Abstract
Background:
Diabetes is one of the most prevalent metabolic diseases. Irisin (FNDC5 protein) is involved in the new strategy of combating type 2 diabetes. In the liver, the antidiabetic mechanism of silymarin at the molecular level is unknown. This study investigated the effects of silymarin on irisin and the related gene expression and oxidative stress status in the liver of type 2 diabetic rats.
Methods:
Thirty-six rats were divided into 6 groups (n=6 each) by simple randomization: control, control+silymarin (60 mg/kg daily in normal saline orally for 60 days), control+silymarin (120 mg/kg daily in normal saline orally for 60 days), diabetic, diabetic+silymarin (60 mg/kg daily for 60 days), and diabetic+silymarin (120 mg/kg daily for 60 days). Biochemical parameters were measured by spectrophotometric and immunoassay methods, and quantitative polymerase chain reaction was used to evaluate gene expression. The data were analyzed by one-way ANOVA, followed by the Tukey test, using SPSS software, version 16.0. The results were considered statistically significant at a P value less than 0.05.
Results:
In the diabetic rats treated with silymarin (60 and 120 mg/kg), by comparison with the diabetic group, body weight (P=0.04 and P=0.02), insulin (P<0.001), expression of PGC-1α (P=0.04 and P=0.02), expression of FNDC5 (P=0.03 and P=0.01), and concentration of irisin in the liver (P=0.02 and P=0.01) and serum (P<0.001) were significantly increased, whereas the levels of glucose (P<0.001), HOMA-IR (P=0.03 and P=0.01), and liver injury markers (P<0.001) were significantly reduced. Oxidative stress status and histopathological changes were improved in the treated groups.
Conclusion:
These results suggest that silymarin because of its ability to upregulate irisin and antioxidant effects can be considered an antidiabetic agent.
Keywords: Silymarin , FNDC5 protein , Diabetes mellitus type 2 , Rat
What’s Known
Several previous studies have shown that silymarin is widely used in liver diseases.
Silymarin is a potent free radical scavenger.
What’s New
This is the first study on the effects of silymarin irisin expression in rats with type 2 diabetes.
PGC-1α and FNDC5 mRNA expressions are induced by silymarin.
Silymarin improves oxidative status in the liver tissue of diabetic rats.
Introduction
Diabetes mellitus (DM), as a chronic disease, is one of the major public health problems. Reduced insulin secretion or resistance to its function has a role in the creation of DM. The incidence of DM in the world was estimated to be 4% in 2010 and is expected to have amounted to 5.4% by 2025.1 DM is the leading cause of liver disorders. In diabetic patients, a wide spectrum of liver diseases such as nonalcoholic fatty liver disease, cirrhosis, and liver cell carcinoma are prevalent.2
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is a multifunctional regulatory transcription factor and plays an important role in the regulation of gluconeogenesis, mitochondrial biogenesis, and fatty acid β-oxidation in the liver. Due to its key role in glucose metabolism, PGC-1α is a very attractive target gene for antidiabetic therapy.3 Studies have shown that PGC-1α mRNA expression in insulin resistance is reduced.4 PGC-1α is associated with an increased expression of fibronectin type III domain containing 5 (FNDC5). Irisin is a product of the proteolytic breakdown of the extracellular domain of FNDC5. Irisin is an antidiabetic hormone and is involved in the liver metabolism. Furthermore, it has been shown that the level of this hormone decreases in people with type 2 DM. Liu et al.5 showed that the circulating irisin level was significantly lower in their diabetic patients than that in their control group. Research has also demonstrated that FNDC5/irisin ameliorates glucose disorders and insulin resistance.6
Recent studies have reported a negative relationship between irisin levels and oxidative stress, and in fact irisin has antioxidant properties.7 Hyperglycemia and insulin resistance cause increased generation of free radicals and reactive oxygen species (ROS). Oxidative stress plays a critical role in the pathogenesis of diabetic complications.8
In recent years, the use of natural products has been considered for the treatment of hyperglycemia and complications of DM. Flavonoids are natural substances available in plants. These compounds have a high potential to treat a variety of disorders, including diabetes.9 Silymarin is a milk thistle (Silybum marianum) extract, which contains a mixture of flavonolignans. The protective effects of silymarin treatment as a potent ROS scavenger have been shown by earlier researchers.10 Further, the hepatoprotective effects of silymarin by attenuating proinflammatory gene expression in the liver have been supported by additional research.11 Although the therapeutic effects of silymarin on DM and liver diseases have been shown in human and animal studies,12,13 its molecular mechanisms remain to be revealed. Therefore, the aim of the present study was to investigate the protective effects of silymarin on irisin expression and oxidative stress in the liver of rats with type 2 diabetes.
Materials and Methods
Animals and Experimental Designs
The current experimental study was performed at the Animal House and Biochemistry Laboratory of Hamadan University of Medical Sciences (2016-2107). Thirty-six adult male Wistar rats (220±10 g) were used. The animals were kept in standard conditions (12-h dark/light cycle at 22±2 °C). The rats was divided into 6 groups (n=6 each) by simple randomization. Group C formed our normal control group. Group C+S60 and Group C+S120 comprised control rats treated with silymarin (Goldaru; Isfahan, Iran) (60 and 120 mg/kg daily, respectively) in normal saline orally for 60 days.14 Group D formed our diabetic control group. Group D+S60 and Group D+S120 comprised diabetic rats treated with silymarin (60 and 120 mg/kg daily, respectively) for 60 days. At the end of the experimental period, body weight was determined. The study was approved by the Medical Ethics Review Board of Hamadan University of Medical Sciences (IR.UMSHA.REC.1395.205).15
For the induction of type 2 diabetes, 15 minutes after the injection of nicotinamide (NIC, Sigma) (120 mg/kg; i.p.), streptozotocin (STZ, Sigma) (60 mg/kg; i.p.) dissolved in 0.1 M of citrate buffer (pH 4.5) was injected in 18 overnight-fasted rats. To confirm type 2 diabetes, after 72 hours, the fasting blood glucose (FBS) level of the rats was measured with a glucometer (Accu-Chek; Roche, Germany). The animals were considered diabetic when their FBS level was above 150 mg/dL.16 After the end of the study, the fasted rats were anesthetized with ketamine (50 mg/kg) and blood samples were collected from their vena cava vein for biochemical analysis and stored at -20 °C. Additionally, the liver tissue was separated from each rat and cleaned with an ice-cold saline solution and frozen in liquid nitrogen immediately after separation and stored at -70°C until analysis. For histopathological evaluations, a small piece of the liver tissue was collected.
Measurement of Serum Biochemical Parameters
Total bilirubin and liver enzymes (ALT, AST, and ALP) were measured ýusing a standard procedure kit (Pars Azmoon kit, Iran). The level of insulin was determined using the rat Insulin ELISA Kit (Mercodia, Uppsala, Sweden). The insulin resistance index (HOMA-IR) was calculated as follows:17 HOMA−IR=fasting insulin (μU/mL)×fasting glucose (mg/dL)/405. The concentrations of tissue and serum irisin were determined using a commercial ELISA kit (ZellBio GmbH, Germany).
Assay of Hepatic Oxidative Stress Parameters
In the liver samples, malondialdehyde (MDA) via the Yagi method, total antioxidant capacity (TAC) via the Benzie and Strain method,18 total thiol molecules (TTM) via the Hu method, and total oxidant status (TOS) with the ferric-xylenol orange 1 (FOX1) reagent via the Erel method were determined. Additionally, the oxidative stress index (OSI) was calculated by dividing TOS by TAC.17
Quantitative Real-Time Polymerase Chain Reaction (PCR)
Total RNA extraction was performed manually from the tissues using the RNX-plus reagent (CinnaGen, Tehran, Iran). Complementary DNA (cDNA) synthesis was carried out using the PrimeScript RT reagent kit (TaKaRa Biotechnology, Japan). Quantitative real-time PCR was performed with SYBR premix Ex TaqTM II (TaKaRa Biotechnology, Japan) on a Roche Light Cycler 96 System (Roche Life Science Deutschland GmbH, Sandhofer, Germany). The characteristics of the forward and reverse primer sequence (5’→3’) were listed as follows: β-actin forward: CCCGCGAGTACAACCTTCT and reverse: CGTCATCCATGGCGAACT, FNDC5 forward: GAGGTGCTGATCATCGTCGT and reverse: GAGCAAGCACTGAAAGGGTTT, and PGC-1α forward: GTGCAGCCAAGACTCTGTATGG and reverse: GTCCAGGTCATTCACATCAAGTTC. The fold change in the gene expression was investigated via the 2-ΔΔCT formula.19
Histological Observation of the Liver Tissues
For histopathological evaluations, a small piece of the liver tissues was fixed in 10% formalin solution, dehydrated in graduate ethanol (50-100%), cleared in xylene, and embedded in paraffin. The liver sections (5 μm) were examined with a photomicroscope after staining with hematoxylin and eosin dye (H&E).
Statistical Analysis
The results were expressed as means±standard deviations (SDs). The data were analyzed by SPSS 16 and Prism 6.0 software (GraphPad, San Diego, CA, USA), and a P value less than 0.05 was considered statistically significant. One-way analysis of variance, followed by the Tukey test, was employed for between-group comparisons. The normality was checked using the Kolmogorov-Smirnov test.
Results
General and Biochemical Parameters
As is indicated in table 1, body weight decreased in the diabetic rats (Group D) compared to the control group (Group C) after 8 weeks of experiment (P<0.001). Treatment with both doses of silymarin (60 mg/kg [P=0.04] and 120 mg/kg [P=0.02]) improved body weight compared to that in Group D.
Table 1.
Effects of silymarin on the rats’ body weight and biochemical parameters in the different studied groups
| Parameter/ Group | C (n=6) | C+S60 (n=6) | C+S120 (n=6) | D (n=6) | D+S60 (n=6) | D+S120 (n=6) |
|---|---|---|---|---|---|---|
| Final body weight (g) | 297.50±18.39 | 288.00±18.69 | 289.00±19.76 | 182.50±16.94 a | 215.6±14.94 b | 219.83±23.69 b |
| FBS (mg/dL) | 83.33±10.93 | 76.50±12.24 | 79.50±13.80 | 299.383±27.05 a | 202.67±45.14 b | 182.50±35.82 b |
| Insulin (µU/mL) | 11.46±0.47 | 11.96±0.67 | 11.65±1.64 | 7.10±1.06 a | 9.60±1.11 b | 9.80±0.96 b |
| HOMA-IR | 2.35±0.27 | 2.24±0.26 | 2.28±0.41 | 5.79±0.90 a | 4.71±0.64 b | 4.35±0.56 b |
| ALT (U/L) | 53.00±6.10 | 47.16±5.26 | 55.00±5.51 | 160.66±7.11 a | 73.50±2.07 b | 70.50±3.04 b |
| AST (U/L) | 108.33±6.59 | 119.16±11.16 | 115.66±9.79 | 239.16±14.17 a | 152.00±6.71 b | 140.50±10.44 b |
| ALP (U/L) | 250.50±19.59 | 267.16±20.74 | 270.33±21.44 | 730.16±41.88 a | 396.50±34.56 b | 431.83±24.92 b |
| Total bilirubin (mg/dL) | 0.43±0.05 | 0.42±0.03 | 0.48±0.06 | 1.03±0.11 a | 0.57±0.06 b | 0.54±0.11 b |
Results are expressed as means±SDs. C: Control; S: Silymarin; D: Diabetic; FBS: Fasting blood sugar; ALT: Alanine transaminase; AST: Aspartate transaminase; ALP: Alkaline phosphatase; HOMA-IR: Insulin resistance index;
Significant compared to the healthy control group;
Significant compared to the diabetic control group; Significant difference between the diabetic and control groups in the all the parameters (P<0.001); Significant difference between the diabetic rats treated with silymarin (60 and 120 mg/kg) and the diabetic group in body weight (P=0.04 and P=0.02), FBS (P<0.001), insulin (P<0.001), HOMA-IR (P=0.03 and P=0.01), and liver injury markers (P=0.00)
To study the possible protective role of silymarin against insulin resistance, we investigated the levels of serum FBS, insulin, and HOMA-IR. Eight weeks after the injection of NIC/STZ, a significant increase in FBS was detected in Group D in comparison to Group C (P<0.001). Treatment with silymarin with both doses of 60 and 120 mg/kg caused a significant reduction in FBS compared to that in Group D (P<0.001) (table 1).
Induced type 2 diabetes caused a reduction in insulin concentration and an increase in HOMA-IR in Group D compared to Group C (P<0.001). Treatment with both doses of silymarin significantly increased the insulin level and reduced HOMA-IR back to the normal value (insulin: P<0.001; HOMA-IR: Group D+S60 and Group D+S120 compared to Group D; P=0.03 and P=0.01, respectively) (table 1).
Liver injury markers (ALT, AST, ALP, and total bilirubin) in the diabetic rats showed a significant rise by comparison with those in Group C (P<0.001). The increased serum liver injury markers in Group D were modulated after the administration of silymarin (P<0.001) (table 1).
Effects of Silymarin on the Oxidative Stress Biomarkers
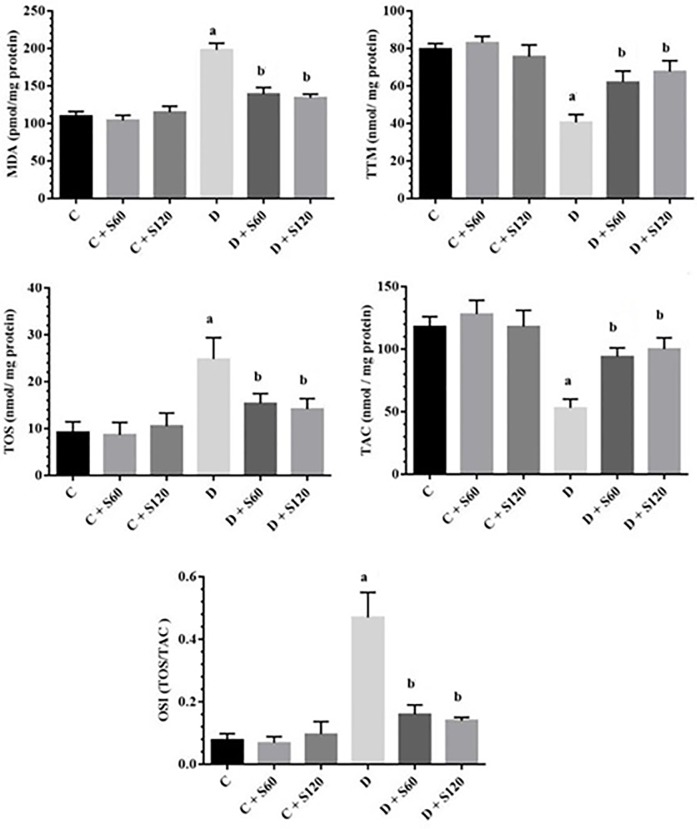
As is depicted in figure 1, significant increases were observed in the MDA, TOS, and OSI levels in Group D versus Group C (P<0.001), whereas treatment with silymarin resulted in decreased levels of MDA, TOS, and OSI compared to Group D (P<0.001) (figure 1). The levels of TAC and TTM were significantly decreased in Group D compared to Group C (P<0.001). Treatment with silymarin significantly reversed TAC in the treated groups (Group D+S60 [P=0.04] and Group D+S120 [P=0.01]) and TTM in the treated groups (Group D+S60 [P=0.01] and Group D+S120 [P<0.001]) compared to Group D.
Figure1.
Effects of silymarin on liver oxidative stress parameters. The results are expressed as means±SDs. Liver MDA (A), TTM (B), TOS (C), TAC (D) and OSI (E) contents. C: Control; S: Silymarin; D: Diabetic; MDA: Malondialdehyde; TTM: Total thiol molecule; TOS: Total oxidant status; TAC: Total antioxidant capacity; OSI: Oxidative stress index; aSignificant compared to the healthy control group; bSignificant compared to the diabetic control group; Significant difference between the diabetic and control groups in the all the parameters (P<0.001) Significant difference between the diabetic rats treated with silymarin (60 and 120 mg/kg) and the diabetic group in MDA, TOS, and OSI (P= 0.00), TAC (P=0.04 and P=0.01), and TTM (P=0.01 and P<0.001)
Effects of Silymarin on Gene Expression
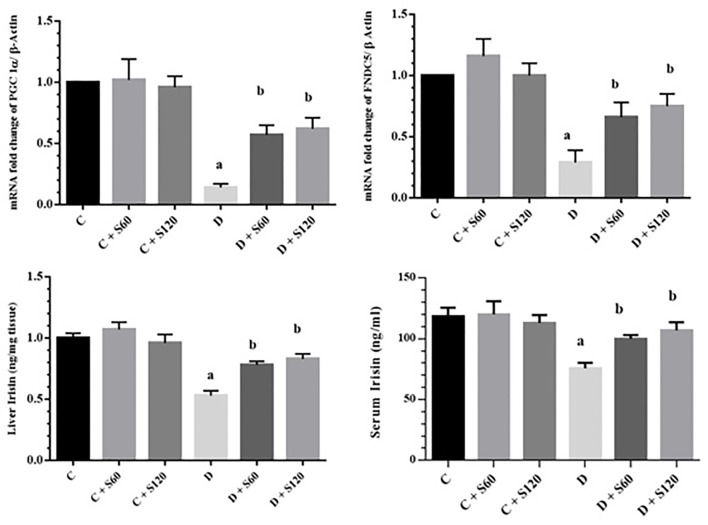
The expressions of PGC-1α and FNDC5 at the mRNA level in the liver tissues of all the groups were assessed. The results indicated that the expression of PGC-1α and FNDC5 in Group D was significantly less than that in Group C (P<0.001). Silymarin caused a significant increase in PGC-1α and FNDC5 gene expression (Group D+S60 vs. Group D, PGC-1α: P=0.04 and FNDC5: P=0.03; Group D+S120 vs. Group D, PGC-1α: P=0.02 and FNDC5: P=0.01) (figure 2).
Figure2.
Effect of silymarin on PGC-1α, FNDC5, and irisin. The results are expressed as means±SDs. A) PGC-1α mRNA levels in the liver; B) FNDC5 mRNA levels in the liver; C) liver irisin, and D) serum irisin concentration. C: Control; S: Silymarin; D: Diabetic; FNDC5: Fibronectin type III domain-containing protein 5; aSignificant compared to the healthy control group; bSignificant compared to the diabetic control group; Significant difference between the diabetic and control groups in the all the parameters (P<0.001); Significant difference between the treated diabetic rats (60 and 120 mg/kg) and the diabetic groups in PGC-1α (P=0.04 and P=0.02), FNDC5 (P=0.03 and P=0.01), liver irisin (P=0.02 and P=0.01), and serum irisin (P<0.001)
Effects of Silymarin on Serum and Tissue Irisin Concentrations
A significant reduction in the irisin concentration in the liver and serum of Group D compared to Group C was identified (P<0.001). There was also a significant rise in the irisin concentration in Group D+S60 and Group D+S120 compared to Group D (liver irisin, Group D+S60 and Group D+S120; P=0.02 and P=0.01, respectively, and serum irisin [P<0.001]) (figure 2).
Histological Observation
The liver tissues of all the control rats showed a normal lobular architecture with central veins, radiating hepatic cords, and portal triads containing the portal vein, hepatic artery, and bile duct (figure 3A).
Figure3.
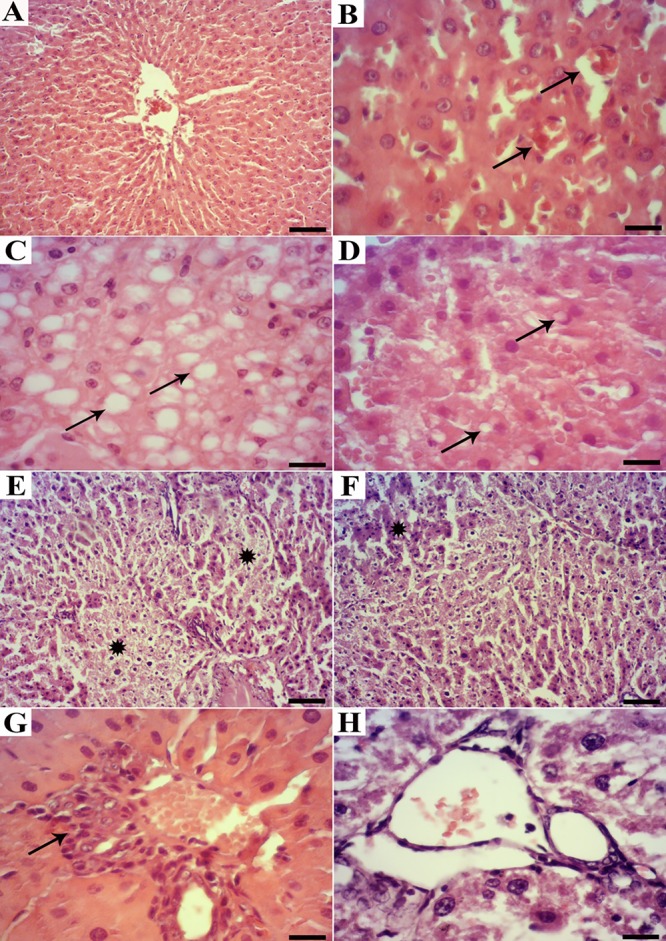
Histopathological observation in the liver tissues of the experimental rats. A) Normal rat: A normal lobular pattern is seen with a centrilobular vein and radiating irregular anastomosing plates of the hepatocytes with intervening sinusoids (H&E, scale bar=100 µm), B) Diabetic rat: Congestion and dilation of the sinusoids (arrows) (scale bar=25 µm), C) Diabetic rat: Disorganization of the hepatic cords and the presence of degenerated hepatocytes with macrovesicular steatosis (stars) (scale bar=25 µm), D) Treated diabetic rat: Cytoplasmic vacuoles in the cytoplasm (scale bar=25 µm), E) Diabetic rat: Focal necrosis (stars) (scale bar=100 µm), F) Treated diabetic rat: Disorganization of the hepatic cords and mild hepatocyte degeneration (star) (scale bar=100 µm), G) Diabetic rat: Mild-to-moderate infiltration of the mononuclear cells around the portal area (arrow) (scale bar=25 µm), H) Treated diabetic rat: Normal portal area without mononuclear cell infiltration (scale bar=25 µm)
Sections of the liver from the diabetic rats (figure 3B-F) showed mild-to-severe lesions represented by disorganization in the hepatic cords, moderate centrilobular hepatocyte degeneration with an accumulation of lipid droplets in the cytoplasm of the hepatocytes, congestion and dilation of the sinusoids and central veins, infiltration of the mononuclear cells in the portal tracts, mild bile duct proliferation, and focal necrosis. Additionally, the presence of giant hepatocytes without distinct cell borders and morphological changes such as pyknosis, karyorrhexis, and chromatolysis were constant features in the centrilobular area.
The livers of the treated diabetic rats (figure 3 G and H) with low and high doses of silymarin showed improvement in the structure of the hepatic tissue compared to those of the untreated diabetic rats.
Discussion
DM is a global problem with a close correlation with liver diseases.2 Silymarin therapies have been shown in some studies to possess antidiabetic and hepatoprotective properties.5 The results of the present study showed that silymarin inhibited NIC/STZ-induced weight loss and oxidative stress, attenuated insulin resistance, reduced deleterious hepatic injuries, and induced the production of PGC-1α and FNDC5/irisin in the hepatic tissues of our diabetic rats.
In our study, the body weight of the untreated diabetic group decreased significantly. This reduction in body weight can be due to a breakdown of tissue proteins in diabetic rats.20 We found that silymarin was able to inhibit body weight reduction during an 8-week period following the administration of NIC/STZ, probably due to the protective role of silymarin in controlling muscle atrophy (i.e., reversal of gluconeogenesis).21 Studies have shown that silymarin can restore weight and improve glucose and insulin levels in diabetic rats.22
Silymarin is widely used in liver diseases all over the world for its hepatoprotective potential. In the last decade, other beneficial capabilities of silymarin administration such as antioxidant, anti-inflammatory, immunomodulatory, and liver-regenerating capacities were emphasized by experimental research and clinical trials. Silymarin inhibits liver injury by maintaining the integrity of the plasma membrane, inhibits the secretion of liver enzymes in blood, and suppresses apoptosis in hepatocytes.10 Shaker et al.23 showed that the use of silymarin (100 mg/kg) in the treatment of carbon tetrachloride-induced liver damage (2 mL/kg) significantly reduced the liver enzyme activity in the rat serum. Similarly, in the current study, we recorded a decrease in the rats’ serum ALT, AST, ALP, and total bilirubin levels after treatment with silymarin.
The liver plays an important role in regulating glucose concentration in physiological and pathological states such as in DM. In type 2 DM, insulin resistance in the liver leads to hyperglycemia and further distortion of glucose metabolism.24 Hyperglycemia inhibits complex III and leads to the production of ROS in the liver and other organs. Oxidative stress also plays a key role in the pathology of diabetes.25 Silymarin possesses antioxidant properties. It prevents lipid peroxidation, inhibits glutathione reduction, and induces antioxidant enzymes activity.26 These effects are determined largely by the presence of a β ring catechol group (dihydroxylated β- ring), capable of donating hydrogen electrons that stabilize ROS, and a high phenolic content. Additionally, the presence of 2’,3’ unsaturation in conjugation with a 4-oxo-function in the C ring and the presence of functional groups capable of binding transition metal ions such as iron may also be responsible for the antioxidant nature of silymarin.5 In the present study, treatment with silymarin reduced the level of oxidative stress markers (MDA, TOS, and OSI) and increased antioxidants (TTM and TAC) in the liver tissue. The antioxidant properties of silymarin are a strong justification for its hepatoprotective effects.27 Oxidative stress plays a key role in triggering hepatic damage by inducing alterations in lipids and proteins as well as structural and pathway changes that control normal and physiological functions. These pathways such as PGC-1α regulate gene transcription, mitochondrial biogenesis, and protein expression in the liver tissue.28,29 Research has shown that the expression of PGC-1α is reduced in muscle samples from patients with type 2 DM. Furthermore, in nondiabetic subjects with a family history of DM, there is a significant 34% reduction in PGC-1α mRNA expression compared to individuals with no family history of diabetes. Therefore, diminished PGC-1α expression levels might be a marker of a prediabetic condition.30 On the other hand, it has been demonstrated that the increased expression of this gene may improve glucose homeostasis and reduce insulin resistance.31 Barbagallo et al.32 reported that the PGC-1α gene expression in adipose tissue-derived mesenchymal stem cells is induced by silibinin. Similarly, in the current study, in line with other studies, we demonstrated that NIC/STZ decreased PGC-1α mRNA expression. We observed that the PGC-1α gene expression was upregulated by silymarin treatment. A possible mechanism for the effects of silymarin on the PGC-1α gene expression is a hypoglycemic and antioxidant effect.
FNDC5 is a downstream molecule and the target of PGC-1α; thus, the expression of FNDC5 and the synthesis of the transmembrane FNDC5 protein are induced by PGC-1α. Irisin is a product of FNDC5. The concentration of irisin in plasma has been reported to decrease by 72% in PGC-1α- deficient mice.33 Irisin has attracted remarkable interest because of its putative therapeutic potential for obesity and diabetes. Several studies have shown an association between decreased levels of circulating irisin and insulin resistance or diabetes.34 Studies have revealed lower concentrations of irisin in patients with type 2 DM.35 In an animal study, Bostrom et al.36 showed that irisin was able to improve glucose tolerance and insulin sensitivity. Huh et al.37 reported a correlation between irisin and glucose in humans. Previous research has shown that irisin inhibits palmitic acid-induced apoptosis in liver cells via inhibiting oxidative stress status and inflammation is mediated by the inhibition of protein arginine methyltransferase-3, indicating a relationship between oxidative stress and irisin.38 Our results in the current study revealed a significant diminution of FNDC5 mRNA and irisin protein in the liver and serum of the diabetic group. For the first time, the findings of the present investigation showed that treatment with silymarin significantly induced mRNA transcripts and the protein of irisin in the hepatic tissue and serum of treated diabetic rats. Previous studies have demonstrated that adenovirus- upregulated irisin improves hepatic steatosis and insulin resistance in genetic-induced obese mice.39 Another study also showed that the upregulation of irisin was associated with a reduction in oxidative stress.7 Recent data have shown that silymarin interacts with transcription factors and subsequent alter gene expression and protein synthesis.22 Therefore, one of the main mechanisms of the hepatoprotective effects of silymarin in the liver may be its antioxidant effects. Indeed, via these properties, silymarin can increase FNDC5/irisin production.
Since irisin is produced and secreted by different tissues, the increased serum irisin concentration in our study may have been due to the effects of silymarin on various tissues including liver, muscle, and heart. This is a topic for further research. In addition, the molecular mechanisms of the expression, secretion, and actions of irisin on the liver tissue remain unknown and require further studies.
The results of histopathological studies strongly support the outcome of our study. Histopathological research has presented no evidence of bile duct proliferation, mononuclear cell infiltration, or parenchymal cell necrosis in the livers of treated diabetic rats. A comparison between low- and high-dose silymarin and the histological alterations were similar in both treatment groups.
In order to select the appropriate doses in the present study, we considered the results of other studies and reports that effects of silymarin are dose dependent.5 We selected the dose of 60 mg/kg because it has been demonstrated to be the suitable dose for hepatoprotective effects.40 The 120 mg/kg dose is the high dose used in other studies to show its ability to reveal diabetic changes.14 Accordingly, we concluded that a comparison between these 2 doses would enable us to find the optimal therapeutic dose for the prevention of DM complications.
In our study, the administration of silymarin (60 and 120 mg/kg) was chronic and long-term. Our normal group rats adapted their body to gradual changes and maintained their homeostasis. We detected no significant difference between the normal groups receiving silymarin and the control group. Had we tried a sudden administration of high doses of silymarin, we might have observed significant changes in the controls receiving silymarin by comparison with the control group. At any rate, our results demonstrated no significant changes in all the factors between the 2 treatment control groups and the untreated control group: this confirms that the silymarin doses used did not have toxic effects on the rats’ livers. Similarly, Soto et al.22 reported no significant difference in insulin and glucose levels after treating their control group with silymarin (200 mg/kg for 30 days) compared to their healthy control group.
The strength of the current study is that it is the first report on the effects of silymarin on the irisin hormone. The major limitation of our study, however, is that financial constraints precluded the use of some additional experiments such as western blotting for PGC-1α, direct assay of ROS, and antioxidant enzyme activity.
Conclusion
Liver injury is a serious complication among patients with DM. We showed that silymarin significantly decreased FBS and increased the insulin level; hence, it was able to improve HOMA-IR. Furthermore, silymarin augmented oxidative stress status in the liver tissue. The present study also showed that not only did silymarin increase the PGC-1α and FNDC5 gene expression in diabetic rat liver tissues but also it raised the irisin concentration in the liver and serum of diabetic rats. We concluded that one of the principle mechanisms of the hepatoprotective effects of silymarin on the liver might be its antioxidant effects. However, more research is required to elucidate the molecular mechanisms of the effects of silymarin.
Acknowledgement
The present study was financially supported by Hamadan University of Medical Sciences (No: 9505052617). We would like to thank T. Ghiasvand, Sh. Heidari, and M. Taheri for their assistance. The results presented in this article were a part of a PhD thesis by N. Kheiripour.
Conflict of Interest:None declared.
References
- 1.Bayat A, Azizi-Soleiman F, Heidari-Beni M, Feizi A, Iraj B, Ghiasvand R, et al. Effect of Cucurbita ficifolia and Probiotic Yogurt Consumption on Blood Glucose, Lipid Profile, and Inflammatory Marker in Type 2 Diabetes. Int J Prev Med. 2016;7:30. doi: 10.4103/2008-7802.175455. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zoppini G, Fedeli U, Gennaro N, Saugo M, Targher G, Bonora E. Mortality from chronic liver diseases in diabetes. Am J Gastroenterol. 2014;109:1020–5. doi: 10.1038/ajg.2014.132. [DOI] [PubMed] [Google Scholar]
- 3.Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR, et al. Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. Proc Natl Acad Sci U S A. 2012;109:9635–40. doi: 10.1073/pnas.1207287109. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voroneanu L, Nistor I, Dumea R, Apetrii M, Covic A. Silymarin in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Diabetes Res. 2016;2016:5147468. doi: 10.1155/2016/5147468. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aydin S, Kuloglu T, Aydin S, Eren MN, Celik A, Yilmaz M, et al. Cardiac, skeletal muscle and serum irisin responses to with or without water exercise in young and old male rats: cardiac muscle produces more irisin than skeletal muscle. Peptides. 2014;52:68–73. doi: 10.1016/j.peptides.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Zhu D, Wang H, Zhang J, Zhang X, Xin C, Zhang F, et al. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J Mol Cell Cardiol. 2015;87:138–47. doi: 10.1016/j.yjmcc.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Ghadermazi R, Khoshjou F, Hossini Zijoud SM, Behrooj H, Kheiripour N, Ganji M, et al. Hepatoprotective effect of tempol on oxidative toxic stress in STZ-induced diabetic rats. Toxin Rev. 2018;37:82–6. doi: 10.1080/15569543.2017.1313277. [DOI] [Google Scholar]
- 9.Pandey KB, Rizvi Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–8. doi: 10.4161/oxim.2.5.9498. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vargas-Mendoza N, Madrigal-Santillan E, Morales-Gonzalez A, Esquivel-Soto J, Esquivel-Chirino C, Garcia-Luna YG-RM, et al. Hepatoprotective effect of silymarin. World J Hepatol. 2014;6:144–9. doi: 10.4254/wjh.v6.i3.144. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovelace ES, Wagoner J, MacDonald J, Bammler T, Bruckner J, Brownell J, et al. Silymarin Suppresses Cellular Inflammation By Inducing Reparative Stress Signaling. J Nat Prod. 2015;78:1990–2000. doi: 10.1021/acs.jnatprod.5b00288. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazazis CE, Evangelopoulos AA, Kollas A, Vallianou NG. The therapeutic potential of milk thistle in diabetes. Rev Diabet Stud. 2014;11:167–74. doi: 10.1900/RDS.2014.11.167. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedighifard Z, Roghani F, Bidram P, Harandi SA, Molavi S. Silymarin for the Prevention of Contrast-Induced Nephropathy: A Placebo-Controlled Clinical Trial. Int J Prev Med. 2016;7:23. doi: 10.4103/2008-7802.174762. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheela N, Jose MA, Sathyamurthy D, Kumar BN. Effect of silymarin on streptozotocin-nicotinamide-induced type 2 diabetic nephropathy in rats. Iran J Kidney Dis. 2013;7:117–23. [PubMed] [Google Scholar]
- 15.Touitou Y, Smolensky MH, Portaluppi F. Ethics, standards, and procedures of animal and human chronobiology research. Chronobiol Int. 2006;23:1083–96. doi: 10.1080/07420520601055308. [DOI] [PubMed] [Google Scholar]
- 16.Rezaei Farimani A, Saidijam M, Goodarzi MT, Yadegar Azari R, Asadi S, Zarei S, et al. Effect of Resveratrol Supplementation on the SNARE Proteins Expression in Adipose Tissue of Stroptozotocin-Nicotinamide Induced Type 2 Diabetic Rats. Iran J Med Sci. 2015;40:248–55. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahabadi M, Mohammadalipour A, Karimi J, Sheikh N, Solgi G, Goudarzi F, et al. Hepatoprotective effect of parthenolide in rat model of nonalcoholic fatty liver disease. Immunopharmacol Immunotoxicol. 2017;39:233–42. doi: 10.1080/08923973.2017.1327965. [DOI] [PubMed] [Google Scholar]
- 18.Zanganeh N, Siahpoushi E, Kheiripour N, Kazemi S, Goodarzi MT, Alikhani MY. Brucellosis Causes Alteration in Trace Elements and Oxidative Stress Factors. Biol Trace Elem Res. 2018;182:204–8. doi: 10.1007/s12011-017-1102-3. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Pournaghi P, Sadrkhanlou RA, Hasanzadeh S, Foroughi A. An investigation on body weights, blood glucose levels and pituitary-gonadal axis hormones in diabetic and metformin-treated diabetic female rats. Vet Res Forum. 2012;3:79–84. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi EJ, Kim EK, Jeoung NH, Kim SH. Effect of silymarin on gluconeogenesis and lactate production in exercising rats. Food Sci Biotechnol. 2016;25:119–24. doi: 10.1007/s10068-016-0108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soto C, Mena R, Luna J, Cerbon M, Larrieta E, Vital P, et al. Silymarin induces recovery of pancreatic function after alloxan damage in rats. Life Sci. 2004;75:2167–80. doi: 10.1016/j.lfs.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Shaker E, Mahmoud H, Mnaa S. Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem Toxicol. 2010;48:803–6. doi: 10.1016/j.fct.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Mohamed J, Nazratun Nafizah AH, Zariyantey AH, Budin SB. Mechanisms of Diabetes-Induced Liver Damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ Med J. 2016;16:e132–41. doi: 10.18295/squmj.2016.16.02.002. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khazaei M, Karimi J, Sheikh N, Goodarzi MT, Saidijam M, Khodadadi I, et al. Effects of Resveratrol on Receptor for Advanced Glycation End Products (RAGE) Expression and Oxidative Stress in the Liver of Rats with Type 2 Diabetes. Phytother Res. 2016;30:66–71. doi: 10.1002/ptr.5501. [DOI] [PubMed] [Google Scholar]
- 26.Surai PF. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants (Basel) 2015;4:204–47. doi: 10.3390/antiox4010204. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freitag AF, Cardia GF, da Rocha BA, Aguiar RP, Silva-Comar FM, Spironello RA, et al. Hepatoprotective Effect of Silymarin (Silybum marianum) on Hepatotoxicity Induced by Acetaminophen in Spontaneously Hypertensive Rats. Evid Based Complement Alternat Med. 2015;2015:538317. doi: 10.1155/2015/538317. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singal AK, Jampana SC, Weinman SA. Antioxidants as therapeutic agents for liver disease. Liver Int. 2011;31:1432–48. doi: 10.1111/j.1478-3231.2011.02604.x. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93:884S–90. doi: 10.3945/ajcn.110.001917. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patti ME. Gene expression in the pathophysiology of type 2 diabetes mellitus. Curr Diab Rep. 2004;4:176–81. doi: 10.1007/s11892-004-0020-x. [DOI] [PubMed] [Google Scholar]
- 31.Summermatter S, Shui G, Maag D, Santos G, Wenk MR, Handschin C. PGC-1alpha improves glucose homeostasis in skeletal muscle in an activity-dependent manner. Diabetes. 2013;62:85–95. doi: 10.2337/db12-0291. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbagallo I, Vanella L, Cambria MT, Tibullo D, Godos J, Guarnaccia L, et al. Silibinin Regulates Lipid Metabolism and Differentiation in Functional Human Adipocytes. Front Pharmacol. 2015;6:309. doi: 10.3389/fphar.2015.00309. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Y, Li H, Shen SW, Shen ZH, Xu M, Yang CJ, et al. Swimming exercise increases serum irisin level and reduces body fat mass in high-fat-diet fed Wistar rats. Lipids Health Dis. 2016;15:93. doi: 10.1186/s12944-016-0263-y. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ural UM, Sahin SB, Tekin YB, Cure MC, Sezgin H. Alteration of maternal serum irisin levels in gestational diabetes mellitus. Ginekol Pol. 2016;87:395–8. doi: 10.5603/GP.2016.0013. [DOI] [PubMed] [Google Scholar]
- 35.Perakakis N, Triantafyllou GA, Fernandez-Real JM, Huh JY, Park KH, Seufert J, et al. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol. 2017;13:324–37. doi: 10.1038/nrendo.2016.221. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8. doi: 10.1038/nature10777. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise Metabolism 2012;61:1725–38. doi: 10.1016/j.metabol.2012.09.002. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park MJ, Kim DI, Choi JH, Heo YR, Park SH. New role of irisin in hepatocytes: The protective effect of hepatic steatosis in vitro. Cell Signal. 2015;27:1831–9. doi: 10.1016/j.cellsig.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Mo L, Shen J, Liu Q, Zhang Y, Kuang J, Pu S, et al. Irisin Is Regulated by CAR in Liver and Is a Mediator of Hepatic Glucose and Lipid Metabolism. Mol Endocrinol. 2016;30:533–42. doi: 10.1210/me.2015-1292. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaker ME, Shiha GE, Ibrahim TM. Comparison of early treatment with low doses of nilotinib, imatinib and a clinically relevant dose of silymarin in thioacetamide-induced liver fibrosis. Eur J Pharmacol. 2011;670:593–600. doi: 10.1016/j.ejphar.2011.08.041. [DOI] [PubMed] [Google Scholar]