 5.0 mm. The hardness, chewiness, cohesiveness and gumminess were evaluated.
Microstructural analysis was performed according to the protocols of Matumoto-Pintro et al. (2011). The FC samples were frozen with liquid nitrogen, lyophilized (Christ Alpha 1–4 LD plus, Marin Christ, Germany), mounted in an aluminum stub on carbon tape and coated with a gold layer (Sputter coater, Baltec SCD 050, Balzers, Liechtenstein). Observations were recorded using a scanning electron microscope (SEM) (Quanta 250, FEI, Hillsboro, OR, EUA) operated at 15 kV and 10 µm.
Sensory analysis included evaluation of the acceptability of FC5, FC10 and FC20. This analysis was approved by the Research Ethics Committee of the State University of Maringa (CAAE: 55,919,816.4.0000.0104).
To simulate market conditions, the cheese was salted (15%; w/v with common salt) for 10 min before analysis. Triangular-shaped samples (3.5 cm high × 1.35 cm of base) were wrapped in aluminum foil and coded with random three-digit numbers. The acceptability was evaluated according to the methodology described by Meilgaard et al. (1999) with 117 consumers including students, professors, employees and visitors of the State University of Maringa. Consumers evaluated the attributes of color, flavor, odor, texture and global appearance, using a semi-structured hedonic scale of nine points. The acceptance index (AI) was calculated for each sample according to Dick et al. (2011) (Eq. 3):
| 3 |
where x = mean score of the sample, and n = highest score of the sample.
Microbiological analyses were performed to ensure hygiene and sanitary quality were maintained during the manufacturing and storage processes of FC that was intended for sensory analysis. The number of total coliforms, Staphylococcus aureus and the presence or absence of Salmonella spp were determined using 3 M Petrifilm™ (St. Paul, MN 55144, USA) according to the manufacturer’s instructions.
Statistical analysis
Each experiment was performed in triplicate. Analysis of variance (ANOVA) was performed using the general linear model with SPSS (v.19.0) (IBM SPSS Statistics, SPSS Inc., Chicago, USA) for Windows. Means and standard deviations were calculated for each variable. Concentrations of YM and storage time were considered fixed factors in the factorial design. Differences were considered significant at p < 0.05 using the Tukey test. Sensory analysis data were evaluated by ANOVA and Tukey’s test for the means comparison (p < 0.05). Principal Components Analysis (PCA) was used to verify correlations between treatments and acceptability attributes.
Results and discussion
Phenolic compounds and antioxidant activity of the YM
The high antioxidant activity of yerba mate is mainly due to its phenolic compounds (Riachi and Maria 2017). The present study revealed the TPC content to be 99.26 ± 7.42 mg GAE/g (Table 1), which is in agreement with the findings of Valerga et al. (2012) who studied leaf extracts of plants from Corrientes (Argentina) produced using a mixture of acetone and water (TPC content: 96.07 mg GAE/g dry leaves). Mejía et al. (2010) evaluated the phenolic compounds of I. paraguariensis originating from different places and obtained values of 90.40 mg GAE/g of dried leaves for plants from Buenos Aires, and 133.00 mg GAE/g of dried leaves for plants from Paraguay. These differences in the concentrations of phytochemicals are likely due to differences that arise according to maturity, climatic conditions and cultivation (Kumar et al. 2017).
Table 1.
Radical scavenging by free radical scavenging
| Storage (days) | Treatments | TPC1 (mg GAE5/g) | ABTS2 (%) | DPPH3 (%) | FRAP4 (mg GAE/g) |
|---|---|---|---|---|---|
| 1 | YM | 99.26 ± 7.42 | 14.35 ± 0.09 | 0.21 ± 0.006 | 22.17 ± 0.92 |
| Control | 0.62 ± 0.07aC | 14.59 ± 0.57aD | 2.93 ± 0.10aD | 0.14 ± 0.01aC | |
| FC5 | 1.08 ± 0.04aBC | 29.12 ± 0.28aC | 36.78 ± 0.19aC | 0.44 ± 0.03aB | |
| FC10 | 1.40 ± 0.25aB | 38.76 ± 2.18aB | 67.30 ± 1.35aB | 0.66 ± 0.11aB | |
| FC20 | 2.49 ± 0.00aA | 61.24 ± 1.04aA | 94.89 ± 0.10bA | 1.24 ± 0.04aA | |
| 21 | Control | 0.44 ± 0.05bB | 10.61 ± 0.19aC | 2.25 ± 0.67bD | 0.11 ± 0.02bC |
| FC5 | 1.00 ± 0.05bAB | 21.56 ± 1.83aB | 32.52 ± 3.34bC | 0.38 ± 0.02bB | |
| FC10 | 1.21 ± 0.33bA | 36.53 ± 0.87bA | 66.55 ± 3.37bB | 0.59 ± 0.00bAB | |
| FC20 | 1.27 ± 0.02bA | 34.22 ± 0.67bA | 95.57 ± 0.29aA | 0.66 ± 0.11bA |
Control: without YM; FC5: enriched with 0.50% of YM; FC10: enriched with 1.0% of YM; FC20: enriched with 2.0% of YM. 1TPC: total phenolic compounds; 2ABTS: ABTS radical scavenging; 3DPPH: DPPH free radical scavenging; 4FRAP: ferric reduction antioxidant power; 5GAE: gallic acid equivalent. Results are expressed as mean ± standard deviation. Different lowercase letters in the same column are significantly different (p < 0.05) for storage days. Different uppercase letters in the same column are significantly different (p < 0.05) for treatments. 6For YM the result was expressed as IC50 (μg/mL)(inhibitory minimum concentration of compound activity by 50%)
Chlorogenic acids such as caffeoylquinic, dicaffeoylquinic and feruloylquinic and caffeine acids are present in high quantities in YM (Riachi and Maria 2017). The six most abundant compounds are the mono-(3-O-caffeoylquinic acid, 5-O-caffeoylquinic acid and 4-O-caffeoylquinic acid) and di-caffeoylquinic acids (3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid and 4,5-dicaffeoylquinic acid) (Cardozo Junior and Morand 2016).
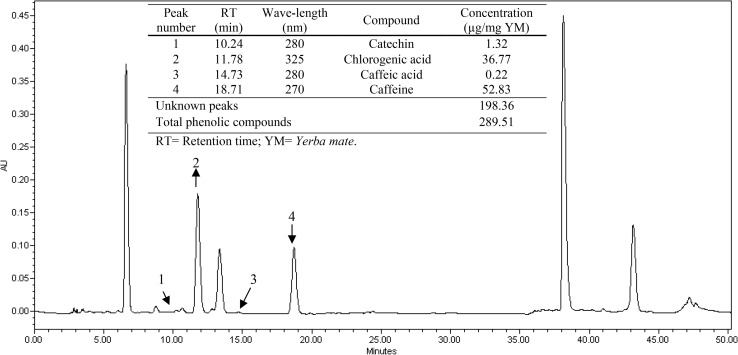
The YM extracts were also analyzed by HPLC (Fig. 1). Catechin [retention time (rt) = 10.24 min], 5-O-caffeoylquinic acid (chlorogenic acid; rt = 11.78 min), caffeic acid (rt = 14.73 min) and caffeine (rt = 18.71 min) were identified in the extract. The concentrations of caffeine, chlorogenic acid, catechin, and caffeic acid were calculated to be 52.83, 36.77, 1.32 and 0.22 μg/mg YM, respectively. Some peaks were only quantified.
Fig. 1.
Chromatographic profiles (HPLC) of YM extract
Deladino et al. (2013) found that aqueous extracts of I. paraguariensis (2% w/v) had lower concentrations of chlorogenic acid (20.90 ± 1.1 mg/g YM extract) and caffeine (14.7 ± 0.7 mg/g YM extract), while Filip et al. (2000b) reported the most abundant compound to be chlorogenic acid (39.0 ± 1.4 mg/100 g dry leaves) with only low levels of caffeic acid (2.0 ± 1.4 mg/100 g dry leaves) from analysis of the aqueous extract of I. paraguariensis (30% w/v).
The ABTS and FRAP assays in the present study revealed the antioxidant properties of YM extract, by demonstrating the free radical scavenging ability to be 14.35% ± 0.09 and 22.17 ± 0.92 mg GAE/g, respectively. A small concentration of YM extract (0.21 μg/mL) was able to scavenge 50% of the DPPH free radical. This is in contrast to previously published results, in which Schinella et al. (2009) reported that a high concentration of aqueous extract of I. paraguariensis (7.7 μg/mL) was required to achieve 50% scavenging of the same radical. According to Guangrong et al. (2008) the IC50 value for 2.6-bis(1.1-dimethylethyl)-4-methylphenol (BHT), a synthetic antioxidant widely used in the food industry, was 19.4 μg/mL. This is higher than the IC50 value for YM presented in this study, which indicates that higher concentrations of synthetic antioxidants are required to sequester 50% of the radical compared with natural compounds.
Our results show that the high antioxidant activity of YM make it suitable for use as a natural antioxidant, to replace synthetic compounds or to be used in combination in order to reduce consumption of synthetic chemicals.
Phenolic compounds and antioxidant activity of fresh cheese
The concentrations of bioactive compounds and antioxidant activity were influenced by storage time (Table 1), with a reduction in both parameters observed at day 21 (p < 0.05). This may due to formation of polyphenol-protein complexes, and consequent reduction in polyphenol recovery (Lamothe et al. 2014). Significant differences were observed between FC that included YM and the control, with the TPC and antioxidant activity or FC found to be positively correlated with the concentration of YM (p < 0.05) in both periods analyzed (1 and 21 days). This indicates that the phenolic compounds and antioxidant activity of YM were retained in FC, highlighting YM as a good candidate for food enrichment.
Supplementation of low-fat ultra-filtered soft cheese with rosemary extract (a natural antioxidant) at concentrations of 1, 2, 3, and 5% (v/v) has been reported; and the cheese fortified with 1% rosemary extract was found to retain a greater proportion of the TPC and antioxidant activity of rosemary (Hala et al. 2010). These values decreased following 30 days of storage, as was observed in the case of FC enriched with YM in the present study. Cheddar-type cheese enriched with green tea extract has been shown to exhibit increased antioxidant activity by 25–44% compared with cheese without enrichment (Giroux et al. 2013). In other dairy products such as cream cheese, the antioxidant properties of Agaricus bohusii have been exploited to preserve the cheese (Reis et al. 2012).
The use of bioactive compounds as natural antioxidants in food products has been extensively studied, and YM has been added to dairy products in order to increase the antioxidant activity. An example of this is light yogurt, in which lyophilized YM extract added in small concentrations (0.125 and 0.25%) was able to confer antioxidant activity to the product, inhibiting its lipid oxidation (Preci et al. 2011). In fermented milk, the addition of 1% lyophilized herbal extract, composed of 87.5% clove and 12.5% YM, increased the antioxidant activity and TPC of the milk compared with natural fermented milk (Ramos et al. 2017).
Physical characterization of fresh cheese
The TPA of FC revealed a significant increase (p < 0.05) in hardness up to 14 days of storage for all treatments (Table 2), while the hardness of FC20 continued to increase for the full 21 days of storage. This can be explained by the interaction of the phenolic compounds and milk proteins, as the strength of the bonds and force required to compress the FC increases due to the greater cohesion between the particles. The cohesiveness was significantly different (p < 0.05) between the treatments after 14 days of the storage, with FC10 and FC20 exhibiting the highest cohesiveness.
Table 2.
Texture profile analysis (TPA) of the fresh cheese (FC) enriched with yerba mate (YM)
| Treatments | Storage (days) | |||
|---|---|---|---|---|
| 1 | 7 | 14 | 21 | |
| Hardness (g) | ||||
| Control | 615.00 ± 7.07cB | 922.50 ± 38.89bA | 1387.50 ± 53.03aA | 1020.00b ± 21.21bC |
| FC5 | 740.00 ± 63.64cB | 1067.50 ± 95.45bA | 1455.00 ± 14.14aA | 1027.50 ± 3.53bC |
| FC10 | 702.50 ± 3.53bB | 1140.00 ± 289.91abA | 1340.00 ± 0.00aA | 1320.00 ± 77.78aB |
| FC20 | 935.00 ± 7.07cA | 1260.00 ± 49.50bA | 1300.00 ± 113.14bA | 1785.00 ± 77.78aA |
| Chewiness (mJ) | ||||
| Control | 18.50 ± 0.85cB | 31.60 ± 1.84bA | 40.70 ± 0.99aA | 22.45 ± 0.21cC |
| FC5 | 23.75 ± 2.47aAB | 33.15 ± 0.64aA | 36.15 ± 7.00aA | 28.20 ± 1.70aC |
| FC10 | 20.00 ± 0.14 dB | 27.25 ± 1.06cA | 43.15 ± 0.78bA | 48.80 ± 0.14aB |
| FC20 | 27.20 ± 0.14cA | 38.10 ± 6.22bcA | 44.65 ± 1.48bA | 59.00 ± 2.54aA |
| Cohesiveness | ||||
| Control | 0.73 ± 0.02abA | 0.76 ± 0.01aA | 0.70 ± 0.00bB | 0.57 ± 0.01cC |
| FC5 | 0.71 ± 0.01abA | 0.74 ± 0.00aA | 0.69 ± 0.21abB | 0.67 ± 0.01bB |
| FC10 | 0.74 ± 0.01abA | 0.72 ± 0.00bA | 0.76 ± 0.01aA | 0.74 ± 0.01abA |
| FC20 | 0.73 ± 0.01aA | 0.73 ± 0.03aA | 0.78 ± 0.02aA | 0.77 ± 0.01aA |
| Gumminess (g) | ||||
| Control | 461.00 ± 7.07cB | 692.00 ± 60.81bA | 971.00 ± 41.01aA | 582.00 ± 5.66bcC |
| FC5 | 526.00 ± 50.91cB | 758.5 ± 30.40bA | 1041.00 ± 66.47aA | 634.00 ± 15.56bcC |
| FC10 | 522.00 ± 7.07bB | 843.50 ± 174.65abA | 1009.5 ± 33.23aA | 884.00 ± 94.75abB |
| FC20 | 715.50 ± 6.36cA | 951.00 ± 110.31bcA | 1120.00 ± 83.44bA | 1423.50 ± 21.92aA |
Control: without YM; FC5: enriched with 0.50% of YM; FC10: enriched with 1.0% of YM; FC20: enriched with 2.0% of YM. Results are expressed as mean ± standard deviation. Different lowercase letters in the same line are significantly different (p < 0.05). Different uppercase letters in the same column are significantly different (p < 0.05)
The chewiness increased in line with storage time, except for the control and FC5 samples, which decreased on day 21. Gumminess showed the same trend, with values increasing significantly (p < 0.05) up to day 14 and decreasing at day 21 for all samples except FC20, which showed increased gumminess at all time points. The decrease in chewiness and gumminess on day 21 of storage can be explained by proteolysis of the cheese and the beginning of degradation processes. At day 21, the pH was lower in all samples compared with the first day, reaching a value of 5.28. The results showed that YM was able to interact with FC to change the texture parameters, resulting in higher cohesiveness and gumminess. Green tea extract (0.1%) has also been used for cheese enrichment to increase the parameters of hardness, cohesiveness and springiness (Lamothe et al. 2014). Furthermore, green tea extract has been shown to increase the hardness of cheddar-type cheese and decrease the cohesiveness and springiness (Giroux et al. 2013).
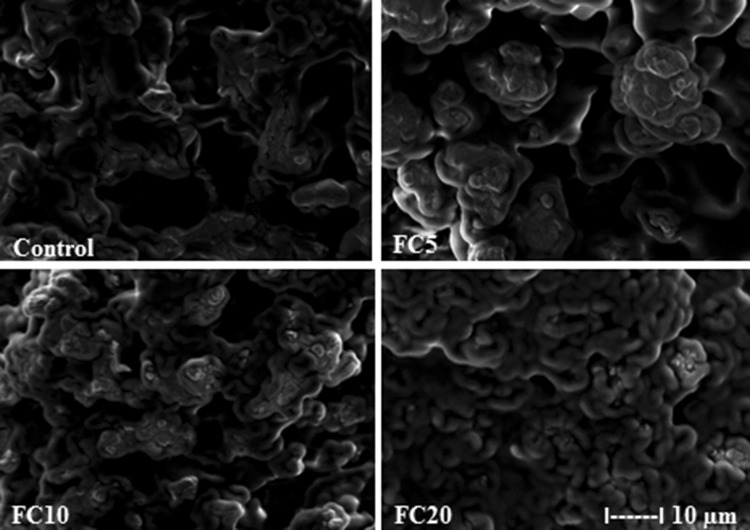
The effects of YM on the structure of FC are illustrated in Fig. 2. Microscopy analysis revealed the structure of the control sample to be more continuous, with larger voids observed between the protein networks compare with YM-added samples. With increasing YM concentration, the voids in the FC structure became smaller. The FC5, FC10 and FC20 preparations exhibited a particularly compact protein network, which is probably due to the interaction between milk proteins and polyphenols of the YM (Livney 2010; Vital et al. 2015; Yuksel et al. 2010). This effect was most obvious in FC20, presumably due to the higher amount of polyphenols from the increased concentration of YM. Similar observations have been reported regarding the microstructure of yogurt supplemented with Pleurotus ostreatus (PO) aqueous extract, in which the control displayed a branched network with large void spaces, while more compact microstructures were observed with increasing concentrations of PO extract (Vital et al. 2015).
Fig. 2.
Scanning electron microscopy (SEM) images of fresh cheese (FC) enriched with yerba mate (YM). Control: without YM; FC5: enriched with 0.50% of YM; FC10: enriched with 1.0% of YM; FC20: enriched with 2.0% of YM
Color and sensory acceptability of fresh cheese
When using natural antioxidants in foods, the sensory impact and quality of the final product is an important consideration. During storage, all samples showed the same behavior with respect to color (p < 0.05), indicating that the storage does not significantly influence the product color. However, the L* and a* values decreased as concentrations of YM in the FC increased, while b * increased. The result was that higher concentrations of YM caused the cheese to have a light green color, characteristic of YM (data not shown).
Microbiology analysis that was conducted prior to sensory evaluation showed no microorganism counts for coliforms, S. aureus and Salmonella spp for any of the formulations. The acceptability was not found to be significantly different (p < 0.05) between FC5 and FC10 for any of the attributes (Table 3). However, FC20 was rated by consumers to be less acceptable (p < 0.05) in all attributes except for texture (which was deemed to be similar to F5). In general, all samples were scored between 6 (“like lightly”) and 7 (“like moderately”); except in the case of FC20, where the color, flavor and global acceptance attributes were scored below 6. The acceptance indexes were 76.16, 72.55 and 61.63% for FC5, FC10 and FC20 respectively; demonstrating that all formulations had an acceptance of more than 50%. Another study in which YM extract was used to supplement a mature cheese (Prato cheese) at low concentrations (0.1 and 0.2%) reported an acceptance of 80% (after 30 days maturation of cheese with 0.1% of extract) (Faion et al. 2015).
Table 3.
Sensory attributes of fresh cheese (FC) enriched with yerba mate (YM)
| Sensory attributes | FC5 | FC10 | FC20 |
|---|---|---|---|
| Color | 7.00 ± 1.23a | 6.55 ± 1.46a | 5.56 ± 1.92b |
| Flavor | 6.62 ± 1.78a | 6.38 ± 1.99a | 5.40 ± 2.28b |
| Odor | 6.83 ± 1.36a | 6.79 ± 1.37a | 6.17 ± 1.78b |
| Texture | 6.80 ± 1.91ab | 7.00 ± 1.66a | 6.36 ± 2.07b |
| Global acceptance | 6.85 ± 1.60a | 6.53 ± 1.70a | 5.55 ± 2.12b |
FC5: enriched with 0.50% of YM; FC10: enriched with 1.0% of YM; FC20: enriched with 2.0% of YM. Results are expressed as mean ± standard deviation. Different lowercase letters in the same line are significantly different (p < 0.05)
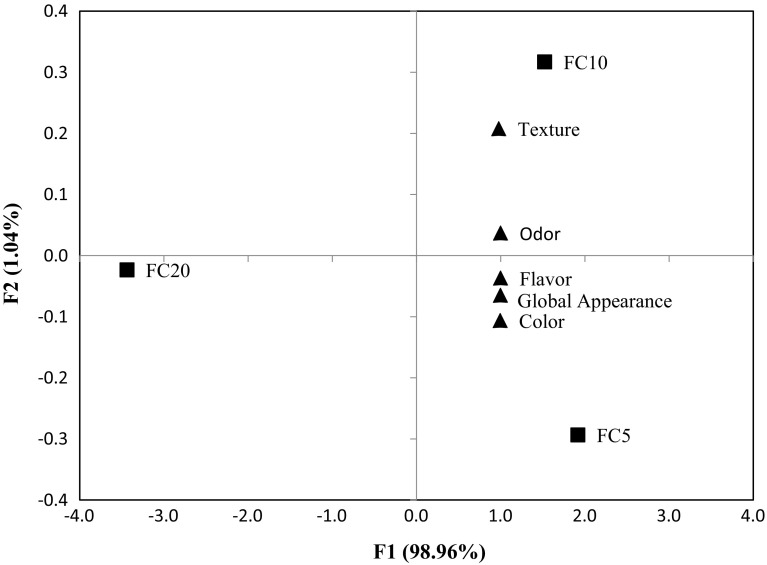
In the PCA (Fig. 3); flavor, global appearance and color are on the right side of F1, located close to FC5. The location of FC10 is also on the right side of F1, close to texture and odor. The FC20 sample is placed on the left of F1, indicating inverse relationships to the sensory attributes. This demonstrates the greater influence of attributes in the FC5 and FC10 formulations, which were evaluated to be more acceptable. The two principal components axes explained 100% of the total variance.
Fig. 3.
Principal component analysis of the scores for color, flavor, odor, texture and global appearance of fresh cheese (FC) enriched with yerba mate (YM). ■: FC treatments (FC5 = enriched with 0.50% of YM; FC10 = enriched with 1.0% of YM; FC20 = enriched with 2.0% of YM). ▲: sensory attributes
Conclusion
Yerba mate was found to contain a high content of phenolic compounds and exhibit antioxidant activity; properties which were conferred to fresh cheese which was supplemented with YM. Both the concentration of YM that was added to FC and the storage time modified the texture of the cheese, increasing its hardness due to interactions between polyphenols and milk proteins. The structure of the protein network and color of the FC were also altered by the addition of YM, and sensory analysis showed that supplementation with low concentrations of YM (FC5 and FC10) were well acceptable to consumers. The YM represent a source of bioactive compounds to improve the biological activity of FC.
Acknowledgements
We thank National Council for Scientific and Technological Development (CNPq) for scholarship.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bandyopadhyay P, Ghosh AK, Ghosh C. Recent developments on polyphenol–protein interactions: effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. 2012;3:592. doi: 10.1039/c2fo00006g. [DOI] [PubMed] [Google Scholar]
- Brewer MS. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Saf. 2011;10:221–247. doi: 10.1111/j.1541-4337.2011.00156.x. [DOI] [Google Scholar]
- Cardozo Junior EL, Morand C. Interest of mate (Ilex paraguariensis A. St.-Hil.) as a new natural functional food to preserve human cardiovascular health—a review. J Funct Foods. 2016;21:440–454. doi: 10.1016/j.jff.2015.12.010. [DOI] [Google Scholar]
- Deladino L, Teixeira A, Reta M, Molina García AD, Navarro AS, Martino MN. Major phenolics in yerba mate extracts (Ilex paraguariensis) and their contribution to the total antioxidant capacity. Food Nutr Sci. 2013;4:154–162. [Google Scholar]
- Dick M, de Jong EV, de Souza JP. Análise sensorial de carne de frango pré-cozida e embalada em bandeja de cartão após aquecimento em forno micro-ondas e forno convencional. UNOPAR Cient Ciênc Biol Saúde. 2011;13:39–44. [Google Scholar]
- Faion AM, Beal P, Ril FT, Cichoski AJ, Casian RL, Valdurga AT, Oliveira D, Valdurga E. Influence of the addition of natural antioxidant from mate leaves (Ilex paraguariensis St. Hill) on the chemical, microbiological and sensory characteristics of different formulations of Prato cheese. J Food Sci Technol. 2015;52:1516–1524. doi: 10.1007/s13197-013-1045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip R, Giberti G, Coussio J, Acevedo C, Ferraro G. Estudio fitoquímico y farmacológico de Ilex theezans C. Dominguezia vol: Martius ex Reisseck; 2000. p. 16. [Google Scholar]
- Filip R, Lotito SB, Ferraro G, Fraga CG. Antioxidant activity of Ilex paraguariensis and related species. Nutr Res. 2000;20:1437–1446. doi: 10.1016/S0271-5317(00)80024-X. [DOI] [Google Scholar]
- Giroux HJ, De Grandpré G, Fustier P, Champagne CP, St-Gelais D, Lacroix M, Britten M. Production and characterization of Cheddar-type cheese enriched with green tea extract. Dairy Sci Technol. 2013;93:241–254. doi: 10.1007/s13594-013-0119-4. [DOI] [Google Scholar]
- Guangrong H, Jiaxin J, Dehui D. Antioxidative and antibacterial activity of the methanol extract of Artemisia anomala S. Moore. Afr J Biotechnol. 2008;7:1335–1338. [Google Scholar]
- Gul K, Singh AK, Jabeen R. Nutraceuticals and functional foods: the foods for the future world. Crit Rev Food Sci Nutr. 2016;56:2617–2627. doi: 10.1080/10408398.2014.903384. [DOI] [PubMed] [Google Scholar]
- Hala MF, Ebtisam ED, Sanaa I, Badran MA, Marwa AS, Said ME. Manufacture of low fat UF-soft cheese supplemented with rosemary extract (as natural antioxidant) J Am Sci. 2010;6:570–579. [Google Scholar]
- Han J, Britten M, St-Gelais D, Champagne CP, Fustier P, Salmieri S, Lacroix M. Effect of polyphenolic ingredients on physical characteristics of cheese. Food Res Int. 2011;44:494–497. doi: 10.1016/j.foodres.2010.10.026. [DOI] [Google Scholar]
- Han J, Britten M, St-Gelais DP, Champagne C, Fustier P, Salmieri S, Lacroix M. Polyphenolic compounds as functional ingredients in cheese. Food Chem. 2011;124:1589–1594. doi: 10.1016/j.foodchem.2010.08.021. [DOI] [Google Scholar]
- Kumar S, Yadav A, Yadav M, Yadav JP. Effect of climate change on phytochemical diversity, total phenolic content and in vitro antioxidant activity of Aloe vera (L.) Burm.f. BMC Res Notes. 2017;10:60. doi: 10.1186/s13104-017-2385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamothe S, Azimy N, Bazinet L, Couillard C, Britten M. Interaction of green tea polyphenols with dairy matrices in a simulated gastrointestinal environment. Food Funct. 2014;5:2621–2631. doi: 10.1039/C4FO00203B. [DOI] [PubMed] [Google Scholar]
- Li W, Hydamaka AW, Lowry L, Beta T. Comparison of antioxidant capacity and phenolic compounds of berries, chokecherry and seabuckthorn. Central Eur J Biol. 2009;4:499–506. [Google Scholar]
- Livney YD. Milk proteins as vehicles for bioactives. Curr Opin Colloid Interface Sci. 2010;15:73–83. doi: 10.1016/j.cocis.2009.11.002. [DOI] [Google Scholar]
- Lucey JA. ADSA foundation scholar award. Formation and physical properties of milk protein gels. J Dairy Sci. 2002;85:281–294. doi: 10.3168/jds.S0022-0302(02)74078-2. [DOI] [PubMed] [Google Scholar]
- Matumoto-Pintro PT, Rabiey L, Robitaille G, Britten M. Use of modified whey protein in yoghurt formulations. Int Dairy J. 2011;21:21–26. doi: 10.1016/j.idairyj.2010.07.003. [DOI] [Google Scholar]
- Meilgaard MC, Carr BT, Civille GV. Sensory evaluation techniques. 3. Boca Raton: CRC Press; 1999. [Google Scholar]
- Mejía EG, Song YS, Heck CI, Ramírez-Mares M. Yerba mate tea (Ilex paraguariensis): phenolics, antioxidant capacity and in vitro inhibition of colon cancer cell proliferation. J Funct Foods. 2010;2:23–34. doi: 10.1016/j.jff.2009.12.003. [DOI] [Google Scholar]
- Preci D, Cichoski AJ, Valduga AT, et al. Desenvolvimento de iogurte light com extrato de erva-mate (Ilex paraguariensis ST. HIL) e adição de probióticos. Alim Nutr. 2011;22:27–38. [Google Scholar]
- Ramos LR, Santos JS, Daguer H, Valese AC, Cruz AG, Granato D. Analytical optimization of a phenolic-rich herbal extract and supplementation in fermented milk containing sweet potato pulp. Food Chem. 2017;221:950–958. doi: 10.1016/j.foodchem.2016.11.069. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Reis FS, Stojković D, Soković M, Glamočlija J, Ćirić A, Barros L, Ferreira ICFR. Chemical characterization of Agaricus bohusii, antioxidant potential and antifungal preserving properties when incorporated in cream cheese. Food Res Int. 2012;48:620–626. doi: 10.1016/j.foodres.2012.06.013. [DOI] [Google Scholar]
- Riachi LG, Maria CAB. Yerba mate: an overview of physiological effects in humans. J Funct Foods. 2017;38:308–320. doi: 10.1016/j.jff.2017.09.020. [DOI] [Google Scholar]
- Schinella G, Fantinelli JC, Tournier H, Prieto JM, Spegazzini E, Debenedetti S, Mosca SM. Antioxidant and cardioprotective effects of Ilex brasiliensis: a comparative study with Ilex paraguariensis (yerba mate) Food Res Int. 2009;42:1403–1409. doi: 10.1016/j.foodres.2009.07.004. [DOI] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Valerga J, Reta M, Lanari MC. Polyphenol input to the antioxidant activity of yerba mate (Ilex paraguariensis) extracts. LWT Food Sci Technol. 2012;45:28–35. doi: 10.1016/j.lwt.2011.07.022. [DOI] [Google Scholar]
- Vital ACP, Goto PA, Hanai LN, Gomes-da-Costa SM, de Abreu Filho BA, Nakamura CV, Matumoto-Pintro PT. Microbiological, functional and rheological properties of low fat yogurt supplemented with Pleurotus ostreatus aqueous extract. LWT Food Sci Technol. 2015;64:1028–1035. doi: 10.1016/j.lwt.2015.07.003. [DOI] [Google Scholar]
- Yıldırım A, Mavi A, Kara AA. Determination of Antioxidant and Antimicrobial Activities of Rumex crispus L. extracts. J Agric Food Chem. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- Yuksel Z, Avci E, Erdem YK. Characterization of binding interactions between green tea flavanoids and milk proteins. Food Chem. 2010;121:450–456. doi: 10.1016/j.foodchem.2009.12.064. [DOI] [Google Scholar]
- Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001;49:5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- Zhu QY, Hackman RM, Ensunsa JL, Holt RR, Keen CL. Antioxidative activities of Oolong tea. J Agric Food Chem. 2002;50:6929–6934. doi: 10.1021/jf0206163. [DOI] [PubMed] [Google Scholar]