Abstract
Jujube (Ziziphus jujuba Mill.) extract has been commonly used as a flavoring ingredient due to its unique aroma. In this study, solid phase micro extraction method was used to extract the volatile compounds in fresh jujube, with the aid of GC–MS for further chemical separation and identification. As a result, 33 volatile compounds, including aldehydes, alcohols, acids, ketones and esters, were identified. Among them, hexanal (276.5–1314 μg/100 g FW), (E)-2-hexanal, (145.1–1876 μg/100 g FW), nonanal (188.2–1047 μg/100 g FW), and n-decanoic acid (58.42–1268 μg/100 g FW) were found to be the major volatile compounds in fresh jujube fruit. Based on the type and amounts of the volatile compounds, 15 Chinese jujube cultivars were divided into 5 clusters through hierarchical cluster analysis and principal component analysis (PCA). Fiver clusters include cluster 1 (LB, HP, LZ, NP, JS, PZ, and YL), cluster 2 (BJ, DB), cluster 3 (PB, BZ, JD and XZ), cluster 4 (JB) and cluster 5 (YZ). According to the PCA, the clusters 1, 2 and 3 could not be discriminated from each other, but clusters 4 and 5 could be separated very well from each other.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-03689-7) contains supplementary material, which is available to authorized users.
Keywords: Jujube, Volatile compounds, SPME, Hierarchical cluster analysis, Principal component analysis
Introduction
Chinese jujube (Ziziphus jujuba Mill.) is mainly grown in the northern China. It is commonly consumed as a fresh fruit, also used as a traditional medicine because of its rich amount of bioactive chemicals (Gao et al. 2013; Ji et al. 2017; Kao and Chen 2015). In addition, jujube extract is widely used as a desirable flavoring ingredient in food industry due to its unique and desirable aroma.
However, many factors, such as regional climate, conditions of soil, plantation, and storage in the postharvest stage, etc., can affect the type and amount of volatile compounds in fruits (Gomes et al. 2016). For instance, it was found that, along with the reduction of crop load of jujube fruit, the amounts of trans-2-hexenal and benzaldehyde in its fresh fruit increased, while those of hexanal, heptanal, and nonanal decreased (Galindo et al. 2015).
Extraction is an essential step for volatile compound analysis. Liquid–liquid extraction (LLE), solid phase micro-extraction (SPME), and simultaneous distillation and extraction (SDE) are three common methods for volatile extraction. Among them, SPME is relatively simple, rapid, and easy to be performed. In addition, this solvent free method is suitable to analyze samples in gaseous, solid or aqueous status (Aziz-Zanjani and Mehdinia 2014).However, it is well known that different extraction methods may significantly impact the flavor analysis (Sanchez-Palomo et al. 2009). For example, Wang et al. studied the effects of different extraction methods on jujube aromas, including LLE, SDE, ultrasound-assisted solvent extraction (UAE) and head space solid-phase micro-extraction (HS-SPME). They found that LLE and UAE could extract similar compounds, but the volatile compounds extracted by SDE, HS-SPME, and LLE were significantly different (Wang et al. 2014).
Identification of volatile compounds is usually performed by gas chromatography (GC), which is commonly connected to a mass spectrometer (MS) due to its high sensitivity and high resolution (Farajzadeh et al. 2014). With the aid of SPME–GC–MS, flavor analysis can be conducted under a rapid extraction and analysis with improved analytical precision and accuracy (Spietelun et al. 2013).
The flavor compounds in the jujube fruits have been analyzed by the aforementioned techniques. The volatile compounds of the Spain jujube were reported to include aldehydes, terpenes, esters, ketones and hydrocarbons (Hernández et al. 2016). Regarding the volatile compounds in jujube wine and jujube leaves, the former were mainly composed of esters and acids (Li et al. 2016), while the latter contained some other components, such as z-ocimene and 1,1-dimethyl-3-methylene-2-ethenyl-cyclohexane acetate(Yang et al. 2011).
In this study, the SPME method was adopted to extract the volatile compounds in the jujube fruits. Subsequently, the PCA was used to classify the 15 cultivars of Chinese jujubes based on their volatile compounds. In more detail, the specific aims of this study were to: (1) extract and identify the main volatile chemicals in the jujube fruit; and (2) investigate the differences of the aroma profiles between the different cultivars of Chinese jujube.
Materials and methods
Sample collection
All the jujube fruit samples were collected from a farm in Shanxi province, China, in October 2015. The fruits were carefully picked up to avoid any broken part, and kept in the same shape. After the fresh jujube fruits were picked up from the trees, they were transported to the lab and immediately frozen at − 80 °C. The investigated jujube cultivars include Ziziphus jujuba Mill. cv.Banzao (BZ), Ziziphus jujuba Mill. cv.Dabailing (DB), Ziziphus jujuba Mill. cv.Cang county Jinsixiaozao (JS), Ziziphus jujuba Mill. cv.Huping (HP), Ziziphus jujuba Mill. cv.Lingbao (LB), Ziziphus jujuba Mill. cv.Yuanling (YL), Ziziphus jujuba Mill. cv.Jidan (JD), Ziziphus jujuba Mill. cv.Lizao (LZ), Ziziphus jujuba Mill. cv.BaodeYouzao (YZ), Ziziphus jujuba Mill. cv. Bin county Jinzao (BJ), Ziziphus jujuba Mill. cv.Junzao (JB), Ziziphus jujuba Mill. cv.PingshunJunzao (PB), Ziziphus jujuba Mill. cv.Xiangzao (XZ), Ziziphus jujuba Mill. cv.Pozao (PZ), and Ziziphus jujuba Mill. cv.Neihuangbianhesuan (NP).
Chemicals
A mixture of alkane standard (C8–C20) and the internal standard, 6-methyl-5-hepten-2-ol, were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Sample preparation
The sample preparation method was based on a previous report (González et al. 2009) with minor modifications. All the fresh jujube samples were carefully peeled with simultaneous removal of their stones. After the fruit was cut into small pieces in approximately same size (3 mm), an amount of 3.0 g of the sample was weighed and extracted by the HS-SPME method for volatile compound analysis.
Optimization of the SPME method
The jujube sample NP was selected at first to optimize the SPME method. In this study, the hybrid coating fiber DVB/CAR/PDMS (50/30 μm) that was purchased from Supleco (Aldrich, Bellefonte, PA, USA) was used for the volatile extraction. The extraction condition included the following parameters: incubation temperature (40, 60, 80, 100 °C), incubation time (15, 30, 45 and 60 min), and extraction time (5, 15, 25, 35 min), which were tested to optimize the extraction condition. Based on the optimization result, the extraction condition was fixed at the following parameters: incubation at 80 °C for 30 min and extracted by 25 min for all the samples.
GC–MS method to identify volatile compounds
Trace 1300 Gas Chromatograph with Tri plus RSH auto-sampler was connected to an ISQ single quadrupole mass spectrometer (Thermo Scientific, USA) with a Thermo TR-5 capillary column (30 m × 0.25 mm × 0.25 μm). The flow rate of Helium was controlled at 1 mL/min. Injection port was in a splitles mode at 250 °C. Temperature program began at 40 °C, hold for 2 min, then increased to 180 °C at the rate of 5 °C/min, hold for 5 min, and then ramped to 240 °C at the rate of 10 °C/min hold for 10 min. The MS detector adopted an electronic ionization (EI) mode, of which the electron impact energy was set at 70 eV. The temperature of ion source and MS transfer line were 280 °C. The scan range of MS was 40–700 m/z. NIST library (2.0 version) and the retention index (RI) were used for chemical identification. RI was calculated by the following equation:
where t is the retention time of a detected compound; tn is the retention time of an alkane standard which was eluted before the detected compound, n is the number of carbons of that standard; tN is the retention time of an alkane standard which was eluted after the detected compound, N is the number of carbons of the standard.
Quantification of the compounds was calculated based on the peak area of the sample and internal standard (6-methyl-5-hepten-2-ol). All the samples were run in triplicate.
Statistics
The data was expressed as mean value ± standard error, which were conducted by one-way variance analysis (ANOVA). Significant level was obtained by the Tukey test (p < 0.05) by JMP software. Principle component analysis (PCA) and hierarchical cluster analysis (HCA) were also operated by the JMP software.
Results and discussion
Optimization of the SMPE method
In this study, the volatile compounds were divided into the following chemical classes including acid, aldehyde, ketone, ester, and alcohol according to their chemical structures. In order to find out the optimized condition for the volatile chemical extraction, the total peak areas of different chemical groups and the sum of total peak areas of the chemical groups were compared so as to determine the best chemical absorption by the SPME.
Effect of temperature on extraction efficiency
Temperature is an important factor which can affect the efficiency of sampling from matrix to the SPME fiber. The diffusion of volatile compounds from the matrix to the head space could be dynamically achieved by heating because the volatile compounds need energy to overcome some barriers in the matrix (Ma et al. 2013). Generally speaking, for the less volatility the more energy is needed. Figure S1shows the values of the total peak areas of all the volatile compounds extracted at different temperatures. The highest values of the peak areas of acids and esters were obtained at 100 °C, while those for the chemical groups of the aldehydes and ketones were achieved at 80 °C; the highest value of peak areas of the alcohols was at 60 °C, when 2,3-butanediol contributed about 92% of all the alcohol peak area. It is clear that different chemicals were extracted to their respective highest values under different temperatures. Since the aldehydes were the major volatile compounds in the jujube fruit, 80 °C was chosen as the optimized temperature in this study.
Effect of incubation time on extraction efficiency
In order to have sufficient volatile compounds vaporized from the matrix into the head space of the vial, the incubation time was also investigated since it is another important factor which can affect the extraction efficiency. As shown in the Figure S2, the total peak area of the detected chemicals approaches to its highest value at 30 min of incubation, which means the most analytes were evaporated from the solid sample into the gas phase, and adsorbed by the fiber. In regards of the total peak areas of different groups of the volatile compounds, such as aldehydes, acids, esters and ketones, the total peak areas of the aldehydes, acids, esters and the sum of these chemical groups were all at their highest values at 30 min, though the total peak areas of ketones and alcohols were obtained at different time. Since the ketones and alcohols only accounted for less than 5% of volatile compounds in jujube, the incubation time for 30 min was selected as another optimized parameter.
Effect of extraction time on extraction efficiency
Under the fixed conditions of other parameters (i.e., 80 °C for incubation temperature and 30 min for incubation time), it was found that SPME could absorb the most amounts of the volatile chemicals, particularly the aldehydes, acids and ketones, when the SPME was exposed in the head space after 25 min (see Figure S3). As a result, 25 min was chosen as the optimized parameter for the SPME extraction.
Identification and quantification of volatile compounds by GC–MS method
Volatile compounds that were identified by GC–MS are listed in Table S1. A number of 33 chemicals, including 18 aldehydes, 2 alcohols, 3 ketones, 5 acids and 5 esters, were detected, but not all of them were detected in all the cultivars. Except the cultivar HP that contained all the 6 detected esters, other cultivars only contained a few of them. Aldehydes and acids were the major volatile compounds in the jujube fruits, which together accounted for more than 95% of the volatile compounds in most cultivars (see Table S2). Alcohols and ketones were identified in most cultivars, but the sum of their combined contents only accounted for a small percentage.
Aldehydes was the largest group of the volatile compounds in the jujube fruits. According to the comparison of their mass spectra and retention index with those in the NIST library and standards, 18 aldehydes were identified, including (E)-2-pentenal, hexanal, (E)-2-hexenal, heptanal, (Z)-2-heptenal, benzaldehyde, 2-phentyl furan, octanal, benzeneacetaldehyde, (E)-2-octenal, nonanal, (E)-2-nonenal, decanal, (E)-2-decenal, 10-undecenal, undecanal, 2-undecenal and dodecanal (Table S1). According to Table S2, hexanal, (E)-2-hexenal, nonanal, and decanal were the major aldehydes in the jujube fruits. This result is in agreement with another study (Hernández et al. 2016). In addition, according to the Galindo’s report (Galindo et al. 2015), hexanal, trans-2-hexanal and benzaldehyde were the major compounds in jujube. Moreover, a previous report about the jujube flavors that were analyzed by GC–MS with electronic nose claimed that hexanal and (E)-2-hexenal were the main volatile chemicals (Chen et al. 2018), although the concentrations of the aldehydes were in significant difference (p < 0.05). For example, the concentration of hexanal was in a range from 276.5 to 1314 μg/100 g FW; (E)-2-hexenal varied from 145.1 to 1876 μg/100 g FW; nonanal was from 188.2 to 1047 μg/100 g FW; the content of decanal was from 73.77 to 246.1 μg/100 g FW, which was not as high as the other three aldehydes mentioned above, but still higher than other aldehydes detected in jujube. In addition, benzeneacetaldehyde was not detected in the cultivars of BJ, DB, HP, JD, LZ and NP. 10-Undecenal was not detected in the HP, JB, JS, LB, PZ and YL. 2-Undecenal was not found in the HP, JB, LB and YL; dodecanal was not detected in JB, LB and YL, and 2-pentenal was detected in all the cultivars except JB. Except the major aldehydes including hexanal, (E)-2-hexenal, nonanal and decanal, the contents of the other aldehydes in most cultivars were less than 100 μg/100 g FW (Table 1).
Table 1.
Concentration of Volatile Compounds in Jujube Fruits, μg/100 g FW
| BJ | BZ | DB | HP | JB | JD | JS | LB | |
|---|---|---|---|---|---|---|---|---|
| Aldehydes | ||||||||
| (E)-2-Pentenal | 22.66 ± 1.90 b | 27.71 ± 0.42 a | 14.12 ± 0.91 de | 11.77 ± 0.48 defg | n.d. | 15.38 ± 1.39 cd | 13.43 ± 0.92 def | 14.19 ± 0.80 de |
| Hexanal | 1314 ± 28 a | 830.7 ± 21.3 b | 763.1 ± 20.5 b | 372.7 ± 11.3 ef | 61.25 ± 3.02 h | 814.6 ± 12.0 b | 562.0 ± 10.2 d | 575.5 ± 21.8 d |
| (E)-2-Hexenal | 1524 ± 12 c | 1694 ± 53 b | 1392 ± 27 d | 741.8 ± 15.6 efg | 145.1 ± 2.37 i | 774.7 ± 20.8 ef | 570.4 ± 9.7 h | 1510 ± 33 cd |
| Heptanal | 77.57 ± 1.91 b | 67.74 ± 1.63 bc | 64.07 ± 2.94 bcd | 39.13 ± 2.12 defg | 67.39 ± 2.20 bc | 64.45 ± 3.74 bcd | 44.09 ± 1.48 cdefg | 57.82 ± 2.33 bcdef |
| (Z)-2-Heptenal | 53.34 ± 4.83 cde | 87.21 ± 2.81 b | 63.82 ± 2.78 c | 45.43 ± 1.88 def | 25.15 ± 1.35 g | 114.3 ± 6.7 a | 62.15 ± 3.61 cd | 37.33 ± 0.46 efg |
| Benzaldehyde | 342.5 ± 12.4 b | 106.1 ± 5.2 cde | 455.7 ± 8.4 a | 190.1 ± 8.6 c | 512.0 ± 13.5 a | 48.58 ± 0.44 def | 84.11 ± 3.04 def | 57.88 ± 2.96 def |
| Furan, 2-pentyl- | 56.52 ± 5.05 de | 116.8 ± 1.5 b | 65.74 ± 1.06 d | 40.04 ± 3.96 f | 20.25 ± 1.00 g | 84.56 ± 2.07 c | 53.65 ± 2.10 e | 59.28 ± 1.80 de |
| Octanal | 356.9 ± 10.3 a | 104.1 ± 1.8 d | 108.2 ± 4.1 d | 37.49 ± 1.19 f | 134.5 ± 4.1 c | 173.1 ± 3.6 b | 110.1 ± 4.8 d | 122.5 ± 2.4 cd |
| Benzeneacetaldehyde | n.d. | 10.89 ± 0.43 c | n.d. | n.d. | 32.13 ± 1.27 a | 3.96 ± 0.36 e | n.d. | 7.39 ± 0.12 d |
| (E)-2-Octenal | 87.08 ± 6.78 de | 111.2 ± 3.7 bc | 124.3 ± 2.3 ab | 103.4 ± 3.5 cd | 81.27 ± 3.17 e | 115.9 ± 5.2 abc | 80.21 ± 2.85 e | 57.14 ± 1.45 f |
| Nonanal | 635.0 ± 21.3 b | 516.6 ± 16.5 cd | 440.9 ± 17.0 de | 188.2 ± 6.5 i | 555.7 ± 16.6 bc | 649.2 ± 16.1 b | 284.3 ± 15.9 gh | 411.1 ± 13.7 ef |
| (E)-2-Nonenal | 47.79 ± 3.71 bcd | 50.16 ± 0.57 bcd | 71.98 ± 2.24 bc | 42.26 ± 2.50 cd | 77.24 ± 2.00 bc | 83.73 ± 1.18 b | 44.50 ± 1.22 cd | 35.41 ± 1.06 d |
| Decanal | 131.5 ± 4.4 cde | 210.3 ± 3.8 b | 215.6 ± 11.7 ab | 100.6 ± 3.2 ef | 192.8 ± 6.5 b | 192.2 ± 2.9 b | 151.4 ± 12.6 c | 150.3 ± 4.9 c |
| (E)-2-Decenal | 6.57 ± 0.71 b | 11.14 ± 0.43 b | 8.83 ± 0.19 b | 11.17 ± 0.33 b | 13.95 ± 1.58 b | 12.76 ± 1.60 b | 5.71 ± 0.38 b | 7.31 ± 0.32 b |
| 10-Undecenal | 4.24 ± 0.35 d | 24.64 ± 2.09 a | 4.33 ± 0.22 | n.d. | n.d. | 7.40 ± 0.22 bcd | n.d. | n.d. |
| Undecanal | 15.55 ± 1.24 g | 50.51 ± 4.28 b | 27.93 ± 1.63 ef | 16.53 ± 0.33 g | 37.98 ± 0.72 cd | 27.97 ± 3.12 ef | 26.12 ± 0.54 f | 19.68 ± 1.82 fg |
| 2-Undecenal | 4.67 ± 0.51 d | 7.47 ± 0.24 c | 7.67 ± 0.56 c | n.d. | n.d. | 11.38 ± 0.42 b | 4.13 ± 0.10 d | n.d. |
| Dodecanal | 5.21 ± 0.85 g | 15.93 ± 0.12 b | 9.70 ± 0.59 de | 8.49 ± 0.21 ef | n.d. | 13.65 ± 0.88 bc | 8.74 ± 0.69 de | n.d. |
| Alcohols | ||||||||
| 1-Octen-3-ol | 10.34 ± 0.15 c | 7.59 ± 0.48 de | 10.02 ± 0.63 c | 16.33 ± 0.59 b | 14.05 ± 0.70 b | 3.78 ± 0.22 gh | 2.60 ± 0.20 h | n.d. |
| Benzyl alcohol | 85.66 ± 5.24 a | n.d. | 95.38 ± 3.80 a | 21.09 ± 1.29 e | 38.22 ± 4.03 c | 7.52 ± 0.22 f | 34.66 ± 1.18 cd | 19.91 ± 2.17 e |
| Acids | ||||||||
| Hexanoic acid | 116.8 ± 5.42 ef | 222.6 ± 20.1 a | 189.5 ± 5.7 abc | 155.4 ± 5.1 cd | n.d. | 94.09 ± 1.77 fg | 54.51 ± 1.61 h | 197.1 ± 6.6 ab |
| Octanoic Acid | n.d. | 83.88 ± 5.15 c | n.d. | 42.03 ± 3.54 de | 99.38 ± 1.84 b | 19.34 ± 2.51 f | 35.80 ± 2.40 e | 18.25 ± 1.32 f |
| Nonanoic acid | n.d. | 9.46 ± 1.16 c | n.d. | n.d. | n.d. | 14.83 ± 1.53 b | 3.81 ± 0.14 ef | n.d. |
| n-Decanoic acid | 333.5 ± 8.3 e | 1189 ± 129 a | 354.2 ± 13.9 de | 244.2 ± 16.3 ef | 674.6 ± 12.9 bc | 852.7 ± 17.4 b | 1106 ± 56 a | 274.4 ± 9.2 e |
| Dodecanoic acid | 139.9 ± 1.8 defg | 249.4 ± 1.9 d | 195.1 ± 7.2 de | 29.39 ± 2.03 fgh | 1048 ± 24 b | 693.3 ± 7.4 c | 151.4 ± 4.0 def | 112.2 ± 2.6 efgh |
| Esters | ||||||||
| Hexanoic acid, methyl ester | n.d. | 5.26 ± 0.10 a | n.d. | 2.67 ± 0.04 c | n.d. | n.d. | n.d. | 5.43 ± 0.25 a |
| Hexanoic acid, ethyl ester | n.d. | n.d. | n.d. | 37.35 ± 1.30 a | n.d. | n.d. | n.d. | 16.49 ± 1.14 b |
| Benzoic acid, ethyl ester | 2.02 ± 0.08 c | n.d. | n.d. | 424.2 ± 23.8 b | 822.9 ± 21.2 a | n.d. | n.d. | 8.74 ± 0.81 c |
| Octanoic acid, ethyl ester | n.d. | n.d. | n.d. | 10.59 ± 0.66 c | 31.02 ± 2.47 a | n.d. | n.d. | 14.37 ± 1.03 b |
| Dodecanoic acid, methyl ester | n.d. | 10.60 ± 0.87 d | 195.1 ± 7.2 a | 2.34 ± 0.17 de | 31.26 ± 1.54 b | 3.46 ± 0.19 de | 2.57 ± 0.06 de | 6.42 ± 0.34 de |
| Ketones | ||||||||
| 2-Nonanone | 3.93 ± 0.08 i | 14.62 ± 0.32 fgh | 8.61 ± 0.37 hi | 57.11 ± 1.61 c | 98.71 ± 3.91 a | 10.66 ± 0.19 ghi | 8.66 ± 0.12 hi | 87.99 ± 2.59 b |
| 2-Undecanone | 3.94 ± 0.32 ij | 15.65 ± 1.01 ef | 6.38 ± 0.11 hi | 24.37 ± 1.78 d | 80.16 ± 2.65 a | 13.86 ± 1.22 fg | 13.16 ± 0.56 fg | 21.55 ± 1.62 de |
| 5,9-Undecadien-2-one, 6,10-dimethyl- | 18.09 ± 1.28 hi | 66.92 ± 3.24 c | 39.38 ± 1.57 efg | 36.34 ± 2.27 fgh | 91.47 ± 10.49 b | 59.58 ± 1.15 cde | 28.32 ± 1.15 ghi | 16.99 ± 1.71 hi |
| LZ | NP | PB | PZ | XZ | YL | YZ | |
|---|---|---|---|---|---|---|---|
| Aldehydes | |||||||
| (E)-2-Pentenal | 15.79 ± 0.71 cd | 9.27 ± 0.40 fg | 27.79 ± 0.66 a | 10.67 ± 0.69 efg | 18.74 ± 0.41 bc | 8.47 ± 0.09 g | 4.03 ± 0.25 h |
| Hexanal | 541.6 ± 15.0 d | 431.1 ± 12.2 e | 675.5 ± 20.1 c | 438.7 ± 10.9 e | 558.8 ± 18.1 d | 303.3 ± 5.3 fg | 276.5 ± 15.8 g |
| (E)-2-Hexenal | 707.5 ± 16.8 fg | 624.6 ± 14.2 gh | 1557 ± 19 c | 842.6 ± 11.4 e | 1876 ± 30 a | 531.9 ± 13.1 h | 263.5 ± 14.9 i |
| Heptanal | 30.60 ± 3.13 g | 31.68 ± 0.63 fg | 60.36 ± 16.78 bcde | 37.55 ± 0.95 efg | 131.9 ± 5.6 a | 36.11 ± 1.40 efg | 27.00 ± 2.51 g |
| (Z)-2-Heptenal | 50.18 ± 2.35 cde | 29.47 ± 1.51 fg | 94.92 ± 6.47 b | 30.04 ± 1.25 fg | 58.11 ± 1.16 cd | 27.85 ± 0.50 g | 26.59 ± 3.34 g |
| Benzaldehyde | 177.6 ± 61.1 c | 136.4 ± 4.7 cd | 109.5 ± 4.7 cde | 10.24 ± 0.36 f | 68.51 ± 3.01 def | 44.87 ± 0.96 ef | 13.47 ± 0.60 f |
| Furan, 2-pentyl- | 56.95 ± 1.26 de | 38.42 ± 1.81 f | 164.0 ± 3.2 a | 28.78 ± 1.41 fg | 67.09 ± 0.70 d | 25.18 ± 0.35 g | 59.66 ± 1.07 de |
| Octanal | 70.36 ± 2.40 e | 136.6 ± 6.1 c | n.d. | 182.7 ± 3.4 b | 63.92 ± 2.31 e | 172.4 ± 3.0 b | 22.25 ± 1.63 f |
| Benzeneacetaldehyde | n.d. | n.d. | 15.72 ± 0.31 b | 4.48 ± 0.16 e | 8.79 ± 0.38 d | 5.30 ± 0.06 e | 4.67 ± 0.23 e |
| (E)-2-Octenal | 80.49 ± 1.65 e | 56.85 ± 2.05 f | 131.4 ± 5.38 a | 41.35 ± 3.01 f | 53.73 ± 1.24 f | 57.55 ± 2.61 f | 49.09 ± 0.79 f |
| Nonanal | 264.1 ± 16.4 ghi | 333.8 ± 10.3 fg | 228.3 ± 3.7 hi | 399.6 ± 8.5 ef | 330.8 ± 2.7 fg | 228.0 ± 3.4 hi | 1047 ± 50 a |
| (E)-2-Nonenal | 41.34 ± 2.38 cd | 27.13 ± 0.78 d | 61.72 ± 15.98 bcd | 26.92 ± 0.68 d | 50.74 ± 1.94 bcd | 41.18 ± 0.99 cd | 201.6 ± 20.5 a |
| Decanal | 110.8 ± 2.9 de | 139.2 ± 2.9 cd | 246.1 ± 9.6 a | 121.2 ± 5.1 cde | 200.3 ± 9.0 b | 143.3 ± 2.6 cd | 73.77 ± 4.33 f |
| (E)-2-Decenal | 6.42 ± 0.16 b | 6.65 ± 0.32 b | 13.08 ± 0.77 b | 4.36 ± 0.28 b | 6.06 ± 0.24 b | 9.31 ± 0.24 b | 64.58 ± 6.71 a |
| 10-Undecenal | 5.16 ± 0.22 cd | 4.87 ± 0.17 cd | 8.57 ± 0.67 bc | n.d. | 26.53 ± 1.62 a | n.d. | 11.12 ± 0.43 b |
| Undecanal | 21.62 ± 0.53 fg | 28.92 ± 2.09 def | 36.33 ± 1.41 de | 15.04 ± 1.00 g | 46.82 ± 1.04 bc | 82.86 ± 0.85 a | 81.03 ± 1.34 a |
| 2-Undecenal | 4.70 ± 0.03 d | 3.87 ± 0.14 de | 8.39 ± 0.59 c | 1.85 ± 0.07 ef | 3.44 ± 0.19 de | n.d. | 27.45 ± 1.06 a |
| Dodecanal | 11.42 ± 0.97 cd | 5.80 ± 0.18 fg | 21.51 ± 1.08 a | 5.68 ± 0.31 fg | 5.60 ± 0.21 fg | n.d. | 4.07 ± 0.25 g |
| Alcohols | |||||||
| 1-Octen-3-ol | 4.05 ± 0.02 gh | 6.50 ± 0.16 def | 27.50 ± 0.86 a | 4.83 ± 0.13 fgh | 8.76 ± 0.29 cd | 5.95 ± 0.12 efg | 15.80 ± 0.77 b |
| Benzyl alcohol | 25.24 ± 1.01 de | 59.48 ± 1.62 b | 4.41 ± 0.14 f | 2.47 ± 0.16 f | n.d. | n.d. | n.d. |
| Acids | |||||||
| Hexanoic acid | 151.2 ± 3.2 de | 74.84 ± 2.95 gh | 189.4 ± 5.3 abc | 56.41 ± 2.15 h | 205.5 ± 7.1 ab | 172.4 ± 3.0 bcd | 57.93 ± 5.23 h |
| Octanoic Acid | 29.39 ± 2.17 ef | 79.17 ± 2.87 c | 42.75 ± 1.93 de | 52.09 ± 1.99 d | n.d. | 136.4 ± 5.1 a | 21.12 ± 1.36 f |
| Nonanoic acid | 7.50 ± 0.13 cd | 1.89 ± 0.04 fg | 8.95 ± 0.19 c | 5.35 ± 0.17 de | 17.58 ± 0.42 a | 12.81 ± 0.25 b | n.d. |
| n-Decanoic acid | 419.6 ± 20.4 de | 828.1 ± 17.2 b | 665.3 ± 8.7 bc | 713.3 ± 11.4 bc | 558.9 ± 14.4 cd | 1268 ± 50 a | 58.42 ± 1.50 f |
| Dodecanoic acid | 127.3 ± 7.7 defgh | 93.20 ± 1.58 efgh | 115.7 ± 9.2 efgh | 126.9 ± 5.2 defgh | 17.00 ± 0.57 gh | 1319 ± 89 a | 8.52 ± 0.04 h |
| Esters | |||||||
| Hexanoic acid, methyl ester | 1.96 ± 0.05 d | n.d. | 3.66 ± 0.17 b | n.d. | 2.50 ± 0.19 c | 2.83 ± 0.09 c | n.d. |
| Hexanoic acid, ethyl ester | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Benzoic acid, ethyl ester | n.d. | n.d. | 17.70 ± 0.96 c | 3.85 ± 0.20 c | n.d. | n.d. | 18.67 ± 1.33 c |
| Octanoic acid, ethyl ester | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Dodecanoic acid, methyl ester | 3.24 ± 0.17 de | 7.88 ± 0.57 de | 5.75 ± 0.20 de | n.d. | 9.33 ± 0.13 de | 20.88 ± 0.46 c | n.d. |
| Ketones | |||||||
| 2-Nonanone | 10.17 ± 0.34 ghi | 11.22 ± 0.38 gh | 32.99 ± 0.88 d | 7.51 ± 0.24 hi | 21.89 ± 1.33 e | 21.06 ± 0.21 ef | 16.57 ± 0.54 efg |
| 2-Undecanone | 7.85 ± 0.25 ghi | 9.99 ± 1.05 fgh | 14.24 ± 0.35 f | 5.74 ± 0.27 hij | 33.74 ± 0.37 c | 62.03 ± 1.74 b | n.d. |
| 5,9-Undecadien-2-one, 6,10-dimethyl- | 41.30 ± 0.46 defg | 56.80 ± 1.55 cdef | 57.41 ± 1.56 cde | 12.12 ± 0.33 i | 23.79 ± 1.32 ghi | 60.64 ± 0.77 cd | 277.5 ± 10.1 a |
Data were represented by mean value ± standard error
n.d. means not detected
The same letters in the same row followed the data means no significant difference, (p < 0.05)
Only two alcohols in the fresh jujube fruit were identified. They were 1-octen-3-ol and benzyl alcohol (Table 1). The former was identified in all cultivars except LB, and its content was in a range from 2.60 to 16.33 μg/100 g FW. The latter was identified in all cultivars except the BZ, XZ, YL and YZ. Its content varied from 2.47 to 95.38 μg/100 g FW (Table 1).
Ketones were not the major compounds in the jujube fruits. Only three ketones were identified. They were 2-nonanone, 2-undecanone and 6, 10-dimethyl-5, 9-undecadien-2-one. As shown in Table 1, 2-nonanone and 6,10-dimethyl-5,9-undecadien-2-one were found in all cultivars, which ranged from 3.93 to 98.71 μg/100 g FW and 12.12 to 277.5 μg/100 g FW, respectively. 2-Undecanone was identified in all cultivars except YZ, with a range from 3.94 to 80.16 μg/100 g FW.
Five short chain organic acids were identified in the jujube fruits (Table 1), including hexanoic acid, octanoic acid, nonanoic acid, n-decanoic acid, and dodecanoic acid. Some of these acids were also found in other cultivars of jujube fruits, such as Ziziphus jujuba Mill. cv. Pingdingdazao, Muzao and Yuanzao (Chen et al. 2018). Among them, octanoic acid was detected in all cultivars except BJ, DB and XZ. Nonanoic acid was not detected in the BJ, DB, HP, JB, LB and YZ (Table 1). n-Decanoic acid and dodecanoic acid were identified as the major acids in all the cultivars. The former was measured in a range from 58.42 (YZ) to 1268 (YL) μg/100 g FW, while the latter had a relatively higher amount of 1319 and 693 μg/100 g FW, in two cultivars, YL and JD, respectively. However, its content in other cultivars was lower than that of n-decanoic acid. Besides, it is worthy of mention that the acids was reported to be the major group among all the volatile chemicals in dried jujube(Chen et al. 2017).
Esters were thought to be the most important aroma compounds in the jujube brandy wine (Zhang et al. 2016), which contributed the largest portion in 81.7% of all the volatile compounds (Shu et al. 2014). However, esters only accounted for a small portion in the fresh jujube fruit. As shown in Table 1, hexanoic acid methyl ester, hexanoic acid ethyl ester, benzoic acid ethyl ester, octanoic acid ethyl ester, and dodecanoic acid methyl ester were identified, but they were only found in a few cultivars (Table 1), and their contents in the jujube fruits were very low, except those in the cultivar HP and JB, in which the total amount of esters in all volatile compounds accounted for 15.64% and 17.50%, respectively (Table S2).
Hierarchical cluster analysis and principal component analysis
In multivariate statistics, principal component analysis (PCA) and hierarchical cluster analysis (HCA) are unsupervised methods, which do not request the prior information (Sena et al. 2002). These two methods were used in this study to determine the similarity of the cultivars based on the contents of the identified volatile chemicals, so as to classify the jujube cultivars based on their geographic origins.
The HCA method was calculated based on Ward’s method. As profiled in Figure S4 and Figure S5, 15 cultivars were classified into five groups. Cluster 1 that includes the cultivars LB, HP, LZ, NP, JS, PZ, and YL contains relatively lower contents of volatile compounds. Cluster 2 includes the BJ and DB, which had a higher concentration of aldehydes than cluster 1. Cluster 3 includes the PB, BZ, JD and XZ, which contains similar contents of aldehydes, ketones, alcohols and esters as cluster 2, but higher content of acids than cluster 2. Cluster 4 only has the cultivar JB because it had very low contents of some aldehydes but higher contents of esters. Similarly, cluster 5 only has the cultivar YZ because of its low contents of aldehydes, acids and esters. According to a previous report (Chen et al. 2018), Zizphus jujuba Mill. cv. Banzao (BZ) and Xiangzao (XZ) were categorized into the same group, but they were different from the cultivar Junzao (JB). Our result is in agreement with this classification. However, different environmental conditions might influence the classification. For instance, the cultivars Jinsixiaozao (JS) and Junzao (JB) were clustered in the same group in Chen’s report, but they are separated in two different groups in this study, which is ascribed to different sources of the samples. In this study, JS was collected from the Shanxi province, which is different from the sample of JS that was collected from Hebei province in Chen’s study.
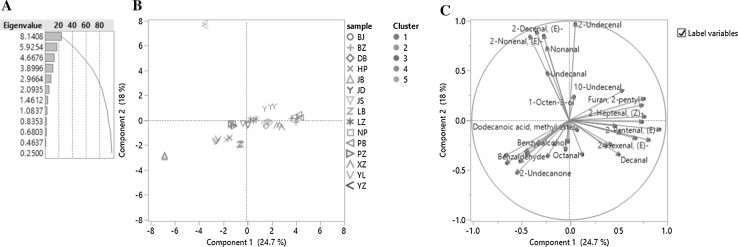
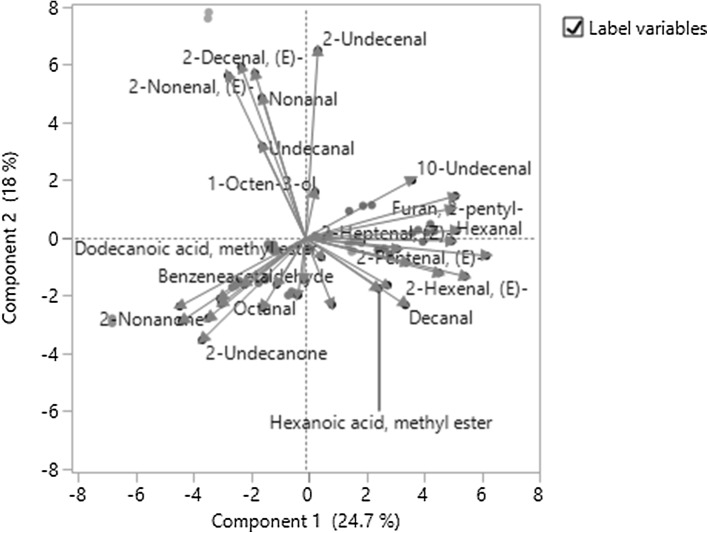
PCA was used to decrease the dimension of data variance, which was calculated based on the correlation of contents of volatile compounds in this study. According to the eigenvalues shown in the Fig. 1a, there were eight eigenvalues large than 1, which meant there could be eight principal components for further data analysis after the reduced dimension. However, only two dimensions (or top two principal components, i.e., PC1 and PC2) were used in this study in order to simplify the statistical analysis and obtain a planner score plot of PCs. According to Fig. 1b, PC1 and PC2 together can explain 42.7% of total data variance that means some information of the volatile compounds have been lost during the statistical re-modeling. As shown in the loading plot (Fig. 1c), the shorter the arrow, the more information has been lost in PCA. For example, alcohols (i.e., benzyl alcohol and 1-octen-3-ol) and esters were the major groups of the volatile compounds that have been suffered the most with the loss of their information. In Fig. 1B, clusters 1, 2, 3 were close to each other, which cannot be distinguished from each other according to score plot in this PCA. However, based on the same PCA, the cluster 4 and cluster 5 were separated very well. In more details shown in the biplot (Fig. 2), it is clear that benzoic acid ethyl ester, octanoic acid ethyl ester and 2-undecanone are positively related to the cluster 4 (JB); (E)-2-decenal and (E)-2-nonenal are positively related to the cluster 5 (YZ); 10-undecenal, hexanal and (E)-2-pentenal are positively related to the cluster 3; In addition, (E)-2-pentenal is also found to be positively related to the cluster 2. This result explains why the cluster 2 and the cluster 3are significantly overlapped (see Fig. 3); octanal, Octanoic acid and benzeneacetaldehyde are positively related to the cluster 1. These correlations in this study have formed the foundations for the classification of different cultivars into different clusters.
Fig. 1.
Principal component analysis of 15 cultivars of Jujube [a eigenvalues of principal components, b score plot of first two principal components (the same color in the score plot means they were in the same cluster, which were cataloged by HCA), c loading plot of different variances], legend of cultivars in b: different marks represented different cultivars, the colors of the mark were meaningless; legend of cluster in b: different color means different cluster (color figure online)
Fig. 2.
Biplot of principal component analysis
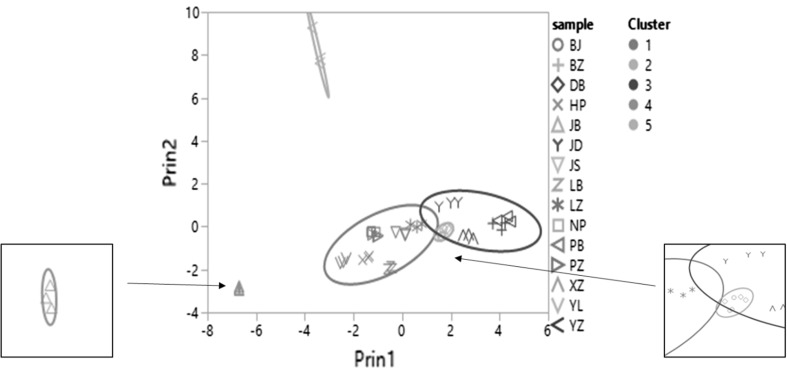
Fig. 3.
Bivariate fit of principal component 2 by principal component 1, legend of cultivars: different marks represented different cultivars, the colors of the mark were meaningless; legend of cluster: different color means different cluster. Density ellipses were shown in cluster grouping, p = 0.95 (color figure online)
Figure 3 reveals how the clusters are close to each other. The density ellipse of each cluster was calculated under the 95% CI. Obviously, the clusters 1, 2 and 3 are crossed over together so that they cannot be discriminated from each other. However, the cluster 4 and cluster 5 are separated far way, demonstrating they are significantly different from other three clusters. Therefore, only cultivar JB in the cluster 4 and YZ in the cluster 5 can be distinguished from other 13 cultivars based on the volatile compounds analysis.
Moreover, since the investigated different cultivars of jujube in this study were planted in the same orchard, the effect of environmental factors on the fruit flavors could be eliminated in a large extent. However, based on the HCA and PCA results, most cultivars could not be differentiated well according to amount and chemical types of the identified volatile compounds. Therefore, classification of the jujube samples with additional consideration of other factors, such as soil (or metal elements), non-volatile chemicals, etc., might be helpful in the further study.
Conclusion
In summary, based on the SPME method, a total of 33 volatile compounds were identified by the GC–MS, which included aldehydes, alcohols, esters, acids and ketones. The major volatile compounds of fresh jujube fruits included the aldehydes and acids, such as hexanal, (E)-2-hexenal, nonanal, and n-decanoic acid, based on their measured contents. In comparison, the contents of alcohols and esters were very low in the investigated jujube fruits. In addition, based on the HCA and PCA, the 15 jujube cultivars could be classified into five clusters. The cluster 1, cluster 2 and cluster 3 could not be discriminated, while cluster 4 and cluster 5 could be discriminated from each other, also from the other three clusters. The results from this study expand the knowledge of volatile compounds in jujube, which could help the food industry select more suitable jujube cultivars for flavoring ingredient extraction. In addition, it shows that HCA and PCA are effective ways for classification analysis, which could be used for functional ingredient classification in other fruits or vegetables.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- FW
Fresh weight
- SPME
Solid phase micro extraction
- LLE
Liquid–liquid extraction
- SDE
Simultaneous distillation and extraction
- UAE
Ultrasound-assisted extraction
- GC–MS
Gas chromatography–mass spectrometry
- PCA
Principle component analysis
- HCA
Hierarchical cluster analysis
Conflict of interest
All authors declare that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lina Wang, Phone: 864-656-5702, Email: lina@g.clemson.edu.
Feng Chen, Phone: 864-656-5702, Email: fchen@clemson.edu.
References
- Aziz-Zanjani MO, Mehdinia A. A review on procedures for the preparation of coatings for solid phase microextraction. Microchim Acta. 2014;181:1169–1190. doi: 10.1007/s00604-014-1265-y. [DOI] [Google Scholar]
- Chen K, Gao L, Li Q, Li H-R, Zhang Y. Effects of CO2 pretreatment on the volatile compounds of dried Chinese jujube (Zizyphus jujuba Miller) Food Sci. Technol (Campinas) 2017;37:578–584. doi: 10.1590/1678-457x.20016. [DOI] [Google Scholar]
- Chen Q, Song J, Bi J, Meng X, Wu X. Characterization of volatile profile from ten different varieties of Chinese jujubes by HS-SPME/GC–MS coupled with E-nose. Food Res Int. 2018;105:605–615. doi: 10.1016/j.foodres.2017.11.054. [DOI] [PubMed] [Google Scholar]
- Farajzadeh MA, Nouri N, Khorram P. Derivatization and microextraction methods for determination of organic compounds by gas chromatography. TrAC Trends Anal Chem. 2014;55:14–23. doi: 10.1016/j.trac.2013.11.006. [DOI] [Google Scholar]
- Galindo A, Noguera-Artiaga L, Cruz ZN, Burló F, Hernández F, Torrecillas A, Carbonell-Barrachina ÁA. Sensory and physico-chemical quality attributes of jujube fruits as affected by crop load. LWT-Food Sci Technol. 2015;63:899–905. doi: 10.1016/j.lwt.2015.04.055. [DOI] [Google Scholar]
- Gao Q-H, Wu C-S, Wang M. The jujube (Ziziphus Jujuba Mill.) fruit: a review of current knowledge of fruit composition and health benefits. J Agric Food Chem. 2013;61:3351–3363. doi: 10.1021/jf4007032. [DOI] [PubMed] [Google Scholar]
- Gomes BL, Fabi JP, Purgatto E. Cold storage affects the volatile profile and expression of a putative linalool synthase of papaya fruit. Food Res Int. 2016;89:654–660. doi: 10.1016/j.foodres.2016.09.025. [DOI] [PubMed] [Google Scholar]
- González M, Gaete-Eastman C, Valdenegro M, Figueroa CR, Fuentes L, Herrera R, Moya-León MA. Aroma development during ripening of Fragaria chiloensis fruit and participation of an alcohol acyltransferase (FcAAT1) gene. J Agric Food Chem. 2009;57:9123–9132. doi: 10.1021/jf901693j. [DOI] [PubMed] [Google Scholar]
- Hernández F, Noguera-Artiaga L, Burló F, Wojdyło A, Carbonell-Barrachina ÁA, Legua P. Physico‐chemical, nutritional, and volatile composition and sensory profile of Spanish jujube (Ziziphus jujuba Mill.) fruits. J Sci Food Agric. 2016;96(8):2682–2691. doi: 10.1002/jsfa.7386. [DOI] [PubMed] [Google Scholar]
- Ji X, Peng Q, Yuan Y, Shen J, Xie X, Wang M. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): a review. Food Chem. 2017;227:349–357. doi: 10.1016/j.foodchem.2017.01.074. [DOI] [PubMed] [Google Scholar]
- Kao TH, Chen BH. Functional components in Zizyphus with emphasis on polysaccharides. Polysacch Bioact Biotechnol. 2015;1:795–827. [Google Scholar]
- Li S-G, Mao Z-Y, Wang P, Zhang Y, Sun P-P, Xu Q, Yu J. Brewing jujube brandy with Daqu and yeast by solid state fermentation. J Food Process Eng. 2016;39(2):157–165. doi: 10.1111/jfpe.12208. [DOI] [Google Scholar]
- Ma Q, Hamid N, Bekhit A, Robertson J, Law T. Optimization of headspace solid phase microextraction (HS-SPME) for gas chromatography mass spectrometry (GC–MS) analysis of aroma compounds in cooked beef using response surface methodology. Microchem J. 2013;111:16–24. doi: 10.1016/j.microc.2012.10.007. [DOI] [Google Scholar]
- Sanchez-Palomo E, Alanon ME, Diaz-Maroto MC, Gonzalez-Vinas MA, Perez-Coello MS. Comparison of extraction methods for volatile compounds of Muscat grape juice. Talanta. 2009;79:871–876. doi: 10.1016/j.talanta.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Sena M, Frighetto R, Valarini P, Tokeshi H, Poppi R. Discrimination of management effects on soil parameters by using principal component analysis: a multivariate analysis case study. Soil Till Res. 2002;67:171–181. doi: 10.1016/S0167-1987(02)00063-6. [DOI] [Google Scholar]
- Shu Y, Zhang Z, Wang Z, Ren H, Wang H (2014) Research on characteristic aAromatic compounds in jujube brandy. In: Proceedings of the 2012 international conference on applied biotechnology (ICAB 2012). Springer, pp 499–506
- Spietelun A, Marcinkowski Ł, de la Guardia M, Namieśnik J. Recent developments and future trends in solid phase microextraction techniques towards green analytical chemistry. J Chromatogr A. 2013;1321:1–13. doi: 10.1016/j.chroma.2013.10.030. [DOI] [PubMed] [Google Scholar]
- Wang H, Li P, Sun S-H, Zhang Q-D, Su Y, Zong Y-L, Xie J-P. Comparison of liquid–liquid extraction, simultaneous distillation extraction, ultrasound-assisted solvent extraction, and headspace solid-phase microextraction for the determination of volatile compounds in jujube extract by gas chromatography/mass spectrometry. Anal Lett. 2014;47:654–674. doi: 10.1080/00032719.2013.845899. [DOI] [Google Scholar]
- Yang L-J, Li X-G, Liu H-X. Herbivore-induced plant volatiles in the leaves of Ziziphus jujuba from China. Chem Nat Compd. 2011;47:820–822. doi: 10.1007/s10600-011-0073-4. [DOI] [Google Scholar]
- Zhang W, Zhang L, Xu C. Chemical and volatile composition of jujube wines fermented by Saccharomyces cerevisiae with and without pulp contact and protease treatment. Food Sci Technol (Campinas) 2016;36:204–209. doi: 10.1590/1678-457X.0011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.