Abstract
Background
Cocaine addiction is a chronic brain disease affecting neurotransmission. Muscarinic cholinergic receptors modulate dopaminergic signaling in the reward system, and muscarinic receptor stimulation can block direct reinforcing effects of cocaine. Here, we tested the hypothesis that specific muscarinic M4 receptor stimulation can attenuate the discriminative stimulus effects and conditioned rewarding effects of cocaine, measures believed to predict the ability of cocaine and cocaine-associated cues to elicit relapse to drug taking.
Methods
We tested the M4-selective positive allosteric modulators VU0152100 and VU0467154 in a drug discrimination assay and a conditioned place preference assay, including extinction and reinstatement of place preference. Specificity of the cocaine discrimination effect was verified using knockout mice lacking either M1 or M4 receptors (M1−/−, M4−/−). We also replicated previous findings in cocaine-induced locomotor hyperactivity and striatal dopamine microdialysis assays.
Results
VU0152100 attenuated the discriminative stimulus effect of cocaine in wild-type mice and M1−/− mice, but not in M4−/− mice, without affecting rates of responding. As previously shown with VU0152100, VU0467154 almost eliminated cocaine-induced hyperactivity and striatal dopamine efflux. VU0467154 failed to attenuate acquisition of cocaine-conditioned place preference, but facilitated extinction and prevented reinstatement of the conditioned place preference.
Conclusions
These findings further support the notion that M4 receptors are promising targets for the treatment of cocaine addiction, by showing that results can be replicated using distinct ligands, and that in addition to blocking reinforcing effects of cocaine relevant to ongoing drug taking, M4 positive allosteric modulators can also attenuate subjective and conditioned effects relevant to relapse.
Keywords: muscarinic cholinergic, psychostimulant, abuse, addiction, discriminative stimulus, classical conditioning
1. Introduction
Cocaine addiction remains a serious public health problem for which no effective treatments are available. Muscarinic cholinergic systems have been shown to modulate the effects of drugs of abuse including cocaine, thereby emerging as potential targets for the development of treatments (Kruse et al., 2014). The orthosteric binding site on muscarinic acetylcholine receptors is highly conserved between subtypes, but in the last decade, highly subtype-selective ligands have been developed by targeting allosteric binding sites, making it possible to investigate the functions of muscarinic receptor subtypes (Nickols and Conn, 2014; Thal et al., 2016).
We previously reported that activation of the M4 subtype by a positive allosteric modulator (PAM) inhibited behavioral and neurochemical effects of cocaine in rodents. Specifically, the M4-selective PAM VU0152100 decreased the reinforcing effects of cocaine in a single-session (acquisition) self-administration procedure, cocaine-induced locomotor activation, and cocaine-stimulated increases in extracellular striatal dopamine (Dencker et al., 2012). Combined M1/M4 receptor stimulation also decreased cocaine self-administration in rats and mice, after both acute and chronic treatment (Thomsen et al., 2014, 2012). Conversely, knockout mice lacking M4 receptors (M4−/− mice) self-administered more cocaine, and showed higher cocaine-induced increases in extracellular dopamine levels, relative to wild-type mice (Schmidt et al., 2011). Taken together, those findings indicate that M4 receptors exert an inhibitory effect on the reinforcing effects of cocaine.
Although candidate medications that reduce the direct reinforcing effects of cocaine, and maintain this effect when administered chronically, have shown the most promise in terms of reducing cocaine use clinically (Czoty et al., 2016; Haney and Spealman, 2008), there is also an interest in treatments that reduce the ability of drug-associated stimuli to trigger relapse to drug use. Medications that attenuate both the reinforcing and conditioned effects of cocaine may be the most effective. Therefore, we here extended our previous studies to test the hypothesis that selective M4 receptor stimulation can also attenuate conditioned or “subjective” effects of cocaine in addition to attenuating its reinforcing effects, using a drug discrimination assay and a conditioned place preference (CPP) assay in mice, including extinction and reinstatement tests. We previously found that combined stimulation of M1 and M4 receptors decreased the discriminative stimulus effects of cocaine, an effect that was diminished in M1−/− mice and M4−/− mice and was absent in M1−/−M4−/− double-knockout mice, indicating the involvement of both receptor subtypes (Thomsen et al., 2012, 2010). Here, we again verified the specificity of the drug discrimination effect using knockout mice lacking either the M4 receptor or the M1 receptor. In order to further strengthen confidence in the generality of the findings, we also verified that we could replicate previous results – reduction of cocaine-induced locomotor activation and dopamine efflux – using a newer, longer-acting, M4 receptor-selective PAM, VU0467154, which was then applied in the CPP studies. Since both PAMs were expected to produce comparable effects, it was not a goal of the investigation to compare the two ligands across endpoints, e.g., for drug medication development lead compound optimization or other purposes.
2. Materials and Methods
2.1. Animals
Drug discrimination studies were conducted at the McLean Hospital in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the McLean Hospital Institutional Animal Care and Use Committee. Male M1−/− and M4−/− mice bred at Taconic Farms (Germantown, NY) were generously provided by J. Wess (Gomeza et al. 1999; Miyakawa et al. 2001; backcrossed 11 generations to C57BL/6NTac), male age-matched C57BL/6NTac wild-type mice were purchased from Taconic Farms. The mice were acquired at 4–8 weeks of age and were acclimated to the housing facilities for at least 7 days before experiments began. Most mice had been tested previously with muscarinic receptor ligands (M1 agonist, scopolamine and/or other antagonists) before the tests reported here. Mice were group housed in a 12-h light/dark cycle at ~22°C. Water was accessible ad libitum and food (rodent diet 5001; PMI Feeds, Inc., St. Louis, MO) was provided daily after training/testing sessions, 4 g/mouse/day. Rodent “treats”, nesting material, and exercise/nesting devices were provided for enrichment. All testing was conducted during the light phase of the circadian cycle to allow comparison with previous studies.
All other studies were conducted at the Laboratory of Neuropsychiatry in accordance with guidelines from the Animal Experimentation Inspectorate, Denmark and the European Communities Council Directive of 24 November 1986 (86/609/EEC). Experimentally naïve male C57BL/6 mice (Taconic, Denmark) were acquired at 10 weeks of age and were acclimated to the housing facilities for at least 7 days before experiments began. Mice were group housed in cages enriched with housing and nesting material, and were kept at 22–24°C, on a reversed 12-h light/dark cycle (lights on at 7 pm), with free access to water and food. All testing was conducted during the dark phase of the circadian cycle, except the microdialysis experiment, which was conducted in the middle of the light cycle.
2.2. Apparatus
Behavioral studies were conducted in equipment from Med Associates (St Albans, VT, USA): mouse modular operant-conditioning chambers (ENV-307A) for drug discrimination studies (Thomsen et al., 2010, 2012), and open field activity arena (OFA 510) for locomotor activity and CPP studies. Both types of apparatus were individually enclosed in sound-attenuating cubicles equipped with a light, and, for the operant-conditioning chambers, a ventilation fan. The operant-conditioning chambers contained two nose-poke holes each fitted with a photocell and a yellow cue light, and a plate into which liquid food was delivered from a syringe pump.
The open field activity chambers (27 × 27 × 30 cm) fitted with a beam-break movement detection system consisting of three 16 × 16 arrays of infrared photobeams, arranged to detect movement along X, Y, and Z dimensions. For locomotor activity studies, the chambers had white floors and clear walls. For CPP studies, a partition of clear red plastic (not transparent for mice) was used to create two compartments each measuring 27 × 13.5 × 30 cm. One compartment had white walls, a smooth white floor, and a clear plastic lid, and the other compartment had black and grey striped walls, a dark Lego® plate floor, and a dark plastic lid. During habituation, testing, extinction, and reinstatement phases, the partition had a 4 × 4 cm opening allowing the mice free movement between compartments. During conditioning, the partition had no opening, constraining the mouse to the compartment it was placed in.
2.3. Drug discrimination
The experimental procedure was as previously described (Thomsen et al., 2012, 2010; Thomsen and Caine, 2016). In brief, mice were trained to discriminate 10 mg/kg cocaine from saline, under an FR 10 schedule of food reinforcement. Stable discrimination was defined as at least 7 of 8 consecutive sessions satisfying the following criteria: 1) ≥10 reinforcers earned per session, 2) ≥80% correct responses for the first reinforcer, and 3) ≥90% correct total responses. Once criteria were met, mice were tested with saline and 0.32, 1.0, 3.2, 10, and 18 mg/kg cocaine to generate dose-effect functions. In pretreatment tests, VU0152100 (0.1–3.2 mg/kg) was administered 30 min. before cocaine, in the same mice (within-subjects). Doses were tested within subjects in a pseudorandom order, counterbalanced between subjects and genotypes. At least one training session was interspersed between each test session, and tests were only performed when mice satisfied discrimination criteria.
2.4. Open Field Locomotor Activity
Mice were habituated to the test room for at least 45 min before beginning a session. Each mouse was placed in an open field chamber and was allowed to habituate for 90 min before the first injection was given, either vehicle or VU0467154 (0.3, 1 or 3 mg/kg); 30 min later, saline or cocaine (30 mg/kg) was administered, and locomotor activity was recorded for an additional 120 min. For time course analysis of the locomotor activity data, ambulation was recorded as distance travelled in meters per 10 min bin over the 4-h session. Total activity data were calculated as the total distance travelled from the time of cocaine administration (t=120 min) to the end of the experiment (t=240 min). In addition, total activity recorded from the time of cocaine administration was obtained for vertical counts (breaks of elevated beams), small movements activity counts that may represent stereotypies (beam breaks within a predefined area surrounding the mouse), ambulatory episodes (number of periods with movement), resting time (periods in-between ambulatory episodes in seconds), and velocity (speed in cm/s averaged over ambulatory episodes).
2.5. Microdialysis studies
The microdialysis methods have been described previously (Dencker et al. 2012). Briefly, mice were anaesthetized with sevoflurane, body temperature was maintained using a thermoregulatory heating pad (CMA/150 temperature controller, CMA/Microdialysis, Stockholm, Sweden), and the long-acting analgesic Metacam (5 mg/kg) was administered subcutaneously immediately before surgery. Guide cannulas were stereotaxically implanted into the brain to allow positioning of a CMA/7 microdialysis probe with 2 mm membrane length in the right ventral striatum at the coordinates AP+1, L+1.2 relative to bregma, and V-3 mm relative to dura, and the incisor bar −3.2 mm (Franklin and Paxinos 1997). Probe placement was verified histologically at the end the experiment. Mice were allowed to recover in individual cages for at least 24 hours. Then, in freely moving mice, microdialysis samples were collected in 20 min intervals using a CMA/142 microfraction collector (CMA/Microdialysis). The first three 20-min samples were discarded, and the subsequent three samples were collected for analysis of basal extracellular levels of dopamine. Subsequently, mice were injected with VU0467154 (1mg/kg) or vehicle 60 min before administration of cocaine (30 mg/kg) or saline. Concentrations of dopamine in the microdialysis samples were determined by reversed-phase high-performance liquid chromatography (RP-HPLC) with electrochemical detection as previously described (Weikop et al. 2004; Dencker et al. 2012).
2.6. Cocaine Conditioned Place Preference (CPP)
Mice were habituated to the test room for at least 30 min before beginning of the session. During habituation (day 1), pre-conditioning (days 2–4), post-conditioning test (day 11), extinction (days 13–21), and reinstatement test (day 22), mice were allowed to move freely between the two compartments for 20 min. During conditioning sessions (days 5–10), mice were confined to the white or dark compartment for 30 min. A biased design was used: all mice displayed preference for the dark compartment during pre-conditioning, and the white compartment was therefore designated as the cocaine-paired environment for all mice. During conditioning sessions, VU0467154 (1 mg/kg) or vehicle was administered 30 min before the start of the session, and cocaine (30 mg/kg) or saline was administered on alternating days, immediately before the mouse was placed in the designated compartment (total of six once-daily pairings). Control groups received saline injections paired with both compartments, and either VU0467154 (1 mg/kg) or vehicle pretreatments. This resulted in four treatment groups: vehicle pretreatment + saline conditioning (Veh+Sal), vehicle pretreatment + cocaine conditioning (Veh+Coc), VU0467154 pretreatment + saline conditioning (VU+Sal), and VU0467154 pretreatment + cocaine conditioning (VU+Coc). Mice were assigned to a treatment group stratified for time spent in the light compartment during pre-conditioning (averaged over the three days). No injections were given before the pre-conditioning or post-conditioning test sessions. On extinction days, mice were administered VU0467154 or vehicle only, 30 min before the sessions. On the reinstatement day, mice were administered VU0467154 or vehicle and cocaine or saline as during conditioning. Activity and time spent in the different compartments were recorded throughout all sessions.
During the experiment, two mice had to be euthanized; one in the vehicle-cocaine group and one in the vehicle-vehicle group, and their data were excluded from all analyses. In addition, one mouse from the vehicle-cocaine group was excluded at the pre-conditioning stage because it failed to explore, but stayed 100% of the time in one compartment (this was also confirmed as statistical outlier using Grubbs’ test).
2.7. Drugs, chemicals, and reagents
The M4 receptor-selective, brain-penetrant PAMs VU0152100 (3-amino-N-(4-methoxybenzyl)-4,6-dimethylthieno[2,3-b]pyridine carboxamide) and VU0467154 (5-amino-3,4-dimethyl-N-(4-((trifluoromethyl)sulfonyl)benzyl)thieno[2,3-c]- pyridazine-6-carboxamide) were used, both developed at Vanderbilt University (Brady et al., 2008; Bubser et al., 2014). VU0152100 was synthesized at Vanderbilt University, and was dissolved in Tween80 by gentle heating and diluted to 5% Tween in sterile water (prepared fresh daily). VU0467154 was purchased from Regency (Australia) and was similarly prepared in 2% Tween in saline (referred to as “vehicle” in the rest of the manuscript). Cocaine hydrochloride was supplied by the National Institute on Drug Abuse, National Institutes of Health (Bethesda, MD; McLean studies) or purchased from the Copenhagen University Hospital pharmacy (Copenhagen, Denmark; Copenhagen studies), and was dissolved in 0.9% saline. All drugs were administered intraperitoneally in a volume of 10 ml/kg, except for the microdialysis experiment, in which cocaine/saline was administered subcutaneously.
For microdialysis, dopamine HCl was obtained from Sigma (St. Louis, MO, USA). Acetonitrile and all other chemicals were purchased from Merck (Darmstadt, Germany) and Fluka Chemie (Buchs, Switzerland). Deionized water was obtained from a MiliQ water purification system (Milipore, MA, USA).
2.8. Data Analysis
For drug discrimination, percent cocaine-appropriate responding and response rate (responses/s) were analyzed by two-way ANOVA with cocaine dose and VU0152100 dose as repeated-measures factors, within each genotype. If responding was suppressed to fewer than 10 responses, the quantity of behavior was considered insufficient to evaluate the percentage of cocaine-appropriate responses and that calculation was not included in the data presentation or analysis. This occurred for four data points: two with 18 mg/kg cocaine alone, and two in the VU0152100 dose-finding experiment. Therefore, in addition to ANOVA analysis (which is susceptible to loss of power due to missing values), A50 values were calculated, i.e., the dose of cocaine estimated to produce 50% cocaine-appropriate responding. A50 values were obtained in each mouse by interpolation of the dose-effect curves, and group means and 95% confidence intervals were calculated. The effect of VU0152100 pretreatment on A50 values was assessed in each genotype by two-tailed, paired-sample t-test.
For microdialysis data, basal extracellular levels of dopamine were calculated in each group as the mean of three fractions collected before the drug or vehicle administration, and were set to 100%. Other values were expressed on a relative scale as percentage of basal levels. Open field total-session activity measures (total distance traveled, ambulatory episodes, stereotypic and vertical counts, resting time, velocity) were each analyzed by one-way ANOVA with drug treatment as between-subjects factor, followed if significant by Bonferroni’s multiple comparisons test. Distance traveled per 10-min bin, percent-transformed dopamine levels, and time spent in the cocaine-paired compartment, were analyzed by two-way ANOVA with time/test day as a repeated-measures factor and treatment group as between-subjects factor. Analyses were conducted using GraphPad Prism 6 or StataSE 13. Data are presented as group means ± one SEM, significance level was set at P<0.05.
3. Results
3.1. Cocaine discrimination
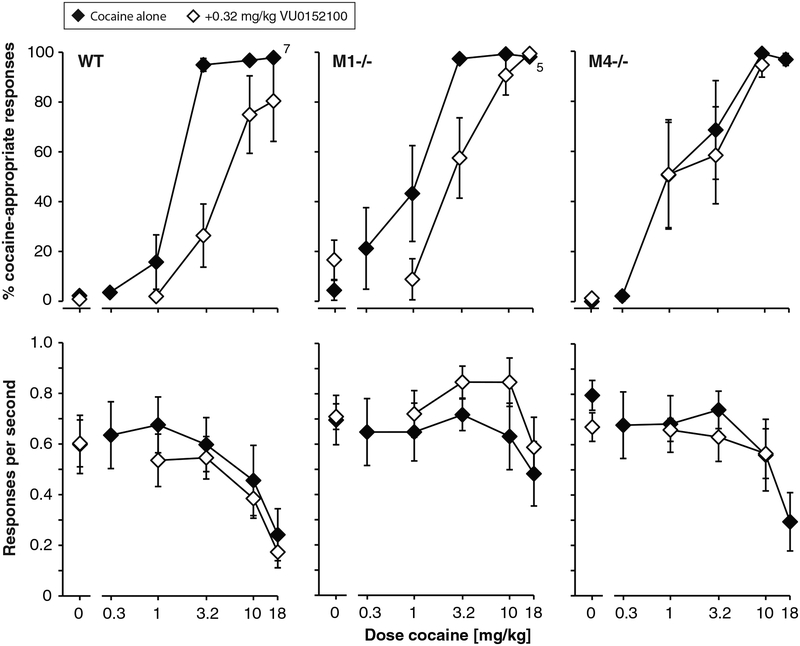
The percentages of cocaine-appropriate responding for the whole session are presented, but comparable effects were observed in percent cocaine-appropriate responding for the first reinforcer. Initially, dose-finding studies were conducted in wild-type mice (n=7), M1−/− mice (n=6), and M4−/− mice (n=6), with 0.1–3.2 mg/kg VU0152100 tested as a pretreatment to 10 mg/kg cocaine (data not shown). In wild-type mice, VU0152100 reduced cocaine-appropriate responding at intermediate doses with the most effective dose varying between subjects, creating a shallow U shaped curve. Four of seven wild-type mice showed decreases to <5% cocaine-appropriate responding. VU0152100 had no effect in the M4−/− mice (none showed less than 70% cocaine-appropriate responding at any dose), while the effect was more variable in M1−/− mice. VU0152100 did not significantly affect rates of responding in any genotype.
Based on those data, a dose of 0.32 mg/kg VU0152100 was selected for further testing, as pretreatment to a range of cocaine doses (Figure 1). Percent cocaine-appropriate responding increased as function of cocaine dose in all comparisons (all P<0.0001). VU0152100 pretreatment shifted the cocaine discrimination curve to the right and/or down in the wild-type mice (Figure 1A), supported by a significant effect of pretreatment [F(1,60)=19.0, P<0.0001] and a pretreatment by cocaine dose interaction [F(4,60)=4.32, P<0.01]. VU0152100 similarly shifted the cocaine curve to the right in the M1−/− mice (Figure 1B), with a significant effect of pretreatment [F(1,44)=6.16, P<0.05] and a significant pretreatment by cocaine dose interaction [F(4,44)=3.25, P<0.05]. In contrast, in M4−/− mice, cocaine-appropriate responding was related only to cocaine, with no effect of VU0152100 pretreatment (Figure 1C). Measured as changes in A50 values, VU0152100 pretreatment also decreased the potency of cocaine as a discriminative stimulus (i.e., increased A50 values) significantly in the wild-type mice and in the M1−/− mice, but had no effect in the M4−/− mice (see Table 1). Rates of responding were related to cocaine dose, but were not significantly modified by VU0152100 pretreatment in any genotype.
Figure 1. Effect of pretreatment with 0.32 mg/kg VU0152100 on the discriminative stimulus effect of cocaine.
Percent cocaine-appropriate responding as a function of cocaine dose, in wild-type mice (WT, panel A), M1−/− mice (B), and M4−/− mice (C). Abscissae: dose cocaine in mg/kg; ordinates: percentage cocaine-appropriate responses (top); response rate in responses per second (bottom). Data are groups means ± one S.E.M. Group sizes: wild-type mice: n=8, M1−/− mice and M4−/− mice: n=6.
Table 1.
M4 PAM-induced shifts in cocaine dose-effect functions
| A50 cocaine alone | A50 +VU0152100 | Group size | |
|---|---|---|---|
| Wild-type | 1.51 [1.16 – 1.97] | 5.69 [3.62 – 8.96] *** | 8 |
| M1−/− | 0.89 [0.43 – 1.86] | 3.47 [1.81 – 6.66] *** | 6 |
| M4−/− | 1.46 [0.60 – 3.55] | 1.53 [0.59 – 3.97] | 6 |
Values are group means, with 95% confidence limits indicated in bracket.
P<0.001 vs. cocaine alone, paired-sample two-tailed t-test, calculated on log values.
3.2. Locomotor activity in the open field arena
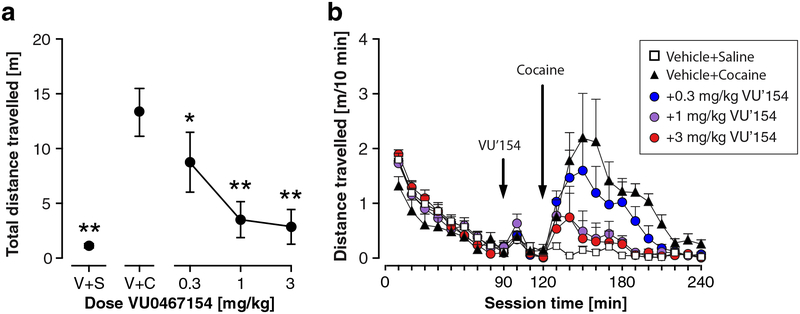
Locomotor activity, measured as total distance travelled, differed significantly by treatment group [F(4,35)=19.6, P<0.001] (Figure 2A). Specifically, cocaine (30 mg/kg) induced a significant increase in locomotor activity compared to Vehicle+Saline (P<0.01), which was significantly reduced by pretreatment with VU0467154 (0.3–3 mg/kg, P<0.01 to P<0.05). Measured as 10-min bins over the course of the session, cocaine significantly increased locomotor activity compared to Vehicle+Saline 140–200 min after session start, i.e., 20–80 min after cocaine administration (P<0.0001 to P<0.01; Figure 2B). This cocaine-induced hyperactivity was reduced by VU0467154 at 1 and 3 mg/kg, 140–200 min after session start (P<0.0001 to P<0.05) and for 0.3 mg/kg VU0467154, 150–160 min after session start (all P<0.01). A two-way repeated measure ANOVA confirmed significant effects of treatment [F(4,35) =5.24, P<0.001] and time [F(23,805)=21.8, P<0.0001], and significant treatment by time interaction [F(92,805)=2.86, P<0.0001].
Figure 2. Effect of pretreatment with VU0467154 on cocaine-stimulated locomotor activity.
(A) Total distance travelled after cocaine administration. V+S: vehicle +saline, V+C: vehicle + 30 mg/kg cocaine, other points: doses of VU0467154 + cocaine. *P<0.05; **P<0.01 vs. the vehicle+cocaine group, Bonferroni’s multiple comparisons test after significant ANOVA. (B) Distance as a function of time. Abscissa: time in minutes; ordinate: distance travelled in meters. Group sizes: n=8.
Other parameters in the open field (120–240 min) were analyzed as well (Table 2). Cocaine increased ambulatory episodes relative to Vehicle+Saline, and VU0467154 attenuated this increase, reaching significance at 1 and 3 mg/kg (main effect of treatment group: [F(4,35)=6.49, P<0.001], see Table 2 for post-hoc significance levels). The same was observed for small movements that may represent stereotypic activity ([F(4,35)=13.9, P<0.0001, Table 2). Conversely, cocaine caused a decrease in resting time, which was attenuated by VU0467154 pretreatment ([F(4,35)=17.1, P<0.0001] and Table 2). No significant differences were found in velocity, or in vertical activity counts (representing rearing or jumps), neither as a function of cocaine nor VU0467154 administration.
Table 2.
Additional activity measures in the open field activity monitoring arena
| Saline+Vehicle | Cocaine+Vehicle | Cocaine + doses of VU0467154 | |||
|---|---|---|---|---|---|
| 0.3 mg/kg | 1 mg/kg | 3 mg/kg | |||
| Ambulatory episodes | 53.0 ± 10.1 ** | 582.4 ± 82.4 | 393.5 ± 129 | 169.6 ± 83.0 * | 131.9 ± 77.2 ** |
| “Stereotypic” counts | 4417 ± 672.2 *** | 16155 ± 799. | 10610 ± 1303 | 8626 ± 1874 ** | 5945 ± 1136 *** |
| Vertical counts | 115.3 ± 67.7 | 277.6 ± 87.5 | 162.5 ± 83.5 | 228.9 ± 89.2 | 160.3 ± 59.8 |
| Resting time | 7308 ± 66.7 *** | 5908 ± 87.7 | 6523 ± 191.4 * | 6882 ± 147.4 *** | 7123 ± 139.7 *** |
| Velocity | 323.3 ± 47.8 | 298.2 ± 13.4 | 221.9 ± 27.9 | 279.0 ± 48.9 | 288.5 ± 43.3 |
Data represent total-session counts after cocaine administration, as group means ± one SEM.
P<0.05,
P<0.01,
P<0.001 vs. cocaine+vehicle, Bonferroni’s multiple comparisons test after significant ANOVA. Group sizes: n=8.
3.3. Dopamine microdialysis
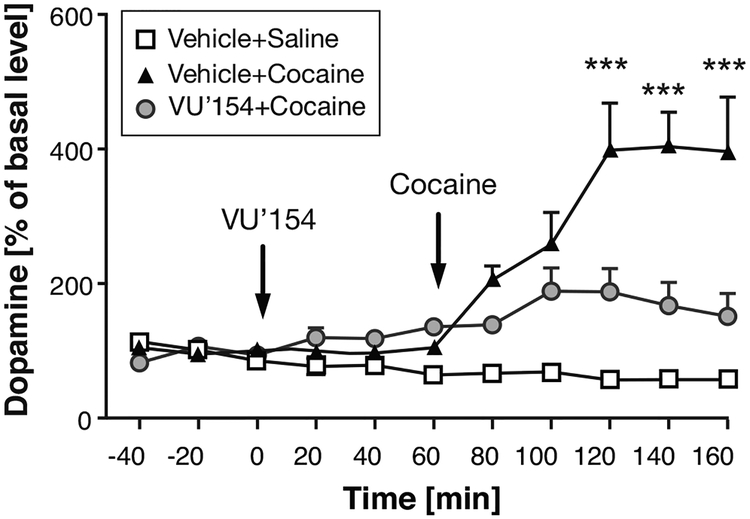
Systemic administration of 30 mg/kg cocaine produced a 4-fold increase in extracellular striatal dopamine levels, which was significantly blunted by 1 mg/kg VU0467154 (dose selected based on locomotor activity results) relative to vehicle-pretreated mice (Figure 3). ANOVA confirmed a significant effect of time [F(10,170)=7.80, P<0.0001], and treatment [F(2,170)=9.12, P<0.01], with a significant time by treatment interaction [F(20,170)=5.57, P<0.0001]. Posthoc analysis revealed that dopamine levels were significantly reduced in the VU0467154+Cocaine group relative to the Vehicle+Cocaine group at 120–160 min after VU0467154 administration. Comparisons of the dopamine levels in the VU0467154+Cocaine group relative to Vehicle+Saline did not reach significance at any time point.
Figure 3. Effect of pretreatment with 1 mg/kg VU0467154 on cocaine-stimulated increases in extracellular dopamine in the ventral striatum.
Abscissa: time in minutes; ordinate: dopamine level as %basal level. ***P<0.001 VU0467154+cocaine vs. vehicle+cocaine, Bonferroni’s multiple comparisons test after significant ANOVA. Group sizes: VU0467154+cocaine, n=13; vehicle+cocaine, n=4, vehicle+saline, n=3.
3.4. Conditioned place preference
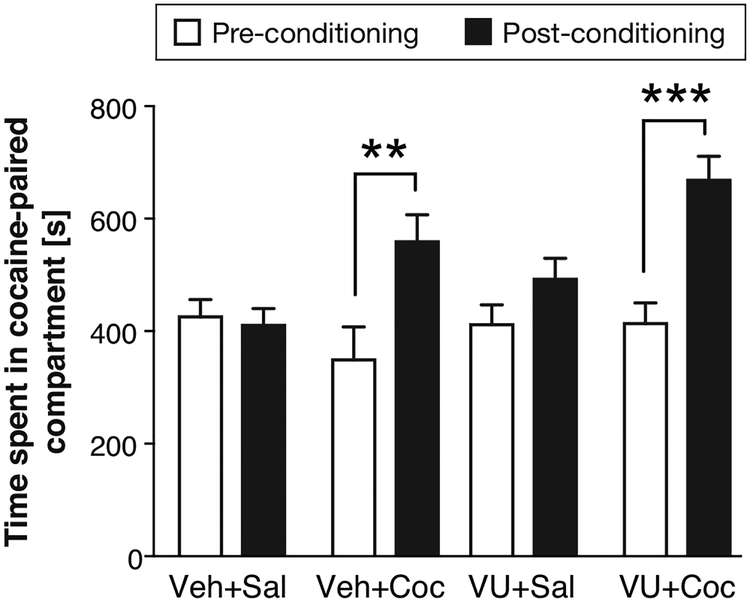
Cocaine produced place preference, as measured by an increase in time spent in the cocaine-paired compartment post-conditioning relative to pre-conditioning, in both vehicle-pretreated mice (P<0.01, Bonferroni post hoc analysis, Figure 4) and VU0467154-pretreated mice (P<0.001). Two-way ANOVA showed a main effect of conditioning phase [F(1,41)=44.3; P<0.0001] and a significant treatment by conditioning interaction [F(3,41)=9.54; P<0.0001], while the main effect of treatment just missed significance (P=0.052). There was no significant difference between the two cocaine-conditioned groups in the time spent in the cocaine-paired compartment in the post-conditioned test, i.e., 1 mg/kg VU0467154 did not attenuate cocaine CPP. The saline conditioned groups also did not differ significantly, and there was no indication that VU0467154 alone caused either place preference or aversion.
Figure 4. Effect of pretreatment with 1 mg/kg VU0467154 on cocaine-conditioned place preference (CPP).
Ordinate: time spent in the cocaine-paired compartment, in seconds. **P<0.01, ***P<0.001 post-conditioning vs. pre-conditioning, Bonferroni’s multiple comparisons test after significant ANOVA. Group sizes n=10–12.
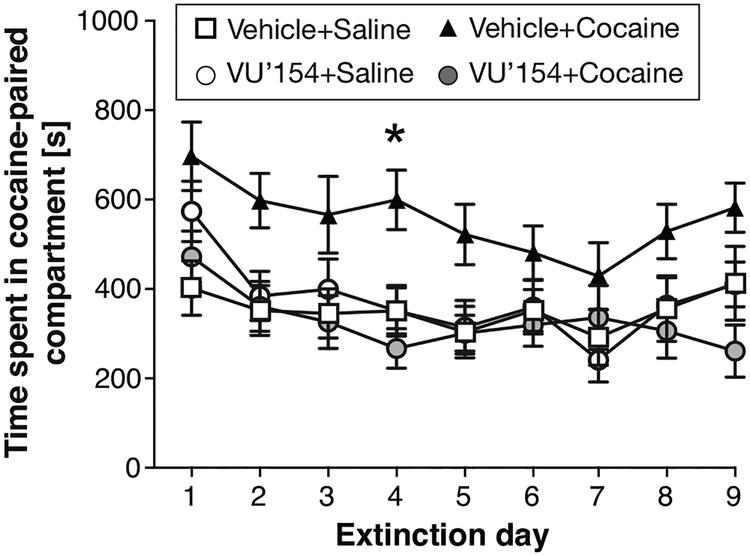
Following post-conditioning test, extinction was initiated (Figure 5). Administration of vehicle or VU0467154 (1 mg/kg) was continued throughout the extinction phase, but cocaine was never administered. Time in the cocaine-paired compartment declined slowly over days, and the Veh+Coc group appeared to spend more time in the cocaine-paired compartment throughout extinction; a two-way repeated measure ANOVA confirmed that both treatment group [F(3,41)=3.85; P<0.05] and time [F(8,328)=10.6; P<0.0001] affected behavior, with no significant interaction. Post-hoc, the Veh+Coc to VU+Coc comparison reached statistical significance on extinction day 4 (P<0.05). The Veh+Coc group also spent significantly more time in the cocaine-paired compartment compared to the VU+Sal group, on days 2, 3, 4 and 9 (P<0.01 to P<0.05), and compared to the Veh+Sal group, on days 1, 2, and 4 (P<0.01 to P<0.05; significance levels not shown in Figure 5 to avoid crowding).
Figure 5. Effect of pretreatment with 1 mg/kg VU0467154 on the extinction of cocaine CPP.
Ordinate and group size as in Figure 4. *P<0.05 Veh+Coc vs. VU+Coc, Bonferroni’s multiple comparisons test after significant ANOVA (see text for additional significant comparisons).
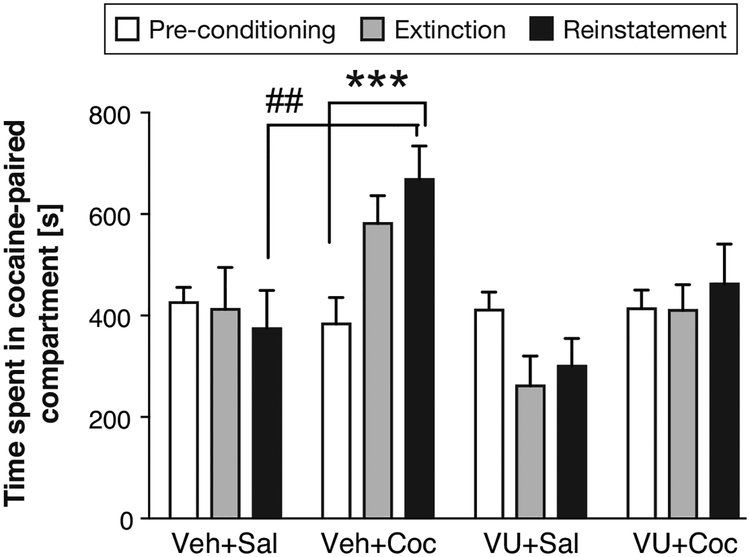
After nine extinction days, we tested whether CPP could be reinstated, and whether VU0467154 could affect reinstatement (Figure 6). VU0467154 or vehicle pretreatment was administered as previously, and mice were given a single injection of cocaine (10 mg/kg) or saline before the session, as during conditioning. Time spent in the cocaine-paired compartment was related to treatment group [F(3,41)=3.61; P<0.05], with a significant interaction of treatment and test day (i.e., reinstatement vs. pre-conditioning vs. last day of extinction) [F(6,82)=4.67; P<0.001], while the main effect of test day was not significant. Compared to the Veh+Sal group, only the Veh+Coc group spent more time in the cocaine-paired compartment during reinstatement (P<0.01). Furthermore, only the Veh+Coc group spent significantly more time in the cocaine-paired compartment during the reinstatement test compared to its own pre-conditioning level (P<0.001). Time spent in the cocaine-paired compartment during reinstatement was not significantly higher compared to the last day of extinction, indicating that either incomplete extinction or reinstatement could account for the effect.
Figure 6. Effect of pretreatment with 1 mg/kg VU0467154 on the reinstatement of cocaine CPP.
Ordinate and group size as in Figure 4. ##P<0.01 Veh+Coc vs. Veh+Sal; ***P<0.001 reinstatement vs. pre-conditioning, Bonferroni’s multiple comparisons test after significant ANOVA.
4. Discussion
We previously showed that M1-selective agonists/PAMs and the M1/M4-preferring agonist xanomeline attenuated the discriminative stimulus effect of cocaine, and studies in knockout mice indicated that both M1 and M4 receptors mediated the effect of xanomeline (Thomsen et al., 2012, 2010). Here, we directly tested the hypothesis that selective stimulation of M4 receptors would similarly attenuate cocaine’s subjective effects. VU0152100 produced an almost 4-fold rightward shift in the cocaine discrimination curve in both wild-type mice and M1−/− mice, but had no effect in M4−/− mice, confirming that M4 receptors were necessary to mediate the effect, with no apparent involvement of M1 receptors. VU0152100 did not suppress food-maintained responding at effective doses. As previously observed for M1 agonists, the attenuation of cocaine’s discriminative stimulus effect by VU0152100 was of smaller magnitude than the reduction in cocaine’s reinforcing effects, as both VU0152100 and M1 agonists reduced cocaine self-administration to saline intake levels (Dencker et al., 2012; Thomsen et al., 2010). The large effect in self-administration is more directly relevant to the addictive properties of cocaine, because discriminative stimulus effects are not limited to “positive” effects, but encompass all perceivable sensations produced by cocaine (e.g., cardiovascular, anxiogenic, jitteriness).
We then used the longer-acting M4 PAM VU0467154 to further substantiate our previous findings that VU0152100 attenuated (almost abolished) cocaine-induced locomotor hyperactivity and cocaine-induced increases in extracellular striatal dopamine levels (Dencker et al., 2012). The two PAMs have shown comparable effects with no obvious differences. Both VU0152100 and VU0467154 also attenuated amphetamine-induced hyperactivity in rats and wild-type mice, but not in M4−/− mice (Brady et al., 2008; Bubser et al., 2014; Byun et al., 2014), confirming that both PAMs work through M4 receptors. Those data also indicate that M4 receptor stimulation is equally effective at attenuating effects of monoamine reuptake inhibitors and releasers, which is important in terms of potential clinical applications. In the present study, qualitative aspects of activity were evaluated in more detail than previously. Neither cocaine nor VU0467154 affected velocity, consistent with the interpretation that VU0467154 specifically inhibited the cocaine-induced increases in activity, rather than producing sedation or impairing motor function (see also Brady et al. 2008; Dencker et al. 2012; Bubser et al. 2014 for lack of effect on the rotarod test).
Finally, we tested the hypothesis that M4 receptor stimulation would attenuate the rewarding effects of cocaine. VU0467154, at a dose that was effective in the locomotor activity and microdialysis experiments, did not attenuate acquisition of classical cocaine conditioning. This is perhaps surprising given that VU0467154 reduced striatal dopamine to levels statistically not different from no-cocaine. It is possible that higher doses of VU0467154 would have prevented acquisition of cocaine CPP, and it would be of interest to test whether an M4 PAM can suppress the expression of cocaine CPP after acquisition, i.e. administered on the test day rather than during conditioning. VU0467154 appeared to facilitate extinction and prevent reinstatement of CPP. The vehicle-pretreated mice failed to completely extinguish to pre-conditioning levels, differing from previous experiments in C57BL/6 mice in our laboratory, but consistent with slow extinction typically observed with this strain in cocaine self-administration experiments (Thomsen and Caine, 2011, 2006; Ward et al., 2009). Interestingly, M4−/− mice showed protracted extinction of cocaine self-administration, consistent with M4 receptors promoting extinction (Schmidt et al., 2011). Whether the vehicle-pretreated mice showed actual reinstatement or simply failed to extinguish, taken together, the data suggest that the M4 PAM weakened the conditioned effects of cocaine, further supporting the potential therapeutic usefulness of M4 PAMs.
M4 receptors are widely expressed in the brain (Levey et al., 1991; Vilaró et al., 1991), and precisely how the M4 PAMs modulate cocaine effects is still unclear, but striatal M4 receptors are most likely key mediators. Indeed, infusion of the non subtype-selective muscarinic agonist oxotremorine into the nucleus accumbens reduced cocaine self-administration in rats (Mark et al., 2006). Striatal M4 receptors are expressed on glutamatergic cortical/thalamic input projections, cholinergic interneurons, and medium spiny neurons (MSNs), predominantly on the dopamine D1 receptor-expressing direct pathway neurons (Ince et al., 1997; Weiner et al., 1990; Yan et al., 2001). A combination of pharmacological, electrophysiological, optogenetic, and genetic (knockout) approaches showed that presynaptic M4 receptor stimulation modulates corticostriatal glutamatergic transmission to both direct- and indirect-pathway MSNs (Pancani et al., 2014). Striatal dopamine release is inhibited by M4 receptors on direct-pathway MSNs through endocannabinoid signaling (Foster et al., 2016), and modulated by M4 receptors on cholinergic interneurons, via nicotinic cholinergic signaling (Bonsi et al., 2008; Shin et al., 2015; Threlfell et al., 2010; Threlfell and Cragg, 2011). Finally, postsynaptic M4 receptors on MSNs are thought to act as a functional antagonist of D1 receptor-mediated cyclic AMP-dependent signaling (Jeon et al., 2010; Onali and Olianas, 2002). All those mechanisms may contribute to the observed reduction of neurochemical and behavioral effects of cocaine by M4 PAMs, and future studies are required to determine the relative importance of each receptor population/mechanism. While antipsychotic effects of M4 receptor stimulation appear to be mediated overwhelmingly through D1-expressing direct-pathway MSNs (Dencker et al., 2011; Foster et al., 2016), inhibition of the abuse-related effects of cocaine appear to depend only partially on D1-expressing neurons, suggesting the involvement of other receptor populations as well (Dencker et al., 2012).
In conclusion, we further substantiated our findings on the “cocaine-blocking” effects of M4 receptor stimulation using a different ligand. We also extended findings to show that in addition to blocking the direct reinforcing effects of cocaine, M4 PAMs can blunt the subjective effects of cocaine. Surprisingly, M4 receptor failed to prevent acquisition of cocaine conditioning, but appeared to weaken the conditioning, facilitating extinction and preventing reinstatement of the conditioned response. Taken together, these findings further support the potential usefulness of targeting M4 receptors in the treatment of cocaine addiction.
Acknowledgments
This study was funded by the Psychiatric Center Copenhagen, and grant DA027825 from the National Institute on Drug Abuse, National Institutes of Health (MT). Discovery and characterization of the PAMs was supported by the Molecular Libraries Probe Production Centers Network (U54MH084659, PJC). We thank Professor Jurgen Wess, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, for providing the knockout mice. We thank Jeffrey Wessell, Lauren Joseph, Anne-Marie Paulsen, and Saiy Kiasari for technical assistance.
References
- Bonsi P, Martella G, Cuomo D, Platania P, Sciamanna G, Bernardi G, Wess J, Pisani A, 2008. Loss of Muscarinic Autoreceptor Function Impairs Long-Term Depression But Not Long-Term Potentiation in the Striatum. J. Neurosci 28, 6258–6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AE, Jones CK, Bridges TM, Kennedy JP, Thompson AD, Heiman JU, Breininger ML, Gentry PR, Yin H, Jadhav SB, Shirey JK, Conn PJ, Lindsley CW, 2008. Centrally Active Allosteric Potentiators of the M4 Muscarinic Acetylcholine Receptor Reverse Amphetamine-Induced Hyperlocomotor Activity in Rats. J. Pharmacol. Exp. Ther 327, 941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubser M, Bridges TM, Dencker D, Gould RW, Grannan M, Noetzel MJ, Lamsal A, Niswender CM, Daniels JS, Poslusney MS, Melancon BJ, Tarr JC, Byers FW, Wess J, Duggan ME, Dunlop J, Wood MW, Brandon NJ, Wood MR, Lindsley CW, Conn PJ, Jones CK, 2014. Selective Activation of M 4 Muscarinic Acetylcholine Receptors Reverses MK-801-Induced Behavioral Impairments and Enhances Associative Learning in Rodents. ACS Chem. Neurosci 5, 920–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun NE, Grannan M, Bubser M, Barry RL, Thompson A, Rosanelli J, Gowrishankar R, Kelm ND, Damon S, Bridges TM, Melancon BJ, Tarr JC, Brogan JT, Avison MJ, Deutch AY, Wess J, Wood MR, Lindsley CW, Gore JC, Conn PJ, Jones CK, 2014. Antipsychotic Drug-Like Effects of the Selective M4 Muscarinic Acetylcholine Receptor Positive Allosteric Modulator VU0152100. Neuropsychopharmacology 39, 1578–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Stoops WW, Rush CR, 2016. Evaluation of the “Pipeline” for Development of Medications for Cocaine Use Disorder: A Review of Translational Preclinical, Human Laboratory, and Clinical Trial Research. Pharmacol. Rev 68, 533–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencker D, Weikop P, Sørensen G, Woldbye DPD, Wörtwein G, Wess J, Fink-Jensen A, 2012. An allosteric enhancer of M4 muscarinic acetylcholine receptor function inhibits behavioral and neurochemical effects of cocaine. Psychopharmacology (Berl) 224, 277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencker D, Wörtwein G, Weikop P, Jeon J, Thomsen M, Sager TN, Mørk A, Woldbye DPD, Wess J, Fink-Jensen A, 2011. Involvement of a subpopulation of neuronal M4 muscarinic acetylcholine receptors in the antipsychotic-like effects of the M1/M4 preferring muscarinic receptor agonist xanomeline. J. Neurosci 31, 5905–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Wilson JM, Remke DH, Mahmood MS, Uddin MJ, Wess J, Patel S, Marnett LJ, Niswender CM, Jones CK, Xiang Z, Lindsley CW, Rook JM, Conn PJ, 2016. Antipsychotic-like Effects of M4 Positive Allosteric Modulators Are Mediated by CB2 Receptor-Dependent Inhibition of Dopamine Release. Neuron 91, 1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KJB.; Paxinos G, 1997. The mouse brain in stereotaxic coordinates Academic Press, New York. [Google Scholar]
- Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C.-x., Wess J, 1999. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M4 muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci 96, 10483–10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Spealman R, 2008. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 199, 403–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince E, Ciliax BJ, Levey AI, 1997. Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse 27, 357–366. [DOI] [PubMed] [Google Scholar]
- Jeon J, Dencker D, Wörtwein G, Woldbye DPD, Cui Y, Davis AA, Levey AI, Schütz G, Sager TN, Mørk A, Li C, Deng C-X, Fink-Jensen A, Wess J, 2010. A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors. J. Neurosci 30, 2396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse AC, Kobilka BK, Gautam D, Sexton PM, Christopoulos A, Wess J, 2014. Muscarinic acetylcholine receptors: novel opportunities for drug development. Nat. Rev. Drug Discov 13, 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey A, Kitt C, Simonds W, Price D, Brann M, 1991. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J. Neurosci 11, 3218–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark GP, Kinney AE, Grubb MC, Zhu X, Finn DA, Mader SL, Berger SP, Bechtholt AJ, 2006. Injection of oxotremorine in nucleus accumbens shell reduces cocaine but not food self-administration in rats. Brain Res 1123, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Yamada M, Duttaroy A, Wess J, 2001. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J. Neurosci 21, 5239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickols HH, Conn PJ, 2014. Development of allosteric modulators of GPCRs for treatment of CNS disorders ☆. Neurobiol. Dis 61, 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onali P, Olianas MC, 2002. Muscarinic M4 receptor inhibition of dopamine D1-like receptor signalling in rat nucleus accumbens. Eur. J. Pharmacol 448, 105–111. [DOI] [PubMed] [Google Scholar]
- Pancani T, Bolarinwa C, Smith Y, Lindsley CW, Conn PJ, Xiang Z, 2014. M4 mAChR-mediated modulation of glutamatergic transmission at corticostriatal synapses. ACS Chem. Neurosci 5, 318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LS, Thomsen M, Weikop P, Dencker D, Wess J, Woldbye DPD, Wortwein G, Fink-Jensen A, 2011. Increased cocaine self-administration in M4 muscarinic acetylcholine receptor knockout mice. Psychopharmacology (Berl) 216, 367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Adrover MF, Wess J, Alvarez VA, 2015. Muscarinic regulation of dopamine and glutamate transmission in the nucleus accumbens. Proc. Natl. Acad. Sci. U. S. A 112, 8124–8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DM, Sun B, Feng D, Nawaratne V, Leach K, Felder CC, Bures MG, Evans DA, Weis WI, Bachhawat P, Kobilka TS, Sexton PM, Kobilka BK, Christopoulos A, 2016. Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature 531, 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB, 2016. Effects of dopamine D1-like and D2-like antagonists on cocaine discrimination in muscarinic receptor knockout mice. Eur. J. Pharmacol 776, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB, 2011. False positive in the intravenous drug self-administration test in C57BL/6J mice. Behav. Pharmacol 22, 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB, 2006. Cocaine self-administration under fixed and progressive ratio schedules of reinforcement: comparison of C57BL/6J, 129X1/SvJ, and 129S6/SvEvTac inbred mice. Psychopharmacology (Berl) 184, 145–154. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Conn PJ, Lindsley C, Wess J, Boon JY, Fulton BS, Fink-Jensen A, Caine SB, 2010. Attenuation of Cocaine’s Reinforcing and Discriminative Stimulus Effects via Muscarinic M1 Acetylcholine Receptor Stimulation. J. Pharmacol. Exp. Ther 332, 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Fulton BS, Caine SB, 2014. Acute and chronic effects of the M1/M4-preferring muscarinic agonist xanomeline on cocaine vs. food choice in rats. Psychopharmacology (Berl) 231, 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Lindsley CW, Conn PJ, Wessell JE, Fulton BS, Wess J, Caine SB, 2012. Contribution of both M1 and M4 receptors to muscarinic agonist-mediated attenuation of the cocaine discriminative stimulus in mice. Psychopharmacology (Berl) 220, 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Clements MA, Khodai T, Pienaar IS, Exley R, Wess J, Cragg SJ, 2010. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J. Neurosci 30, 3398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Cragg SJ, 2011. Dopamine signaling in dorsal versus ventral striatum: the dynamic role of cholinergic interneurons. Front. Syst. Neurosci 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilaró MT, Wiederhold K-H, Palacios JM, Mengod G, 1991. Muscarinic cholinergic receptors in the rat caudate-putamen and olfactory tubercle belong predominantly to the m4 class: In situ hybridization and receptor autoradiography evidence. Neuroscience 40, 159–167. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Rosenberg M, Dykstra LA, Walker EA, 2009. The CB1 antagonist rimonabant (SR141716) blocks cue-induced reinstatement of cocaine seeking and other context and extinction phenomena predictive of relapse. Drug Alcohol Depend 105, 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikop P, Egestad B, Kehr J, 2004. Application of triple-probe micro- dialysis for fast pharmacokinetic/pharmacodynamic evaluation of dopamimetic activity of drug candidates in the rat brain. J Neurosci Methods 140, 59–65. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR, 1990. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc. Natl. Acad. Sci. U. S. A 87, 7050–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Flores-Hernandez J, Surmeier D., 2001. Coordinated expression of muscarinic receptor messenger RNAs in striatal medium spiny neurons. Neuroscience 103, 1017–1024. [DOI] [PubMed] [Google Scholar]