Abstract
Cancer vaccines, including peptide-based vaccines, have been considered a key tool of effective and protective cancer immunotherapy because of their capacity to provide long-term clinical benefit for tumors. Among a large number of explorations of peptide antigen-based vaccines, cancer-testis antigens (CTAs), which are activated in cancers but silenced in normal tissues (except testis tissue), are considered as ideal targets. Currently, personalized treatment for cancer has become a trend due to its superior clinical efficacy. Thus, we envisage rational selection of CTA peptides to design “personalized” CTA peptide vaccines. This review summarizes the advances in CTA peptide vaccine research and discusses the feasibility of establishing “personalized” CTA peptide vaccines.
The field of cancer vaccines, including peptide-based vaccines, has moved forward drastically during the last 2-3 decades after the demonstration that in both animal models and, later, in patients, it is possible to generate antitumor immune responses [1]. Naive T cells can be induced to proliferate and be activated by antigen-presenting cells, particularly dendritic cells (DCs), which present tumor antigens via the major histocompatibility complex (MHC). As T cells recognize and kill tumor cells that express these antigens on their surface, selecting appropriate tumor antigens as targets is crucial for the preparation of peptide vaccines.
Tumor antigens have been grouped into three categories: tumor-associated antigens (TAAs); cancer-specific antigens, which are also called neoantigens; and cancer-testis antigens (CTAs) [2]. The unfavorable tumor specificity of TAAs carries the risk of inducing autoimmunity against corresponding normal tissues. The neoepitopes generated by somatic mutations can be recognized by T cells and therefore are regarded as ideal cancer vaccine targets [3]. Nonetheless, each tumor has a unique combination of mutations, with only a small fraction shared among cases [4], resulting in the difficulty of identifying neopeptides and preparing mutanome vaccines. In contrast, as CTAs are normally expressed in the testis but are also highly expressed across cancers [5], [6] and associated with disease stage, an unfavorable prognosis, and cancer invasion, CTAs may constitute potentially promising targets and allow convenient establishment of vaccines based on “off-the-shelf” CTA peptides. This review summarizes the recent advances in CTA peptide-based cancer vaccines. In particular, we describe the details of our novel immunotherapeutic approach, called the CTA personalized peptide vaccine.
“Conventional” CTA Peptide Vaccines
Accumulating experimental evidence favors CTAs as highly suitable antigens for cancer vaccination. Compared to DC vaccines, peptide vaccines do not require the use of autologous cells, which has resulted in strong interest in CTA peptide vaccines in recent years. However, the complexity and diversity of cancer antigens and tumor cell characteristics seem to limit the clinical application of this knowledge. Below, we discuss the progress thus far in the identification of CTA immunogenic peptides and the clinical trials of peptide vaccines, as summarized in Table 1, Table 2. In addition, advances in developing effective cancer peptide vaccines are reviewed.
Table 1.
Peptide-Based Vaccination Trials
| Phase | Indication | CTAs | Year of Publication |
|---|---|---|---|
| I | Prostate cancer | NY-ESO-1 | 2014 |
| I | Advanced malignancies | NY-ESO-1 | 2007 |
| I | Neuroblastoma and sarcoma | MAGE-A1, MAGE-A3, NY-ESO-1 | 2015 |
| II | Metastatic melanoma | MAGE-A3, GP100, Tyrosinase, MAGE-A2, MAGEA1 | 2012 |
| III | NSCLC | MAGE-A3 | 2009 |
| III | Melanoma skin cancer | MAGE-A3 | 2010 |
| II | Gastric cancer | DEPDC1, URLC10, FoxM1, Kif20A, VEGFR1 | 2017 |
| I/II | Urothelial carcinoma of the bladder | DEPDC1, MPHOSPH1 | 2016 |
| I/II | Esophageal squamous cell carcinoma | TTK, LY6K, IMP3 | 2009/2012 |
| I | Gastric cancer | LY6K | 2014 |
| I | Gastric cancer | URLC10, VEGFR1 | 2014 |
| I | NSCLC | CDCA1, LY6K, VEGFR1, VEGFR2 | 2013 |
| I | Pancreatic cancer | CDCA1, Kif20A, VEGFR1, VEGFR2 | 2013 |
| II | HNSCC | CDCA1, LY6K, IMP3 | 2015 |
| I | ESCC | TTK, URLC10, KOC1, VEGFR1, VEGFR2 | 2014 |
| I | Advanced solid cancer | KOC1, TTK, URLC10, DEPDC1, MPHOSPH1 | 2016 |
Table 2.
Previously Identified CTA Peptides
| CTAs | Amino Acid Sequence (mer) | Start Position | HLA Type |
|---|---|---|---|
| NY-ESO-1 | SLLMWITQC | 157 | HLA-A*0201 |
| YLAMPFATPME | 91 | HLA-A*2402 | |
| LLMWITQCF | 158 | HLA-A*2402 | |
| MAGE-A3 | FLWGPRALV | 271 | HLA-A*0201 |
| KVAELVHFL | 112 | HLA-A*0201 | |
| TFPDLESEF | 97 | HLA-A*2402 | |
| VAELVHFLL | 113 | HLA-A*2402 | |
| DEPDC1 | FLDLPEPLL | 282 | HLA-A*0201 |
| EYYELFVNI | 294 | HLA-A*2402 | |
| LY6K | RYCNLEGPPI | 177 | HLA-A*2402 |
| CDCA1 | YMMPVNSEV | 65 | HLA-A*0201 |
| KLATAQFKI | 351 | HLA-A*0201 | |
| KTVNELQNL | 508 | HLA-A*2402 | |
| IMP3 | NLSSAEVVV | 515 | HLA-A*0201 |
| RLLVPTQFV | 199 | HLA-A*0201 | |
| KTVNELQNL | 508 | HLA-A*2402 | |
| TTK | SYRNEIAYL | 567 | HLA-A*2402 |
| KK-LC-1 | RQKRILVNL | 78 | HLA-B*1501 |
CTA Peptide-Based Clinical Trials
Among a large number of explorations of CTA peptide-based vaccines, the investigations of the widely expressed tumor antigens MAGE-A3 and NY-ESO-1 are noteworthy as paradigms. The melanoma antigen gene (MAGE) protein family is a large, highly conserved group of proteins that share a common MAGE homology domain [7], [8]. MAGE-A3, as one of the most immunogenic MAGE proteins, is restricted in expression to reproductive tissues but is aberrantly expressed in a wide variety of cancer types [9]. NY-ESO-1 is a CTA that was first described in a patient with esophageal cancer in 1997 [10] and has subsequently been reported to be expressed in a wide range of tumor types [11], [12], [13], [14], [15]. Humoral immune responses and cellular immune responses against NY-ESO-1 and MAGE-A3 have been detected [16], and the restricted epitopes have been identified as the recognition sites for CD8+ cytotoxic T lymphocytes (CTLs) [17], [18]. Numerous preclinical and clinical studies have indicated that MAGE-A3 peptide vaccines can trigger immune responses, and promising findings have been achieved in cancer subjects [19], [20], [21], [22], [23], [24]. Two phase III clinical trials, known as DERMA and MAGRIT, have been approved for patients with melanoma and non–small cell lung cancer (NSCLC) [25], [26]. However, the MAGRIT trial was stopped in 2014 due to a lack of clinical benefit for NSCLC patients. Despite the disappointing result, other ongoing clinical trials remain, and the field anticipates satisfactory outcomes. However, cancer vaccines based on NY-ESO-1–restricted immunogenic peptides combined with various adjuvants exhibit antitumor potential [27], [28], [29], [30].
In addition to the CTAs described above, other CTAs could be ideal targets, such as DEPDC1 [31], [32], [33], CDCA1 [34], LY6K [35], [36], IMP3 [37], [38], [39], and TTK [40], [41]. These antigens are all members of a class of CTAs with specific expression characteristics. With the identification of immunogenic epitopes, particularly HLA-A*24–restricted peptides, studies of these CTA multipeptide-based vaccines continue and are achieving promising results. For example, Wataru Obara et al. [42] reported the DEPDC1 immunogenic peptide 294EYYELFVNI302 and successfully constructed the peptide vaccine S-288310. In the ensuing phase I/II clinical trial, S-288310 was found to be well tolerated and to effectively increase survival time for patients with advanced urothelial carcinoma of the bladder. Additionally, 177RYCNLEGPPI186, 567SYRNEIAYL575, 508KTVNELQNL516, and 56VYGIRLEHF64 (derived from LY6K, TTK, IMP3, and CDCA1, respectively) have been reported to be promising HLA-A*24–restricted epitope peptides [43], [44]. A clinical cancer vaccination study, based on the peptides above, demonstrated satisfactory safety and good disease control in patients with solid tumors [45], [46], [47], [48], [49]. Remarkably, due to a lack of effective and standard treatment, pancreatic carcinoma is associated with a high mortality rate, but a phase I clinical cancer vaccination trial with a combination of peptides derived from CDCA1, KIF20A, VEGFR2, and VEGFR1 showed a detectable clinical benefit in four patients, suggesting this vaccine as a novel treatment for pancreatic cancer [50]. In general, CTA peptide-based vaccines can elicit a potent immune response against cancers, particularly solid tumors. Because additional studies have explored and established other HLA-restricted immunogenic epitopes [51], [52], [53], [54], a CTA peptide-based vaccine is expected to have wide applications for patients.
Modified Vaccine Strategies
Since CTA peptide vaccines seem to be an effective treatment to combat cancers, strategies to modify vaccines to improve their clinical efficacy have attracted great interest for superior clinical benefits. A pressing concern for CTA peptide vaccines is the considerable heterogeneity of CTA tumor expression. Thus, further studies are required to explore new CTAs as candidates. For instance, Kita-Kyushu lung cancer antigen-1 (KK-LC-1) [55], [56], [57] and sperm protein 17 (SP17) [58], [59], [60] are reportedly expressed frequently in tumors and carry epitope peptides recognized by CTLs [61], [62], [63], making them new candidate tumor biomarkers and immunotherapy targets. Additionally, many other CTAs are expected to be ideal targets for peptide vaccines, and further exploration is needed. Moreover, various adjuvants have been tested for their ability to enhance cytotoxic CD8+ T lymphocyte activity. These adjuvants include GM-CSF, Flt3-ligand, incomplete Freund's adjuvant, and saponin-based adjuvant (ISCOMATRIX) [64], [65]. Stronger adjuvants have been shown to improve the frequency of eliciting T cells [66].
Another strategy to enhance vaccination efficacy is to induce CD4+ immune responses to support the priming and maintenance of CD8+ CTLs [67]. Interestingly, an NY-ESO-1 peptide containing the HLA-DP4–restricted epitope can also generate HLA-A2–restricted CD8+ T cells [68], suggesting that this peptide may be used as a cancer vaccine to induce both CD4+ and CD8+ T cell responses, which is a promising direction for the development of peptide vaccines and constitutes a beneficial situation for future immunotherapies [69]. DNA vaccination has become a favored strategy for inducing immunity [70]. It offers the opportunity to engineer peptide antigen expression with more detailed design and delivery parameters [71]. Recombinant vectors encoding CTAs and even short hairpin RNA have been used in preclinical models to enhance immune system activation [72]. Other modifications to the vaccine approach currently in clinical trials include the use of combinatorial treatment. The microenvironment is an important factor due to the impact on the outcome of immune-modulating treatments. Therefore, multiple combined therapeutic strategies [73] have the potential for efficient control of tumor burden and improvement of the tumor microenvironment, and the details will be discussed below.
“Personalized” CTA Peptide Vaccines
In the clinical trials and advances of CTA peptide vaccines described above, multipeptide vaccines exhibited potent antitumor ability, though the immune response induced by the vaccination suggested that the potential could be improved. Currently, personalized treatment for cancer has become a trend after the demonstration of its feasibility, safety, and immunotherapeutic activity against individual tumors. However, the published experiments were conducted using a combination of multiple peptide vaccines rather than individualized administration of peptide vaccines. Therefore, for maximal efficacy, we envisage the establishment of a “personalized” CTA peptide vaccine for eligible patients.
Selection of Target Peptides for Vaccine Design
Despite issues with immunogenicity and specificity, CTAs are receiving attention as potential antineoplastic targets. Unfortunately, the immunogenicity of most CTAs is too low to induce antitumor responses, and the poor specificity can result in off-target toxicity. To address these limitations, additional studies are needed to explore new CTAs with high immunogenicity and specificity that can serve as ideal targets for cancer immunotherapy, and CTA peptide vaccines may thus be universal in clinical application. Accordingly, a critical challenge for personalized CTA peptide vaccines is accurate and comprehensive construction of a CTA peptide library to select the most suitable target peptides for optimal immune responses. The processing and presentation of antigens are complex processes. Protease cleavage products of thousands of proteins compete for binding in the pockets of MHC molecules [74], though only a portion of MHC-presented peptides can induce an effective T cell response. MHC-peptide stability can be predicted through epitope prediction algorithms including BIMAS, NetMHC, and NetCTL. These algorithms employ different prediction models but have all been trained using characterized epitope/MHC combinations, resulting in the prediction of the likelihood of short peptide sequences binding to a given HLA-allele. However, compared to MHC class I molecules, accurate prediction of ligands able to bind to MHC class II molecules is more difficult due to the variable lengths of binding peptides and the high abundance of MHC class II binding epitopes [75]. Moreover, considering that CD8+ CTLs have been demonstrated to recognize peptide epitopes derived from CTAs that are presented on MHC class I molecules and to kill tumor cells, the establishment of CTA MHC class I-restricted peptides is worthy of exploration.
Manufacturing of “Personalized” CTA Peptide Vaccines
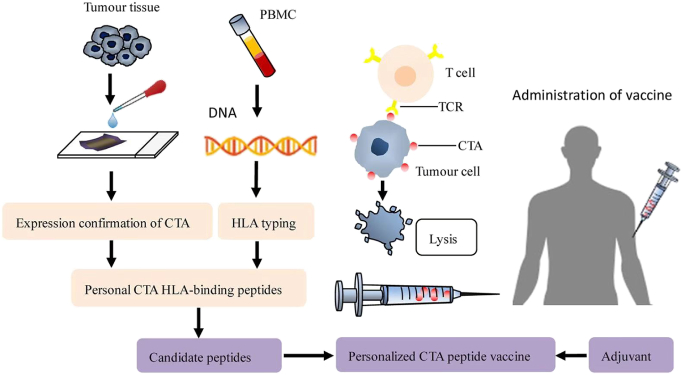
A comprehensive CTA peptide library can be conveniently employed to design individual CTA peptide vaccines. Tissue samples are diagnosed as positive for certain CTAs via immunohistochemical analysis, and simultaneously, peripheral blood mononuclear cells from patients are evaluated using a serotyping technique to determine their HLA type. Certain CTA HLA-binding peptides have thus been selected as target peptides for design of a vaccine that is administered to patients combined with an immune adjuvant (Fig. 1). Nanovaccines represent an emerging area, and advances have been made to improve the delivery system of NY-ESO-1 and provide strong protection against cancer through nanotechnology [76]. In addition, the PC7A nanovaccine is an attractive candidate with potential for T cell activation and synergy with checkpoint inhibition [77]. This nanovaccine platform is expected to be adopted to incorporate peptides and constitute personalized peptide nanovaccines via the CTA peptide library. Compared to the conventional CTA peptide vaccines described above, administration of a personalized CTA peptide vaccine may trigger a stronger immune response for attacking tumor cells and lead to a better prognosis.
Fig. 1.
Workflow for targeted peptide selection and vaccine manufacture.
Certain CTAs have been discovered in tumor tissues through immunohistochemical and HLA typing and determined by serotyping techniques. Thus, personal CTA HLA-binding peptides can be selected as target peptides for vaccine design. Candidate peptides are selected for incorporation into the personalized peptide vaccine, which is administered to patients in combination with an immune adjuvant to trigger an immune response to attack tumor cells.
Developing Combination Therapy
It is also critical to define the most suitable clinical setting for CTA peptide vaccination. A therapeutic vaccine will most likely be highly functional in an adjuvant or minimal residual disease setting. Efficient control of a larger tumor load may require multiple combined therapeutic strategies to eliminate the tumor burden and improve the overall tumor microenvironment. A promising area of study is the combination of peptide vaccines with chemoradiation therapy, including biotherapeutics such as immunomodulating or antivascular antibodies [78]. For instance, in a phase I vaccination trial with combined chemotherapy, the number of regulatory T cells decreased from the baseline value after administration of cyclophosphamide [79], reflecting the safety, flexibility, and superiority of CTA peptide vaccines combined with chemotherapy. Chemoradiation therapy combined with immunotherapy has demonstrated promising outcomes in clinical trials [80] due to its synergistic effect of enhancing antitumor immunity by inducing antigen expression on tumor cells and activating lymphocytes [81], [82]. Immunosuppression in the tumor microenvironment attenuates vaccine-induced immune effectors, and PD-1 is an important inhibitory component in the tumor microenvironment. Thus, combination therapy consisting of immune checkpoint inhibitors and cancer vaccine-enriched populations of CTA-reactive T cells may function synergistically to induce more effective antitumor immune responses. For example, Karyampudi et al. reported that in patients with breast cancer, PD-1 blockade enhances vaccine efficacy by altering both CD8+ T cell and DC components of the tumor microenvironment [83]. Furthermore, cancer vaccines stimulate and enhance active immunity; thus, coadministration of an agent that activates DCs can lead to increased immunogenicity of protein antigens and induction of immune responses [84]. These findings support the use of multiple combined therapeutic approaches that may amplify T cell expansion and increase the durable effect of vaccination, which is necessary to achieve promising results.
Conclusions
The capacity of the immune system to specifically attack cancer cells renders it the most powerful weapon for controlling cancer in the long term. CTAs are promising targets for cancer immunotherapy due to their expression in cancers and their rarity in normal tissues. With the identification of CTA peptides and clinical trials of CTA multipeptide vaccines, establishing personalized CTA peptide vaccines has become possible. Challenges remaining include the search for promising targets, identification of additional immunogenic CTA peptides, choice of suitable clinical settings, and development of feasible combination therapy. It is believed that when these limitations are overcome, personalized CTA peptide vaccines may provide a powerful tool to induce an immune response against cancer and become a universal treatment in cancer immunotherapy.
Acknowledgements
This work was funded by grants from the National Key Research and Development Program of China (No. 2017YFC1308900) and Jiangsu Provincial Medical Youth Talent (No. QNRC2016045). The funding sources had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
No conflict of interest to disclose.
References
- 1.Paulis LE, Mandal S, Kreutz M, Figdor CG. Dendritic cell-based nanovaccines for cancer immunotherapy. Curr Opin Immunol. 2013;25:389–395. doi: 10.1016/j.coi.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Tagliamonte M, Petrizzo A, Tornesello ML, Buonaguro FM, Buonaguro L. Antigen-specific vaccines for cancer treatment. Hum Vaccin Immunother. 2014;10:3332–3346. doi: 10.4161/21645515.2014.973317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 4.Sahin U, Tureci O. Personalized vaccines for cancer immunotherapy. Science. 2018;359:1355–1360. doi: 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- 5.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann O, Caballero OL, Stevenson BJ, Chen YT, Cohen T, Chua R, Maher CA, Panji S, Schaefer U, Kruger A. Genome-wide analysis of cancer/testis gene expression. Proc Natl Acad Sci U S A. 2008;105:20422–20427. doi: 10.1073/pnas.0810777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weon JL, Potts PR. The MAGE protein family and cancer. Curr Opin Cell Biol. 2015;37:1–8. doi: 10.1016/j.ceb.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coulie PG, Karanikas V, Lurquin C, Colau D, Connerotte T, Hanagiri T, Van Pel A, Lucas S, Godelaine D, Lonchay C. Cytolytic T-cell responses of cancer patients vaccinated with a MAGE antigen. Immunol Rev. 2002;188:33–42. doi: 10.1034/j.1600-065x.2002.18804.x. [DOI] [PubMed] [Google Scholar]
- 9.Esfandiary A, Ghafouri-Fard S. MAGE-A3: an immunogenic target used in clinical practice. Immunotherapy. 2015;7:683–704. doi: 10.2217/imt.15.29. [DOI] [PubMed] [Google Scholar]
- 10.Chen YT, Scanlan MJ, Sahin U, Tureci O, Gure AO, Tsang S, Williamson B, Stockert E, Pfreundschuh M, Old LJ. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forghanifard MM, Gholamin M, Farshchian M, Moaven O, Memar B, Forghani MN, Dadkhah E, Naseh H, Moghbeli M, Raeisossadati R. Cancer-testis gene expression profiling in esophageal squamous cell carcinoma: identification of specific tumor marker and potential targets for immunotherapy. Cancer Biol Ther. 2011;12:191–197. doi: 10.4161/cbt.12.3.15949. [DOI] [PubMed] [Google Scholar]
- 12.Yin B, Liu G, Wang XS, Zhang H, Song YS, Wu B. Expression profile of cancer-testis genes in transitional cell carcinoma of the bladder. Urol Oncol. 2012;30:886–892. doi: 10.1016/j.urolonc.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Zhang WM, Xiao G, Xie D, Zhang M, Guo AL, Wen JM. Correlation of NY-ESO-1 gene and protein expression to metastasis and clinicopathologic features of hepatocellular carcinoma. Ai Zheng. 2005;24:622–626. [PubMed] [Google Scholar]
- 14.Shigematsu Y, Hanagiri T, Shiota H, Kuroda K, Baba T, Mizukami M, So T, Ichiki Y, Yasuda M, So T. Clinical significance of cancer/testis antigens expression in patients with non–small cell lung cancer. Lung Cancer. 2010;68:105–110. doi: 10.1016/j.lungcan.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Odunsi K, Jungbluth AA, Stockert E, Qian F, Gnjatic S, Tammela J, Intengan M, Beck A, Keitz B, Santiago D. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003;63:6076–6083. [PubMed] [Google Scholar]
- 16.Thomas R, Al-Khadairi G, Roelands J, Hendrickx W, Dermime S, Bedognetti D, Decock J. NY-ESO-1 based immunotherapy of cancer: current perspectives. Front Immunol. 2018;9:947. doi: 10.3389/fimmu.2018.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi H, Tanaka F, Ohta M, Inoue H, Mori M. Identification of HLA-A24-restricted CTL epitope from cancer-testis antigen, NY-ESO-1, and induction of a specific antitumor immune response. Clin Cancer Res. 2004;10:890–896. doi: 10.1158/1078-0432.ccr-1086-3. [DOI] [PubMed] [Google Scholar]
- 18.Eikawa S, Kakimi K, Isobe M, Kuzushima K, Luescher I, Ohue Y, Ikeuchi K, Uenaka A, Nishikawa H, Udono H. Induction of CD8 T-cell responses restricted to multiple HLA class I alleles in a cancer patient by immunization with a 20-mer NY-ESO-1f (NY-ESO-1 91-110) peptide. Int J Cancer. 2013;132:345–354. doi: 10.1002/ijc.27682. [DOI] [PubMed] [Google Scholar]
- 19.Oiso M, Eura M, Katsura F, Takiguchi M, Sobao Y, Masuyama K, Nakashima M, Itoh K, Ishikawa T. A newly identified MAGE-3-derived epitope recognized by HLA-A24-restricted cytotoxic T lymphocytes. Int J Cancer. 1999;81:387–394. doi: 10.1002/(sici)1097-0215(19990505)81:3<387::aid-ijc12>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.N. Miyagawa, K. Kono, K. Mimura, H. Omata, H. Sugai, H.J.O. Fujii, A newly identified MAGE-3-derived, HLA-A24-restricted peptide is naturally processed and presented as a CTL epitope on MAGE-3-expressing gastrointestinal cancer cells, 70 (2006) 54–62. [DOI] [PubMed]
- 21.P. Bruggen, Van Der, J. Bastin, T. Gajewski, P.G. Coulie, P. Bo?L, C. Smet, De, C. Traversari, A. Townsend, T. Boon, . %J Immunogenetics, A peptide encoded by human gene MAGE-3 and presented by HLA-A2 induces cytolytic T lymphocytes that recognize tumor cells expressing MAGE-3, 43 (1996) 377–383. [DOI] [PubMed]
- 22.I. Kawashima, S.J. Hudson, V. Tsai, S. Southwood, K. Takesako, E. Appella, A. Sette, E.J.H.I. Celis, The multi-epitope approach for immunotherapy for cancer: identification of several CTL epitopes from various tumor-associated antigens expressed on solid epithelial tumors ☆, 59 (1998) 1–14. [DOI] [PubMed]
- 23.Sang M, Wang L, Ding C, Zhou X, Wang B, Wang L, Lian Y. B.J.C.L. Shan. Melanoma-associated antigen genes — an update. 2011;302:85–90. doi: 10.1016/j.canlet.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 24.O. Chie, T. Masako, K. Akiko, M. Haruo, A. Tadashi, I. Akira, K. Yoshio, Y. Shusuke, T. Ryuji, Y.J.O.R. Naoya, Dendritic cell-based vaccination in metastatic melanoma patients: phase II clinical trial, 28 (2012) 1131. [DOI] [PMC free article] [PubMed]
- 25.Preeta T. M.J.C.L.C. Beloo. MAGRIT: the largest-ever phase III lung cancer trial aims to establish a novel tumor-specific approach to therapy. 2009;10:371–374. doi: 10.3816/CLC.2009.n.052. [DOI] [PubMed] [Google Scholar]
- 26.Y.H. Cheng, E.W. Wong, C.Y.J.S. Cheng, Cancer/testis (CT) antigens, carcinogenesis and spermatogenesis, 1 (2011) 209–220. [DOI] [PMC free article] [PubMed]
- 27.S. Guru, W. Mingjun, L.E. Peterson, H.Y. Wang, J. Teresa, M.P. Mims, K. Dov, M.M. Ittmann, T.M. Wheeler, A.P. Gee, %J Investigational New Drugs, HLA-restricted NY-ESO-1 peptide immunotherapy for metastatic castration resistant prostate cancer, 32 (2014) 235–242. [DOI] [PMC free article] [PubMed]
- 28.A. Bender, J. Karbach, A. Neumann, D. Jäger, S.E. Al-Batran, A. Atmaca, E. Weidmann, M. Biskamp, S. Gnjatic, L.J.C.I.A.J.o.t.A.o.C.I. Pan, LUD 00-009: phase 1study of intensive course immunization with NY-ESO-1 peptides inHLA-A2 positive patients with NY-ESO-1-expressing cancer, 7 (2007) 16. [PMC free article] [PubMed]
- 29.D.K. Krishnadas, S. Shusterman, F. Bai, L. Diller, J.E. Sullivan, A.C. Cheerva, R.E. George, K.G.J.C.I.I.C. Lucas, A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma, 64 (2015) 1251–1260. [DOI] [PMC free article] [PubMed]
- 30.S. Gnjatic, H. Nishikawa, A.A. Jungbluth, A.O. Güre, G. Ritter, E. Jäger, A. Knuth, Y.T. Chen, L.J.J.A.i.C.R. Old, NY-ESO-1: Review of an immunogenic tumor antigen, 95 (2006) 1–30. [DOI] [PubMed]
- 31.Y. Sheng-Guang, L. Wei-Jia, Y. Jian-Jun, H. Guo-Jin, H.J.A.P.J.C.P. Zhao-Quan, DEP domain containing 1 is a novel diagnostic marker and prognostic predictor for hepatocellular carcinoma, 15 (2015) 10917–10922. [DOI] [PubMed]
- 32.K. Céline, S.K. Anja, S. Friederike, S. Winfried, P.M. Schlag, K.J.M.C. Wolfgang, 10,1, Identification of early molecular markers for breast cancer, 10 (2011) 15. [DOI] [PMC free article] [PubMed]
- 33.H. Yosuke, K. Mitsugu, F. Yoshiko, T. Ryo, S. Taro, M. Tsuneharu, F. Tomoaki, N. Yusuke, K.J.C.R. Toyomasa, Cell-permeable peptide DEPDC1-ZNF224 interferes with transcriptional repression and oncogenicity in bladder cancer cells, 70 (2010) 5829. [DOI] [PubMed]
- 34.H. Satoshi, D. Yataro, K. Tatsuya, I. Nobuhisa, Y. Takumi, M. Masaki, I. Tomoo, T. Eiju, K. Satoshi, N. Yusuke, Activation of CDCA1-KNTC2, members of centromere protein complex, involved in pulmonary carcinogenesis, 66 (2006) 10339–10348. [DOI] [PubMed]
- 35.I. Nobuhisa, T. Atsushi, Y. Wataru, I. Kouki, N. Hitoshi, I. Hiroyuki, M. Yohei, N. Haruhiko, F. Masahiro, H.J.C.R. Masao, Cancer-testis antigen lymphocyte antigen 6 complex locus K is a serologic biomarker and a therapeutic target for lung and esophageal carcinomas, 67 (2007) 11601–11611. [DOI] [PubMed]
- 36.N.V. Dalen, G.A.M.S. Dongen, Van, S.J. Smeets, E.J.C. Nieuwenhuis, S.V.W. Marijke, G.B. Snow, R.H. Brakenhoff, %J International Journal of Cancer, Characterization of the human Ly-6 antigens, the newly annotated member Ly-6K included, as molecular markers for head-and-neck squamous cell carcinoma, 103 (2003) 768–774. [DOI] [PubMed]
- 37.C. Li, K. Rock, Ba, Z. Jiang, A. Fraire, K.J.M.P. Dresser, IMP3 is a novel biomarker for adenocarcinoma in situ of the uterine cervix: an immunohistochemical study in comparison with p16(INK4a) expression, 20 (2007) 242. [DOI] [PubMed]
- 38.Zhang J, Yingfu OU, Yibing MA, Zheng L, Zhang X, Xia R, Kong F, Shen Y, Wang S, Lin LJOL. 1927-1933. Clinical implications of insulin-like growth factor II mRNA-binding protein 3 expression in non-small cell lung carcinoma, 9 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.E.A.M. Damasceno, C. Fabiana Pirani, A.V.O.D. Magalh?Es, M.D.V. Carneiro, G.H.S. Takano, L.M.D.S. Vianna, H.B.K. Seidler, M.J.J.J.o.C.R. Maria Imaculada, C. Oncology, IMP3 expression in gastric cancer: association with clinicopathological features and HER2 status, 140 (2014) 2163–2168. [DOI] [PubMed]
- 40.G.B. Mills, R. Schmandt, ., M. Mcgill, ., A. Amendola, ., M. Hill, ., K. Jacobs, ., C. May, ., A.M. Rodricks, S. Campbell, ., D. Hogg, . %J Journal of Biological Chemistry, Expression of TTK, a novel human protein kinase, is associated with cell proliferation, 267 (1992) 16000–16006. [PubMed]
- 41.D.C. Guillermo, P.D.C. Ignacio, M.J.C.M.C. Marcos, Targeting cell cycle kinases for cancer therapy, 14 (2007) -. [DOI] [PubMed]
- 42.W. Obara, M. Eto, H. Mimata, K. Kohri, N. Mitsuhata, I. Miura, T. Shuin, T. Miki, T. Koie, H.J.A.o.O.O.J.o.t.E.S.f.M.O. Fujimoto, A phase I/II study of cancer peptide vaccine S-288310 in patients with advanced urothelial carcinoma of the bladder, 28 (2016) 798. [DOI] [PubMed]
- 43.Takako S, Takuya T, Yataro D, Yusuke N, Hideaki TJCS. 1803-1808. Identification of human leukocyte antigen-A24-restricted epitope peptides derived from gene products upregulated in lung and esophageal cancers as novel targets for immunotherapy, 98 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.H.J.J.o.T.M. Suzuki, Multiple therapeutic peptide vaccines consisting of combined novel cancer testis antigens and anti-angiogenic peptides for patients with non–small cell lung cancer, 11 (2013) 97–97. [DOI] [PMC free article] [PubMed]
- 45.Koji K, Yoshiki M, Yataro D, Atsushi T, Ken M, Koji Y, Takuya T, Yoshihiko K, Yusuke N. F.J.C.S. Hideki. Vaccination with multiple peptides derived from novel cancer-testis antigens can induce specific T-cell responses and clinical responses in advanced esophageal cancer. 2010;100:1502–1509. doi: 10.1111/j.1349-7006.2009.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.K. Kono, H. Iinuma, Y. Akutsu, H. Tanaka, N. Hayashi, Y. Uchikado, T. Noguchi, H. Fujii, K. Okinaka, R.J.J.o.T.M. Fukushima, 10,1, Multicenter, phase II clinical trial of cancer vaccination for advanced esophageal cancer with three peptides derived from novel cancer-testis antigens, 10 (2012) 141–141. [DOI] [PMC free article] [PubMed]
- 47.H. Ishikawa, M. Imano, O. Shiraishi, A. Yasuda, Y.F. Peng, M. Shinkai, T. Yasuda, H. Imamoto, H.J.G.C. Shiozaki, Phase I clinical trial of vaccination with LY6K-derived peptide in patients with advanced gastric cancer, 17 (2014) 173–180. [DOI] [PubMed]
- 48.H. Yoshie, K. Junko, N. Akihito, I. Kentaro, K. Masae, K. Tomohiro, S. Nobuko, O. Taro, W.J.G. Sumio, Phase I clinical trial of peptide vaccination with URLC10 and VEGFR1 epitope peptides in patients with advanced gastric cancer, 140 (2014) S-677-S-678. [DOI] [PMC free article] [PubMed]
- 49.Yoshihiro Y, Daiki F, Akira Y, Masatoshi H, Hideki N, Takuya T, Masashi N, Yasuo T, Kenta K. N.J.C.C.R.A.O.J.o.t.A.A.f.C.R. Yoshihiro. Phase II clinical trial of multiple peptide vaccination for advanced head and neck cancer patients revealed induction of immune responses and improved OS. 2015;21:312–321. doi: 10.1158/1078-0432.CCR-14-0202. [DOI] [PubMed] [Google Scholar]
- 50.R. Okuyama, A. Aruga, T. Hatori, K. Takeda, M.J.O. Yamamoto, Immunological responses to a multi-peptide vaccine targeting cancer-testis antigens and VEGFRs in advanced pancreatic cancer patients, 2 (2013) -. [DOI] [PMC free article] [PubMed]
- 51.A. Tosi, S. Dalla Santa, E. Cappuzzello, C. Marotta, D. Walerich, G. Del Sal, P. Zanovello, R. Sommaggio, A.J.O. Rosato, Identification of a HLA-A*0201-restricted immunogenic epitope from the universal tumor antigen DEPDC1. [DOI] [PMC free article] [PubMed]
- 52.T. Yusuke, Y. Akira, T. Hirotake, S. Satoru, Y. Sachiko, O. Ryuji, K. Yasuhiro, H. Masatoshi, I. Atsushi, H.J.I.J.o.C. Akinobu, Identification of CDCA1-derived long peptides bearing both CD4+ and CD8+ T-cell epitopes: CDCA1-specific CD4+ T-cell immunity in cancer patients, 134 (2013) 352–366. [DOI] [PubMed]
- 53.H. Michiko, H. Shinya, I. Atsushi, S. Satoru, N. Tetsuya, K. Hiroyuki, I. Yoshiaki, Y. Kazunori, I. Katsunori, I.J.I.J.o.C. Mitsuhiro, HLA-A2-restricted CTL epitopes of a novel lung cancer-associated cancer testis antigen, cell division cycle associated 1, can induce tumor-reactive CTL, 123 (2010) 2616–2625. [DOI] [PubMed]
- 54.T. Yusuke, H. Michiko, S. Satoru, I. Katsunori, H. Shinya, I. Atsushi, I. Mitsuhiro, H. Yuki, Y. Kentaro, S.J.C.S. Kenji, Peptides derived from human insulin-like growth factor-II mRNA binding protein 3 can induce human leukocyte antigen-A2-restricted cytotoxic T lymphocytes reactive to cancer cells, 102 (2011) 71–78. [DOI] [PMC free article] [PubMed]
- 55.Shigematsu Y, Hanagiri TH, Kuroda K, Baba T, Mizukami M, So T, Ichiki Y, Yasuda M, So T. M.J.L.C. Takenoyama. Clinical significance of cancer/testis antigens expression in patients with non-small cell lung cancer. 2010;68:105–110. doi: 10.1016/j.lungcan.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 56.S. Akiko, F. Nobue, F. Takashi, I. Yoshinobu, T. Yoshihito, N. Yatsushi, K. Noritada, Y. Hitoshi, W.J.A.R. Masahiko, Frequent High Expression of Kita-Kyushu Lung Cancer Antigen-1 (KK-LC-1) in Gastric Cancer, 35 (2015) 3575. [PubMed]
- 57.T. Fukuyama, N. Futawatari, Y. Ichiki, A. Shida, T. Yamazaki, Y. Nishi, H. Nonoguchi, Y. Takahashi, H. Yamazaki, N.J.V. Kobayashi, Correlation between expression of the cancer/testis antigen KK-LC-1 and Helicobacter pylori infection in gastric cancer, 31 (2017) 403. [DOI] [PMC free article] [PubMed]
- 58.L. Mirandola, E. Pedretti, J.A. Figueroa, R. Chiaramonte, M. Colombo, C. Chapman, F. Grizzi, F. Patrinicola, W.M. Kast, D.D.J.O. Nguyen, Cancer testis antigen sperm protein 17 as a new target for triple negative breast cancer immunotherapy, 8 (2017) 74378–74390. [DOI] [PMC free article] [PubMed]
- 59.C.A. Schutt, L. Mirandola, J.A. Figueroa, D.D. Nguyen, J. Cordero, K. Bumm, B.L. Judson, M.J.O. Chirivainternati, The cancer-testis antigen, sperm protein 17, a new biomarker and immunological target in head and neck squamous cell carcinoma, 8 (2017) 100280–100287. [DOI] [PMC free article] [PubMed]
- 60.Chiriva-Internati M, Mirandola L, Figueroa JA, Yu Y, Grizzi F, Kim M, Jenkins M, Cobos E, Jumper C, Alalawi RJC. 2014. Selective expression and immunogenicity of the cancer/testis antigens SP17, AKAP4 and PTTG1 in non–small cell lung cancer: new candidates for active immunotherapy. [DOI] [PubMed] [Google Scholar]
- 61.F. Takashi, H. Takeshi, T. Mitsuhiro, I. Yoshinobu, M. Makiko, S. Tetsuya, S. Masakazu, S. Tomoko, S. Kenji, Y.J.C.R. Kosei, Identification of a new cancer/germline gene, KK-LC-1, encoding an antigen recognized by autologous CTL induced on human lung adenocarcinoma, 66 (2006) 4922–4928. [DOI] [PubMed]
- 62.K. Ait-Tahar, A.P. Anderson, M. Barnardo, G.P. Collins, C.S.R. Hatton, A.H. Banham, K.J.A.i.H. Pulford, ,, Sp17 protein expression and major histocompatibility class I and II epitope presentation in diffuse large B cell lymphoma patients, 2017 (2017) 1–9. [DOI] [PMC free article] [PubMed]
- 63.S.D. Xiang, G. Qian, K.L. Wilson, A. Heyerick, M.J.V. Plebanski, Mapping T and B cell epitopes in sperm protein 17 to support the development of an ovarian cancer vaccine, 33 (2015) 5950–5959. [DOI] [PubMed]
- 64.I.D. Davis, C. Weisan, J. Heather, P. Phillip, S. Mark, H. Wendie, C. Qiyuan, D. Nektaria, L. Tina, M.J.P.o.t.N.A.o.S.o.t.U.S.o.A. Roger, Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans, 101 (2004) 10697–10702. [DOI] [PMC free article] [PubMed]
- 65.E. J?Ger, ., S. Gnjatic, ., Y. Nagata, ., E. Stockert, ., D. J?Ger, ., J. Karbach, ., A. Neumann, ., J. Rieckenberg, ., Y.T. Chen, G. Ritter, . %J Proceedings of the National Academy of Sciences of the United States of America, Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers, 97 (2000) 12198–12203. [DOI] [PMC free article] [PubMed]
- 66.R. Thomas, G. Alkhadairi, J. Roelands, W. Hendrickx, S. Dermime, D. Bedognetti, J.J.F.i.I. Decock, NY-ESO-1 based immunotherapy of cancer: current perspectives, 9 (2018) 947-. [DOI] [PMC free article] [PubMed]
- 67.Sun Z, Chen F, Meng F, Jia W. B.J.C.L. Liu. MHC class II restricted neoantigen: a promising target in tumor immunotherapy. 2017;392:17–25. doi: 10.1016/j.canlet.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 68.Z. Gang, L. Yong, E.G. Mona, S. John, S. Alexandro, W. Rong-Fu, S.A. Rosenberg, P.F. Robbins, %J Cancer Research, Generation of NY-ESO-1-specific CD4+ and CD8+ T cells by a single peptide with dual MHC class I and class II specificities: a new strategy for vaccine design, 62 (2002) 3630–3635. [PMC free article] [PubMed]
- 69.K. Kazuhiro, I. Midori, U. Akiko, W. Hisashi, S. Eiichi, D. Yuichiro, N. Jun, S. Yasuyuki, Y. Tomoki, N.J.I.J.o.C. Yoshio, A phase I study of vaccination with NY-ESO-1f peptide mixed with picibanil OK-432 and montanide ISA-51 in patients with cancers expressing the NY-ESO-1 antigen, 129 (2011) 2836–2846. [DOI] [PubMed]
- 70.K.R. Porter, K.J.C.I.i.M.B. Raviprakash, DNA vaccine delivery and improved immunogenicity, 22 (2016) 129. [DOI] [PubMed]
- 71.L. Li, N.J.E.R.o.V. Petrovsky, Molecular mechanisms for enhanced DNA vaccine immunogenicity, 15 (2015) 1. [DOI] [PMC free article] [PubMed]
- 72.G.F. Soudeh, G.F.J.I. Somayyeh, siRNA and cancer immunotherapy, 4 (2012) 907–917. [DOI] [PubMed]
- 73.Krishnadas DK, Bai F, Lucas KGJI. Therapy. Cancer testis antigen and immunotherapy. 2013;2:11–19. doi: 10.2147/ITT.S35570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joffre OP, Segura E, Savina A. S.J.N.R.I. Amigorena. Cross-presentation by dendritic cells. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 75.B. Seliger, M. Kloor, S.J.O. Ferrone, HLA class II antigen-processing pathway in tumors: Molecular defects and clinical relevance, 6 (2017) -. [DOI] [PMC free article] [PubMed]
- 76.P.C. de Faria, L.I. dos Santos, J.P. Coelho, H.B. Ribeiro, M.A. Pimenta, L.O. Ladeira, D.A. Gomes, C.A. Furtado, R.T.J.N.L. Gazzinelli, Oxidized multiwalled carbon nanotubes as antigen delivery system to promote superior CD8(+) T cell response and protection against cancer, 14 (2014) 5458. [DOI] [PubMed]
- 77.M. Luo, H. Wang, Z. Wang, H. Cai, Z. Lu, Y. Li, M. Du, G. Huang, C. Wang, X.J.N.N. Chen, A STING-activating nanovaccine for cancer immunotherapy, 12 (2017) 648. [DOI] [PMC free article] [PubMed]
- 78.Fourcade J, Sun Z, Pagliano O, Chauvin JM, Sander C, Janjic B, Tarhini AA, Tawbi HA, Kirkwood JM, Moschos S. PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8(+) T cells induced by melanoma vaccines. Cancer Res. 2014;74:1045–1055. doi: 10.1158/0008-5472.CAN-13-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murahashi M, Hijikata Y, Yamada K, Tanaka Y, Kishimoto J, Inoue H, Marumoto T, Takahashi A, Okazaki T. K.J.C.I. Takeda. Phase I clinical trial of a five-peptide cancer vaccine combined with cyclophosphamide in advanced solid tumors. 2016;166-167:48–58. doi: 10.1016/j.clim.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 80.H. Iinuma, R. Fukushima, T. Inaba, J. Tamura, T. Inoue, E. Ogawa, M. Horikawa, Y. Ikeda, N. Matsutani, K.J.J.o.T.M. Takeda, Phase I clinical study of multiple epitope peptide vaccine combined with chemoradiation therapy in esophageal cancer patients, 12 (2014) 84. [DOI] [PMC free article] [PubMed]
- 81.Dalgleish AG. %J Immunotherapy. Rationale for combining immunotherapy with chemotherapy. 2015;7:309–316. doi: 10.2217/imt.14.111. [DOI] [PubMed] [Google Scholar]
- 82.Teng F, Kong L, Meng X, Yang J. J.J.C.L. Yu. Radiotherapy combined with immune checkpoint blockade immunotherapy: Achievements and challenges. 2015;365:23–29. doi: 10.1016/j.canlet.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 83.Karyampudi L, Lamichhane P, Scheid AD, Kalli KR, Shreeder B, Krempski JW, Behrens MD, Knutson KL. Accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Res. 2014;74:2974–2985. doi: 10.1158/0008-5472.CAN-13-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.M.V. Dhodapkar, S. Mario, Z. Biwei, W. Ding, R.D. Carvajal, M.L. Keohan, C. Ellen, R.E. Sanborn, L. Jose, P.J.S.T.M. John, Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205, 6 (2014) 232–251. [DOI] [PMC free article] [PubMed]