Abstract
As data on prevalence and etiology of dementia in American Indians are limited, we sought to determine rates and patterns of memory loss among American Indian veterans with vascular risk factors. Sixty consecutive outpatient American Indian veterans with a mean age of 64 years (range 50–86), without prior dementia or mild cognitive impairment (MCI), and with ≥ 2 vascular risk factors were enrolled. The Montreal Cognitive Assessment (MoCA) and the Beck Depression Inventory-II were used to screen for cognitive impairment and depression. Patients with MoCA scores < 26 were referred for additional evaluation, including imaging, serology, and neuropsychological testing. Overall rates, types, and distribution of cognitive impairment were determined. Most prevalent vascular risk factors included hypertension (92%), hyperlipidemia (88%), diabetes (47%), and smoking (78%). Eight patients (13%) with severe depression were excluded, leaving 23/52 with abnormal MoCA scores (44%, 95%CI 30%–59%). Fifteen completed additional evaluation for memory loss, including four with normal MoCA scores who requested evaluation based on symptoms. Results were adjudicated as normal (4), non-amnestic MCI (4), vascular MCI (5), and vascular dementia (2). These results show that rates of undiagnosed cognitive impairment among American Indian veterans with vascular risk factors exceed rates previously published in non-American Indian cohorts. The most common etiology is vascular. Our findings support the need to improve vascular risk reduction in this understudied population.
Keywords: Vascular risk factors, Vascular dementia, American Indians, Mild cognitive impairment
Introduction
As the impact of vascular disease on cognitive impairment has become increasingly recognized, a convergence of mechanisms that involve vascular and neurodegenerative processes has been proposed to cause impairment of cognition (Gorelick et al. 2011). Vascular contributions to cognitive impairment and dementia (VCID) represent a field of research focusing on the brain damage caused by a variety of ischemic and hemorrhagic vascular injuries leading to cognitive decline (Gorelick et al. 2011; Corriveau et al. 2016). Risk factors associated with vascular cognitive impairment are similar to those linked to stroke, including hypertension, diabetes, and hypercholesterolemia (Gorelick et al. 2011; Corriveau et al. 2016).
Previous research has consistently reported higher prevalence of vascular risk factors among American Indians over the past two decades (Go et al. 2014; Howard et al. 1999; Rhoades et al. 2007), which would in turn lead to an increased risk for development of vascular cognitive impairment (Gorelick et al. 2011). Although racial and ethnic inequalities in dementia incidence in USA have been noted (Mayeda et al. 2016), data on incidence, prevalence, and etiology of dementia in this population are limited (Jervis and Manson 2002; Mehta and Yeo 2017). These disparities, coupled with the relative paucity of cerebrovascular disease research focusing on American Indians, have prompted calls for further investigations in this area (Mehta and Yeo 2017; Cruz-Flores et al. 2011).
Building upon a recently created Native American Navigator Program through the Oklahoma Veterans Affairs Healthcare system, we initiated a pilot project focused on assessing the prevalence and patterns of memory loss in American Indian veterans with vascular risk factors and without a prior diagnosis of cognitive impairment.
Research design and methods
Study participants
The study was approved by the Institutional Review Board of the University of Oklahoma Health Sciences Center and the local Veterans Affairs Research and Development Committee rules and regulations. The study was carried out in accordance with the Helsinki Declaration of 1975 (and as revised in 1983). Individual informed consent was obtained for all study participants.
Recruitment consisted of a two-step process: (1) Information about the study was provided through the Native American Navigator program, American Indian patient groups and the Oklahoma City VA Cardiovascular clinics to outpatients seen at our local facility, followed by (2) self-referral by American Indian veterans who became aware of the study and decided to participate. The Native American Navigator Program was recently initiated at our facility in response to an increased number of American Indian veterans establishing medical care through the VA in our state. The program facilitates coordination of care for patients new to the VA healthcare system with complex medical problems, including multiple vascular risk factors, and is working very closely with American Indian patient support groups.
Inclusion criteria consisted of (1) American Indian race as self-identified by the patient, (2) the presence of at least two vascular risk factors, and (3) willingness to participate in the study.
Exclusion criteria were determined a priori and included (1) prior diagnosis of any cognitive impairment, (2) use of memory loss medications, (3) recent (< 6 months) history of stroke, and (4) self-reported intake of narcotic medications, sedatives, alcohol, or illicit drugs within 2 h before cognitive screening.
After consent, the patients underwent a complete physical examination in the Vascular Medicine Clinic. Hospitalization records, imaging studies, prescription medications, outpatient clinic notes, and consultation reports were reviewed.
All study participants were screened by trained research personnel for cognitive impairment using the Montreal Cognitive Assessment (MoCA) test (Nasreddine et al. 2005) and for depression using the Beck Depression Inventory-II (BDI-II) (Beck et al. 1996). All patients noted to have moderate or severe depression were offered the option of urgent referral to Mental Health Services if such referral was not already in place.
Patients with MoCA scores < 26 were offered additional evaluation in an academic memory loss clinic located at the same facility. The evaluation consisted of a neurological examination, including repeat cognitive screening, laboratory tests recommended to screen for comorbidities associated with memory loss (including vitamin B12 and thyroid studies), brain imaging, and neuropsychological evaluation. Prior to the referral and/or performing any of the imaging studies, laboratory tests, or neuropsychological evaluation, the patients were reminded that they have the option to opt out. Final cognitive status was assigned by blinded adjudication as part of clinical consensus management conferences based on published criteria (Gorelick et al. 2011; McKhann et al. 2011; McKeith et al. 2017; Bang et al. 2015).
Results of the clinical examinations, blood tests, imaging, neuropsychological evaluations, and final diagnoses were reviewed with each patient through follow-up visits and, if agreed upon by the patient, with their immediate family members. These results were readily available to the patients’ primary care and/or other subspecialty providers through electronic records.
Statistical analysis
Descriptive statistics were used to summarize the distribution of clinical variables and to compare baseline characteristics of patients with abnormal (< 26) and normal MoCA scores (≥ 26). Means were compared between groups using a two-sample t test or Wilcoxon rank sum test for groups with small sample sizes. Proportions were compared between groups using a chi-square test, or Fisher’s exact test when the expected frequency count was low. A Cochrane-Armitage test for trend was used to compare the distribution of ordered categorical variables between groups, where the exact form of the test was used for comparisons with small expected cell counts. All data analyses were generated using SAS software, Version 9.2, of the SAS System for Windows (SAS Institute Inc., Cary, NC, USA). A two-sided 0.05 alpha level was used to define statistical significance.
Results
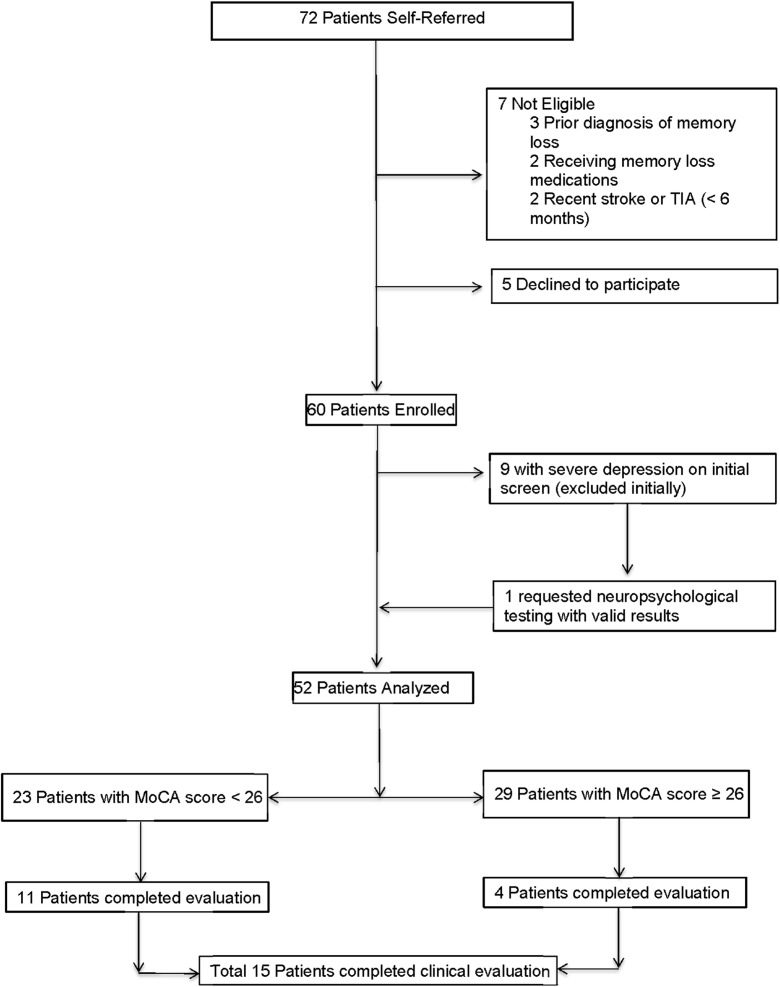
Sixty consecutive community-dwelling patients, with a mean age of 64 years, were screened over a 12-month period. A flow diagram of patients enrolled and analyzed is presented in Fig. 1.
Fig. 1.
Flow diagram of subjects screened, enrolled, and analyzed
Demographic variables, vascular risk factors, pertinent medications, and cognitive and depression status for all patients and subgroups of patients separated by MoCA scores are presented in Table 1. All patients were veterans of the United States Armed Forces, resulting in an over-representation of males. The most prevalent vascular risk factors included hypertension (92%), hyperlipidemia (88%), diabetes (47%), and prior/current smoking (78%). Most (95%) had at least a high-school education, with 22% achieving either college or advanced degrees.
Table 1.
Demographics, risk factors, and pertinent medications in all patients (N = 60) and in those separated by initial cognitive screening scores. Data include mean/standard deviation for continuous measures and frequency/percentages for categorical measures
| Characteristic | All participants (n = 60) | MoCA < 26 (n = 23) | MoCA ≥ 26 (n = 29) | P † | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | ||
| Age (years) | 50 | 86 | 64 | 7.1 | 51 | 86 | 65.3 | 7.3 | 50 | 75 | 63.3 | 7.3 | 0.31 |
| Count | % | Count | % | Count | % | ||||||||
| Male gender | 54 | 90 | 23 | 100 | 24 | 83 | 0.059* | ||||||
| Education | 0.40** | ||||||||||||
| < High school | 3 | 5 | 2 | 9 | 0 | 0 | |||||||
| High school | 16 | 27 | 5 | 22 | 8 | 28 | |||||||
| Some college | 28 | 47 | 12 | 52 | 14 | 48 | |||||||
| College/advanced degree | 13 | 22 | 4 | 17 | 7 | 24 | |||||||
| Smoking status | 0.084 | ||||||||||||
| Never | 13 | 22 | 6 | 26 | 4 | 14 | |||||||
| Former | 22 | 36 | 5 | 22 | 15 | 52 | |||||||
| Current | 25 | 42 | 12 | 52 | 10 | 34 | |||||||
| Medication | |||||||||||||
| Antiplatelets | 43 | 72 | 18 | 78 | 19 | 66 | 0.31 | ||||||
| Statins | 46 | 77 | 19 | 83 | 23 | 79 | > 0.9* | ||||||
| ACE inhibitor | 28 | 47 | 8 | 35 | 17 | 59 | 0.09 | ||||||
| ARBs | 7 | 12 | 3 | 13 | 3 | 10 | > 0.9* | ||||||
| Beta blocker | 32 | 53 | 15 | 65 | 13 | 45 | 0.14 | ||||||
| Calcium channel blocker | 22 | 36 | 10 | 43 | 11 | 38 | 0.69 | ||||||
| Diuretic | 25 | 42 | 12 | 52 | 11 | 38 | 0.30 | ||||||
| Comorbidities | |||||||||||||
| Coronary artery disease | 20 | 34 | 7 | 30 | 10 | 36 | 0.69 | ||||||
| Atrial fibrillation | 1 | 2 | 0 | 0 | 0 | 0 | – | ||||||
| Peripheral artery disease | 6 | 10 | 2 | 9 | 2 | 7 | > 0.9* | ||||||
| Hypercholesterolemia | 53 | 88 | 21 | 91 | 25 | 86 | 0.68* | ||||||
| Hypertension | 55 | 92 | 22 | 96 | 25 | 86 | 0.37* | ||||||
| Diabetes | 28 | 47 | 10 | 43 | 13 | 45 | 0.92 | ||||||
ACE inhibitor angiotensin-converting enzyme inhibitor, ARBs angiotensin II receptor blockers
†P value reflects comparison of means, using a two-sample t test, for continuous measures and a comparison of proportions, using a chi-square test, for categorical measures, unless otherwise indicated, between groups defined by initial cognitive screening scores
*Fisher’s exact test
**Cochran-Armitage trend test
At the initial screening visit, nine patients (15%) were found to have severe depression, without suicidal ideation. Of these patients, one individual, who was already under the care of a psychiatrist, requested evaluation for memory loss including neuropsychological testing. The results showed the presence of cognitive impairment in addition to depression and were interpreted as valid by the neuropsychologist. He was included in subsequent analyses. The remaining eight patients (13%) with severe depression were excluded from subsequent analyses. Six of these eight patients were already seen, treated and followed by a psychiatrist at our facility. The remaining two patients declined Mental Health Clinic referral because they already had an established physician-patient relationship with a mental health professional in their community.
After excluding the eight severely depressed patients, 23 patients of the remaining 52 had abnormal MoCA scores (44%, 95%CI 30–59%). There were no significant differences between patients with (N = 23) and without (N = 29) abnormal MoCA for demographic characteristics, educational status, vascular risk factors, or pertinent medications (Table 1). Trends towards significance were noted for sex and smoking, with a higher percentage of males and current smokers among patients with cognitive impairment compared to those without cognitive impairment (Table 1).
A total of 15 patients agreed to undergo additional evaluation for memory loss in our memory loss clinic. These 15 included 11 of the 23 patients with MoCA scores < 26 as well as an additional four patients with normal MoCA scores who requested evaluation based on subjective symptoms. The evaluation consisted of a complete neurological examination, repeat cognitive screening, standard laboratory tests, and brain imaging. Neuropsychological testing was obtained for those patients who were agreeable. Final cognitive status was assigned by adjudication as part of the memory loss clinic consensus management conference and resulted in the following results: (a) normal (N = 4), (b) non-amnestic MCI (N = 4), (c) vascular MCI (N = 5), or (d) vascular dementia (N = 2).
Table 2 provides a summary of the association between the initial cognitive status defined by the MoCA score, with patients classified as having abnormal (MoCA < 26) or normal (MoCA ≥ 26) cognitive status, and the final cognitive assessment (adjudicated status). MoCA correctly identified cognitive status in 87% of cases [Kappa coefficient of 0.66 (95% CI 0.23–1.00)].
Table 2.
Association between initial cognitive status defined by the MoCA score and the final cognitive assessment (adjudicated status)
| Adjudicated final cognitive status | Initial cognitive status by MOCA score | ||
|---|---|---|---|
| Impaired | Normal | Total (N) | |
| No cognitive impairment | 1 | 3 | 4 |
| Non-amnestic MCI | 4 | 0 | 4 |
| Vascular MCI | 4 | 1 | 5 |
| Vascular dementia | 2 | 0 | 2 |
| Total | 11 | 4 | 15 |
Discussion
Our results show that American Indian veterans with multiple vascular risk factors have high rates of undiagnosed, most often vascular, cognitive impairment, and exceeding rates of cognitive impairment documented in previously published older non-American Indian cohorts (Mehta and Yeo 2017; Hugo and Ganguli 2014; Knopman et al. 2016). A major strength of our study is that all patients evaluated for memory loss underwent brain imaging, allowing for confirmation of ischemic changes typical of vascular cognitive impairment (Gorelick et al. 2011). In addition, none of the individuals who completed formal clinical evaluation for memory loss were found to meet criteria for amnestic MCI, a prodrome of Alzheimer disease (AD), or Alzheimer-type dementia. While the potential for an additional contribution from neurodegenerative or multifactorial causes cannot be entirely excluded in some of the patients with non-amnestic MCI, these results strongly suggest that vascular pathology, instead of the classic neurodegenerative pathology, is the main contributing factor for cognitive impairment in this population.
The increased recognition of the impact of vascular risk factors on cognitive impairment and the overlap between vascular risk factors, aging and classic neurodegenerative diseases, such as AD, have generated interest in deciphering mechanisms involved (Gorelick et al. 2011; Corriveau et al. 2016). High-resolution imaging has resulted in better definition of key vascular lesions linked to vascular cognitive impairment, such as cerebral microhemorrhages, white matter hyperintensities, and brain infarction (Gorelick et al. 2011; Corriveau et al. 2016). A complex relationship has been reported between these vascular lesions revealing an association between the presence of cerebral microhemorrhages and increased risk for intracerebral hemorrhage after ischemic stroke. Additionally, the presence and progression of white matter hyperintensities have been demonstrated to precede the appearance of microhemorrhages (Ungvari et al. 2017). Among the vascular risk factors involved, hypertension in the elderly has been shown to substantially increase the the risk for both vascular cognitive impairment and AD by alterations in the neurovascular coupling processes and microvascular injury (Csiszar et al. 2017), with increasing evidence for the causal role of neurovascular uncoupling in later development of cognitive decline (Tarantini et al. 2017). Hypertension, in addition to aging and AD, has also been shown to be strongly associated with an increased risk for cerebral microhemorrhages, and development of susebquent cognitive decline (Ungvari et al. 2017). While mechanisms underlying the development of cerebral microhemorrhages are not completely understood, there is evidence that alterations in extracellular matrix of cerebral vasculature, abnormalities in vascular smooth muscle cells, arterial stiffening, increased pulse pressure, and impaired myogenic protection are all significant contributors (Ungvari et al. 2017). Age-related dysregulation of cerebral microvasculature leads to blood-brain-barrier disruption, neuroinflammation, exacerbation of neurodegeneration, development of cerebral microhemorrhages, microvascular rarefaction, ischemic neuronal dysfunction, and subsequent neuronal damage (Toth et al. 2017). These complex, multifaceted mechanisms involved in the pathogenesis of vascular cognitive impairment support the need to study populations at risk for developing cognitive impairment, ideally focusing on the stage preceding the development of dementia (Gorelick et al. 2011; Corriveau et al. 2016). Furthermore, these efforts should strive to include broad racial and ethnic backgrounds to account for differing contributions from varying genetic and vascular risk profiles.
Previous studies examining the impact of race and ethnicity in the prevalence and incidence of dementia in USA dementia focused on comparing rates of dementia among African-Americans and Hispanics to rates of dementia among whites and consistently found higher rates of dementia and AD among racial and ethnic minorities as compared to whites (Folstein et al. 1991; Berkman and Gurland 1998; Haerer et al. 1987; Perkins et al. 1997; Prineas et al. 1995; Schoenberg et al. 1985; Still et al. 1990; Teresi et al. 1999). This finding was confirmed even in a large population-based longitudinal study of elderly New York City residents examining the incidence of AD among three ethnic/racial groups (Tang et al. 2001), demonstrating a significantly higher incidence rate for AD among non-Hispanic black and Caribbean Hispanic elders as compared to non-Hispanic whites, even after correcting for potential confounders such as differences in years of formal education (Tang et al. 2001).
Our current findings are consistent with prior research supporting the existence of significant differences in dementia etiology between American Indians and whites. In a study conducted in Canada, Hendrie et al. compared 192 elderly Cree Indians and an age-stratified sample of 241 English-speaking whites (Hendrie et al. 1993). The authors found a significant lower age-adjusted prevalence of AD among the Cree Indians (0.5%) as compared to whites (3.5%), while the age-adjusted prevalence of dementia was similar between the two groups (Hendrie et al. 1993). A separate study of Cherokee Indians living in northeastern Oklahoma compared 26 individuals over the age of 65 with AD to an equal number of normal controls (Rosenberg et al. 1996). The degree of ethnic ancestry for each participant was assessed by genealogical records provided by the Cherokee Nation Tribal Registration Department, and presence of the ε4 allele of the apolipoprotein E gene was determined. Interestingly, the authors found that elders with more than 50% genetic Cherokee ancestry were less likely to be in the AD group than the control group, further supporting a greater tendency for vascular rather than AD-type causes for cognitive impairment among American Indian populations (Rosenberg et al. 1996).
Despite the absence of comprehensive national dementia prevalence data for American Indians, prior publications have suggested a higher risk for non-Alzheimer dementia (Jervis and Manson 2002) and an increased risk for dementia at a younger age in American Indians compared to the general population (Garrett et al. 2015; Jacklin et al. 2012). Furthermore, with the increased prevalence of vascular risk factors (Rhoades et al. 2007; Howard et al. 1999) and the growing number of American Indians 65 years and older (Garrett et al. 2015), the number of American Indians with vascular dementia is likely to increase in upcoming decades. Thus, we argue that improved vascular risk reduction earlier in life and detection of deficits in the mild stages of cognitive impairment would be highly beneficial in preventing vascular dementia in this at risk population.
Our pilot study has several limitations, including a small sample size and under-representation of women. The sample size does not allow us to adequately determine specific associations between cognitive impairment and individual vascular risk factors. Another potential limitation is the lack of analysis of the degree of ancestry, as patients were classified based on self-identification as American Indian. Lastly, only a portion of the patients who scored positive for cognitive impairment agreed to complete formal testing to characterize the most likely etiology for memory loss. As a result, although a high level of agreement between MoCA and cognitive status was noted, the confidence interval for the Kappa coefficient is wide.
The present study provides further support of the concept that incidence of AD is likely lower in American Indians and provides critical preliminary evidence that vascular cognitive impairment may be more prevalent in this ethnic group. These results support the need for further, larger studies, with close involvement of the American Indian patient support groups and local communities. Goals of future studies should include determining prevalence and etiology of cognitive impairment, maximizing management strategies for vascular risk reduction and elucidating specific mechanisms leading to the development of vascular cognitive deficits in American Indians to improve care in this underserved and understudied population.
Acknowledgments
We thank Mrs. Candy Klump, the Oklahoma City VA Native American program coordinator, for assistance with recruitment of patients. We also thank Adrienne Elias, Daniel Garcia-Martino, and Eka Makharoblidze, for their assistance with cognitive and depression screening procedures. We thank all our veterans for participation.
Authors contributions
A. Kirkpatrick: study concept and design, acquisition of data, analysis, and interpretation of data and preparation of manuscript. J. Stoner: analysis and interpretation of data and preparation of manuscript. F. Donna-Ferreira: acquisition of data, interpretation of data, and preparation of manuscript. George C. Malatinszky: acquisition of data, interpretation of data, and preparation of manuscript. Leslie D. Guthery: acquisition of data, interpretation of data, and preparation of manuscript. J. Scott: interpretation of data and preparation of manuscript. C. Prodan: study concept and design, acquisition of data, interpretation of data, and preparation of manuscript.
Funding information
This work was supported by grants from the United States Department of Veterans Affairs (1I01CX000340), American Heart Association (15GRNT25270010), and the National Institute of General Medical Sciences (U54GM104938).
Compliance with ethical standards
The study was approved by the Institutional Review Board of the University of Oklahoma Health Sciences Center and the local Veterans Affairs Research and Development Committee rules and regulations. The study was carried out in accordance with the Helsinki Declaration of 1975 (and as revised in 1983). Individual informed consent was obtained for all study participants.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet. 2015;386:1672–1682. doi: 10.1016/S0140-6736(15)00461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio: Psychological Corporation; 1996. [Google Scholar]
- Berkman CS, Gurland BJ. The relationship between ethnoracial group and functional level in older persons. Ethn Health. 1998;3:175–188. doi: 10.1080/13557858.1998.9961860. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Bosetti F, Emr M, Gladman JT, Koenig JI, Moy CS, Pahigiannis K, Waddy SP, Koroshetz W. The science of vascular contributions to cognitive impairment and dementia (VCID): a framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell Mol Neurobiol. 2016;36:281–288. doi: 10.1007/s10571-016-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Flores S, Rabinstein A, Biller J, Elkind MS, Griffith P, Gorelick PB, et al. Racial-ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2091–2116. doi: 10.1161/STR.0b013e3182213e24. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Tarantini S, Fulop GA, Kiss T, Valcarcel-Ares MN, Galvan V et al (2017) Hypertension impairs neurovascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. Geroscience. 10.1007/s11357-017-9991-9 [DOI] [PMC free article] [PubMed]
- Folstein MF, Bassett SS, Romanoski AJ, Nestadt G. The epidemiology of delirium in the community: the eastern Baltimore mental health survey. Int Psychogeriatr. 1991;3:169–176. doi: 10.1017/S1041610291000637. [DOI] [PubMed] [Google Scholar]
- Garrett MD, Baldridge D, Benson W, Crowder J, Aldrich N. Mental health disorders among an invisible minority: depression and dementia among American Indian and Alaska native elders. Gerontologist. 2015;55:227–236. doi: 10.1093/geront/gnu181. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/CIRCULATIONAHA.113.003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haerer AF, Anderson DW, Schoenberg BS. Survey of major neurologic disorders in a biracial United States population: the Copiah County Study. South Med J. 1987;80:339–343. doi: 10.1097/00007611-198703000-00016. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, Hall KS, Pillay N, Rodgers D, Prince C, Norton J, Brittain H, Nath A, Blue A, Kaufert J, Shelton P, Postl B, Osuntokun B. Alzheimer’s disease is rare in Cree. Int Psychogeriatr. 1993;5:5–14. doi: 10.1017/S1041610293001358. [DOI] [PubMed] [Google Scholar]
- Howard BV, Lee ET, Cowan LD, Devereux RB, Galloway JM, Go OT, Howard WJ, Rhoades ER, Robbins DC, Sievers ML, Welty TK. Rising tide of cardiovascular disease in American Indians. The strong heart study. Circulation. 1999;99:2389–2395. doi: 10.1161/01.CIR.99.18.2389. [DOI] [PubMed] [Google Scholar]
- Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med. 2014;30:421–442. doi: 10.1016/j.cger.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacklin KM, Walker JD, Shawande M. The emergence of dementia as a health concern among first nations populations in Alberta, Canada. Can J Public Health. 2012;104:e39–e44. doi: 10.1007/BF03405652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jervis LL, Manson SM. American Indians/Alaska natives and dementia. Alzheimer Dis Assoc Disord. 2002;16(Suppl 2):S89–S95. doi: 10.1097/00002093-200200002-00011. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, et al. Mild cognitive impairment and dementia prevalence: the atherosclerosis risk in communities neurocognitive study (ARIC-NCS) Alzheimers Dement. 2016;2:1–11. doi: 10.1016/j.dadm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12:216–224. doi: 10.1016/j.jalz.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, el-Agnaf O, Feldman H, Ferman TJ, ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O'Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology. 2017;89:88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement. 2017;13:72–83. doi: 10.1016/j.jalz.2016.06.2360. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Perkins P, Annegers JF, Doody RS, Cooke N, Aday L, Vernon SW. Incidence and prevalence of dementia in a multiethnic cohort of municipal retirees. Neurology. 1997;49:44–50. doi: 10.1212/WNL.49.1.44. [DOI] [PubMed] [Google Scholar]
- Prineas RJ, Demirovic J, Bean JA, Duara R, Gomez-Marin O, Loewenstein D, et al. South Florida program on aging and health. Assessing the prevalence of Alzheimer’s disease in three ethnic groups. J Fla Med Assoc. 1995;82:805–810. [PubMed] [Google Scholar]
- Rhoades DA, Welty TK, Wang W, Yeh F, Devereux RB, Fabsitz RR, et al. Aging and the prevalence of cardiovascular disease risk factors in older American Indians: the strong heart study. J Am Geriatr Soc. 2007;55:87–94. doi: 10.1111/j.1532-5415.2006.01018.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg RN, Richter RW, Risser RC, Taubman K, Prado-Farmer I, Ebalo E, et al. Genetic factors for the development of Alzheimer disease in the Cherokee Indian. Arch Neurol. 1996;53:997–1000. doi: 10.1001/archneur.1996.00550100071017. [DOI] [PubMed] [Google Scholar]
- Schoenberg BS, Anderson DW, Haerer AF. Severe dementia. Prevalence and clinical features in a biracial US population. Arch Neurol. 1985;42:740–743. doi: 10.1001/archneur.1985.04210090004002. [DOI] [PubMed] [Google Scholar]
- Still CN, Jackson KL, Brandes DA, Abramson RK, Macera CA. Distribution of major dementias by race and sex in South Carolina. J S C Med Assoc. 1990;86:453–456. [PubMed] [Google Scholar]
- Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/WNL.56.1.49. [DOI] [PubMed] [Google Scholar]
- Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–58. doi: 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teresi JA, Albert SM, Holmes D, Mayeux R. Use of latent class analyses for the estimation of prevalence of cognitive impairment, and signs of stroke and Parkinson’s disease among African-American elderly of Central Harlem: results of the Harlem aging project. Neuroepidemiology. 1999;18:309–321. doi: 10.1159/000026226. [DOI] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–H20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A, Prodan CI. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol. 2017;312:H1128–H1143. doi: 10.1152/ajpheart.00780.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]