Abstract
Water-induced crystallisation of amorphous core-cyclodextrin (CD) complex is an essential step in a solid encapsulation process and removal of added water is a challenging. Ethanol addition is expected to shorten the complex dehydration time. This study investigated crystallisation of amorphous spray-dried α-, β- and γ-CD powders by direct mixing 15% (w/w) of ethanol:water mixture (0:100, 20:80, 40:60, 60:40, 80:20 and 100:0) over 72 h period. The results showed α- and β-CD powders crystallised at all concentrations of ethanol solutions. Especially mixed with 0:100 and 20:80 ethanol:water solutions, the crystallisation behaviour of α- and β-CD powders was similar to that of commercial crystalline counterparts. γ-CD powders exhibited a crystallisation sign as mixed with 0:100 and 20:80 ethanol:water solutions only. In the study of fish oil encapsulation using the mixture of water and ethanol to induce the complex crystallisation, only γ-CD powder was able to form complex with fish oil.
Keywords: Cyclodextrin powder, Fish oil, Amorphous powder, Ethanol-induced crystallisation, Solid encapsulation
Introduction
Cyclodextrins (CDs), cyclic oligosaccharides, can be distinguished in α-, β-, and γ-CDs consisting of 6, 7 and 8 d-glucose units, respectively. These construction units are connected through α-1,4 linkages to form CD molecules which are shaped like a truncated, thick walled bucket with a hydrophobic cavity and hydrophilic exterior. This cavity is the reason for the possibility to form inclusion complexes with parts or even whole guest molecules inside the cavity (Reineccius et al. 2002). Moreover, CD powders have been categorized as generally recognized as safe (GRAS) in the USA, natural products in Japan, and as novel food in Australia, New Zealand and EU countries (FSANZ 2004; Irie and Uekama 1997). Hence, they are being widely used in food production to encapsulate many hydrophobic compounds, especially bioactive compounds aiming to solubilize, stabilize or control release rate of these components (Szente and Szejtli 2004; Hedges 1998; Ho et al. 2014).
The increasing demand for fish oil (FO) as a functional and healthy food is caused by the fact that FO is a very rich source of omega-3 polyunsaturated fatty acids. In particular, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are promoted for their beneficial effects on human health, especially in cardiovascular and brain disorders, mental health, eye health and joint health (USDA 2016). In food processing, the major problems associated with the incorporation of FO into food products are insolubility in water, susceptibility to oxidative deterioration, and development of undesirable rancid odour and taste (Cameron-Smith et al. 2015; Bellitz et al. 2009). In this case, the encapsulation of FO leads to a limitation in the access of oxygen to the active centres, which subsequently helps to protect them from oxidation. The complexation of FO using CD powders has been well reported (Choi et al. 2010; Hădărugă et al. 2016; Na et al. 2011). In these studies, the CD-FO complexes were typically prepared by co-precipitation (self-assembly aggregation) and paste (kneading) methods. For the co-precipitation approach, the resultant complex powder is relatively stable at normal environmental conditions. However, a very low yield and long processing time (> 24 h) of this method, along with other disadvantages such as high volume material handling problems make it unsuitable for commercialization. Meanwhile, the kneading method is quite simple and easy to accomplish, but it requires a large scale mixing equipment, extensive dehydration of pasty complexes and poor reproducibility with some molecules (Marques 2010; Shrestha et al. 2017). Therefore, both reported FO complexation methods are likely unsuitable for large scale applications unless the mentioned drawbacks are addressed.
In a recent report by Ho (2017), an innovative encapsulation approach using CD powder which addresses the disadvantages of co-precipitation and paste methods was reported. In this approach (known as solid encapsulation), the CD powders must be in the amorphous structure, instead of the crystalline CD powders which are used in co-precipitation and paste methods. In crystalline structure, the molecules are aligned in a long range order, forming a tightly packed molecular structure which is a barrier for diffusion of guests to the cavity of CD molecules. In contrast, in amorphous structure, the molecules are randomly arranged to form a loose structure, thereby allowing guests to diffuse easily into molecular cavities (Szejtli 1998). Completely amorphous CD powders can be easily produced by spray drying of CD solution (Ho et al. 2017a, b). Using the characteristics of amorphous CD powders, the solid encapsulation approach is accomplished via direct mixing of guest molecules with amorphous CD powders, followed by addition of water with an amount close to or higher than a crystallisation-inducing level of amorphous CD powders. In this method, the encapsulation and crystallisation of the amorphous complex powder can occur almost simultaneously. Crystallisation of the amorphous complex powders during encapsulation process causes the guest molecules to be locked inside the CD cavity making them stable. This encapsulation approach was initially developed for CO2 gas encapsulation using amorphous α-CD powder (Ho 2017). It was also successfully applied for tea tree oil encapsulation using amorphous β-CD powder (Shrestha et al. 2017).
However, an exclusion of water due to encapsulation and crystallisation of amorphous powders in solid encapsulation resulted in a high water amount on the surface of the complex powder particles, making them unstable and easy to be spoiled by micro-organisms (Astray et al. 2009). Thus, the dehydration of the complex powder is required to improve its stability during storage. A long dehydration time of the complex can result in a decrease of encapsulation efficiency (i.e. the amount of guest molecules entrapped into cyclodextrin cavities over its initial amount used to prepare the complex), especially for volatile guest molecules. In order to address the problems of solid encapsulation, crystallisation of amorphous CD powders with food-grade organic solvents (e.g. ethanol) or a mixture of solvent and water, instead of only water could be an alternative. The use of solvent reduces the dehydration process of the complex due to very easy evaporation of solvent and water at normal conditions, and facilitates the penetration of the active compounds into the cavity of CD molecules because of dissolution of the active compounds into solvent. To the best of our knowledge, there is no such study reported. Thus, in this study, crystallisation behaviour of amorphous α-, β- and γ-CD powders using the mixture of ethanol and water with and without complexation with fish oil was investigated.
Materials and methods
Materials
The crystalline α- and β-CD powders were purchased from Wacker Biochem Group (Wacker Chemie AG, Germany) while crystalline γ-CD powder was obtained from Ensuiko Sugar Refining Co. Ltd (Tokyo, Japan). Absolute ethanol, gas chromatography grade hexane and fish oil (derived from the Menhaden fish.) were purchased from Sigma–Aldrich (Sigma–Aldrich Co. LLC, Australia). All other chemicals used in this study were of analytical reagent grade.
Production of amorphous α-, β- and γ-CD powders
The amorphous form of CD powders was produced using spray drying method as reported by Ho et al. (2015). Amorphous α- and β-CD powders were produced by using an Anhydro spray dryer (The University of Queensland, Australia), which was equipped with a twin fluid nozzle and has a water evaporation capacity of about 4 L/h. The compressed air inlet of atomizer was set at 40 kPa. The feed concentration was 10% (w/w) for α-CD and 4% (w/w) for β-CD powder. The inlet and outlet temperature of the drying air were controlled at 180 and 80 °C, respectively. For γ-CD powder, due to very high cost of crystalline γ-CD powder, and high loss of powder and high water evaporation capacity (which requires a large amount of sample solution for operation) in Anhydro spray dryer, a Buchi spray dryer (B-290, Labortechnik AG, Switzerland) with a lower water evaporation capacity (0.5 L/h) was used to produce amorphous γ-CD powder to maximize the yield of obtained powder. The solubility of γ-CD powder at normal conditions (e.g. 23 °C) is 22.6% (w/w). In order to achieve completely amorphous spray-dried γ-CD powder, the CD powder in feed solution must be completely dissolved into water without any chance of crystallization during feeding. Therefore, the feed solution at a lower concentration (20%, w/w) than its saturation limit was prepared for spray drying. The air inlet and outlet temperature were set at 180 and 80 °C, respectively. The spray-dried powders were collected from the cyclone and stored in vacuum sealed aluminium pouches for further experiments.
Crystallisation behaviour of amorphous CDs through the addition of different ratios of ethanol–water mixture
Crystallisation characteristics of spray-dried amorphous α-, β-and γ-CD powders mixed with the mixture of ethanol:water at different ratios (0:100, 20:80, 40:60, 60:40, 80:20, and 100:0; hereafter, known as 0, 20, 40, 60, 80 and 100% ethanol solutions) over 72 h was investigated via direct mixing CD powders at 15% (v/w) of the mixture. To describe in detail, 10 g of each type of amorphous CD powders (α-, β- or γ-CDs) was weighed into an aluminium pouch, then 1.5 mL solution of ethanol–water at different ratios was added under vigorous mixing. Once the addition was completed, the pouch was sealed and the mixing was continued for at least 15 min by manually kneading through the sealed pouch. The pouches were stored at 23 ± 1 °C for crystallisation over 72 h. After each time interval (4, 8, 12, 24, 48 and 72 h), a small amount of sample was taken for X-ray diffraction (XRD), differential scanning calorimetry (DSC) and polarized light microscopy (PLM) analyses.
Analytical approaches
X-ray diffraction
X-ray diffractograms of the powder samples were obtained by a Bruker Advance AXS D8 X-Ray diffractometer (Bruker AXS GmbH, Karlsruhe, Germany). The scanning settings were according to Ho et al. (2015) with a voltage of 40 kV, a current of 40 mA using Cu radiation, and a scanning speed of 1°/min. The angular range was 2θ = 3°–30°, whereas step size and time constant were 0.02° and 1 s, respectively.
Differential scanning calorimetry
Water evaporation behaviour during the heating of samples was evaluated by differential scanning calorimetry (DSC, Mettler Toledo, Switzerland). About 5 mg of the powder was sealed in a 40 µL aluminium pan by a pin-hole lid. The temperature program ran from 25 to 250 °C with a heating rate of 10 °C/min (Ho et al. 2015). The purge gas was nitrogen. The calorimeter was calibrated by indium before performing the analysis.
Polarized light microscopy
Images of α-, β- and γ-CD powders under polarized light were acquired using a prism and optical microscope (Scientific Instruments and Optical Sales, Australia) with polarizer filters fitted with a 5.0 MP camera system using TSView7 software (Fuzhou Tucsen Image Technology Co., Ltd., China) and connected to the video entry port of a computer. Isopropanol was used to disperse the powder particles on the slides. Images were taken with a 4-X objective.
Complexation of amorphous cyclodextrins with fish oil
The complexation of amorphous α-, β- and γ-CD powders with FO was investigated via the solid encapsulation approach and the ethanol:water mixture was used to crystallise the amorphous inclusion complex. As shown in the X-ray results in Fig. 4, all CD powders showed a signal of crystallisation with 20% ethanol solution (20:80 ethanol:water), thus the complexation of fish oil with amorphous CD powders was initially carried out with this ethanol solution. The ethanol and water solutions were added separately to maximize the encapsulation efficiency and crystallisation of the amorphous complex. The amorphous powders (20 g) was initially mixed with FO at a ratio of 1:0.1 (CD:FO, w/w) using an overhead stirrer (400 RPM, Heidolph RZR 2050, Kelheim, Germany). Then, the absolute ethanol was directly added to the CD-FO mixture at 3% (w/w, based on the weight of CD powder) to assist the FO molecules to access the CD cavity. In order to accelerate the crystallisation of amorphous FO-CD complex, water was mixed with 12% (w/w, based on the weight of CD powder). The mixing in a closed vessel was continued for another 5 min and then the samples were stored in the sealed aluminium pouches for at least 4 h for crystallisation. Finally, the samples were dried under atmospheric conditions at about 23 ± 1 °C for 1 h to remove ethanol prior to subjecting to the surface oil determination.
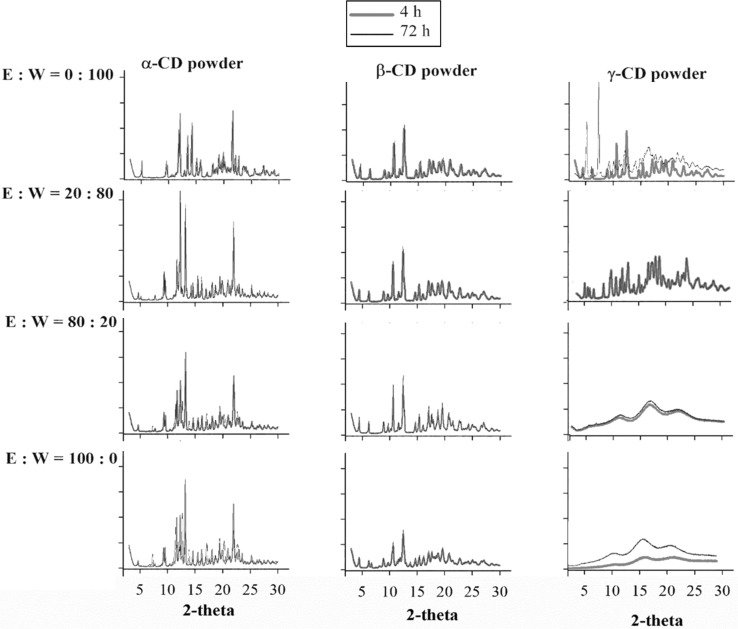
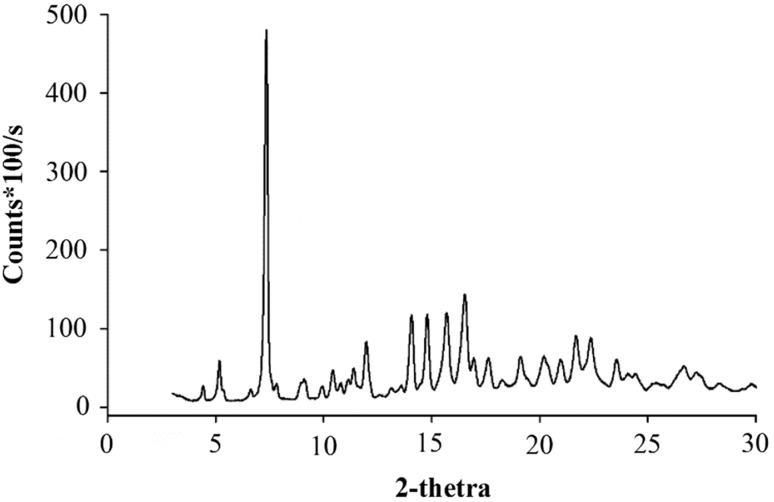
Fig. 4.
XRD curves of spray-dried amorphous α-, β- and γ-CD powders mixed with the mixture of ethanol (E):water (W) with different ratios (w/w) after stored in the airtight bag for 4
 and 72 h
and 72 h
 . Due to insignificant differences in characteristics of XRD curves at different time intervals (4, 8, 12, 24, 48, and 72 h) and those mixed with 40 and 60% ethanol solutions (E:W = 40:60 and E:W = 60:40), only the XRD results for 0% (E:W = 0:100), 20% (E:W = 20:80), 80% (E:W = 80:20) and 100% (E:W = 100:0) ethanol solutions at 4 and 72 h were shown
. Due to insignificant differences in characteristics of XRD curves at different time intervals (4, 8, 12, 24, 48, and 72 h) and those mixed with 40 and 60% ethanol solutions (E:W = 40:60 and E:W = 60:40), only the XRD results for 0% (E:W = 0:100), 20% (E:W = 20:80), 80% (E:W = 80:20) and 100% (E:W = 100:0) ethanol solutions at 4 and 72 h were shown
Determination of surface oil of the CD-FO complex powder
The amount of FO on the particle surface of the complex powder was determined using the hexane solvent method previously described by Bhandari et al. (1999). About 5 g of the complexed powder was mixed with 20 mL hexane and shaken gently for 10 min. Subsequently, the slurry was filtered through a filter paper (90 mm ϕ) to an Erlenmeyer flask and the residuals on the filter were rewashed with another 10 mL hexane. The filtrate (e.g. FO containing hexane) in Erlenmeyer flask was purged with N2 until the weight of Erlenmeyer flask was constant (approximately 60 min). The easily extractable surface FO amount was calculated as the difference of the previous and the final weight of the Erlenmeyer flask. The surface FO (%) was calculated by the following Eq. (1).
| 1 |
In order to ensure the hexane washing method providing reasonable data, the free FO content on particle surface was also determined by UV–visible spectrophotometry for several experiments. This method was reported in detail by Choi et al. (2010). The surface FO values of both methods were in the same range (not shown data), however due to complexities in UV–visible spectrophotometry method, the hexane solvent method was used in this study.
Statistical analysis
The experiments were performed following a fully randomized design with three replications. Experimental data were subjected for analysing of the variance (ANOVA) at a significance level of p = 0.05 using the Minitab Express statistical programme (Minitab Inc., USA). For the characterization of inclusion complexes, each criterion was repeated at least two times.
Results and discussion
Characterization of spray-dried CD powders
The moisture content of spray-dried powders was 5.41 ± 0.15 (w/w, wet basis) while that of commercial CD powders was 9.56 ± 0.19 (w/w, wet basis).
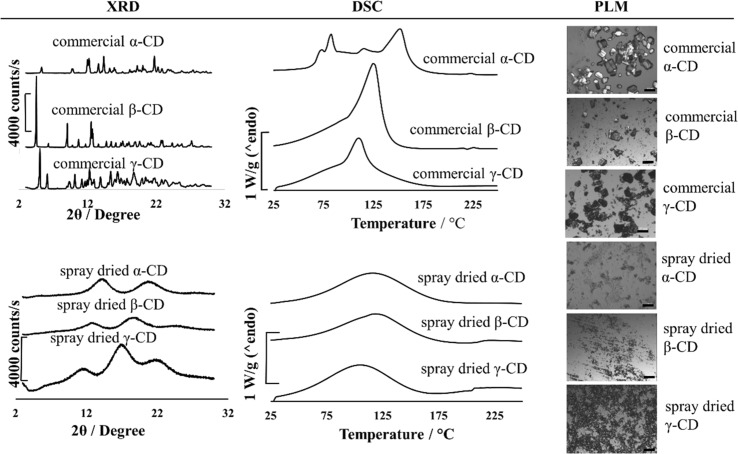
The analytical results of XRD, DSC and PLM of commercial and spray dried α-, β- and γ-CD powders are showed in Fig. 1. The X-ray diffractograms of commercial α-, β- and γ-CD powders showed many peaks, whereas those of spray-dried α-, β- and γ-CD powders only had the humps. In spray drying, water molecules are evaporated so rapidly that the solutes does not have enough time to arrange into an ordered structure. Typically, it takes several seconds (e.g. 2–5 s) for the solutes to pass through drying zone (Gharsallaoui et al. 2007). Thus, spray drying is considered as the most efficient approach, as compared to freeze-drying or milling, in production of amorphous powders (Ho et al. 2017b). Similar results for the production of amorphous α- and β-CD powders by spray drying were also reported by Ho et al. (2015), Shrestha et al. (2017), Franco et al. (2017) and Hong et al. (2011).
Fig. 1.
XRD patterns, DSC thermograms and polarized light microscopic (PLM) images of commercial and spray-dried α-, β- and γ-CD powders. For images, scale bar
 = 200 μm
= 200 μm
In DSC analysis, one or several sharp endothermic peaks associated with water evaporation were observed on the thermal curves of all commercial CD powders whereas those of all spray-dried CD powders only showed a broad hump caused by water evaporation or structural relaxation. The amorphous powders with porous and open structure allow water molecules to diffuse out easily, which results in an endothermic hump over the whole range of temperature. On the other hand, the commercial crystalline powders releases the water through evaporation in several steps because of the compact structure of crystalline CD powders, especially α-CD ones (Coey 1974). The disappearance of the endothermic peaks in the thermograms in the second DSC scan for CD powders (data not shown) confirmed that these peaks were associated with water evaporation.
The polarized light microscopic images of commercial crystalline and spray-dried amorphous α-, β- and γ-CD powders are shown Fig. 1. Crystalline materials with anisotropic properties under polarized light showed a birefringent phenomenon which appeared colourful and bright with regions of different contrasts depending on their orientation. On the other hand, amorphous powders with isotropic properties appeared in a similar colour to the background which results in a dark colour (Taylor 2015). There were other differences observed in the polarized light images of amorphous and crystalline CD powders. Amorphous CD powder had a homogenously dark background and no shape of crystals whereas crystalline powders showed coloured bright crystals with different shapes.
Overall, the XRD, DSC and PLM results of commercial and spray-dried α-, β- and γ-CD powders showed that the commercial powders are completely crystalline while corresponding spray-dried powders are amorphous.
Crystallisation properties of amorphous α-, β- and γ-CD powders
The analytical results of differential scanning calorimetry, polarized light microscopy and X-ray diffraction showed that after 4 h, the crystallisation process of amorphous α-, β- and γ-CD powders was almost completed as there was no significant changes in XRD, DSC and PLM results. This means that the XRD curves, DSC scans and PLM of samples collected at 8, 12, 24 and 48 h were similar to those obtained at 4 and 72 h. In order to simplify the data presentation, only results at 4 and 72 h for XRD, DSC and PLM are shown.
Differential scanning calorimetry (DSC)
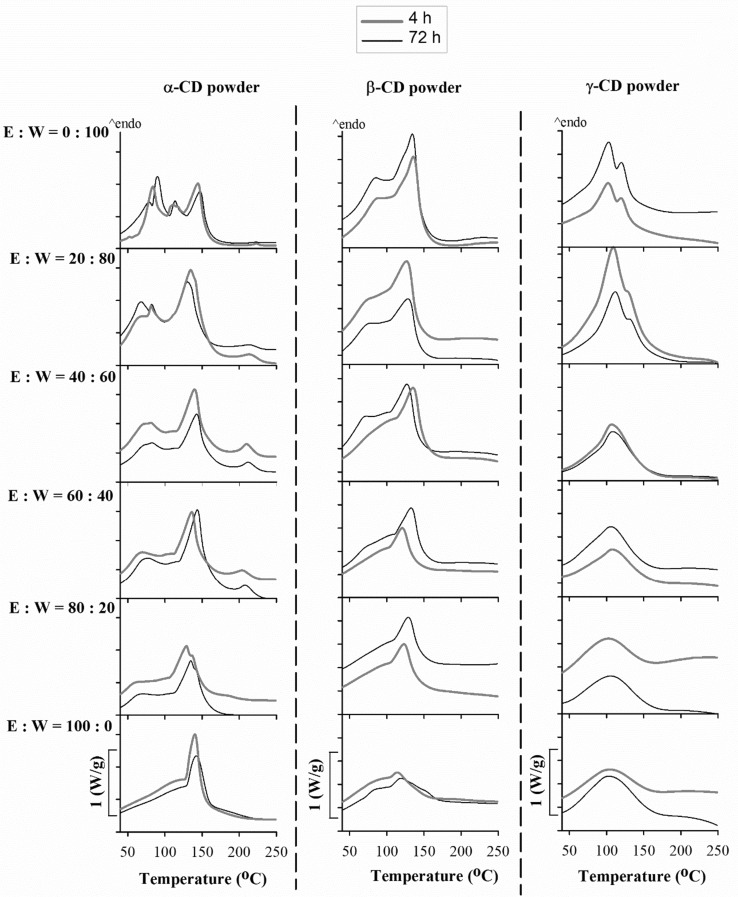
The DSC is a commonly-used method to verify the formation of crystals in the solid state (Karathanos et al. 2007). The differences in DSC curves between purely amorphous and crystalline CD powders discussed in previous section would be a basis to investigate the crystallisation behaviour of amorphous CD powders. The DSC results at 4 and 72 h are shown in Fig. 2. Each peak of the DSC curve symbolizes an energy consumption, due to the evaporation of water and ethanol. The more and the sharper the peaks are, the higher is the resistance of the powder structure to release water and ethanol out of the CD molecules. The progress of crystallisation of CD powders can be determined by comparing the changes of the DSC peaks. These peaks can be shifted, disappeared or even broadened. The method to determine the changes in powder structure from amorphous to crystalline and vice versa due to DSC analysis was previously summarized and described by Ho et al. (2017a).
Fig. 2.
DSC thermograms of spray-dried amorphous α-, β- and γ-CD powders mixed with mixture of ethanol (E):water (W) with different ratios (w/w) after stored in the airtight bag for 4
 and 72 h
and 72 h
 . Due to insignificant differences in characteristics of DSC curves at different time intervals (4, 8, 12, 24, 48, and 72 h), only the DSC results for 0% (E:W = 0:100), 20% (E:W = 20:80), 40% (E:W = 40:60), 60% (E:W = 60:40), 80% (E:W = 80:20) and 100% (E:W = 100:0) ethanol solutions at 4 and 72 h were shown
. Due to insignificant differences in characteristics of DSC curves at different time intervals (4, 8, 12, 24, 48, and 72 h), only the DSC results for 0% (E:W = 0:100), 20% (E:W = 20:80), 40% (E:W = 40:60), 60% (E:W = 60:40), 80% (E:W = 80:20) and 100% (E:W = 100:0) ethanol solutions at 4 and 72 h were shown
As can be seen in Fig. 2, for all CD powders, the DSC curves at 0% ethanol solution (ethanol:water = 0:100 mixture) were very resemble to those of commercial crystalline CD powders, in which many endothermic peaks were found (Fig. 1). This result indicated that all amorphous CD powders completely crystallise with the ethanol:water = 0:100 mixture. An increase of ethanol ratio in the ethanol–water mixture led to a decline in the number of the sharp peaks and/or resulted in a broadening of the peaks. This is possibly caused by the fact that water molecules play a more important role in assistance of CD molecules to rearrange into an ordered structure than ethanol molecules do. Among CD powders, α-CD powder with 6 membered sugar ring molecules tends to crystallise more easily and requires less amount of “lubricant” than β- and γ-CD powders which have 7 and 8 corresponding ring molecules, respectively. With 100% ethanol solution (ethanol:water = 100:0), sharp peaks were observed on the DSC curve of α-CD powder while CD powders constructed with higher amounts of glucose molecules, especially γ-CD powders, did not exhibit any sharp peak on the DSC curve at the same concentration of ethanol solution. However, for all CD powders, the number of such sharp peaks observed on the DSC curves increased with the decrease of ethanol ratio in ethanol–water mixture (e.g. at higher water content).
Moreover, the similarities in the DSC curves of all samples at 4 and 74 h indicated that the crystallisation of all CD powders was almost completed after 4 h and an extending of crystallisation time could not enhance powders further crystallinity. For all CD powders, the DSC curves of CD powders mixed with 20% ethanol solution (ethanol:water = 20:80) was similar to those of CD powders added 0% ethanol solution (ethanol:water = 0:100).
Polarized light microscopy (PLM)
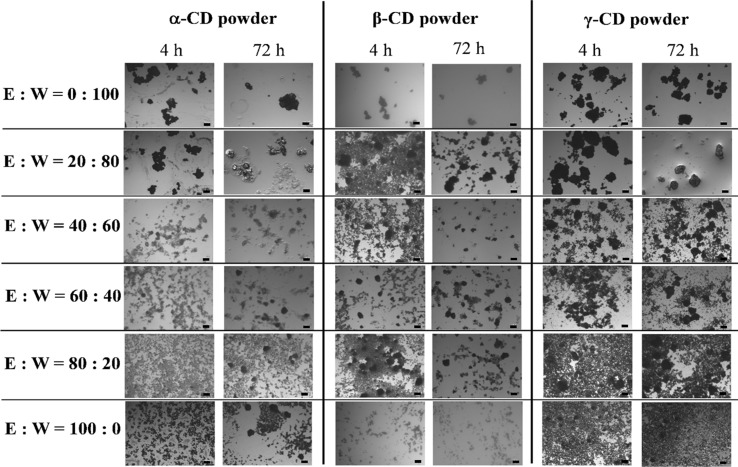
As shown in Fig. 1, amorphous CD powders had a smooth well uniformly distributed surface without any crystal structure under PLM, unlike commercial crystal CD powders which had a lumped and partially separated structure of crystals and often surrounded or capped by additionally amorphous powder. Moreover, the size of the crystalline CD powders was bigger than that of the amorphous ones, thus it was possible to distinguish the crystalline and amorphous CD powders structure under PLM easily.
As can be seen in Fig. 3, the crystallisation behaviour can be compared on the basis of the visual appearance of the CD powder particles. All characteristics of CD crystals can be observed for all CD powders mixed with only water (e.g. ethanol:water = 0:100 mixture). PLM images show that crystallisation rate of amorphous CD powders increases with a decrease of the ethanol ratio in the added mixture, by which a steady increase in crystal size was observed for all CD powders, while dark amorphous powder particles get less and distinctive crystals appear more. After 4 h crystallisation, the estimated size of the crystal aggregates differed from α-, β- and γ-CD powders, which were 100–150 µm, 100–250 µm and 150–350 µm, respectively. The differences in PLM images of all CD powders mixed with 20–100% concentration of ethanol solution between 4 and 72 h crystallisation time in Fig. 3 could be due to the aggregation of some small crystals.
Fig. 3.
Polarized light microscopic (PLM) images of spray-dried amorphous α-, β- and γ-CD powders mixed with the mixture of ethanol (E):water (W) with different ratios (w/w) after stored in the airtight bag for 4 and 72 h. Due to insignificant differences in characteristics of PLM images at different time intervals (4, 8, 12, 24, 48, and 72 h), only the PLM results for 0% (E:W = 0:100), 20% (E:W = 20:80), 40% (E:W = 40:60), 60% (E:W = 60:40), 80% (E:W = 80:20) and 100% (E:W = 100:0) ethanol solutions at 4 and 72 h were shown. Scale bar
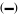 = 100 μm
= 100 μm
However, the appearance of powder particles under PLM mixed with 20% ethanol solution was the same as that of CD powders mixed with only water (e.g. ethanol:water = 0:100 mixture), which had all characteristics of CD crystals. The results showed that the replacement of water by ethanol above 20% can significantly reduce the molecular mobility of the CD powders, resulting in less crystallisation of cyclodextrins.
X ray diffraction (XRD)
As mentioned, to simplify the evaluation of the data, only 4 and 72 h complexation time intervals are presented in this study. Furthermore, there were similar results observed for 40 and 60% ethanol solutions, hence XRD analysis only focused on 0, 20, 80 and 100% ethanol solutions.
The XRD results in Fig. 4 showed that regardless the concentrations of ethanol in the added ethanol–water solution, both α- and β-CD powders crystallised almost completely. While the rate of crystallisation of the α-CD powder was almost the same for all concentrations of ethanol, there was a steady progress in the rate of crystallisation of β-CD powder with a decrease of ethanol concentration. The XRD peaks of β-CD powders became more distinctive and sharper at lower ethanol concentrations. However, for both α- and β-CD powders, crystallisation behaviour at 4 and 72 h time intervals did not show any significant differences. For γ-CD powders, it can be seen that 80 and 100% of ethanol solutions were not able to induce the crystallisation of γ-CD powders whereas γ-CD samples mixed with the 20% ethanol solutions, many sharp peaks were observed on their XRD curves. The results indicated that the interaction of ethanol to the CD molecules which were possibly between hydroxyl groups of the alcohol and the hydroxyl groups of CD by hydrogen bonds was able to increase the mobility of the complex to initiate nucleation and crystallisation. In previous study, crystallisation of amorphous complexes between tea tree oil and amorphous β-CD powders was also reported (Shrestha et al. 2017).
As compared to DSC and PLM analytical results, XRD is the most sensitive to detect the crystallisation of amorphous CD powders. In many cases (e.g. α- and β-CD powders with 100% ethanol solutions) in which both DSC and PLM were unable to detect the crystallisation of CD powders, XRD showed it clearly.
Complexation of fish oil by solid amorphous CD powders (solid encapsulation method)
The amount of FO not included in the CD molecule cavity in the complexation process is referred as the surface oil. A high surface oil indicates low encapsulation efficiency of the FO in the powder and vice versa. Table 1 shows the amount of surface oil extracted from the complexes prepared from α-, β-, and γ-CD powders and their encapsulation efficiency. Surface oil of the complex powders prepared from α-CD and β-CD powders was 61.10 ± 2.10 and 65.90 ± 2.70%, respectively, which was much higher than that measured in the complex powder produced from γ-CD powder (13.70 ± 5.10% surface oil). These values indicate that there are differences in the FO complexation efficiency of α-, β- and γ-CD powders. The high SO values led to the assumption that no effective molecular complexation occurs. An explanation could be the size of the cavity of CD molecules. The fatty-acid glycerides do not fit either fully or partly into the cavity of α-CD and β-CD molecules while a lager cavity of γ-CD molecules offers them a better complexation ability with FO. In preliminary experiment, effects of different concentrations of FO to CD, amount of water and ethanol on encapsulation efficiency of β-CD powder was investigated and the results found no effect on surface oil. A very high surface oil value was found for all conditions (> 50%, w/w) indicating that fish oil is not complexed effectively by β-CD powder.
Table 1.
The amount of surface oil and encapsulation efficiency of the complexes prepared from α-, β-, and γ-CD powders
| FO complexation of CD powder | ||
|---|---|---|
| CD | Surface oil (%) | Encapsulation efficiency (%) |
| α-CD | 61.10 ± 2.10 | 38.90 ± 2.10 |
| β-CD | 65.90 ± 2.70 | 34.10 ± 3.12 |
| γ-CD | 13.70 ± 3.10 | 86.30 ± 4.34 |
It was noticed that the FO complexation of γ-CD powder was accomplished with 3% (w/w) absolute ethanol and 12% (w/w) water. It seems that this amount of water and ethanol fails to induce complete crystallisation of the FO-γ-CD complex to lock the FO into the crystalline structure of the complex. The X-ray results in Fig. 4 confirmed that γ-CD powders exhibited some extent of crystallisation at the same addition. Moreover, crystallisation of γ-CD powder in the presence of FO might be different to that of pure γ-CD powder. Thus, at this complexation condition, the stable FO-γ-CD complex is possibly not produced. In order to further investigate this assumption, the experiments were carried out with a higher amount of water, e.g. 20 and 25% (w/w) water in combination with and without 5% (w/w) ethanol to optimise FO encapsulation efficiency of γ-CD powder. The results showed that without ethanol combination, an increase of amount added water from 20 to 25% (w/w), the surface oil markedly reduced from 10.70 ± 1.60 to 3.80 ± 1.70%. This can be explained by enhancement of crystallisation rate of FO-γ-CD complex at high amount of added water. Interestingly, with the presence of 5% (w/w) ethanol, surface oil was reduced to much lower values. Surface oil extracted from the FO-γ-CD complex powder prepared with 20% water and 5% ethanol was only about 1.00%. The structure of this FO-γ-CD complex powder was almost completely crystalline (Fig. 5). The results showed that the addition of ethanol effectively supported the crystallisation, complexation and inclusion properties of the CD-FO complexes.
Fig. 5.
XRD patterns of γ-CD-FO complex powder. The complex was prepared from amorphous γ-CD powder and FO with a ratio of 1:0.1 (w/w) and crystallised with 5% ethanol and 20% water by weight of CD powder
These results are comparable to the one reported by Na et al. (2011) in-which γ-CD was used to encapsulate FO by liquid encapsulation method and reported that complexation with γ-CD has a lower SO than with β-CD. Moreover, a comparison to other complexation methods of FO using different wall materials showed that SO analysed in this study were markedly lower than SO reported in previous studies (> 10%) (Chung et al. 2010; Klinkesorn et al. 2006; Chan 2011).
The benefits of the water–ethanol mixtures on the encapsulation efficiency in this study showed that water and ethanol molecules act as high energy molecules and get replaced during complexation by the apolar guest molecules inside the apolar CD-cavity, as recently reported by Shrestha (2017) and Astray et al. (2009). Patanay (1987) found that it was even possible for the ethanol to be part of the complexes, as the hydroxyl groups of the alcohol bind to the CD hydroxyl groups through hydrogen bondings. This helps to improve the inclusion efficiency because FO can be transported to and complexed in the cavities by ethanol easily. Ethanol molecules are small enough to evaporate quickly out of the cavities, even if the crystallisation is completed (Aree and Chaichit 2008).
Conclusion
The investigation of the crystallisation behavior of spray-dried amorphous α-, β- and γ-CD powders in solid state by the addition of variable ratio of ethanol:water mixture showed that both α- and β-CD powders crystallized at all concentrations of ethanol while γ-CD only crystallised with mixture solutions with less than 20% of ethanol. The XRD, DSC and PLM results indicated that crystallisation behaviour of amorphous CD powders was almost unchanged after 4 h. The complete crystallisation of CD powder helps to entrap guest molecules more effectively. The solid encapsulation of FO using amorphous CD powders, followed by crystallisation of amorphous complex using ethanol solutions showed that among three common types of CD powders, only γ-CD powders were capable to entrap higher amount of FO. At the same encapsulation conditions, encapsulation efficiency of α- and β-CD powders was less than 40% (w/w), being much lower than that of γ-CD powder which was about 86.0% (w/w). Interestingly, by optimisation on the amount of water and ethanol used to prepare the complex powder with γ-CD powder, encapsulation efficiency of as high as 99.0% (w/w) can be achieved. This study has developed a new and simple technique to encapsulate fish oils, which has high potential for large scale production of fish oil powder due to high yield (almost 100%), short processing time and high encapsulation efficiency. Moreover, in the encapsulated powder form, incorporation of fish oils into food products become easier. Further studies on characterisation and stability of the complexes of FO and γ-CD powders during storage at different conditions will be undertaken.
Acknowledgements
The authors acknowledge the facilities, and the scientific and technical assistance, of School of Agriculture and Food Sciences and the Australian Microscopy and Microanalysis Research Facility at the Centre for Microscopy and Microanalysis, The University of Queensland.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aree T, Chaichit N. Crystal form III of beta-cyclodextrin-ethanol inclusion complex: layer-type structure with dimeric motif. Carbohydr Res. 2008;34:2285–2291. doi: 10.1016/j.carres.2008.04.028. [DOI] [PubMed] [Google Scholar]
- Astray G, Gonzalez-Barreiro C, Mejut JC, Rial-Otero R, Simal-Gandar J. A review on the use of cyclodextins in foods. Food Hydrocoll. 2009;23:1631–1640. doi: 10.1016/j.foodhyd.2009.01.001. [DOI] [Google Scholar]
- Bellitz H, Grosch W, Schieberle P. Food chemistry. Berlin: Springer; 2009. [Google Scholar]
- Bhandari BR, D’Arcy BR, Padukka I. Encapsulation of lemon oil by paste method using beta-cyclodextrin: encapsulation efficiency and profile of oil volatiles. J Agric Food Chem. 1999;47(12):5194–5197. doi: 10.1021/jf9902503. [DOI] [PubMed] [Google Scholar]
- Cameron-Smith D, Albert B, Cutfield W. Fishing for answers: is oxidation of fish oil supplements a problem? J Nutr Sci. 2015;4:1–2. doi: 10.1017/jns.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E. Preparation of Ca-alginate beads containing high oil content: influence of process variables on encapsulation efficiency and bead properties. Carbohydr Polym. 2011;84:1267–1275. doi: 10.1016/j.carbpol.2011.01.015. [DOI] [Google Scholar]
- Choi MJ, Ruktanonchai U, Min SG, Chun JY, Soottitantawat A. Physical characteristics of fish oil encapsulated by beta-cyclodextrin using an aggregation method or polycaprolactone using an emulsion-diffusion method. Food Chem. 2010;119(4):1694–1703. doi: 10.1016/j.foodchem.2009.09.052. [DOI] [Google Scholar]
- Chung C, Sanguansri L, Augustin M. Resistant starch modification: effects on starch properties and functionality as co-encapsulant in sodium caseinate-based fish oil microcapsules. J Food Sci. 2010;75(9):636–642. doi: 10.1111/j.1750-3841.2010.01857.x. [DOI] [PubMed] [Google Scholar]
- Coey JMD. Amorphous solids: a review of the applications of the Mössbauer effect. J Phys Colloq. 1974;35:C6-89–C6-105. [Google Scholar]
- Franco M, Araújo D, de Paula E. Cavalcanti L, Yokaichiya F (2017) X-ray scattering techniques applied in the development of drug delivery systems. X-ray Scattering, Alicia Esther Ares, IntechOpen, 10.5772/65326. https://www.intechopen.com/books/x-ray-scattering/x-ray-scattering-techniques-applied-in-the-development-of-drug-delivery-systems. Accessed 26 June 2015
- FSANZ (2004) Final assessment report: Alpha-cyclodextrin as a novel food. http://www.foodstandards.gov.au/code/applications/Documents/A494_Alpha-Cyclodextrin_as_a_novel_food_IAR.pdf. Accessed 25 Aug 2014
- Gharsallaoui A, Roudaut G, Chambi O, Voilley A, Saurel R. Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Res Int. 2007;40:1107–1121. doi: 10.1016/j.foodres.2007.07.004. [DOI] [Google Scholar]
- Hădărugă D, Ünlüsayin M, Gruia A, Birău C, Rusu G, Hădărugă N. Thermal and oxidative stability of Atlantic salmon oil (Salmo salar L.) and complexation with beta-cyclodextrin. Beilstein J Org Chem. 2016;12:179–191. doi: 10.3762/bjoc.12.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges AR. Industrial applications of cyclodextrins. Chem Rev. 1998;98:2035–2044. doi: 10.1021/cr970014w. [DOI] [PubMed] [Google Scholar]
- Ho, TM (2017) Studies of the encapsulation and release of carbon dioxide from amorphous and crystalline alpha-cyclodextrin powders and its application in food systems, Ph.D. Thesis, School of Agriculture and Food Sciences, The University of Queensland. 10.14264/uql.2018.85
- Ho TM, Howes T, Bhandari BR. Encapsulation of gases in powder solid matrices and their applications: a review. Powder Technol. 2014;259:87–108. doi: 10.1016/j.powtec.2014.03.054. [DOI] [Google Scholar]
- Ho TM, Howes T, Bhandari BR. Characterization of crystalline and spray dried amorphous alpha-cyclodextrin powders. Powder Technol. 2015;17(06):585–594. doi: 10.1016/j.powtec.2015.06.027. [DOI] [Google Scholar]
- Ho TM, Truong T, Bhandari BR. Spray-drying and non-equilibrium states/glass transition. In: Bhandari BR, Roos YH, editors. Non-equilibrium states and glass transitions in foods—processing effects and product-specific implications, Chapter 5. New York: Woodhead Publishing; 2017. pp. 111–136. [Google Scholar]
- Ho TM, Truong T, Bhandari BR. Methods to characterize the structure of food powders—a review. Biosci Biotechnol Biochem. 2017;12(1):1–21. doi: 10.1080/09168451.2016.1274643. [DOI] [PubMed] [Google Scholar]
- Hong J, Shah J, McGonagle M. Effect of cyclodextrin derivation and amorphous state of complex on accelerated degradation of ziprasidone. J Pharm Sci. 2011;100(7):2703–2716. doi: 10.1002/jps.22498. [DOI] [PubMed] [Google Scholar]
- Irie T, Uekama K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J Pharm Sci. 1997;86:147–162. doi: 10.1021/js960213f. [DOI] [PubMed] [Google Scholar]
- Karathanos V, Mourtzinos I, Yannakopoulou K, Andikopoulos N. Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with beta-cyclodextrin. Food Chem. 2007;101:652–658. doi: 10.1016/j.foodchem.2006.01.053. [DOI] [Google Scholar]
- Klinkesorn U, Sophanodora P, Chinachoti P, Decker E, McClements J. Characterization of spray-dried tuna oil emulsified in two-layered interfacial membranes prepared using electrostatic layer-by-layer deposition. Food Res Int. 2006;39(4):449–457. doi: 10.1016/j.foodres.2005.09.008. [DOI] [Google Scholar]
- Marques HMC. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr J. 2010;25(5):313–326. doi: 10.1002/ffj.2019. [DOI] [Google Scholar]
- Na H, Kim J, Kim J, Lee K. Encapsulation of fish oil using cyclodextrin and whey proteine concentrate. Biotechnol Bioprocess Eng. 2011;16(6):1077–1082. doi: 10.1007/s12257-011-0099-2. [DOI] [Google Scholar]
- Patonay G, Fowler K, Shapira A, Nelson G, Warner IM. Cyclodextrin complexes of polyaromatic hydrocarbons in the presence of aliphatic alcohols. J Incl Phenom. 1987;5(6):717–723. doi: 10.1007/BF00656591. [DOI] [Google Scholar]
- Reineccius T, Reineccius G, Peppard T. Encapsulation of flavours using cylcodextrins: comparison of flavor retention in alpha, beta and gamma types. Food Chem Toxicol. 2002;67:3271–3279. [Google Scholar]
- Shrestha M, Ho TM, Bhandari BR. Encapsulation of tea tree oil by amorphous beta-cyclodextrin powder. Food Chem. 2017;221:1474–1483. doi: 10.1016/j.foodchem.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem Rev. 1998;98(5):1743–1753. doi: 10.1021/cr970022c. [DOI] [PubMed] [Google Scholar]
- Szente L, Szejtli J. Cyclodextrins as food ingredients. Trends Food Sci Technol. 2004;15(3):137–142. doi: 10.1016/j.tifs.2003.09.019. [DOI] [Google Scholar]
- Taylor LS. Physical stability and crystallization inhibition. In: Newman A, editor. Pharmaceutical amorphous solid dispersions. Hoboken: Wiley; 2015. pp. 179–217. [Google Scholar]
- USDA (2016) National nutrient database for standard reference – fish oil. United States Department of Agriculture. https://ndb.nal.usda.gov/ndb/foods/show/726?n1=%7BQv%3D1%7D&fgcd=&man=&lfacet=&count=&max=50&sort=default&qlookup=04590&offset=&format=Full&new=&measureby=&Qv=1&ds=&qt=&qp=&qa=&qn=&q=&ing=. Accessed 01 Aug 2017