Abstract
Chemical, physical, rheological and sensory properties of biscuits fortified with shrimp protein hydrolysate powder (SHP) at various levels (0–7.5%, flour substitution) were investigated. The addition of 7.5% SHP slightly decreased diameter of biscuit and thickness was decreased as SHP at levels of 2.5–7.5% was incorporated. Hardness and fracturability of biscuit decreased as the levels of SHP was higher than 1.25 and 2.5%, respectively. Surface of biscuit became more reddish and yellowish as SHP was added. The addition of 5% SHP increased likeness score of resulting biscuit. Fortification of SHP affected the viscoelastic properties of dough. Deformation resistance and strength of dough decreased with increasing levels of SHP. Additional volatile compounds including aldehyde, ketone, alkane and ether, were detected when 5% SHP was incorporated, thus more likely contributing to odor and flavor of resulting biscuit. The addition of 5% SHP into biscuit resulted in the increased protein content with reduction of carbohydrate. Therefore, the biscuit could be fortified with 5% SHP and had higher nutritional value and increased sensory properties.
Keywords: Protein hydrolysate, Cephalothorax, Biscuit, Nutritional value, Fortification
Introduction
Highly demanded health promoting products brings about the fortification of high nutritional proteinaceous ingredient, fibre, antioxidant and other active components in several food products. Biscuit fortified with shrimp oil rich in polyunsaturated fatty acids along with astaxanthin (Takeungwongtrakul and Benjakul 2017), sponge cake fortified with cabbage leaf powder (Prokopov et al. 2015) and cookies fortified with milk protein hydrolysates (Gani et al. 2015) have been developed successfully. Numerous sources of health promoting ingredients are the processing byproducts, which are commonly discarded without full exploitation. An increasing interest in the recovery of target compounds from those low market value resources is gained. Coincidentally, the novel products containing a high nutritive ingredients have been developed (Takeungwongtrakul and Benjakul 2017).
Protein hydrolysates from seafood processing by-product have become one of potential ingredients for high nutritional and health-promoting functional foods owing to their biologically active peptides (Cao et al. 2009; Sae-leaw et al. 2016). Protein is one of important constituents of the human diet due to the presence of essential amino acids (Gani et al. 2015). Shrimp cephalothorax, a by-product generated from shrimp manufacturing, has protein content in the range of 50–65% and also serves as a source of lipid (11% dry weight basis), chitin (11% dry weight basis), enzymes and other nutritive components (Cao et al. 2009; Takeungwongtrakul et al. 2012). Utilization of shrimp processing byproducts has gained increasing interest as the source of active components or nutrients (Cao et al. 2009).
Biscuit is a popular bakery product because of its variety as well as the convenient and ready-to-eat nature. It also has an extended shelf-life (Gandhi et al. 2001). Biscuit is a dry and crunchy in texture, which is made from wheat flour, sugar, and fat (Maache-Rezzoug et al. 1998). Commercial biscuit generally contains 7–8% protein, which is low in content (Iqbal et al. 2006). To enhance its nutritive value, proteinaceous ingredient can be further added as the supplement. Gani et al. (2015) added milk protein hydrolysate to increase protein content in cookie. However, the amount of added ingredients could affect dough formulation. In general, wheat flour, fat, sugar as well as water content are considered as the crucial parameters influencing the viscoelastic properties, the resistance to stretch, elasticity, and gas holding ability of dough (Sudha et al. 2007). The different rheological characteristics directly influenced the structure, mouth-feel and acceptability of biscuit (Lazaridou et al. 2007). Since protein hydrolysate from shrimp cephalothorax had high levels of essential amino acids with nutritional quality (Sinthusamran et al. 2018), it can be used to enhance the nutritional value as well as sensory property of biscuit. Nevertheless, no information regarding the biscuit fortified with protein hydrolysate from shrimp cephalothorax exists. This study aimed to develop biscuit fortified with shrimp protein hydrolysate at various levels. Characteristic, rheological, nutritional and sensory properties of resulting biscuits were also examined, compared to the typical biscuit.
Materials and methods
Chemicals
All-purpose flour (Imperial, KCG Corp. Co., Ltd., Bangkok, Thailand), soybean oil (Angoon, Thai Vegetable oil Co., Ltd., Nakornpathom, Thailand), baking soda (McGarrett, JR F&B Co., Ltd., Bangkok, Thailand) and other ingredients were procured from a local market in Hat Yai, Songkhla, Thailand. Alcalase from Bacillus licheniformis (20 unit/g dry matter) was from Novozyme (Bagsvaerd, Denmark).
Preparation of protein hydrolysate from shrimp cephalothorax
Shrimp hydrolysate was prepared from cephalothorax of Pacific white shrimp as tailored by Sinthusamran et al. (2018). Cephalothorax was firstly ground with the aid of a blender (HR2163, Phillips, Guangzhou, Guangdong, China) at high speed for 5 min. To the ground sample, distilled water was added at sample/distilled water ratio of 1:1 (w/v). The mixture was then subjected to homogenization at ~ 11,000 rpm for 2 min using a homogenizer model T25 digital (IKA®-Werke GmbH & CO. KG., Stanfen, Germany). Homogenate was adjusted to pH 8.0 with 1.0 M NaOH or 1.0 M HCl. The homogenate (pH 8.0) was firstly incubated at 50 °C for 3 h for initial autolysis. After autolysis, the mixture was further incubated at 60 °C for totally 15 min, followed by the addition of Alcalase (1.0% w/w). Hydrolysis was continued for 2 h before being terminated by heating in hot water (90 °C) for approximately 15 min. The mixture was filtered through two layers of cheesecloth, in which undigested matter was removed. The resulting filtrate was centrifuged at 4000×g at 4 °C using a centrifuge model Avanti® J-E (Beckman Coulter, Inc., Palo Alto, CA, USA) for 15 min. Then, the supernatant was dried using a freeze-dryer (CoolSafe 55, ScanLaf A/S, Lynge, Denmark) for 72 h. Shrimp hydrolysate powder was referred to as “SHP”.
Effect of SHP on properties and quality of biscuit
Preparation of biscuit fortified with SHP
Biscuits were made using the selected formulation: all-propose flour (Imperial, KCG Corporation Co., Ltd., Bangkok, Thailand) (130 g), oil (Angoon, Thai Vegetable oil Co., Ltd., Nakornpathom, Thailand) (20 g), sugar (Mitr Phol Sugar, Mitr Phol Sugar Co., Ltd., Bangkok, Thailand) (30 g), baking soda (McGarrett, JR F&B Co., Ltd., Bangkok, Thailand) (1.5), salt (Prung Thip iodized refined salt, Thai Refined Salt Co., Ltd., Bangkok, Thailand) (3.5 g) and water (48 g). SHP was added into the dough formulation at different levels [1.25, 2.5, 5 and 7.5% (w/w) flour substitutions]. Firstly, dry ingredients were mixed uniformly and the oil was subsequently added. The mixture was mixed thoroughly in a dough mixer (KitchenAid casserole multifunctional 5 k, KitchenAid, Benton Harbor, MC, USA) for 3 min. To the obtained mixture, water was added. The mixture was gently kneaded manually to form dough for 10 min. Dough sheet was prepared with the aid of a rolling pin to obtain a thickness of 3 mm. Using a cutter having a diameter of 30 mm, biscuits with the desired shape were obtained. The shaped biscuit dough was subjected to baking in an electric oven (Mamaru MR-1214, Mamaru Co., Ltd., Bangkok, Thailand) at 200 °C for 15 min. After being baked, biscuits were left at room temperature for 30 min. Biscuit samples were referred to as ‘BH-1.25’, ‘BH-2.5’, ‘BH-5’ and ‘BH-7.5’ for those containing 1.25, 2.5, 5 and 7.5% flour substitution, respectively. The control biscuit without SHP addition was also prepared and named as “CON”. All the biscuits were analyzed.
Analyses
Dimension of biscuit
Diameter (mm) and thickness (mm) of ten biscuit were measured using a vernier caliper and recorded.
Color parameters
Color of sample surface was determined using a Hunter lab colorimeter (Color Flex, Hunter Lab Inc., Reston, VA, USA). The colorimeter was warmed up for 10 min and calibrated with a white standard. L*, a* and b* values indicating lightness/brightness, redness/greenness and yellowness/blueness, respectively, were recorded. Total difference of color (∆E*) and chroma difference (∆C*) was determined as detailed by Takeungwongtrakul et al. (2015).
where ΔL*, Δa* and Δb* are the differences between the corresponding color parameters of the sample and that of the control biscuit (L* = 68.87, a* = 8.20 and b* = 33.12).
Textural properties
Texture property of biscuit was determined at 25 °C using a texture analyzer (Stable Mico System, Godalming, Surrey, UK). A load cell of 10 kg and a speed of 0.5 mm/s were used for testing. A spherical plunger having 5 mm diameter was used for measurement of hardness. The maximum force needed to disrupt the biscuit samples was recorded. Fracturability, defined as the distance needed to break the sample, was examined. Ten biscuits were used for each treatment.
Sensory evaluation
Acceptability of the samples was assessed by 80 untrained panelists, the students and staffs from the Department of Food Technology, Prince of Songkla University. Appearance, color, odor, flavor, texture and overall acceptability of biscuits were evaluated using a nine-point hedonic scale in which a score of 1 = not like very much, 5 = neither like nor dislike and 9 = like extremely. The samples were labeled with random three-digit codes. Panelists were instructed to rinse their mouth with water after each sample evaluation and the order of presentation of the samples was randomized (Meilgaard et al. 2006).
Rheological parameters
Temperature sweep
Rheological characteristics of dough were determined using a RheoStress RS1 rheometer (HAAKE, Karlsruhe, Germany). Measuring geometry included a stainless steel having 60 mm diameter parallel plate. The gap was controlled at 1.0 mm. Dough samples prepared as described previously were placed on the peltier plate (25 °C). Testing was run from 25 to 90 °C with the fixed heating rate (5 °C/min) at the constant strain (0.5%) and frequency (1 Hz). The loss factor (tan δ) was recorded throughout the temperature sweep.
Creep and recovery measurements
Creep measurement of dough samples was carried out at constant stress of 100 Pa with frequency of 1 Hz for 300 s. Subsequently, a recovery test was continued at a stress of 0 Pa for another 300 s. Measurements were conducted at 25 °C. Creep-recovery curves were recorded and analyzed.
Characterization of the selected biscuit fortified with SHP
Biscuit added with SHP rendering the highest sensory property or acceptability was selected and characterized in comparison with the control biscuit (without SHP addition).
Volatile compounds analysis
Biscuit samples were analyzed for volatiles using a solid-phase microextraction gas chromatography mass spectrometry (SPME GC–MS) as tailored by Intarasirisawat et al. (2015). All the volatiles detected were expressed as the abundance (peak area).
Chemical compositions and energy value
Moisture, fat, protein, ash and cholesterol contents of samples were determined by the AOAC method No. of 925.45, 948.15, 981.10, 923.03 and 976.26, respectively (AOAC 2002). Total carbohydrate was calculated following the method of (Benjakul and Karnjanapratum 2018). Based on protein, fat and carbohydrate contents, energy of samples was calculated by the Atwater factor (Prokopov et al. 2015). Total sugar content was measured by the Munson-Walker gravimetric method (Barry 2000). Water activity was determined using a 4TEV water activity meter (Aqualab, Pullman, WA, USA).
Statistical analysis
All the experiments were done in triplicate. Analysis of variance (ANOVA) was carried out and means were compared using the Duncan’s multiple range tests (Steel and Torrie 1986). A Randomized Complete Block Design (RCBD) was used for analysis of acceptance test. T-test was used for pair comparison. SPSS software (IBM software, New York, NY, USA) was used for all analyses.
Results and discussion
Compositions, characteristics and properties of biscuit fortified with SHP at different levels
Diameter and thickness of biscuit
Diameter and thickness of biscuit samples are presented in Table 1. Biscuits containing SHP at all levels showed the similar diameter (3.33–3.47 mm). However, BH-7.5 sample (3.33 cm) had lower diameter than that of the control biscuit (3.51 cm) (P < 0.05). The diameter was decreased when the amount of SHP was greater than 5% (P < 0.05). The decrease in thickness of sample was noticeable as the SHP added was higher than 1.25% (P < 0.05). The lowest thickness of biscuit was found when 7.5% SHP was incorporated (P < 0.05). SHP, particularly at high level, affected the expansion of biscuit during baking process. Protein hydrolysate containing small peptides might interfere dough matrix formation. The network with disconnection of dough proteins could not be puffed properly during baking (Gani et al. 2015). As a result, less expansion of biscuit fortified with SHP was obtained. Takeungwongtrakul and Benjakul (2017) found that thickness of biscuit was decreased with the addition of encapsulated oil at higher level. Expansion ratio of biscuit varied with the formulation of dough, which was governed by the competition of ingredients for the free water. Flour and other ingredient generally absorbed water during dough mixing (Takeungwongtrakul and Benjakul 2017). Benjakul and Karnjanapratum (2018) documented that expansion of cracker was decreased when the amount of bio-calcium of tuna bone was increased. Thus, the addition of SHP into dough directly influenced the thickness and expansion of resulting biscuits. Furthermore, the amount of SHP was a key factor affecting the appearance of biscuits.
Table 1.
Characteristics, color and textural properties of biscuit fortified with SHP at different levels
| Sample | CON | BH-1.25a | BH-2.5 | BH-5 | BH-7.5 |
|---|---|---|---|---|---|
| Diameter (cm) | 3.51 ± 0.14b | 3.47 ± 0.15ab | 3.44 ± 0.10ab | 3.39 ± 0.10ab | 3.33 ± 0.07a |
| Thickness (cm) | 0.56 ± 0.01c | 0.55 ± 0.01c | 053 ± 0.01b | 0.50 ± 0.02b | 0.46 ± 0.01a |
| Color | |||||
| L* | 68.87 ± 0.20e | 65.83 ± 0.10d | 63.97 ± 0.13c | 62.63 ± 0.07b | 62.33 ± 0.05a |
| a* | 8.20 ± 0.07a | 9.36 ± 0.09b | 10.70 ± 0.42c | 10.81 ± 0.34c | 11.47 ± 0.17d |
| b* | 33.12 ± 0.46a | 34.56 ± 0.46b | 35.82 ± 0.09c | 36.13 ± 0.14c | 36.35 ± 0.09d |
| ∆E* | – | 2.12 ± 0.39a | 3.69 ± 0.05b | 4.18 ± 0.13c | 4.52 ± 0.07d |
| ∆C* | – | 0.60 ± 0.09a | 1.40 ± 0.24b | 1.72 ± 0.10c | 1.81 ± 0.04c |
| Texture properties | |||||
| Hardness (N) | 36.13 ± 1.89d | 34.96 ± 1.89d | 28.56 ± 0.62c | 25.02 ± 2.27b | 22.11 ± 3.31a |
| Fracturability (mm) | 5.28 ± 0.26c | 5.20 ± 0.31c | 4.80 ± 0.21bc | 4.34 ± 0.47ab | 4.20 ± 0.45a |
| Moisture | 1.16 ± 0.08a | 1.68 ± 0.10a | 2.39 ± 0.09b | 2.74 ± 0.05c | 3.09 ± 0.07c |
| a w | 0.27 ± 0.05a | 0.28 ± 0.01a | 0.33 ± 0.01b | 0.34 ± 0.01b | 0.37 ± 0.02c |
Values are presented as mean ± SD (n = 3). Different lowercase letters within the same row indicate significant difference (P < 0.05)
CON, biscuit without SHP (control); BH, biscuit fortified with SHP
aNumber: The level of SHP (% flour substitution)
Color
Surface color of biscuit fortified with SHP at varying levels is given in Table 1. The control biscuit had the highest lightness (L* value) with the lowest redness (a* value) and yellowness (b* value), compared to those containing SHP (P < 0.05). Lower L* value was attained in the biscuit when the amount of SHP increased (P < 0.05). On the other hand, both a* and b* values of biscuit increased with increasing SHP amounts (P < 0.05). However, there were no differences in a* and b* values between BH-2.5 and BH-5 samples (P > 0.05). Both ∆E* and ∆C* values of biscuit increased as the levels of SHP increased (P < 0.05). Nevertheless, there was no difference in ∆C* value between BH-5 and BH-7.5 (P > 0.05). In general, the color of biscuit is developed from two reactions during baking. Those included the Maillard reaction and caramelization (Takeungwongtrakul and Benjakul 2017). Maillard reaction is crucial for color development of biscuits, in which brown-yellow color is formed during baking (Takeungwongtrakul and Benjakul 2017). The result suggested that SHP rich in peptides directly affected the color of biscuit, in which browner and more yellowish color was formed (Fig. 1). This was more likely associated with higher degree of non-enzymatic browning or Maillard reaction. Maillard reaction is a chemical reaction between an amino group and a reducing sugar (Martins et al. 2000). Peptides in SHP with a large amount of free amino groups could serve as the reactants, especially for browning reaction. Free amino groups from SHP easily underwent Maillard reaction during baking at high temperature. As a result, biscuit containing SHP, particularly at higher level, turned to be more brownish on surface as shown by higher a* and b* values but lower L* value. Gani et al. (2015) reported that milk protein hydrolysate affected the color of cookies to a higher extent than milk protein concentrate (P < 005). Therefore, the level of SHP had the profound influence on the color of biscuit, mostly by enhancing brown or yellowish color.
Fig. 1.
The appearance of biscuits fortified with SHP at different levels. CON, biscuit without SH powder (control); BH-1.25, BH-2.5, BH-5 and BH-7.5, biscuit fortified with SH powder at 1.25, 2.5, 5 and 7.5% (flour substitution), respectively
Moisture content and water activity (Aw)
The lowest moisture content (1.16%) and Aw (0.27) were found in the control biscuit (P < 0.05) as presented in Table 1. However, both parameters of BH-1.25 sample were similar to those of control (P > 0.05). When SHP was higher than 1.25%, the increases in both moisture content and Aw were found (P < 0.05). There were no differences in both parameters between BH-5 and BH-7.5 and between BH-2.5 and BH-5, respectively (P > 0.05). The highest Aw (0.37) was attained for BH-7.5 sample (P < 0.05). Protein hydrolysate with short peptides mostly contained water soluble hydrophilic peptides. When SHP was incorporated into biscuit, a number of hydrophilic sites was increased. Free water, which reacted with hydrophilic domains of hydrolysate, was trapped in biscuit dough as shown by higher moisture content. Aw of biscuit was generally related with moisture content (Mieszkowska and Marzec 2016). Moisture content generally contributed to the texture and mouth-feel characteristic of biscuit, while Aw is commonly used to evaluate the storage stability of biscuit (Benjakul and Karnjanapratum 2018).
Hardness and fracturability
All the samples containing SHP exhibited lower hardness (22.11–34.96 N), compared to the control biscuit (36.13 N) (P < 0.05), except BH-1.25, which had no difference with the control (P > 0.05) (Table 1). Hardness of biscuit containing SHP generally decreased with increasing level of SHP (P < 0.05). A similar trend was noticed in fracturability of biscuit added with SHP. However, the addition of SHP lower than 5% had no impact on the fracturability of biscuit (P > 0.05). Thus the fortification of SHP resulted in the softer and less brittle texture in comparison with the control. SHP might dilute gluten content. Moreover, SHP plausibly interacted with wheat protein. Consequently, biscuit dough became weaker and less elastic (Zadow 1981). This resulted in the decrease in hardness and fracturability of resulting biscuits. The hardness of biscuits was dependent on the porosity of biscuit dough (Umesha et al. 2015). However, Gani et al. (2015) documented that the hardness of cookies added with milk protein hydrolysate increased with increasing level of hydrolysate up to 15%. Moreover, moisture content was contributed to the decrease hardness and fracturability of biscuit. This was associated with less solid content in dough. The result was in line with Takeungwongtrakul and Benjakul (2017). Thus, the addition of SHP porosity caused the softer texture of resulting biscuit.
Sensory properties
Sensory properties of biscuits fortified with SHP at different levels are presented in Table 2. No differences in likeness scores for appearance and color between the control and the sample added with SHP at all levels added were noticeable (P > 0.05). Nevertheless, higher BH-5 had higher color likeness score than the control (P < 0.05). The higher color likeness score of BH-5 was related with the increased a* and b* values of biscuit, which was reflected by yellowish brown in color. The brown color of biscuit was most likely governed by the reaction between amino acid and reducing sugar, named the Maillard reaction (Takeungwongtrakul et al. 2015). SHP at the amount lower than 5% had no impact on odor likeness of biscuit (P > 0.05). BH-5 sample showed the higher odor likeness score than the control (P < 0.05). The lowest odor likeness was found for BH-7.5 (P < 0.05). The higher odor likeness of BH-5 might be attributed to typical odor of SHP (Simpson et al. 1998) and some products generated from browning reaction during baking (Pasqualone et al. 2014). Some amino acids affected the shrimp flavor. Those included alanine, arginine, glycine and glutamic acid (Cao et al. 2009), which were found at high proportion in SHP from cephalothorax of Pacific white shrimp (Sinthusamran et al. 2018). Furthermore, biscuit containing SHP lower than 5% had no impact on likeness score of flavor and texture, compared to the control (P > 0.05). Nevertheless, no difference in texture likeness was observed among all biscuit containing SHP (P > 0.05). Higher likeness score of texture was more likely related with decreases in hardness and fracturability values of biscuit (Table 1). The incorporation of SHP rendered the biscuit with softened texture. The highest flavor likeness was found in BH-5 sample, compared to others (P < 0.05). The higher flavor likeness score of biscuit fortified with SHP was possibly due to shrimp flavor of SHP added. Dey and Dora (2014) reported that protein hydrolysate obtained from shrimp head had high content of flavoring amino acids involving glutamic acid, aspartic acid, glycine and alanine, which contributed to the good flavor of biscuit fortified with SHP. Biscuits added with 5% SHP exhibited the highest overall acceptability compared to other samples (P < 0.05). This was more likely related with better odor and flavor of biscuit added with the appropriate level of SHP. Therefore, SHP up to 5% could be fortified into biscuit to improve the sensory properties with higher nutritive value and acceptability.
Table 2.
Sensory properties of biscuit fortified with SHP at different levels
| Sample | Appearance | Color | Odor | Flavor | Texture | Overall acceptability |
|---|---|---|---|---|---|---|
| CON | 7.62 ± 0.48a | 7.40 ± 0.58a | 7.45 ± 0.42b | 7.29 ± 0.49b | 7.32 ± 0.38a | 7.35 ± 0.41b |
| BH-1.25a | 7.58 ± 0.38a | 7.42 ± 0.39a | 7.35 ± 0.51bc | 7.32 ± 0.55b | 7.57 ± 0.41ab | 7.50 ± 0.48b |
| BH-2.5 | 7.72 ± 0.42a | 7.67 ± 0.63ab | 7.45 ± 0.53bc | 7.35 ± 0.62b | 7.60 ± 0.50ab | 7.62 ± 0.55b |
| BH-5 | 7.94 ± 0.60a | 8.12 ± 0.55b | 7.94 ± 0.66c | 7.91 ± 0.56c | 7.85 ± 0.37b | 8.24 ± 0.44c |
| BH-7.5 | 7.44 ± 0.56a | 7.22 ± 0.74a | 6.55 ± 0.70a | 6.43 ± 0.42a | 7.50 ± 0.95ab | 6.53 ± 0.61a |
Values are presented as mean ± SD (n = 3). The number of people who assessed the biscuits is 80 people. Different lowercase letters within the same column indicate significant difference (P < 0.05)
CON, biscuit without SHP (control); BH, biscuit fortified with SHP
aNumber: The level of SHP (% flour substitution)
Dynamic viscoelastic properties of dough
Viscoelastic changes of dough during heating
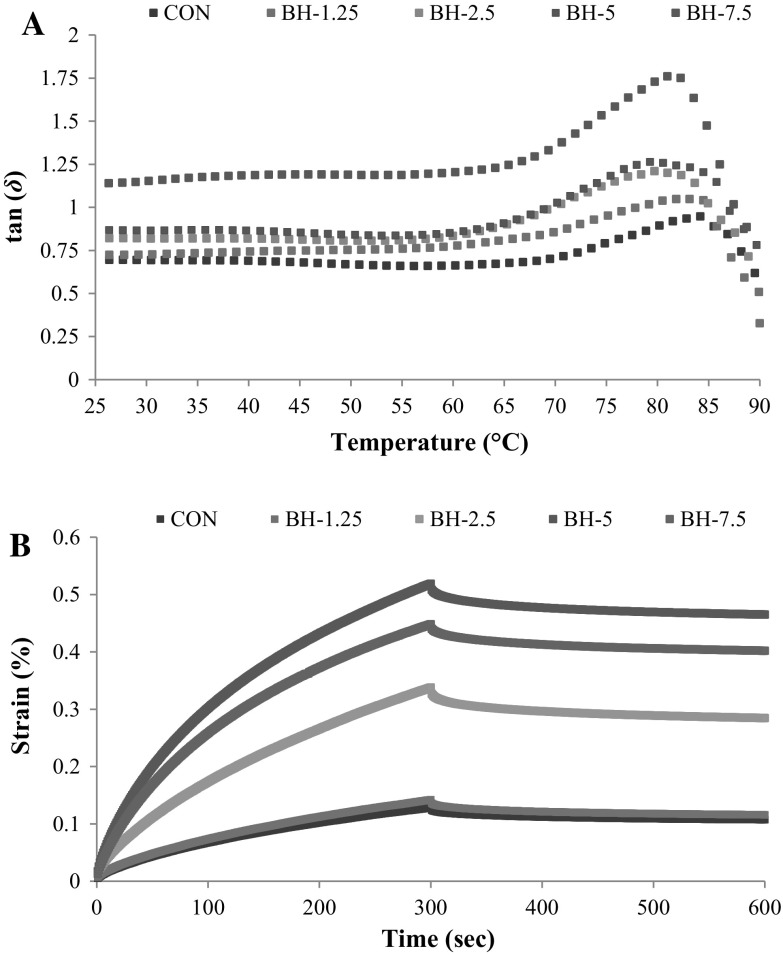
Starch gelatinization process of dough containing SHP was monitored by the viscoelasticity as function of temperatures (Sanz et al. 2015). Loss factor (tan δ) is one of parameter that exhibits the ratio of loss modulus (G″) and elastic modulus (G′) (Jekle et al. 2016). With higher tan δ, dough has higher viscosity. For tan δ < 1, G′ is higher than G″, indicating a predominance of elastic over viscous properties in dough (Zhang et al. 2017). Effect of heating temperature on tan δ value of dough fortified with SHP at different levels is depicted in Fig. 2a. Generally, tan δ values of dough added with SHP increased when SHP levels increased, regardless of temperature. This suggested that the fortification of SHP resulted in the increased viscosity in network structure of dough (Lazaridou et al. 2007). Tan δ value of BH-7.5 was higher than 1, indicating that G″ was higher than G′. SHP at higher amounts resulted in an increase in dough viscosity (Maache-Rezzoug et al. 1998). During the first stage of heating (from 25 to 60 °C), tan δ obtained from all dough samples remained constant. This was associated with water swelling of starch granules. This led to an increase in dough volume and initial softening of dough (Rosell et al. 2007). Tan δ value sharply increased as temperature was increased from 60 to 85 °C. The increased tan δ suggested that starch granular structure started to disintegrate and crystalline structure was molten (Jekle et al. 2016). As a result, amylose was liberated from starch granules and resulted in an increase in viscosity associated with a network formation (Jekle et al. 2016). Subsequently, the sharp decrease in tan δ was observed as temperature increased from 85 to 90 °C. This revealed that dough became more fragile at the later stage of heating, associated with the loosen structure in the final product (Zhang et al. 2017). Tan δmax has been used for determination of the start of gelatinization (Jekle et al. 2016). The onset temperatures of the control, BH-1.25, BH-2.5, BH-5 and BH-7.5 samples were 84.14, 83.23, 82.32, 81.89 and 80.96 °C, respectively. The control and BH-1.25 samples with higher proportion of gluten content demonstrated more elastic character at the onset of gelatinization of dough matrix (tan δmax lower than 1). Jekle et al. (2016) also reported that the amount of gluten has an effect on the elasticity of biscuit dough. Moreover, the different amount of gluten in starch influenced the onset of gelatinization. The addition of SHP to replace flour to some extent could lower the concentration of gluten, leading to slight shift of onset temperature of dough. The viscoelastic properties of dough likely contributed the texture characteristic of biscuit during baking (Sanz et al. 2015).
Fig. 2.
Dynamic viscoelastic loss factor (tan δ) (a) during heating (25–90 °C) and creep-recovery curves (b) of dough fortified with SHP at different levels. Caption: see Fig. 1
Creep and recovery curve
Creep-recovery measurements of doughs without and with SHP at different levels exhibited typical viscoelastic properties as shown in Fig. 2b. The maximum deformation creep test correlated with the strength of dough (Zhang et al. 2017). The greater deformation resistance was found in dough sample with stronger dough structure (Wang and Sun 2002). Dough without SHP (control) and added with 1.25% SHP showed higher resistance to deformation (strain), compared with other dough samples added with SHP at level higher than 1.25% (P < 0.05). The incorporation of SHP higher than 1.25% decreased the resistance to deformation of dough as evidenced by the increase in strain value (%). The result indicated that SHP affected the firmness texture of dough by increasing softness in structure. The maximum strain value was found in BH-7.5, compared to other dough samples (P < 0.05), indicating the softest texture of dough. The decrease in strength of dough when SHP was added might be due to the interference of protein hydrolysate or peptide in the sulfhydryl/disulfide reaction occurring during dough formation (Gani et al. 2015). This led to the decrease of strength of dough network. Furthermore, the addition of SHP also caused the dilution of starch as well as gluten protein (Gani et al. 2015). Short chain peptides themselves could not form the strong gel network. Zhang et al. (2017) documented that the deformation of the dough is markedly dependent upon the stability of network. Therefore, the incorporation of SHP into biscuit dough decreased firmness of dough as evidenced by higher deformation (strain value). As a consequence, the modified dough network or structure resulted in the varying textural property of the resulting biscuits (Table 1).
Volatile compound, chemical compositions and nutritional value of biscuit fortified with 5% SHP
Volatile compounds
Volatile compounds of the control biscuit (CON) and those added with 5% SHP (BH-5) are shown in Table 3. Most flavoring volatiles were associated with lipid oxidation, Maillard reaction and caramelization occurring during baking (Mildner et al. 2009). Volatile compounds in the CON sample were generally less abundant, compared to those of BH-5 sample. The major volatile compounds found in CON sample consisted of 1-hydroxy-2-propanone, pentanal, 1-(acetyloxy)-2-propanone, 2-furanmethanol and 2(5H)-furanone. Those compounds were also detected in BH-5 sample. Takeungwongtrakul and Benjakul (2017) reported that all biscuits fortified without and with micro-encapsulated shrimp oil contained 2-furanmethanol as the major volatile compound. 2-Furanmethanol was considered as the volatile marker for baking process (Mildner et al. 2009). Additionally, alcohols including 1-chlorobutylacetate, 1-butanol, 1-pentanol, 1-octenol and 2-ethylhexanol were found in CON sample. The oxidation and degradation of lipids during baking process generated several aldehyde, ketone and alcohol (Takeungwongtrakul and Benjakul 2017). The result indicated that lipid oxidation of oil possibly occurred in biscuits to some degree during biscuit baking. Furthermore, aldehydes including butanal, 2-methylbutanal, 3-methylbutanal, and benzaldehyde, 1-(1-piperidyl)ethanone, 5-(2-methylpropyl)nonane, trichloromethane, 3-methoxypropene and caprolactam were found only in BH-5 sample. Those volatile compounds might contribute to the new aroma, which was related to higher odor or flavor likeness scores (Table 2). 3-Methylbutanal, 2-methylbutanal, 5-(2-methylpropyl)nonane, caprolactam and trichloromethane were also obtained at the high abundance in BH-5 sample. 3-Methylbutanol had a wine-like odor and might contribute to the odor of shrimp products (Pan and Kuo 1994). 2,2,4,6,6-Pentamethyl heptane, 5-(2-methylpropyl)nonane and trichloromethane were distributed in BH-5 sample. Additionally, 3-methoxypropene was only found in BH-5 sample. The incorporation of SHP could provide or generate new aroma and flavor volatile compounds, which might be related with higher degree of Maillard reaction and lipid oxidation taken place during baking process.
Table 3.
Volatile compounds of control biscuit (CON) and the biscuit fortified with 5% SHP (BH-5)
| Compounds | Peak abundance (× 106) | |
|---|---|---|
| CON | BH-5 | |
| Alcohols | ||
| 1-Chlorobutylacetate 1-butanol | 5.19 | 4.15 |
| 1-Pentanol | 8.39 | 11.25 |
| 1-Octenol | 4.64 | 3.33 |
| 2-Ethylhexanol | 4.27 | 4.98 |
| 2-Furanmethanol | 11.30 | 30.11 |
| Aldehyde | ||
| Butanal | ND | 9.17 |
| Pentanal | 65.06 | 42.28 |
| Hexanal | 5.58 | 44.5 |
| 2-Methylbutanal | ND | 174.16 |
| 3-Methylbutanal | ND | 337.06 |
| Heptenal | 3.13 | 4.12 |
| 3-Hydroxypropanal | 2.01 | 3.22 |
| Benzaldehyde | ND | 81.85 |
| Ketone | ||
| 2-Propanone | 9.17 | 2.22 |
| 1-(1-Piperidyl)Ethanone | ND | 41.31 |
| 1-Methoxy-2-propanone | 11.3 | 8.16 |
| 1-Hydroxy-2-propanone | 88.08 | 68.08 |
| 1-Hydroxy-2-butanone | 5.62 | 9.56 |
| 1-(acetyloxy)-2-propanone | 47.97 | 16.77 |
| Ethanone, 1-(1H-pyrrol-2-yl)- | 2.6 | 30.05 |
| 2(5H)-furanone | 10.07 | 20.68 |
| Carboxylic acids | ||
| Acetic acid | 9.44 | 13.62 |
| Methylenecyclopropanecarboxylic acid | 17.11 | ND |
| 3-Methylbutanoic acid | ND | 19.11 |
| Phenolic acid | 2.3 | 8.09 |
| Alkane | ||
| Cyclohexane | 2.14 | 1.55 |
| Methane | 8.74 | 7.77 |
| 2,2,4,6,6-Pentamethyl heptane | 3.13 | 110.84 |
| 5-(2-Methylpropyl)nonane | ND | 133.67 |
| Trichloromethane | ND | 114.25 |
| Ether | ||
| 3-Methoxypropene | ND | 104.75 |
| Dimethyl ether | 2.7 | 10.9 |
| Other | ||
| Caprolactam | ND | 131.74 |
Chemical composition and energy values
Chemical compositions of BH-5 in comparison with CON samples are presented in Table 4. The main component found in the CON sample was carbohydrate (78.46 g/100 g), followed by protein (9.33 g/100 g). The lower contents of fat (7.48 g/100 g) and ash (3.03 g/100 g) were noted. Sugar (12.27 g/100 g) was more likely from added sugar in formulation. When 5% SHP was incorporated into biscuit, protein content was increased up to 11.82 g/100 g (P < 0.05), which was coincidental with the reduction of carbohydrate to 76.86 g/100 g. The fortification of SHP into biscuit formulation could enhance the nutritional value with significant increase in protein content. SHP had high protein content (86.04%) and low amount of lipid content (0.63%) (Sinthusamran et al. 2018). SHP was rich in glutamic acid/glutamine, aspartic acid/asparagine, arginine and leucine. It had high content of essential amino acids (346.65 mg/g dry sample) (Sinthusamran et al. 2018). Cao et al. (2008) also reported that the hydrolysate from shrimp cephalothorax was an excellent source of nutrients. The nutritional value of SHP was ascertained by high protein efficiency ratio (PER) (2.99) value, which was higher than that from beef muscle protein (2.81) (Dey and Dora 2014). Consequently, the nutritional value of biscuit fortified with 5% SHP was increased. No differences in fat, ash and cholesterol were found between both samples (P > 0.05). Therefore, the fortification of SHP augmented the nutritional value of biscuit by increasing protein content.
Table 4.
Chemical composition and energy value of control biscuit (CON) and the biscuit fortified with 5% SHP
| Chemical composition | CON | BH-5 |
|---|---|---|
| Protein (g/100 g) | 9.33 ± 0.08a | 11.82 ± 0.21b |
| Total fat (g/100 g) | 7.48 ± 0.22a | 7.22 ± 0.14a |
| Total carbohydrate (g/ 100 g) | 78.46 ± 2.45b | 76.86 ± 3.12a |
| Total sugar (g/100 g) | 12.27 ± 0.78a | 12.53 ± 0.55a |
| Ash (g/100 g) | 3.03 ± 0.31a | 3.27 ± 0.17a |
| Cholesterol (mg/100 g) | 0.75 + 0.02a | 0.74 ± 0.02a |
| Energy value (kcal/100 g) | 423.48 ± 11.81b | 410.15 ± 10.11a |
Values are presented as mean ± SD (n = 3) Different lowercase letters within the same row indicate significant difference (P < 0.05)
CON, biscuit without SHP (control); BH-5, biscuit fortified with 5% SHP
The energy values of biscuits were calculated using the Atwater factor (Prokopov et al. 2015) as shown in Table 4. The energy value (410.15 kcal/100 g) of BH-5 sample was slightly lower than that of the CON sample (423.48 kcal/100 g). This was due to the non-significantly lower fat content found in BH-5 sample.
Conclusion
Protein hydrolysate powder produced from shrimp cephalothorax (SHP) could be fortified in biscuit. The addition of SHP influenced the physical and quality properties of biscuit, depending on the level used. Sensory properties of biscuit were significantly increased, when SHP at 5% was fortified, compared to the control biscuit. The addition of SHP affected rheological properties of dough via softening. Additionally, the addition of SHP developed the odor and flavor of resulting biscuit as shown by the release or formation of new volatile compounds. SHP up to 5% could be added into biscuit for improvement of nutritive value as well as acceptability. Therefore, SHP fortified biscuit could be a new product rich in protein and enhanced sensory property.
Acknowledgements
The financial support from Prince of Songkla University is acknowledged.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AOAC . Offical methods of analysis. 16. Washington: Association of Official Analytical Chemists; 2002. [Google Scholar]
- Barry C. Fruits and fruit products: sucrose in fruit products. In: Horwitz W, editor. Methods of analysis of AOAC international. Gaithersburg: AOAC International; 2000. p. 17. [Google Scholar]
- Benjakul S, Karnjanapratum S. Characteristics and nutritional value of whole wheat cracker fortified with tuna bone bio-calcium powder. Food Chem. 2018;259:181–187. doi: 10.1016/j.foodchem.2018.03.124. [DOI] [PubMed] [Google Scholar]
- Cao W, Zhang C, Hong P, Ji H. Response surface methodology for autolysis parameters optimization of shrimp head and amino acids released during autolysis. Food Chem. 2008;109:176–183. doi: 10.1016/j.foodchem.2007.11.080. [DOI] [PubMed] [Google Scholar]
- Cao W, Zhang C, Hong P, Ji H, Hao J, Zhang J. Autolysis of shrimp head by gradual temperature and nutritional quality of the resulting hydrolysate. LWT-Food Sci Technol. 2009;42:244–249. doi: 10.1016/j.lwt.2008.05.026. [DOI] [Google Scholar]
- Dey SS, Dora KC. Optimization of the production of shrimp waste protein hydrolysate using microbial proteases adopting response surface methodology. J Food Sci Technol. 2014;51:16–24. doi: 10.1007/s13197-011-0455-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi A, Kotwaliwale N, Kawalkar J, Srivastav D, Parihar V, Nadh PR. Effect of incorporation of defatted soyflour on the quality of sweet biscuits. J Food Sci Technol. 2001;38:502–503. [Google Scholar]
- Gani A, Broadway AA, Ahmad M, Ashwar BA, Wani AA, Wani SM, Masoodi FA, Khatkar BS. Effect of whey and casein protein hydrolysates on rheological, textural and sensory properties of cookies. J Food Sci Technol. 2015;52:5718–5726. doi: 10.1007/s13197-014-1649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intarasirisawat R, Benjakul S, Vissessanguan W, Maqsood S, Osako K. Skipjack roe protein hydrolysate combined with tannic acid increases the stability of fish oil upon microencapsulation. Eur J Lipid Sci Technol. 2015;117:646–656. doi: 10.1002/ejlt.201400247. [DOI] [Google Scholar]
- Iqbal A, Khalil LA, Ateeq N, Sayyar Khan M. Nutritional quality of important food legumes. Food Chem. 2006;97:331–335. doi: 10.1016/j.foodchem.2005.05.011. [DOI] [Google Scholar]
- Jekle M, Mühlberger K, Becker T. Starch–gluten interactions during gelatinization and its functionality in dough like model systems. Food Hydrocoll. 2016;54:196–201. doi: 10.1016/j.foodhyd.2015.10.005. [DOI] [Google Scholar]
- Lazaridou A, Duta D, Papageorgiou M, Belc N, Biliaderis CG. Effects of hydrocolloids on dough rheology and bread quality parameters in gluten-free formulations. J Food Eng. 2007;79:1033–1047. doi: 10.1016/j.jfoodeng.2006.03.032. [DOI] [Google Scholar]
- Maache-Rezzoug Z, Bouvier J-M, Allaf K, Patras C. Effect of principal ingredients on rheological behaviour of biscuit dough and on quality of biscuits. J Food Eng. 1998;35:23–42. doi: 10.1016/S0260-8774(98)00017-X. [DOI] [Google Scholar]
- Martins SIFS, Jongen WMF, van Boekel MAJS. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci Technol. 2000;11:364–373. doi: 10.1016/S0924-2244(01)00022-X. [DOI] [Google Scholar]
- Meilgaard MC, Carr BT, Civille GV. Sensory evaluation techniques. Boca Raton: CRC Press; 2006. [Google Scholar]
- Mieszkowska A, Marzec A. Effect of polydextrose and inulin on texture and consumer preference of short-dough biscuits with chickpea flour. LWT-Food Sci Technol. 2016;73:60–66. doi: 10.1016/j.lwt.2016.05.036. [DOI] [Google Scholar]
- Mildner SS, Zawirska WR, Obuchowski W, Gośliński M. Evaluation of antioxidant activity of green tea extract and its effect on the biscuits lipid fraction oxidative stability. J Food Sci. 2009;74:362–370. doi: 10.1111/j.1750-3841.2009.01313.x. [DOI] [PubMed] [Google Scholar]
- Pan BS, Kuo JM. Flavour of shellfish and kamaboko flavorants. In: Shahidi F, Botta JR, editors. Seafoods: chemistry, processing technology and quality. Boston: Springer; 1994. pp. 85–114. [Google Scholar]
- Pasqualone A, Bianco AM, Paradiso VM, Summo C, Gambacorta G, Caponio F. Physico-chemical, sensory and volatile profiles of biscuits enriched with grape marc extract. Food Res Int. 2014;65:385–393. doi: 10.1016/j.foodres.2014.07.014. [DOI] [Google Scholar]
- Prokopov T, Goranova Z, Saeva M, Slava A, Galanakis CM. Effect of powder from white cabbage outer leaves on sponge cake quality. Int Agrophys. 2015;29:1–8. doi: 10.1515/intag-2015-0055. [DOI] [Google Scholar]
- Rosell CM, Collar C, Haros M. Assessment of hydrocolloid effects on the thermo-mechanical properties of wheat using the Mixolab. Food Hydrocoll. 2007;21:452–462. doi: 10.1016/j.foodhyd.2006.05.004. [DOI] [Google Scholar]
- Sae-leaw T, O’Callaghan YC, Benjakul S, O’Brien NM. Antioxidant activities and selected characteristics of gelatin hydrolysates from seabass (Lates calcarifer) skin as affected by production processes. J Food Sci Technol. 2016;53:197–208. doi: 10.1007/s13197-015-1989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz T, Laguna L, Salvador A. Biscuit dough structural changes during heating: influence of shortening and cellulose ether emulsions. LWT-Food Sci Technol. 2015;62:962–969. doi: 10.1016/j.lwt.2015.02.036. [DOI] [Google Scholar]
- Simpson BK, Nayeri G, Yaylayan V, Ashie INA. Enzymatic hydrolysis of shrimp meat. Food Chem. 1998;61:131–138. doi: 10.1016/S0308-8146(97)00131-3. [DOI] [Google Scholar]
- Sinthusamran S, Benjakul S, Kijroongrojana K, Prodpran T, Kishimura H. Protein hydrolysates from cephalothorax of Pacific white shrimp manufactured with different processes: compositions, characteristics and antioxidative activity. Waste Biomass Valoriz. 2018 [Google Scholar]
- Steel RGD, Torrie JH (1986) Principles and procedures of statistics: a biometrical approach. McGraw-Hill
- Sudha ML, Srivastava AK, Vetrimani R, Leelavathi K. Fat replacement in soft dough biscuits: its implications on dough rheology and biscuit quality. J Food Eng. 2007;80:922–930. doi: 10.1016/j.jfoodeng.2006.08.006. [DOI] [Google Scholar]
- Takeungwongtrakul S, Benjakul S. Biscuits fortified with micro-encapsulated shrimp oil: characteristics and storage stability. J Food Sci Technol. 2017;54:1126–1136. doi: 10.1007/s13197-017-2545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeungwongtrakul S, Benjakul S, Aran H. Lipids from cephalothorax and hepatopancreas of Pacific white shrimp (Litopenaeus vannamei): compositions and deterioration as affected by iced storage. Food Chem. 2012;134:2066–2074. doi: 10.1016/j.foodchem.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Takeungwongtrakul S, Benjakul S, Aran H. Characteristics and oxidative stability of bread fortified with encapsulated shrimp oil. Ital J Food Sci. 2015;27:476–486. doi: 10.1007/s13197-017-2545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesha SS, Manohar RS, Indiramma AR, Akshitha S, Naidu KA. Enrichment of biscuits with microencapsulated omega-3 fatty acid (Alpha-linolenic acid) rich Garden cress (Lepidium sativum) seed oil: physical, sensory and storage quality characteristics of biscuits. LWT-Food Sci Technol. 2015;62:654–661. doi: 10.1016/j.lwt.2014.02.018. [DOI] [Google Scholar]
- Wang FC, Sun XS. Creep-recovery of wheat flour doughs and relationship to other physical dough tests and breadmaking performance. Cereal Chem. 2002;79:567–571. doi: 10.1094/CCHEM.2002.79.4.567. [DOI] [Google Scholar]
- Zadow JG. Measurement of the effect of whey protein concentrates on fermenting doughs by the Instron Tester. Aust J Dairy Technol. 1981;36:56–59. [Google Scholar]
- Zhang D, Mu T, Sun H. Comparative study of the effect of starches from five different sources on the rheological properties of gluten-free model doughs. Carbohydr Polym. 2017;176:345–355. doi: 10.1016/j.carbpol.2017.08.025. [DOI] [PubMed] [Google Scholar]