Abstract
Enhancement of natural based polymeric membranes for active packaging takes the attention of scientists. Their biological activities can be obtained by adding essential oils, which are natural extracts with antimicrobial and antioxidant properties. The target of current work aimed to produce bio-active membranes from cellulose acetate incorporated with Rosemary and Aloe Vera oil. The developed film’s chemical structures and morphologies were investigated using FT-IR and SEM characterization tools. The impact of essential oils incorporation on water uptake, wettability behavior, and mechanical properties were explored. The results displayed that antimicrobial activity against Escherichia coli (E. coli) and Bacillus subtilis (B. subtilis) increased as Rosemary and Aloe Vera oil percentage increases in cellulose acetate membranes. In addition, higher activity against B. subtilis compared to E. coli was also observed. Moreover, free radical scavenger activity (ABTS and DPPH) of cellulose acetate membranes, improved by increasing the essential oil content in the feed mixture. The obtained results provide a high potential for production of an efficient food packaging membrane from cellulose acetate containing Rosemary and Aloe Vera oil.
Keywords: Cellulose acetate, Antibacterial, Antioxidant, Essential oils
Introduction
Excessive use of synthetic polymers in different types has caused great concern about their disposal ways. Economically suitable biodegradable polymers which can be simply formed into different shapes are the better reply to this issue. Over the past years, there are many bio-based polymers were used in food-related packaging (El-Fawal 2014). Cellulose represents one of those polymers which is renewable and widely available in large quantities from various plant sources (Voicu et al. 2016). Recently, the researchers have been concentrated on the evolution of cellulose derivatives to be applied in food packaging applications (Niu et al. 2018). Cellulose acetate (CA)—among cellulosic derivatives—has a specific interest due to its biodegradable nature, greater optical clarity, and simplicity of preparing by melting techniques or solvent-casting method (Stoja et al. 2017). The unique cellulose acetate mechanical properties enable it to be used in many applications include water treatment and filtration, fuel cell applications and packaging (Mendes et al. 2018; Niu et al. 2018).
Essential oils (EO) are mixtures of complex volatile compounds with a strong smell that are created in numerous plant tissues (Bárbara et al. 2013). Essential oils have various therapeutic applications in human medicine because of its antibacterial, anticancer, antiphlogistic, antioxidant, antinociceptive, and antiviral properties (Buchbauer 2010). The antibacterial, antifungal and antioxidant properties of various EO have been considered so far (Jhang and Lee 2017). The EO impacts the microbial cells through several mechanisms, including the enzyme systems disruption, bacteria genetic material compromising and interactions with the cell membrane phospholipid (Burt et al. 2007). Moreover, use of essential oils is becoming common to increase food products shelf-life because of the customers are more aware of the health problems caused by numerous synthetic preservatives (Stoja et al. 2017). Application of EO as food preservatives are restricted by their strong flavor, but incorporating them into a polymer matrix act as an alternative means of decreasing their sensory influence.
Aloe Vera and Rosemary oils are two examples of EO which have been used as an antibacterial and antioxidant agent in many different medical applications (Jhang and Lee 2017). Aloe Vera oil has been used in traditional medicine for centuries as it has antibacterial and antimicrobial ability (Jhang and Lee 2017). Aloe Vera has excellent antioxidant and antitumor properties; it is valuable in the treatment of burns and wounds, as an analgesic and a blood purifier; and it is used as a skin moisturizer (Casian et al. 2007). Recently, it has been used as a supplement for functional food. Rosemary oil is considered a natural antioxidant and antimicrobial agent which has been used in different biomedical applications (Aleksandar et al. 2014). In addition, it is able to expand the shelf-life of food products and preserve their quality during storage.
The present work aims to develop natural based polymeric membranes for food packaging applications. Herein, two essential oils, Rosemary and Aloe Vera were incorporated separately into CA membranes with different concentrations. In that way, the effects of Rosemary and Aloe Vera oils content on CA membranes were characterized. As well as antibacterial activity and antioxidant of the obtained CA-Rosemary and CA-Aloe Vera oils system was investigated.
Materials and methods
Materials
Cellulose acetate (Degree of acetylation 40%) was supplied by Sigma- Aldrich Chemie Gmbh. (USA). Rosemary and Aloe Vera oil, acetic acid, sulfuric acid; phenolphthalein, acetone, and sodium hydroxide were of analytical grade and obtained from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). Folin Ciocalteu and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid, ABTS) were purchased from Sigma Aldrich Co., Ltd. (USA).
Methods
Preparation of membrane
Cellulose acetate (CA) based membrane was prepared using traditional casting evaporation method. Briefly, 0.5 g of CA was dispersed in 25 mL of acetone and stirred overnight. Rosemary (RM) or Aloe Vera (AV) oil were added separately to CA solution with final concentrations of 20, 50, and 80% (v/w) (based on CA weight) and marked as CA/RM1, CA/RM2, CA/RM3 and CA/AV1, CA/AV2, CA/AV3, respectively. The mixtures were kept under continuous stirring at room temperature for 20 min to obtain homogeneous solutions followed by casting into a clean Petri dish and dried at room temperature for about 72 h. The dried membranes were gently separated and rinsed with 20 mL of distilled water. Finally, the membranes were attached to the clean glass support with clamps and allowed to dry at room temperature for 24 h.
Membrane characterization
Fourier-transform infrared spectroscopy
Infrared spectra were performed by Fourier transform infrared spectrophotometer (Shimadzu FTIR–8400 S, Japan) to examine the structure of the developed membranes.
Water uptake
Water uptake (%) measuring was performed by placing a dried sample in distilled water for 150 min to reach the equilibrium swelling state, the membrane was filtered off, carefully bolted with a filter paper and weighted. The water uptake was calculated by the following equation:
where Mo is the weight of the dry sample and M is the weight of the swelled sample.
Mechanical properties
Mechanical properties of CA membranes incorporated with varying amounts of oil (Rosemary or Aloe Vera) were investigated using a universal testing machine (AG-1S, Shimadu, Japan) according to ASTM D- 882 standards. These properties included the membrane thickness and maximum stress and strain to failure. The electronic digital micrometer was used to measure the membrane thickness. All measurements were carried in triplicate.
Morphological analysis
The surface morphology of CA membranes was examined by scanning electron microscope (JEOL Ltd., Tokyo, Japan) operated at 10 kv voltage acceleration. CA membrane was fixed on stainless steel stubs with double face tape, a layer of gold (10–15 nm) was sputtered on the samples by JFC-1100E sputter (JOEL Ltd., Tokyo, Japan).
Antimicrobial activity determination
Antimicrobial activity of CA membranes incorporated with different amounts of Rosemary or Aloe Vera oils was measured according to a method reported earlier (Skyttä and Mattila 1991). Briefly, the bacteria were incubated in Luria–Bertani medium (LB medium, 1% peptone, 0.5% meat extract, and 1% NaCl, pH = 7). The inoculation was conducted at 37 °C for 24 h under shaking. The obtained bacterial suspension was diluted with the previous peptone medium solution. Then, 0.1 mL of diluted bacteria suspension was cultured in 10 mL liquid peptone medium. Then, 50 mg of CA membranes was added to the bacterial suspension. The inoculated medium, incubated at 37 °C for 24 h. After incubation, the antibacterial activity was monitored by measuring the optical density of the culture medium at 620 nm and calculating the inhibition percentage using the following equation (Vásquez et al. 2017):
where ODb and ODs are the optical density of bacterial culture with and without testing membranes.
In situ evaluation method
For this experiment, European light cheese slices obtained commercially were used for the analysis. The evaluation was measured according to a method reported earlier (Lee et al. 2015). Briefly, the cheese slices was removed from their commercial packages and cut into certain dimensions (3 × 3 cm2 and thickness ≈ 1.5 mm) then packaged with cellulose membranes containing different amounts of oil (Rosemary or Aloe Vera). The slices were sterilized by exposure to ultraviolet light for 15 min on both sides. After that, they were experimentally contaminated on both sides, by the spreading 50 μL of a cell suspension (106 CFU/mL) of B. subtilis or E. coli on the cheese surface. After drying the inoculum, the slices were placed in contact with films which containing oils. The system was stored for 7 days at 4 °C and was analyzed at 0, 1, 3, 5 and 7 days. At measuring time intervals, the cheese slice was immersed in diluted LB (10 mL) for 5 min with shaking. After that 50 µL of bacterial culture (from diluted LB) were spread on nutrient agar plates. Then, the agar plates were incubated at 37 °C for 24 h, and the numbers of colonies were counted. All measurements were carried in triplicate.
Assay for phenolic compounds
The total phenolic content of the CA membranes was calculated based on the reduction of Folin Ciocalteu reagent from yellow to blue colored compound (Navajas et al. 2013). A membrane sample of 50 mg was immersed in 5 mL ethanol to extract phenolic compounds. A volume of 0.5 mL membrane extract was added to 2.0 mL Folin Ciocalteu reagent (10%, v/v) followed by the addition of 2 mL 7.5% (w/v) sodium carbonate solution. The mixture was incubated at 50 °C for 5 min. Finally, the absorption was measured at 760 nm using a spectrophotometer (UV-1200S, China). Gallic acid solutions (0–100 µg) were used to obtain the standard curve. The total phenolic content was expressed as microgram gallic acid equivalent per gram membrane (μg GAE/g membrane).
Antioxidant activity assay
Antioxidant activities of CA membranes incorporated with different amounts of Rosemary or Aloe Vera oils were determined by two different test systems namely ABTS and DPPH assays.
ABTS radical scavenging assay
For ABTS radical scavenging assay, the radical cations were pre-formed by reaction of K2S2O8 aqueous solution (3.30 mg) in water (5 mL) with ABTS (17.2 mg). The resulting radical cation solution (bluish green) was kept overnight in the dark below 0 °C. Then 1 mL the solution was diluted to 60 mL with distilled water. The sample extract was obtained as described in the determination of total phenolic content. A volume of 0.1 mL membrane extract was added to 2.0 mL of ABTS solution in the test tube. Then, the test tube was incubated for 30 min (at room temperature) in the dark. The absorbance was measured at 734 nm using a spectrophotometer (UV-1200S, China). The radicals inhibition percentage was calculated using the following formula:
where Acontrol is the absorbance of ABTS solution without extract and Asample is the absorbance of the sample with ABTS solution.
DPPH radical scavenging activity
The antioxidant activity of the extract was measured by the DPPH method http://www.sciencedirect.com/science/article/pii/S1021949813001348—bib18with slight modifications (Dehpour et al. 2009). The DPPH solution was prepared by dissolving 6 mg DPPH in 50 ml methanol (about 0.3 mM). The sample extract was obtained as described in the determination of total phenolic content section. The extract (2.5 mL) and DPPH solution (2.5 mL) was mixed together in a test tube. Then, the test tube was incubated for 30 min (at room temperature) in the dark. The absorbance was measured at 517 nm using a spectrophotometer (UV-1200S, China). The radicals inhibition percentage was calculated using the following formula:
where Acontrol is the absorbance of DPPH solution without extract and Asample is the absorbance of the sample with DPPH solution.
Results and discussion
Fourier transform infrared analysis
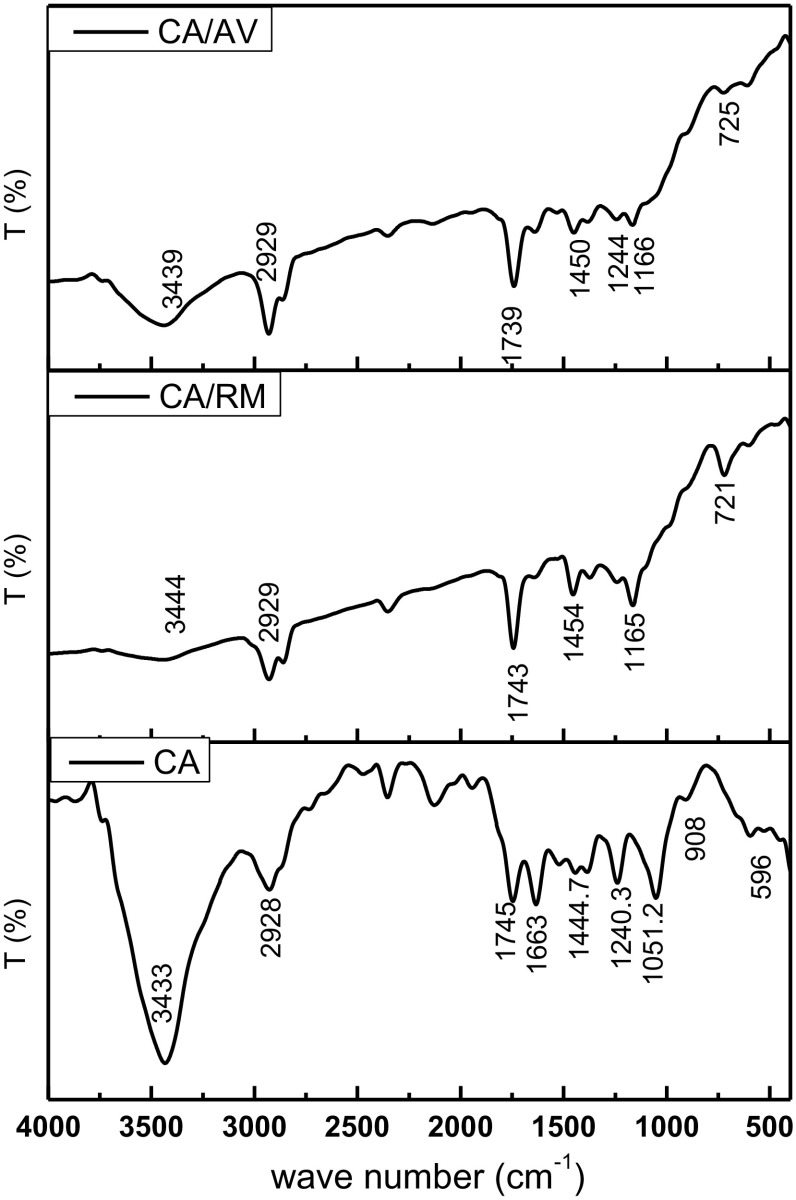
Figure 1 displays the FT-IR chart of cellulose acetate incorporated with Rosemary and Aloe Vera oil. In the case of CA membrane (blank), The broad absorption band at 3433 cm−1 is appointed to O–H stretching of a hydroxyl group (Zafar et al. 2012; Waheed et al. 2014). The bands at wavenumber 2928 cm−1 is assigned to stretching of C–H bond (Khalf et al. 2015). The peak at 1745 cm−1 is attributed to the stretching mode of the C=O group (Dehkordi et al. 2015; Waheed et al. 2014; Zafar et al. 2012). The bands at 1224 and 1444 cm−1 arise from C–H bending, wagging and rocking vibrations, respectively (Khalf et al. 2015; Waheed et al. 2014). The peak at 1051 cm−1 assigned to ether linkage (C–O–C) from glycosidic component followed by a peak at 908 cm−1 come from saccharide (Waheed et al. 2014). The FT-IR spectrum of the CA membranes impregnated with Rosemary or Aloe Vera oils displayed insignificant changes compared to control sample spectrum. The presence of Rosemary or Aloe Vera oils affects the intensity of the O–H stretching of the hydroxyl group as it decreased. This indicates the interaction between O–H of CA and oil function groups (Zafar et al. 2012; Waheed et al. 2014). It has been reported that the intensity of hydroxyl group vibration in the FT-IR spectrum of CA/phenols blends decreases as the concentration of phenols in blend increase (Gaibler et al. 2004). All the other peaks were similar to the peaks existing in the blank membrane.
Fig. 1.
FT-IR of cellulose acetate membranes incorporated with Rosemary and Aloe Vera oil extracts
Membranes microstructure
The microstructure of membranes, which rely on the interactions between drying conditions and membrane components, extremely affects the physical properties of the final membranes. In membranes containing EO, the final structure is influenced by the stability of the film-forming emulsion. The final EO droplets size and distribution in the matrix depend on several emulsion destabilization mechanisms (coalescence, flocculation, and creaming) that happen through film drying step (Fabra et al. 2009; Villalobos et al. 2005).
Figure 2a shows the SEM micrographs for CA membranes and that contain essential oils. SEM observations show that CA membranes exhibited roughness structures. This roughness may come from drying conditions in the air. EO adding increase irregularities on the membrane surface because of the low miscibility of these compounds in the polymer matrix. EO are dispersed in the film-forming solution and coalescence, creaming and flocculation phenomena happen during the membrane drying process. This lead to a portion of the EO droplets moves to the membrane surface (during the drying process), wherever the EO evaporates and leaving holes on the surface (Sandra et al. 2016). Distribution variation of EO in the polymer matrix is associated with the volatility, different composition and interactions (affinity) between them and the polymer matrix. Those factors influence the initial emulsion stability and EO distributed throughout the film drying step. Similar results earlier have been observed for polymer and EO like starch films (Jimenez et al. 2012), gelatin films (Ahmad et al. 2012) and starch-gelatin blend films (Sandra et al. 2016).
Fig. 2.
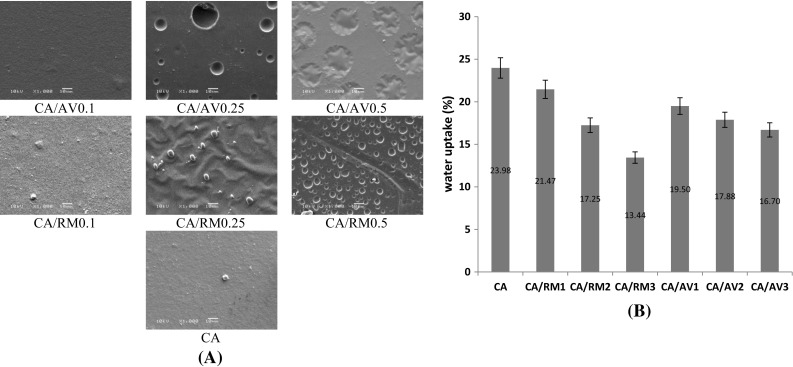
a SEM, b water uptake of cellulose acetate membranes incorporated with Rosemary and Aloe Vera oil extracts
Mechanical properties
Table 1 illustrates mechanical properties of CA membranes with and without Rosemary and Aloe Vera Oil. According to the Table, the tensile strength (TS) of the CA membrane was decreased with increasing oil content. For example, TS of CA (blank) was 166.5 N/mm2 and it decreased to 114.7 and 69.9 N/mm2 by using 0.5% of Rosemary and Aloe Vera oils, respectively. The TS of CA membranes refers to the intermolecular hydrogen bonds between its functional groups (i.e. hydroxyl) along the polymer backbone. So the addition of essential oils causes the reduction in TS as it penetrates between the polymer chains and hinders the intermolecular interactions between CA functional groups (Kalaycıoglu et al. 2017). Furthermore, the additions of oils make strain percent changed randomly as this additive effect on the arrangement of polymer chains. The incorporation of EO into polymeric matrices have represented different effects as represented by earlier studies. Kechichian et al. (2010) found that there was a reduction in the mechanical properties of the starch membranes after integrating clove and cinnamon powder. Sanchez-Gonzalez et al. (2011) found an alike trend in membranes based on chitosan and HPMC after using different EO concentrations. In addition, Sandra et al. (2016) found a similar trend in membranes based on starch-gelatin blend films after using different concentrations of cinnamon, clove, and oregano. All the previous results indicate that the EO effect on the membrane’s mechanical properties relies on the polymer matrix type, the kind and concentration of EO, and the interactions among the components (Ahmad et al. 2012), which define the efficient adhesion forces at the oil-polymer interface.
Table 1.
Mechanical properties of cellulose acetate membranes incorporated with Rosemary and Aloe Vera oil extract
| Membranes | Mechanical properties | |
|---|---|---|
| Stress (N/mm2) | Strain (%) | |
| CA | 166.5 ± 8.1 | 17.9 ± 5.4 |
| CA/RM1 | 226.8 ± 10.5 | 12.7 ± 0.5 |
| CA/RM2 | 148.3 ± 8.4 | 15.6 ± 4.2 |
| CA/RM3 | 114.7 ± 5.3 | 22.9 ± 3.1 |
| CA/AV1 | 162.4 ± 6.4 | 20.8 ± 3.5 |
| CA/AV2 | 148.3 ± 5.2 | 32 ± 5.2 |
| CA/AV3 | 69.9 ± 3.4 | 13.4 ± 3.7 |
Water uptake percent
Water uptake of CA membranes and that incorporated with Rosemary and Aloe Vera oil is shown in Fig. 2b. A decrease in water uptake was noticed by increasing the oil content in the feed mixture, i.e. the addition of EO to CA membranes caused a decrease in the water uptake and contact angle data (Table 2). This means that the addition of EO to CA membranes lead to increase the hydrophobicity. The hydrophobic phenolic ingredients of Rosemary and Aloe Vera oil can explain the behavior. CA has both hydrophilic groups (i.e. hydroxyl groups) and hydrophobic groups (i.e.; acetate groups); ratio and distribution of both groups control the wettability behavior of membranes. The addition of oil extracts influences this balance toward the hydrophobic nature. The acetyl groups of CA matrix (hydrophobic region) interacting with poly-aromatic ingredients of the essential oil and so the water uptake changed depending on this interaction. In addition, water molecules should disperse mostly through the uninterrupted polymeric phase, but the existence of EO droplets acts discontinuities that cause an efficient, increase in the tortuosity parameter for water transfer in the matrix and so hydrophobicity increased. The effect of EO integration on the water uptake and contact angle properties of the membranes has been studied in earlier studies and different results have been described (Ahmad et al. 2012; Wu et al. 2013). This effect relies on various factors, such as the polymer kind, the amount and composition of the oil added and the interactions between the oil and the matrix (Sanchez-Gonzalez et al. 2011).
Table 2.
Contact angle measurement of cellulose acetate membranes incorporated with Rosemary and Aloe Vera oil extracts
| Membranes | Contact angle (degree) |
|---|---|
| CA | 52.8 ± 0.4 |
| CA/RM1 | 55.2 ± 0.2 |
| CA/RM2 | 72.9 ± 0.2 |
| CA/RM3 | 78.9 ± 0.3 |
| CA/AV1 | 64.1 ± 0.3 |
| CA/AV2 | 71.3 ± 0.3 |
| CA/AV3 | 74.8 ± 0.8 |
Antibacterial activity determination
Evaluation of the antibacterial activity is essential to investigate the activity of membrane against pathogen bacterial for different applications. Figure 3 shows the antibacterial activity of CA membranes against E. coli (Gram-negative) and B. subtilis (Gram-positive) bacteria. The antibacterial activity was determined by the growth turbidity method. The results demonstrated that the CA membrane has no antibacterial activity. This result agrees with previous work (Stoja et al. 2017). However, the membranes acquire antimicrobial activity by adding Rosemary and Aloe Vera oils to it. Antimicrobial activity of essential oils has been referred to the existence of phenolic compounds. The inhibitory effect of phenol compounds is clarified by their interaction with the microorganisms cell membrane (Ben Arfa et al. 2006). Furthermore, antimicrobial activity was raised by increasing oil extract content (Stoja et al. 2017). The differences in antibacterial activity between CA/AV and CA/RO membranes may be related to the ratio of polymer: EO in the membrane, the composition and the nature of the EO and potential interactions between active compounds and polymers which can impact their diffusion in the medium (Sanchez-Gonzalez et al. 2011). The designation of the natural antimicrobial compounds and the future evolution of these compounds via structure/activity studies offer a promising way of a research for novel antimicrobials.
Fig. 3.
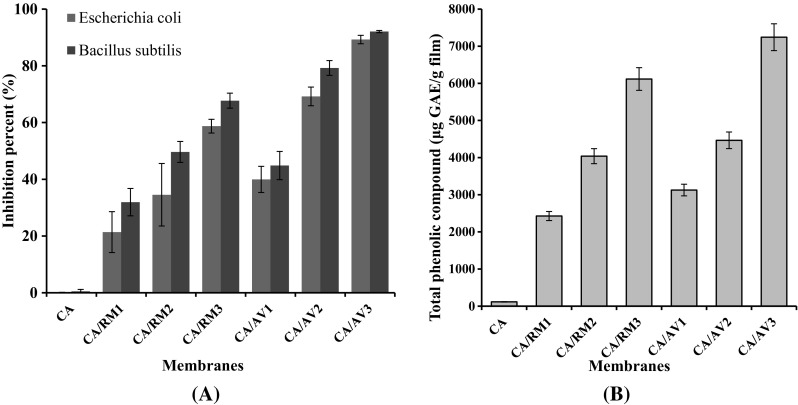
a Antibacterial activity of cellulose acetate membranes incorporated with Rosemary and Aloe Vera oil extracts against E. coli and B. subtilis., b Total phenolic compounds
In situ evaluation method
Using of sliced cheese to evaluate the tested membranes showed that the CA membrane has no antibacterial activity (Table 3). This result agrees with previous work (Stoja et al. 2017). In addition, untreated cheese slices show the high colony number of B. subtilis and E. coli. On the other hand, the membranes of CA incorporated with Rosemary and Aloe Vera oils have excellent antimicrobial activity at all concentrations tested. The count of B. subtilis and E. coli was significantly reduced in cheese slices by increasing oil concentration. And so the best membranes are CA/AV3 and CA/RM3 as there is no bacterial growth observed. The significant decrease in the development of bacterial and the long-term control of antibacterial inhibition was attributed to the antimicrobial activity of oil ingredients.
Table 3.
Antibacterial activity of cellulose acetate membranes incorporated with Rosemary and Aloe Vera oil extracts against E. coli and B. subtilis in sliced cheese
| Membranes | Strains | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B. subtilis (Gram-positive) | E. coli (Gram-negative) | |||||||||
| Time (day) | ||||||||||
| 0 | 1 | 3 | 5 | 7 | 0 | 1 | 3 | 5 | 7 | |
| Control | 310 ± 6.2 | 337 ± 6.2 | 349 ± 7 | 363 ± 7.2 | 381 ± 7.6 | 430 ± 8.6 | 437 ± 8.7 | 429 ± 8.5 | 463 ± 9 | 481 ± 9.6 |
| CA | 342 ± 6.9 | 332 ± 5.2 | 337 ± 5.1 | 337 ± 5.4 | 339 ± 5.6 | 472 ± 9.4 | 479 ± 9.6 | 464 ± 9.2 | 472 ± 9.2 | 469 ± 9.2 |
| CA/RM1 | 223 ± 4.5 | 235 ± 4.8 | 267 ± 5.8 | 253 ± 5 | 271 ± 5.5 | 282 ± 5.5 | 273 ± 5.2 | 275 ± 5.3 | 272 ± 5.1 | 278 ± 5.3 |
| CA/RM2 | 94 ± 2 | 88 ± 1.8 | 97 ± 2.5 | 119 ± 2.3 | 103 ± 2 | 88 ± 1.8 | 83 ± 1.6 | 74 ± 1.3 | 98 ± 1.9 | 93 ± 1.8 |
| CA/RM3 | 0 | 0 | 0 | 0 | 0 | 9 ± 0.2 | 5 ± 0.1 | 0 | 0 | 0 |
| CA/AV1 | 290 ± 5.5 | 281 ± 5.3 | 294 ± 6 | 305 ± 6 | 317 ± 5.9 | 342 ± 6.8 | 332 ± 6.7 | 337 ± 6.7 | 337 ± 6.7 | 339 ± 6.8 |
| CA/AV2 | 104 ± 2.5 | 138 ± 4.2 | 197 ± 3.9 | 169 ± 3.4 | 203 ± 4 | 143 ± 2.8 | 135 ± 2.6 | 137 ± 2.6 | 123 ± 2.4 | 141 ± 2.7 |
| CA/AV3 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 |
Controle treatment refer to infected cheese uncovered by membranes
Total phenolic content
The antioxidant activity of Rosemary and Aloe Vera oils was described to be resulting partly from the presence of phenolic groups (Viuda-Martos et al. 2010). As the specific structure of phenolic compounds enables it to scavenge free radicals and perform as antioxidants (Lopez et al. 2003).
Total phenolic compounds of membrane extracts were estimated based on the reduction of the Folin Ciocalteu reagent and the data are given in Fig. 3b. Results demonstrated that the soaking of the developed membranes in ethanol for extracting the included phenolic components leads to the expansion of their structural integrity and liberated Rosemary and Aloe Vera oil content. The data confirmed that the total phenolic content of the CA membrane (blank) is very small and it starts to increase after the addition of Aloe Vera and Rosemary oils. Moreover, total phenolic content increased as the oil amount increased in the CA membranes. This indicates that the oxygenated monoterpenes, maybe monoterpenoid ketones with recognized antioxidant properties, may have the utmost influence to the antioxidant capacity of the oils. Our results are in agreement with previous data reported on the antioxidant activity of Rosemary and Aloe Vera EO (Viuda-Martos et al. 2010; Aleksandar et al. 2014).
Antioxidant determination
DPPH radical scavenging activity determination
An in vitro design systems have been applied for evaluating the antioxidant potency of essential oil via measuring its free radical scavenging activity. DPPH assay based on 2,2-diphenyl-1-picrylhydrazyl radical for examining radical scavenging activity of the phenolic ingredients of essential oil or plant extracts. The radical form of DPPH has violet color until accepting an electron from the antioxidant compound, the violet color of the DPPH radical was reduced to yellow colored diphenyl-picrylhydrazine radical that was estimated colorimetrically (Dehpour et al. 2009).
Figure 4a presented the inhibition percent of DPPH dye by CA incorporated with a different percent of Rosemary or Aloe Vera oil. The CA membrane has slightly radical scavenging activity because of the presence of the polysaccharide hydroxyl groups. The increase of Aloe Vera or Rosemary oil in membranes shows a regular increase of free radical scavenging activity. In addition, the results display that the antioxidant properties of the Rosemary oil are higher than that of the Aloe Vera oil. This can be clarified by the existence of different ingredients and phenolic compounds in both oils (Sanchez-Gonzalez et al. 2011). The obtained results have been agreed with that published earlier (Jhang and Lee 2017; Vidic et al. 2014).
Fig. 4.
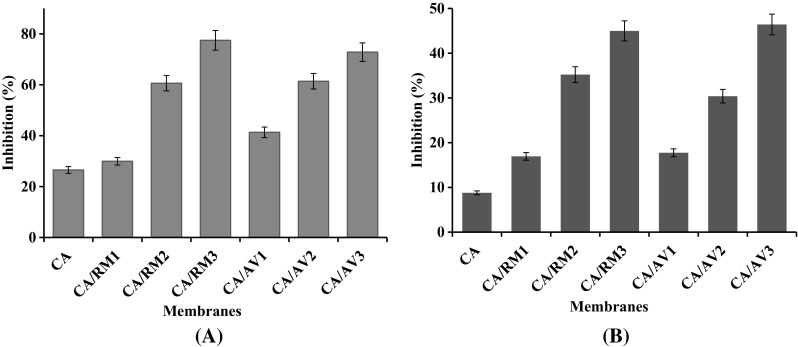
a DPPH scavenging activity; b ABTS·+cation scavenging activity of cellulose acetate membranes incorporated with Rosemary and Aloe Vera oil extract
ABTS radical scavenging activity determination
The antioxidant of CA membranes incorporated with Rosemary and Aloe Vera oil was determined by ABTS cation scavenging activity assay- which measures mostly water-soluble antioxidants with Single Electron Transfer (SET) mechanism and the results are shown in Fig. 4b.
It can be seen from Fig. 4b that CA membrane (control) showed the lowest ABTS radical scavenging activity. However, ABTS radical scavenging activity significantly increased after the addition of both Rosemary and Aloe Vera oils. By raising the oil amount from 20 to 80% (v/w) the activity was promoted. This can be referred to the highest phenolic content of the oils compared with CA. The presence of phenolic compounds in Rosemary and Aloe Vera oils allowed it to donate an electron and decolorized ABTS color. Our results are in agreement with previous data reported (Jhang and Lee 2017; Vidic et al. 2014; Stoja et al. 2017).
Conclusion
Cellulose acetate membranes were successfully formed, but such membranes have no antibacterial activity. Aloe Vera and Rosemary essential oils were integrated into the membranes. SEM analysis exhibited that the membranes containing essential oils were non-uniform (more roughness). Moreover, the prepared membranes exhibited a hydrophobic character by using essential oils. Antioxidant an activity assay (DPPH and ABTS assay) indicated that the membranes containing essential oils have an excellent antioxidant ability compared to blank cellulose acetate membrane. Moreover, the prepared membranes showed decent antimicrobial activity against Gram-negative (E. coli) and Gram-positive (B. subtilis) bacteria and this activity increased with increasing the used essential oil contents. The obtained results clearly suggested that the newly developed cellulose acetate membranes could be applied effectively as bio-active materials for food packaging fields.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmad M, Benjakul S, Prodpran T, Agustini TW. Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leather jacket incorporated with essential oils. Food Hydrocoll. 2012;28:189–199. doi: 10.1016/j.foodhyd.2011.12.003. [DOI] [Google Scholar]
- Aleksandar R, Isidora M, Nebojša P, Tatjana Ć, Saša V, Momir M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement Altern Med. 2014;14:225–234. doi: 10.1186/1472-6882-14-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárbara T, António M, Cristina R, Nuno RN, José MN, Jorge AS, Maria LN. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind Crops Prod. 2013;43:587–595. doi: 10.1016/j.indcrop.2012.07.069. [DOI] [Google Scholar]
- Ben Arfa A, Combes S, Preziosi-Belloy L, Gontard N, Chalier P. Antimicrobial activity of carvacrol related to its chemical structure. Lett Appl Microbiol. 2006;43:149–154. doi: 10.1111/j.1472-765X.2006.01938.x. [DOI] [PubMed] [Google Scholar]
- Buchbauer G. Biological activities of essential oils. In: Başer KHC, Buchbauer G, editors. Handbook of essential oils: science, technology, and applications. Boca Raton: CRC Press/Taylor & Francis Group; 2010. pp. 235–280. [Google Scholar]
- Burt SA, Van DZ, Koets AP, De Graaff AM, Van Knapen F, Gaastra W, Haagsman HP, Veldhuizen EJ. Carvacrol induces heat shock protein 60 and inhibits synthesis of flagellin in Escherichia coli O157:H7. Appl Environ Microbiol. 2007;73:4484–4490. doi: 10.1128/AEM.00340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casian OR, Parvu M, Vlase L, Tamas M. Antifungal activity of Aloe vera leaves. Fitoterapia. 2007;78:219–222. doi: 10.1016/j.fitote.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Dehkordi FS, Pakizeh M, Mahboub MN. Properties and ultrafiltration efficiency of cellulose acetate/organically modified Mt (CA/OMMt) nanocomposite membrane for humic acid removal. Appl Clay Sci. 2015;105–106:178–185. doi: 10.1016/j.clay.2014.11.042. [DOI] [Google Scholar]
- Dehpour AA, Ebrahimzadeh MA, Nabavi SF, Nabavi SM. Antioxidant activity of methanol extract of Ferula assafoetida and its essential oil composition. Grasas Aceites. 2009;60:405–412. doi: 10.3989/gya.010109. [DOI] [Google Scholar]
- El-Fawal G. Preparation, characterization and antibacterial activity of biodegradable films prepared from carrageenan. J Food Sci Technol. 2014;51:2234–2239. doi: 10.1007/s13197-013-1255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabra MJ, Talens P, Chiralt A. Microstructure and optical properties of sodium caseinate films containing oleic acid—beeswax mixtures. Food Hydrocoll. 2009;23:676–683. doi: 10.1016/j.foodhyd.2008.04.015. [DOI] [Google Scholar]
- Gaibler DW, Rochefort WE, Wilson JB, Kelley SS. Blends of cellulose ester/phenolic polymers-chemical and thermal properties of blends with polyvinyl phenol. Cellulose. 2004;11:225–237. doi: 10.1023/B:CELL.0000025425.00668.de. [DOI] [Google Scholar]
- Jhang LJ, Lee DJ. Compressional-puffing pretreatment for enhanced antioxidant compounds extraction from Aloe vera. J Taiwan Inst Chem Eng. 2017;81:170–174. doi: 10.1016/j.jtice.2017.09.009. [DOI] [Google Scholar]
- Jimenez A, Fabra MJ, Talens P, Chiralt A. Effect of re-crystallization on tensile, optical and water vapour barrier properties of corn starch films containing fatty acids. Food Hydrocoll. 2012;26:302–310. doi: 10.1016/j.foodhyd.2011.06.009. [DOI] [Google Scholar]
- Kalaycıoglu Z, Torlak E, Evingur GA, Ozen I, Erim FB. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int J Biol Macromol. 2017;101:882–888. doi: 10.1016/j.ijbiomac.2017.03.174. [DOI] [PubMed] [Google Scholar]
- Kechichian V, Ditchfield C, Veiga-Santos P, Tadini C. Natural antimicrobial ingredients incorporated in biodegradable films based on cassava starch. LWT Food Sci Technol. 2010;43:1088–1094. doi: 10.1016/j.lwt.2010.02.014. [DOI] [Google Scholar]
- Khalf A, Singarpu K, Madihally SV. Cellulose acetate core–shell structured electro spun fiber fabrication and characterization. Cellulose. 2015;22:1384–1400. doi: 10.1007/s10570-015-0555-9. [DOI] [Google Scholar]
- Lee J, Lee J, Song K. Development of a chicken feet protein film containing essential oils. Food Hydrocoll. 2015;46:208–215. doi: 10.1016/j.foodhyd.2014.12.020. [DOI] [Google Scholar]
- Lopez M, Martınez F, Del Valle C, Ferrit M, Luque R. Study of phenolic compounds as natural antioxidants by a fluorescence method. Talanta. 2003;60:609–616. doi: 10.1016/S0039-9140(03)00191-7. [DOI] [PubMed] [Google Scholar]
- Mendes G, Faria M, Carvalho A, Gonçalves MC, Pinho MN. Structure of water in hybrid cellulose acetate-silica ultrafiltration membranes and permeation properties. Carbohyd Polym. 2018;189:342–351. doi: 10.1016/j.carbpol.2018.02.030. [DOI] [PubMed] [Google Scholar]
- Navajas YR, Martos MV, Sendra E, Alvarez JAP, López F. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control. 2013;30(2):386–392. doi: 10.1016/j.foodcont.2012.07.052. [DOI] [Google Scholar]
- Niu X, Liu Y, Song Y, Han J, Pan H. Rosin modified cellulose nanofiber as a reinforcing and co-antimicrobial agents in polylactic acid/chitosan composite film for food packaging. Carbohyd Polym. 2018;183:102–109. doi: 10.1016/j.carbpol.2017.11.079. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gonzalez L, Chafer M, Gonzalez-Martínez C, Chiralt A, Desobry S. Study of the release of limonene present in chitosan films enriched with bergamot oil in food simulants. J Food Eng. 2011;105:138–143. doi: 10.1016/j.jfoodeng.2011.02.016. [DOI] [Google Scholar]
- Sandra A, Amparo C, Pilar S, Josefa R, Chelo GM, Maite C. Antifungal films based on starch-gelatin blend, containing essential oils. Food Hydrocoll. 2016;61:233–240. doi: 10.1016/j.foodhyd.2016.05.008. [DOI] [Google Scholar]
- Skyttä E, Mattila ST. A quantitative method for assessing bacteriocins and other food antimicrobial by automated turbidometry. J Microbiol Methods. 1991;14:77–88. doi: 10.1016/0167-7012(91)90036-P. [DOI] [Google Scholar]
- Stoja M, Tijana A, Ksenija A, Dusan M, Jasna I, Irena Z (2017) Cellulose acetate based material with antibacterial properties created by supercritical solvent impregnation. Int J Polym Sci. Article ID 8762649
- Vásquez MJM, Buitimea ELV, Jatomea MP, Encinas JCE, Félix FR, Valdes SS. Functionalization of chitosan by a free radical reaction: characterization, antioxidant and antibacterial potential. Carbohydr Polym. 2017;155:117–127. doi: 10.1016/j.carbpol.2016.08.056. [DOI] [PubMed] [Google Scholar]
- Vidic D, Tarić E, Alagić J, Maksimović M. Determination of total phenolic content and antioxidant activity of ethanol extracts from Aloe spp. BCTBH. 2014;42:5–10. [Google Scholar]
- Villalobos R, Chanona J, Hernández P, Gutierrez G, Chiralt A. Gloss and transparency of hydroxypropilmethylcellulose films containing surfactants as affected by their microstructure. Food Hydrocoll. 2005;19:53–61. doi: 10.1016/j.foodhyd.2004.04.014. [DOI] [Google Scholar]
- Viuda-Martos M, Ruiz NY, Sánchez ZE, Fernández-López J, Pérez-Álvarez JA. Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Frag J. 2010;25:13–19. doi: 10.1002/ffj.1951. [DOI] [Google Scholar]
- Voicu SI, Condruz RM, Mitran V, Cimpean A, Miculescu F, Andronescu C, Miculescu M, Thakur VK. Sericin covalent immobilization onto cellulose acetate membrane for biomedical applications. ACS Sustain Chem Eng. 2016;4:1765–1774. doi: 10.1021/acssuschemeng.5b01756. [DOI] [Google Scholar]
- Waheed S, Ahmad A, Khan SM, Gul S, Jamil T, Islam A, Hussain T. Synthesis, characterization, permeation and antibacterial properties of cellulose acetate/polyethylene glycol membranes modified with chitosan. Desalination. 2014;351:59–69. doi: 10.1016/j.desal.2014.07.019. [DOI] [Google Scholar]
- Wu J, Chen S, Ge S, Miao J, Li J, Zhang Q. Preparation, properties and antioxidant activity of an active film from silver carp (Hypophthalmichthys molitrix) skin gelatin incorporated with green tea extract. Food Hydrocol. 2013;32:42–51. doi: 10.1016/j.foodhyd.2012.11.029. [DOI] [Google Scholar]
- Zafar M, Ali M, Khan SM, Jamil T, Butt MTZ. Effect of additives on the properties and performance of cellulose acetate derivative membranes in the separation of isopropanol/water mixtures. Desalination. 2012;285:359–365. doi: 10.1016/j.desal.2011.10.027. [DOI] [Google Scholar]