Abstract
AIM
To analyze the expression of uncoupling protein 2 (UCP2) in retinal pigment epithelium (RPE) cells at the different human age, further explore the possible new target of RPE cells protection.
METHODS
Adult retinal pigment epithelial-19 (ARPE-19) cells and the primary RPE cells at the different age (9-20y, 50-55y, 60-70y, >70y) were cultured and harvested. The expression of UCP2 in these cells was detected by reverse transcription-polymerase chain reaction (RT-PCR), Western blot and confocal microscopy.
RESULTS
Cells from the donors more than 60y are larger and more fibroblastic in appearance compared to ARPE-19 cells and those primary cultures obtained from the younger individuals by using phase-contrast micrographs. Results of RT-PCR, Western blot and confocal microscopy all showed that UCP2 was highly expressed in ARPE-19 cells and in the younger primary cultured human RPE cells at the age of 9-20y and 50-55y, whereas lower expression of UCP2 was measured in the older primary cultured human RPE cells at the age more than 60y.
CONCLUSION
Expression of UCP2 gene is decreased in aged RPE cells, promoting the lower ability of anti-oxidation in these cells. It is indicated that UCP2 gene might be a new target for protecting the cells from oxidative stress damage.
Keywords: retinal pigment epithelium cells, aging, uncoupling protein 2, oxditive stress, anti-oxidation
INTRODUCTION
Retinal pigment epithelial (RPE) is one monolayer pigmented cell located in the retina and exposed to a highly oxidative environment, partly due to increased oxygen partial pressure from the choriocapillaris and digestion of polyunsaturated fatty acid laden photoreceptor outer segments. It is known that aging RPE cells are an underlying cause in the progression of age-related retinal diseases such as age-related macular degeneration (AMD), in part, from cumulative oxidative damage to the RPE[1]–[7].
Potential antioxidants have been reported for RPE cells protection in an oxidative environment. Shen et al[8] discovered that riluzole protected RPE cells from apoptosis and this protection mechanism could be from stabilizing mitochondrial Δψm and preventing the release of cytochrome C (Cyt-c). They also proved that changes in TRAAK expression might help to protect RPE cells in oxidative stress. Sachdeva et al[9] found a higher expression of the major regulators of antioxidant Nrf2-targeted genes in aging RPE, suggesting an age-related increase in oxidative stress. The study indicated that the aging RPE was vulnerable to oxidative stress damage due to impaired Nrf2 signaling[9]. There is also a study which identified PGC-1α as a potent driving factor for mitochondrial function and antioxidant capacity of RPE[10]. In addition, a present study showed that isorhamnetin, a 3-O-methylated metabolite of quercetin, exhibited antioxidant effects. They certified that isorhamnetin protected human RPE cells from oxidative stress-induced cell death, inhibiting H2O2-induced reactive oxygen species (ROS) production and caspase-3 activation in RPE cells. And this effect was associated with activation of the PI3K/Akt signaling pathway[11].
A few studies showed that uncoupling protein 2 (UCP2) played an important role in reducing ROS and inhibiting cell death under oxidative stress. UCP2 was a proton transport protein in the inner membrane of mitochondria, and its essence was H+ channel on the inner membrane of the mitochondria. UCP2 occupied 6%-8% of all mitochondrial proteins and 14% of a membrane protein, increasing the proton leakage of the inner mitochondrial membrane. This resulted in the reduction of transmembrane gradient dependent on the mitochondrial proton on the synthesis of adenosine triphosphate (ATP), leading to the decrease in the efficiency of synthesizing ATP by adenosine diphosphate phosphorylation, oxidation, and phosphate dissolving coupling. With the higher ROS level, the expression of UCP2 increased and the formation of ROS decreased, thus preventing the apoptosis of mitochondrial pathway[12]–[13].
Previous studies had reported that UCP2 was significantly increased in the cells of human colon cancer and was proportional to the deterioration extent of cancer cells[14]. It suggested that UCP2 might be used as an adaptive tool in cancer cells, which could reduce oxidative stress and resist apoptosis. Considering the exposure of RPE cells in a high oxidative environment, it is important to analyze the expression of UCP2 in these cells. The purpose of this study was to detect the expression of UCP2 in RPE cells at different ages.
SUBJECTS AND METHODS
Ethical Approval
Adult retinal pigment epithelial-19 (ARPE-19) cell line and primary RPE cells (9-20y, 50-55y, 60-70y, >70y) were donated by Professor Dr. Joyce Tombran-Tink of the University of Wisconsin and the University of Pennsylvania in the United States. The study followed the principles of the Declaration of Helsinki. The experimental procedures have been approved by the Xi'an Medical University Use Committee.
Cell Culture
Culture medium for RPE cells in vitro included: DMEM/F12 medium, inactivated fetal calf serum, 0.25% pancreatin filtered by 0.22-micron filter, a double antibody of penicillin and streptomycin. Cells were cultured in a moist incubator with 5% CO2 at 37°C. Digestion and passaging were performed by 0.25% trypsin when the cells grew to 85%-95% every 2-3d. The morphology of cells was observed by phase contrast microscope.
Characterization of Retinal Pigment Epithelial Cells
An RPE cell marker RPE65 was examined to assess RPE homogeneity in the cultures. Immunofluorescence method was used to identify the cells. Cells were sowed in 6-well plates slide. When the density of adhered cells reached 85 to 95 percent, the cells were removed from the incubator and washed three minutes each time by phosphate buffered saline (PBS) three times. Cells were fixed by 4% multi polyformaldehyde indoor for 30min, then washed three minutes each time by PBS three times. The membrane was permeabilized by Triton for 20min and was blocked by 5% goat serum for one hour. Diluted 1:250 anti-mouse monoclonal RPE65 antibody was dropped and hatched overnight at 4°C, then washed three minutes each time by PBS three times. FITC labeled secondary antibody was dropped and incubated for 1h at 37°C in the avoidance of light, then washed three minutes each time by PBS three times. Nuclear staining was performed for 7min using 10 µg/mL DAPI, then washed three minutes each time by PBS three times, and mounted using 50% glycerol. Normal mouse and rabbit serum (1:1000) were used instead of the RPE65 antibody in some experiments to serve as negative controls. Labeled cells were visualized by confocal microscopy.
Reverse Transcription-Polymerase Chain Reaction
Total RNA was extracted using RNA RNeasy kit (Qiagen, Valencia, CA, USA). The first chain reverse transcription cDNA of total RNA was proposed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA), and the reverse transcription-polymerase chain reaction (RT-PCR) for DNA was amplified using the iTaq polymerase kit (Bio-Rad, Hercules, CA, USA) at an annealing temperature of 60°C for 35 cycles. PCR products were analyzed on 1% agarose gel electrophoresis. UCP2 primers: forward CTACAAGACCATTGCACGAGAGG and reverse AGCTGCTCATAGGTGACAAACAT were used. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal RNA loading control.
Western Blot Analysis
Protein samples were collected using nuclear protein and cell plasma protein extraction kit (Bio-Rad, Hercules, CA, USA). Of 30 µg of protein was separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). After blocking with 5% (w/v) non-fat dried milk, the membranes were incubated with primary antibodies (1:1000) for 3h at room temperature (RT), followed by washing and incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1h at RT. Bound antibody was determined using the Bio-Rad ECL detection system, and Image J software was used to quantitate the protein levels[15].
Expression of Uncoupling Protein 2 in Retinal Pigment Epithelial Cells by Confocal Microscopy
UCP2 was measured by fluorescent immunohistochemistry. Mitochondria were labeled using Mito Tracker Deep Red, and the actin protein was used for cytoskeleton. The immunofluorescence intensity of UCP2 in these cells was observed by confocal microscope. Cells were cultured and inoculated in Petri culture dish with the density of one hundred thousand cells per milliliter. Then the cells were fixed using 4% formaldehyde at RT for 30min and washed twice with PBS. Actin protein labeled by PBS labeled mitochondria or phalloidin containing 400 nmol/L Mito Tracker Deep Red (1:1000) was added in and incubated at RT for 30min in light, and then washed twice with PBS. Normal serum blocking fluid diluted with PBS (1:50) was added and was incubated in the light at RT for 20min, and then the liquid was dumped without wishing. The first pAb Anti-UCP (one gamma per milliliter dilution) was added and incubated in the light at RT for 1h, and then washed twice with PBS. Then, FITC-goat anti-mouse immunoglobulin G (1:500) diluted with PBS was added and incubated in light for 30min at RT, and then washed twice with PBS for 5min each time. Five hundred microliter PBS was added, and the confocal microscopy analysis was done immediately.
Statistical Analysis
All assays were performed using 6-10 independent primary cultures of RPE cells between passages 4-8, and each experiment was done in triplicate. All data were expressed as means±standard error (SE). One-way ANOVA test was performed, and statistical significance was set at P<0.05.
RESULTS
ARPE-19 and Primary Human Retinal Pigment Epithelial Cell Cultures and Identification
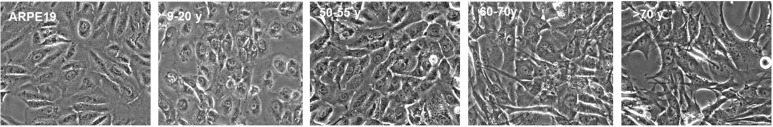
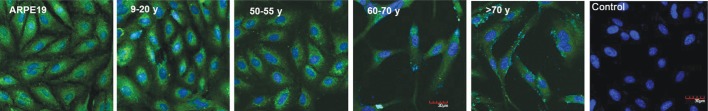
Phase-contrast micrographs of ARPE-19 cells and RPE cells showed that RPE cells from the 9-20y and 50-60y donors were more uniform and rounded in morphology and closely arranged like pebbles with round nucleus and clear cytoplasm. While cells derived from the 60-70y and >70y donor cells were longer than the fibroblast state, and the cell body was slightly larger than that of the young donor. ARPE-19 cells and RPE cells from the younger donor grew as a monolayer of tightly packed cobblestone-like cells in culture whereas those from individuals >60y were larger and more fibroblastic in appearance (Figure 1). With increased donor age, RPE cells appeared larger in size and showed greater cytoplasmic spreading. The identity of the cells in the cultures was established by visual observation of the pigmented cells by phase contrast microscopy and using RPE65 as an expression marker. Visual observation and immunocytochemistry indicated that greater than 99% of the cells in ARPE-19 and the primary cultures were pigmented and expressed RPE65 (Figure 2). Images of confocal microscopy showed that the RPE65 was uniformly expressed in the cytoplasm.
Figure 1. Phase-contrast micrographs of ARPE-19 and RPE cells from various donor ages.
ARPE-19 cells and RPE cells from the younger donors (9-20y) grew fast, the morphology was uniform and polygonal, had round nucleus, clear nucleolus and cytoplasm. The density of the cells reached above 90% within two to three days and then passaged. The cells were larger with increased aging, but all cultures showed robust pigmentation. Scale bar=30 µm.
Figure 2. Greater than 99% of cells in ARPE-19 and the primary RPE cultures from all donor ages expressed RPE65 (green fluorescence), a specific marker for these cells.
Scale bar=30 µm.
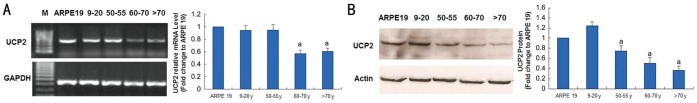
Expression of UCP2 in RPE Cells at Different Ages Detected by RT-PCR and Western Blot
The mRNA level of UCP2 in ARPE-19 cells and RPE cells at the younger donors (9-20y) were higher than that from the older donors (>60y) (Figure 3A). The protein level of UCP2 in ARPE-19 cells and RPE cells at 9-20y donors was relatively higher, while the expression of UCP2 in RPE cells more than 60y was decreased gradually with age (Figure 3B).
Figure 3. Expression of UCP2 in RPE cells with increased chronological age.
A: RT-PCR showed that there was a consistent decrease in mRNA levels of UCP2 with aging at an approximate 0.94 and 0.57 fold changes, respectively between the youngest and oldest donors; B: The protein levels of UCP2 decreased at an approximate 1.24 and 0.36 fold changes with aging in Western blot, respectively between the youngest and oldest donors. Results are expressed as the mean±SE of 3 independent experiments done in triplicates. aSignificantly different from 9-20y (P<0.05).
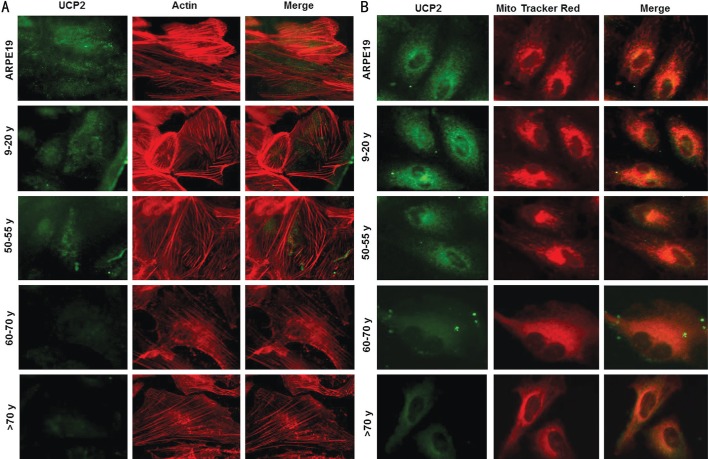
Expression of UCP2 in RPE Cells at Different Ages Detected by Confocal Microscopy
The expression of UCP2 in ARPE-19 cells and RPE cells decreased with age as shown in Figure 4A-4B. Cytoskeleton (Figure 4A) and mitochondria (Figure 4B) were labeled by actin and Mito Tracker Red in red, respectively, whereas UCP2 was labeled in green. The green staining under the confocal microscope was lost gradually in RPE cells with the increased donor ages.
Figure 4. Expression of UCP2 in RPE cells derived from human primary cells at different ages detected by confocal microscopy.
UCP2 antibody (green), actin (A; red) and Mito Tracker Red (B) showed the lost UCP2 antibody green staining in the RPE cells from the older donors by confocal microscopy. Scale bar=30 µm.
DISCUSSION
RPE cells are layers of epithelial cells containing melanin between the neural retina and choroid. RPE is a monolayer of hexagonal cells that are closely connected to each other, which could block the free travel of water and ions. Oxidative stress could damage the integrity of the closely linked structure in RPE, and therefore the RPE cells declined when barrier function is impaired, which was a key pathogenic factor of AMD[16]–[27].
As the main producer of cellular superoxide, mitochondria are the target of intracellular oxidative stress damage. Therefore, excessive oxidative stress could lead to mitochondrial damage, which further leads to the dysfunction of vascular endothelial cells[28] and gangliocyte ganglion cells[29]. In the process of aging, UCP was responsible for physiological uncoupling and could cause the decline of mitochondrial ROS production. UCP2 was a key subtype of human and a key protein to prevent oxidative stress damage in cultured glomerular mesangial cells[30]. UCP2 played an important role in the protection of oxidative stress of human sperm by reducing the production of ROS[31]. UCP2 could promote cell proliferation and inhibit the cell apoptosis induced by high glucose through up-regulating Bcl-2 and down-regulating caspase-3 and Cyt-c, which arrived at a conclusion that UCP2 might be a new protective factor for diabetic complications (such as diabetic retinopathy). In contrast to young mice, the deficiency of UCP2 in the elderly mice was more harmful to acute pancreatitis, which was hypothesized to be caused by an imbalance inflammatory response[32]. Oxidative stress was believed to support many aging processes, and the oxidative stress theory of aging was the most widely recognized theory[33]. Some researchers showed that induction of UCP could reduce cell damage due to the production of excessive ROS in the process of aging[34]. UCP2 could also prolong the lifespan, and was an important metabolic protein of the mitochondria connected to longevity[35]. In order to analyze the expression of antioxidant protein UCP2 in human RPE cells at the different ages, we cultured ARPE-19 cells and primary RPE cells, and then detected the expression of UCP2 in these cells by RT-PCR, Western blot, and laser scanning confocal microscopy. All the results showed that the expression of UCP2 in ARPE-19 cells and RPE cells from the younger donors was higher than those from the older donors, which suggested that the resistant ability to oxidative stress in RPE cells from the elderly people decreased, while apoptosis and oxidative damage increased significantly. Therefore, we proposed that the incidence of age-related pathological changes (AMD, etc.) in RPE cells in the older population increased significantly, with the lower ability of anti-oxidation, partly due to the decreased expression of UCP2.
UCP2, as a protein in the mitochondrial membrane, could prevent the apoptosis of the mitochondrial pathway and inhibit oxidative stress. We proposed the increased expression of UCP2 by regulation could enhance the ability of aged RPE cells to resist the oxidant damage. UCP2 was expected to be a new protective target to RPE cells with either diabetic retinopathy or aging and might become a new target for treating aging and degenerative diseases.
In conclusion, expression of the UCP2 gene was decreased in aged RPE cells, promoting the lower ability of anti-oxidation in these cells. It is indicated that the UCP2 gene might be a new target of RPE cells, protecting these cells from oxidative stress damage.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81100665; No.81770929).
Conflicts of Interest: He Y, None; Wang X, None; Liu X, None; Ji Z, None; Ren Y, None.
REFERENCES
- 1.D'Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9(4):645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- 2.Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, Vollrath D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet. 2000;26(3):270–271. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- 3.Dorey CK, Wu G, Ebenstein D, Garsd A, Weiter JJ. Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Invest Ophthalmol Vis Sci. 1989;30(8):1691–1699. [PubMed] [Google Scholar]
- 4.Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100(10):1519–1535. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- 5.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45(2):115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 6.Dunaief JL, Dentchev T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120(11):1435–1442. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- 7.Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Molr Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- 8.Shen C, Ma W, Zheng W, Huang H, Xia R, Li C, Zhu X. The antioxidant effects of riluzole on the APRE-19 celll model injury-induced by t-BHP. BMC Ophthalmol. 2017;17(1):210. doi: 10.1186/s12886-017-0614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sachdeva MM, Cano M, Handa JT. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp Eye Res. 2014;119:111–114. doi: 10.1016/j.exer.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iacovelli J, Rowe GC, Khadka A, Diaz-Aguilar D, Spencer C, Arany Z, Saint-Geniez M. PGC-1α induces human RPE oxidative metabolism and antioxidant capacity. Invest Ophthalmol Vis Sci. 2016;57(3):1038. doi: 10.1167/iovs.15-17758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Gong HM, Zou HH, Liang L, Wu XY. Isorhamnetin prevents H2O2-induced oxidative stress in human retinal pigment epithelial cells. Mol Med Rep. 2018;17(1):648–652. doi: 10.3892/mmr.2017.7916. [DOI] [PubMed] [Google Scholar]
- 12.Hong Y, Fink BD, Dillon JS, Sivitz WI. Effects of adenoviral overexpression of uncoupling protein-2 and -3 on mitochondrial respiration in insulinoma cells. Endocrinology. 2001;142(1):249–256. doi: 10.1210/endo.142.1.7889. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Axelsson J, Nordfors L, Qureshi AR, Avesani C, Barany P, Schalling M, Heimbürger O, Lindholm B, Stenvinkel P. Changes in fat mass after initiation of maintenance dialysis is influenced by the uncoupling protein 2 exon 8 insertion/deletion polymorphism. Nephrol Dial Transplant. 2007;22(1):196–202. doi: 10.1093/ndt/gfl504. [DOI] [PubMed] [Google Scholar]
- 14.Horimoto M, Resnick MB, Konkin TA, Routhier J, Wands JR, Baffy G. Expression of uncoupling protein-2 in human colon cancer. Clin Cancer Res. 2004;10(18 Pt1):6203–6207. doi: 10.1158/1078-0432.CCR-04-0419. [DOI] [PubMed] [Google Scholar]
- 15.He Y, Leung KW, Ren Y, Pei JZ, Ge J, Tombran-Tink J. PEDF improves mitochondrial function in RPE cells during oxidative stress. Invest Ophthalmol Vis Sci. 2014;55(10):6742–6755. doi: 10.1167/iovs.14-14696. [DOI] [PubMed] [Google Scholar]
- 16.Dong X, Li ZR, Wang W, Zhang WJ, Liu SZ, Zhang XM, Fang J, Maeda H, Matsukura M. Protective effect of canolol from oxidative stress-induced cell damage in ARPE-19 cells via an ERK mediated antioxidative pathway. Mol Vis. 2011;17:2040–2048. [PMC free article] [PubMed] [Google Scholar]
- 17.Lin H, Qian J, Castillo AC, Long B, Keyes KT, Chen G, Ye Y. Effect of miR-23 on oxidant-induced injury in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2011;52(9):6308–6314. doi: 10.1167/iovs.10-6632. [DOI] [PubMed] [Google Scholar]
- 18.Zhuge CC, Xu JY, Zhang J, Li W, Li P, Li Z, Chen L, Liu X, Shang P, Xu H, Lu Y, Wang F, Lu L, Xu GT. Fullerenol protects retinal pigment epithelial cells from oxidative stress-induced premature senescence via activating SIRT1. Invest Ophthalmol Vis Sci. 2014;55(7):4628–4638. doi: 10.1167/iovs.13-13732. [DOI] [PubMed] [Google Scholar]
- 19.Cruz-Guilloty F, Saeed AM, Duffort S, Cano M, Ebrahimi KB, Ballmick A, Tan YH, Wang H, Laird JM, Salomon RG, Handa JT, Perez VL. T cells and macrophages responding to oxidative damage cooperate in pathogenesis of a mouse model of age-related macular degeneration. PLoS One. 2014;9(2):e88201. doi: 10.1371/journal.pone.0088201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Promsote W, Veeranan-Karmegam R, Ananth S, Shen DF, Chan CC, Lambert NA, Ganapathy V, Martin PM. L-2-oxothiazolidine-4-carboxylic acid attenuates oxidative stress and inflammation in retinal pigment epithelium. Mol Vis. 2014;20:73–88. [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C, Cano M, Wang JJ, Li J, Huang C, Yu Q, Herbert TP, Handa JT, Zhang SX. Role of unfolded protein response dysregulation in oxidative injury of retinal pigment epithelial cells. Antioxid Redox Signal. 2014;20(14):2091–2106. doi: 10.1089/ars.2013.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheu SJ, Liu NC, Ou CC, Bee YS, Chen SC, Lin HC, Chan JY. Resveratrol stimulates mitochondrial bioenergetics to protect retinal pigment epithelial cells from oxidative damage. Invest Ophthalmol Vis Sci. 2013;54(9):6426–6438. doi: 10.1167/iovs.13-12024. [DOI] [PubMed] [Google Scholar]
- 23.Plafker SM, O'Mealey GB, Szweda LI. Mechanisms for countering oxidative stress and damage in retinal pigment epithelium. Int Rev Cell Mol Biol. 2012;298:135–177. doi: 10.1016/B978-0-12-394309-5.00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jitsanong T, Khanobdee K, Piyachaturawat P, Wongprasert K. Diarylheptanoid 7-(3, 4 dihydroxyphenyl)-5-hydroxy-1-phenyl-(1E)-1-heptene from Curcuma comosa Roxb. protects retinal pigment epithelial cells against oxidative stress-induced cell death. Toxicol In Vitro. 2011;25(1):167–176. doi: 10.1016/j.tiv.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Faghiri Z, Bazan NG. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp Eye Res. 2010;90(6):718–725. doi: 10.1016/j.exer.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang ZY, Shen LJ, Tu L, Hu DN, Liu GY, Zhou ZL, Lin Y, Chen LH, Qu J. Erythropoietin protects retinal pigment epithelial cells from oxidative damage. Free Radic Biol Med. 2009;46(8):1032–1041. doi: 10.1016/j.freeradbiomed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 27.Davis MD, Gangnon RE, Lee LY, Hubbard LD, Klein BE, Klein R, Ferris FL, Bressler SB, Milton RC, Age-Related Eye Disease Study Group The age-related eye disease study severity scale for age-related macular degeneration: AREDS report no. 17. Arch Ophthalmol. 2005;123(11):1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102(4):488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 29.Noh YH, Kim KY, Shim MS, Choi SH, Choi S, Ellisman MH, Weinreb RN, Perkins GA, Ju WK. Inhibition of oxidative stress by coenzyme Q10 increases mitochondrial mass and improves bioenergetic function in optic nerve head astrocytes. Cell Death Dis. 2013;4:e820. doi: 10.1038/cddis.2013.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Castro S, Scarpino S, Marchitti S, Bianchi F, Stanzione R, Cotugno M, Sironi L, Gelosa P, Duranti E, Ruco L, Volpe M, Rubattu S. Differential modulation of uncoupling protein 2 in kidneys of stroke-prone spontaneously hypertensive rats under high-salt/low-potassium diet. Hypertension. 2013;61(2):534–541. doi: 10.1161/HYPERTENSIONAHA.111.00101. [DOI] [PubMed] [Google Scholar]
- 31.Fu ZH, Zhou YH, Zhu WJ, Chen XM, Li XM, Tan Z. Uncoupling protein 2 combats oxidative damage to human sperm. Zhonghua Nan Ke Xue. 2010;16(6):516–519. [PubMed] [Google Scholar]
- 32.Müller S, Kaiser H, Krüger B, Fitzner B, Lange F, Bock CN, Nizze H, Ibrahim SM, Fuellen G, Wolkenhauer O, Jaster R. Age-dependent effects of UCP2 deficiency on experimental acute pancreatitis in mice. PLoS One. 2014;9(4):e94494. doi: 10.1371/journal.pone.0094494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harper ME, Bevilacqua L, Hagopian K, Weindruch R, Ramsey JJ. Ageing, oxidative stress, and mitochondrial uncoupling. Acta Physiol Scand. 2004;182(4):321–331. doi: 10.1111/j.1365-201X.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 34.Dietrich MO, Horvath TL. The role of mitochondrial uncoupling proteins in lifespan. Pflugers Arch. 2010;459(2):269–275. doi: 10.1007/s00424-009-0729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrews ZB. Uncoupling protein-2 and the potential link between metabolism and longevity. Curr Aging Sci. 2010;3(2):102–112. doi: 10.2174/1874609811003020102. [DOI] [PubMed] [Google Scholar]