Abstract
The effects of pre-harvest applications of calcium nitrate at different concentrations were investigated on storability and quality attributes of plum fruits under low temperature storage. Uniform and healthy trees of plum cv. Satluj Purple were sprayed with calcium nitrate (1.0%, 1.5% and 2.0%) in 2nd and 3rd week of April and control trees were water sprayed only. Fruits from treated and control trees were harvested at colour break stage, and then stored at low temperature conditions (0–1 °C and 90–95% RH) for 35 days. Evaluation of physico-chemical parameters and enzymatic activities were made on the day of harvest and after 7, 14, 21, 28 and 35 days of storage. The colour of the fruits with respect to a* and b* values (green–red and blue–yellow spectrum, respectively) improved progressively up to 35 days of storage, whereas, fruit firmness decreased. Total sugars and pectin methylesterase (PME) and cellulase activities increased up to 28 days of storage before showing a declining trend. Two superimposed sprays of calcium nitrate (2.0%) enhanced the storage life of plum fruits up to 28 days by reducing physiological loss in weight (PLW), PME and cellulase activities, delaying development of fruit colour & anthocyanin and retaining sensory quality, firmness and total sugars in plum fruits.
Keywords: Anthocyanin, Cellulase, Fruit firmness, Plum, PME, Total sugars
Introduction
Subtropical plums (Prunus salicina) are generally cultivated in the plains of north-western India. There are several varieties of Japanese plums amongst which ‘Satluj Purple’ is the commercial variety grown in Punjab. Due to its early ripening behaviour, low chill requirement, better size and excellent colour and quality it has become popular among the fruit growers of this region. It fetches a high price in the region due to its early availability i.e. in the month of May, when there is little competition from other fruits in the market. But, plum fruit has a short shelf-life due to its climacteric pattern of ripening, exhibiting various physiological and biochemical changes even after harvest, resulting into the fast decay of fruit, rapid deterioration in quality and thus lowering its marketing value. Fruit is highly susceptible to textural softening associated with breakdown of pectic substances, cellulose and hemi-cellulose in the middle lamella, which weakens cell wall and reduces cohesive forces binding cells together (Wills et al. 1998). Loss of firmness in the fruits is initiated by the action of cellulolytic enzymes and pectolytic enzymes that are involved in subsequent changes of texture (Elhassan 2016). Polygalacturonase (PG) and Pectin methylesterase (PME) are the principal enzymes participating in pectin degradation (Kays 1997). During ripening, PME removes methyl ester groups from the cell wall pectin constituents, which are accessible to depolymerisation by PG, reducing intercellular adhesiveness and tissue rigidity (Alonso et al. 1997). Earlier, attempts have been made to store the surplus pear fruits under cold storage conditions (Pasquariello et al. 2013) but to reduce the losses (physiological/pathological) during cold storage there is need to develop some safer and eco-friendly techniques.
Much research evidence indicates that calcium has ability to delay ripening and senescence, reduce rate of respiration and various physiological disorders, prolong shelf-life and also preserve the quality parameters of fruits. Calcium plays an important role in maintaining the structure and integrity of the cell wall by inhibiting the germination of fungal spores and cell wall degrading enzymatic activities, thus contributing to the firmness of fruit (Biggs 1999). An increase in calcium concentration in fruit delays the fruit ripening, by reducing ethylene emissions and slightly retarding the climacteric rise (Hansford 1994). It strengthens the structure of cells by maintaining the fibrillar packaging in the cell walls thus reinforcing the cell to cell contact which is related to the formation of calcium pectate and counteracts the pectin methylesterase activity as observed in calcium treated pear fruits (Alandes et al. 2009). Faust (1989) asserted that calcium in the apoplast exerts a binding effect in the complex of polysaccharides and proteins comprising the cell wall and that cytoplasmic calcium may regulate several enzyme activities. However, application of excessive calcium concentration led to salinity stress that caused external spotting in apple fruit (Sharples and Johnson 1976) and also reduced growth and photosynthetic rate in cucumber (Dabuxilatu, 2005).But, as evidenced by recent research reports, pre-harvest sprays of calcium nitrate has been found to improve various physico-chemical properties of fruit like retaining fruit firmness, delaying softening, reducing physiological loss in weight and titratable acidity and increasing total soluble solids, reducing sugar and pectin contents and enhancing the shelf-life of guava cv. Lucknow-49 (Jayachandran et al. 2005). In addition, calcium nitrate was found to reduce the dessication and decay in grapes and thus prolonged its shelf-life (Kalloo 2003). Therefore, considering the positive effect of calcium compounds on fresh horticultural commodities, the present research was conducted to investigate the influence of pre-harvest sprays of calcium nitrate on storability and quality attributes of plum fruits cv. Satluj Purple under low temperature storage.
Materials and methods
Experimental procedure
For the experiment, six-years-old, twenty-eight uniform and healthy trees of plum cv. Satluj Purple were selected at the Fruit Research Farm, Department of Fruit Science, Punjab Agricultural University, Ludhiana (India) in the year 2017. T-1, T-2 and T-3 (twelve trees) were sprayed once with calcium nitrate (1.0%, 1.5% and 2.0%) in 2nd week of April, while T-4, T-5 and T-6 (twelve trees) were sprayed twice with calcium nitrate (1.0%, 1.5% and 2.0%) in 2nd and 3rd week of April and T-7 (four trees) were water sprayed using 5 L of solution for each replication. Fruits from treated and untreated trees were harvested at colour break stage (last week of April). The fruits of uniform size, apparently free from diseases and bruises were selected, washed and air dried under shade before packaging. The experiment comprised of seven treatments with four replications in each treatment. One kg of fruit from every replication of each treatment was packed in corrugated fibreboard (CFB) boxes (5% perforation) with paper lining and kept at low temperature conditions (0–1 °C and 90–95% RH) for 35 days. For study of storage behaviour, fruit samples were analysed after 7, 14, 21, 28 and 35 days of storage for various physico-chemical characteristics.
Physico-chemical analysis
Physiological loss in weight
The weight of fruits after each interval of cold storage was recorded and per cent physiological loss in weight was calculated as follows:
Sensory quality
Sensory quality of fruits were assessed on the basis of colour, appearance, texture and taste and were rated by panel of 5 judges using nine point hedonic scale (1–9) as described by Amerine et al. (1965).
Fruit firmness
Firmness of ten randomly selected fruits was measured with the help of fruit pressure tester Model FT-327, Italy. About one square centimetre of the peel in each fruit from the shoulder end on both sides was removed with the help of a peeler and firmness of pulp was recorded and expressed in terms of Newtons (N).
Peel colour
The skin colour of sample fruit was recorded on colour coordinates as L*, a* and b* from opposite positions of each fruit in Commission International de I’Eclairage (CIE) units using a ColorFlex spectrophotometer (HunterLab ColorFlex, Hunter Associates Inc., Reston, VA, USA) with the head of 15 mm diameter to fit fruit surface (Hunter 1975).
Total sugars
Total sugars were estimated by Lane and Eynon’s titration method as reported by Ranganna (1986) and expressed in percentage (%).
Fruit calcium content
Five gram of fresh weight samples were taken and dried in hot air oven. These samples were later ground and 0.5 g of powder was put into digestion tubes to which 10 ml of diacid (Nitric acid: Perchloric acid; 4:1) was added. The tubes were left overnight for digestion. Next day, the tubes were put on digester with low heat till dense brown fumes appeared. When the fumes at the neck of the flask condensed and left with 1–2 mL solution, it was removed. After cooling, the volume was made 100 mL with double distilled water and the solution was filtered through Whatman’s filter paper no. 1 and filtrate was stored in polyethylene bottles, calcium was analyzed by Atomic Absorption Spectrophotometer (AAnalyst 200, Perkin Elmer) and expressed as ppm (FW).
Anthocyanins
To measure anthocyanins of pulp as well as peel of plum a revised version of the method proposed by Zheng and Tian (2006) was followed. Five gram of fruit was placed in 40 mL of methanol extract (with 1% HCl for extraction). The solution was subjected to oscillation extraction for 4 h before being filtered; the volume of filtrate was set at 50 mL. A spectrophotometer was then employed to measure the absorption of filtrate at wavelengths of 530 nm, 620 nm, and 650 nm. The anthocyanin content was expressed as mg/100 g FW.
Pectin methylesterase (PME) and cellulase activities
Enzyme extraction for PME and cellulsae enzymes
For the extraction of both enzymes, twenty grams of fruit pulp was blended in 60–100 mL NaCl solution (0.15 M). After that it was filtered through two layers of cheesecloth and centrifuged at 2000 rpm for 30 min at 4 °C. The supernatant was used as an enzyme source (Mahadevan and Sridhar 1982).
PME assay
PME activity was determined by measuring the increase in acidity after the hydrolysis of pectin by the enzyme. For PME assay, 20.0 mL of 1.0% pectin solution was taken in 50 mL beaker and its pH was adjusted to 7.0. Then 10.0 mL of enzyme solution was added to it and immediately adjusted to 7.0 pH with 1 mol/L of NaOH. This was noted as zero time. Further the beaker was put in water bath maintained at temperature 30 °C for 15 min and pH was checked at regular interval of 15 min and adjusted to 7.0 by using 0.02 mol/L of NaOH, with continuous stirring of contents. The volume of alkali used at each interval was noted and the enzyme activity was expressed as mL of 0.02 N NaOH used.
Cellulase assay
For assay 4.0 mL of carboxy methyl cellulose (CMC) solution, 1.0 mL of sodium acetate acetic acid buffer (pH 5.2) and 2.0 mL of enzyme source (extract) were pipetted into the viscometer and the contents were mixed by drawing air rapidly through the large arm of viscometer by suction. Then, suction was applied to viscometer through the small arm and the efflux time of the mixture was determined and considered as the zero time. The efflux time of mixture was determined at various intervals and cellulase activity was expressed as per cent reduction in viscosity of the substrate (Mahadevan and Sridhar 1982).
Statistical analysis
The data were statistically analyzed by Factorial Completely Randomized Design (CRD) using statistical package SAS 9.3 (The SAS system for Windows, Version 9.3, SAS Institute, Cary, NC, USA) and significant effects (p ≤ 0.05) were noted. Significant difference amongst the means was determined by least significant difference (LSD). Results were expressed as mean ± standard error.
Results and discussions
Physiological loss in weight (PLW)
The physiological loss in weight progressively increased with the advancement of storage irrespective of the treatments (Table 1). However, this increase was relatively slower in calcium treated fruits. Decline in loss of weight in fruits during storage might be due to the oxidation and respiration processes and transpiration losses. Maximum weight loss was obtained in untreated fruits as compared to fruits treated with calcium nitrate. The data indicates that the fruits treated with two sprays of Ca(NO3)2 @ 2.0% recorded minimum percentage loss in weight from 1.82 to 5.47% under 35 days of storage period, whereas in untreated fruits it ranged from 2.92 to 6.51%. Fruits treated with two sprays of Ca(NO3)2 @ 2.0% exhibited significantly lower weight loss as compared to other treatments due its efficacy in maintaining the fruit firmness and rigidity of the cell wall. These results are in agreement with the findings of Alcaraz-Lopez et al. in peach (2004), Goutam et al. (2010) in guava and Singh et al. (2013) in ber fruits.
Table 1.
Physiological loss in weight (PLW) % of plum fruits subjected to various treatments of calcium chloride during cold storage (0–1 °C, 90–95% RH)
| Parameters | Calcium chloride Conc. (%) | No. of sprays | Storage interval (days) | ||||
|---|---|---|---|---|---|---|---|
| Physiological loss in weight (PLW) % | 0 | 7 | 14 | 21 | 28 | ||
| 1.0 | 1 | 2.72 ± 0.09a | 3.48 ± 0.06ab | 4.27 ± 0.06a | 5.42 ± 0.09ab | 6.33 ± 0.16ab | |
| 1.5 | 1 | 2.45 ± 0.04b | 3.27 ± 0.06b | 4.15 ± 0.04a | 5.31 ± 0.04bc | 6.05 ± 0.06bc | |
| 2.0 | 1 | 2.21 ± 0.08c | 3.12 ± 0.09bc | 4.04 ± 0.09ab | 5.28 ± 0.04bc | 5.94 ± 0.03bcd | |
| 1.0 | 2 | 1.98 ± 0.04d | 2.78 ± 0.14c | 3.67 ± 0.04bc | 5.14 ± 0.09cd | 5.62 ± 0.12cde | |
| 1.5 | 2 | 1.87 ± 0.04d | 2.35 ± 0.13d | 3.26 ± 0.12cd | 5.02 ± 0.06d | 5.51 ± 0.21de | |
| 2.0 | 2 | 1.82 ± 0.04d | 2.28 ± 0.11d | 3.21 ± 0.08d | 4.98 ± 0.06d | 5.47 ± 0.18e | |
| Control | – | 2.92 ± 0.06a | 3.68 ± 0.15a | 4.40 ± 0.26a | 5.54 ± 0.07a | 6.51 ± 0.08a | |
| P | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | ||
| LSD (< 0.05) | 0.2094 | 0.3779 | 0.4139 | 0.2267 | 0.4537 | ||
Means in a column with the same letter are not significantly different at (p ≤ 0.05) according to LSD
Sensory quality
Pre-harvest spray with different concentrations of calcium nitrate had a significant effect on the sensory quality of plum fruits. From the data (Table 3) it is clear that fruits treated with two sprays of Ca(NO3)2 (1%, 1.5% and 2%) maintained the acceptable sensory quality up to 28 days of storage, whereas fruits treated with one spray of Ca(NO3)2 (1%, 1.5% and 2%) and control retained the acceptable sensory quality only up to 21 days of storage period. At the end of 35 days of storage period, maximum sensory quality rating (7.60) was recorded in fruits treated with two sprays of Ca(NO3)2 @ 2.0% as compared to control fruits, where minimum sensory rating (5.04) was recorded. Similar trend was reported by Singh and Mandal (2000) in litchi and Bhat et al. (2012) in pear fruits.
Table 3.
Sensory quality, total sugars and calcium content (ppm) of plum fruits subjected to various treatments of calcium nitrate during cold storage (0–1 °C, 90–95% RH)
| Parameters | Calcium nitrate Conc. (%) | No. of sprays | Storage interval (days) | |||||
|---|---|---|---|---|---|---|---|---|
| Sensory quality (1–9) | 0 | 7 | 14 | 21 | 28 | 35 | ||
| 1.0 | 1 | 5.89 ± 0.05b | 7.36 ± 0.08ab | 7.84 ± 0.04b | 8.47 ± 0.18a | 6.96 ± 0.10c | 5.54 ± 0.15cd | |
| 1.5 | 1 | 5.80 ± 0.05bc | 7.12 ± 0.03bc | 7.69 ± 0.04c | 8.38 ± 0.10ab | 7.25 ± 0.07bc | 5.89 ± 0.19bc | |
| 2.0 | 1 | 5.75 ± 0.04bc | 6.93 ± 0.03cd | 7.65 ± 0.05c | 8.35 ± 0.17ab | 7.44 ± 0.03b | 5.95 ± 0.18bc | |
| 1.0 | 2 | 5.59 ± 0.13cd | 6.68 ± 0.06d | 7.49 ± 0.04d | 8.02 ± 0.05ab | 8.32 ± 0.07a | 6.34 ± 0.16b | |
| 1.5 | 2 | 5.45 ± 0.04d | 6.41 ± 0.13e | 7.17 ± 0.05e | 7.81 ± 0.05bc | 8.54 ± 0.14a | 7.43 ± 0.12a | |
| 2.0 | 2 | 5.43 ± 0.06d | 6.39 ± 0.11e | 7.15 ± 0.05e | 7.79 ± 0.11c | 8.60 ± 0.13a | 7.60 ± 0.17a | |
| Control | – | 6.15 ± 0.05a | 7.52 ± 0.05a | 8.11 ± 0.03a | 8.35 ± 0.04c | 6.21 ± 0.03d | 5.04 ± 0.03d | |
| p | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | ||
| LSD (< 0.05) | 0.2313 | 0.2629 | 0.1476 | 0.3828 | 0.3035 | 0.5127 | ||
| Total sugars (%) | 1.0 | 1 | 9.69 ± 0.13b | 9.86 ± 0.06b | 10.13 ± 0.13ab | 10.34 ± 0.14ab | 9.35 ± 0.11d | 8.66 ± 0.09c |
| 1.5 | 1 | 9.43 ± 0.14c | 9.56 ± 0.17c | 9.79 ± 0.16bc | 9.96 ± 0.23bc | 9.59 ± 0.09c | 8.79 ± 0.04bc | |
| 2.0 | 1 | 9.37 ± 0.24c | 9.50 ± 0.16c | 9.70 ± 0.05bc | 9.89 ± 0.24bc | 9.66 ± 0.13c | 8.83 ± 0.08bc | |
| 1.0 | 2 | 9.14 ± 0.40d | 9.27 ± 0.18d | 9.43 ± 0.14cd | 9.56 ± 0.25c | 9.95 ± 0.07b | 9.06 ± 0.08ab | |
| 1.50 | 2 | 9.06 ± 0.17d | 9.14 ± 0.16de | 9.35 ± 0.19cd | 9.44 ± 0.17c | 10.08 ± 0.45a | 9.32 ± 0.16a | |
| 2.0 | 2 | 8.99 ± 0.12d | 9.07 ± 0.19e | 9.23 ± 0.12d | 9.35 ± 0.18c | 10.16 ± 0.54a | 9.36 ± 0.16a | |
| Control | – | 9.87 ± 0.15a | 10.09 ± 1.26a | 10.41 ± 014a | 10.67 ± 0.04a | 9.09 ± 0.20e | 8.45 ± 0.15c | |
| p | 0.001 | 0.001 | 0.001 | 0.003 | 0.001 | 0.001 | ||
| LSD (< 0.05) | 0.73 | 1.69 | 0.48 | 0.65 | 0.98 | 0.38 | ||
| Fruit calcium content (ppm) on fresh weight basis | 1.0 | 1 | 49.65 ± 1.32de | 51.45 ± 1.49de | 53.70 ± 1.31de | 56.10 ± 1.53cd | 58.65 ± 1.75bc | 61.35 ± 1.08d |
| 1.5 | 1 | 52.65 ± 1.38cd | 54.30 ± 1.39d | 56.40 ± 1.12d | 58.65 ± 1.43bcd | 60.00 ± 1.52bc | 62.40 ± 1.24cd | |
| 2.0 | 1 | 54.60 ± 1.47bc | 55.95 ± 1.74cd | 57.75 ± 1.25cd | 59.70 ± 1.53bc | 60.75 ± 1.76bc | 63.00 ± 1.31cd | |
| 1.0 | 2 | 58.80 ± 1.36b | 59.70 ± 1.27bc | 61.05 ± 1.35bc | 62.55 ± 1.83ab | 63.90 ± 1.66ab | 65.70 ± 1.37bc | |
| 1.5 | 2 | 63.60 ± 1.21a | 64.20 ± 1.28ab | 65.25 ± 1.31ab | 66.45 ± 1.41a | 67.80 ± 1.57a | 69.30 ± 1.35ab | |
| 2.0 | 2 | 65.25 ± 1.40a | 65.70 ± 1.36a | 66.45 ± 1.06a | 67.50 ± 1.36a | 68.70 ± 1.55a | 70.05 ± 4.18a | |
| Control | – | 47.10 ± 1.42e | 49.20 ± 1.33e | 51.75 ± 1.47e | 54.45 ± 1.55d | 57.00 ± 1.55c | 60.15 ± 1.24d | |
| p | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | ||
| LSD (< 0.05) | 4.65 | 4.81 | 4.32 | 5.19 | 5.52 | 6.68 | ||
Means in a column with the same letter are not significantly different at (p ≤ 0.05) according to LSD
Fruit firmness
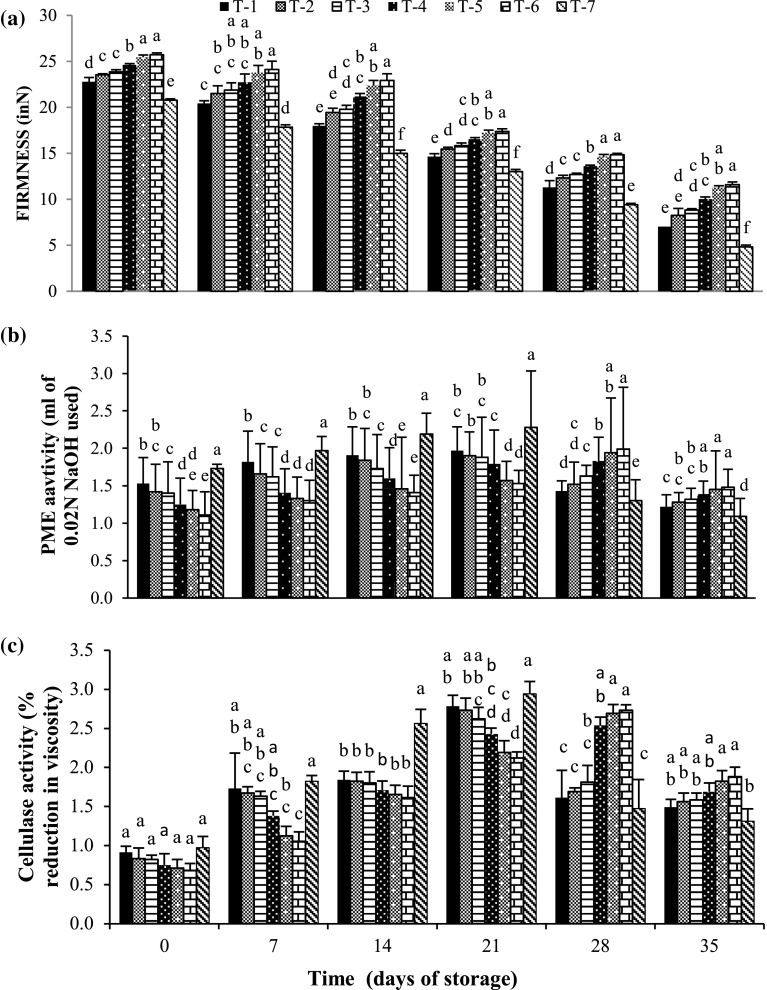
Fruit firmness is one of the most important factors that determines the post-harvest quality and physiology of fruits. Softening of fruit is generally associated with the breakdown of pectin in the fruit cell wall (Bal 2013). In the present study, fruit firmness in plum fruits decreased with the increase in storage period (Fig. 1a). Two sprays of Ca(NO3)2 @ 2.0% were found effective in maintaining the firmness of fruits. On the day of harvesting, maximum firmness (25.72 N) was found in fruits treated with two sprays of Ca(NO3)2 @ 2.0% and minimum firmness (20.78 N) was observed in control fruit.. During the entire storage period (35 days), maximum fruit firmness (25.72–11.61 N) was retained in two sprays of Ca(NO3)2 @ 2.0%, which was at par with two sprays of Ca(NO3)2 @ 1.5%, whereas, minimum fruit firmness (20.78–4.42 N) was recorded in control fruit. Calcium compounds may delay the reduction in the firmness of fruit during storage due its ability to stabilize the membrane systems and form calcium pectate, which enhances the rigidity of the middle lamella of the cell wall and increases resistance against polygalactouronase activity (Amal et al. 2010). Manganaris et al. (2005) also described, that the fruits treated with calcium salts showed higher firmness as compared to control fruits. These findings are in close conformity with the findings of Rombaldi et al. (2001) in peaches and Wojcik and Lewandowski (2003) in strawberry.
Fig. 1.
Variation in a fruit firmness, b PME activity and c cellulase activity in plum cv. Satluj Purple during cold storage (0–1 °C, 90–95% RH) in relation to pre-harvest treatment with different concentration of calcium nitrate. Vertical bars represent ± Standard error of mean of 4 replicates, T-1: one spray Ca(NO3)2 (1.0%),T-2: one spray Ca(NO3)2 (1.5%), T-3: one spray Ca(NO3)2 (2.0%), T-4: two spray Ca(NO3)2 (1.0%), T-5: two spray Ca(NO3)2 (1.5%), T-6: two spray Ca(NO3)2 (2.0%) and T-7: control
Peel colour
Fruit peel colour is an important quality attribute as it affects the physical appearance of the fruit as well as increases the market acceptability. L* value indicates the luminosity of fruits, which decreased progressively with the advancement of storage period (Table 2). Fruits treated with two sprays of Ca(NO3)2 @ 2.0% registered maximum L* value (62.38–44.98), followed by two sprays of Ca(NO3)2 @ 1.5%, whereas, minimum L* value (54.14–32.37) was reported in control fruits.
Table 2.
Peel colour L*, a* and b* values of plum fruits subjected to various treatments of calcium nitrate during cold storage (0–1 °C, 90–95% RH)
| Parameters | Calcium nitrate Conc. (%) | No. of sprays | Storage interval (days) | |||||
|---|---|---|---|---|---|---|---|---|
| L* value | 0 | 7 | 14 | 21 | 28 | 35 | ||
| 1.0 | 1 | 56.34 ± 1.18de | 51.91 ± 1.40d | 47.64 ± 0.90d | 44.7 ± 0.94de | 38.26 ± 1.17d | 34.86 ± 0.76d | |
| 1.5 | 1 | 57.82 ± 0.17cd | 55.79 ± 0.18c | 48.15 ± 0.34d | 45.67 ± 0.21cde | 40.67 ± 0.14c | 35.32 ± 0.29d | |
| 2.0 | 1 | 58.83 ± 0.43bc | 56.34 ± 0.46bc | 48.53 ± 0.42d | 46.11 ± 0.46bcd | 41.14 ± 0.44c | 35.72 ± 0.46cd | |
| 1.0 | 2 | 60.85 ± 0.29ab | 57.09 ± 2.25ab | 51.33 ± 0.83c | 48.56 ± 1.80abc | 43.41 ± 0.18b | 37.65 ± 0.25c | |
| 1.5 | 2 | 61.12 ± 0.74ab | 57.65 ± 0.41a | 53.97 ± 0.45b | 48.84 ± 0.37ab | 45.32 ± 0.33a | 42.59 ± 0.19b | |
| 2.0 | 2 | 62.38 ± 0.29a | 58.26 ± 0.22a | 56.55 ± 0.97a | 49.46 ± 0.95a | 46.17 ± 0.65a | 44.98 ± 1.10a | |
| Control | – | 54.14 ± 1.05e | 50.26 ± 2.39e | 45.09 ± 0.17e | 42.98 ± 0.20e | 35.42 ± 0.20e | 32.37 ± 0.71e | |
| p | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | ||
| LSD (< 0.05) | 2.38 | 4.66 | 2.21 | 3.01 | 1.89 | 2.1 | ||
| a* value | 1.0 | 1 | − 8.99 ± 0.28b | 9.25 ± 0.18b | 16.43 ± 0.18b | 20.83 ± 0.19b | 28.56 ± 0.18b | 31.87 ± 0.22b |
| 1.5 | 1 | − 10.23 ± 0.13c | 8.93 ± 0.21b | 15.95 ± 0.19b | 20.37 ± 0.20b | 26.54 ± 0.19c | 30.89 ± 0.24c | |
| 2.0 | 1 | − 10.40 ± 0.21c | 8.67 ± 0.19bc | 15.82 ± 0.19b | 19.68 ± 0.19c | 26.41 ± 0.16c | 30.83 ± 0.23c | |
| 1.0 | 2 | − 11.61 ± 0.18d | 8.25 ± 0.19cd | 15.14 ± 0.21c | 19.05 ± 0.20c | 24.09 ± 0.18d | 27.35 ± 0.23d | |
| 1.50 | 2 | − 13.39 ± 0.18e | 7.60 0.20de | 14.11 ± 0.22d | 17.81 ± 0.20d | 23.32 ± 0.14e | 25.52 ± 0.24e | |
| 2.0 | 2 | − 13.47 ± 0.20e | 7.51 ± 0.20e | 14.08 ± 0.20d | 17.75 ± 0.19d | 23.27 ± 0.18e | 25.43 ± 0.23e | |
| Control | – | − 5.19 ± 0.22a | 10.63 ± 0.20a | 17.33 ± 0.19a | 23.46 ± 0.20a | 31.52 ± 0.23a | 32.94 ± 0.21a | |
| p | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | ||
| LSD (< 0.05) | 0.69 | 0.67 | 0.68 | 0.67 | 0.62 | 0.78 | ||
| b* value | 1.0 | 1 | 9.23 ± 0.27b | 11.70 ± 0.24b | 15.98 ± 0.27b | 17.45 ± 0.28b | 21.23 ± 0.27b | 22.19 ± 0.27b |
| 1.5 | 1 | 9.07 ± 0.28bc | 11.53 ± 0.23bc | 15.45 ± 0.27bc | 17.23 ± 0.26b | 19.39 ± 0.25c | 21.44 ± 0.26b | |
| 2.0 | 1 | 9.04 ± 0.26bc | 11.37 ± 0.26bcd | 14.83 ± 0.27cd | 17.12 ± 0.27b | 19.25 ± 0.27cd | 21.36 ± 0.23bc | |
| 1.0 | 2 | 8.22 ± 0.27cd | 10.75 ± 0.27cde | 14.08 ± 0.28de | 15.74 ± 0.29c | 18.86 ± 0.27cd | 20.54 ± 0.26cd | |
| 1.5 | 2 | 7.95 ± 0.28d | 10.51 ± 0.28de | 13.42 ± 0.28e | 15.41 ± 0.28c | 18.53 ± 0.23cd | 20.23 ± 0.25d | |
| 2.0 | 2 | 7.89 ± 0.28d | 10.42 ± 0.27e | 13.33 ± 0.28e | 15.25 ± 0.27c | 18.42 ± 0.26d | 20.17 ± 0.27d | |
| Control | – | 10.34 ± 0.27a | 13.95 ± 0.27a | 17.68 ± 0.26a | 21.73 ± 0.27a | 28.04 ± 0.27a | 30.11 ± 0.26a | |
| P | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | ||
| LSD (< 0.05) | 0.93 | 0.88 | 0.92 | 0.93 | 0.88 | 0.87 | ||
Means in a column with the same letter are not significantly different at (p ≤ 0.05) according to LSD
The a* and b* values increased with the advancement in storage period in all the treatments indicating improvement in varietal characteristic colour development of the fruits. The negative a* value signifies green colour of the fruit as marked on the day of harvest which gradually increased and acquired positive a* value indicating the intensity of redness of ripe fruits. Maximum b* values were noted after 35 days of storage which indicates the fruit colour development. On the day of harvesting, maximum a* and b* values (− 5.19 and 10.34, respectively) were noticed in control fruits, whereas, minimum a* and b* values (− 13.47 and 7.89, respectively) were observed in two sprays of Ca(NO3)2 @ 2.0%, followed by two sprays of Ca(NO3)2 @ 1.5%. After 35 days of storage, highest a* and b* (32.94 and 30.11, respectively) values were noticed in control fruits and minimum (25.43 a* and 20.17 b*, respectively) in two sprays of Ca(NO3)2 @ 2.0%, followed by two sprays of Ca(NO3)2 @ 1.5%. The colour development was much faster in control fruits. However, fruits treated with two sprays of calcium nitrate (2.0% and 1.5%) showed a significantly slower rate of colour development than control fruits, indicating delayed ripening of fruits. Increase in a* and b* values may be due to degradation of chlorophyll content and synthesis of colouring compounds like carotenoids and anthocyanins. Pre-harvest spray of calcium nitrate retarded the colour development in persimmon fruits (Agusti et al. 2004). The decrease in L* value with advancement of storage period was also recorded in plum fruit by Steffens et al. (2013). Increasing trend of a* and b* values with increase in storage period is in accordance with the observations of Majeed and Jawandha (2016) in ‘Satluj Purple’ plum.
Total sugars
Sugar content is the most relevant factor for consumer perception of maturity and it is closely related to the stage of maturity in plum fruits (Manganaris et al. 2008). Plums contain three predominant sugars: glucose, fructose, sucrose and sugar alcohol sorbitol and their content varies with cultivar (Wilford et al. 1997). In the present investigation, total sugars content of fruits progressively increased up to 21 days of storage and thereafter declined in all the treatments except in fruits treated with two sprays of Ca(NO3)2 (1.0%, 1.5% and 2.0%) (Table 3). However, fruits from two sprays of calcium nitrate (1.0, 1.5 and 2.0%) treatments resulted in increase in total sugars up to 28 days of cold storage and declined thereafter. This increase in total sugars with the advancement of storage period may be due to the breakdown of complex organic metabolites into simpler molecules and at the later stages of storage a decline in sugar content may be attributed to the utilization of sugars along with other organic acids as substrate in respiration process (Patel et al. 2011). After 35 days of cold storage, in all the treatments a decline in total sugars was recorded. Maximum total sugars (9.36%) were recorded in two sprays of calcium nitrate (2.0%) and minimum in control fruits. Results of the present study are similar to the findings of Mahajan and Dhatt (2004) in plum fruits where total sugars increased at a faster rate in control fruits and then declined after 2 weeks of storage, whereas, calcium treated fruit maintained minimum total sugars up to 3 weeks and declined gradually and maintained highest total sugars at the end of storage period. Similar trend was found in the experiments conducted by Jayachandran et al. (2005) in guava and Jawandha et al. (2008) in ber fruits.
Fruit calcium content
Calcium maintains the integrity of the cell membrane, delays senescence and prevents softening of the fruits which ultimately enhances the shelf-life. On the day of harvesting, maximum calcium content (65.25 ppm) was found in fruits treated with two sprays of Ca(NO3)2 @ 2.0%, followed by two sprays of Ca(NO3)2 @ 1.5% (Table 3). The minimum calcium content (47.10 ppm) was observed in untreated fruits. Similar trend was followed throughout the storage period with maximum calcium content (65.25–70.05 ppm) in fruits treated with two sprays of Ca(NO3)2 @ 2.0%, which was at par with two sprays of Ca(NO3)2 @ 1.5%, whereas minimum calcium content (47.10–60.15 ppm) was found in control fruits. Calcium content increased with the increase in storage period in all the treatments, as prolonged storage of fruit results in the loss of moisture which leads to the manifestation of increased calcium content. The elevated level of calcium content in fruits treated with higher concentration of calcium compounds may be due to its greater absorption and deposition. With ripening of fruit, there is an increase in level of water-soluble pectins which attribute to the creation of channels of discontinuity and openings for calcium penetration (Manganaris et al. 2007). These results are in accordance with the findings of Singh et al. (1982) in peach and Madani et al. (2014) in papaya, where an increase in calcium content was shown with increase in the concentration of calcium chloride application. A similar trend of increase in calcium content was found by Chardonnet et al. (2003) in apples and Serrano et al. (2004) in peaches.
Anthocyanins
The anthocyanin content in cold stored plum fruits increased progressively with the advancement of storage period (Table 4). This increase in anthocyanins in plum with the advancement of storage period might be attributed to post-harvest ripening and development of red colour (Sharma et al. 2013). On the day of harvesting, maximum anthocyanin content (12.49 mg/100 g FW) was found in control fruits and minimum anthocyanin content (11.21 mg/100 g FW) in fruits treated with two sprays of Ca(NO3)2 @ 2.0%. Similar trend was followed throughout the storage period (35 days). The present study showed that calcium salts play an important role in the cell biochemistry, as it is evident from the data that fruit treated with calcium compounds delayed the progression of anthocyanin content which is directly proportional to the ripening process. Results are in accordance with the findings of Amal et al. (2010) in strawberry and Turmanidze et al. (2016) in blackberry, raspberry and strawberry, that showed a relatively slow increase in anthocyanin content with post-harvest treatment of calcium chloride as compared to control.
Table 4.
Anthocyanin content of plum fruits subjected to various treatments of calcium nitrate during cold storage (0–1 °C, 90–95% RH)
| Parameters | Calcium nitrate Conc. (%) | No. of sprays | Storage interval (Days) | |||||
|---|---|---|---|---|---|---|---|---|
| Anthocyanin (mg/100 g FW) | 0 | 7 | 14 | 21 | 28 | 35 | ||
| 1.0 | 1 | 12.07 ± 0.10ab | 13.18 ± 0.11b | 14.11 ± 0.24b | 16.23 ± 0.24ab | 17.34 ± 0.25ab | 18.17 ± 0.17ab | |
| 1.5 | 1 | 11.81 ± 0.08bc | 12.97 ± 0.14bc | 13.97 ± 0.22bc | 16.07 ± 0.22bc | 17.18 ± 0.24ab | 18.09 ± 0.14ab | |
| 2.0 | 1 | 11.73 ± 0.08bcd | 12.84 ± 0.18bcd | 13.93 ± 0.19bc | 15.68 ± 0.24bcd | 17.07 ± 0.24ab | 17.93 ± 0.18ab | |
| 1.0 | 2 | 11.47 ± 0.14cd | 12.42 ± 0.25cd | 13.64 ± 0.25bc | 15.31 ± 0.25cde | 16.92 ± 0.25ab | 17.71 ± 0.23b | |
| 1.5 | 2 | 11.29 ± 0.21cd | 12.31 ± 0.21cd | 13.43 ± 0.20bc | 14.95 ± 0.19de | 16.84 ± 0.23ab | 17.63 ± 0.26b | |
| 2.0 | 2 | 11.21 ± 0.25d | 12.27 ± 0.22d | 13.35 ± 0.23c | 14.82 ± 0.26e | 16.78 ± 0.28ab | 17.56 ± 0.30b | |
| Control | – | 12.49 ± 0.24a | 13.98 ± 0.24a | 14.86 ± 0.19a | 16.95 ± 0.14a | 17.69 ± 0.35a | 18.52 ± 0.19a | |
| p | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | ||
| LSD (< 0.05) | 1.04 | 0.88 | 0.88 | 0.89 | 0.93 | 0.89 | ||
Pectin methylesterase (PME)
Pre-harvest treatment with two sprays of calcium nitrate (2.0%) significantly lowered the PME activity up to 21 days of storage as compared to control fruits. On the day of harvesting, maximum PME activity (1.73 mL 0.02 N NaOH used) was observed in control fruits and minimum PME activity (1.11 mL 0.02 N NaOH used) was recorded in fruits treated with two sprays of Ca(NO3)2 @ 2.0%, followed by two sprays of Ca(NO3)2 @ 1.5% (Fig. 1b). A similar trend was followed after 7, 14 and 21 days of storage period. After 21 days of storage, PME activity declined in all the treatments except in two sprays of Ca(NO3)2 (1.0%, 1.5% and 2.0%) where it continued to increase up to 28 days of storage period. After 35 days of storage, a decline in PME activity was found in all the treatments and maximum PME activity (1.48 mL 0.02 N NaOH used) was retained by two sprays of Ca(NO3)2 @ 2.0%, which was at par with two sprays of Ca(NO3)2 @ 1.5% and minimum PME activity (1.09 mL 0.02 N NaOH used) was found in control fruits. This might be due to the presence of high substrate level for PME activity at later stages of storage in fruits treated with two sprays of Ca(NO3)2 @ 2.0%, conversely, in other treatments the availability of substrate was lowered at later stages of storage due to the decomposition of substrate in rapid metabolic processes during early storage period. Similar trend was recorded for PME activity during cold storage of ber (Jawandha et al. 2012), plum (Majeed and Jawandha 2016) and apple (Mahajan 1994) fruits. The positive affect of calcium compound on the suppression of fruit softening enzyme might be due to its ability to strengthen the structure of cells by maintaining the fibrillar packaging in cell wall thus reinforcing the cell to cell contact which is related to formation of calcium pectate and counteracts the PME activity (Alandes et al. 2009).
Cellulase activity
All the treatments showed progressive increase in cellulase activity with the advancement of storage period up to 21 days of storage (Fig. 1c). However, cellulase activity increased up to 28 days of storage period, only in fruits treated with two sprays of calcium nitrate (1.0, 1.5 and 2.0%). On the day of harvesting, maximum cellulase activity (0.97% reduction in viscosity) was found in control fruits and minimum cellulase activity (0.68% reduction in viscosity) was recorded in fruits treated with two sprays of Ca(NO3)2 @ 2.0%, followed by two sprays of Ca(NO3)2 @ 1.5%. Similar trend was recorded after 7, 4 and 21 days of storage. Cell walls consist of strong cellulose microfibrils, hemicelluloses, pectins, calcium and structural proteins. External application of calcium enhances the rigidity of the cell wall that may lead to more resistance to the activities of cellulase enzyme. But, at the end of storage, maximum cellulase activity (1.88% reduction in viscosity) was retained by fruits treated with two sprays of Ca(NO3)2 @ 2.0% and minimum cellulase activity (1.31% reduction in viscosity) was found in control fruits. This might be due to the presence of high substrate level for cellulase activity in two sprays of calcium nitrate (2.0%) as compared to other treatments, as the same was decomposed to a greater extent in other treatments within 21 days of storage. Similar results were reported by (Mahajan 1994) in apple and Jawandha et al. (2009) in ber fruits.
Correlations
Fruit calcium content showed a significant negative correlation with PLW (r = − 0.771, p ≤ 0.01), PME (r = − 0.773, p ≤ 0.01) and cellulase enzymes (r = − 0.643, p ≤ 0.01) and positive correlation with firmness (r = 0.767, p ≤ 0.01) (Table 5). Consequently, calcium compounds form cross bridges with the carboxyl group of the pectic substances in the middle lamella which results in strengthening as well as increasing the rigidity of cell wall. This binding further restricts the activities of cell wall degrading enzymes thus reducing the pace of softening during storage. Positive correlation between fruit calcium content and firmness (r = 0.767, p ≤ 0.01) may be due to the covalent bond formation between calcium and pectin which controls the cell separation characteristics of the cell and helps to maintain the firmness of the tissues (Siddiqui and Bangerth 1995). Negative correlation was obtained between L* value and anthocyanin content as the fruits become darker with progression of storage period, while a* and b* values of colour showed a significant positive correlation with anthocyanins which indicates that anthocyanin content increases with the increase in a* and b* values as the fruits attained characteristic colour.
Table 5.
Correlations
| Variables compared | Pearson correlation coefficient (r) |
|---|---|
| Calcium versus PLW | − 0.771** |
| Calcium versus PME | − 0.773** |
| Calcium versus cellulase | − 0.643** |
| Calcium versus firmness | 0.767** |
| Lightness versus anthocyanin | − 0.907** |
| a* value versus anthocyanin | 0.908** |
| b* value versus anthocyanin | 0.933** |
**Correlation is significant at the 0.01 level (2-tailed)
Conclusion
It can be concluded from the study that two pre-harvest sprays of Ca(NO3)2 @ 2.0% were most effective in maintaining sensory quality, fruit firmness, total sugars and calcium content and reducing physiological loss in weight and activities of PME and cellulase enzymes in plum fruits during cold storage. It also delayed colour development and anthocyanin formation which indicates the delay in ripening and metabolic activities of the fruits. This treatment efficiently extended the storage life of plum fruits under low temperature storage up to 28 days.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agusti M, Juan M, Martinez-Fuentes A, Mesejo C, Almela V. Calcium nitrate delays climacteric of persimmon fruit. Ann Appl Biol. 2004;144:65–69. doi: 10.1111/j.1744-7348.2004.tb00317.x. [DOI] [Google Scholar]
- Alandes L, Perez I, Llarca E, Quiles A, Hernondo I. Use of calcium lactate to improve structure of ‘Flor de Invierno’ fresh cut pears. Postharvest Biol Technol. 2009;53:145–151. doi: 10.1016/j.postharvbio.2009.03.006. [DOI] [Google Scholar]
- Alcaraz-Lopez C, Botia M, Alcaraz CF, Riquelme F. Effect of foliar sprays containing calcium, magnesium and titanium on peach (Prunus persica L.) fruit quality. J Sci Food Agric. 2004;84:949–954. doi: 10.1002/jsfa.1703. [DOI] [PubMed] [Google Scholar]
- Alonso J, Howell N, Canet W. Purification and characterization of two pectin methylesterse from persimmon (Diospyros kaki) J Sci Food Agric. 1997;75:352–358. doi: 10.1002/(SICI)1097-0010(199711)75:3<352::AID-JSFA885>3.0.CO;2-G. [DOI] [Google Scholar]
- Amal SH, El-Mogy MM, Aboul-Anean HE, Alsanius BW. Improving strawberry fruit storability by edible coating as a carrier of thymol or calcium chloride. J Hortic Sci Ornam Plants. 2010;2:88–97. [Google Scholar]
- Amerine MA, Pangborn RM, Roessler EB. Principles of sensory evaluation of food. London: Academic press; 1965. p. 5. [Google Scholar]
- Bal E. Postharvest application of chitosan and low temperature storage affect respiration rate and quality of plum fruits. J Agric Sci Technol. 2013;15:1219–1230. [Google Scholar]
- Bhat MY, Ahsan H, Banday FA, Dar MA, Wani I, Hassan GI. Effect of harvest dates, pre harvest calcium sprays and storage period on physico-chemical characteristics of pear cv. Bartlett. J Agric Res Dev. 2012;2:101–106. [Google Scholar]
- Biggs AR. Effects of calcium salts on apple bitter rot caused by two Colletotrichum spp. Plant Dis. 1999;83:1001–1005. doi: 10.1094/PDIS.1999.83.11.1001. [DOI] [PubMed] [Google Scholar]
- Chardonnet CO, Charron CS, Sams CE, Conway WS. Chemical changes in the cortical tissue and cell walls of calcium-infiltrated ‘Golden Delicious’ apples during storage. Postharvest Biol Technol. 2003;28:97–111. doi: 10.1016/S0925-5214(02)00139-4. [DOI] [Google Scholar]
- Dabuxilatu IM. Interactive effect of salinity and supplemental calcium application on growth and ionic concentration of soybean and cucumber plants. Soil Sci Plant Nutr. 2005;51:549–555. doi: 10.1111/j.1747-0765.2005.tb00063.x. [DOI] [Google Scholar]
- Elhassan SYM. Role of cellulase enzyme in fruit softening during muskmelon fruit ripening. Am J Sci Ind Res. 2016;7:98–105. [Google Scholar]
- Faust M (1989) Utilization of major nutrients by fruit trees. In: Physiology of temperate zone fruit trees. Wiley, New York, pp 83–95
- Goutam M, Dhaliwal HS, Mahajan BVC. Effect of pre-harvest calcium sprays on post-harvest life of winter guava (Psidium guajava L.) J Food Sci Technol. 2010;47:501–506. doi: 10.1007/s13197-010-0085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. Role of calcium in respiratory control. Med Sci Sports Exerc. 1994;26:44–51. doi: 10.1249/00005768-199401000-00009. [DOI] [PubMed] [Google Scholar]
- Hunter S. The measurement of appearance. New York: Wiley; 1975. pp. 304–305. [Google Scholar]
- Jawandha SK, Randhawa JS, Gill PPS, Singh J. Effect of post-harvest treatment on storage quality in ‘Umran’ ber fruit. J Hortic Sci. 2008;3:48–52. [Google Scholar]
- Jawandha SK, Mahajan BVC, Gill PPS. Effect of pre-harvest treatments on the cellulase activity and quality of ber fruits under cold storage. Not Sci Biol. 2009;46:88–91. doi: 10.15835/nsb113536. [DOI] [Google Scholar]
- Jawandha SK, Gupta N, Randhawa JS. Effect of post-harvest treatments on enzyme activity and quality of cold stored ber fruit. Not Sci Biol. 2012;4:86–89. doi: 10.15835/nsb448181. [DOI] [Google Scholar]
- Jayachandran KS, Srihari D, Reddy RY. Pre-harvest sprays of different sources of calcium to improve the shelf life of guava. Indian J Hortic. 2005;62:68–70. [Google Scholar]
- Kalloo G. Production technology for promoting export potential of horticultural crops. Indian Hortic. 2003;48:6–11. [Google Scholar]
- Kays SJ. Postharvest physiology of perishable plant product. Athens: Exon Press; 1997. [Google Scholar]
- Madani B, Muda Mohamed MT, Biggs AR, Kadir J, Awang Y, Tayebimeigooni A, Shojaei TR. Effect of pre-harvest calcium chloride applications on fruit calcium level and post-harvest anthracnose disease of papaya. Crop Prot. 2014;55:55–60. doi: 10.1016/j.cropro.2013.10.009. [DOI] [Google Scholar]
- Mahadevan A, Sridhar R. Methods in physiological plant pathology. Madras: Sivagami Pub; 1982. [Google Scholar]
- Mahajan BVC. Biochemical and enzymatic changes in apple during cold storage. J Food Sci Technol. 1994;31:142–144. [Google Scholar]
- Mahajan BVC, Dhatt AS. Effect of post-harvest treatments on the quality and storage behaviour of subtropical plum cv. Satluj Purple. Acta Hortic (ISHS) 2004;662:379–384. doi: 10.17660/ActaHortic.2004.662.57. [DOI] [Google Scholar]
- Majeed R, Jawandha SK. Enzymatic changes in plum (Prunus salicina L.) subjected to some chemical treatments and cold storage. J Food Sci Technol. 2016;53:2372–2379. doi: 10.1007/s13197-016-2209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganaris GA, Vasilakakis M, Diamantidis G, Mignani I. Cell wall cationic composition and distribution in chilling-injured nectarine fruit. Postharvest Biol Technol. 2005;37:72–80. doi: 10.1016/j.postharvbio.2005.02.009. [DOI] [Google Scholar]
- Manganaris GA, Vasilakakis M, Diamantidis G, Mignani I. The effect of post-harvest calcium application on tissue calcium concentration, quality attributes, incidence of flesh browning an cell wall physicochemical aspects of peach fruits. Food Chem. 2007;100:1385–1392. doi: 10.1016/j.foodchem.2005.11.036. [DOI] [Google Scholar]
- Manganaris GA, Vicente AR, Crisosto CH (2008) Effect of pre-harvest and post- harvest conditions and treatments on plum fruit quality. CAB reviews: perspectives in agriculture, veterinary science, nutrition and natural resources, pp 1–9
- Pasquariello MS, Rega P, Migliozzi T, Capuano LR, Scortichini M, Petriccione M. Effect of cold storage and shelf life on physiological and quality traits of early ripening pear cultivars. Sci Hortic. 2013;162:341–350. doi: 10.1016/j.scienta.2013.08.034. [DOI] [Google Scholar]
- Patel N, Naik AG, Arbat Shakti S. Response of post-harvest chemical treatments on shelf-life and quality of custard apple cv. Balanagar. Ind J Hortic. 2011;68:547–550. [Google Scholar]
- Ranganna S. Handbook of analysis and quality control for fruit and vegetables. 2. New Delhi: Tata McGraw-Hill publishing company limited; 1986. [Google Scholar]
- Rombaldi CV, Silva JA, Machado LD, Parussolo A, Kaster LC, Girardi CL, Danieli R. Harvesting stage and cold storage influences on the quality of Chiripa peaches (Prunus persica L.) Cienc Rural. 2001;31:19–25. doi: 10.1590/S0103-84782001000100004. [DOI] [Google Scholar]
- Serrano M, Martinez-Romero D, Castillo S, Guillen F, Valero D. Effect of pre-harvest sprays containing calcium, magnesium and titanium on the quality of peaches and nectarines at harvest and during post-harvest storage. J Sci Food Agric. 2004;84:1270–1276. doi: 10.1002/jsfa.1753. [DOI] [Google Scholar]
- Sharma S, Sharma RR, Pal RK. Effect of ethylene absorbents on compression injury and quality of ‘Santa Rosa’ Japanese plum (Prunus salicina) during transportation. Indian J Agric Sci. 2013;83:223–226. [Google Scholar]
- Sharples RO, Johnson DS. Post-harvest chemical treatments for the control of storage disorders of apples. Ann Appl Biol. 1976;83:157–167. doi: 10.1111/j.1744-7348.1976.tb01704.x. [DOI] [Google Scholar]
- Siddiqui S, Bangerth F. Differential effect of calcium and strontium on flesh firmness and properties of cell wall in apple. J Hortic Sci. 1995;70:949–954. doi: 10.1080/14620316.1995.11515370. [DOI] [Google Scholar]
- Singh JP, Mandal BK. Role of wrappers and post-harvest application of calcium nitrate on the storage behaviour of sub-tropical litchi cv Manaraji. J App Biol. 2000;10:37–42. [Google Scholar]
- Singh BP, Gupta OP, Chauhan KS. Effect of preharvest calcium nitrate on the storage of peach fruits. J Agric Sci. 1982;4:235–239. [Google Scholar]
- Singh SK, Singh RS, Awasthi OP. Influence of pre- and post-harvest treatments on shelf life and quality attributes of ber fruits. Indian J Hortic. 2013;70:610–613. [Google Scholar]
- Steffens CA, Amarante CVD, Erlani OA, Brackmann A. Fruit quality preservation of ‘Laetita’ plums under controlled atmosphere storage. An Acad Brasil Cienc. 2013;86:485–494. doi: 10.1590/0001-3765201420130237. [DOI] [PubMed] [Google Scholar]
- Turmanidze T, Gulua L, Jgenti M, Wicker L. Effect of calcium chloride treatments on quality characteristics of Blackberry, Raspberry and Strawberry fruits after cold storage. Turk J Agric Food Sci Technol. 2016;4:1127–1133. [Google Scholar]
- Wilford LE, Sabarez H, Price WE. Kinetics of carbohydrate change during dehydration of d’Agen Prunes. Food Chem. 1997;59:149–155. doi: 10.1016/S0308-8146(96)00272-5. [DOI] [Google Scholar]
- Wills R, McGlasson B, Graham D, Joyce D (1998) Postharvest. In: An introduction to the physiology and handling of fruit, vegetables and ornamentals, 4th edn. CAB International, Wallingford, p 262
- Wojcik P, Lewandowski M. Effect of calcium and boron sprays on yield and quality of ‘Elsanta’ strawberry. J Plant Nutr. 2003;26:671–682. doi: 10.1081/PLN-120017674. [DOI] [Google Scholar]
- Zheng XL, Tian SP. Effect of oxalic acid on control of postharvest browning of litchi fruit. Food Chem. 2006;96:519–523. doi: 10.1016/j.foodchem.2005.02.049. [DOI] [Google Scholar]