Abstract
Objectives
To characterize US patients with ankylosing spondylitis (AS) who were treated with secukinumab and to assess rheumatologist-reported reasons for prescribing treatment in clinical practice.
Methods
This descriptive analysis of data from a US retrospective medical chart review included patients aged ≥ 18 years diagnosed with AS who initiated secukinumab after 15 January 2016. Eligible rheumatologists used online forms to collect patient demographics, disease characteristics, co-morbidity profile, and treatment history prior to or on the index date, defined as the date of the first secukinumab prescription recorded in the medical chart. Information on physician-level characteristics and reasons for secukinumab prescription and dosing were also collected.
Results
Medical charts from 78 patients with AS who initiated secukinumab were reviewed by 25 rheumatologists between 7 July 2017 and 11 August 2017. Overall, 76.9% of patients were male, mean (SD) age was 39.8 (10.8) years, and 34.6% were biologic naïve. The most common reasons for secukinumab initiation among biologic-naïve and biologic-experienced patients, respectively, were efficacy/effectiveness (77.8%) and failure of other prior biologics (84.3%). Nearly all patients (94.9%) received a loading dose, including 150 mg every week (39.7%), 300 mg every week (53.8%), and other (1.3%). Overall, 73 patients (93.6%) received ≥ 1 maintenance secukinumab dose, of whom 56.2% and 43.8% received 150 mg and 300 mg, respectively, every 4 weeks.
Conclusions
In this US medical chart review of patients with AS who initiated secukinumab, approximately one-third were biologic naïve, and secukinumab efficacy/effectiveness and failure of other biologics were the most common reasons for initiating secukinumab.
Electronic supplementary material
The online version of this article (10.1007/s40801-018-0146-9) contains supplementary material, which is available to authorized users.
Key Points
| In this medical chart review of US patients with ankylosing spondylitis, approximately one-third of patients (34.6%) were biologic naïve, and another 53.8% of patients had received only one biologic prior to initiating secukinumab, suggesting that rheumatologists are prescribing secukinumab early in the treatment algorithm when considering biologic therapy. |
| The most common reasons for secukinumab initiation among biologic-naïve and biologic-experienced patients, respectively, were efficacy/effectiveness of secukinumab (77.8%) and failure of other prior biologics (84.3%). |
| These findings will provide information to rheumatologists on how secukinumab is used by their peers for the treatment of ankylosing spondylitis in US clinical practice. |
Introduction
Ankylosing spondylitis (AS) is a chronic, immune-mediated disease characterized by involvement of the axial skeleton and sacroiliac joints [1, 2]. Inflammation of the vertebrae is often associated with chronic pain and stiffness of the entire spine and sacroiliac joints, and in severe cases can lead to structural changes in the spine—including fusion of the spine and sacroiliac joints—and new bone formation [1, 2]. AS has an estimated prevalence of 0.2–0.5% in the US and is associated with considerable physical, emotional, and economic burden [3–5].
The goals of treatment in patients with AS are to reduce and/or control inflammation and symptoms of pain, stiffness, and fatigue; maintain spinal flexibility and normal posture; reduce functional limitations; preserve or normalize social participation; maintain work ability; and decrease complications of disease (e.g., uveitis) [6, 7]. Current treatment guidelines recommend nonsteroidal anti-inflammatory drugs (NSAIDs) as first-line therapy in patients with active AS who have pain and stiffness. In addition, local glucocorticoids may be used for patients with peripheral arthritis, enthesitis, or isolated sacroiliitis. Analgesics (e.g., acetaminophen and opioids) may be considered in patients in whom previously recommended treatments have failed and resulted in residual pain, are contraindicated, and/or are poorly tolerated. In patients with active AS despite NSAID treatment, use of a biologic is recommended. Current practice suggests use of a tumor necrosis factor inhibitor (TNFi) as first-line biologic therapy; however, approximately 40% of patients do not achieve a response, as defined by the Assessment of SpondyloArthritis international Society criteria for 20% improvement (ASAS 20) [8]. If the initial TNFi therapy fails, switching to another TNFi or an interleukin (IL)-17 inhibitor should be considered [7]. Although switching TNFis may be effective for some patients, subsequent TNFis may not be as effective [9–11], highlighting the unmet patient needs for biologics with different mechanisms of action.
Secukinumab is a fully human anti-IL-17A monoclonal antibody and is the first and only non-TNFi approved for the treatment of AS in the US. With patients and physicians having more options available for treatment, it is important to understand why therapies are being started or stopped and how they are being prescribed; however, there are a limited number of studies describing why or how secukinumab is being prescribed in real-world settings. The objectives of this analysis were to describe the clinical and treatment characteristics of patients with AS who were prescribed secukinumab in US clinical practice and to describe rheumatologist-reported reasons for prescribing secukinumab.
Materials and Methods
Data Source and Study Design
This was a retrospective medical chart review of patients in the US diagnosed with AS and aged ≥ 18 years who initiated secukinumab treatment after its approval date of 15 January 2016. Rheumatologists who were from different geographic areas and represented different demographics and practice settings were recruited from an external vendor to participate in this study. The selected vendor has the largest panel of medical specialists in the US. Physicians in this panel cover all states of the US and have similar age, gender, and practice type distribution to the American Medical Association (AMA)-registered physicians. The vendor’s access to panelists is facilitated by the online services provided by the vendor to physicians, such as medical education, ethical drug promotion, clinical development, job recruitment, and clinic appointment services. Rheumatologists were screened based on the following eligibility criteria: (1) completed their residency and fellowship training; (2) treated ≥ 1 patient with AS in the 12 months prior to the chart review; and (3) had prescribed secukinumab for the treatment of AS. Eligible rheumatologists were then invited to provide patient information using an online chart abstraction form.
This study was designed and implemented in accordance with the Guidelines for Good Pharmacoepidemiology Practices of the International Society for Pharmacoepidemiology, the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines, and with the ethical principles laid down in the Declaration of Helsinki [12, 13]. An institutional review board exemption was obtained for the study prior to initiation of data collection.
Study Population
Eligible patients were required to meet the following criteria: (1) received a diagnosis of AS; (2) were prescribed secukinumab for the treatment of AS by a participating rheumatologist in a real-world setting (i.e., outside of an interventional clinical trial) on or after 15 January 2016; (3) were aged ≥ 18 years at the time of secukinumab initiation; and (4) had medical records containing information on AS diagnosis, co-morbidities, and treatment history prior to the secukinumab initiation that were accessible to the participating rheumatologists.
Measures and Outcomes
The chart abstraction form was used to collect patient-level information abstracted by rheumatologists from eligible patients’ medical charts, such as demographics (age, sex, race, and insurance type), disease characteristics (disease duration, physician-assessed disease severity, AS symptoms experienced, and co-morbidities), treatment history (previous treatments and rheumatologist-reported reasons for discontinuation of treatment immediately preceding secukinumab), and characteristics of secukinumab use (duration of treatment, dosing, current secukinumab treatment status, rheumatologist-reported reasons for secukinumab prescription, and reasons for secukinumab discontinuation [if applicable]). The screening section was used both to confirm that participating rheumatologists had ≥ 1 chart for a patient with AS that met all study selection criteria and to collect physician-level data such as demographics (age and sex), practice characteristics (type of practice and region of practice), and experience treating patients with AS (years of experience, number of patients with AS seen in the previous year, number of AS charts contributed to the study, and tools and references used to assist AS diagnosis).
Data Analysis
Descriptive statistics are shown for all patient demographics, disease characteristics, treatment history, and characteristics of secukinumab use, as well as physician-level characteristics. Rheumatologist-reported reasons for secukinumab prescription were also examined separately among biologic-naïve and biologic-experienced patients. Categorical variables were summarized using frequency counts and percentages. Continuous variables were summarized by means and SDs or medians and interquartile ranges (IQRs).
All data analyses were conducted using Statistical Analysis System 9.4 software.
Results
Rheumatologist Characteristics
Medical charts for 78 patients with AS who initiated secukinumab were reviewed by 25 rheumatologists between 7 July 2017, and 11 August 2017. On average, rheumatologists contributed a mean (SD) of 3.1 (1.6) AS medical charts to the analysis.
Characteristics of the participating rheumatologists are presented in Table 1. Overall, the rheumatologists had been in practice for a mean (SD) of 12.8 (9.2) years; 19 of 25 rheumatologists were from a private practice setting and 11 of 25 were from the Northeast region of the US. Most rheumatologists (n = 19) used established criteria to assist in the diagnosis of AS, including the ASAS criteria (n = 15), the 1984 modified New York criteria for AS (n = 13), or both (n = 9). In addition, nearly all rheumatologists (n = 24) referenced published international treatment guidelines to assist with AS treatment, including those published by the American College of Rheumatology (ACR) (n = 22), ASAS/European League Against Rheumatism (EULAR) (n = 9), or both (n = 7).
Table 1.
Characteristics of rheumatologists participating in the medical chart review of patients with AS (N = 25)
| Characteristic | Rheumatologists (N = 25) |
|---|---|
| Age, mean (SD), years | 45.8 (12.4) |
| Male, n | 18 |
| Type of practice, n | |
| Private practice | 19 |
| Academic institution | 6 |
| Region of practice, n | |
| Northeast | 11 |
| Midwest | 4 |
| South | 6 |
| West | 4 |
| Years in practice, mean (SD) | 12.8 (9.2) |
| No. of AS patient medical charts contributed by physician, mean (SD) | 3.1 (1.6) |
| Tools referenced to assist AS diagnosis, na | |
| New York criteria | 13 |
| ASAS criteria | 15 |
| New York criteria and ASAS criteria | 9 |
| Other | 0 |
| No criteria | 6 |
| AS treatment guidelines referenced, nb | |
| ACR | 22 |
| ASAS/EULAR | 9 |
| ACR and ASAS/EULAR | 7 |
| Other | 0 |
| No guidelines | 1 |
ACR American College of Rheumatology, AS ankylosing spondylosis, ASAS Assessment of SpondyloArthritis international Society, EULAR European League Against Rheumatism
aMultiple tools could be selected by a single physician except when “no criteria” was selected
bMultiple guidelines could be selected by a single physician except when “no guidelines” was selected
Patient Demographic and Disease Characteristics at the Time of Secukinumab Initiation
Among the 78 patients with AS whose charts were reviewed, 76.9% were male and the mean (SD) age was 39.8 (10.8) years (Table 2). At the time of secukinumab initiation, 14.1% of patients had a disease duration of < 1 year and 7.7% had a duration of > 10 years. Overall, most patients (91.0%) had moderate or severe rheumatologist-assessed AS disease severity. The most common symptoms were lower back pain (91.0%), morning stiffness (83.3%), and limited spinal mobility/range of motion (61.5%). Nearly two-thirds of patients (65.4%) had co-morbidities, the most common of which were hypertension (25.6%), anemia (20.5%), digestive disorders (15.4%), and anxiety (11.5%); 5.1% of patients also had psoriasis (Table 2).
Table 2.
Demographic and disease characteristics at the time of secukinumab initiation of patients with AS (N = 78)
| Characteristic | Patients with AS (N = 78) |
|---|---|
| Age | |
| Mean (SD), years | 39.8 (10.8) |
| Median (IQR), years | 38.0 (32.0–45.0) |
| Male, n (%) | 60 (76.9) |
| Race/ethnicity, n (%) | |
| White/non-Hispanic | 54 (69.2) |
| Hispanic | 13 (16.7) |
| Black/non-Hispanic | 8 (10.3) |
| Asian/Pacific Islander | 3 (3.8) |
| Insurance type, n (%) | |
| Commercial/private | 56 (71.8) |
| Medicaid | 13 (16.7) |
| Medicare | 9 (11.5) |
| Military | 1 (1.3) |
| No insurance | 1 (1.3) |
| Unknown/not sure | 1 (1.3) |
| Duration of AS, n (%) | |
| < 1 year | 11 (14.1) |
| 1–2 years | 26 (33.3) |
| 3–5 years | 22 (28.2) |
| 6–10 years | 13 (16.7) |
| > 10 years | 6 (7.7) |
| Physician-assessed disease severity, n (%) | |
| Mild | 7 (9.0) |
| Moderate | 56 (71.8) |
| Severe | 15 (19.2) |
| AS symptoms experienced, n (%)a | 78 (100) |
| Lower back pain | 71 (91.0) |
| Morning stiffness | 65 (83.3) |
| Limited spinal mobility and range of motion | 48 (61.5) |
| Fatigue (e.g., low energy/tired) | 47 (60.3) |
| Nocturnal pain | 45 (57.7) |
| Neck pain | 38 (48.7) |
| Hip pain | 26 (33.3) |
| Enthesitis | 23 (29.5) |
| Peripheral arthritis | 20 (25.6) |
| Dactylitis | 10 (12.8) |
| Inflammatory eye disease | 6 (7.7) |
| Otherb | 1 (1.3) |
| Co-morbidities, n (%)c | |
| Hypertension | 20 (25.6) |
| Anemia | 16 (20.5) |
| Digestive disorders | 12 (15.4) |
| Anxiety | 9 (11.5) |
| Hyperlipidemia | 8 (10.3) |
| Depression | 6 (7.7) |
| Inflammatory eye diseases (e.g., uveitis) | 5 (6.4) |
| Crohn disease | 4 (5.1) |
| Diabetes | 4 (5.1) |
| Psoriasis | 4 (5.1) |
| Ulcerative colitis | 4 (5.1) |
| Malignancyd | 2 (2.6) |
AS ankylosing spondylosis
aMultiple AS symptoms could be selected for each patient except when “None of the above” was selected
bOther symptoms included “psoriasis”
cMultiple co-morbidities could be selected for each patient except if “none of the above” was selected
dMalignancy included “melanoma”
AS Treatment History
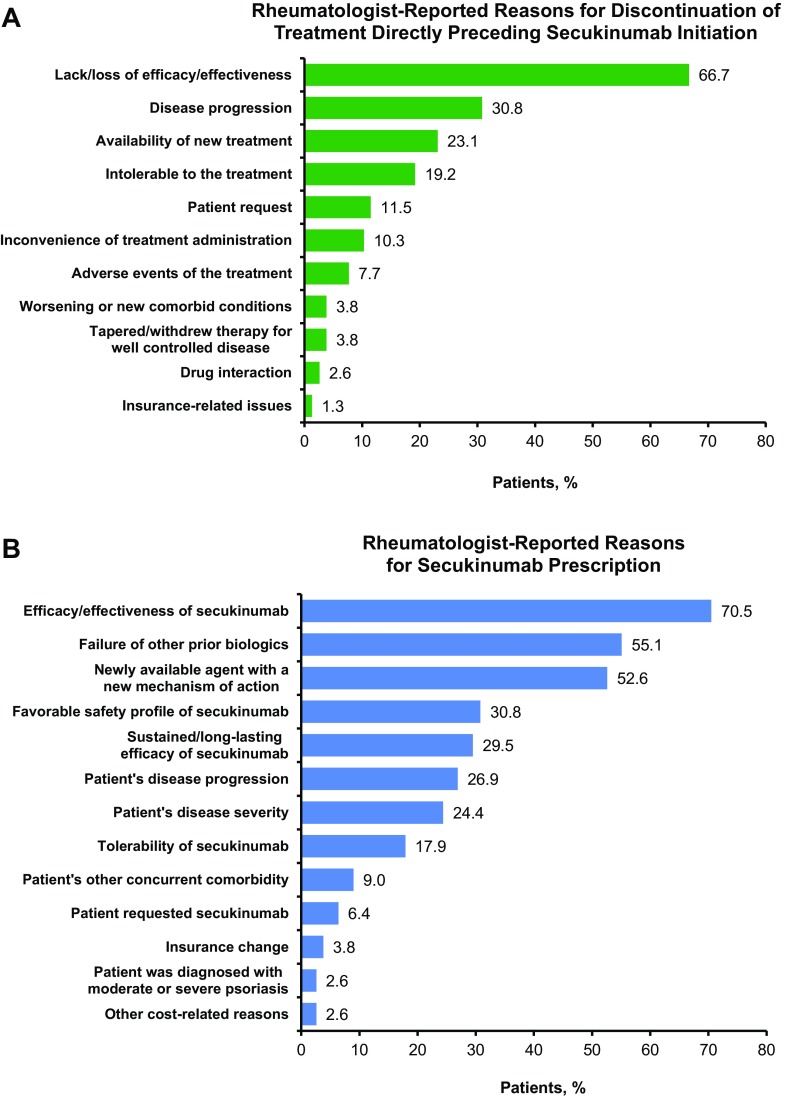
All 78 patients with AS received treatment prior to initiating secukinumab (Table 3). The median (IQR) time from initiation of patients’ first treatment to initiation of secukinumab was 18.1 (9.0–33.1) months, and patients received a mean (SD) of 2.5 (1.3) treatments prior to initiating secukinumab. Patients received a mean (SD) of 0.8 (0.7) prior biologics at any time prior to secukinumab initiation, including 42 patients (53.8%) who received one prior biologic, eight (10.3%) who received two prior biologics, and one (1.3%) who received ≥ three prior biologics. Over one-third of patients (n = 27; 34.6%) were biologic naïve. Etanercept (29.5%) and adalimumab (28.2%) were the most common biologics used prior to secukinumab. NSAIDs (52.6%) and biologics (51.3%) were the most commonly used classes of treatments directly preceding secukinumab. The most common rheumatologist-reported reasons for discontinuation of treatment directly preceding secukinumab were lack/loss of efficacy/effectiveness (66.7%), disease progression (30.8%), and availability of new treatment (23.1%) (Fig. 1a).
Table 3.
Treatment history of patients with AS (N = 78)
| Characteristic | Patients with AS (N = 78) |
|---|---|
| Received treatment prior to secukinumab, n (%) | 78 (100.0) |
| Time since initiation of first treatment to initiation of secukinumab, median (IQR), months | 18.1 (9.0–33.1) |
| No. of treatments received prior to secukinumaba | |
| Mean (SD) | 2.5 (1.3) |
| 1, n (%) | 16 (20.5) |
| 2, n (%) | 32 (41.0) |
| ≥ 3, n (%) | 30 (38.5) |
| No. of biologics received prior to secukinumaba | |
| Mean (SD) | 0.8 (0.7) |
| 1, n (%) | 42 (53.8) |
| 2, n (%) | 8 (10.3) |
| ≥ 3, n (%) | 1 (1.3) |
| Treatments directly preceding secukinumab, n (%)b | |
| NSAIDs | 41 (52.6) |
| Any biologic | 40 (51.3) |
| Nonbiologic DMARDs | 22 (28.2) |
| Analgesics and opioids | 6 (7.7) |
| Glucocorticoids | 5 (6.4) |
AS ankylosing spondylitis, DMARD disease-modifying antirheumatic drug, IQR interquartile range, NSAID nonsteroidal anti-inflammatory drug
aThe number of treatments received prior to secukinumab was calculated among patients who had received ≥ 1 treatment prior to secukinumab. The number of all previous distinct treatments was considered, regardless of treatment combinations
bMultiple treatments directly preceding secukinumab could be selected for each patient
Fig. 1.
a Rheumatologist-reported reasons for discontinuing the treatment that directly preceded secukinumab initiation in patients with AS and b rheumatologist-reported reasons for secukinumab prescription in patients with AS (N = 78). AS ankylosing spondylitis
Rheumatologist-Reported Reasons for Prescription of Secukinumab and Characteristics of Secukinumab Use
The most common rheumatologist-reported reasons for secukinumab prescription were efficacy/effectiveness of secukinumab (70.5%), failure of other prior biologics (55.1%), and secukinumab’s status as a newly available agent with a new mechanism of action (52.6%) (Fig. 1b). When stratified by use of prior biologics (i.e., whether biologic naïve or biologic experienced), the most common reason for secukinumab prescription in biologic-naïve patients was efficacy/effectiveness of secukinumab (77.8%), followed by favorable safety profile of secukinumab (51.9%), and perceived sustained/long-lasting efficacy of secukinumab (51.9%) (Supplemental Fig. S1). Failure of other prior biologics (84.3%), efficacy/effectiveness of secukinumab (66.7%), and secukinumab’s status as a newly available agent with a new mechanism of action (62.7%) were the most common reasons for secukinumab prescription in biologic-experienced patients.
At the time of chart abstraction, patients had received secukinumab for a mean (SD) of 4.7 (4.6) months (Table 4). Only 39.7% of patients with AS received an initial loading dose of 150 mg every week, while 53.8% received an initial loading dose of 300 mg every week and 5.1% did not receive a loading dose. Of the 73 patients who received an initial maintenance dose of secukinumab, more than half (56.2%) received an initial maintenance dose of 150 mg every 4 weeks, with the remaining 43.8% of patients receiving an initial maintenance dose of 300 mg every 4 weeks. Nearly all patients (94.9%) were still receiving secukinumab at the time of chart abstraction, while three patients (3.8%) had discontinued treatment, and one patient (1.3%) had an unknown treatment status. Among the three patients who discontinued treatment, one patient discontinued due to lack of efficacy/effectiveness, one due to patient request and lack of efficacy/effectiveness, and one due to death unrelated to secukinumab treatment.
Table 4.
Characteristics of secukinumab use of patients with AS (N = 78)
| Characteristic | Patients with AS (N = 78) |
|---|---|
| Duration of treatment with secukinumaba | |
| Mean (SD), months | 4.7 (4.6) |
| Median (IQR), months | 3.5 (1.0–6.0) |
| Dose | |
| Initial loading dose, n (%) | |
| 150 mg every weekb | 31 (39.7) |
| 300 mg every weekc | 42 (53.8) |
| Otherd | 1 (1.3) |
| No loading dose | 4 (5.1) |
| Received maintenance dose, n (%) | |
| Initial maintenance dosee | |
| 150 mg every 4 weeks | 41 (56.2) |
| 300 mg every 4 weeks | 32 (43.8) |
| Current use of secukinumab, n (%) | |
| Patient still receiving secukinumab | 74 (94.9) |
| Patient discontinued secukinumab | 3 (3.8) |
| Reason for secukinumab discontinuation, n (%)f | |
| Lack/loss of efficacy/effectiveness | 2 (66.7) |
| Patient request | 1 (33.3) |
| Otherg | 1 (33.3) |
| Unknown status of secukinumab use | 1 (1.3) |
AS ankylosing spondylitis, IQR interquartile range
aDuration of treatment with secukinumab was calculated among all patients; the calculated duration underestimates the true duration because most patients were still receiving secukinumab at the time of the survey
bAmong the 31 patients who initiated secukinumab at 150 mg every week, 24 received an initial maintenance dose of 150 mg every 4 weeks, five received 300 mg every 4 weeks, and two did not remain on the therapy long enough to receive maintenance treatment
cAmong the 42 patients who initiated secukinumab at 300 mg every week, 14 received an initial maintenance dose of 150 mg every 4 weeks, 26 received 300 mg every 4 weeks, and two did not remain on the therapy long enough to receive maintenance treatment
dOther initial loading dose included “methotrexate”
eThe proportions of initial maintenance doses were calculated among patients who received a maintenance dose
fThe proportions of reasons for discontinuation were calculated among patients who discontinued secukinumab. Multiple reasons could be selected for each patient
gOther reason included “patient deceased”
Discussion
In this retrospective chart review of 78 patients who initiated secukinumab for the treatment of AS in US clinical practice, nearly all patients (91.0%) had moderate or severe disease as assessed by their rheumatologist, approximately three-quarters had received an AS diagnosis ≤ 5 years ago, and hypertension and anemia were the most commonly reported co-morbidities. Prior to secukinumab initiation, NSAIDs and biologics were the most common classes of medication used in these patients; approximately one-third of patients were biologic naïve. There are a limited number of real-world studies that have characterized patients in clinical practice who initiated secukinumab for AS. A cross-sectional, web-based panel survey evaluated real-world treatment experience and satisfaction with secukinumab in 200 US patients with AS, 98.5% of whom had received some treatment for AS prior to initiation of secukinumab, and included patients who were primarily male (60.0%) and White (66.0%), with a mean (SD) age of 34.4 (10.6) years [14]. A retrospective analysis from the Symphony Health Solutions, LLC, national medical claims and pharmacy database described the demographic and clinical characteristics of 543 patients with AS who initiated secukinumab in routine clinical practice between 15 January 2016, and 31 December 2016 [15]. Compared with the Symphony database, which also did not collect information on HLA-B27 and based diagnoses on ICD-9-CM or ICD-10 codes, the patients in our chart review were younger (39.8 vs. 46.4 years) and more likely to be male (76.9% vs. 48.4%). However, both cohorts had similar proportions of patients with hypertension as the top co-morbidity (≈ 25%), as well as the proportion of patients who were biologic naïve prior to initiation of secukinumab (≈ 34%). Due to the limitations of healthcare claims data, no information on disease severity was captured in the Symphony database; thus, no comparisons could be made across real-world cohorts regarding clinical characteristics. Although in our study disease severity was assessed on the basis of the participating rheumatologists’ clinical expertise, the high proportion of patients with moderate and severe disease is consistent with patients treated with secukinumab enrolled in phase III randomized controlled trials [16–18]. In contrast with patients enrolled in clinical trials, the patients in our study were younger, were more likely to be male, had a shorter duration of disease, and were less likely to be biologic naïve. Additional research is needed to better characterize baseline clinical features and to evaluate outcomes with secukinumab use as first-line biologic therapy for the treatment of AS in clinical practice versus clinical trials, but overall, these findings are among the first to provide rheumatologists with information on characteristics of and real-world dosing in patients with AS who initiate secukinumab in the US.
In this study, rheumatologists prescribed secukinumab to patients as maintenance therapy at the approved 150-mg dose in more than one-half of patients with AS, with the remaining approximately 40% of patients receiving maintenance therapy at the higher 300-mg dose. Similar proportions of patients in the Symphony database reported initiating secukinumab at doses of 150 mg and 300 mg (59.1% and 40.9%, respectively) [15]. The safety and efficacy of secukinumab 300 mg following an intravenous (IV) loading regimen was investigated in the phase III MEASURE 3 study, which found that the proportion of patients achieving an ASAS 20 response was significantly higher in both the IV-300 mg and IV-150 mg treatment groups than in the placebo group (60.5% and 58.1% vs. 36.8%; p < 0.05 for both comparisons), with no differences in the overall safety profiles of the two dosing regimens [18]. A second study estimating the clinical difference between secukinumab 300 mg and 150 mg following dose escalation to 300 mg (NCT03350815) is currently ongoing. It is possible that some physicians included in our study also treat patients with psoriatic arthritis and psoriasis and are comfortable with prescribing secukinumab at a dose of 300 mg. A dose-ranging study of secukinumab in patients with psoriasis found that patients weighing < 90 kg achieved better responses across all treatment groups compared with those patients weighing ≥ 90 kg, suggesting a potential benefit of weight-based dosing. Although body mass index was not captured in our study, the increasing prevalence of obesity in the US population may contribute to increased use of 300-mg dosing in patients with AS seen in clinical practice [19]. Further research is needed to understand rheumatologist preferences for prescribing one dose over the other; however, the data suggest that in clinical practice for the treatment of AS, there may be flexibility in the dosing of secukinumab used.
The rheumatologists in our study had been in practice for approximately 13 years and most (76.0%) used established criteria to assist in the diagnosis of AS. Although our retrospective chart review provided information on AS symptoms and co-morbidities, no information on specific measures of AS severity was available. Nevertheless, the proportion of patients with moderate AS in this study who initiated secukinumab is in line with recommendations to use biologics, including an IL-17 inhibitor, in patients who do not respond to nonbiologic therapies [7].
The most frequently cited reasons for secukinumab initiation among rheumatologists treating patients with AS were its efficacy/effectiveness, failure of other biologics, and its status as a newly available agent with a new mechanism of action. Although data on some AS symptoms, such as pain and stiffness, were available in this study, other key patient-reported outcome measures were not available in medical charts, which otherwise might have provided additional information and insight into patient experiences to support reasons why secukinumab was prescribed and/or why prior treatments were discontinued [20–22]. Among patients with AS who were biologic naïve, efficacy/effectiveness remained the most common reason for secukinumab initiation, while failure of other prior biologics was the most common reason among biologic-experienced patients. Other reasons were fairly balanced between the two groups, including the desire to initiate a newly available treatment agent with a new mechanism of action. These reasons were primarily focused on the benefits related to efficacy, safety, and tolerability of treatment that have been demonstrated in randomized clinical trials [16, 18]. Additionally, concerns about secukinumab-associated adverse events and insurance-related issues were less frequently cited than other reasons, suggesting that reported payer barriers were not particularly high or were deemed a lower priority. The decision to discontinue treatment could potentially be due to clinical versus insurance reasons. At the time of chart abstraction, patients had received secukinumab for a mean (SD) of 4.7 (4.6) months—this is comparable in duration to the 16-week placebo-controlled period of the MEASURE 1 and 2 pivotal clinical trials of secukinumab in patients with AS. Only three patients in our study had discontinued treatment at the time of chart abstraction, none of whom reported adverse events as a reason for discontinuation. By comparison, at week 16, 1.2% of secukinumab-treated patients in MEASURE 1 and 4.8% of secukinumab-treated patients in MEASURE 2 discontinued treatment due to adverse events. It is possible that these rates could have been elevated because patients and investigators were blinded to study treatment in clinical trials. In addition, discontinuations due to adverse events may have been observed in our study with longer follow-up [16].
As with any retrospective observational study, our study has some limitations that should be considered when interpreting the results. The sample size was relatively small (N = 78), particularly for patients with milder rheumatologist-assessed disease severity, and this may reduce the generalizability of the findings to a larger US population of patients with AS; however, all regions of the US were well represented by participating rheumatologists. Because the study relied on self-report by the participating rheumatologists, these findings may be affected by reporting bias or recall bias in the physician screening portion of the study (e.g., bias in favor of secukinumab use, bias in favor of specific practice per guideline recommendations, or selection of “unknown/not sure” response options), as well as non-random missing data in the chart abstraction forms. In addition, rheumatologists contributed data from their own eligible patients into the study; while rheumatologists were to include all eligible patients, this may have been affected by recall bias from the rheumatologist and/or selection bias. Although patients’ medical charts included information on AS diagnosis and three-quarters of rheumatologists used established criteria to aid in the diagnosis of AS, HLA-B27 was not collected by the chart abstraction forms, potentially limiting the generalizability of data to patients with confirmed AS. Furthermore, assessments of disease severity and treatment effectiveness in real-world settings may have been based on clinical expert opinion, using heterogeneous criteria, rather than validated and/or quantifiable measures of disease activity or severity. Lastly, although this study only included patients who initiated secukinumab, the choice of biologic and the decision of when to use secukinumab may differ based on the formulary of each payer/employer group. Despite these limitations, our study offers rheumatologists timely clinical data on patients with AS who initiated secukinumab. Future studies with proper follow-up will allow for additional real-world insights into the duration of secukinumab use, clinical outcomes, and patient-reported experiences while receiving treatment among patients with AS.
Conclusion
These findings provide insight into the characteristics of patients with AS in US clinical practice who are initiating secukinumab, the first and only non-TNFi approved for the treatment of AS in the US, within the first 2 years of approval and the factors leading to prescription of secukinumab by their treating rheumatologists. Approximately one-third of patients were biologic naïve and one-half of patients had received only one biologic prior to initiating secukinumab, which is surprising, given formulary restrictions typically imposed on newer biologic therapies. Lack/loss of efficacy/effectiveness and disease progression/failure of other biologics were the most common rheumatologist-reported reasons for discontinuing preceding treatments and initiating secukinumab in patients with AS. Because more than one-half of patients in our study received secukinumab at an initial loading dose of 300 mg, and > 40% of patients received secukinumab at a maintenance dose of 300 mg, future studies are needed to understand the dosing considerations weighed by rheumatologists who prescribe secukinumab for the treatment of AS.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Support for third-party writing assistance for this manuscript, furnished by Eric Deutsch, Ph.D., CMPP, of Health Interactions, Inc, was provided by Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Funding
This study was sponsored by Novartis Pharmaceuticals Corporation, East Hanover, NJ.
Conflict of interest
R. Joshi has received consulting fees from Novartis. D. Latremouille-Viau, M. K. Meiselbach, and J. Xie are employees of Analysis Group, Inc. Y. Park is an employee of Novartis Pharmaceuticals Corporation. P. Sunkureddi has received consulting and speakers’ bureau fees from Novartis, and is an investigator of a clinical trial sponsored by Novartis.
Research involving human participants
An institutional review board exemption was obtained for the study prior to initiation of data collection.
Informed consent
Informed consent was not required in this study because the data collected did not contain personal identifiers.
References
- 1.Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. 2016;374:2563–2574. doi: 10.1056/NEJMra1406182. [DOI] [PubMed] [Google Scholar]
- 2.Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–1390. doi: 10.1016/S0140-6736(07)60635-7. [DOI] [PubMed] [Google Scholar]
- 3.Reveille JD. Epidemiology of spondyloarthritis in North America. Am J Med Sci. 2011;341:284–286. doi: 10.1097/MAJ.0b013e31820f8c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reveille JD, Witter JP, Weisman MH. Prevalence of axial spondylarthritis in the United States: estimates from a cross-sectional survey. Arthritis Care Res (Hoboken). 2012;64:905–910. doi: 10.1002/acr.21621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reveille JD, Weisman MH. The epidemiology of back pain, axial spondyloarthritis and HLA-B27 in the United States. Am J Med Sci. 2013;345:431–436. doi: 10.1097/MAJ.0b013e318294457f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward MM, Deodhar A, Akl EA, et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2016;68:282–298. doi: 10.1002/art.39298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978–991. doi: 10.1136/annrheumdis-2016-210770. [DOI] [PubMed] [Google Scholar]
- 8.Sepriano A, Regel A, van der Heijde D, et al. Efficacy and safety of biological and targeted-synthetic DMARDs: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open. 2017;3:e000396. doi: 10.1136/rmdopen-2016-000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deodhar A, Yu D. Switching tumor necrosis factor inhibitors in the treatment of axial spondyloarthritis. Semin Arthritis Rheum. 2017;47:343–350. doi: 10.1016/j.semarthrit.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Ciurea A, Exer P, Weber U, et al. Does the reason for discontinuation of a first TNF inhibitor influence the effectiveness of a second TNF inhibitor in axial spondyloarthritis? Results from the Swiss Clinical Quality Management Cohort. Arthritis Res Ther. 2016;18:71016-0969-2. doi: 10.1186/s13075-016-0969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lie E, van der Heijde D, Uhlig T, et al. Effectiveness of switching between TNF inhibitors in ankylosing spondylitis: data from the NOR-DMARD register. Ann Rheum Dis. 2011;70:157–163. doi: 10.1136/ard.2010.131797. [DOI] [PubMed] [Google Scholar]
- 12.ISPE Guidelines for good pharmacoepidemiology practices (GPP) Pharmacoepidemiol Drug Saf. 2008;17:200–208. doi: 10.1002/pds.1471. [DOI] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Magrey M, Bozyczko M, Wolin D, et al. Treatment experience and satisfaction in ankylosing spondylitis patients treated with secukinumab: results from a US web-based survey [abstract] Ann Rheum Dis. 2018;77:1014. [Google Scholar]
- 15.Oelke KR, Garg R, Li Y, et al. Real-world use of secukinumab among biologic-naïve and biologic-experienced patients with ankylosing spondylitis in the United States [abstract] Arthritis Rheumatol. 2017;69:A1524. [Google Scholar]
- 16.Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373:2534–2548. doi: 10.1056/NEJMoa1505066. [DOI] [PubMed] [Google Scholar]
- 17.Sieper J, Deodhar A, Marzo-Ortega H, et al. Secukinumab efficacy in anti-TNF-naïve and anti-TNF-experienced subjects with active ankylosing spondylitis: results from the MEASURE 2 Study. Ann Rheum Dis. 2017;76:571–592. doi: 10.1136/annrheumdis-2016-210023. [DOI] [PubMed] [Google Scholar]
- 18.Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285. doi: 10.1186/s13075-017-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412–421. doi: 10.1111/bjd.12110. [DOI] [PubMed] [Google Scholar]
- 20.Deodhar A, Mittal M, Reilly P, et al. Ankylosing spondylitis diagnosis in US patients with back pain: identifying providers involved and factors associated with rheumatology referral delay. Clin Rheumatol. 2016;35:1769–1776. doi: 10.1007/s10067-016-3231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danve A, Deodhar A. Screening and referral for axial spondyloarthritis—need of the hour. Clin Rheumatol. 2015;34:987–993. doi: 10.1007/s10067-015-2958-2. [DOI] [PubMed] [Google Scholar]
- 22.López-Medina C, Garrido-Castro JL, Castro-Jiménez J, et al. Evaluation of quality of life in patients with axial spondyloarthritis and its association with disease activity, functionality, mobility, and structural damage. Clin Rheumatol. 2018;37:1581–1588. doi: 10.1007/s10067-018-4112-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.