Abstract
Sulfated polysaccharide (fucoidan) was isolated from Nizamuddinia zanardinii by enzyme (alcalase), ultrasonic and enzyme-ultrasonic methods. The extracted fucoidans were assessed for their chemical compositions, molecular characteristics, anticancer and immunomodulatory activities. Enzyme-ultrasonic isolated fucoidan showed the maximum extraction yield (7.87%) while that obtained by ultrasonic had the minimum value (3.6%). fucoidans were composed of different levels of carbohydrates (52.78–58.65%), proteins (6.98–8.91%), sulfates (21.78–29.6%) and uronic acids (0.42–1.08%). The weight mean average molecular weight of fucoidans varied between 443.7 and 1020.85 kDa. The polysaccharide chains were consisted of fucose, galactose, glucose, mannose and xylose. All the recovered fucoidans showed strong growth inhibition against HeLa and Hep-G2 cancer cells. The isolated fucoidans were non-toxic and considerably stimulated the macrophage cells to release nitric oxide. Enzyme extraction produced fucoidan with the most macrophage stimulation capacity (> 42 µmol). These results suggested that enzyme treatment preserved more sulfate groups in fucoidan structure influencing its anticancer and immunostimulatory activities.
Keywords: Nizamuddinia zanardinii, Fucoidan, Enzyme extraction, Molecular weight, Sulfate group
Introduction
At present, consumers prefer the natural products with less additives to improve their health and life-style. In this regard, during the last decades seaweeds has been attracted the great attention of many researchers for isolation of natural substances such as polysaccharides and their sulfated derivatives, polyphenols, polyunsaturated fatty acids (PUFAs), protein, pigments, vitamins, tocopherols and phycocyanins with potential application in deferent industries like alimentary, pharmaceutical and cosmetic products (Kadam et al. 2015).
Fucoidan is a sulfated polysaccharide found in cell wall of brown seaweeds and animal species. This polysaccharide has various bioactivities including antioxidant, anti-tumor, antimicrobial, anti-viral, anti-inflammatory, antidiabetic, anti-obesity and anti-coagulant (Wang et al. 2008; Lee et al. 2012; Takeda et al. 2012; Hayashi et al. 2008; Wang et al. 2013; Kim and Lee 2013; Nishino et al. 1991; Kantachumpoo and Chirapart 2010). Fucoidan bioactivity depends on the species, seaweed compositional, structural and molecular properties, sulfate content and the purity of the final product (Cho et al. 2014; Borazjani et al. 2017). Furthermore, extraction methodology has a crucial effect on the structural characteristics and consequently the biological activities of the extracted molecules (Rostami et al. 2017).
Traditionally, the isolation of polysaccharides is mainly carried out by heating, boiling and refluxing of raw material in organic solvents. These methods suffer from several disadvantages namely long extraction time, high operation temperature, usage of high amount of organic solvents as well as low extraction yields (Cheng et al. 2015). Recently, different non-conventional extraction methods such as ultrasonic, microwave, enzymatic and supercritical fluid has been promoted in order to overcome these disadvantages. Among them, ultrasound (UAE) and enzymatic techniques gained more interests and attentions because they are highly efficient, environment-friendly, easily operational and also they can lead to the high extraction yield of natural products from raw materials (Wu et al. 2014).
Previously, various combined extraction methods have been carried out for high-added value compounds extraction from terrestrial materials such as ultrasonic-microwave extraction, ultrasound-assisted enzymatic extraction, supercritical co2-microwave-hydrothermal extraction, pressurized liquids-ultrasound, and microwave-assisted enzymatic extraction (Sumere et al. 2018; Shang et al. 2018; Zeng et al. 2015; Fan et al. 2015; Cheng et al. 2015; Quitain et al. 2013). However, there are few reports and most of them recent with respect to combination of non-conventional extraction methods for isolating bioactive substances from seaweeds (Quitain et al. 2013; Guillard et al. 2015, 2016). Moreover, effects of enzymatic, UAE and combination of these techniques on the extraction yield and properties of Nizimuddinia zanardinii Fucoidan have not been previously reported.
Therefore, the aim of this study was to examine the combination strategy in the extraction of fucoidan from N. zanardinii using UAE, enzyme and enzyme-UAE methods and evaluate their implications on yield, chemical and molecular structure, anticancer and immunostimulatory properties.
Materials and methods
Materials
Nizamuddinia Zarnardinii samples were freshly collected from the rocky substrate of Chabahr in the Sistan and Baluchestan province of Iran (Oman Sea) in February 2016. The collected samples were washed, dried (40 °C), milled and maintained at freezer until use.
Extraction procedure
Pre-treatment of N. zanardinii
50 g of seaweed was suspended in 500 mL of 85% EtOH and stirred for 24 h at room temperature to remove pigments and small molecule compounds. Then the seaweed residue was rinsed with acetone and finally dried under laminar hood at 22 ± 2 °C.
Enzyme-assisted extraction
The dried and pre-treated seaweed (50 g) was treated with alcalase (2.5 mL/dry material weight, pH 7, solid-to-solvent ratios 1:30 g/mL) for 24 h at 50 °C. Choosing the alcalase in this methods is based on our previous work about the fucoidan extraction by different carbohydrases and proteases enzymes which among them alcalase extracted fucoidan showed higher yield and better biological activity. After incubation time, the mixture was put in water bath (95 °C) for 15 min in order to inactivate the enzyme and then cooled with ice bath. After cooling, the suspension was centrifuged (10 min at 9000 rpm) and collected supernatant part was concentrated by rotary evaporator (60 °C). Alginic acid was removed by the addition of calcium chloride (1%) to the concentrated extracts after maintaining the mixture overnight at 4 °C and centrifugation (10 min at 9000 rpm). Fucoidan was precipitated by ethanol addition to reach the final concentration of 70%. Centrifugation (6000 rpm, 15 min) was used for recovering the fucoidan. The recovered fucoidan was washed with ethanol (three times) and acetone (twice) and finally dried under laminar hood (22 ± 2 °C). The fucoidan was weighed and stored at − 20 °C until analyzed. The yields of fucoidan were calculated based on the dried seaweed treated with 85% EtOH and acetone.
Ultrasound-assisted extraction
The dried and pre-treated N. zanardinii samples were placed into a glass beaker containing distilled water (1:76 g/mL) and suspensions were sonicated by means of a high-power ultrasonic probe system with a titanium horn (frequency 20 kHz, max power 400 W, Ø = 1.3 cm) at 196 W and 70 °C for 59 min. This UAE condition is based on our previous work about optimization the fucoidan extraction by response surface methodology. The supernatant was separated from the solid part using centrifugation (9000 rpm, 10 min) and concentrated. The alginic acid was removed using calcium chloride (1%) and fucoidan was recovered with EtOH.
Enzyme-ultrasound assisted extraction
The dried and pre-treated seaweed (50 g) was initially treated with alcalase (2.5 mL/dry material weight, pH 7, temperature 50 °C, solid-to-solvent ratios 1:30 g/mL) for 23 h and then followed by sonication (power 196 W, temperature 70 °C, time 59 min). After treatment, the collected supernatant was concentrated and alginic acid was removed using calcium chloride (1%). Finally, fucoidan was precipitated by EtOH addition to reach the final concentration of 70%.
Chemical composition
The determination of neutral sugar amount of the isolated polysaccharides was carried out using the phenol–sulfuric acid method and d-glucose as a standard, the absorbance was recorded at 490 nm (Dubois et al. 1956). Lowry method was employed to measure the amount of protein using a DC protein assay kit (Bio-Rad, CA, USA), the absorbance was recorded at 720 nm (Lowry et al. 1951). The sulfate content was determined by the BaCl2 gelatin method and the absorbance was recorded at 360 nm (Dodgson and Price 1962). The level of uronic acid was measured by a sulfamate/m-hydroxydiphenyl assay and glucuronic acid as a standard, the absorbance was recorded at 525 nm (Filisetti-Cozzi and Carpita 1991).
Determination of monosaccharide composition
The monosaccharide composition of extracted fucoidan were determined by GC-MAS (gas chromatography mass spectrometry). Sample preparation was performed according to the procedure previously reported by Rostami et al. (2018). The monosaccharide standards including fucose, rhamnose, xylose, mannose, galactose and glucose were applied according to the reference.
FT-IR spectroscopy
For FT-IR analysis, sulfated polysaccharides were mixed with KBr powder and pressed into a pellet. Then, the pellets were scanned using a FT-IR spectrophotometer (Bruker Instruments, Billerica, USA) in the wavelength of 400–4000 cm−1 at room temperature.
Determination of molecular properties
Molecular properties of the sulfated polysaccharides were determined by HPSEC–UV–MALLS–RI system. Sample preparation was performed according to procedure previously reported by Bahramzadeh et al. (2019). Molecular properties including average molecular weight (Mw), number average molecular weight (Mn), polydispersity and radius of gyration (Rg) of extracted fucoidan were determined using ASTRA 5.3 software (Wyatt Technology Corp.). Following equation was used for calculation of the specific volume of gyration (SVg):
in which N is Avogadro’s number (6.02 × 1023/mol) and the units for SVg, Mw and Rg were cm3/g, kDa and kDa respectively.
Scanning electron microscopy (SEM)
SEM analysis (Philips XL 30, Netherlands) at a 20.0 kV accelerating voltage was applied to investigate the effect of different extraction methods on the microstructure of extracted fucoidan. Samples first coated with gold and then observed under different magnifications.
Anticancer activity
HeLa and HepG2 cells were plated (1 × 104 cells/well) in a 96-well plate and the plates were put in incubator (4 h, 37 °C, 5% CO2). After incubation, different concentrations of polysaccharide samples (100, 200 and 400 µg/mL) were added to each well and then plates put again in the incubator (72 h, 37 °C, 5% CO2). Finally, the WST-1 colorimetric assay kit was employed for determination the anticancer activity of the samples. 5-fluorouracil (5-Fu, 10 µg/mL) was considered as a positive control.
Macrophage proliferation activity and nitric oxide releasing capacity
100 μL of RAW264.7 cells were seeded (1 × 104 cells/well) in 96-well plates and incubated for 24 h at 37 °C with 5% CO2. After incubation, 100 μL of the polysaccharide samples (10, 25 and 50 μg/mL) were added to each well and plate incubated at 37 °C. After 3 days 20 μL of WST-1 solution were added to each well and plates incubated at 37 °C. After 4 h absorbance of samples was determined at 450 nm using a microplate reader (Borazjani et al. 2018).
RAW264.7 cells were seeded (1 × 105 cells/well) in a 96-well plate and treated with different concentration of polysaccharide (10, 25 and 50 μg/mL) and lipopolysaccharide (LPS, 1 μg/mL). After incubation at 37 °C for 18 h, the separated supernatant was mixed with Griess reaction solution and maintained in room temperature. After 10 min, absorbance of samples was determined at 540 nm using a microplate reader. Nitric oxide (NO) production of macrophage cells was quantified by matching with a sodium nitrite standard curve (Green et al. 1982).
Statistical analyses
All experiments were expressed as mean values ± standard deviations. One way ANOVA and Duncan’s test (p < 0.05) was performed for the calculating the differences between the extraction methods and concentrations of Fucoidan.
Results and discussion
Effect of extraction methods on the yield of fucoidan
The effect of different extraction methods (enzyme, UAE, enzyme-UAE) on fucodian yields is shown in Table 1. The type of extraction clearly affected the fucoidan yield and it varied from 3.6 to 7.87%. Among the tested methods, enzyme-UAE exhibited the highest fucodian yield (7.87%) while the lowest value was obtained in the UAE extraction (3.6%). The higher yields in EAE (5.58%) compared to UAE could be due to better disintegration of cell wall matrix by alcalase enzyme in a rather prolonged reaction time. You et al. (2013) reported that enzyme-assisted method (EAE) had the higher Cornus officinalis polysaccharides yield compared to UAE methods. Combination the enzyme and UAE led to achieve the heights fucoidan yields (7.87%). The higher yields of the enzyme-UAE compared to those of the EAE and UAE could be explained by the fact that when alcalase was added into the extraction suspension, initially the enzyme catalyzes the cell wall and then sonication produces high cavitation intensity leading to more solvent penetration. These sequential events can accelerate the release of intracellular polysaccharide into the solvent and consequently increase the extraction efficiency. Synergistic effect between enzymes and UAE for extraction polysaccharides was reported by Easson et al. (2011) and Wu et al. (2014). Overall, results of the current study showed that the enzyme-UAE could be an appropriate and effective extraction technique for the isolation of fucoidan from N. zanardinii with high extraction yield.
Table 1.
Yield, chemical and monosaccharide composition of different fucoidans isolated from N. zanardinii
| Fucoidan | Yield (%) | Protein (%) | Carbohydrate (%) | Uronic acid (%) | Sulfate (%) |
|---|---|---|---|---|---|
| UAE | 3.6 ± 0.31c | 8.53 ± 0.45a | 58.65 ± 0.17a | 1.08 ± 0.06a | 22.97 ± 0.29b |
| Enzyme | 5.58 ± 0.35b | 6.98 ± 0.25b | 53.55 ± 0.17b | 0.42 ± 0.02c | 29.6 ± 0.6a |
| Enzyme-UAE | 7.87 ± 0.72a | 8.91 ± 0.25a | 52.78 ± 0.27b | 0.73 ± 0.02b | 21.78 ± 1.11b |
| Fucoidan | Monosaccharide composition | ||||
|---|---|---|---|---|---|
| Fucose (%) | Galactose (%) | Glucose (%) | Mannose (%) | Xylose (%) | |
| UAE | 32.33 ± 0.16b | 29.95 ± 0.25a | 3.19 ± 0.06a | 27.97 ± 0.11a | 6.56 ± 0.13a |
| Enzyme | 32.93 ± 0.37ab | 30.8 ± 0.20a | 2.54 ± 0.24b | 26.75 ± 0.83a | 6.97 ± 0.40a |
| Enzyme-UAE | 33.86 ± 0.57a | 31.32 ± 0.81a | 2.04 ± 0.24b | 27.31 ± 0.21a | 5.47 ± 0.18b |
The letters a,b,c,d indicate significant difference at p < 0.05
Chemical compositional analysis
The contents of carbohydrate, protein, uronic acid and sulfates in N. zanardinii fucoidans extracted by enzyme, UAE and enzyme-UAE methods are showed in Table 1. The carbohydrate contents in fucoidan extracted by enzyme, UAE and enzyme-UAE were 53.55%, 58.65% and 52.78%, respectively. As can be seen in Table 1, UAE (8.53%) and enzyme-UAE (8.91%) fucoidans contained higher amounts of proteins compared with that of EAE (6.98%). Uronic acid contents in enzyme, UAE and enzyme-UAE were 0.42%, 1.08% and 0.73%, respectively. The sulfate content was the highest in enzyme (29.6%), followed by UAE (22.97%) and enzyme-UAE (21.78%). This discrepancy in the chemical composition of different fucoidans might be related to the type of extraction method that used (Dong et al. 2016).
Monosaccharide compositions of enzyme, UAE and enzyme-UAE extracted fucoidans are shown in Table 1. In all extracted fucoidans the fucose was the main monosaccharide and other monosaccharides are in the following order from high to low content: Mannose > Galactose > Xylose > Glucose. Rhamnose and Arabinose were not found in the extracted fucoidans. This monosaccharide composition was reported previously for fucoidan extracted from Sargassum augustifolium with different ratios (Borazjani et al. 2018). These results indicated that not only extraction yields differ from one isolation technique to another, but also their chemical constituents are significantly divergent.
Infrared spectroscopy analysis
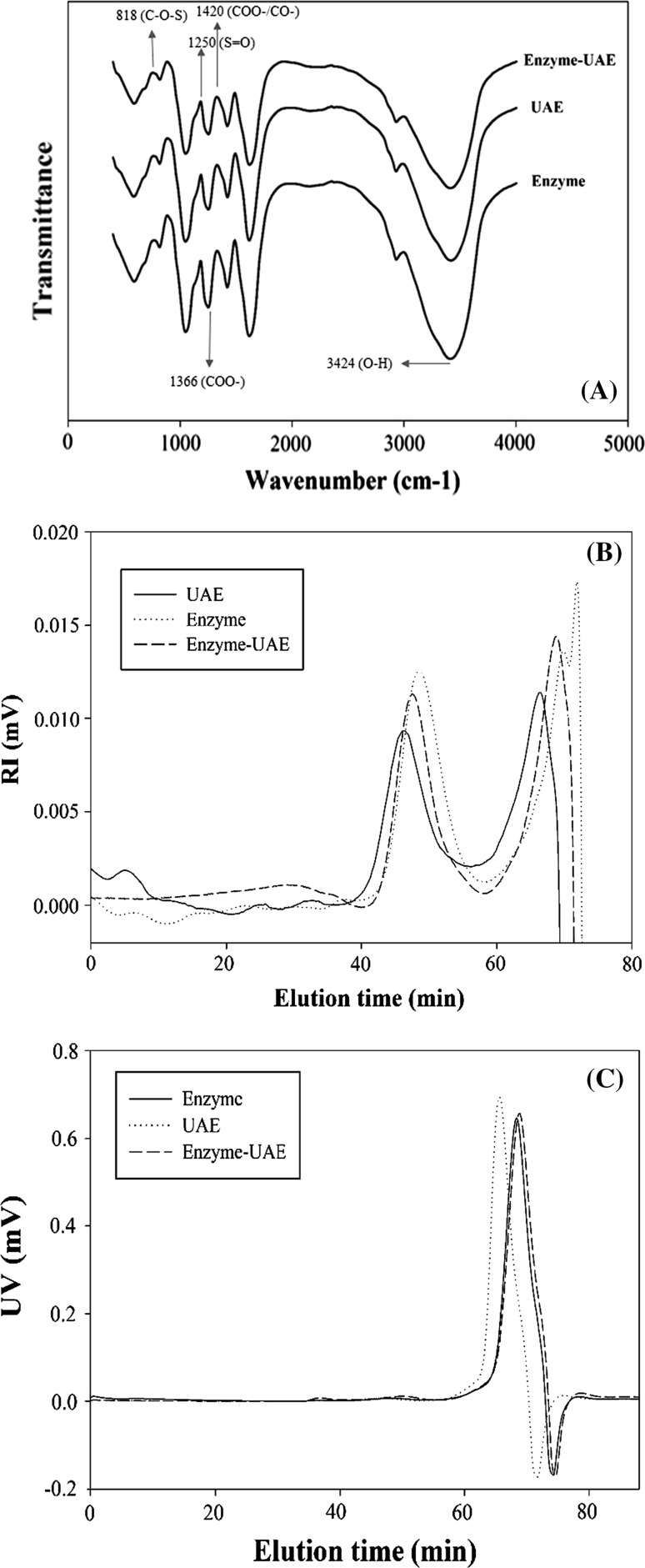
Figure 1a show the infrared spectra of fucoidans extracted by enzyme, UAE and enzyme-UAE from N. zanardinii. Like the other seaweed fucoidans, the FT-IR spectra of different fucoidans showed a strong absorbance band at 3424 cm−1 that is attributed to O–H stretching vibration. The band at 1420 cm−1 and 1366 cm−1 represent the presence of the asymmetrical bending vibration of CH3, and symmetrical bending vibration of CH3, respectively (Lim et al. 2014). The band at 1250 cm−1 and 818 cm−1 were attributed to the sulfate esters (S=O) and sulfate group (C–O–S), respectively (Huang et al. 2016).
Fig. 1.
FT-IR spectra (a), RI (b) and UV chromatograms (c) of different fucoidans
Molecular characteristics
The RI superimposed chromatograms for fucoidans extracted by enzyme, UAE and enzyme-UAE methods are showed in Fig. 1b. As shown in the RI chromatogram, the extracted fucoidans had one major peak at the elution times of 42–55 min. The peak at the elution time of 66 min was related to the calcium chloride. As can be seen in Fig. 1c, enzyme extracted fucoidans had lower UV peak levels than those extracted by UAE and enzyme-UAE, corresponding to their protein contents. This result indicated that the enzyme treatment removed the proteins from the extracted fucoidan more than to the others. These results were in agreement with protein content of extracted fucoidan. The average molecular weights of enzyme, UAE and enzyme-UAE were 642.85, 1020.85 and 443.70 kDa, respectively (Table 2). The Rg values, which indicate the size of the molecules, of enzyme, UAE and enzyme-UAE were 56.5, 62.05 and 52.05 nm, respectively. The SVg values for different fucoidan ranged from 0.29 to 0.80 cm3/g. It could be seen that UAE had the relatively more compact compared to enzyme and enzyme-UAE.
Table 2.
Weight average molecular weight (Mw), number average molecular weight (Mn), radius of giration (Rg), specific volume of gyration (SVg) and polydispersity of different extracted Fucoidan
| Fucoidan | Mw (kDa) | Mn (kDa) | Polydispersity (Mw/Mn) | Rg (nm) | SVg (cm3/g) |
|---|---|---|---|---|---|
| UAE | 1020.85 ± 31.75a | 806.15 ± 17.47a | 1.27 ± 0.01a | 62.05 ± 4.03a | 0.29 ± 0.01c |
| Enzyme | 642.85 ± 7.85b | 642.2 ± 13.44b | 1 ± 0.01b | 31.05 ± 0.91c | 0.41 ± 0.03b |
| Enzyme-UAE | 443.7 ± 52.61c | 345.7 ± 34.93c | 1.28 ± 0.02a | 52.05 ± 0.83b | 0.80 ± 0.05a |
The letters a,b,c,d indicate significant difference at p < 0.05
Morphological analysis
Figure 2 presents the scanning electron micrographs (SEM) of fucoidans extracted by enzyme, UAE and enzyme-UAE at different magnifications (200, 500 and 1000 fold exaggeration conditions). As can be seen, under a 200 fold exaggeration condition, all fucoidans exhibited a distributed fluffy powder. They also showed an irregular shape with no uniform size and plenty of pores at 500 and 1000 fold exaggeration.
Fig. 2.
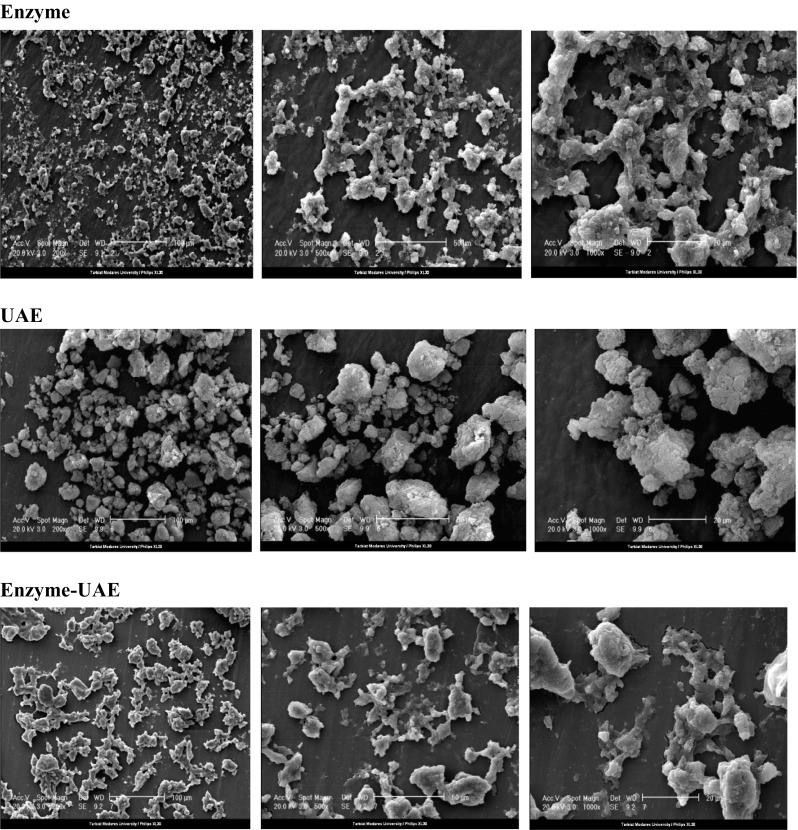
Scanning electron micrographs of the different fucoidans (× 200; × 500; × 1000, left to right respectively)
Anticancer activity
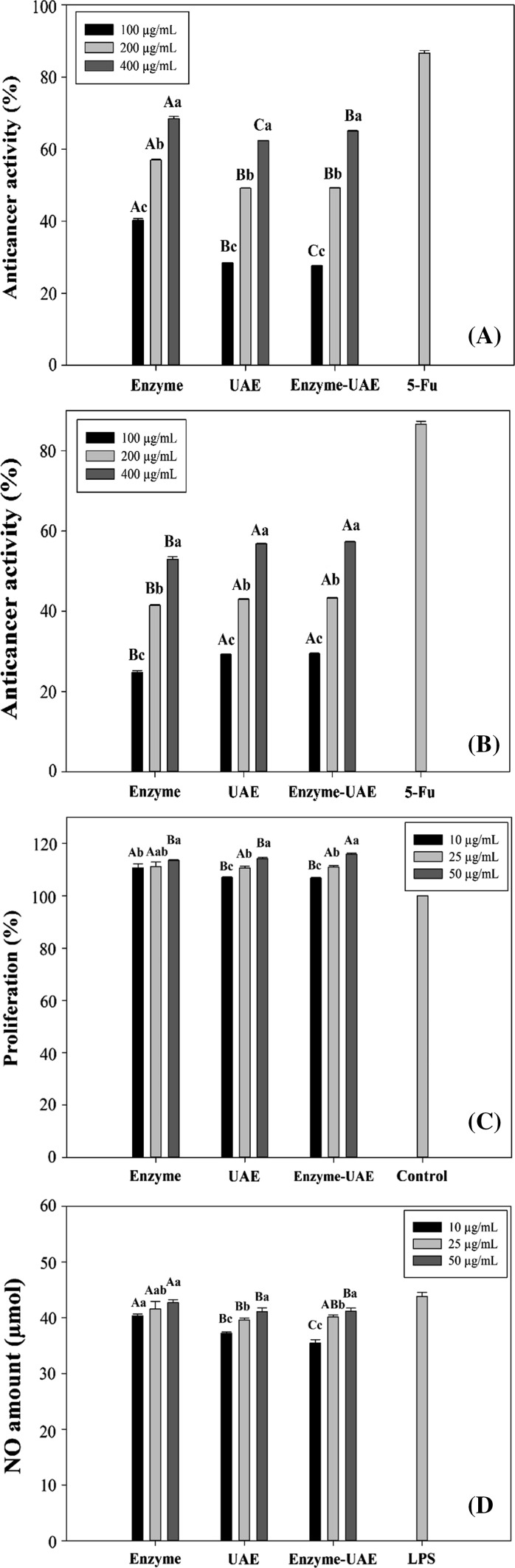
In the present study, the anticancer activities of fucoidans extracted from N. zanardinii by enzyme, UAE and enzyme-UAE were tested at three concentrations ranging from 100 to 400 µg/mL. The fucoidans showed the anticancer activity ranging from 28.40 to 68.46% for HeLa cells and 24.72 to 57.29% (Fig. 3a) for Hep-G2 cells (Fig. 3b). The anticancer activity of the fucoidan extracted by UAE was lower than those extracted by enzyme and enzyme-UAE. A previous report (Zhao et al. 2016) showed that anticancer activity of bioactive polysaccharides is attributable to the various factors such as sulfate content, monosaccharide composition, molecular weight and amount of impurity of protein and total phenol. Also, Yang et al. (2008) proposed that anticancer activities of brown seaweed fucoidans depend on species and growing conditions of brown seaweeds, extraction and purification methods and as well as the use of different cancer cell lines.
Fig. 3.
Effects of different fucoidans on proliferation of HeLa (a) and HepG2 cancer cells (b), proliferation (c) and NO production (d) in RAW264.7 macrophages cells (mean ± SD). The letters a, b, c, d indicate a significant difference (p < 0.05) between the concentrations of each fucoidan, and A,B,C,D indicate a significant difference (p < 0.05) between different fucoidans at a constant concentration
Immunomodulatory activity
As can be seen in Fig. 3c, compared to the control proliferations of RAW264.7 cells were significantly increased in the presence of all the extracted fucoidans. These results suggested that N. zanardinii sulfated polysaccharides are nontoxic and could stimulate the growth of RAW 264.7 cells. The nitric oxide (NO) productions of RAW264.7 cells in the presence of different concentrations of different fucoidans (10, 25 and 50 μg/mL) were investigated and the results are shown in the Fig. 3d. When RAW264.7 cells were cultured in medium containing enzyme-UAE, a minimum amount of NO was released, whereas treating the cells with enzyme extracted fucoidans resulted in significant increase of NO production. The higher stimulating potential of fucoidan isolated using enzyme could be attributed to its greater sulfate content. The direct and favorable relationships between sulfate contents of polysaccharides with their capacities to induce macrophage NO release have been previously reported in fucoidans isolated from Sargassum angustifolium and Undaria pinnatifida (Borazjani et al. 2018; Cho et al. 2011).
Conclusion
The yields, chemical composition, molecular properties, anticancer and immunomodulatory activities of fucoidan extracted from N. zanardinii by enzyme, ultrasound and enzyme-altrasound methods were evaluated. The highest and lowest yields were obtained by enzyme-ultrasound and ultrasound methods, respectively. Different extraction methods resulted in obtaining fucoidans with various chemical compositions and molecular weights. Enzyme-ultrasound isolated fucoidans showed the lowest amount of sulfate group while those obtained by enzyme had the highest levels of sulfate content. Fucose, galactose, mannose, glucose and xylose were the monosaccharides present in all extracted fucoidans. All the isolated fucoidans exhibited appropriate macrophage stimulating properties and also anticancer activity against HeLa and HepG2 cells in vitro.
Acknowledgements
Authors would like to thank the Iran National Science Foundation (INSF) for support of this research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bahramzadeh S, Tabarsa M, SangGuan You S, Li C, Bita S. Purification, structural analysis and mechanism of murine macrophage cell activation by sulfated polysaccharides from Cystoseira indica. Carbohydra Polym. 2019;205:261–270. doi: 10.1016/j.carbpol.2018.10.022. [DOI] [PubMed] [Google Scholar]
- Borazjani NJ, Tabarsa M, You S, Rezaei M. Improved immunomodulatory and antioxidant properties of unrefined fucoidans from Sargassum angustifolium by hydrolysis. J Food Sci Technol. 2017;54(12):4016–4025. doi: 10.1007/s13197-017-2867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borazjani NJ, Tabarsa M, You S, Rezaei M. Purification, molecular properties, structural characterization, and immunomodulatory activities of water soluble polysaccharides from Sargassum angustifolium. Int J Biol Macromol. 2018;109:793–802. doi: 10.1016/j.ijbiomac.2017.11.059. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Song H, Yang Y, Liu Y, Liu Z, Hu H, Zhang Y. Optimization of microwave-assisted enzymatic extraction of polysaccharides from the fruit of Schisandra chinensis Baill. Int J Biol Macromol. 2015;76:161–168. doi: 10.1016/j.ijbiomac.2015.01.048. [DOI] [PubMed] [Google Scholar]
- Cho ML, Lee BY, You SG. Relationship between oversulfation and conformation of low and high molecular weight fucoidans and evaluation of their in vitro anticancer activity. Molecules. 2011;16(1):291–297. doi: 10.3390/molecules16010291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M, Lee DJ, Kim JK, You S. Molecular characterization and immunomodulatory activity of sulfated fucans from Agarum cribrosum. Carbohydr Polym. 2014;113:507–514. doi: 10.1016/j.carbpol.2014.07.055. [DOI] [PubMed] [Google Scholar]
- Dodgson KS, Price RG. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J. 1962;84:106–110. doi: 10.1042/bj0840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Lin S, Zhang Q, Chen H, Lan W, Li H, He J, Qin W. Effect of extraction methods on the properties and antioxidant activities of Chuanminshen violaceum polysaccharides. Int J Biol Macromol. 2016;93:179–185. doi: 10.1016/j.ijbiomac.2016.08.074. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Easson MW, Condon B, Dien BS, Iten L, Slopek R, Yoshioka-Tarver M, Lambert A, Smith J. The application of ultrasound in the enzymatic hydrolysis of switchgrass. Appl Biochem Biotechnol. 2011;165:1322–1331. doi: 10.1007/s12010-011-9349-1. [DOI] [PubMed] [Google Scholar]
- Fan T, Hu J, Fu L, Zhang L. Optimization of enzymolysis-ultrasonic assisted extraction oF polysaccharides from Momordica charabtia L. by response surface methodology. Carbohydr Polym. 2015;115:701–706. doi: 10.1016/j.carbpol.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Filisetti-Cozzi TMCC, Carpita NC. Measurement of uronic acids without interference from neutral sugars. Anal Biochem. 1991;197:157–162. doi: 10.1016/0003-2697(91)90372-Z. [DOI] [PubMed] [Google Scholar]
- Green LC, Wanger DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal Biochem. 1982;126:131–136. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- Guillard CL, Dumay J, Donnay-Moreno C, Bruzac S, Ragon JY, Fleurence J, Bergé JP. Ultrasound-assisted extraction of R-phycoerythrin from Grateloupia turuturu with and without enzyme addition. Algal Res. 2015;12:522–528. doi: 10.1016/j.algal.2015.11.002. [DOI] [Google Scholar]
- Guillard CL, Bergé JP, Donnay-Moreno C, Bruzac S, Ragon JY, Baron R, Fleurence J, Dumay J. Soft liquefaction of the red seaweed Grateloupia turuturu Yamada by ultrasound-assisted enzymatic hydrolysis process. J Appl Phycol. 2016;28(4):2575–2585. doi: 10.1007/s10811-015-0788-x. [DOI] [Google Scholar]
- Hayashi K, Nakano T, Hashimoto M, Kanekiyo K, Hayashi T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int Immunopharm. 2008;8(1):109–116. doi: 10.1016/j.intimp.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Huang CY, Wu SJ, Yang WN, Kuan AW, Chen CY. Antioxidant activities of crude extracts of Fucoidan extracted from Sargassum glaucescens by a compressional-puffing-hydrothermal extraction process. Food Chem. 2016;197:1121–1129. doi: 10.1016/j.foodchem.2015.11.100. [DOI] [PubMed] [Google Scholar]
- Kadam SU, Tiwari BK, Smyth TJ, O’Donnell CP. Optimization of ultrasound assisted extraction of bioactive components from brown seaweed Ascophyllum nodosum using response surface methodology. Ultrason Sonochem. 2015;23:308–316. doi: 10.1016/j.ultsonch.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Kantachumpoo A, Chirapart A. Components and antimicrobial activity of polysaccharides extracted from Thai brown seaweeds. Kasetsart J. 2010;44:220–233. [Google Scholar]
- Kim KG, Lee BY. Fucoidan from the sporophyll of Undaria pinnatifida suppresses adipocyte differentiation by inhibition of inflammation-related cytokines in 3T3-L1 cells. Nutr Res. 2013;32(6):439–447. doi: 10.1016/j.nutres.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Lee SH, Ko CI, Ahn G, You S, Kim JS, Heu MS, Kim J, Jee Y, Jeon YJ. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr Polym. 2012;89(2):599–606. doi: 10.1016/j.carbpol.2012.03.056. [DOI] [PubMed] [Google Scholar]
- Lim SJ, Aida WMW, Maskat MY, Mamot S, Ropien J, Mohd DM. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocol. 2014;42(2):280–288. doi: 10.1016/j.foodhyd.2014.03.007. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Nishino T, Aizu Y, Nagumo T. The relationship between the molecular weight and the anticoagulant activity of two types of fucan sulfates from the brown seaweed Ecklonia kurome. Agric Biol Chem. 1991;55(3):791–796. [Google Scholar]
- Quitain AT, Kai T, Sasaki M, Goto M. Microwave − hydrothermal extraction and degradation of fucoidan from supercritical carbon dioxide Deoiled Undaria pinnatida. Ind Eng Chem Res. 2013;52(23):7940–7946. doi: 10.1021/ie400527b. [DOI] [Google Scholar]
- Rostami Z, Tabarsa M, You S, Rezaei M. Relationship between molecular weights and biological properties of alginates extracted under different methods from Colpomenia peregrina. Process Biochem. 2017;58:289–297. doi: 10.1016/j.procbio.2017.04.037. [DOI] [Google Scholar]
- Rostami Z, Tabarsa M, You S, Rezaei M. Structural characterization and RAW264.7 murine macrophage stimulating activity of a fucogalactoglucan from Colpomenia peregrina. J Food Sci Technol. 2018;55(11):4650–4660. doi: 10.1007/s13197-018-3406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang H, Chen S, Li R, Zhou H, Wu H, Song H. Influences of extraction methods on physicochemical characteristics and activities of Astragalus cicer L. polysaccharides. Process Biochem. 2018;73:220–227. doi: 10.1016/j.procbio.2018.07.016. [DOI] [Google Scholar]
- Sumere BR, de Souza MC, dos Santos MP, Bezerra RMN, da Cunha DT, Martinez J, Rostagno MA. Combining pressurized liquids with ultrasound to improve the extraction of phenolic compounds from pomegranate peel (Punica granatum L.) Ultrason Sonochem. 2018;48:151–162. doi: 10.1016/j.ultsonch.2018.05.028. [DOI] [PubMed] [Google Scholar]
- Takeda K, Tomimori K, Kimura R, Ishikawa C, Nowling TK, Mori N. Anti-tumor activity of fucoidan is mediated by nitric oxide released from macrophages. Int J Oncol. 2012;40:251–260. doi: 10.3892/ijo.2011.1168. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Q, Zhang Z, Li Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int J Biol Macromol. 2008;42(2):127–132. doi: 10.1016/j.ijbiomac.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Wang J, Jin W, Zhang W, Hou Y, Zhang H, Zhang Q. Hypoglycemic property of acidic polysaccharide extracted from Saccharina japonica and its potential mechanism. Carbohydr Polym. 2013;95(1):143–147. doi: 10.1016/j.carbpol.2013.02.076. [DOI] [PubMed] [Google Scholar]
- Wu H, Zhu J, Diao W, Wang C. Ultrasound-assisted enzymatic extraction and antioxidant activity of polysaccharides from pumpkin (Cucurbita moschata) Carbohydr Polym. 2014;113:314–324. doi: 10.1016/j.carbpol.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Yang C, Chung D, Shin IS, Lee H, Kim J, Lee Y, You S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int J Biol Macromol. 2008;43:433–437. doi: 10.1016/j.ijbiomac.2008.08.006. [DOI] [PubMed] [Google Scholar]
- You Q, Yin X, Zhao Y. Enzyme assisted extraction of polysaccharides from the fruit of Cornus officinalis. Carbohydr Polym. 2013;98(1):607–610. doi: 10.1016/j.carbpol.2013.06.036. [DOI] [PubMed] [Google Scholar]
- Zeng H, Zhang Y, Lin S, Jian Y, Miao S, Zheng B. Ultrasonic–microwave synergistic extraction (UMSE) and molecular weight distribution of polysaccharides from Fortunella margarita (Lour.) Swingle. Sep Purif Technol. 2015;144:97–106. doi: 10.1016/j.seppur.2015.02.015. [DOI] [Google Scholar]
- Zhao YM, Wang J, Wu ZG, Yang JM, Li W, Shen LX. Extraction, purification and anti-proliferative activities of polysaccharides from Lentinus edodes. Int J Biol Macromol. 2016;93:136–144. doi: 10.1016/j.ijbiomac.2016.05.100. [DOI] [PubMed] [Google Scholar]