Abstract
The demand for duck meat and eggs in Asian countries increases every year. Duck egg albumen has become an important ingredient in the food industry alongside its hen counterpart, because of its excellent nutritive and functional properties. The major proteins in duck albumen are ovalbumin, ovomucoid, ovomucin, conalbumin, and lysozyme. Comparing with hen albumen, lower contents of ovalbumin, conalbumin, lysozyme and ovoflavoprotein are found in duck albumen. Nevertheless, duck albumen shows better gelling and foaming properties than hen albumen. During storage, duck albumen gel properties are enhanced, while foam volume and foam stability are decreased. Moreover, the changes in quality indices of duck egg including the thinning of the albumen, an increase in albumen pH, loss of water and carbon dioxide occur as storage time is increased. Some processes such as alkaline treatment also cause the loss in nutritive value of egg albumen. In this review, the composition and functional properties of duck albumen and how they are affected by processing conditions are also addressed, in comparison with hen albumen. A better understanding of duck egg albumen would be beneficial so that the food processing industry can exploit the potential of this avian protein.
Keywords: Drying methods, Duck egg, Foaming property, Gelation, Physicochemical property, Chemical composition, Storage conditions
Introduction
Egg and egg products are consumed by people in Asian countries, regardless of their culture and religion. Eggs contain almost all essential nutrients including lipids, minerals, proteins, and all essential vitamins (excluding vitamin C) (Abeyrathne et al. 2013). In the food industry, egg products are used not only as nutrients but predominantly for their sensorial and functional properties (Phillips and Williams 2011). Based on world ranking, Thailand is one of the ten major countries for egg production (Fouad et al. 2018; Huang and Lin 2011). According to Aendo et al. (2018) in 2015, 13,548,366 ducks were used to produce eggs in Thailand, of which 51.86% were from free-grazing duck farms located, mainly in the western and central areas of Thailand. Globally, hen eggs are most popular, while duck and quail eggs are also utilized for consumption. In general, the composition of the egg white proteins in the duck and hen eggs is different. Duck albumen contains about 60% methionine, by 30% threonine, by 25% tryptophan and by 45% sulfur amino acids higher than that of hen egg. Thus, duck egg may be a better source of those amino acids (Pikul 1998). Duck eggs, both fresh and processed, are popular in China and South-East Asia, representing 10–30% of total egg consumption in those regions (Pingle 2009). Among commercial avian products, salted eggs are popular in Asian countries especially in South-East Asia and China (Ganesan et al. 2014).
An egg contains two important parts for human consumption, the egg yolk and albumen. Albumen contains numerous biological substances including ovotransferrin, ovomucoid, lysozyme, cystatin, and ovoinhibitor (Saxena and Tayyab 1997). Ovomucoid, ovoinhibitor, and cystatin are known to be protease inhibitors. Ovotransferrin, avidin, and ovoflavoprotein are functionally classified as mineral and vitamin binding agents (Rossi et al. 2013). Duck albumen has several functional properties including emulsifying, foaming and gelling properties and water holding capacity (Chen et al. 2009). Moreover, albumen proteins and their derivative-proteins play a major role through their antimicrobial (Anton et al. 2006), antioxidant (Ren et al. 2014) and anti-cancer properties (Lee et al. 2017). However, eggs have a limited shelf-life and are prone to quality loss. During stockpiling or storage, many changes occur. Those alterations include the thinning of the albumen, an increase in albumen pH, loss of water and carbon dioxide, increase in yolk water content, debilitating and extending of the vitelline membrane, as well as changes in protein conformation (Qiu et al. 2012) and these changes are likely to affect the albumen functional properties. Additionally, processing e.g. salting, drying, and high-pressure processing, also influence the resulting egg albumen proteins (Katekhong and Charoenrein 2018; Quan and Benjakul 2018b; Yang et al. 2016). In pidan egg albumen, alkaline treatment causes the formations of lysinoalanine and lanthionine, as well as the racemization of amino acids (Chang et al. 1999). Some amino acids such as leucine, asparagine, and glutamine were increased during pidan making, while there was no report regarding the changes in amino acid compositions of salted duck egg white during salting process (Ganesan et al. 2014).
Several reviews or studies of the physicochemical and functional properties of hen albumen have been published. However, less information regarding the changes in duck albumen physicochemical and functional properties during storage and processing and about its applications exists. In this paper the composition and functional properties of duck albumen as affected by storage and processing conditions are reviewed.
Duck albumen proteins and their characteristics
Duck albumen composition
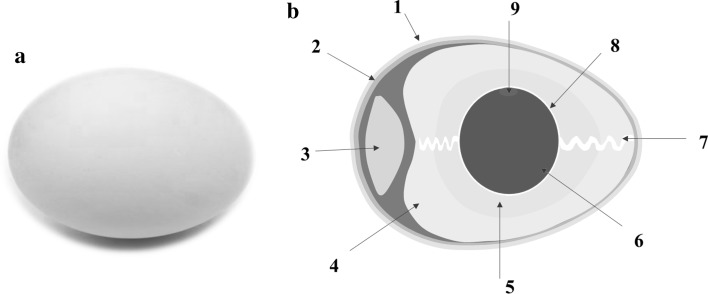
Eggs generally consist of three important parts: the yolk, the albumen or white, and the eggshell with the eggshell membrane. The yolk is encompassed by the albumen and both are further wrapped by the eggshell membrane within the outer covering of a hard eggshell (Hincke et al. 2012) (Fig. 1). Because of the many varieties of laying duck species worldwide, duck egg weight ranges from approximately 60 to 90 g. The egg yolk, albumen, and eggshell account for 28–35%, 45–58%, and 11–13% (based on the whole-egg weight), respectively (Huang and Lin 2011). Duck eggs have a relatively higher percentage of egg yolk, compared to other avian eggs. Congjiao et al. (2017) studied the divergent proteomic patterns of egg white proteins from chickens, turkeys, ducks, quails, pigeons, and geese using tandem mass tag quantification technology, and totals of 148, 162, 138, 183, 179, and 150 proteins, respectively, were identified. Generally, duck and goose albumen had the highest similarity of protein composition (about 78%). Furthermore, dBPS1 and dBPS2 were the two basic proteins isolated from duck egg albumen, which share a basic lineage with the cygnin and meleagrin of swan, turkey and chicken eggs (Naknukool et al. 2008).
Fig. 1.
Duck egg (a) and cross section of duck egg (b); 1: eggshell; 2: shell membrane; 3: air cell; 4: thin albumen; 5: thick albumen; 6: egg yolk; 7: chalaza; 8: vitelline membrane; 9: germinal disc
Duck albumen has water (88.3%) as its main component and it is rich in protein (8.8%). The remainder includes ash (0.53%), and trace amounts of lipids (0.13%) (Huang and Lin 2011). In descending order, the main albumen proteins are ovalbumin (40% of dry matter), ovomucoid (10%), ovomucin (3%), ovotransferrin (2.0%), and lysozyme (1.2%). The major proteins in duck albumen are ovalbumin, ovomucoid, ovomucin, conalbumin, and lysozyme. Comparing protein compositions between hen and duck albumen, there are slight differences. Their compositions and biologically functional properties are presented in Table 1 (Belitz et al. 2009; Huang and Lin 2011). However, Hu et al. (2016) did not detect extra-cellular fatty acid-binding protein precursor (ex-FABP), ovomucoid, prostaglandin D2 synthase (PG D2 synthase) or clusterin in duck albumen proteins. Nevertheless, two main proteins, vitellogenin-2 and the “deleted in malignant brain tumors 1” protein (DMBT1), were found in duck albumen based on a 2-DE gel map. The diversity of proteins determines the gelling, emulsifying, and foaming properties of egg albumen, which are the critical factors governing the quality of food using albumen as an ingredient (Congjiao et al. 2017).
Table 1.
Composition and biological functions of the major duck and hen egg albumen proteins
| Protein | Source of albumen | Ovalbumin | Ovomucoid | Conalbumin (ovotransferrin) | Ovomucin | Lysozyme | Ovomacroglobulin | Flavoprotein | Avidin | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Percent of the total proteina | Duck | 40.0 | 10.0 | 2.0 | 3.0 | 1.2 | 1.0 | 0.3 | 0.03 | Mine (1995) |
| Hen | 54 | 11 | 12 | 3.5 | 3.4 | 0.5 | 0.8 | 0.05 | Huang and Lin (2011) | |
| Molecular weight (kDa) | Duck | 43.49 | 28.0 | 70.0 | ND | 15.0 | ND | 33.0 | 48.1 | Hu et al. (2016), Hytönen et al. (2003), Miguel et al. (2005) |
| Hen | 44.5 | ~28.0 | 77.7 | 5.5–8.3 × 103 | 14.0 | 769.0 | 32.0 | 60.1 | Hytönen et al. (2003), Miguel et al. (2005), Mine (1995) | |
| Biological functionalities | Phosphoglyco-protein | Inhibits trypsin | Binds metallic ions | Staloprotein; viscous | Lyses some bacteria | Inhibits serine and cysteine proteases | Binds riboflavin | Binds biotin | Mine (1995), Belitz et al. (2009) | |
ND not determined
aAverage values
Several protease inhibitors are present in hen egg albumen and can inhibit serine protases such as ovomucoid and ovoinhibitor, while cystatin is an inhibitor of thiol proteases (Réhault 2007; Stevens 1991). However, those protease inhibitors could not be found in duck egg albumen by 2-dimensional polyacrylamide gel electrophoresis (2-DE) (Hu et al. 2016). Quan and Benjakul (2018b) reported protease inhibitor from duck albumen with molecular weight of 44 kDa based on inhibitory activity staining. This protein was plausibly ovalbumin, which had inhibitory activity toward trypsin (Takenawa et al. 2015). Ovalbumin is known as a member of serpin family and shares sequence homology with α1-protease inhibitor, antithrombin III and angiotensinogen (Saxena and Tayyab 1997). In food industry, protease inhibitors have been used as the food additives to improve textural property of several food products e.g. surimi, meat ball, and sausage, etc. (Klomklao et al. 2016). Duck albumen also showed higher inhibitory activity toward trypsin than hen counterpart (Quan and Benjakul 2018a). Therefore, duck egg albumen could be used as the substitute for hen egg albumen in some surimi-based products as the source of protease inhibitor, in which the protein degradation can be prevented (Quan and Benjakul 2018a).
Functional properties of egg albumen
Gelling property
Due to the superior foaming and gelling capacities of albumen in food systems, its use is preferred in food products to whole egg or egg yolk. Gelation has an essential role in a number of widely available products, e.g. imitation crab, reformulated meat products, tofu, fish ball, and surimi (Alleoni 2006). Egg albumen is a regularly used ingredient for improving gel strength or the water-holding capacity of many food products. The rheological and textural properties of many products depend on the gelling properties or heat coagulation of egg proteins (Ren et al. 2010). Some foods (e.g. meringues and angle cakes) require egg albumen as a foaming agent. However, the gelling characteristics and coagulation temperature of duck and hen eggs are quite different. Pikul (1998) noted that the gelling temperature of duck albumen was 67.5 °C, while that of hen albumen was found to be 75.0 °C. Further, Pikul (1998) found that gel from duck eggs was firmer than that of hen eggs when the same temperature and heating time were applied. The highest hardness of gel from duck albumen was obtained at 80 °C, while that of hen eggs reached its maximum at 85 °C. Moreover, gels from duck albumen showed higher cohesiveness and higher water binding than those from hen eggs. Thus, duck albumen is superior to the hen counterpart in improving gelation in food products. In addition, because egg albumen also contains protease inhibitors, these can help prevent protein degradation during gel formation in some muscle foods, such as surimi, etc. Recently, Quan and Benjakul (2018a) documented that sardine surimi gel with duck albumen added had higher hardness, breaking force, chewiness, and gumminess than that with hen albumen when the same level of albumen was incorporated due to the higher efficiency of duck albumen in preventing autolysis of surimi gel. Thus, duck albumen can be considered as protein additive in some products, where duck albumen protein is used a gelling agent to alleviate gel weakening.
Mechanism of egg albumen gel formation
The gelation of egg albumen is considered to be a two-step process. Firstly, some proteins are denatured, while the second step involves the aggregation of the denatured proteins. The extent of denaturation is associated with the unfolding of the proteins, the nature of the interactions or bondings, and the kinetics of the aggregation process, and these factors determine the type and characteristics of the resulting gels (Campbell et al. 2003). For gelation, complete denaturation of proteins is not required because of the formation of insoluble precipitates mediated by extensive hydrophobic interactions or hydrogen-bonding between the unfolded protein chains (Campbell et al. 2003). The partially unfolded egg proteins are able to form complexes, resulting in the formation of a gel (i.e. the ordered formation of a 3-dimensional network) or a coagulum (a random interaction between egg proteins) depending on the gelling conditions (Ren et al. 2010). With varying environmental conditions, such as protein concentration, heating conditions, ionic strength and pH, a transparent/turbid solution and transparent/turbid gel may be formed. Gel hardness is generally maximal under conditions that induce the formation of a slightly turbid or transparent gel (Phillips and Williams 2011).
The oxidation of SH groups into S–S bonds and the disulfide (S–S)/sulfhydryl (SH) interchange reaction are the key factors determining the intermolecular S–S bonds in the avian protein gel network. Ovalbumin (OVA) contains both S–S bonds and SH groups in its molecule. Native OVA has one S–S bond in each species. Nevertheless, avian species contain different SH groups, with hen albumen containing four OVA (Cys11, Cys30, Cys367, and Cys382), and duck albumen containing two (Cys11 and Cys331). Only one SH radical of the hen OVA molecule is readily available for heat induced aggregation (Sun and Hayakawa 2002). The differences in number of free SH groups in albumen probably affect the heat-induced gelation and gelling properties of hen and duck albumen.
Factors affecting the gelling properties of egg albumen
Albumen forms a 3-dimensional gel network as a result of the interaction between the protein chains. The physicochemical conditions of the medium such as its ionic strength, type of salts, pH, protein concentration, and its interaction with other components have an impact on the quality of the gel (Croguennec et al. 2002; Raikos et al. 2007).
pH
The pH is an essential factor affecting the net charge of proteins. At high pH values, the reactivity of the SH group is augmented. The pHs of egg albumen increases after eggs are stockpiled and stored. Those changes are coincidental with increases in the viscosity index, penetration force, and gel elasticity (Croguennec et al. 2002). The increase in pH from 8.66 to 9.11 found by Quan and Benjakul (2018e) after 3 days of storage was in line with the increased cohesiveness, chewiness, gumminess, and hardness of duck albumen gel. The alkaline pH of duck egg albumen is able to induce the unfolding of protein molecules as a result of enhanced repulsion between negatively charge domains. As a consequence, the interactions between unfolded proteins favor the formation of inter-junction zones in the gel network (Quan and Benjakul 2018e). The lowest cohesiveness and gel strength were found at pH values in the range of 6–7 as a result of the low net charge of albumen proteins. Gel strength increased with increasing pH, and the highest gel strength for duck albumen was observed at pH 9 by Ren et al. (2010), while at a low pH (pH < 6), the resulting gels were more brittle but firmer with poor water-binding properties and low elasticity. Houska et al. (2004) investigated the effects of dry matter content and pH on the gel strength of native albumen and the gel strength was found to increase with increasing pH and dry matter content. Raikos et al. (2007) also reported that a higher gel strength was found among egg samples at pH 5 and 8 than at pH 2. At high ionic strength and near the isoelectric point, coagula or soft/turbid gels of egg albumen were formed. The presence of these coagula within the gel network led to the formation of opaque gels. Nevertheless, the maximum gel hardness was obtained at the critical point, where the interaction between the linear protein chains was balanced by electrostatic repulsive forces (Phillips and Williams 2011). Hence, duck egg was suggested to be stored at 4 °C not longer than 6 days, in which albumen from duck egg could be used as the potential binder and gelling agent for food applications.
Salts and type of salts
Salts can alter the initial ionic nature of a gel. Excessive coagulation of albumen proteins can be induced by NaCl, thus causing the gel to become weaker (Raikos et al. 2007). Gels were found to weaken when 0.9 M NaCl solution was added. However, the inclusion of NaCl up to 0.1 M resulted in slight increases in elasticity and gel strength (Woodward 1990). Iwashita et al. (2015) investigated the effects of inorganic salts including NaCl, Na2SO4, and NaSCN on the thermal aggregation of albumen. Na2SO4 and NaSCN are kosmotropic and chaotropic, respectively. The surface tension of the solution was increased more by the kosmotrope than the chaotrope. This might be due to the fact that chaotrope destabilizes protein tertiary structure, leading to a decrease in the denaturation temperature, while kosmotropes stabilizes protein structure. At salt concentrations greater than 0.5 M, the salting out of protein molecules dominated. The anions of salts at low concentrations bind to the positively-charged domains of lysozyme, causing an increase in solubility, regardless of the type of ions (Iwashita et al. 2017). Croguennec et al. (2002) also reported that the water release of hen albumen gel was higher with the addition of NaCl at higher levels. The maximum amount of water was liberated in the presence of 120 mM NaCl. The excessive heat-induced aggregation of albumen proteins was related to the higher NaCl concentration, most likely via ionic interaction or hydrophobic interaction. For coagulum type gels from cooked salted duck albumen, NaCl plays a key role in the properties and appearance of the gel. A gel with opaque color and a coarse texture is formed when NaCl at high amounts is present (Kaewmanee et al. 2011). The hardness, springiness, gumminess, chewiness, and resilience of salted duck albumen gel was noted to decrease with increasing salting time.
The type of salts also affects heat-induced and alkaline-induced gelation of egg albumen. Croguennec et al. (2002) revealed that Ca2+ and Mg2+ modified the viscoelastic properties of egg albumen gel, in which the gel was less homogeneous with particles clustered in random aggregates, and had no string of beads structures. The addition of cations increased heat stability of albumen proteins, while CuSO4 markedly softened coagulum gel of albumen. FeCl3 and A1C13 did not affect gel firmness. These changes were the result of the shielding of the negative charge of albumen proteins (Beveridge and Ko 1984; Croguennec et al. 2002). Under the extreme pH for pidan production, egg albumen gel could not be formed, properly due to extensive electrostatic repulsion, lowering protein–protein interactions, and preventing gel formation. The addition of selected ions to protein solution diminishes the repulsive forces, and protein–protein association occurs, thus forming a self-supporting gel (Ganasen and Benjakul 2011a). Zhao et al. (2014a) reported that 0.2% CuSO4 exhibited the optimal effect on the strength of alkaline-induced duck albumen gel. Gel strength was increased by 31.92%. MgCl2, ZnSO4, PbO, and CaCl2 also exhibited gel strengthening effect, while the effect of Fe2(SO4)3 was negligible. This was in agreement with the results of Ganasen and Benjakul (2011a) who reported that the presence of Pb4+, Zn2+ and Ca2+ at a level of 0.2% could enhance the solidification of pidan albumen. Those cations might lower the dissociation of protein network, although slightly higher alkaline pH was obtained during aging.
Protein concentration
The gel hardness of albumen generally increases logarithmically with increasing protein concentration. The gel fracturability was found to increase when the protein concentration was increased, due to favorably enhanced aggregation of albumen (Woodward 1990). Iwashita et al. (2015) found that a high concentration of albumen was easily gelled by heat treatment. They noted that with a restricted attractive hydrophobic interaction but strong electrostatic repulsion, strings of beads described as ordered soluble linear aggregates of denatured ovalbumin molecule, are more likely to be formed. At high protein concentrations, a 3-dimensional gel network is developed via interconnections of these soluble linear aggregates. However, a viscous transparent sol instead of a gel network is formed at low protein concentrations (Ren et al. 2010). In general, conditions that lower the electrostatic repulsive forces between the protein molecules favor gel formation. At a sufficiently high protein concentration, high ionic strength and pH values close to zero net charge (the isoelectric point) are the key factors determining the formation of a 3-dimensional gel network (Campbell et al. 2003). Moreover, protein concentration has also been found to impact the rheological characteristics and gelling temperature of albumen gel. Both gelling temperature and storage modulus (G′) were noted to be concentration-dependent and G′ increased as albumen concentrations increased. This increase is generally attributed to a consolidation of attractive forces such as van der Waals and hydrogen bonding between the protein particles within the gel network. Additionally, as the protein concentration increased, the gelation temperature decreased (Eleya and Gunasekaran 2002).
Foaming property
Egg albumen has been used as a functional protein ingredient in a number of bakery products, such as cookies, bread, meringues and cakes as well as in ice cream, due to its excellent foaming properties (Phillips and Williams 2011). The interaction between the thermally induced coagulates and the various constituents are responsible for the unique foaming capacity of albumen, which results in stable foams (Mine 1995). These properties are governed by the ability of proteins in the albumen to form a cohesive viscoelastic film via their intermolecular interactions, and their rapid adsorption at the air–liquid interface during bubbling or whipping. The foaming capacity and stability of albumen mainly depend on storage time, temperature, egg species, dry heating and pH (Lomakina and Mikova 2006).
During storage, losses of foam volume and foam stability were found to be related to a lower thick albumen height. This directly caused a decrease in the viscosity of egg albumen (Ren et al. 2010). When the pH increases with extended storage, native ovalbumin is converted to S-ovalbumin, which is more heat-stable (less hydrophobic) than native ovalbumin. This may decrease foam stability as a result of the interference of S-ovalbumin with the cohesive film formation at the air–water interface. Overall, a positive correlation between the volume of drained liquid and S-ovalbumin content has been documented (Lomakina and Mikova 2006). Nevertheless, the acidity of albumen strongly influences the durability and volume of albumen foam. The lowest drainage and the largest volume were recorded by Bovšková and Mikova (2011) for both pasteurized and non-pasteurized egg albumen in an acidic pH (pH 4.5). At such a pH, some proteins precipitate and form thin film surrounding the air bubbles, resulting in the decrease in surface energy and surface tension. In addition, differences of foaming properties between hen and duck albumen have also been reported. Khaki Campbell Duck egg albumen showed a lower foaming ability than hen egg counterpart and the foam stability of the duck albumen was better than that of hen egg albumen because of higher hydrophobic amino acid found in duck albumen than hen egg counterpart (Pikul 1998). Spray-drying has also been adopted to improve the foaming properties of powders, especially when spray-dried with an inlet temperature at 160 °C. Quan and Benjakul (2018d) reported the exposed hydrophobic domains formed during drying, which was in turn related to increased surface activities. Therefore, spray-dried duck albumen with inlet temperature at 160 °C can be used as foaming agent.
Changes in the quality and physicochemical properties of duck albumen during storage
Changes in quality of duck egg
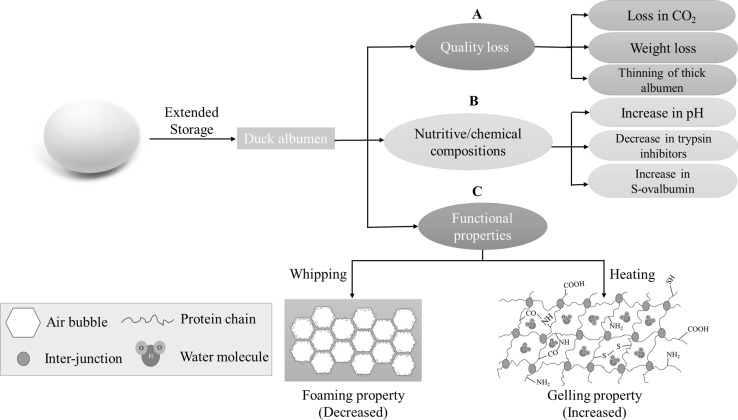
The quality of eggs is influenced by storage conditions and time. Such a quality loss is associated with changes in physicochemical structure and chemical compositions of egg albumen during storage (Fig. 2). Good quality egg generally has thick and transparent albumen. The height of albumen is one of the principal characteristics used to evaluate interior egg quality (Lokaewmanee 2017). Eggs undergo deterioration as storage time increases. As a consequence, they may not be suitable for human consumption. However, loss of quality in eggs can be retarded significantly by lowering the storage temperature. In general, quality deterioration occurs at a faster rate at high temperatures than at refrigerated temperatures. The main factors affecting egg quality are storage time, temperature, humidity, airflow, and handling processes (Akter et al. 2014). Storage time and temperature were found to have a significant impact on the yolk index, yolk color, albumen index and Haugh unit (HU). Pandian et al. (2012) studied the influence of various storage periods (1–5 weeks) on duck egg quality at 75% relative humidity and 18 °C. Storage time had significant effects on the yolk index, yolk color, HU and albumen index. The yolk index value decreased significantly when the storage period was lengthened. Quan and Benjakul (2018e) also found that the HU of duck eggs decreased as both storage time and temperature increased. According to the USDA standard, fresh duck egg with an HU of 81 is graded as “AA” quality. The HU of duck eggs decreased to 68 after 9 days of storage, which were then considered to be grade A. However, the rate of change was found to be lower at 4 °C, compared to that of eggs stored at room temperature. In addition, the shape index, albumen index, yolk index, shell color, specific gravity, HU and yolk color of Khaki Campbell duck eggs were remarkably affected by storage time (Lokaewmanee 2017). Khaki Campbell duck eggs still had acceptable quality for human consumption after storage for 11 days at 30 °C and 78% humidity (Lokaewmanee 2017).
Fig. 2.
Schematic illustration of changes in quality of duck egg (A) chemical compositions (B), and functional properties (C) of egg albumen during storage
During storage, albumen thinning, an important change in albumen, occurs. The degradation of the ovomucin complex results in the breakdown of a thick white to a thin white, especially during improper transportation and prolonged storage (Phillips and Williams 2011; Woodward 1990). During storage, the ovomucin–lysozyme complex undergoes destruction. This phenomenon is related to a reduction of thick albumen height and a decrease in HU (Quan and Benjakul 2018e). Nevertheless, duck eggs have better stability during storage at room temperature than hen eggs. Storage time was found not to greatly affect duck egg yolk and albumen quality indices, including the HU, while such changes occurred in chicken eggs. (Jalaludeen et al. 2009). Duck egg quality was acceptable up to 28 days of storage at 4 °C, while they could be stored for up to only 14 days at room temperature (Akter et al. 2014). Jin et al. (2011) revealed that the HU of hen eggs decreased from 87.62 to 60.92 at 29 °C and from 91.30 to 72.63 at 21 °C after 10 days of storage. Nonetheless, this deterioration did not take place at 5 °C and the HU, egg weight, and albumen height were only slightly decreased when the cold storage time was extended. However, the average HU value was still in the range of grade A after 10 weeks of storage (Jones and Musgrove 2005). Hen eggs lost their interior quality much faster than duck eggs during extended storage, which might be due to higher duck eggs’ ability to resist bacterial spoilage (Brown et al. 1965). Moreover, the thickness of the domestic duck eggshell, which is considered as a physical and bacterial barrier, is thicker than that of hen eggs. Therefore, duck eggs are more resistant to an aggressive external environment than hen eggs (Solomon 2010; Wellman-Labadie et al. 2008). Practically, duck egg should be stored at 4 °C to maintain the quality and functional properties of albumen during storage.
Changes in the chemical composition of duck albumen
Multiple changes in the chemical composition of duck albumen take place during storage, especially alteration of the pH, moisture content, and protein patterns. The pH of freshly laid duck egg albumen increased from 8.66 to 9.11 within the first 3 days of storage at room temperature as the dissolved carbon dioxide was released from the eggs (Quan and Benjakul 2018e). However, Lokaewmanee (2017) demonstrated that albumen pH of Khaki Campbell duck eggs remained unchanged after 11 days of storage at 30 °C and 78% relative humidity. A decrease in CO2 of stored quail and hen eggs has however been found (Akter et al. 2014; Jin et al. 2011; Khan et al. 2013). Changes in protein patterns were also found to be accelerated by a high storage temperature and pH. S-ovalbumin, an extremely and irreversibly heat-stable form, is regularly converted from ovalbumin (Deleu et al. 2015; Huang et al. 2012). Qiu et al. (2012) reported that the decrease in protease inhibitors became more pronounced when eggs were stored at a higher temperature. Additionally, lipocalin family proteins were decreased, while the formation of ovalbumin complexes was increased with increasing storage temperature. Those phenomena are related to a thermally promoted change in hen eggs. During storage at high temperature, clusterin decreases. This change can be considered as an effective biomarker for egg quality assessment. However, duck albumen protein patterns were not greatly changed after 15 days of storage at low temperature (Quan and Benjakul 2018e). The denaturation of some proteins such as trypsin inhibitors occurred at varying rates during storage for duck and hen eggs, which might have been caused by differences in stability and the molecular properties of proteins between the two types of eggs (Qiu et al. 2012; Quan and Benjakul 2018e). Schäfer et al. (1999) observed no significant changes in the content of conalbumin and lysozyme in hen albumen during storage. However, S-ovalbumin which is associated with a change in the isoelectric point of ovalbumin, was formed when the pH became slightly more acidic during storage (Schäfer et al. 1999). Storage of hen eggs for up to 4 weeks at 15 °C was found to decrease the activity of serine protease inhibitors by 50%, and the activity of lysozyme was decreased by approximately 10%. The activity of cystatin was detected only at a trace level (Kopeć et al. 2005). Eggs stored at refrigerated temperature could therefore maintain bioactive components, especially trypsin inhibitors during storage.
Changes in the physicochemical properties of duck albumen as affected by processing
Salting
Salted duck egg is a popular traditional egg product in China and other Asian countries. Fresh duck eggs are pickled in a saline solution or in salted clay. During the salting process, the salt diffuses and penetrates into the egg yolk and albumen through the eggshell and the eggshell membrane. Some proteins in the salted duck eggs are degraded to amino acids and small peptides compared to fresh eggs. As a result, a higher amount of inorganic salts can permeate into the eggs (Lian et al. 2014). In general, fresh duck eggs are treated with a high salt concentration (25%, w/v), which is the typical requirement for salted duck egg production, in order to develop the unique “fresh, fine, tender, loose, gritty and oily texture” yolk characteristic (Kaewmanee et al. 2009; Xu et al. 2017). Commonly, salted eggs are made from duck eggs because they offer more desirable characteristics with richer flavor than hen eggs (Ji et al. 2013). Salting for 15 days and at least 30 days for ripening are essential to attain the desired features, particularly for aroma and flavor. The pickling period is dependent on the salt content in the pickling solution or salted clay and is also affected by the environmental temperature (Huang and Lin 2011; Kaewmanee et al. 2009).
During the salting process, the yolk normally becomes hardened and solidified because of water migration from the yolk to the albumen. Thereafter, water passes through the eggshell and leaves the egg. Meanwhile, the egg albumen becomes watery and loses viscosity when the salt migrates into the egg (Lian et al. 2014). Kaewmanee et al. (2009) reported that salting induced the weight proportion of the egg albumen to increase, while the yolk proportion decreased. With increasing salting time, the moisture contents of both egg yolk and albumen gradually decrease, associated with increases in the ash and salt contents. It was reported that the amount of precipitated protein increased with salting time (Huang et al. 1999). Those aggregates might be stabilized by weak bonds such as ionic interaction, etc., which could be destroyed by SDS used for electrophoresis. As a consequence, no differences in protein patterns were observed (Quan and Benjakul 2018b). Quan and Benjakul (2018b) noted that the moisture content decreased, whereas the salt content and surface hydrophobicity of duck egg albumen increased as the salting time increased. Moreover, trypsin inhibitory activity and specific activity in duck egg albumen continuously decreased throughout the salting time of 30 days (Quan and Benjakul 2018b). For the nutritive compositions of salted duck albumen, proteins, fat, carbohydrate, and minerals were found to be higher, as compared to those of fresh duck albumen (Ganesan et al. 2014). It was suggested that salting process greatly alters the nutritive value of salted duck egg albumen.
In general, salted duck egg albumen powder contains a high NaCl content (30%) and possesses hygroscopic properties. This makes it less suitable for application in food products. Thus, the desalination of salted duck albumen could be beneficial for the duck-egg industry and a new source of protein can be gained with the environmental effects being minimized (Zhou et al. 2014). Salted duck albumen is a potential ingredient for producing food and it has already been used in the production of high protein Frankfurter sausages and noodles (Zhou et al. 2014). Tan et al. (2016) studied the utilization of salted duck albumen as an alternative source of salt for manufacturing yellow alkaline noodles. Yellow alkaline noodles with salted duck albumen (15.21 g of salted duck egg albumen/100 g of wheat flour) added had a significantly higher protein content and were more yellow and lighter compared to a control product. Under sensory evaluation, salted duck albumen-yellow alkaline noodles showed high acceptability because of improved color and textural properties (Tan et al. 2016). Therefore, the potential use of salted duck egg albumen as an alternative source to table salt in some food products such as surimi or meat ball should be more investigated.
Drying
Desugarization before dehydration
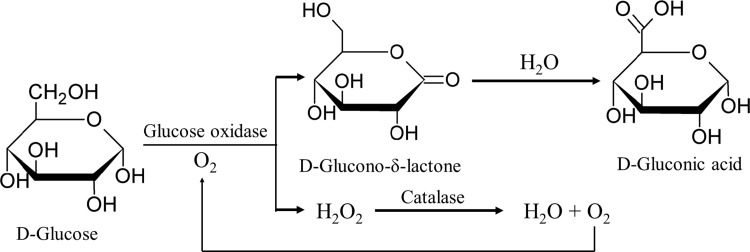
Dried egg albumen powder or egg white powder is prone to undesirable browning known as a Maillard reaction. The reaction involves reducing sugars, mainly glucose, which constitute about 4 g/L in the original liquid form, and amino groups in the albumen. The reaction between a cephalic amino group and the aldehydes of glucose (glucose-cephalin reaction) is responsible for the discoloration and off-flavor development in egg albumen. The reaction also causes a loss of protein solubility (Sisak et al. 2006; Wu 2014). In practice, desugarization is a general requirement before dehydration in order to remove the glucose from the egg albumen. This aims to prevent the browning of the product obtained. Additionally, desugarization improves resistance to microbial attack in the powder obtained, thus increasing the storage stability of the egg albumen powder (Sisak et al. 2006). Desugarization can be conducted either by yeast or bacterial fermentation, in which different organic acids are produced via the conversion of glucose. The reducing sugar content of liquid whole hen egg was completely depleted within 12 h following fermentation by baker’s yeast (Sharma et al. 2012). However, these processes pose some bacteriological problems. The use of commercial glucose oxidase containing catalase is a common practice, which converts glucose into gluconic acid (Fig. 3) (Woods and Swinton 1995). The enzymatic desugarization process is reproducible and straightforward besides yielding final products with consistently high organoleptic properties (Lechevalier et al. 2013). Recently, Quan and Benjakul (2018d) investigated the optimum conditions for the desugarization of duck albumen using response surface methodology (RSM), which were found to be: glucose oxidase (31.24 units/mL), catalase (781 units/mL) and an incubation time of 6.55 h at 30 °C. The foaming capacity (FC) and foam stability (FS) of the desugarized duck albumen were enhanced. In the absence of sugar, proteins with increased surface hydrophobicity could migrate to air interface effectively. Proteins could adsorb more easily at the interface because of their higher flexibility (Quan and Benjakul 2018d). More importantly, desugarization was able to improve the lightness of powders produced by spray-drying process and resulted in a lower browning index (A294 and A420). In conclusion, desugarization is a crucial step before dehydration to obtain perfectly white egg albumen powders.
Fig. 3.
Enzymatic removal of glucose from egg albumen. Firstly, glucose is converted to gluconic acid by glucose oxidase. Hydrogen peroxide formed is further transformed to oxygen and water by catalase
Effect of drying methods on the functional properties of egg albumen powder
Dehydration can retard chemical reactions and microbial growth. Therefore, it is a successful means for preserving egg albumen. Numerous dried egg albumen products are available in the market. Dried egg white products are produced by hot air drying, spray drying, microwave vacuum drying, infrared drying, and freeze-drying (Ma et al. 2013; Phillips and Williams 2011). However, the quality, and physical and functional properties of egg albumen powder are directly influenced by the conditions and drying methods used.
Freeze drying
Freeze drying is considered as being one of the best drying technologies for maintaining the quality of products. This technology also has other advantages, such as good rehydration capacity and the aqueous solubility of the powders obtained. Moreover, it is the most appropriate method for the drying of heat-sensitive materials. Nevertheless, freeze drying involves high capital costs as well as processing costs and the drying process takes a long time (Chen et al. 2012; Zhou et al. 2014). To overcome this limitation, a combined method involving microwave vacuum-drying and freeze drying of duck albumen was developed (Zhou et al. 2014). Drying time of fresh duck albumen and desalted duck albumen was significantly decreased by this combination of methods, compared to a freeze-drying process alone. Fresh duck albumen protein dried in this way had a better color, a higher emulsifying index, and a lower apparent density than that produced by freeze drying alone (Zhou et al. 2014). Katekhong and Charoenrein (2018) dried hen egg albumen with two drying methods, freeze drying and hot air drying. Drying egg albumen using hot air induced changes in color and protein conformation, mainly due to protein aggregation in the resulting powder.
Spray drying
Spray drying is the most popular drying technology used in the pharmaceutical, chemical, and food industries for the preparation of protein powder (Asghar and Abbas 2015). During the spray drying process, a foodstuff material in liquid form is exposed to hot air with a temperature in the range of 100–300 °C. The hot air evaporates the liquid in the foodstuff material. The product obtained from spray drying is in dried form, either agglomerates or granules. The shape of the product obtained depends on the physical and chemical properties of the foodstuff material, and the design and operation of the dryer. Evaporation through droplets is facilitated by vapor and heat transfer during the spray-drying process (Asgar and Abbas 2012). Ayadi et al. (2008) investigated the optimum operating conditions for drying whole egg and egg albumen using a pilot-scale spray dryer. Spray drying of egg white at moderate conditions (air inlet temperature ranged from 110 to 125 °C) resulted in a product that enhanced water holding capacity of resulting gels. In addition, a gel prepared from the dried samples was firmer than that of the fresh samples. Ma et al. (2013) found that the optimized conditions for hen egg albumen spray drying were as follows: flow 22 mL/min, inlet-air temperature 178.2 °C and feeding temperature 39.8 °C.
Quan and Benjakul (2018d) compared the physicochemical properties of duck albumen powders produced by spray drying and freeze drying. Lower trypsin inhibitory activity was found in the spray-dried albumen powder than in its freeze-dried counterpart. Freeze-dried albumen powder had the highest lightness (L*) as compared to that subjected to spray drying with different inlet temperatures (140–180 °C). For the gelling properties of duck albumen powders, the hardness, chewiness, gumminess and springiness of the gel decreased as spray-drying temperatures were increased. Conversely, freeze-drying yielded gels with higher textural properties, e.g. hardness and springiness, etc. (Quan and Benjakul 2018c). Hence, spray-dried albumen powder with a moderate inlet temperature (160 °C) could maintain high solubility and foaming properties as well as the bioactive compounds, especially protease inhibitors in duck albumen.
Pidan processing
Pidan, or century egg is at a high demand in China and many South-East Asian countries, such as Malaysia, Thailand, and Vietnam (Zhao et al. 2014b). There are three methods for processing pidan: immersion, coating and a combination of both methods (Huang and Lin 2011). Chicken, duck, or quail eggs are preserved in a mixture of sodium hydroxide or sodium carbonate and lime, along with black tea, metal ions and salt from some weeks to some months, depending upon the processing methods (Tu and Zhao 2017). During pickling, the alkali diffuses and migrates through the egg shell and membrane and induces changes in the chemical components of the egg, particularly via a Maillard reaction (Ganasen and Benjakul 2011b). Ji et al. (2013) studied the changes in structure and chemical compositions of albumen during pidan pickling by vacuum technology. The disulfide bond content decreased with increased pickling time, whereas increases in surface hydrophobicity and sulfhydryl groups were recorded. For the secondary structure, increases in random coils and β-sheets were accompanied by decreases in β-turns and α-helices since albumen proteins underwent unfolding and depolymerization under strong alkaline solution treatments (Ji et al. 2013).
The preserved duck albumen is rich in minerals, such as Fe, Mg, Ca, Zn, Cu, Na, and K. Some water-soluble vitamins, amino acids, and the moisture content of the egg albumen dramatically decrease when fresh duck eggs are processed into preserved eggs. Chang et al. (1999) reported that the formation of lysinoalanine and the racemization (d-serine and d-aspartic acid) in Pidan albumen occurred during the pickling process. However, most inorganic elemental contents, the free amino acid content, and the pH of the egg albumen significantly increased (Zhao et al. 2014b). In general, the loss of lysine and formation of racemized amino acids lead to the subsequent loss in nutritive value of pidan (Ganesan et al. 2014). Therefore, the pidan processing needs further studies to reduce the formation of lysinoalanine and the racemization of some amino acids.
Conclusion
Duck egg albumen is a good source of protein. It possesses excellent gelling and foaming properties making it an essential food ingredient. Also, it contains protease inhibitors, and can thus be used as an additive to prevent proteolysis in some foods, particularly surimi gel, preventing the degradation of muscle protein and improving the gel property. Nevertheless, the functional properties or target activity can be altered when duck eggs are stored or processed. Therefore, duck albumen should be considered as a potential protein additive to replace hen albumen in food products.
Acknowledgements
This work was supported by the Higher Education Research Promotion and a scholarship from the Thailand Education Hub for Southern Region of ASEAN Countries Project Office of the Higher Education Commission.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abeyrathne EDNS, Lee HY, Ahn DU. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents—a review. Poult Sci. 2013;92:3292–3299. doi: 10.3382/ps.2013-03391. [DOI] [PubMed] [Google Scholar]
- Aendo P, Netvichian R, Tippayalak S, Sanguankiat A, Khuntamoon T, Songserm T, Tulayakul P. Health risk contamination of heavy metals in yolk and albumen of duck eggs collected in central and western Thailand. Biol Trace Elem Res. 2018;184:501–507. doi: 10.1007/s12011-017-1202-0. [DOI] [PubMed] [Google Scholar]
- Akter Y, Kasim A, Omar H, Sazili AQ. Effect of storage time and temperature on the quality characteristics of chicken eggs. J Food Agric Environ. 2014;12:87–92. [Google Scholar]
- Alleoni ACC. Albumen protein and functional properties of gelation and foaming. Sci Agric. 2006;63:291–298. doi: 10.1590/S0103-90162006000300013. [DOI] [Google Scholar]
- Anton M, Nau F, Nys Y. Bioactive egg components and their potential uses. Worlds Poult Sci J. 2006;62:429–438. doi: 10.1079/WPS2005105. [DOI] [Google Scholar]
- Asgar A, Abbas M. Dried egg powder utilization, a new frontier in bakery products. Agric Biol J N Am. 2012;3:493–505. doi: 10.5251/abjna.2012.3.12.493.505. [DOI] [Google Scholar]
- Asghar A, Abbas M. Effect of spray dried whole egg powder on physicochemical and sensory properties of cake. Am J Sci Ind Res. 2015;6:97–102. [Google Scholar]
- Ayadi M, Khemakhem M, Belgith H, Attia H. Effect of moderate spray drying conditions on functionality of dried egg white and whole egg. J Food Sci. 2008;73:281–287. doi: 10.1111/j.1750-3841.2008.00811.x. [DOI] [PubMed] [Google Scholar]
- Belitz HD, Grosch W, Schieberle P. Eggs. In: Belitz HD, editor. Food chemistry. New York: Springer; 2009. pp. 546–562. [Google Scholar]
- Beveridge T, Ko S. Firmness of heat-induced whole egg coagulum. Poult Sci. 1984;63:1372–1377. doi: 10.3382/ps.0631372. [DOI] [Google Scholar]
- Bovšková H, Mikova K. Factors influencing egg white foam quality. Czech J Food Sci. 2011;29:322–327. doi: 10.17221/435/2010-CJFS. [DOI] [Google Scholar]
- Brown W, Baker R, Naylor H. Comparative susceptibility of chicken, duck and turkey eggs to microbial invasion. J Food Sci. 1965;30:886–892. doi: 10.1111/j.1365-2621.1965.tb01860.x. [DOI] [Google Scholar]
- Campbell L, Raikos V, Euston SR. Modification of functional properties of egg-white proteins. Mol Nutr Food Res. 2003;47:369–376. doi: 10.1002/food.200390084. [DOI] [PubMed] [Google Scholar]
- Chang H-M, Tsai C-F, Li C-F. Changes of amino acid composition and lysinoalanine formation in alkali-pickled duck eggs. J Agric Food Chem. 1999;47:1495–1500. doi: 10.1021/jf980951k. [DOI] [PubMed] [Google Scholar]
- Chen Y-C, Chang H-S, Wang C-T, Cheng F-Y. Antioxidative activities of hydrolysates from duck egg white using enzymatic hydrolysis. Asian Australas J Anim Sci. 2009;22:1587–1593. doi: 10.5713/ajas.2009.90119. [DOI] [Google Scholar]
- Chen C, Chi Y-J, Xu W. Comparisons on the functional properties and antioxidant activity of spray-dried and freeze-dried egg white protein hydrolysate. Food Bioproc Technol. 2012;5:2342–2352. doi: 10.1007/s11947-011-0606-7. [DOI] [Google Scholar]
- Congjiao S, Junnian L, Wenbo L, Guiyun X, Ning Y. Divergent proteome patterns of egg albumen from domestic chicken, duck, goose, turkey, quail and pigeon. Proteomics. 2017;17:1700145–1700157. doi: 10.1002/pmic.201700145. [DOI] [PubMed] [Google Scholar]
- Croguennec T, Nau F, Brule G. Influence of pH and salts on egg white gelation. J Food Sci. 2002;67:608–614. doi: 10.1111/j.1365-2621.2002.tb10646.x. [DOI] [Google Scholar]
- Deleu LJ, Wilderjans E, Van Haesendonck I, Courtin CM, Brijs K, Delcour JA. Storage induced conversion of ovalbumin into S-ovalbumin in eggs impacts the properties of pound cake and its batter. Food Hydrocoll. 2015;49:208–215. doi: 10.1016/j.foodhyd.2015.03.014. [DOI] [Google Scholar]
- Eleya MMO, Gunasekaran S. Gelling properties of egg white produced using a conventional and a low-shear reverse osmosis process. J Food Sci. 2002;67:725–729. doi: 10.1111/j.1365-2621.2002.tb10666.x. [DOI] [Google Scholar]
- Fouad AM, Ruan D, Wang S, Chen W, Xia W, Zheng C. Nutritional requirements of meat-type and egg-type ducks: what do we know? J Anim Sci Biotechnol. 2018;9:1–11. doi: 10.1186/s40104-017-0217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganasen P, Benjakul S. Chemical composition, physical properties and microstructure of pidan white as affected by different divalent and monovalent cations. J Food Biochem. 2011;35:1528–1537. doi: 10.1111/j.1745-4514.2010.00475.x. [DOI] [Google Scholar]
- Ganasen P, Benjakul S. Effects of green tea and chinese tea on the composition and physical properties of pidan white. J Food Process Preserv. 2011;35:907–916. doi: 10.1111/j.1745-4549.2011.00544.x. [DOI] [Google Scholar]
- Ganesan P, Kaewmanee T, Benjakul S, Baharin BS. Comparative study on the nutritional value of pidan and salted duck egg. Korean J Food Sci Anim Resour. 2014;34:1–6. doi: 10.5851/kosfa.2014.34.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hincke MT, Nys Y, Gautron J, Mann K, Rodriguez-Navarro AB, McKee MD. The eggshell: structure, composition and mineralization. Front Biosci. 2012;17:1266–1280. doi: 10.2741/3985. [DOI] [PubMed] [Google Scholar]
- Houska M, Kyhos K, Novotna P, Landfeld A, Strohalm J. Gel strength of the native egg white. Czech J Food Sci. 2004;22:58–66. doi: 10.17221/3407-CJFS. [DOI] [Google Scholar]
- Hu S, Qiu N, Liu Y, Zhao H, Gao D, Song R, Ma M. Identification and comparative proteomic study of quail and duck egg white protein using 2-dimensional gel electrophoresis and matrix-assisted laser desorption/ionization time-of-flight tandem mass spectrometry analysis. Poult Sci. 2016;95:1137–1144. doi: 10.3382/ps/pew033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JF, Lin CC. Production, composition, and quality of duck eggs. In: Nys YBM, Van IF, editors. Improving the safety and quality of eggs and egg products: volume 1: egg chemistry, production and consumption. 1. Cambridge: Woodhead Publishing Ltd.; 2011. pp. 487–504. [Google Scholar]
- Huang J-J, Tsai J-S, Pan BS. Pickling time and electrodialysis affects functional properties of salted duck egg white. J Food Biochem. 1999;23:607–618. doi: 10.1111/j.1745-4514.1999.tb00589.x. [DOI] [Google Scholar]
- Huang Q, Qiu N, Ma M, Jin Y, Yang H, Geng F, Sun S. Estimation of egg freshness using S-ovalbumin as an indicator. Poult Sci. 2012;91:739–743. doi: 10.3382/ps.2011-01639. [DOI] [PubMed] [Google Scholar]
- Hytönen VP, Laitinen OH, Grapputo A, Kettunen A, Savolainen J, Kalkkinen N, Marttila AT, Nordlund HR, Nyholm TKM, Paganelli G, Kulomaa MS. Characterization of poultry egg-white avidins and their potential as a tool in pretargeting cancer treatment. Biochem J. 2003;372:219–225. doi: 10.1042/bj20021531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita K, Inoue N, Handa A, Shiraki K. Thermal aggregation of hen egg white proteins in the presence of salts. Protein J. 2015;34:212–219. doi: 10.1007/s10930-015-9612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita K, Handa A, Shiraki K. Co-aggregation of ovalbumin and lysozyme. Food Hydrocoll. 2017;67:206–215. doi: 10.1016/j.foodhyd.2017.01.014. [DOI] [Google Scholar]
- Jalaludeen A, Churchill A, Joseph L, Anitha P (2009) Duck meat, egg, and their products. In: Souvenir of the IV world waterfowl conference, Thrissur, India, Kerala Agricultural University, 2009. World’s Poultry Science Association, pp 57–64
- Ji L, Liu H, Cao C, Liu P, Wang H, Wang H. Chemical and structural changes in preserved white egg during pickled by vacuum technology. Food Sci Technol Int. 2013;19:123–131. doi: 10.1177/1082013212442186. [DOI] [PubMed] [Google Scholar]
- Jin Y, Lee K, Lee W, Han Y. Effects of storage temperature and time on the quality of eggs from laying hens at peak production. Asian Australas J Anim Sci. 2011;24:279–284. doi: 10.5713/ajas.2011.10210. [DOI] [Google Scholar]
- Jones D, Musgrove M. Effects of extended storage on egg quality factors. Poult Sci. 2005;84:1774–1777. doi: 10.1093/ps/84.11.1774. [DOI] [PubMed] [Google Scholar]
- Kaewmanee T, Benjakul S, Visessanguan W. Changes in chemical composition, physical properties and microstructure of duck egg as influenced by salting. Food Chem. 2009;112:560–569. doi: 10.1016/j.foodchem.2008.06.011. [DOI] [Google Scholar]
- Kaewmanee T, Benjakul S, Visessanguan W. Effect of NaCl on thermal aggregation of egg white proteins from duck egg. Food Chem. 2011;125:706–712. doi: 10.1016/j.foodchem.2010.09.072. [DOI] [Google Scholar]
- Katekhong W, Charoenrein S. Influence of spray drying temperatures and storage conditions on physical and functional properties of dried egg white. Drying Technol. 2018;36:169–177. doi: 10.1080/07373937.2017.1307218. [DOI] [Google Scholar]
- Khan MJ, Khan SH, Bukhsh A, Abbass MI, Javed M. Effect of different storage period on egg weight, internal egg quality and hatchability characteristics of Fayumi eggs. Ital J Anim Sci. 2013;12:e51. doi: 10.4081/ijas.2013.e51. [DOI] [Google Scholar]
- Klomklao S, Benjakul S, Kishimura H, Osako K, Simpson BK. Trypsin inhibitor from yellowfin tuna (Thunnus albacores) roe: effects on gel properties of surimi from bigeye snapper (Priacanthus macracanthus) LWT Food Sci Technol. 2016;65:122–127. doi: 10.1016/j.lwt.2015.07.074. [DOI] [Google Scholar]
- Kopeć W, Skiba T, Korzeniowska M, Bobak Ł, Trziszka T. Activity of protease inhibitors and lysozyme of hen’s egg white depending on feed modification and egg storage. Pol J Food Nutr Sci. 2005;14:79–83. [Google Scholar]
- Lechevalier V, Nau F, Jeantet R. Powdered egg. In: Bhandari B, Bansal N, Zhang M, Schuck P, editors. Handbook of food powders. Cambridge: Woodhead Publishing Ltd.; 2013. p. 660. [Google Scholar]
- Lee JH, Moon SH, Kim HS, Park E, Ahn DU, Paik HD. Antioxidant and anticancer effects of functional peptides from ovotransferrin hydrolysates. J Sci Food Agric. 2017;97:4857–4864. doi: 10.1002/jsfa.8356. [DOI] [PubMed] [Google Scholar]
- Lian Z, Qiao L, Zhu G, Deng Y, Qian B, Yue J, Zhao Y. Use of sodium dodecyl sulfate pretreatment and 2-stage curing for improved quality of salted duck eggs. J Food Sci. 2014;79:354–361. doi: 10.1111/1750-3841.12361. [DOI] [PubMed] [Google Scholar]
- Lokaewmanee K. Storage stability of Khaki Campbell duck (Anas platyrhynchos domesticus) eggs at room temperature. Int J Poult Sci. 2017;16:393–402. doi: 10.3923/ijps.2017.393.402. [DOI] [Google Scholar]
- Lomakina K, Mikova K. A study of the factors affecting the foaming properties of egg white—a review. Czech J Food Sci. 2006;24:110–118. doi: 10.17221/3305-CJFS. [DOI] [Google Scholar]
- Ma S, Zhao S, Zhang Y, Yu Y, Liu J, Xu M. Quality characteristic of spray-drying egg white powders. Mol Biol Rep. 2013;40:5677–5683. doi: 10.1007/s11033-013-2669-1. [DOI] [PubMed] [Google Scholar]
- Miguel M, Manso MA, López-Fandiño R, Ramos M. Comparative study of egg white proteins from different species by chromatographic and electrophoretic methods. Eur Food Res Technol. 2005;221:542–546. doi: 10.1007/s00217-005-1182-8. [DOI] [Google Scholar]
- Mine Y. Recent advances in the understanding of egg white protein functionality. Trends Food Sci Technol. 1995;6:225–232. doi: 10.1016/S0924-2244(00)89083-4. [DOI] [Google Scholar]
- Naknukool S, Hayakawa S, Sun Y, Ogawa M. Structural and physicochemical characteristics of novel basic proteins isolated from duck egg white. Biosci Biotechnol Biochem. 2008;72:2082–2091. doi: 10.1271/bbb.80178. [DOI] [PubMed] [Google Scholar]
- Pandian C, Sundaresan A, Sangilimadan K, Omprakash AV, Babu M, Prabakaran R. Effect of different storage periods on egg quality traits of ducks. J Life Sci. 2012;6:871–873. [Google Scholar]
- Phillips GO, Williams PA. Egg proteins. In: Strixner T, Kulozik U, editors. Handbook of food proteins. Oxford: Woodhead Publishing Ltd.; 2011. pp. 150–209. [Google Scholar]
- Pikul J. Characteristics of duck eggs and the quality of duck eggs products. Arch Geflugelk. 1998;62:72–82. [Google Scholar]
- Pingle H (2009) Waterfowl production for food security. In: The IV world waterfowl conference, Thrissur, India, Kerala Agricultural University. World’s Poultry Science Association, pp 5–15
- Qiu N, Ma M, Zhao L, Liu W, Li Y, Mine Y. Comparative proteomic analysis of egg white proteins under various storage temperatures. J Agric Food Chem. 2012;60:7746–7753. doi: 10.1021/jf302100m. [DOI] [PubMed] [Google Scholar]
- Quan TH, Benjakul S. Comparative study on the effect of duck and hen egg albumens on proteolysis and gel property of sardine surimi. Int J Food Prop. 2018;20:S2786–S2797. doi: 10.1080/10942912.2017.1374290. [DOI] [Google Scholar]
- Quan TH, Benjakul S. Compositions, protease inhibitor and gelling property of duck egg albumen as affected by salting. Korean J Food Sci Anim Resour. 2018;38:14–25. doi: 10.5851/kosfa.2018.38.1.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan TH, Benjakul S. Gelling properties of duck albumen powder as affected by desugarization and drying conditions. J Texture Stud. 2018;49:520–527. doi: 10.1111/jtxs.12339. [DOI] [PubMed] [Google Scholar]
- Quan TH, Benjakul S (2018d) Impacts of desugarization and drying methods on physicochemical properties of duck albumen powder. Dry Technol. 10.1080/07373937.2018.1469509
- Quan TH, Benjakul S. Quality, protease inhibitor and gelling property of duck egg albumen as affected by storage conditions. J Food Sci Technol. 2018;55:513–522. doi: 10.1007/s13197-017-2960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikos V, Campbell L, Euston SR. Rheology and texture of hen’s egg protein heat-set gels as affected by pH and the addition of sugar and/or salt. Food Hydrocoll. 2007;21:237–244. doi: 10.1016/j.foodhyd.2006.03.015. [DOI] [Google Scholar]
- Réhault S. Antiproteases. In: Huopalahti R, Anton M, López-Fandiño R, Schade R, editors. Bioactive Egg Compounds. Heidelberg: Springer; 2007. pp. 85–92. [Google Scholar]
- Ren Y, Wu J, Renema R. Nutritional and health attributes of eggs. In: Guerrero-Legarreta I, editor. Handbook of poultry science and technology. Hoboken: Wiley; 2010. pp. 533–578. [Google Scholar]
- Ren Y, Wu H, Li X, Lai F, Xiao X. Purification and characterization of high antioxidant peptides from duck egg white protein hydrolysates. Biochem Biophys Res Commun. 2014;452:888–894. doi: 10.1016/j.bbrc.2014.08.116. [DOI] [PubMed] [Google Scholar]
- Rossi M, et al. Developments in understanding and assessment of egg and egg product quality over the last century. Worlds Poult Sci J. 2013;69:414–429. doi: 10.1017/S0043933913000408. [DOI] [Google Scholar]
- Saxena I, Tayyab S. Protein proteinase inhibitors from avian egg whites. Cell Mol Life Sci. 1997;53:13–23. doi: 10.1007/PL00000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer A, Drewes W, Schwägele F. Effect of storage temperature and time on egg white protein. Food/Nahr. 1999;43:86–89. doi: 10.1002/(SICI)1521-3803(19990301)43:2<86::AID-FOOD86>3.0.CO;2-O. [DOI] [Google Scholar]
- Sharma HK, Singh J, Sarkar BC, Singh B, Premi M. Statistical optimization of desugarization process parameters of liquid whole egg using response surface methodology. LWT Food Sci Technol. 2012;47:208–212. doi: 10.1016/j.lwt.2011.08.017. [DOI] [Google Scholar]
- Sisak C, Csanádi Z, Ronay E, Szajáni B. Elimination of glucose in egg white using immobilized glucose oxidase. Enzyme Microb Technol. 2006;39:1002–1007. doi: 10.1016/j.enzmictec.2006.02.010. [DOI] [Google Scholar]
- Solomon SE. The eggshell: strength, structure and function. Br Poult Sci. 2010;51:52–59. doi: 10.1080/00071668.2010.497296. [DOI] [PubMed] [Google Scholar]
- Stevens L. Egg white proteins. Comp Biochem Physiol B Biochem Mol Biol. 1991;100:1–9. doi: 10.1016/0305-0491(91)90076-P. [DOI] [PubMed] [Google Scholar]
- Sun Y, Hayakawa S. Heat-induced gels of egg white/ovalbumins from five avian species: thermal aggregation, molecular forces involved, and rheological properties. J Agric Food Chem. 2002;50:1636–1642. doi: 10.1021/jf0109975. [DOI] [PubMed] [Google Scholar]
- Takenawa T, Takahashi K, Le-Chang S, Okazaki E, Osako K (2015) The effect of ovalbumin on the protease activity. In: International symposium on aquatic product processing, Bogor, Indonesia, pp 39–41
- Tan TC, Phatthanawiboon T, Mat Easa A. Quality, textural, and sensory properties of yellow alkaline noodles formulated with salted duck egg white. J Food Qual. 2016;39:342–350. doi: 10.1111/jfq.12203. [DOI] [Google Scholar]
- Tu Y, Zhao Y. Inorganic Elements in Preserved Egg. In: Hester PY, editor. Egg Innovations and Strategies for Improvements. San Diego: Academic Press; 2017. pp. 427–434. [Google Scholar]
- Wellman-Labadie O, Picman J, Hincke MT. Antimicrobial activity of cuticle and outer eggshell protein extracts from three species of domestic birds. Br Poult Sci. 2008;49:133–143. doi: 10.1080/00071660802001722. [DOI] [PubMed] [Google Scholar]
- Woods LFJ, Swinton SJ. Enzymes in the starch and sugar industries. In: Tucker GA, Woods LFJ, editors. Enzymes in food processing. 1. Dordrecht: Springer; 1995. p. 319. [Google Scholar]
- Woodward SA. Egg protein gels. In: Harris P, editor. Food gels. Netherlands: Springer; 1990. pp. 175–199. [Google Scholar]
- Wu J. Eggs and egg products processing. In: Clark S, Jung S, Lamsa B, editors. Food processing: principles and applications. second. Edition. Chichester: Wiley; 2014. pp. 437–455. [Google Scholar]
- Xu L, Zhao Y, Xu M, Yao Y, Nie X, Du H, Tu Y-G. Effects of salting treatment on the physicochemical properties, textural properties, and microstructures of duck eggs. PLoS ONE. 2017;12:1–17. doi: 10.1371/journal.pone.0182912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Jin Y, Xu Y, Bin Y, Xu X. Effect of pressure cooking on physicochemical properties of salted eggs. RSC Adv. 2016;6:97089–97095. doi: 10.1039/C6RA18737D. [DOI] [Google Scholar]
- Zhao Y, et al. Effects of alkaline concentration, temperature, and additives on the strength of alkaline-induced egg white gel. Poult Sci. 2014;93:2628–2635. doi: 10.3382/ps.2013-03596. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tu Y, Xu M, Li J, Du H. Physicochemical and nutritional characteristics of preserved duck egg white. Poult Sci. 2014;93:3130–3137. doi: 10.3382/ps.2013-03823. [DOI] [PubMed] [Google Scholar]
- Zhou B, Zhang M, Fang Z, Liu Y. A combination of freeze drying and microwave vacuum drying of duck egg white protein powders. Drying Technol. 2014;32:1840–1847. doi: 10.1080/07373937.2014.952380. [DOI] [Google Scholar]