Abstract
The bioaccessibilities of polyphenols and polysaccharides in green tea powders (GTPs) with different particle sizes of 564.24 µm, 74.85 µm, 34.62 µm and 15.10 µm and their antioxidant activities were investigated using an in vitro simulated gastrointestinal digestion model. The results showed that particle size significantly affected the bioaccessibilities of polyphenols and polysaccharides before and after digestion, except for the bioaccessibility of polysaccharides after gastric plus intestinal (GI) digestion, thus significantly affecting the antioxidant activity of GTPs. Compared with the undigested initial amount, the bioaccessibilities of polyphenols in all GTPs were approximately 59.98–71.00% after gastric digestion and 9.69–15.57% after GI digestion, and the bioaccessibilities of polysaccharides were approximately 71.10–79.51% after gastric digestion and 113.78–190.38% after GI digestion. With the decrease in particle size, the FRAP value of GTP before digestion was significantly increased and that of 15.10 µm was the largest (4.96 mmol Fe2+/g). Both the FRAP and DPPH values after digestion showed a trend of first increasing and subsequently decreasing; 74.85 µm GTP had the largest FRAP (4.11 mmol Fe2+/g) and DPPH (156.61 mg VCE/g) values after gastric digestion, and 34.62 µm GTP had the largest FRAP (0.16 mmol Fe2+/g) and DPPH (1.43 mg VCE/g) values after GI digestion. This study suggested that the bioaccessibilities of polyphenols and polysaccharides in GTPs and their antioxidant activity can be improved by properly reducing the particle size such that TGPs can exert more beneficial health effects.
Keywords: Green tea powder, Bioaccessibility, Antioxidant activity, Polyphenols, Polysaccharides, Digestion
Introduction
Green tea is one of the most widely consumed beverages worldwide, and its consumption has been increasing due to health benefits such as anti-carcinogenetic (Dreosti et al. 1997), anti-hypercholesterolemic (Deka et al. 2011), anti-hypertensive (Hodgson et al. 2005) and hypoglycemic (Yu et al. 2006) activities. It has been reported that polyphenols and polysaccharides are the main active constituents of green tea (Monobe et al. 2010) and are largely responsible for these activities. Numerous studies have reported a relationship between tea consumption and beneficial health effects on cardiovascular diseases, cancer, diabetes, etc. based on the antioxidant and radical scavenging activities of polyphenols and polysaccharides (Dreosti et al. 1997; Yu et al. 2006; Chen et al. 2013).
Green tea powder (GTP) is defined as green tea ground through microparticulation (Hu et al. 2012). GTP contains all of the ingredients of green tea, such as polyphenols, amino acid, saccharide, and caffeine, and also offers several advantages. For example, GTP possesses good solubility, dispersibility, and flowability (Barth 1984), and it can deliver more nutritional and functional components to consumers than green tea or green tea extract (Park et al. 2001). Therefore, GTP has been widely used in drinks and foods (e.g., yoghurt, ice cream, bread, cake, etc.) with green tea flavor as potential antioxidant additive or nutritional supplement (Park et al. 2001; Lu et al. 2010).
Some people believe that the finer the size of GTP, the better its quality because it possesses higher solubility, dispersibility, and flowability (Barth 1984). Xiao et al. (2017) also reported that reduction of the particle size of black tea powder resulted in an increase of its solubility and dispersibility. However, the quality of GTP is also related to its bioaccessibility and functional activity, and no relevant studies have been performed on the effect of the particle size of GTP on its bioaccessibility and functional activity.
Similar to green tea, the bioaccessibility and functional activity of GTP are closely related to the bioaccessibilities of polyphenols and polysaccharides, which are defined as the amounts of polyphenols or polysaccharides released from GTP after digestion compared with the original amount present in GTP (Daly et al. 2010). However, no published information on the bioaccessibility of polyphenols or polysaccharides in GTP is available, although several studies have reported that the bioaccessibility of polyphenols in tea extracts or beverages is low for weak digestive stability (Shim et al. 2012; Chen et al. 2013). Few studies of the effect of particle size on the bioaccessibilities of these materials have been performed except for an investigation by Maeda-Yamamoto et al. (2011) that examined the effect of green tea powder particle size on the absorption of catechins in rats after intragastric administration. Zhao et al. (2018) reported that the particle size of insoluble dietary fiber (IDF) in rice bran affected its phenolic profiles, bioaccessibility and functional properties and that superfine IDF powder exhibited higher phenolic bioaccessibility and antioxidant property than its coarse powder or fine powder.
The current study aimed to assess the bioaccessibilities of polyphenols and polysaccharides in GTPs with different particle sizes and the effect of particle size on their bioaccessibilities and antioxidant activities using an in vitro simulated gastrointestinal digestion model.
Materials and methods
Materials
The materials of (−)-epicatechin (EC), (+)-catechin (C), (−)-epigallocatechin (EGC), (+)-gallocatechin (GC), (−)-epicatechin gallate (ECG), (+)-catechin gallate (CG), (−)-epigallocatechin gallate (EGCG), (+)-gallocatechin gallate (GCG), pepsin, lipase and Folin–Ciocalteu phenol reagent were obtained from Sigma Aldrich (St. Louis, MO). Pancreatin, mucin and 2, 2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Shanghai Yuanye Bio-Technology Co., Ltd (China). The 2, 4, 6-tri (2-pyridyl)-s-triazine (TPTZ) was purchased from Shanghai Aladdin Reagent Co., Ltd (China). All other reagents were obtained from Sinopharm Chemical Reagent Co., Ltd (China).
Green tea powder sample
Fresh tea leaves of Camellia sinensis L. were harvested from tea garden located in Huazhong Agricultural University in Wuhan, China, and manufactured into green tea via steam fixation for 1 min and drying at 80 °C for 2 h. The green tea was roughly ground with a disc mill and sieved through a 40-mesh stainless steel sieve. The coarse powder was ground using a XQM ball mill (Changsha Tianchong Powder Technology Co., Ltd., Hunan, China) at 20 ± 2 °C for 0 h, 3 h, 5 h, and 10 h, and four GTPs were obtained. The particle size distributions were measured using a Mastersizer 3000 laser diffraction particle analyzer (Malvern Instruments Ltd., Worcestershire, UK) and were obtained from the mean values of triplicate determinations. The value of the particle diameter at 50% in the cumulative distribution D50 was used to characterize the median diameter. The D50 values were 564.24 ± 12.32 µm (GTP1), 74.85 ± 6.33 µm (GTP2), 34.62 ± 0.15 µm (GTP3), and 15.10 ± 0.16 µm (GTP4).
Preparation of green tea powder infusion
The GTP infusion was prepared as described previously by Maeda-Yamamoto et al. (2011) with modifications. Briefly, 1.0 g of GTP was extracted with 100 mL of distilled water at 80 °C for 30 min with stirring. After centrifugation at 4000×g, the supernatant was used as the infusion and stored at − 20 °C for analysis.
In vitro digestion procedure
Simulated gastric, duodenal, and bile juices were prepared, and the in vitro digestion model was applied according to the method of Flores et al. (2014) with slight modifications. Briefly, 0.2 g of GTP was transferred to stoppered 125 mL conical flasks and mixed with 12 mL of gastric juice. The mixture was incubated in a shaking water bath at 37°C and 100 rpm for 2 h. After gastric digestion, the pH was increased to 5.3 with 1 M NaOH, followed by the addition of 12 mL of duodenal juice and 6 mL of bile juice. The mixture was again incubated at 37°C and 100 rpm for another 2 h. A blank was prepared with identical chemicals but without GTP and was subjected to the same conditions. After gastric and intestinal digestion, 2 mL of each sample was collected, centrifuged at 4000×g and stored at − 20 °C until analysis.
Determination of total polyphenol content
The total polyphenol content (TPC) in the above sample was determined by the Folin–Ciocalteu method using gallic acid (10–100 μg/mL) as a standard (Flores et al. 2013). The results were expressed as milligrams of gallic acid equivalent (GAE) per gram dry sample (mg GAE/g).
Catechin analysis by HPLC
The catechin content in the above sample was identified by HPLC according to the method of Shim et al. (2012) with some modifications. Briefly, chromatographic separation was performed on an Agilent TC-C18 (250 × 4.6 mm, 5 μm) column at a column temperature of 35 °C with a mobile phase of solvents A and B (V:V, 0.1% formic acid in water: 0.1% formic acid in methanol). Gradient elution was performed by varying the proportion of solvents A and B with a flow rate of 1.0 mL/min with an initial phase of 20% solvent B. UV detection was performed at 278 nm.
Determination of polysaccharide content
The polysaccharides in 2 mL of the above sample were precipitated by adding three volumes of 95% ethanol and were stored at 4 °C for 24 h. The precipitated polysaccharides were collected and dissolved in 10 mL of distilled water. The polysaccharide content was determined by anthrone-sulfuric acid assay using glucose (25–250 μg/mL) as a standard (Somani et al. 1987). The results were expressed as milligrams of glucose equivalent (GE) per gram dry sample (mg GE/g).
Antioxidant activity evaluation
The antioxidant activity of the above sample was measured by investigating the ferric reducing antioxidant power (FRAP) and the scavenging activity of the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical. The FRAP assay was conducted according to the procedure of Flores et al. (Flores et al. 2013) using ferrous sulfate heptahydrate solution (0.2–2 mM) as a standard, and results were expressed as mmol of Fe2+ equivalent per gram dry sample (mmol Fe2+/g). The scavenging activity of the DPPH free radical was determined according to the method of Chen et al. (2013) using ascorbic acid solution (0.10–0.35 mg/ml) as a standard, and results were expressed as milligrams of vitamin C equivalent (VCE) per gram dry sample (mg VCE/g).
Statistical analyses
All data were expressed as the mean ± standard deviation of three independent replications. Statistical analysis was performed using one-way analysis of variance (ANOVA) and the LSD test in SPSS 17.0, and the correlation coefficient was calculated using Excel 2007. Differences were considered significant at P < 0.05.
Results and discussion
Effect of particle size on the bioaccessibilities of polyphenols and polysaccharides in GTPs before digestion
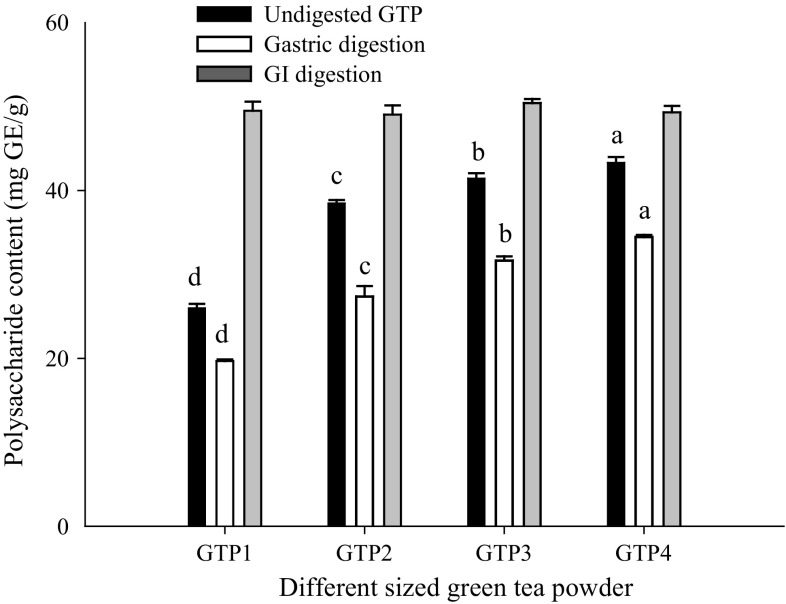
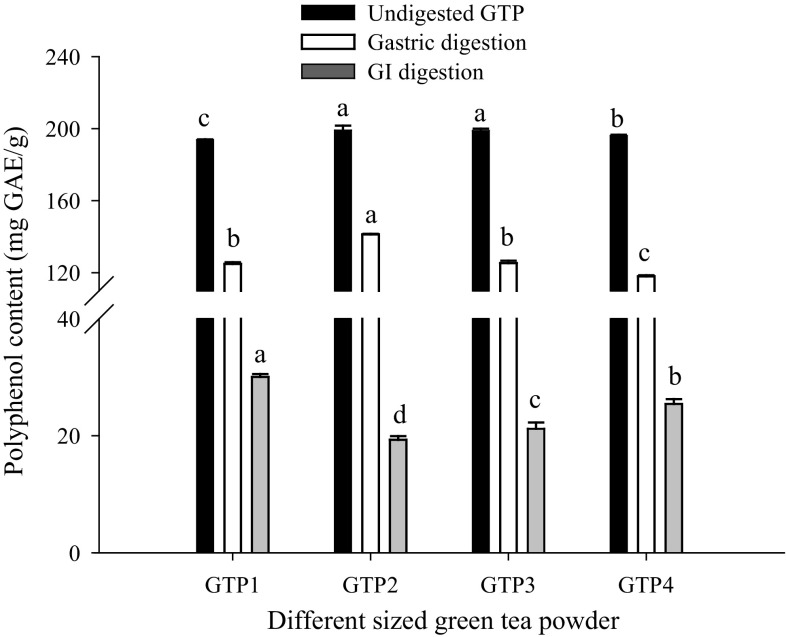
The bioaccessibilities of polyphenols (including catechins, the main components in polyphenols) and polysaccharides in GTPs with different particle sizes before in vitro simulated digestion were studied, and the results showed that the particle size significantly (P < 0.05) affected their bioaccessibilities. The accessible polysaccharides were significantly (P < 0.05) increased with the decrease in particle size (Fig. 1), which was consistent with the report of Hu et al. (2012) that the extraction rate of polysaccharides in GTP increased markedly with decreasing particle size during superfine grinding. The increase might occur because some bonds of cellulose, hemicellulose, and pectin are broken in grinding. However, the accessible polyphenols (Fig. 2) and catechins (Table 1) initially increased and subsequently reduced with decreasing particle size, and 74.85 µm GTP had the highest accessible polyphenols and total catechins, which was consistent with the study of Zaiter et al. (2016) that the maximum concentrations of total phenolic content and all catechins in GTPs were found for granulometric classes between 100 and 180 µm. This phenomenon might be due to the heat generated during superfine grinding, which leads to oxidation of polyphenols and catechins (Hu et al. 2012) or due to powder agglomeration, thus affecting dissolution. Pintauro (1977) has reported that fine tea particles can cause clumping when the powder is exposed to hot water. Therefore, the view that the finer the size of GTP, the better its quality is unacceptable.
Fig. 1.
The contents of polysaccharides in green tea powders with different particle sizes before and after in vitro digestion. Total polysaccharide content is determined using the anthrone-sulfuric acid assay. The results are expressed as milligrams of glucose equivalent (GE) per gram dry sample (mg GE/g). Different letters above the bars indicate significant difference at P < 0.05
Fig. 2.
Total polyphenol contents in green tea powders with different particle sizes before and after in vitro digestion. Total polyphenol content is determined using the Folin–Ciocalteu method. The results are expressed as milligrams of gallic acid equivalent (GAE) per gram dry sample (mg GAE/g). Different letters above the bars indicate significant difference at P < 0.05
Table 1.
The compositions and contents of catechins in green tea powders with different particle sizes (mg/g)
| Catechin | GTP1 | GTP2 | GTP3 | GTP4 |
|---|---|---|---|---|
| GC | 3.90 ± 0.01ab | 3.94 ± 0.03a | 3.85 ± 0.06b | 3.83 ± 0.03b |
| EGC | 42.19 ± 0.14a | 42.08 ± 0.61a | 40.46 ± 0.60b | 39.27 ± 1.10b |
| C | 5.32 ± 0.28b | 5.66 ± 0.02a | 4.66 ± 0.01c | 4.58 ± 0.15c |
| EGCG | 76.84 ± 0.69 | 77.81 ± 1.30 | 77.55 ± 1.42 | 76.66 ± 1.36 |
| GCG | 2.14 ± 0.01 | 2.14 ± 0.04 | 2.13 ± 0.04 | 2.12 ± 0.05 |
| EC | 11.72 ± 0.41 | 12.08 ± 0.13 | 12.02 ± 0.13 | 11.68 ± 0.21 |
| ECG | 18.72 ± 0.12 | 18.85 ± 0.28 | 18.75 ± 0.31 | 18.24 ± 0.51 |
| Total | 160.84 ± 1.31a | 162.57 ± 1.17a | 159.43 ± 2.55ab | 156.39 ± 2.90b |
Data are expressed as the mean ± standard deviation of three replications. Different letters in the same row indicate significant difference at P < 0.05 according to LSD test. GTP1, 564.24 µm; GTP2, 74.85 µm; GTP3, 34.62 µm; GTP4, 15.10 µm
Effect of particle size on the bioaccessibilities of polyphenols and polysaccharides in GTPs after digestion
As shown in Figs. 1 and 2, we found that that the particle size significantly (P < 0.05) affected the bioaccessibilities of polyphenols (including catechins) and polysaccharides after digestion. After gastric digestion, the bioaccessibilities of polyphenols and polysaccharides showed a changing trend similar to their initial amounts in undigested GTPs with a decrease in particle size. However, after GI digestion, the bioaccessible polyphenols were significantly (P < 0.05) decreased initially and subsequently increased significantly (P < 0.05) with decreasing particle size, and no significant difference in the bioaccessible polysaccharides was observed. The bioaccessibilities of total catechins after both gastric digestion and GI digestion were significantly (P < 0.05) increased initially with decreasing particle size and then tended to remain stable when the particle size was 34.62 µm. The different effects of particle size on the bioaccessibilities might be attributed to the following factors. Polyphenols and catechins were relatively stable under acidic conditions (Marchese et al. 2014), and their bioaccessibilities after gastric digestion were primarily associated with their releases from different-sized GTPs, whereas polyphenols and catechins were highly sensitive to alkaline conditions (Bermúdez-Soto et al. 2007), and their bioaccessibilities after GI digestion were primarily related to the stability of released polyphenols and catechins (Correa-Betanzo et al. 2014). The bioaccessibility of polysaccharides after gastric digestion was also closely related to their release from different-sized GTPs, whereas the bioaccessibility after GI digestion relied mostly on the digestion and degradation of carbohydrates.
We observed that the releases of polyphenols and polysaccharides after gastric digestion were significantly (P < 0.01) lower than their initial amounts in undigested GTPs and were approximately 59.98–71.00% and 71.10–79.51% (Figs. 1 and 2), respectively, which might be due to incomplete release. A similar result was also observed for the releases of catechins after gastric digestion (Table 2). The releases of all catechins (except for C) were significantly (P < 0.01) lower than their initial amounts, and the releases of EGCG and ECG in particular were only approximately 38.12–52.74% and 33.33–47.64%, respectively. These were in accordance with the study of Marchese et al. (2014), who reported that the contents of EGCG and ECG from green tea beverages were decreased by 50% after simulated gastric digestion. The increase of C indicated degradation of the ester-type catechins such as EGCG and ECG. Despite the degradation, the bioaccessibility of gallic acid (a degradation product of ester catechin) after gastric digestion was still lower than its initial amount (data not shown), which confirmed the incomplete release. The different releases of polyphenols, catechins, and polysaccharides from different particle sizes of TGPs after gastric digestion led to their different bioaccessibilities.
Table 2.
The compositions and contents of catechins in green tea powders with different particle sizes after in vitro digestion (mg/g)
| Catechin | GTP1 | GTP2 | GTP3 | GTP4 | |
|---|---|---|---|---|---|
| Stomach | GC | 3.40 ± 0.07 | 3.35 ± 0.14 | 3.56 ± 0.05 | 3.39 ± 0.06 |
| EGC | 32.89 ± 0.72b | 32.98 ± 0.09b | 34.52 ± 0.83a | 32.96 ± 0.73b | |
| C | 9.45 ± 0.19 | 9.48 ± 0.20 | 9.90 ± 0.24 | 9.72 ± 0.12 | |
| EGCG | 29.29 ± 1.45c | 36.15 ± 1.47b | 40.89 ± 0.80a | 40.43 ± 0.68a | |
| GCG | ND | ND | ND | ND | |
| EC | 10.38 ± 0.24 | 10.20 ± 0.04 | 10.68 ± 0.23 | 10.43 ± 0.18 | |
| ECG | 6.24 ± 0.19c | 7.92 ± 0.33b | 8.93 ± 0.17a | 8.69 ± 0.35a | |
| Total | 91.65 ± 3.02c | 100.08 ± 2.98b | 108.48 ± 2.27a | 105.62 ± 1.96a | |
| Intestine | GC | 1.32 ± 0.11c | 1.24 ± 0.03c | 2.24 ± 0.11b | 3.10 ± 0.87a |
| EGC | 0.98 ± 0.26d | 2.15 ± 0.12c | 2.77 ± 0.21b | 3.19 ± 0.22a | |
| C | 1.60 ± 0.06b | 1.94 ± 0.11a | 1.97 ± 0.12a | 1.70 ± 0.10b | |
| EGCG | 1.02 ± 0.08a | 0.54 ± 0.09c | 0.86 ± 0.04b | 0.79 ± 0.11b | |
| GCG | ND | ND | ND | ND | |
| EC | 0.45 ± 0.02b | 1.04 ± 0.05a | 1.04 ± 0.08a | 0.46 ± 0.07b | |
| ECG | 0.16 ± 0.01a | 0.12 ± 0.01b | 0.11 ± 0.01b | 0.14 ± 0.01a | |
| CG | 0.53 ± 0.01 | 0.52 ± 0.01 | 0.55 ± 0.04 | 0.48 ± 0.06 | |
| Total | 6.05 ± 0.42c | 7.54 ± 0.27b | 9.55 ± 0.38a | 9.86 ± 0.98a |
Data are expressed as the mean ± standard deviation of three replications. Different letters in the same row indicate significant difference at P < 0.05 according to LSD test. GTP1, 564.24 µm; GTP2, 74.85 µm; GTP3, 34.62 µm; GTP4, 15.10 µm. ND, not detected
After GI digestion, the bioaccessible polysaccharides were significantly (P < 0.01) higher than their initial amounts and were approximately 113.78–190.38%, which might be due to the partial digestion and degradation of carbohydrates such as pectin, starch, and hemicelluloses in GTPs. Carnachan et al. (2012) also reported that the yield of insoluble fiber decreased and that of soluble fiber increased after simulated digestion. However, the bioaccessible polyphenols and catechins were greatly decreased after GI digestion because they were highly sensitive to alkaline conditions, and the bioaccessibilities of polyphenols, EGCG, ECG, EGC, EC, C, and GC were decreased by at least 84.49%, 98.67%, 99.15%, 91.88%, 91.35%, 57.73%, and 19.06%, respectively, compared with their initial amounts. These were consistent with the study of Chen et al. (2013), who reported that the total polyphenol content from tea juice was significantly decreased post-duodenal digestion and that of Green et al. (2007), who reported that more than 80% of total catechins was degraded in the simulated digestive condition. No change in the catechin composition was observed except for GCG and CG after GI digestion. The emergence of CG after GI digestion might be due to the degradation of catechins, and a significant increase of released gallic acid confirmed the degradation compared with its initial amount (data not shown). No GCG was detected, perhaps because its content was lower than the detection limit for degradation.
Effect of particle size on the antioxidant activities of GTPs before and after digestion
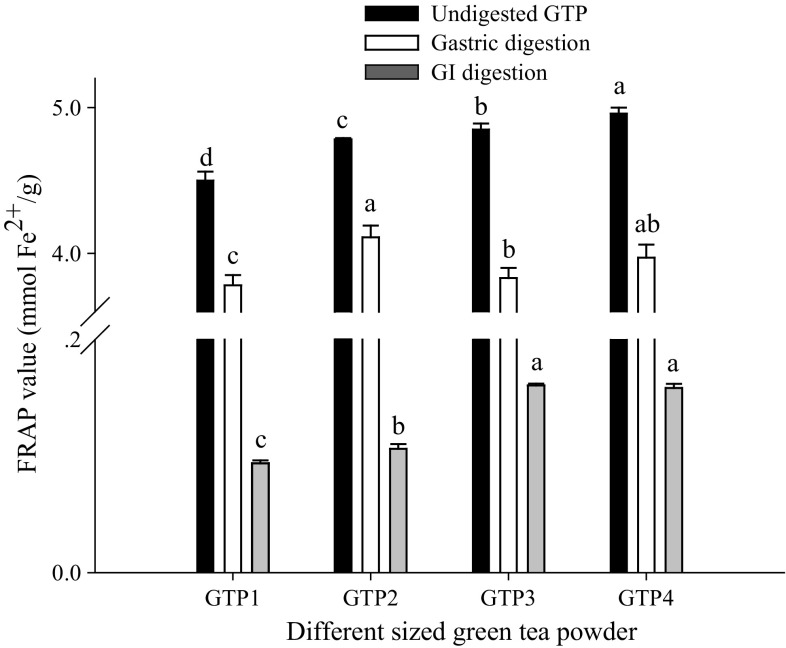
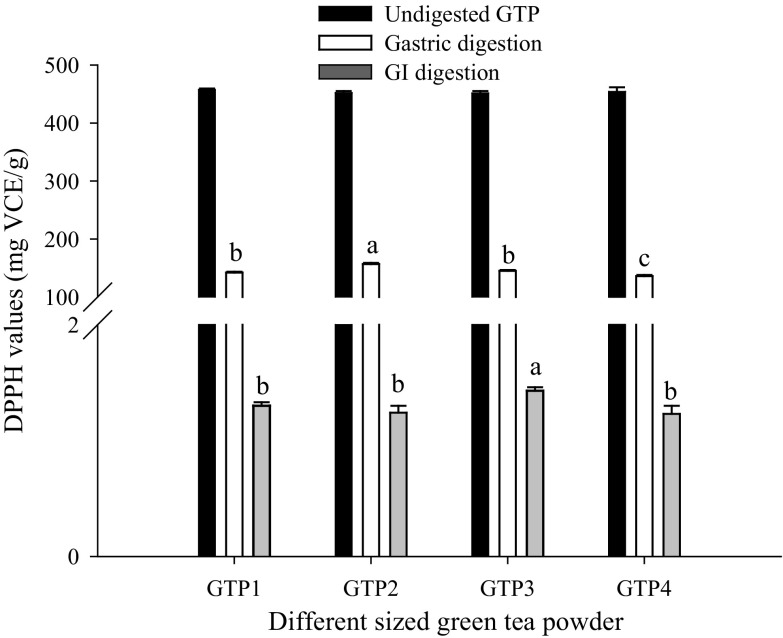
The antioxidant activities of different-sized GTPs before and after digestion based on the FRAP assay and DPPH assay are shown in Figs. 3 and 4. We observed that before digestion, the FRAP value was significantly (P < 0.05) increased with decreasing particle size, and 15.10 µm GTP had the largest FRAP value of 4.96 mmol Fe2+/g, although no significant difference in the DPPH value was observed. After digestion, both the FRAP and DPPH values showed a trend of first increasing and subsequently decreasing with the decrease in particle size. The 74.85 µm GTP had the largest FRAP value of 4.11 mmol Fe2+/g and the largest DPPH value of 156.61 mg VCE/g after gastric digestion, and the 34.62 µm GTP had the largest FRAP value of 0.16 mmol Fe2+/g and the largest DPPH value of 1.43 mg VCE/g after GI digestion. These showed that the particle size significantly affected the antioxidant activities of GTPs before and after digestion, which was in agreement with the report by Zaiter et al. (2016) that the antioxidant activity of green tea powder was dependent on its particle size.
Fig. 3.
Ferric reducing antioxidant power of green tea powders with different particle sizes before and after in vitro digestion. The results are expressed as mmol of Fe2+ equivalent per gram of dry sample (mmol Fe2+/g). Different letters above the bars indicate significant difference at P < 0.05
Fig. 4.
DPPH radical scavenging capacities of green tea powders with different particle sizes before and after in vitro digestion. The results are expressed as milligrams of vitamin C equivalent (VCE) per gram of dry sample (mg VCE/g). Different letters above the bars indicate significant difference at P < 0.05
We also observed that in vitro digestion significantly (P < 0.01) decreased the antioxidant activities of all GTPs. Compared with the initial activities of GTPs before digestion, the FRAP values were decreased by approximately 14.02–21.03% after gastric digestion and by approximately 96.69–97.92% after GI digestion, and the DPPH values were decreased by approximately 65.35–70.09% after gastric digestion and by approximately 99.68–99.73% after GI digestion, respectively. The decrease might be associated with a reduction of the bioaccessible polyphenols and polysaccharides after digestion.
Based on these results, the correlation between the antioxidant activity of GTP and the bioaccessibilities of polyphenols and polysaccharides was calculated. Before digestion, highly positive correlations were observed between the contents of polyphenols (r = 0.552, r = 0.983) and polysaccharides (r = 0.989, r = 0.678) and the values of FRAP and DPPH of GTPs, which showed that the antioxidant potency of GTPs depended primarily on the contents of polyphenols and polysaccharides in them. After gastric digestion, the FRAP and DPPH values of GTPs were highly positive correlated with bioaccessible polyphenols (r = 0.577, r = 0.978) but rather poorly correlated with bioaccessible polysaccharides. After GI digestion, the FRAP and DPPH values of GTPs were moderately positive correlated with bioaccessible polysaccharides (r = 0.332, r = 0.948) but weakly correlated with bioaccessible polyphenols. These results showed that the antioxidant potency of GTP after gastric digestion was based on the bioaccessibility of polyphenols and that the potency after GI digestion was based on the bioaccessibility of polysaccharides. Therefore, the antioxidant activity of 74.85 µm GTP after gastric digestion was the highest despite relatively low values of bioaccessible polysaccharides and that of 34.62 µm GTP after GI digestion was the highest despite a relatively low value of bioaccessible polyphenols. Of course, the interaction between polyphenols and polysaccharides in GTPs and their degradation substance after digestion might affect the antioxidant potency.
The in vitro digestion adversely affected the bioaccessibilities of polyphenols and polysaccharides in GTPs and their antioxidant activities, but GTPs after digestion could still contribute to improvements in human health. For example, digested GTPs might modulate intestinal microbiota (Sun et al. 2018) and still display antioxidant activity, especially in the stomach, by maintaining a redox equilibrium against harmful oxidants and preventing GI tract diseases linked to ROS generation during digestion processes (Bouayed et al. 2011). Moreover, the derivatives of digested polyphenols and polysaccharides are likely to show higher biological activity than the initial substances (Huo et al. 2010).
Conclusion
This research showed that the particle size significantly (P < 0.05) affected the bioaccessibilities of polyphenols and polysaccharides in GTP before and after digestion, thus significantly (P < 0.05) influencing their antioxidant activities. The antioxidant potency of GTP was highly related to the bioaccessibilities of polyphenols and polysaccharides before digestion and was mainly based on the bioaccessibility of polyphenols after gastric digestion and that of polysaccharides after GI digestion. We suggest that the bioavailability of GTP can be improved by properly reducing its particle size and that 34.62 µm GTP after digestion exerts the highest antioxidant activity.
Acknowledgements
This work was financially supported by the Fundamental Research Funds for the Central Universities, China (Grant No. 2662017PY054).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Barth HG. Modern methods of particle size analysis. 1. Hoboken: Wiley; 1984. [Google Scholar]
- Bermúdez-Soto MJ, Tomás-Barberán FA, García-Conesa MT. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007;102:865–874. doi: 10.1016/j.foodchem.2006.06.025. [DOI] [Google Scholar]
- Bouayed J, Hoffmann L, Bohn T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: bioaccessibility and potential uptake. Food Chem. 2011;128:14–21. doi: 10.1016/j.foodchem.2011.02.052. [DOI] [PubMed] [Google Scholar]
- Carnachan SM, Bootten TJ, Mishra S, Monro JA, Sims IM. Effects of simulated digestion in vitro on cell wall polysaccharides from kiwifruit (Actinidia spp.) Food Chem. 2012;133:132–139. doi: 10.1016/j.foodchem.2011.12.084. [DOI] [Google Scholar]
- Chen GL, Hu K, Zhong NJ, Guo J, Gong YS, Deng XT, Huang YS, Chu DK, Gao YQ. Antioxidant capacities and total polyphenol content of nine commercially available tea juices measured by an in vitro digestion model. Eur Food Res Technol. 2013;236:303–310. doi: 10.1007/s00217-012-1897-2. [DOI] [Google Scholar]
- Correa-Betanzo J, Allen-Vercoe E, McDonald J, Schroeter K, Corredig M, Paliyath G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014;165:522–531. doi: 10.1016/j.foodchem.2014.05.135. [DOI] [PubMed] [Google Scholar]
- Daly T, Jiwan MA, O’Brien NM, Aherne SA. Carotenoid content of commonly consumed herbs and assessment of their bioaccessibility using an in vitro digestion model. Plant Foods Hum Nutr. 2010;65:164–169. doi: 10.1007/s11130-010-0167-3. [DOI] [PubMed] [Google Scholar]
- Deka A, Vita JA. Tea and cardiovascular disease. Pharmacol Res. 2011;64:136–145. doi: 10.1016/j.phrs.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreosti IE, Wargovich MJ, Yang CS. Inhibition of carcinogenesis by tea: the evidence from experimental studies. Crit Rev Food Sci Nutr. 1997;37(8):761–770. doi: 10.1080/10408399709527801. [DOI] [PubMed] [Google Scholar]
- Flores FP, Singh RK, Kerr WL, Pegg RB, Kong FB. Antioxidant and enzyme inhibitory activities of blueberry anthocyanins prepared using different solvents. J Agric Food Chem. 2013;61:4441–4447. doi: 10.1021/jf400429f. [DOI] [PubMed] [Google Scholar]
- Flores FP, Singh RK, Kerr WL, Pegg RB, Kong FB. Total phenolics content and antioxidant capacities of microencapsulated blueberry anthocyanins during in vitro digestion. Food Chem. 2014;153:272–278. doi: 10.1016/j.foodchem.2013.12.063. [DOI] [PubMed] [Google Scholar]
- Green RJ, Murphy AS, Schulz B, Watkins BA, Ferruzzi MG. Common tea formulations modulate in vitro digestive recovery of green tea catechins. Mol Nutr Food Res. 2007;51:1152–1162. doi: 10.1002/mnfr.200700086. [DOI] [PubMed] [Google Scholar]
- Hodgson JM, Burke V, Puddey IB. Acute effects of tea on fasting and postprandial vascular function and blood pressure in humans. J Hypertens. 2005;23:47–54. doi: 10.1097/00004872-200501000-00012. [DOI] [PubMed] [Google Scholar]
- Hu JH, Chen YQ, Ni DJ. Effect of superfine grinding on quality and antioxidant property of fine green tea powders. LWT-Food Sci Technol. 2012;45:8–12. doi: 10.1016/j.lwt.2011.08.002. [DOI] [Google Scholar]
- Huo CD, Yang HJ, Cui QZ, Dou QP, Chan TH. Proteasome inhibition in human breast cancer cells with high catechol-omethyltransferase activity by green tea polyphenol EGCG analogs. Bioorg Med Chem Lett. 2010;18:1252–1258. doi: 10.1016/j.bmc.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TM, Lee CC, Mau JL, Lin SD. Quality and antioxidant property of green tea sponge cake. Food Chem. 2010;119:1090–1095. doi: 10.1016/j.foodchem.2009.08.015. [DOI] [Google Scholar]
- Maeda-Yamamoto M, Ema K, Tokuda Y, Monobe M, Tachibana H, Sameshima Y, et al. Effect of green tea powder (Camellia sinensis L. cv. Benifuuki) particle size on O-methylated EGCG absorption in rats; The Kakegawa Study. Cytotechnology. 2011;63:171–179. doi: 10.1007/s10616-010-9331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese A, Coppo EP, Sobolev A, Rossi D, Mannina L, Daglia M. Influence of in vitro simulated gastroduodenal digestion on the antibacterial activity, metabolic profiling and polyphenols content of green tea (Camellia sinensis) Food Res Int. 2014;63:182–191. doi: 10.1016/j.foodres.2014.01.036. [DOI] [Google Scholar]
- Monobe M, Ema K, Azuma K, Maeda-Yamamoto M. Enhancement of phagocytic activity by a crude polysaccharide from tea (Camellia sinensis) extract. Animal Cell Technol: Basic Appl Asp. 2010;16:333–338. [Google Scholar]
- Park DJ, Imm JY, Ku KH. Improved dispersibility of green tea powder by microparticulation and formulation. J Food Sci. 2001;66(6):793–798. doi: 10.1111/j.1365-2621.2001.tb15174.x. [DOI] [Google Scholar]
- Pintauro ND (1977) Agglomeration and aromatization. In: Tea and soluble tea products manufacture. Food Technology Review, No. 38, 1977, Noyes Data Corp. Park Ridge, NJ, pp 39–60
- Shim SM, Yoo SH, Ra CS, Kim YK, Chung JO, Lee SJ. Digestive stability and absorption of green tea polyphenols: influence of acid and xylitol addition. Food Res Int. 2012;45:204–210. doi: 10.1016/j.foodres.2011.10.016. [DOI] [Google Scholar]
- Somani BL, Khanade J, Sinha R. A modified anthrone-sulfuric acid method for the determination of fructose in the presence of certain proteins. Anal Biochem. 1987;167(2):327–330. doi: 10.1016/0003-2697(87)90172-2. [DOI] [PubMed] [Google Scholar]
- Sun HY, Chen YH, Cheng M, Zhang X, Zheng XJ, Zhang ZC. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J Food Sci Technol. 2018;55(1):399–407. doi: 10.1007/s13197-017-2951-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao WH, Zhang Y, Fan CX, Han LJ. A method for producing superfine black tea powder with enhanced infusion and dispersion property. Food Chem. 2017;214:242–247. doi: 10.1016/j.foodchem.2016.07.096. [DOI] [PubMed] [Google Scholar]
- Yu Z, Zhang Y, Ni DJ. Antioxidant and hypoglycemic activity of polysaccharide from tea. Korean J Plant Resour. 2006;19:670–676. [Google Scholar]
- Zaiter A, Becker L, Karam M, Dicko A. Effect of particle size on antioxidant activity and catechin content of green tea powders. J Food Sci Technol. 2016;53(4):2025–2032. doi: 10.1007/s13197-016-2201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Zhang R, Dong L, Huang F, Tang X, Wei Z, Zhang M. Particle size of insoluble dietary fiber from rice bran affects its phenolic profile, bioaccessibility and functional properties. LWT-Food Sci Technol. 2018;87:450–456. doi: 10.1016/j.lwt.2017.09.016. [DOI] [Google Scholar]