Abstract
Background:
Bone marrow stromal cells (BMSCs), as a type of mesenchymal stem cells, and the granulocyte colony-stimulating factor (G-CSF), as a type of growth factor, may recover damaged ovaries. The aim of the present study was to investigate the effects of the coadministration of BMSCs and the G-CSF on damaged ovaries after creating a chemotherapy model with cyclophosphamide (CTX) in rats.
Methods:
The present study was performed in Semnan, Iran, in the late 2016 and the early 2017. BMSCs were cultured and were confirmed using the CD markers of stromal cells. Forty female Wistar rats were randomly divided into 4 groups. The rats were injected intraperitoneally with CTX for 14 days to induce chemotherapy and ovarian destruction. Then, the BMSCs were injected into bilateral ovaries and the G-CSF was injected intraperitoneally, individually and together. Four weeks later, the number of ovarian follicles using H&E staining, the number of apoptotic granulosa cells using the TUNEL assay, the number of produced oocytes from the ovaries, and the levels of serum E2 and FSH using an ELISA reader were assessed. Statistical analysis was done using one-way ANOVA with SPSS, version 16.0.
Results:
The results showed that the effects of the coadministration of 2×106 BMSCs and 70 µg/kg of the G-CSF were significantly more favorable than those in the control group (P<0.001), the BMSC group (P=0.016), and the G-CSF group (P<0.001) on the recovery of damaged ovaries.
Conclusion:
The efficacy of the coadministration of BMSCs and the G-CSF in the recovery of ovaries damaged by chemotherapy was high by comparison with the administration of either of them separately.
Keywords: Mesenchymal stromal cells , Granulocyte colony-stimulating factor , Chemotherapy , Ovary , Regeneration
What’s Known
Chemotherapy with cyclophosphamide (CTX) damages ovaries.
Transplantation of bone marrow stromal cells (BMSCs), as a type of mesenchymal stem cells, repairs damaged ovaries following chemotherapy.
Granulocyte colony-stimulating factor (G-CSF), as a growth factor, has beneficial effects on damaged ovaries.
What’s New
G-CSF may have the potential to regenerate ovaries damaged by chemotherapy with CTX.
Coadministration of BMSCs and the G-CSF may be more effective than either of them alone on ovaries damaged by chemotherapy •with CTX.
Introduction
Ovarian dysfunction is common in premenopausal women receiving chemotherapy, and approximately 30% of women aged less than 35 years and 50% of women aged between 35 and 40 years tend to experience menstrual function cessation after chemotherapy, leading to premature ovarian failure (POF) or menopause.1 POF is described as secondary infertility with elevated gonadotropin levels before age 40, with an estimated incidence rate of 1%.2 The use of chemotherapy increases the cure rate in young cancer patients, but some side effects such as ovarian failure and infertility may occur. POF due to chemotherapy causes cytotoxic effects, which will damage granulosa cells (GCs) and will, thus, possibly lead to folliculogenesis disruption.3 Cyclophosphamide (CTX) is one of the most commonly used chemotherapy drugs, and it directly destroys oocytes and stimulates follicular depletion.4
There are various methods to treat ovarian failure secondary to chemotherapy such as hormone therapy and stem cell therapy.5 Hormone therapy is used to treat common menopausal problems, but it may increase the risk of cancers.2,6 Recent studies on stem cell therapy have indicated that ovarian dysfunction can be treated by different types of stem cell transplantation.7-9 Bone marrow stromal cells (BMSCs) are a type of mesenchymal stem cells which can be differentiated into other cell lines and may have the potential to replace damaged cells.10 Moreover, BMSCs can produce some growth factors and cytokines for mitogenesis, angiogenesis, and anti-apoptosis.11 Various articles have demonstrated that BMSC transplantation can improve ovarian function and structure in rats with chemotherapy-induced ovarian damage.9,12
Colony-stimulating factors (CSFs), as hematopoietic growth factors, can regulate the mobilization, proliferation, and differentiation of bone marrow cells. The granulocyte colony-stimulating factor (G-CSF) is a growth factor with therapeutic roles for cancers, autoimmune diseases, ischemic insults, and degenerative diseases.13 The G-CSF plays key roles in cellular proliferation, anti-inflammatory and anti-apoptotic processes, and mobilization of stem cells to target sites.14 Akdemir et al.15 showed that the G-CSF has the beneficial effect of reducing the severity of ovarian follicle loss in the rat model of cisplatin chemotherapy.15 In several articles, a combination of stem cell transplantation and the G-CSF has been utilized with a view to repairing various parts of the body.16-18 Safari et al.19 achieved fruitful results from combining BMSCs and the G-CSF to treat a rat model of Parkinson’s disease.19
Given that there have been no reports concerning the effects of the G-CSF on ovaries damaged by CTX, in the present study, for the first time, we evaluated the effects of the G-CSF on damaged ovaries after creating a chemotherapy model with CTX and compared the results with the effects of BMSC transplantation into ovaries and the effects of the coadministration of BMSCs and the G-CSF on ovaries damaged by chemotherapy in rats.
Materials and Methods
Animals
The present study was performed in Semnan, Iran, in the late 2016 and the early 2017. Forty female Wistar rats (180-200 g) were used. The animals had free access to food and water under a controlled temperature (25±2 °C). Vaginal smear was obtained daily, and only those showing at least 2 consecutive normal vaginal estrus cycles were used in the experiments. All the procedures were approved by the Research Council of Semnan University of Medical Sciences.
BMSC Culture and Characterization
BMSCs were prepared from an adult female rat. The rat was killed, and its femurs and tibias were dissected out. The bone marrow was ejected with 10 mL of Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Germany) and cultured in the DMEM, containing 10% fetal bovine serum (FBS) (Gibco, Germany) and 1% penicillin/streptomycin (Gibco, Germany). The culture was incubated at 37 °C, 95% humidity, and 5% CO2. After 48 hours, nonadherent cells were removed by replacing the medium. The cells were subcultured 4 times.20,21 The expression of the surface marker of the stem cells was analyzed via the incubation of at least 100 000 cells with fluorescence-labeled monoclonal antibodies against CD29, CD34, CD44, CD45, and CD90 (Sigma, China). Following a 10-minute wash in PBS, the labeled cells were analyzed using a BD FACSCalibur flow cytometry apparatus.21,22
Creating the Chemotherapy Model
The POF model of chemotherapy was created via an intraperitoneal injection of CTX at 50 mg/kg (Sigma, China) diluted in normal saline. Subsequently, CTX was injected at 8 mg/kg daily for 13 consecutive days, for a total of 14 days.7
Procedure of BMSC Transplantation
The rats were randomly divided into 4 groups (n=10 in each group). In the control group, after the induction of the chemotherapy model, 20 µL of the culture medium was directly injected into the bilateral ovaries. In the BMSC group, following the induction of the chemotherapy model, 2×106 BMSCs suspended in 20 µL of the culture medium were directly injected into the bilateral ovaries.23,24 In the G-CSF group, after the induction of the chemotherapy model, 70 µg/kg of the G-CSF was injected intraperitoneally for 5 consecutive days.19 And finally, in the coadministration group of BMSCs and the G-CSF, after the induction of the chemotherapy model and BMSC transplantation, the G-CSF was injected for 5 consecutive days.
BMSC Tracking in the Ovaries
The BMSCs were labeled with DiI (1,1`-dioctadecyl-3,3,3`,3`- tetra methyl in do carbo cyanine perchlorate) (Sigma, China) in the ovaries to show the presence of the transplanted cells after 4 weeks. Briefly, the BMSCs were suspended in the DMEM and 5 µL/mL DiI was added. After incubation for 20 minutes, the cells were centrifuged and washed with PBS and then suspended again for transplantation. Four weeks after transplantation, the prepared paraffin sections and the labeled cells were detected by fluorescence microscopy (Motic, AE31, Spain).20,25
Hormonal Examination
Four weeks after treatment, the serum estradiol (E2) and follicle-stimulating hormone (FSH) levels of all the groups were measured using enzyme-linked immunosorbent assay (ELISA) kits (East Biopharm, China) for rats, according to the manufacturer’s instructions.8,9,26
Evaluation of Ovulation Ability
Four weeks after treatment, the rats were superovulated by an intraperitoneal injection of 150 IU/kg of pregnant mare serum gonadotropin (Sigma, China), followed, 48 hours later, by an intraperitoneal injection of 75 IU/kg of human chorionic gonadotropin (hCG) (Sigma, China).27 The oocytes were obtained from the ampulla portion of the oviduct 14 to 16 hours after the hCG injection.2
Histological Evaluation of the Ovaries
Four weeks after treatment, the ovaries were collected and fixed in 4% paraformaldehyde, dehydrated, paraffin-embedded, and serially sectioned at 5-µm thickness. Five representative sections from each ovary were randomly chosen and routine hematoxylin and eosin (H&E) staining was performed for histological examinations via light microscopy. The number of the primordial, primary, secondary, and antral follicles was measured. Only follicles containing an oocyte were counted. The follicles were thereafter classified. The primordial follicles were identified with having only 1 flat layer of squamous GCs. Primary follicles were defined as those having 1 cuboidal GCs. Secondary follicles were defined as those having multiple layers of GCs without antral spaces. Antral follicles were defined as those having antral spaces.2
Apoptosis Detection
Four weeks after treatment, the apoptotic GCs in the ovaries were evaluated using a TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) assay kit (Roche, Germany). Briefly, the sections were treated with 20 g/mL of proteinase K for 10 minutes and 0.1% Triton X-100 in 0.1% sodium citrate for 2 minutes on ice. The sections were incubated with the TUNEL reaction mixture. Then, the sections were stained with the diaminobenzidine (DAB) solution for 10 minutes at room temperature. Finally, they were counterstained with hematoxylin. At least 100 GCs were counted in 8 random fields under a light microscope (Motic, AE31, Spain) to calculate the percentage of the apoptotic cells.9,26,28
Statistical Analysis
The normality and homogeneity of variance assumptions were verified and then the data were analyzed by one-way analysis of variance (ANOVA), followed by the Tukey test. The obtained data were presented as means±SD, and a P value less than 0.05 was considered statistically significant.
Results
Cultivation and Characterization of the BMSCs
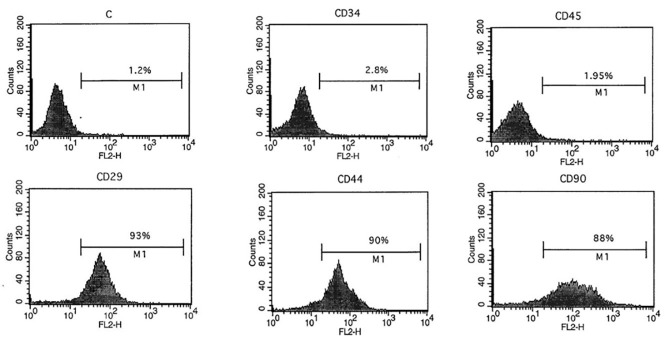
The BMSCs were cultured in T25 flasks. After a few days, the cells appeared to be spindle-shaped. The passages were repeated, and the cells became morphologically homogeneous. Most of the cells expressed the mesenchymal stromal cell markers of CD29, CD44, and CD90, but they did not express the hematopoietic cell markers of CD34 and CD45 (figure 1).
Figure1.
Flow cytometry results show that the bone marrow stromal cells expressed CD29, CD44, and CD90 markers, but not CD34 and CD45 markers.
BMSC Tracking in the Ovaries
The transplanted BMSCs were labeled with DiI as red spots in the sections of the ovaries (figure 2A). The results confirmed the presence of the transplanted cells in the ovaries 4 weeks after transplantation.
Figure2.
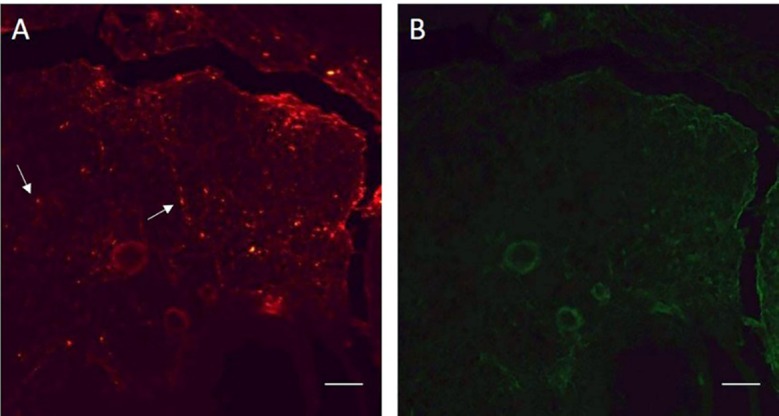
Labeled bone marrow stromal cells (BMSCs) with DiI in a section of the ovary show the presence of the transplanted cells after 4 weeks. A. The labeled BMSCs are visible as red spots. B. In the same section, the labeled BMSCs are not visible with green fluorescence. The arrows show the labeled cells. Scale bars: 100 µm
Levels of Serum E2 and FSH
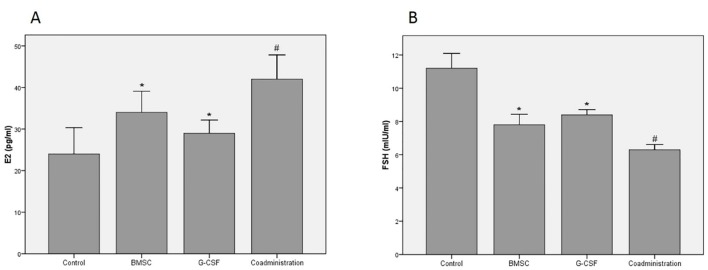
Four weeks after treatment, hormonal examinations by determining the levels of serum E2 and FSH were performed. The results showed that the levels of serum E2 in the experimental groups were significantly higher than those in the control group (P<0.001). The results of the coadministration group were significantly higher than those of the BMSC and G-CSF groups (P=0.016). The results of the BMSC group were significantly higher than those of the G-CSF group (P=0.037) (figure 3A).
Figure3.
Results of the levels of ovarian hormones show that the coadministration of bone marrow stromal cells (BMSCs) and the granulocyte colony-stimulating factor (G-CSF) has significantly improved the damaged ovaries 4 weeks after chemotherapy compared to the other groups. (A) Results of the serum E2 level (B) Results of the serum FSH level; *P<0.05 vs. the control group; #P<0.05 vs. the control, BMSC, and G-CSF groups
The levels of serum FSH in the experimental groups were significantly lower than those in the control group (P<0.001). The results of the coadministration group were significantly lower than those of the BMSCs and G-CSF groups (P<0.001). The results of the BMSC group were significantly lower than those of the G-CSF group (P=0.020) (figure 3B).
Ovulation Ability
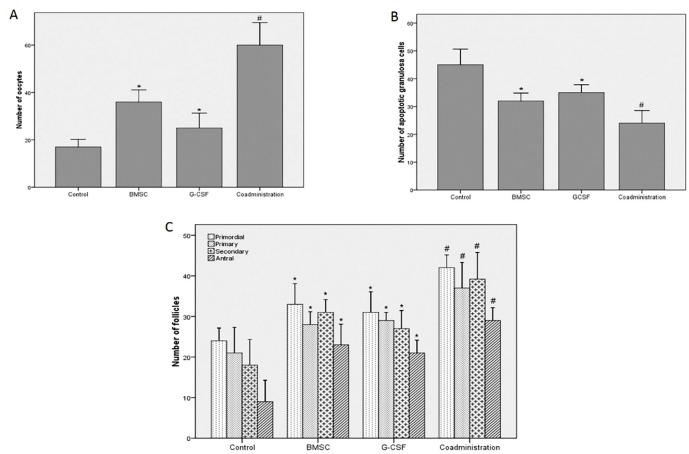
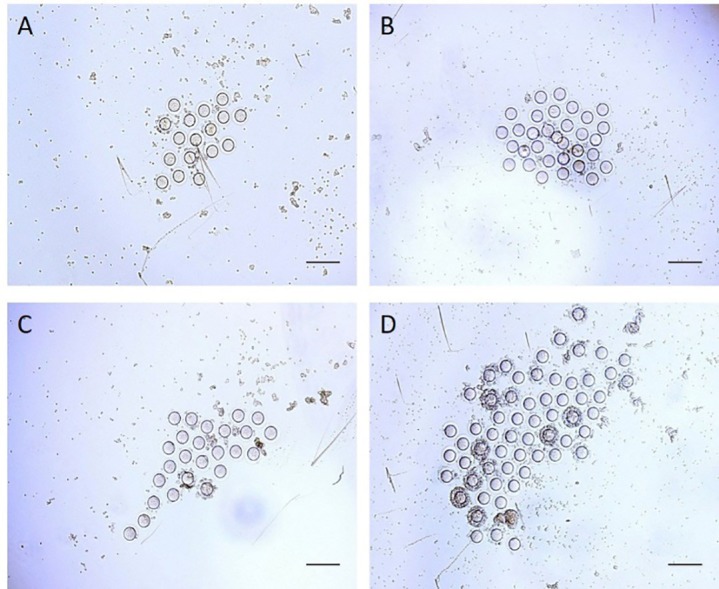
Four weeks after treatment, ovulation ability after superovulation was evaluated by counting the number of the oocytes. The results of ovulation ability showed that the number of the oocytes produced from the ovaries after superovulation in the experimental groups was significantly more than that in the control group (P=0.022). The results of the coadministration group were significantly more than those of the BMSC and G-CSF groups (P<0.001). The results of the BMSC group were significantly higher than those of the G-CSF group (P<0.001) (figure 4A, 5).
Figure4.
Four weeks after treatment, the oocytes are collected from the ovaries following superovulation. Scale bars: 200 µm
Figure5.
Four weeks after treatment, the apoptotic granulosa cells with the TUNEL assay have been marked brown. The arrows show the apoptotic granulosa cells. Scale bars: 50 µm
Apoptosis of the GCs
Four weeks after treatment, the percentage of apoptosis in the GCs was evaluated using the TUNEL assay. The percentage of the TUNEL-positive GCs of the ovaries in the experimental groups was significantly lower than that of the control group (P<0.001). The results of the coadministration group were significantly lower than those of the BMSC and G-CSF groups (P<0.001). There was no statistically significant difference between the BMSC and G-CSF groups (P=0.087) (figure 4B, 6).
Figure6.
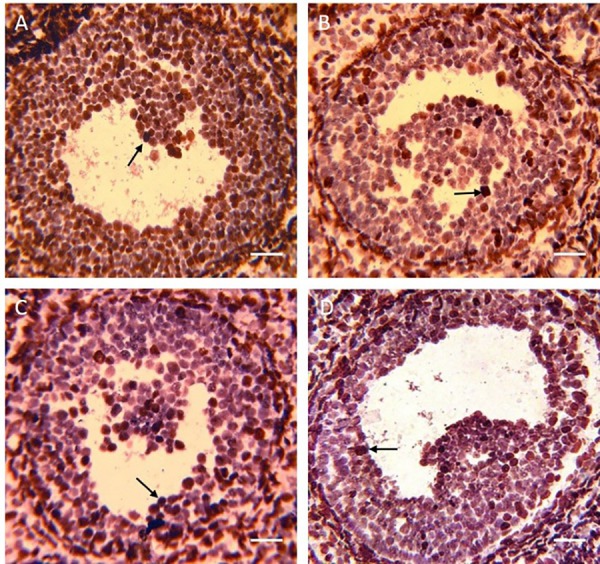
Four weeks after treatment, hematoxylin and eosin (H&E) staining is performed to count the number of the follicles at different stages. Scale bars: 200 µm
Histological Evaluation of the Ovaries
Four weeks after treatment, histological evaluations using H&E staining including the number of the primordial, primary, secondary, and antral follicles were performed. The mean number of the primordial follicles was 24±4 in the control group, 33±6 in the BMSC group, 31±6 in the G-CSF group, and 42±4 in the coadministration group. The mean number of the primary follicles was 21±8 in the control group, 28±4 in the BMSC group, 29±2 in the G-CSF group, and 37±8 in the coadministration group. The mean number of the secondary follicles was 18±8 in the control group, 31±4 in the BMSC group, 27±6 in the G-CSF group, and 39±9 in the coadministration group. The mean number of the antral follicles was 9±7 in the control group, 23±6 in the BMSC group, 21±4 in the G-CSF group, and 29±4 in the coadministration group. The results showed that the number of the follicles in the experimental groups was significantly higher than that of the control group (P<0.001). The results of the coadministration group were significantly higher than those of the BMSC and G-CSF groups (P=0.013). There was no statistically significant difference between the BMSC and G-CSF groups (P=0.063) (figure 4C, 7).
Figure7.
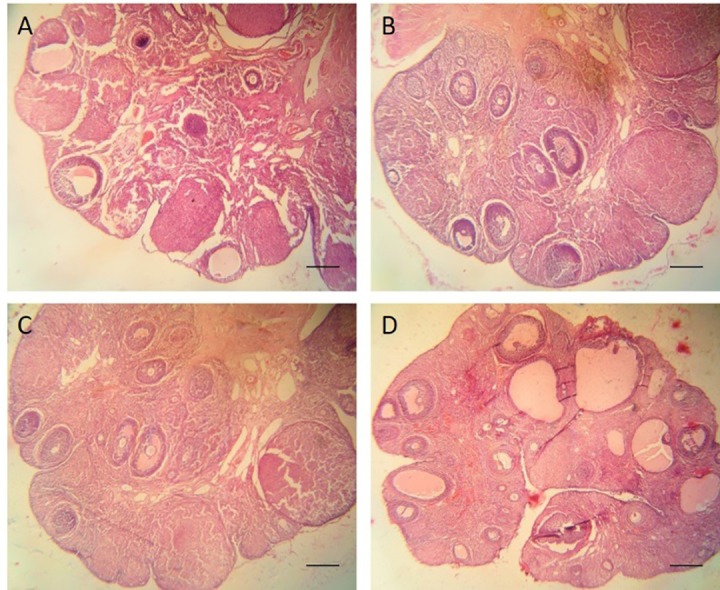
Results show that the coadministration of bone marrow stromal cells (BMSCs) and the granulocyte colony-stimulating factor (G-CSF) has significantly improved the damaged ovaries 4 weeks after chemotherapy compared to the other groups. (A) Results of the number of oocytes after superovulation (B) Results of the number of TUNEL-positive granulosa cells (C) Results of the number of follicles at different stages; *P<0.05 vs. the control group; #P<0.05 vs. the control, BMSC, and G-CSF groups
Discussion
Cancer treatment with chemotherapy may decrease the number of ovarian follicles or may disturb the female hormonal balance.1 Such outcome is disheartening in the case of girls and young women receiving chemotherapy. In the present study, for the first time, we evaluated the effects of the G-CSF, as a type of growth factor, on damaged ovaries after creating a chemotherapy model with CTX. We thereafter compared the results with the effects of BMSC transplantation into ovaries and the effects of the coadministration of BMSCs and the G-CSF on ovaries damaged by chemotherapy in rats. Overall, the results of the coadministration group were significantly more favorable than those of the BMSC, G-CSF, and control groups.
Some studies have shown that BMSC transplantation may repair damaged ovaries following chemotherapy.9,12 Further, the G-CSF may have beneficial effects on ovaries.15,29 However, the effects of the G-CSF, as a growth factor, on ovaries damaged by CTX have yet to be thoroughly assessed. Additionally, the effects of the coadministration of BMSCs and the G-CSF on CTX-damaged ovaries have never been assessed. Comparing the effects of the coadministration of BMSCs and the G-CSF with either of them alone may introduce a novel clinical approach to the recovery of ovaries damaged by chemotherapy.
BMSCs are emerging as strong candidates for cell therapy in damaged ovaries because they produce some growth factors such as the vascular endothelial growth factor, insulin-like growth factor-1 (IGF-1), hepatocyte growth factor, and basic fibroblast growth factor, which may prevent cell apoptosis and may promote functional recovery in the ovary.9,12,30,31 The vascular endothelial growth factor is an angiogenic cytokine that promotes the formation of new capillary networks providing nutrition for GCs.9,12,31 The IGF-1 is a growth hormone that stimulates GC proliferation by regulating DNA replication in theca cells and GCs. Not only does the IGF-1 enhance the function of gonadotropin hormones but also it regulates aromatase activity, promotes follicular antrum formation, and inhibits apoptosis.9,12 The hepatocyte growth factor is a cytokine that promotes follicular maturation and suppresses apoptosis in ovarian follicles and GCs.9 Another growth factor is the basic fibroblast growth factor, which acts as an initiator of folliculogenesis by inducing primordial follicle development.31 Badawy at al.32 showed that BMSCs were able to repair mouse ovarian insufficiency following CTX induction. Fu et al.33 showed that the overexpression of miR-21 in mesenchymal stem cells was able to improve ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit GC apoptosis.
On the other hand, the G-CSF may improve ovaries through several possible mechanisms. One possibility is that the G-CSF has anti-apoptotic properties that induce the expression of Bcl-2, which protects against cell apoptosis and decreases Bax, which induces apoptosis.15,34 Moreover, the G-CSF via the AKT-signaling pathway may balance cell survival and cell death through the phosphorylation of Bad, which is a member of the Bcl-2 family.35,36 The second possibility of the protective mechanism of the G-CSF is that it plays a role in neoangiogenesis. In this regard, Skaznik-Wikiel et al.37 demonstrated that chemotherapy by CTX and busulfan reduced mouse ovarian microvessel density, while in the group which received chemotherapy and the G-CSF, microvessel density was similar to that in the control group. The third possibility of the protective effect of the G-CSF is that the G-CSF, as a growth factor, may help the recovery process of damaged ovaries.15 Finally, the G-CSF may be able to mobilize hematopoietic and bone marrow mesenchymal stem cells into the peripheral blood and these undifferentiated cells may integrate into injured tissues and replace damaged cells.19,38
Due to the benefits of BMSCs and the G-CSF in ovarian tissue repair, we postulated that if BMSCs and the G-CSF were prescribed together in rats with damaged ovaries by creating a chemotherapy model, improvement might be more effective than the administration of either of them alone. We also hypothesized that the coadministration of BMSCs and the G-CSF might be a significant step in the selection of a repair procedure based on ovarian recovery measures.
We cultured BMSCs and transplanted them into rat ovaries after creating a chemotherapy model. The BMSCs expressed CD29, CD44, and CD90, but not CD34 and CD45. This finding was in agreement with the results previously reported by other investigations.9,22 Given that DiI labeling is a straightforward and stable technique to trace cells during in vivo experiments,39 we labeled the BMSCs with DiI and transplanted them into ovaries. We found that the transplanted BMSCs were present in the ovaries after 4 weeks. This result chimes in with other reports.20,25
The histological assessments in the current study comprised H&E staining for counting the number of follicles and the TUNEL assay for calculating the percentage of apoptotic GCs. The functional assessments consisted of superovulation for counting the number of produced oocytes and measuring the levels of serum E2 and FSH with an ELISA reader. Our histological and functional assessments showed that the results of the BMSC and G-CSF groups were significantly more favorable than those of the control group. These findings are concordant with those reported by previous studies.9,12,15,29,37,40
The results of the coadministration group of BMSCs and the G-CSF were significantly more favorable than those of the BMSC, G-CSF, and control groups in our study. The reason is probably related to the combination of useful properties of BMSCs and the G-CSF with different mechanisms of action in the restoration of ovaries after chemotherapy. The results of the functional assessments in the BMSC group were significantly more favorable than those of the G-CSF group, and there was no statistically significant difference in the results of the histological assessments between the BMSC and G-CSF groups. Fu et al.9 showed that BMSC transplantation was able to restore ovarian function after chemotherapy. In the present study, because the BMSCs were injected into the ovaries in situ, the transplanted stem cells may have replaced the damaged cells.10 Moreover, these cells might have produced some growth factors.9,12,30 Accordingly, the recovery of ovarian function after chemotherapy with the in situ transplantation of BMSCs was more favorable than that with the intraperitoneal injection of the G-CSF. Despite its interesting results, our study has some limitations, first and foremost among which is that it has a small number of samples. We, therefore, recommend that future studies be conducted with larger sample sizes with a view to elucidating the molecular mechanisms underlying BMSC and G-CSF function in ovarian repair.
Conclusion
In our study sample, the efficacy of the coadministration of BMSCs and the G-CSF in the recovery of ovaries damaged by chemotherapy with CTX was more effective than that of their separate administration.
Acknowledgement
The current study was supported by a thesis grant from Semnan University of Medical Sciences. We would like to thank the Research Center of Nervous System Stem Cells of Semnan University of Medical Sciences for its cooperation and provision of facilities.
Conflict of Interest:None declared.
References
- 1.Wang F, Wang L, Yao X, Lai D, Guo L. Human amniotic epithelial cells can differentiate into granulosa cells and restore folliculogenesis in a mouse model of chemotherapy-induced premature ovarian failure. Stem Cell Res Ther. 2013;4:124. doi: 10.1186/scrt335. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun M, Wang S, Li Y, Yu L, Gu F, Wang C, et al. Adipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failure. Stem Cell Res Ther. 2013;4:80. doi: 10.1186/scrt231. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dolmans MM, Demylle D, Martinez-Madrid B, Donnez J. Efficacy of in vitro fertilization after chemotherapy. Fertil Steril. 2005;83:897–901. doi: 10.1016/j.fertnstert.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 4.Fleischer RT, Vollenhoven BJ, Weston GC. The effects of chemotherapy and radiotherapy on fertility in premenopausal women. Obstet Gynecol Surv. 2011;66:248–54. doi: 10.1097/OGX.0b013e318224e97b. [DOI] [PubMed] [Google Scholar]
- 5.Liu T, Qin W, Huang Y, Zhao Y, Wang J. Induction of estrogen-sensitive epithelial cells derived from human-induced pluripotent stem cells to repair ovarian function in a chemotherapy-induced mouse model of premature ovarian failure. DNA Cell Biol. 2013;32:685–98. doi: 10.1089/dna.2013.2032. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nandy SB, Gangwani L, Nahleh Z, Subramani R, Arumugam A, de la Rosa JM, et al. Recurrence and metastasis of breast cancer is influenced by ovarian hormone’s effect on breast cancer stem cells. Future Oncol. 2015;11:983–95. doi: 10.2217/fon.14.301. [DOI] [PubMed] [Google Scholar]
- 7.Takehara Y, Yabuuchi A, Ezoe K, Kuroda T, Yamadera R, Sano C, et al. The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Lab Invest. 2013;93:181–93. doi: 10.1038/labinvest.2012.167. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Yu L, Sun M, Mu S, Wang C, Wang D, et al. The therapeutic potential of umbilical cord mesenchymal stem cells in mice premature ovarian failure. Biomed Res Int. 2013;2013:690491. doi: 10.1155/2013/690491. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu X, He Y, Xie C, Liu W. Bone marrow mesenchymal stem cell transplantation improves ovarian function and structure in rats with chemotherapy-induced ovarian damage. Cytotherapy. 2008;10:353–63. doi: 10.1080/14653240802035926. [DOI] [PubMed] [Google Scholar]
- 10.Dawn B, Bolli R. Adult bone marrow-derived cells: regenerative potential, plasticity, and tissue commitment. Basic Res Cardiol. 2005;100:494–503. doi: 10.1007/s00395-005-0552-5. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–9. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 12.Guo JQ, Gao X, Lin ZJ, Wu WZ, Huang LH, Dong HY, et al. BMSCs reduce rat granulosa cell apoptosis induced by cisplatin and perimenopause. BMC Cell Biol. 2013;14:18. doi: 10.1186/1471-2121-14-18. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acosta SA, Tajiri N, Shinozuka K, Ishikawa H, Sanberg PR, Sanchez-Ramos J, et al. Combination therapy of human umbilical cord blood cells and granulocyte colony stimulating factor reduces histopathological and motor impairments in an experimental model of chronic traumatic brain injury. PLoS One. 2014;9:e90953. doi: 10.1371/journal.pone.0090953. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pena I, Borlongan CV. Translating G-CSF as an Adjunct Therapy to Stem Cell Transplantation for Stroke. Transl Stroke Res. 2015;6:421–9. doi: 10.1007/s12975-015-0430-x. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akdemir A, Zeybek B, Akman L, Ergenoglu AM, Yeniel AO, Erbas O, et al. Granulocyte-colony stimulating factor decreases the extent of ovarian damage caused by cisplatin in an experimental rat model. J Gynecol Oncol. 2014;25:328–33. doi: 10.3802/jgo.2014.25.4.328. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nafar M, Parvin M, Sadeghi P, Ghoraishian M, Soleimani M, Tabibi A, et al. Effects of stem cells and granulocyte colony stimulating factor in reperfusion injury. Iran J Kidney Dis. 2010;4:207–13. [PubMed] [Google Scholar]
- 17.England TJ, Abaei M, Auer DP, Lowe J, Jones DR, Sare G, et al. Granulocyte-colony stimulating factor for mobilizing bone marrow stem cells in subacute stroke: the stem cell trial of recovery enhancement after stroke 2 randomized controlled trial. Stroke. 2012;43:405–11. doi: 10.1161/STROKEAHA.111.636449. [DOI] [PubMed] [Google Scholar]
- 18.Elbana AM, Abdel-Salam S, Morad GM, Omran AA. Role of Endogenous Bone Marrow Stem Cells Mobilization in Repair of Damaged Inner Ear in Rats. Int J Stem Cells. 2015;8:146–54. doi: 10.15283/ijsc.2015.8.2.146. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safari M, Jafari B, Zarbakhsh S, Sameni H, Vafaei AA, Mohammadi NK, et al. G-CSF for mobilizing transplanted bone marrow stem cells in rat model of Parkinson’s disease. Iran J Basic Med Sci. 2016;19:1318–24. doi: 10.22038/ijbms.2016.7918. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zarbakhsh S, Moradi F, Joghataei MT, Bahktiari M, Mansouri K, Abedinzadeh M. Evaluation of the Functional Recovery in Sciatic Nerve Injury following the Co-transplantation of Schwann and Bone Marrow Stromal Stem Cells in Rat. Basic Clin Neurosci. 2013;4:291–8. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 21.Haydari S, Safari M, Zarbakhsh S, Bandegi AR, Miladi-Gorji H. Effects of voluntary exercise on the viability, proliferation and BDNF levels of bone marrow stromal cells in rat pups born from morphine- dependent mothers during pregnancy. Neurosci Lett. 2016;634:132–7. doi: 10.1016/j.neulet.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Zarbakhsh S, Goudarzi N, Shirmohammadi M, Safari M. Histological Study of Bone Marrow and Umbilical Cord Stromal Cell Transplantation in Regenerating Rat Peripheral Nerve. Cell J. 2016;17:668–77. doi: 10.22074/cellj.2016.3839. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song D, Zhong Y, Qian C, Zou Q, Ou J, Shi Y, et al. Human Umbilical Cord Mesenchymal Stem Cells Therapy in Cyclophosphamide-Induced Premature Ovarian Failure Rat Model. Biomed Res Int. 2016;2016:2517514. doi: 10.1155/2016/2517514. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su J, Ding L, Cheng J, Yang J, Li X, Yan G, et al. Transplantation of adipose-derived stem cells combined with collagen scaffolds restores ovarian function in a rat model of premature ovarian insufficiency. Hum Reprod. 2016;31:1075–86. doi: 10.1093/humrep/dew041. [DOI] [PubMed] [Google Scholar]
- 25.Harikae K, Miura K, Shinomura M, Matoba S, Hiramatsu R, Tsunekawa N, et al. Heterogeneity in sexual bipotentiality and plasticity of granulosa cells in developing mouse ovaries. J Cell Sci. 2013;126:2834–44. doi: 10.1242/jcs.122663. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, He Y, Liu M, Fu X. Lentivirus-mediated bcl-2 gene therapy improves function and structure of chemotherapy-damaged ovaries in wistar rats. Am J Reprod Immunol. 2013;69:518–28. doi: 10.1111/aji.12048. [DOI] [PubMed] [Google Scholar]
- 27.Kon H, Hokao R, Shinoda M. Fertilizability of Superovulated Eggs by Estrous Stage-independent PMSG/hCG Treatment in Adult Wistar-Imamichi Rats. Exp Anim. 2014;63:175–82. doi: 10.1538/expanim.63.175. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ornberg RL. Proliferation and apoptosis measurements by color image analysis based on differential absorption. J Histochem Cytochem. 2001;49:1059–60. doi: 10.1177/002215540104900815. [DOI] [PubMed] [Google Scholar]
- 29.Pala HG, Pala EE, Artunc Ulkumen B, Aktug H, Yavasoglu A, Korkmaz HA, et al. The protective effect of granulocyte colony-stimulating factor on endometrium and ovary in a rat model of diabetes mellitus. Gynecol Obstet Invest. 2014;78:94–100. doi: 10.1159/000363239. [DOI] [PubMed] [Google Scholar]
- 30.Qiao H, Zhang R, Gao L, Guo Y, Wang J, Zhang R, et al. Molecular Imaging for Comparison of Different Growth Factors on Bone Marrow-Derived Mesenchymal Stromal Cells’ Survival and Proliferation In Vivo. Biomed Res Int. 2016;2016:1363902. doi: 10.1155/2016/1363902. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Ying YF, Ouyang YL, Wang JF, Xu J. VEGF and bFGF increase survival of xenografted human ovarian tissue in an experimental rabbit model. J Assist Reprod Genet. 2013;30:1301–11. doi: 10.1007/s10815-013-0043-9. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badawy A, Sobh MA, Ahdy M, Abdelhafez MS. Bone marrow mesenchymal stem cell repair of cyclophosphamide-induced ovarian insufficiency in a mouse model. Int J Womens Health. 2017;9:441–7. doi: 10.2147/IJWH.S134074. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu X, He Y, Wang X, Peng D, Chen X, Li X, et al. Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. Stem Cell Res Ther. 2017;8:187. doi: 10.1186/s13287-017-0641-z. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solaroglu I, Tsubokawa T, Cahill J, Zhang JH. Anti-apoptotic effect of granulocyte-colony stimulating factor after focal cerebral ischemia in the rat. Neuroscience. 2006;143:965–74. doi: 10.1016/j.neuroscience.2006.09.014. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pastuszko P, Schears GJ, Pirzadeh A, Kubin J, Greeley WJ, Wilson DF, et al. Effect of granulocyte-colony stimulating factor on expression of selected proteins involved in regulation of apoptosis in the brain of newborn piglets after cardiopulmonary bypass and deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2012;143:1436–42. doi: 10.1016/j.jtcvs.2012.01.018. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pastuszko P, Schears GJ, Greeley WJ, Kubin J, Wilson DF, Pastuszko A. Granulocyte colony stimulating factor reduces brain injury in a cardiopulmonary bypass-circulatory arrest model of ischemia in a newborn piglet. Neurochem Res. 2014;39:2085–92. doi: 10.1007/s11064-014-1399-7. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skaznik-Wikiel ME, McGuire MM, Sukhwani M, Donohue J, Chu T, Krivak TC, et al. Granulocyte colony-stimulating factor with or without stem cell factor extends time to premature ovarian insufficiency in female mice treated with alkylating chemotherapy. Fertil Steril. 2013;99:2045–54 e3. doi: 10.1016/j.fertnstert.2013.01.135. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng J, Zou ZM, Zhou TL, Su YP, Ai GP, Wang JP, et al. Bone marrow mesenchymal stem cells can be mobilized into peripheral blood by G-CSF in vivo and integrate into traumatically injured cerebral tissue. Neurol Sci. 2011;32:641–51. doi: 10.1007/s10072-011-0608-2. [DOI] [PubMed] [Google Scholar]
- 39.Li N, Yang H, Lu L, Duan C, Zhao C, Zhao H. Comparison of the labeling efficiency of BrdU, DiI and FISH labeling techniques in bone marrow stromal cells. Brain Res. 2008;1215:11–9. doi: 10.1016/j.brainres.2007.09.095. [DOI] [PubMed] [Google Scholar]
- 40.Bostanci MS, Bakacak M, Inanc F, Yaylali A, Serin S, Attar R, et al. The protective effect of G-CSF on experimental ischemia/reperfusion injury in rat ovary. Arch Gynecol Obstet. 2016;293:789–95. doi: 10.1007/s00404-015-3878-8. [DOI] [PubMed] [Google Scholar]