ABSTRACT
Background
The popularity of nutrition-sensitive interventions calls for high-quality monitoring and evaluation tools. In this context, the Minimum Dietary Diversity for Women of Reproductive Age (MDD-W), validated as a proxy of micronutrient adequacy, does fill a gap. However, because it is a newly endorsed indicator, information on its linkages with other dimensions of food and nutrition security is still scarce.
Objective
The objective of this study was to investigate whether the MDD-W is related to household food insecurity and farm production diversity.
Methods
A cross-sectional survey on a representative sample of 5046 women of reproductive age was conducted in the region of Kayes, Mali, in 2013. Dietary diversity was assessed through qualitative 24-h recall, and MDD-W was computed. MDD-W equaled 1 if the women consumed at least 5 different food groups and 0 otherwise. Food insecurity was measured using the Household Food Insecurity Access Scale and the Household Hunger Scale (HHS), and a farm production diversity score (FPDS) was calculated based on a count of food crops/livestock groups produced. Logistic regressions were used to assess the relation between MDD-W and the indicators of household food security.
Results
Only 27% of women reached the MDD-W. These women consumed animal source foods and/or vitamin A-rich vegetables and fruits more frequently than did other women. Women from extremely food insecure households (moderate to severe hunger according to the HHS) were less likely to reach the MDD-W (OR: 0.70; 95% CI: 0.50, 0.97). One more group in the FPDS increased the odds of attaining the MDD-W (OR: 1.12; 95% CI: 1.06, 1.18).
Conclusion
In the rural region of Kayes, Mali, women's dietary diversity, as measured by the MDD-W, was associated with household-level food security indicators. This study was registered at ISRCTN.org as ISRCTN08435964.
Keywords: women, dietary diversity, nutrition sensitive, household food security, farm production diversity, West Africa, rural
Introduction
Nutrition-sensitive agricultural interventions are promising instruments to accelerate the achievement of nutrition and food security, thus contributing to the second Sustainable Development Goal (SDG-2) (1, 2). Conceptual frameworks propose several hypotheses to explain how agricultural actions can affect nutrition outcomes (3–7). One of these pathways relies on diversification of on-farm production, which should increase food availability and access, and hence increase consumption of diverse foods—a precondition for adequate intake of essential nutrients (8). Consumption of more diverse foods may also be the result of purchases on the market due to higher income resulting from increased production (9). Knowledge of the linkages
between agriculture and nutrition is emerging and sheds light on the importance of focusing on improving access to higher quality diets in projects promoting nutrition-sensitive agriculture (10, 11).
There is clearly a need for high-quality monitoring and evaluation tools in the context of renewed strategies emphasizing dietary diversity (12, 13). However, no indicator in the framework chosen to monitor progress toward nutrition-related SDGs captures diet quality (14). In this respect, the Minimum Dietary Diversity for Women of Reproductive Age (MDD-W), a dichotomous indicator developed and validated as a proxy of micronutrient adequacy (15, 16), does fill a gap. Women of reproductive age are particularly vulnerable to nutritional deficiencies, and it is essential to encourage actions to improve their nutrition that may also improve children's health, particularly through interventions targeting the first 1000 d (from pregnancy to children reaching 24 mo of age). The MDD-W is currently one of the key nutrition-sensitive indicators recommended by FAO (17), and it is also proposed by the new initiative of the Gallup World Poll aimed at providing comprehensive data on the quality of people's diets worldwide (18).
The MDD-W responds to several needs, including gathering accurate and comparable data on women's diet quality at the national or subnational level, making it possible to target at-risk populations, track progress, and measure the impact of programs and policies. To date, the indicator has been tested with regard to whether it is associated with socioeconomic characteristics (19, 20). It has been used to explore linkages between diet and coronary artery disease (21), pregnancy-related outcomes (22, 23), and child growth (24), but also to develop new metrics for food biodiversity in diets (25). In Bangladesh, it has begun to be used for program evaluation (26), and its applicability to pregnant adolescent girls and women has also been tested (27). Farm production diversity was found to be positively associated with the MDD-W in 3 rural settings (24, 28, 29), and 2 studies explored the linkages between MDD-W and household food security indicators (24, 30), but the findings were inconsistent. With the aim of contributing to this emerging literature, we investigated 1) how MDD-W is linked to household food insecurity based on the Household Food Insecurity Access Scale (HFIAS) and the Household Hunger Scale (HHS), 2) how MDD-W is linked to farm production diversity, and 3) whether contextual factors such as household wealth status modify these relations. As a secondary objective, in order to draw conclusions regarding the cost of dichotomization, we also examined whether all these associations held when the number of food groups consumed was used as a continuous variable.
Methods
Data source and design
We used baseline data from a 4-arm cluster randomized controlled trial conducted in 2013 in the region of Kayes in western Mali. The trial was designed to evaluate the impact of a 3-y nutrition-sensitive intervention targeting women and their children during the first 1000 d of each child's life.
Study area
The survey took place in the 3 districts where the intervention was being implemented: Bafoulabé, Diéma, and Yélimané. This area is characterized by a Sahelian climate with negligible rainfall and very frequent droughts. The dry season generally lasts from October to June, and the rainy season, characterized by intense agricultural activities, lasts from July to September. For the most vulnerable households, the lean period usually occurs in April and May. Agriculture is the main occupation, and the main crops cultivated are millet, sorghum, maize, groundnuts, and cowpeas. Livestock is also a significant source of income in the study area. Sedentary farmers belonging to the Manding ethnic group (Soninké, Bambara, and Kassonké) form the majority of the population, which also comprises transhumant pastoralists from nomadic or seminomadic minorities: Fulanis and Maures (31). Despite high agropastoral potential, populations face seasonal food insecurity and high rates of both chronic and acute malnutrition among children (32).
Our study was conducted from 11 November, 2013, to 1 January, 2014, a period that corresponded to the harvest season for sorghum and groundnuts.
Study sample
The sample comprised 5046 mother–child pairs from 4790 households. Mother–child pairs were randomly selected using a multistage cluster selection process: 1) In each of the 76 community health centers (CHCs) of the 3 districts targeted by the intervention, 6 enumeration areas (EAs) were randomly selected; and 2) within each EA, an exhaustive list was drawn up of households with eligible mother–infant pairs—that is, mothers living permanently in the village, having a child aged 12–42 mo. Eleven households/EAs were randomly selected from the list of eligible households. For CHCs covering less than 6 EAs, the number of households selected by EA was adjusted so that 66 mother–child pairs were surveyed in the area covered by each CHC.
Measures
Dietary diversity
Women's dietary intake was assessed through a qualitative 24-h recall. Using a multiple-pass method, mothers were first asked to spontaneously recall all dishes, sauces, snacks, drinks, and other foods they had consumed from the time they woke up to the same time the following day. At the second pass, women were asked to describe the exact composition of all dishes they had eaten. Food items were coded directly in the field by well-trained fieldworkers and classified into a predefined list of 30 food groups, which were further aggregated into the following 10 defined food groups (16): 1) grains, white roots and tubers, and plantains; 2) pulses (beans, peas, and lentils); 3) nuts and seeds; 4) dairy; 5) meat, poultry, and fish; 6) eggs; 7) dark green leafy vegetables; 8) other vitamin A-rich fruits and vegetables; 9) other vegetables; and 10) other fruits. The MDD-W is a dichotomous variable that equals 1 if the women consumed at least 5 different food groups during the past 24 h and 0 otherwise. Women who achieve minimum diet diversity (consuming foods from 5 or more food groups) are expected to have a greater likelihood of meeting their micronutrient needs compared with women who consume foods from fewer food groups. Using a dichotomous indicator with an established cutoff value makes it possible to calculate the prevalence of women who achieve minimum dietary diversity, which has important operational implications. However, for research purposes, in our analysis, we also used the number of food groups consumed as a continuous variable we named the 10-Food Group Women's Dietary Diversity Score (WDDS-10), which ranged from 0 to 10.
Household food security
We used the HFIAS and the HHS to estimate overall perceived household food insecurity and hunger, respectively. The HFIAS comprises a set of 9 questions reflecting 3 different domains of food insecurity: 1) anxiety and uncertainty about food supply, 2) insufficient food quality, and 3) insufficient food intake and its physical consequences. Households were categorized into 4 levels of food insecurity according to recommendations by the US Agency for International Development's Food and Nutrition Technical Assistance III Project (FANTA) (33): food secure and mildly, moderately, and severely food insecure. The HHS was computed from the last 3 questions of the HFIAS that are specifically related to “hunger.” Households were divided into 3 categories based on FANTA recommendations (34): little to no hunger in the household, moderate hunger in the household, and severe hunger in the household. Because only 2% of households were classified as experiencing severe hunger in our study, we combined the “severe hunger” and “moderate hunger” categories.
Farm production diversity
Agricultural biodiversity is usually assessed by a simple count of species (crops, plants, and animals) produced or raised by the household or by the means of indicators such as the Shannon and Simpson indexes that, in addition, capture differences in the quantities of each product but do not take their nutrient composition into account (35, 36). Although there is no standard method for measuring on-farm diversity for nutritional purposes (17), from a nutritional standpoint, diversity implies foods from different food groups. As a result, we chose to build a food production diversity score (FPDS) based on groups rather than species. Heads of households reported details of their farm production, including the types of food crops they cultivated and types of animals they raised, during the past 12 mo. We calculated an FPDS for each household by summing the following crop and livestock groups: 1) cereals; 2) tubers; 3) beans, peas, and pulses; 4) vegetables and fruits; 5) cattle; 6) poultry; 7) goats and sheep; 8) pigs; and 9) camels. The categories of foods were based on available data on crop production, and the livestock groups were computed based on taxonomy (small ruminants, large ruminants, pseudo-ruminants, monogastric, or poultry), like that used in a study in Kenya (37). In our analysis, the FPDS was used as a continuous variable and could range from 1 to 9.
Household wealth status
A wealth index was constructed using a multiple correspondence analysis performed on variables that coded for housing quality (type and size of house, number of persons per room, and floor, wall, and roof material) and facilities (electricity, source of drinking water, type of cooking fuel, and type of toilet facility), for assets (television, phone, mobile phone, refrigerator, radio, torch, and kerosene lamp), and for means of transport (automobile, bicycle, and motorcycle). For each household, the coordinate on the first axis of the correspondence analysis was interpreted as an index of the economic level. We used this wealth index categorized in terciles in subsequent analyses.
Sociodemographic characteristics
For the women, the sociodemographic factors included age, education, occupation, ethnic group, religion, and marital status. For the household, factors included the sex of the head of household and the size of the household.
Data management and statistical analyses
Data were collected using Android tablets. Data quality was ensured by quality checks at data entry and by post-survey data cleaning. Data management and analyses were performed using R software version 3.4.3. All analyses took into account the sampling design (stratification, clustering, and sampling weights) using the Survey package. Unless otherwise specified, the type I error risk was set at 0.05.
A descriptive analysis was conducted of the characteristics of the study sample. MDD-W and WDDS-10 were used as response variables to analyze the relation between women's dietary diversity and household food security and/or farm production diversity. Variables identified as potential confounders for these relations were grouped in 3 dimensions (characteristics of women, characteristics of heads of households, and socioeconomic and demographic characteristics of households). After exploring associations with covariates in each of these 3 dimensions through bivariate analyses (using a P value <0.10 to define significance), we ran a series of multivariate models including the significant covariates:
We obtained ORs, 95% CIs, and P values for each main explanatory variable (i.e., HFIAS, HHS, and FPDS) from separate logistic regression models (A models), with the MDD-W as an outcome.
We obtained a β-coefficient, 95% CIs, and P for each main explanatory variable from separate linear regression models (A models), with the WDDS-10 as an outcome.
In the second step, the household wealth index was added to the previously mentioned models to assess whether the relation between the main explanatory variables and women's dietary diversity held when controlling for the wealth index (B models). In the third step, potential modifier effects were also investigated by including in the models statistical interaction terms (C models) to assess whether the relation between the main explanatory variables and women's dietary diversity was similar across the household wealth terciles.
Finally, to assess whether household food security mediated the relation between farm production diversity and women's dietary diversity, we ran multivariate regression models including HFIAS, HHS, and FPDS as explanatory variables and women's dietary diversity as outcomes.
Ethical considerations
The study was registered at ISRCTN.org (ISRCTN08435964) on 9 December 2013 and received ethical approval from the Committee of the Ministry of Health of Mali. All the participants gave their informed written consent to take part in the study.
Results
Sociodemographic characteristics of the sample
The mean age of the women was 28.56 ± 0.13 y (Table 1). More than 60% of households comprised 4–8 people. Although the households were mostly headed by men, an appreciable proportion (19%) were headed by women. The majority of heads of households were Muslims and practiced polygamy. The level of education was very low among both heads of households and women. The main source of income was agriculture and livestock raising.
TABLE 1.
Descriptive characteristics of the sample
| Characteristics | Mean ± SEM or % |
|---|---|
| Household characteristics, n = 4790 | |
| Household size | |
| 2–3 people | 12.2 |
| 4–8 people | 62.9 |
| ≥9 people | 24.9 |
| Sources of income | |
| Agriculture | 96.3 |
| Livestock | 72.4 |
| Small business | 27.3 |
| Household head characteristics, n = 4790 | |
| Education | |
| No education at all | 74.1 |
| No formal education1 | 13.1 |
| Primary school | 10.4 |
| Secondary school or more | 2.4 |
| Muslim | 99.2 |
| Marital status | |
| Single/divorced/widowed | 1.1 |
| Monogamous | 15.1 |
| Polygamous | 83.8 |
| Female household head | 19.0 |
| Household agriculture practices, n = 4726 | |
| Crops harvested over the past 12 mo | |
| No. of food crop groups | 2.59 ± 0.03 |
| Cereals | 99.2 |
| Tubers | 13.1 |
| Pulses (beans, peas, groundnuts) | 96.3 |
| Vegetables and fruits | 55.6 |
| Livestock owned over the past 12 mo | |
| No. of livestock groups | 1.72 ± 0.05 |
| Household owns at least 1 animal | 77.8 |
| Poultry | 51.0 |
| Cattle | 52.3 |
| Goats and sheep | 66.3 |
| Camel | 2.8 |
| Pigs | 0.4 |
| Farm production diversity score2 | 4.31 ± 0.07 |
| Household food security, n = 4790 | |
| Household Food Insecurity Access Scale | |
| Food secure | 36.6 |
| Moderately food insecure | 16.2 |
| Mildly food insecure | 27.7 |
| Severely food insecure | 19.5 |
| Household Hunger Scale | |
| Little to no hunger | 90.5 |
| Moderate/severe hunger | 9.5 |
| Women's characteristics, n = 5046 | |
| Age, y | 28.56 ± 0.13 |
| Education | |
| No education at all | 93.6 |
| No formal schooling1 | 1.4 |
| Primary school | 4.5 |
| Secondary or more | 0.5 |
| Occupation | |
| Working mothers | 26.3 |
| Housewife | 68.2 |
| Other (student/retired/seeking employment/other) | 5.5 |
Schooling outside the framework of the formal education system (e.g., Koranic School).
Number of crop/livestock groups produced over the past 12 mo.
Household agricultural practices and food security status
Most households had harvested 3 crop groups in the preceding 12 mo (Table 1), mainly cereals (99.2%) and pulses (96.3%); ∼78% of the households had at least 1 farm animal. The animals most frequently raised by households were goats and sheep (66.3%), followed by cattle (52.3%) and poultry (51%). Less than 1% of households reported raising pigs. The FPDS ranged from 1 to 7 crops per livestock groups produced during the past 12 mo, with a mean of 4.31 ± 0.07. According to the HFIAS, nearly 20% of households experienced severe food insecurity; according to the HHS, nearly 10% experienced moderate to severe hunger.
Women's dietary diversity
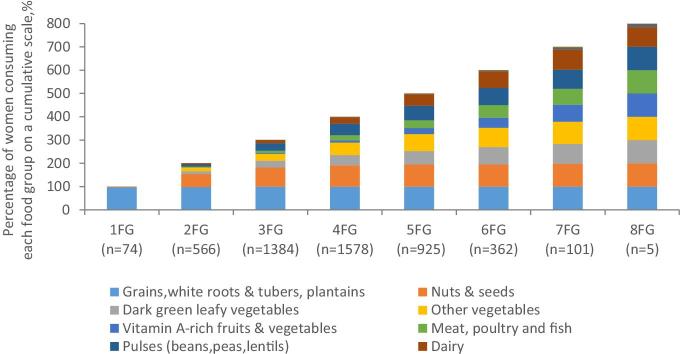
The WDDS-10 ranged from 1 to 8 food groups consumed during the past 24 h, with a mean of 3.82 ± 0.05. Only 27% of women achieved the MDD-W (Figure 1). The diet of the women who consumed only 1 food group basically consisted of starchy staple foods (97%) (Figure 2). Women with a WDDS-10 = 2 generally consumed foods, nuts, and seeds that were added to a diet comprising a starchy staple food (56%) or, less frequently, vegetables other than vitamin A-rich vegetables (15%). As the WDDS-10 increased, the group “nuts and seeds” quickly reached 100%, whereas the consumption of vegetables, peas, and beans increased progressively. The shift from WDDS-10 = 4 food groups (MDD-W = 0) to WDDS-10 ≥ 5 food groups (MDD-W = 1) was mainly driven by the addition of animal source foods (dairy and flesh foods) and vitamin A-rich vegetables and fruits in the diet. For all women, the consumption of eggs and fruits other than vitamin A-rich fruits was very rare.
FIGURE 1.
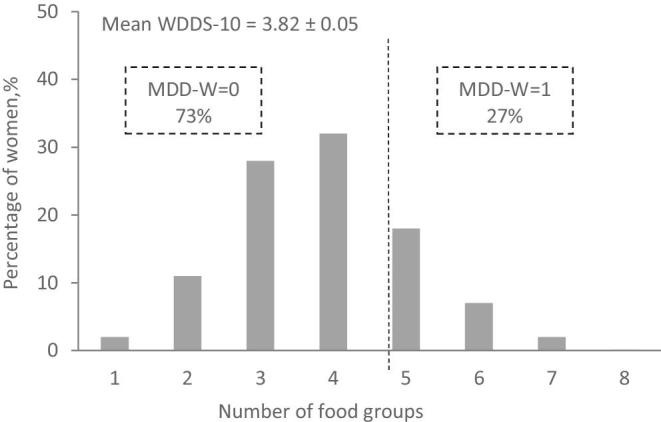
Distribution of the Women's Dietary Diversity Score (WDDS-10) and prevalence of the Minimum Dietary Diversity for Women of Reproductive Age (MDD-W). MDD-W = 1 if the women consumed at least 5 different food groups during the past 24 h and 0 otherwise (N = 4995).
FIGURE 2.
Cumulative percentage of women consuming each food group according to the value of the Women's Dietary Diversity Score (WDDS-10; N = 4995). This way of presenting the data was chosen to show the changes in the actual percentage of women who consume each food group according to the value of WDDS-10, ranging from 1 to 8 food groups. FG, food group.
Bivariate analysis revealed that a number of variables were associated with women's dietary diversity: level of education of the women and of the heads of household, the women's occupation, the sex of the head of household, the number of household members, and the household wealth index (results not shown). In the multivariate regression models presented later, analyses were adjusted for these covariates.
Relation between women's dietary diversity and household food insecurity
In households experiencing either severe food insecurity according to the HFIAS or moderate to severe hunger according to the HHS, only a small proportion of women achieved minimum dietary diversity compared with women in households with no food insecurity or with no or little hunger (OR: 0.76; 95% CI: 0.58, 0.99; P = 0.06; and OR: 0.63; 95% CI: 0.46, 0.87; P < 0.01, respectively; Table 2, A models). Similarly, women presented a lower mean WDDS-10 when they lived in households that experienced severe food insecurity according to the HFIAS (Table 3, A models; β = −0.23, P < 0.01) or in households that experienced moderate to severe hunger (β = −0.34, P < 0.001). When the household wealth index was added to the regression models, the association between the MDD-W or the mean WDDS-10 and the HFIAS or the HHS tended to weaken (Tables 2 and 3, B models). In all models, the household wealth index was a strong independent predictor of women's dietary diversity, with women in the wealthiest households having greater odds of reaching the MDD-W and higher mean WDDS-10. However, the wealth index had no modifying effect on the relation between these variables and the HFIAS or the HHS (interaction terms were all nonsignificant; Tables 2 and 3, C models).
TABLE 2.
Multivariate logistic regression analysis of the association between household food insecurity, farm production diversity, and Minimum Dietary Diversity for Women of Reproductive Age (MDD-W)
| Model A1 | Model B2 | Model C3 | ||||||
|---|---|---|---|---|---|---|---|---|
| n | MDD-W = 1 (%) | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Predictor: Household Food Insecurity Access Scale (HFIAS) | ||||||||
| HFIAS categories | ||||||||
| Food secure | 1759 | 29.2 | Reference | 0.06 | Reference | 0.25 | Reference | 0.53 |
| Moderately food insecure | 773 | 30.2 | 1.09 (0.86, 1.38) | 1.09 (0.86, 1.38) | 1.35 (0.86, 2.11) | |||
| Mildly food insecure | 1297 | 28.6 | 0.99 (0.81, 1.22) | 1.06 (0.86, 1.30) | 1.22 (0.84, 1.78) | |||
| Severely food insecure | 892 | 24.1 | 0.76 (0.58, 0.99) | 0.85 (0.64, 1.11) | 1.09 (0.73, 1.61) | |||
| Wealth index | ||||||||
| 1 (lowest) | 1534 | 22.9 | — | Reference | 0.01 | Reference | 0.01 | |
| 2 | 1600 | 28.9 | — | 1.31 (1.06, 1.63) | 1.64 (1.15, 2.35) | |||
| 3 | 1587 | 32.8 | — | 1.51 (1.16, 1.97) | 1.77 (1.20, 2.60) | |||
| HFIAS × wealth index interaction terms | — | — | 0.37 | |||||
| Predictor: Household Hunger Scale (HHS) | ||||||||
| HHS categories | ||||||||
| Little to no hunger | 4293 | 29.1 | Reference | <0.01 | Reference | 0.03 | Reference | 0.16 |
| Moderate/severe hunger | 428 | 19.8 | 0.63 (0.46, 0.87) | 0.70 (0.50, 0.97) | 0.73 (0.47, 1.13) | |||
| Wealth index | ||||||||
| 1 (lowest) | 1534 | 22.9 | — | Reference | 0.01 | Reference | 0.01 | |
| 2 | 1600 | 28.9 | — | 1.31 (1.05, 1.62) | 1.33 (1.06, 1.67) | |||
| 3 | 1587 | 32.8 | — | 1.50 (1.16, 1.95) | 1.49 (1.14, 1.94) | |||
| HHS × wealth index interaction terms | — | — | 0.63 | |||||
| Predictor: farm production diversity score (FPDS) | ||||||||
| FPDS | 4721 | 27.9 | 1.12 (1.07, 1.18) | <0.001 | 1.12 (1.06, 1.18) | <0.001 | 1.05 (0.97, 1.13) | 0.25 |
| Wealth index | ||||||||
| 1 (lowest) | 1534 | 22.9 | — | Reference | <0.01 | Reference | 0.06 | |
| 2 | 1600 | 28.9 | — | 1.30 (1.05, 1.61) | 0.70 (0.44, 1.12) | |||
| 3 | 1587 | 32.8 | — | 1.54 (1.19, 1.98) | 1.26 (0.76, 2.08) | |||
| FPDS × wealth index interaction terms | — | — | 0.03 | |||||
Adjusted on sociodemographic variables (household size, head of household level of education, sex of the head of household, women's level of education, and women's occupation).
Adjusted on sociodemographic variables + household wealth index.
Adjusted on sociodemographic variables + household wealth index + interaction term (predictor × wealth index).
TABLE 3.
Multivariate linear regression analysis of the association between household food insecurity, farm production diversity, and 10-Food Group Women Dietary Diversity Score
| Model A1 | Model B2 | Model C3 | |||||
|---|---|---|---|---|---|---|---|
| N | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| Predictor: Household Food Insecurity Access Scale (HFIAS) | |||||||
| HFIAS categories | |||||||
| Food secure | 1759 | Reference | <0.01 | Reference | 0.02 | Reference | 0.05 |
| Moderately food insecure | 773 | 0.04 (−0.11, 0.20) | 0.04 (−0.11, 0.19) | 0.17 (−0.08, 0.43) | |||
| Mildly food insecure | 1297 | 0.01 (−0.11, 0.13) | 0.05 (−0.08, 0.17) | 0.14 (−0.05, 0.32) | |||
| Severely food insecure | 892 | −0.23 (−0.39, −0.07) | −0.16 (−0.31, −0.00) | −0.08 (−0.29, 0.13) | |||
| Wealth index | |||||||
| 1 (lowest) | 1534 | — | Reference | <0.001 | Reference | <0.001 | |
| 2 | 1600 | — | 0.22 (0.10, 0.33) | 0.28 (0.11, 0.46) | |||
| 3 | 1587 | — | 0.28 (0.13, 0.42) | 0.38 (0.21, 0.56) | |||
| HFIAS × wealth index interaction terms | — | — | 0.23 | ||||
| Intercept | 3.6 | 3.5 | 3.4 | ||||
| Predictor: Household Hunger Scale (HHS) | |||||||
| HHS categories | |||||||
| Little to no hunger | 4293 | Reference | <0.001 | Reference | <0.01 | Reference | <0.01 |
| Moderate/severe hunger | 428 | −0.34 (−0.49, −0.17) | −0.27 (−0.43, −0.10) | −0.31 (−0.52, −0.09) | |||
| Wealth index | |||||||
| 1 (lowest) | 1534 | — | Reference | <0.001 | Reference | <0.001 | |
| 2 | 1600 | — | 0.21 (0.10, 0.33) | 0.21 (0.09, 0.32) | |||
| 3 | 1587 | — | 0.27 (0.13, 0.41) | 0.26 (0.11, 2.41) | |||
| HHS × wealth index interaction terms | — | — | 0.69 | ||||
| Intercept | 3.6 | 3.5 | 3.5 | ||||
| Predictor: farm production diversity score (FPDS) | |||||||
| FPDS | 4721 | 0.10 (0.06, 0.13) | <0.001 | 0.09 (0.06, 0.12) | <0.001 | 0.09 (0.05, 0.13) | <0.001 |
| Wealth index | |||||||
| 1 (lowest) | 1534 | — | Reference | <0.001 | Reference | 0.12 | |
| 2 | 1600 | — | 0.21 (0.10, 0.32) | 0.21 (−0.02, 0.44) | |||
| 3 | 1587 | — | 0.29 (0.15, 0.43) | 0.28 (−0.02, 0.59) | |||
| FPDS × wealth index interaction terms | — | — | 0.99 | ||||
| Intercept | 3.3 | 3.1 | 3.1 | ||||
Adjusted on sociodemographic variables (household size, head of household level of education, sex of the head of household, women's level of education, and women's occupation).
Adjusted on sociodemographic variables + household wealth index.
Adjusted on sociodemographic variables + household wealth index + interaction term (predictor × wealth index).
Relation between women's dietary diversity and farm production diversity
We found a positive association between women's dietary diversity and farm production diversity: One more food crop per livestock group in the FPDS was associated with greater odds of attaining the MDD-W (OR: 1.12; 95% CI: 1.07, 1.18) and with a 10% increase in the mean WDDS-10 (P < 0.001; Tables 2 and 3, A models). When the household wealth index was added to the regression models, the association between the MDD-W or the mean WDDS-10 and the FPDS remained almost unchanged. There was only a small decrease in the magnitude of the association between the FPDS and the mean WDDS-10 (from β = 0.10 to β = 0.09), but the association remained highly significant (P < 0.001; Table 3, B models). The wealth index did not modify the relation between the FPDS and the mean WDDS-10, but it did modify the relation between the FPDS and the MDD-W (overall P = 0.03 for the interaction term; Table 2, C models). However, the tests for individual coefficients were statistically significant only for the second tercile (P = 0.01).
Finally, we assessed whether the relation between the FPDS and women's dietary diversity was mediated by the food security status of the household by including either the HFIAS or the HHS in regression models B. For both outcomes (MDD-W and WDDS-10), we found only very small changes in coefficients in all the models. Only 1% of the total effect of the FPDS on MDD-W was mediated by either the HFIAS or the HHS, and 0.3% of the total effect of the FPDS on WDDS-10 was mediated by either the HFIAS or the HHS.
Discussion
In the rural region of Kayes in western Mali, both the mean dietary diversity score of women and the proportion of women who reached the MDD-W (27%) were low. In exploring relations between women's dietary diversity, household food insecurity, and farm production diversity, the MDD-W performed well, as did the WDDS-10, and the results led to the same conclusions whichever indicator was used. This finding is reassuring in the sense that in our study, using the MDD-W, which is the recommended dichotomous indicator for operational purposes, did not result in a substantial loss of information regarding the associations with other indicators. Our findings suggest that living in a household experiencing food insecurity is associated with a higher risk of not reaching the MDD-W. This association was particularly clear when the HHS was used as a measure of food insecurity. Women in households that produced a wider variety of food crops and livestock groups had greater odds of reaching minimum dietary diversity. This relation was not mediated by household food insecurity. All these associations remained significant when adjusted for potential confounders, including household wealth.
The MDD-W is a simple tool that can be used to characterize and compare the dietary diversity of women of reproductive age within and across different contexts. Guidelines for measuring women's dietary diversity based on the recommended 10-point food group indicator were published only recently (16), and few studies have reported MDD-W prevalence values. We identified 1 study conducted in the region of Timbuktu in Mali that reported an 8% prevalence of women reaching the MDD-W (38). This is far below the prevalence we found in our study and could be related to the specificity of the area, where more than 40% of households experienced severe food insecurity based on the Food Insecurity Experience Scale (39). The prevalence of MDD-W observed in our study, however, was lower than that found in other Sahelian rural areas in the same season, for example, in 2 rural provinces of Burkina Faso [Sanguie: (49%) and Sourou (30%)] (30). Not surprisingly, we found that women's diet was largely dominated by starchy staples. The high consumption of nuts and seeds was explained by the fact that the survey was conducted during the groundnut harvesting season. Women reaching the MDD-W consumed nutrient-rich foods, such as animal source foods and vitamin A-rich fruits or vegetables, more frequently. Although half of the households reported raising poultry (mainly chickens), eggs were very rarely consumed by women, probably because in rural Mali, eggs are primarily intended for reproduction rather than for human consumption (40). We found that household wealth was an important predictor of the MDD-W, with a gradient in the odds of reaching a minimum dietary diversity and in the mean WDDS-10 as households had a better economic status. These findings are in line with those of previous studies that reported that household wealth is an important driver of women's diet in rural Mali (41, 42) and that household wealth is significantly associated with the MDD-W (19, 30). Although we did not measure household food expenditure, we hypothesize that women in wealthier households benefited from higher income enabling them to diversify their diet through the purchase of varied foods in the market.
Although associations between food insecurity and women's dietary diversity are documented in the literature (43–45), to our knowledge, only 2 studies have examined these associations using the MDD-W (24, 30). In our study, the lowest mean WDDS-10 and the highest risk of not reaching the MDD-W were observed among women living in severely food insecure households (using the HFIAS). In rural Tanzania, no significant relation was found between the MDD-W and the HFIAS (24). The association with household's food insecurity was particularly clear when we used the HHS (i.e., an indicator that captures situations of extreme food insecurity) and remained significant even after adjusting for household wealth. The lower dietary diversity of women living in severe food insecure households was mainly due to the low consumption of nutritious foods, including animal source foods and vitamin A-rich fruits and vegetables. Seasonality may have influenced women's dietary diversity and household food security (46, 47), but seasonal variations could not be accounted for due to the cross-sectional nature of our data. However, in rural Burkina Faso, the MDD-W was found to be sensitive to seasonal changes, and an association between the MDD-W and both HFIAS and HHS was found only during the lean season (30). Other factors may play a confounding role in the relation between food insecurity and women's dietary diversity, including household size, maternal education, dependency ratio, religion, occupation, and marital status (24, 30, 45). We controlled for most of these sociodemographic characteristics, but the association between household food insecurity and women's diet diversity remained significant, thus suggesting a rather direct influence of food security on dietary diversity.
The relation between women's dietary diversity and production diversity observed in our study echoes the considerable evidence in the literature that farm production diversity contributes to household dietary diversity. However, few studies have investigated these associations at the individual woman's level, and to our knowledge, only 4 used the MDD-W (24, 28, 29, 48). Jones (35) recently stressed the importance of using validated and standardized proxy indicators of nutrient intake adequacy in order to provide firm conclusions and guidance on whether and how agriculture can contribute to nutrition. Whereas in Burkina Faso no relation between food crop diversity and the MDD-W was found (48), positive associations between the MDD-W and the diversity of production were found in Tanzania (24), Benin (28), and India (29), but the magnitude of the effect varied depending on the way production diversity was measured. The lack of consensus regarding how to measure production diversity is challenging when trying to compare findings; although further studies using the MDD-W are needed, efforts should also be encouraged to standardize the way farm production diversity (for nutritional purposes) is measured. In our study, we computed the FPDS as a count of crop per livestock groups, a method of calculation that provides an indication of nutritional diversity. Using groups (rather than species) as the unit of measure is crucial to enable appropriate interpretation. If production diversity is measured using a different unit (e.g., species) than the one used to assess dietary diversity (count of groups), associations are less likely to be identified (49). Because of data constraints, the construction of an ideal FPDS based on the exact same groups used for the MDD-W as seen in India (29), or of other relevant indicators such as nutritional functional diversity (50) or modified functional attribute diversity (51), was not possible. In a context in which agricultural policies encourage diversification of production rather than relying on a small number of staple crops (52), our study helps document the relation between individual diet quality and on-farm production diversity assessed from a nutritional perspective.
Finally, several conceptual frameworks (3–7) hypothesize that farm production diversity may affect women's dietary diversity through its positive effects on household food security. Despite the cross-sectional nature of our data, the period of time to which our indicators referred—FPDS during the past 12 mo, HFIAS and HHS during the past month, and MDD-W during the past 24 h—led us to expect we would be able to identify such a plausible pathway. However, our results revealed that food security mediated only a very small part of the total effect of FPDS on women's dietary diversity. In addition, we hypothesize that this pathway may vary with the season.
Our study has some limits. The market may play a role in the relation between agricultural diversification and individual dietary diversity (35), but it was not possible to specifically assess its contribution in the current study. We used baseline data from a randomized controlled trial in which women received either cash transfers or lipid-based nutrient supplements for their children. The baseline survey was conducted before the implementation of these two interventions, but the women were already benefiting from a nutrition program in the region, which included nutrition behavior change communication (BCC) activities. As a result, women's exposure to the BCC activities might have influenced their dietary practices. As mentioned previously, seasonality is known to influence dietary consumption, especially in rural settings in which households rely on their own agricultural production. However, the cross-sectional nature of our data, which were collected during a limited period of time, did not enable us to explore the possible effect of seasonality on the associations we found. Finally, the cross-sectional nature of our data make it impossible to draw conclusions regarding causality in the relations we found. Longitudinal studies are needed to not only identify temporal sequence of exposure and outcome but also study changes in MDD-W prevalence over time.
In conclusion, the latest validated proxy indicator of the micronutrient adequacy of women's diet, MDD-W, revealed strong associations with indicators of food insecurity and of farm production diversity. Using a dichotomous indicator could be expected to be less sensitive in identifying relations with potential determinants compared with a continuous indicator (WDDS-10), but our findings invalidated this hypothesis in our sample. By providing the cutoff point of 5 food groups, the MDD-W is a valuable tool to help identify and characterize populations at greater risk of inadequate nutrient intakes. Nonetheless, it is important to continue investigating the composition of the diet by analyzing the consumption of individual food groups. This will ensure identification of neglected food groups that are potentially the ones whose production should be promoted to achieve greater diversity. All this information is essential to help design efficient interventions to address nutrition through the improvement of dietary quality.
Acknowledgments
We thank our colleague Saidou Magagi from UNICEF for assistance with the training and supervision of the interviewers.
The authors’ responsibilities were as follows—YMP, MS, ALP, and TM: contributed to the conception and design of the study; TM, NEK, and KF: responsible for program implementation; YK, SF, MS, ALP, NEK, and KF: conducted the research; LA and SF: performed statistical analysis; LA, YMP, MS, and ALP: wrote the manuscript; and all authors: read and approved the final manuscript.
Notes
The program implementation was funded by Global Affairs Canada, with support from UNICEF. The research was funded by the European Union through the International Fund for Agricultural Development, UNICEF, World Health Food Program, and Research Institute for Development, with additional support from the CGIAR Research Programs on Agriculture for Nutrition and Health (A4NH) hosted by the International Food Policy Research Institute.
LA received a research allowance from the French Ministry of Higher Education and Research through doctoral school 393 of Pierre and Marie Curie University (Sorbonne University).
Author disclosures: LA, MS, SF, YK, NEK, KF, TM, ALP and YMP, no conflicts of interest.
Abbreviations used:
- CHC
community health center
- EA
enumeration area
- FPDS
farm production diversity score
- HFIAS
Household Food Insecurity Access Scale
- HHS
Household Hunger Scale
- MDD-W
Minimum Dietary Diversity for Women of Reproductive Age
- SDG
Sustainable Development Goal
- WDDS-10
10-Food Group Women's Dietary Diversity Score
References
- 1. Pandya-Lorch R, Fan S.. Reshaping agriculture for nutrition and health. Washington (DC): International Food Policy Research Institute; 2012. [Google Scholar]
- 2. Ruel M, Alderman H.. Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition?. Lancet 2013;382:536–51. [DOI] [PubMed] [Google Scholar]
- 3. Hawkes C, Turner R, Waage J. Current and planned research on agriculture for improved nutrition: a mapping and a gap analysis. Report for the Department of International Development (DFID). London: Leverhulme Centre for Integrative Research on Agriculture and Health; 2012. [Google Scholar]
- 4. Webb P. Impact pathways from agricultural research to improved nutrition and health: literature analysis and research priorities. Rome (Italy): FAO and WHO; 2013. [Google Scholar]
- 5. Gillespie S, Harris J, Kadiyala S. The agriculture–nutrition disconnect in India: what do we know? Discussion Paper 01187. Washington (DC): International Food Policy Research Institute; 2012. [Google Scholar]
- 6. Herforth A, Harris J.. Understanding and applying primary pathways and principles. Brief No. 1. Improving nutrition through agriculture technical brief series. Arlington (VA): USAID/Strengthening Partnerships, Results, and Innovations in Nutrition Globally (SPRING) Project; 2014. [Google Scholar]
- 7. Headey D, Chiu A, Kadiyala S. Agriculture's role in the Indian enigma: help or hindrance to the crisis of undernutrition? Food Secur 2012;4:87–102. [Google Scholar]
- 8. Arimond M, Wiesmann D, Becquey E, Carriquiry A, Daniels M, Deitchler M, Fanou-Fogny N, Joseph M, Kennedy G, Martin-Prevel Y et al.. Simple food group diversity indicators predict micronutrient adequacy of women's diets in 5 diverse, resource-poor settings. J Nutr 2010;140:2059S–69S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koppmair S, Kassie M, Qaim M. Farm production, market access and dietary diversity in Malawi. Public Health Nutr 2016;20:325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruel M, Quisumbing A, Balagamwala M. Nutrition-sensitive agriculture: what have we learned so far?. Glob Food Sec 2018;17:128–53. [Google Scholar]
- 11. Global Panel on Agriculture and Food Systems for Nutrition Food systems and diets: facing the challenges of the 21st century. Washington (DC):International Food Policy Research Institute;2016. [Google Scholar]
- 12. Herforth A, Ballard T. . Nutrition indicators in agriculture projects: current measurement, priorities, and gaps. Glob Food Sec 2016;10:1–10. [Google Scholar]
- 13. Development Initiatives Global nutrition report 2017: nourishing the SDGs. Bristol (UK): Development Initiatives; 2017, p. 41. [Google Scholar]
- 14. Sustainable Development Solutions Network Indicators and a monitoring framework for the Sustainable Development Goals: launching a data revolution for the SDGs [Internet]. 2015. Available from: http://unsdsn.org/resources/publications/indicators
- 15. Martin-Prevel Y, Arimond M, Allemand P, Wiesmann D, Ballard T, Deitchler M, Dop M, Kennedy G, Lartey A, Lee WT et al.. Development of a dichotomous indicator for population-level assessment of dietary diversity in women of reproductive age. Curr Dev Nutr 2017;1:cdn.117.001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. FAO and FHI 360 Minimum dietary diversity for women: A guide for measurement. Rome (Italy): FAO; 2016. [Google Scholar]
- 17. Herforth A, Nicolò G, Veillerette B, Dufour C. Compendium of indicators for nutrition-sensitive agriculture. Rome (Italy): FAO; 2016. [Google Scholar]
- 18. Herforth A, Rzepa A.. Seeking indicators of healthy diets: it is time to measure diets globally. How? Washington (DC): Gallup and Swiss Agency for Development and Cooperation; 2016. [Google Scholar]
- 19. Chakona G, Shackleton C.. Minimum dietary diversity scores for women indicate micronutrient adequacy and food insecurity status in South African towns. Nutrients 2017;9:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morseth M, Grewal N, Kaasa I, Hatloy A, Barikmo I, Henjum S. Dietary diversity is related to socioeconomic status among adult Saharawi refugees living in Algeria. BMC Public Health 2017;17:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fung T, Isanaka S, Hu F, Willett W. International food group-based diet quality and risk of coronary heart disease in men and women. Am J Clin Nutr 2018;107:120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saaka M, Oladele J, Larbi A, Hoeschle-Zeledon I. Dietary diversity is not associated with haematological status of pregnant women resident in rural areas of northern Ghana. J Nutr Metab 2017;2017:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Osman S, Saaka M, Siassi F, Qorbani M, Yavari P, Danquah I, Sotoudeh G. A comparison of pregnancy outcomes in Ghanaian women with varying dietary diversity: a prospective cohort study protocol. BMJ Open 2016;6:e011498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang M, Sudfeld C, Ismail A, Vuai S, Ntwenya J, Mwanyika-Sando M, Fawzi W. Maternal dietary diversity and growth of children under 24 months of age in rural Dodoma, Tanzania. Food Nutr Bull 2018;39(2):219–30. [DOI] [PubMed] [Google Scholar]
- 25. Lachat C, Raneri J, Smith K, Kolsteren P, Van Damme P, Verzelen K, Penafiel D, Vanhove W, Kennedy G, Hunter D et al.. Dietary species richness as a measure of food biodiversity and nutritional quality of diets. Proc Natl Acad Sci USA 2017;115(1):127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nguyen PH, Kim SS, Sanghvi T, Mahmud Z, Tran LM, Shabnam S, Aktar B, Haque R, Afsana K, Frongillo EA et al. Integrating nutrition interventions into an existing maternal, neonatal, and child health program increased maternal dietary diversity, micronutrient intake, and exclusive breastfeeding practices in Bangladesh: results of a cluster-randomized program evaluation. J Nutr 2017;147(12):2326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nguyen PH, Huybregts L, Sanghvi TG, Tran LM, Frongillo EA, Menon P, Ruel MT. Dietary diversity predicts the adequacy of micronutrient intake in pregnant adolescent girls and women in Bangladesh, but use of the 5-group cutoff poorly identifies individuals with inadequate intake. J Nutr 2018;148(5):790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bellon M, Ntandou-Bouzitou G, Caracciolo F. On-farm diversity and market participation are positively associated with dietary diversity of rural mothers in southern Benin, West Africa. PLoS One 2016;11:e0162535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ludwig T. An egg for an egg and a bean for a bean? How production diversity determines dietary diversity of smallholder farmers in rural India. ZEF: Discussion papers on development policy No. 247. Bonn (Germany): Center for Development Research; 2018. [Google Scholar]
- 30. Custodio E, Kayitakire F, Thomas A-C. Exploring the new indicator Minimum Dietary Diversity–Women: results from Burkina Faso [Internet]. Luxembourg (Luxembourg): Publications Office of the European Union; 2016, p. 28.[cited 6 Apr 2018]. Available from: http://publications.jrc.ec.europa.eu/repository/handle/JRC100162. [Google Scholar]
- 31. Traoré B, Traoré M-D.. Les systèmes agriculture-élevage du Mali. In: Hiernaux P, Tarawali G, editors. Improving crop–livestock systems in West and Central Africa: reports from the Workshop on Crop–Livestock Systems in the Dry Savannas of West and Central Africa held at IITA, Ibadan 22–27 novembre 1998. Ibadan (Nigeria); 2002. p. 62–77. [Google Scholar]
- 32. Institut National de la Statistique du Mali (INSTAT) Nutrition et mortalité rétrospectives sondage SMART [Internet]. Bamako (Mali); 2016. Available from: https://docplayer.fr/52552441-Mali-smart-enquete-nutritionnelle-anthropometrique-et-de-mortalite-retrospective.html. [Google Scholar]
- 33. Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for measurement of household food access: indicator guide (v. 3). Washington (DC): Food and Nutrition Technical Assistance Project, Academy for Educational Development; 2007. [Google Scholar]
- 34. Ballard T, Coates J, Swindale A, Deitchler M. Household Hunger Scale: indicator definition and measurement guide. Washington (DC): Food and Nutrition Technical Assistance II Project, FHI 360; 2011. [Google Scholar]
- 35. Jones A. Critical review of the emerging research evidence on agricultural biodiversity, diet diversity, and nutritional status in low- and middle-income countries. Nutr Rev 2017;75:769–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bogard J, Marks G, Wood S, Thilsted S. Measuring nutritional quality of agricultural production systems: application to fish production. Glob Food Sec 2018;16:54–64. [Google Scholar]
- 37. Romeo A, Meerman J, Demeke M, Scognamillo A, Asfaw S. Linking farm diversification to household diet diversification: evidence from a sample of Kenyan ultra-poor farmers. Food Secur 2016;8:1069–85. [Google Scholar]
- 38. Kennedy G, Keding G, Evang E, Nodari GR, Scheerer L. Nutrition baseline survey summary report. Bonn (Germany): Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ); 2017. [Google Scholar]
- 39. Ballard T, Kepple A, Cafiero C. The Food Insecurity Experience Scale: development of a global standard for monitoring hunger worldwide [technical paper]. Rome (Italy): FAO; 2013. [Google Scholar]
- 40. FAO Secteur Avicole Mali: Revues nationales de l’élevage de la division de la production et de la santé animales de la FAO (No. 4). Rome (Italy); 2013.
- 41. Hatløy A, Hallund J, Diarra M, Oshaug A. Food variety, socioeconomic status and nutritional status in urban and rural areas in Koutiala (Mali). Public Health Nutr 2000;3:57. [DOI] [PubMed] [Google Scholar]
- 42. Torheim L, Ouattara F, Diarra M, Thiam F, Barikmo I, Hatløy A, Oshaug A. Nutrient adequacy and dietary diversity in rural Mali: association and determinants. Eur J Clin Nutr 2004;58:594–604. [DOI] [PubMed] [Google Scholar]
- 43. Weigel M, Armijos R, Racines M, Cevallos W. Food insecurity is associated with undernutrition but not overnutrition in Ecuadorian women from low-income urban neighborhoods. J Environ Public Health 2016;2016:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saaka M, Oladele J, Larbi A, Hoeschle-Zeledon I. Household food insecurity, coping strategies, and nutritional status of pregnant women in rural areas of northern Ghana. Food Sci Nutr 2017;5:1154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Na M, Mehra S, Christian P, Ali H, Shaikh S, Shamim A, Labrique A, Klemm R, Wu L, West K. Maternal dietary diversity decreases with household food insecurity in rural Bangladesh: a longitudinal analysis 1–3. J Nutr 2016;146:2109–16. [DOI] [PubMed] [Google Scholar]
- 46. Savy M, Martin-Prével Y, Traissac P, Eymard-Duvernay S, Delpeuch F. Dietary diversity scores and nutritional status of women change during the seasonal food shortage in rural Burkina Faso. J Nutr 2006;136:2625–32. [DOI] [PubMed] [Google Scholar]
- 47. Stevens B, Watt K, Brimbecombe J, Clough A, Judd J, Lindsay D. The role of seasonality on the diet and household food security of pregnant women living in rural Bangladesh: a cross-sectional study. Public Health Nutr 2016;20:121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lourme-Ruiz A, Dury S, Martin-Prével Y. Consomme-t-on ce que l'on sème? Relations entre diversité de la production, revenu agricole et diversité alimentaire au Burkina Faso. Cah Agric 2016;25(6):65001. [Google Scholar]
- 49. Berti P. Relationship between production diversity and dietary diversity depends on how number of foods is counted. Proc Natl Acad Sci USA 2015;112:E5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Remans R, Flynn D, DeClerck F, Diru W, Fanzo J, Gaynor K, Lambrecht I, Mudiope J, Mutuo P, Nkhoma P et al.. Assessing nutritional diversity of cropping systems in African villages. PLoS One 2011;6(6):e21235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Remans R, Wood S, Saha N, Anderman T, DeFries R. Measuring nutritional diversity of national food supplies. Glob Food Sec 2014;3(3–4):174–82. [Google Scholar]
- 52. Cook S. The spice of life: the fundamental role of diversity on the farm and on the plate [discussion paper]. London and The Hague: IIED and Hivos; 2018. [Google Scholar]