Abstract
Background:
Metastasis is the main cause of prostate cancer (PCa) death. The inhibitory effect of N-myc downstream-regulated gene 2 (NDRG2) on the invasiveness properties of PCa cells has been demonstrated previously. However, its underlying mechanisms have not yet been investigated. The present study aimed to investigate the effects of NDRG2 overexpression on the expression of genes involved in epithelial-mesenchymal transition (EMT) including E-cadherin (E-CAD), α- and β-catenins, Slug and Snail, transforming growth factor (TGF)-α and -β, and vascular endothelial growth factor (VEGF).
Methods:
In the present in vitro study, LNCaP cells were divided into three groups, namely NDRG2 group (transfected with PSES-pAdenoVator-PSA-NDRG2-IRES-GFP plasmid), mock group (transfected with mock plasmid), and control group (without transfection). The effect of NDRG2 overexpression on the migration and invasion of LNCaP cells were investigated using the transwell assay. Real-time PCR was used for the evaluation of gene expression. For the statistical analyses, one-way ANOVA, student t test or Mann-Whitney U test were applied using the SPSS software (version 15.0). P values <0.05 were considered statistically significant.
Results:
The results demonstrated that the overexpression of NDRG2 reduced the invasion and migration of LNCaP cells compared to the control and mock groups (P<0.001). A decreased expression of TGF-β (P=0.002), VEGF (P=0.014), Slug (P=0.005), and Snail (P=0.012); and an increased expression of E-CAD (P=0.009) were observed following NDRG2 overexpression in LNCaP cells.
Conclusion:
The results of the present study suggest that NDRG2 inhibits the invasiveness properties of LNCaP cells probably through changes in the expression of genes involved in EMT.
Keywords: NDRG2 protein, human ; Prostatic neoplasms ; Cadherins ; Vascular endothelial growth factors
What’s Known
NDRG2 inhibits the proliferation of many tumor cells through the induction of apoptosis or stopping the cell cycle.
Metastatic behavior of prostate tumor cells is reduced following NDRG2 overexpression
What’s New
NDRG2 overexpression regulated the expression of genes involved in the epithelial-mesenchymal transition of prostate cancer cells, including E-cadherin, TGF-β, Slug, Snail, and VEGF.
Introduction
Prostate cancer (PCa) is the major cause of cancer-related deaths in men.1 Effective treatment of PCa is highly dependent on the stage of the disease. While primary PCa can be treated with conventional therapies, the treatment of metastatic PCa is often unsuccessful. Therefore, understanding the molecular mechanisms of PCa metastasis is crucial in developing novel therapeutic modalities that target PCa metastasis.2
Previous studies have demonstrated a significant reduction in the expression of cell-to-cell and cell-to-matrix adhesion molecules, such as E-cadherin (E-CAD) in the metastatic PCa cells.3 According to previous studies, reduction in the expression of E-CAD in metastatic PCa cells is largely due to the increased expression of the Slug and Snail transcription factors. Slug and Snail are zinc-finger proteins that belong to the superfamily of snail, which down-regulate the expression of E-CAD by binding to E-boxes elements in the promoter region of this gene.4,5 α- and β- catenins are members of the E-cadherin/catenin complex. These proteins are involved in the linkage between E-CAD and actin filaments in the cytoskeleton. The reduction in the expression of α- and β-catenins in tumor tissues is linked to an increase in the invasiveness properties of tumor cells. Furthermore, free cytoplasmic β-catenin acts as regulators of cell signaling and transcription regulation that increase the expression of Slug and Snail by wnt/β-catenin signaling pathway.5 The other signaling pathways that increase the expression of Slug and Snail are TGF-α and TGF-β.7 Vascular endothelial growth factor (VEGF) is the main angiogenic factor secreted by tumor cells and facilitates metastasis of tumor cells through stimulation of angiogenesis. A higher level of VEGF expression in the metastatic PCa compared to localized one has been reported in several studies. Thus, anti-VEGF antibodies (e.g. bevacizumab) have been used in several clinical trials of patients with PCa.8
NDRG2 is a tumor suppressor gene that belongs to the NDRG family of peptides. Reduced expression of NDRG2 has been demonstrated in a variety of tumors, including PCa.9 Furthermore, the role of NDRG2 in inhibiting proliferation of many tumor cells, which is mediated by the induction of apoptosis or stopping cell cycle, has been reported in many studies.10 In addition, a negative association between the level of NDRG2 expression and invasiveness abilities of PCa cells, suggesting the role of this protein in inhibition of PCa metastasis, has been reported.11 Gao et al. has also reported a reduction in the metastatic behaviors of PCa tumor cells following NDRG2 overexpression.12
Several cellular signaling pathways have been described for anti-proliferating effects of NDRG2 in different cells. In colon carcinoma cells, NDRG2 inhibits cell cycle through inhibition of c-Jun/transcriptional activator AP-1/cyclin D1 pathway. However, in HepG2 cells, anti-proliferative effect of NDRG2 is related to P38 mitogen-activated protein kinase/SOCS1 pathway.10 Several signaling pathways have also been described for anti-invasion properties of NDRG2 in various tumors. Among the important mechanisms are: inhibition of phosphatidylinositol 3-kinase (PI3K)/protein kinase B pathway in HSC-3 cells, extracellular signal-regulated kinase (ERK) pathway in HepG2 cells, and glycogen synthase kinase 3β (GSK-3β)/T-cell factor/β-catenin pathway in SW620 cells.10
In prostate cancer cells, although the exact signaling pathway for NDRG2 has not been clearly addressed, it has been suggested that androgen receptor/c-MYC/NDRG2/PSA signaling pathway may involve in anti-proliferative effect of NDRG2.11 To the best of our knowledge, the effects of NDRG2 on major genes involved in EMT of PCa cells have not been investigated yet. Given the importance of this issue and the heterogeneity observed between different cancers, the present study aimed to evaluate the effects of NDRG2 on the expression of major genes involved in the EMT and metastasis of PCa. To this end, we overexpressed NDRG2 gene in LNCaP cell line using PSES-pAdenoVator-PSA-NDRG2-IRES-GFP plasmid and followed its effects on invasion and migration abilities as well as the expression of E-CAD, Slug, Snail, α-catenin, β-catenin, TGF-α, TGF-β, and VEGF using the quantitative real-time PCR method.
Materials and Methods
Plasmid Amplification and Purification
In the present in vitro study, PSES-pAdenoVator-PSA-NDRG2-IRES-GFP plasmid was used to overexpress NDRG2 in LNCaP cells. The plasmid was proliferated in Escherichia coli DH5α competent cells. Plasmid DNA was then extracted using Maxi Extraction Kit (Yekta Tajhiz Azma, Iran) according to the manufacturer’s instructions. The concentration of the extracted DNA was measured using the NanoDrop ND-100 spectrophotometer.
Cell Culture and Transfection
Human LNCaP cell line was obtained from the cell bank of the Pasteur Institute of Iran. The cells were grown in RPMI 1640 (Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 100 unit/ml penicillin-streptomycin (Gibco/Invitrogen, Carlsbad, CA) at 37°C and 5% CO2. LNCaP cells were divided into three groups, including non-transfected cells (control group), transfected with PSES-pAdenoVator-PSA-NDRG2-IRES-GFP (NDRG2 group), transfected with mock plasmid without NDRG2 gene (PSES-pAdenoVator-PSA-IRES-GFP) as the mock group. Transfection was conducted using Lipofectamine 3000 reagent (Invitrogen Inc.) according to the manufacturer’s instructions. After 24, 48, and 72 hours, the transfection efficiency was tested using counting GFP expressing cells under an inverted fluorescence microscope (Hund, Germany).
In Vitro Migration and Invasion Assays
24-well transwell insert (8 µm pore filters, BD Bioscience, Bedford, MA) with and without matrigel-coated membrane was used for the invasion and migration assay, respectively. Briefly, for the migration assays, after filling the lower part of the transwell with RPMI plus 10% FBS, LNCaP cells (5×103) suspended in serum-free RPMI were added to the upper part of the transwell and incubated for 6 hours at 37°C. The cells were allowed to migrate to the bottom of the well through a porous membrane and the cells migrating to the lower surface of the membrane were fixed, stained with 0.1% crystal violet, counted in 10 random visual fields using an inverted microscope (200×), and the mean number of cells per field was calculated. Invasion assay was conducted similar to the migration assay but the membrane was coated with 1 mg/ml of matrigel (BD Bioscience, Bedford, MA) and the incubation time was 24 hours.
RNA Extraction and cDNA Synthesis
Total RNA was isolated from the cells using Trizol (Invitrogen Inc.). The obtained RNA was dissolved in nuclease-free water and its concentration was determined by NanoDrop spectrophotometer. Then, the purified RNA was stored at -80°C until further analysis. cDNA synthesis was performed by 500 ng of total RNA using random hexamers and PrimeScript RT Takara kit (TaKaRa, Japan), according to kit protocol. Thermal cycle settings were reverse transcription at 37ºC for 15 minutes and final denaturation of the enzyme at 85 ºC for 5 seconds to stop the reaction. The final cDNA products were stored at -20ºC until further use.
Quantitative Real-Time PCR
Quantitative real-time PCR was performed with SYBR-Green kit (Takara, Japan) according to the manufacturer’s instructions and using the RotorGene 6000 real-time PCR machine (Qiagen) in a total reaction volume of 20 μl. All reactions were performed in duplicate and the results were normalized to beta-actin (internal control) to correct RNA input in reactions. The sequence of primers for mRNA of NDRG2 and other studied genes are shown in table 1. The primers were designed for exon-exon junction in each mRNA so that they were specifically recognized and bind to target sequence in each mRNA. Relative quantitative method, using beta-actin as a housekeeping reference gene and Pfaffl formula, was applied to calculate relative changes in mRNA expression.13
Table 1.
Primers used for real-time PCR
| Gene | Accession # | Primer sequence (AF; bp) | Gene | Accession # | Primer sequence (AF; bp) |
|---|---|---|---|---|---|
| β-actin | NM_001101.4 | F: 5-GCACAGAGC CTCGCCTTT-3 (230) | α-CAT | NM-001290307.2 | F: 5-AGCTAGCCG CAGAAATGACT-3 (212) |
| R: 5-GCCTCGTCG CCCACATAG-3 | R: 5-TTCAACAGA TGCAGCCAAAAC-3 | ||||
| NDRG2 | NM_001354558.1 | F: 5-CCTCACCTC TTCCATTCC-3 (188) | β-CAT | NM-001330729.1 | F: 5-ACGGAGGAA GGTCTGAGGAG-3 (211) |
| R: 5-TATCACCTC CACGCTCAA-3 | R: 5-AGCCGCTTT TCTGTCTGGTT-3 | ||||
| E-CAD | NM_001317185.1 | F: 5-AGGCAAGGT TTTCTACAGCAT C3 (154) | Snail | NM_005985.3 | F: 5-GGAAGCCTAA CTACAGCGAGC-3 (151) |
| R: 5-TGACACAG CGTGAGAGAAG AGAG-3 | R: 5-AGGACAGAG TCCCAGATGAGC-3 | ||||
| TGF-β | NM_000660.6 | F: 5-AGTGGTTGA GCCGTGGAGG-3 (169) | Slug | NM-003068.4 | F: 5-AAGGACACA TAGAACTCACAC G-3 (195) |
| R: 5GCCATGAGA AGCAGGAAAG-3 | R: 5-CACAGCAGC CAGATTCCTCA-3 | ||||
| VEGF | NM_001171622.1 | F: 5-GGGCAGAAT CATCACGAAGT-3 (238) | TGF-α | NM-001099691.2 | F: 5-CTGGGTATT GTGTTGGCTGC-3 (171) |
| R: 5-GGTGAGGTT TGATCCGCAT-3 | R: 5-TGCTGGCTT G TCCT CCTGC-3 |
NM: Accession number; AF: Size of amplified fragment; bp: Base pair
E: Real-time PCR efficiencies, Target: ACTB, Ct: Threshold cycle, Control: Untreated sample, Sample: treated sample, ref: Reference (beta-actin).
Statistical Analysis
All statistical analyses were carried out using the SPSS 15.0 statistical software package (SPSS, Chicago, IL, USA). Normal distribution of data and homogeneity of variance was checked using Shapiro-Wilk test and Levene’s test, respectively (P<0.05). One-way ANOVA followed by LSD post-hoc test was used to compare the number of invaded and migrated cells between the control, mock, and NDRG2 groups. Student t test or Mann-Whitney U test was used (for parametric and non-parametric variables, respectively) to pairwise comparisons of real-time PCR data between the control and NDRG2 groups. All data were expressed as mean±SD from at least three independent experiments. P values <0.05 were considered statistically significant.
Results
Evaluation of Cell Transfection Efficiency and NDRG2 Expression
Transfection efficiency was evaluated by counting the GFP-expressing LNCaP cells at 24, 48, and 72 hours after PSES-pAdenoVator-PSA-NDRG2-IRES-GFP transfection (figure 1). As shown in figure 1, the expression of GFP increased with increasing the incubation time and reached a maximum of 75% of the cells at 72 hours after transfection and then gradually decreased with increasing the incubation time. The expression of NDRG2 in the control and NDRG2 groups was evaluated using real-time PCR. As shown in figure 1B, NDRG2 expression was significantly increased (about 60-fold) in the NDRG2 group compared to the control group, 72 hours after PSES-pAdenoVator-PSA-NDRG2-IRES-GFP transfection (P<0.001).
Figure1.
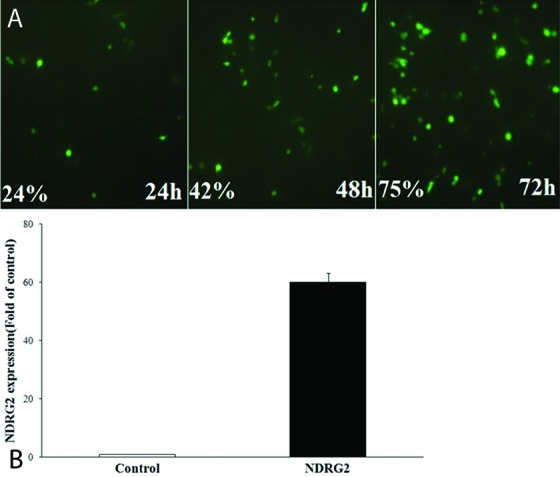
(A) GFP-expressing LNCaP cells and transfection (%) at 24, 48, and 72 hours after transfection with PSES- pAdenoVator-PSA-NDRG2-IRES-GFP plasmid. (B) The results of real-time PCR for NDRG2 expression (mean±SD) in the NDRG2 group compared to the control group, 72 hours after PSES- pAdenoVator-PSA-NDRG2-IRES-GFP transfection (P<0.001).
NDRG2 Overexpression Inhibits Migration and Invasion of LNCap Prostate Cancer Cells
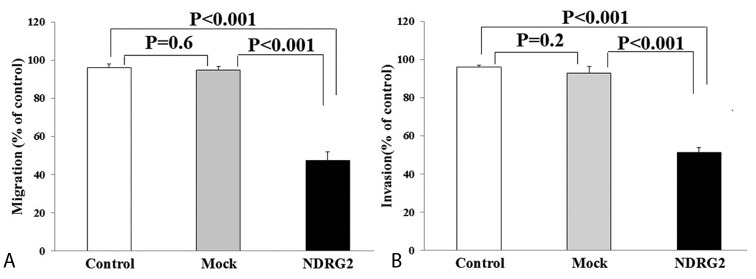
To explore the effect of NDRG2 overexpression on the metastatic activity of LNCaP cells, in vitro transwell migration and invasion assays were performed. The representative micrographs in figure 2 reveal the migrated (2A, 2B, and 2C) and invaded (2D, 2E, and 2F) LNCaP cells at the lower surface of the transwell filter. As shown in figure 3, the number of migrated (3A) and invaded (3B) cells were significantly lower in the NDRG2 group compared to the mock and control groups (P<0.001). No significant differences were observed between the mock and control groups with respect to the number of migrated (P=0.60) and invaded cells (P=0.20).
Figure2.
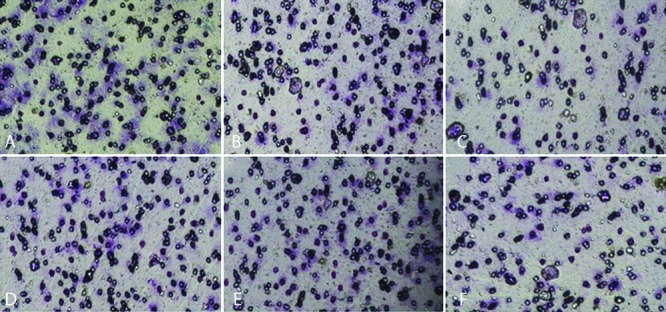
NDRG2 overexpression reduced migration and invasion activities of LNCaP cells in transwell migration assay. The representative micrographs (200×) reveal the migrated and invaded LNCaP cells at the lower surface of the transwell filter in the control group (A and D, respectively) and mock group (B and E, respectively). Overexpression of NDRG2 significantly decreased migration (C) and invasion (F) of LNCaP cells compared to the mock and control groups.
Figure3.
The number of migrated (A) and invaded (B) cells were significantly (P<0.001) lower in the NDRG2 group compared to the control and mock groups. No significant differences were observed between the mock and control groups in the number of migrated (P=0.60) and invaded (P=0.2) cells.
The Effects of NDRG2 Overexpression on the Expression of Major Genes Involved in the EMT of Human Prostate Cancer Cells
The impact of NDRG2 overexpression on the expression of the desired genes was evaluated using real-time PCR technique. The results of the RT-PCR are presented in figure 4. As shown in figure 4A, NDRG2 overexpression caused a significant increase in the expression of E-CAD. Overexpression of NDRG2 in LNCaP also caused a significant decrease in the expression of TGF-β, Slug, Snail, and VEGF genes (figure 4B). NDRG2 expression had no significant effects on α-catenin (P=0.183), β-catenin (P=0.189), and TGF-α (0.289) mRNA levels.
Figure4.
Overexpression of NDRG2 in LNCaP cells increased the expression of E-cadherin (A) and decreased the expression of TGF-β, Slug, Snail, and VEGF (B), significantly. The results are expressed as mean±SD fold of control (A) or mean±SD 1/fold of control (B).
Discussion
The present in vitro study aimed to identify the effect of NDRG2 on the expression of genes involved in the EMT of prostate tumor cells. The results showed a significant reduction in the invasiveness properties of LNCaP cells, including cell invasion and migration abilities following high expression of NDRG2. In addition, NDRG2 overexpression significantly decreased the expression of genes that promote EMT and metastasis, including TGF-β, Slug, Snail, and VEGF while it increased E-CAD expression, a gene involved in the inhibition of EMT. These findings suggest that the anti-metastatic effect of NDRG2 in PCa, at least in part, is mediated through regulating the expression of genes involved in the EMT of epithelial cells.
The selection of a vector that facilitates the specific and high expression of a candidate tumor suppressor gene is crucial for the success of a gene therapy modality. Our results revealed a high expression of NDRG2 following transfection of LNCaP cells with PSES-pAdenoVator-PSA-NDRG2-IRES-GFP plasmid. This plasmid was constructed in our previous study for a strong and specific overexpression of NDRG2 in PCa tumor cells.14 PSES element of this plasmid is a combination of a 189-bp region from prostate-specific antigen (PSA) enhancer (AREc3) and 331-bp region from prostate-specific membrane antigen (PSMA) enhancer (PSME). Tight prostate specificity and strong transcriptional activity (comparable to cytomegalovirus promoter) in the presence or absence of androgen make PSES a useful tool for gene therapy in both androgen-dependent and castration-resistant PCa.15
The results showed a marked inhibition of LNCaP cell invasion and migration following NDRG2 overexpression, which is in line with the results of previous studies.16-18 Degradation of the extracellular matrix mediated by matrix metalloproteinases (MMPs) enzymes, such as MMP2 and MMP9, is one the mechanisms that contributed to the invasive characteristics of malignant cells. Indeed, the anti-invasiveness effect of NDRG2 that mediated through downregulation of MMP2 and MMP9 was demonstrated in our previous studies.16,17 Besides, EMT is another mechanism involved in the initiation of metastasis.19,20 The role of EMT in the promotion of metastasis and induction of drug resistance has been revealed in many tumors, including PCa.20 Downregulation of E-CAD is a key event in the initiation of EMT in PCa and other tumor cells.21 Furthermore, it has been demonstrated that E-CAD overexpression can stop EMT process and induce reverse process, i.e., mesenchymal to epithelial cells transition (MET).22 The results from real-time PCR revealed upregulation of E-CAD gene expression following NDRG2 overexpression in LNCaP cell line, suggesting a possible role of E-CAD in the anti-invasiveness effects of NDRG2. A similar finding was also indicated following NDRG2 overexpression in colon23 and breast cancer,24 suggesting the common role of this pathway in mediating NDRG2 effects in various cancers.
To find the possible underlying mechanisms for the effect of NDRG2 on E-CAD, we evaluated the expression of Snail and Slug transcription repressors following NDRG2 overexpression. The results demonstrated a significant decrease in the expression of both Snail and Slug following NDRG2 upregulation. This finding suggests that the observed induction of E-CAD expression may mediate through decreasing level of Snail and Slug repressors. A positive effect of NDRG2 on the expression of E-CAD, that is mediated through downregulation of Snail expression, has also been reported by Kim et al. in colon cancer cells.23 In breast cancer cell line, inhibition of STAT3 signaling pathway has involved the inhibitory effects of NDRG2 on Snail expression.24 However, whether this pathway has participated in the NDRG2 effects on Snail expression in PCa cells requires further investigation.
The results also showed a significant decrease in the expression of TGF-β following NDRG2 overexpression, suggesting the role of TGF-β signaling pathway in mediating NDRG2 effects in LNCaP cells. TGF-β is a cytokine with regulatory impact on many aspects of tumors, including proliferation, migration, apoptosis, and angiogenesis. In prostate cancer cells, TGF-β stimulates migratory and invasive behavior of tumor cells in the advanced stages of the PCa. In a study conducted on colorectal cancer cells,25 it was demonstrated that TGF-β regulated NDRG2 expression. If the same mechanism operated in PCa cells, our current finding suggests that NDRG2 and TGF-β regulate their expression through a negative feedback mechanism.
Angiogenesis plays an important role in the metastasis of PCa.3 Tumor cells cause angiogenesis by secretion of various proteins, which VEGF is the most important one. The results from real-time PCR revealed a significant decrease in VEGF mRNA following NDRG2 overexpression. It has been demonstrated that activation of TGF-β signaling promotes angiogenesis through increased expression of VEGF.26 Therefore, downregulation of TGF-β may be a possible mechanism behind the effect of NDRG2 on VEGF expression. In breast cancer cell line, in line with the results of the present study, a decrease in VEGF expression by NDRG2 has been reported.27
The role of TGF-α upregulation in the inhibition of E-CAD expression and induction of EMT in PCa has been demonstrated previously.28 Overexpression of NDRG2 in the present study had no significant impact on the expression of TGF-α expression, suggesting that TGF-α signaling is probably not involved in the mediation of NDRG2 effects in LNCaP cells. Wnt/beta-catenin is another signaling pathway which has been indicated in the activation of Snail and Slug.29 The results of the current study showed that overexpression of NDRG2 had no significant effects on the expression of α-catenin and β-catenin, suggesting that this pathway probably has no function in the NDRG2-induced inhibition of Snail and Slug expression.
In the present study, quantitative real-time PCR was used as a sensitive and accurate method for the evaluation of gene expression at mRNA level. Specific primers used in our study were designed for exon-exon junctions that avoid genomic DNA amplification. However, the use of real-time PCR without assessing gene expression at the protein level, using protein detecting techniques (e.g. immune-based assay), is the main limitation of the current study. Hence, one should consider the present investigation as preliminary which requires further confirmation. Moreover, some of the primers that were used in this investigation were designed for the common region of splice variants of each gene. Therefore, the type of splice variants that can be influenced by NDRG2 overexpression was not investigated. Further detailed investigations using specific primers for each splice variant may provide a better understanding of the molecular mechanism of NDRG2 effects in PCa.
Conclusion
The findings of the present study suggest that an increase in the expression of NDRG2 can suppress the metastatic potential of PCa tumor cells. An increase in the expression of E-CAD and a decrease in the expression of Snail, Slug, VEGF, and TGF-β may mediate these effects. Due to downregulation of NDRG2 in PCa and impacts of this protein on inhibition of proliferation and invasive behaviors of prostate tumor cells, gene and protein therapy modalities that upregulate NDRG2 in prostate cancer cells may have therapeutic potential for the treatment of prostate cancer. Further in vivo study is required to confirm the effectiveness of NDRG2 in the treatment of PCa.
Acknowledgement
This manuscript was extracted from the MSc thesis of Mohammad Moradi and was supported by a grant from the Vice Chancellor for Research Affairs of Shiraz University of Medical Sciences. We are also grateful to all staff at Diagnostic Laboratory Sciences and Technology Research Center for their technical assistance.
Conflict of Interest:None declared.
References
- 1.Lin D. Commentary on “The evolutionary history of lethal metastatic prostate cancer.” Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JM, Papaemmanuil E, Brewer DS, Kallio HM, Hognas G, Annala M, Kivinummi K, Goody V, Latimer C, O’Meara S, Dawson KJ, Isaacs W, Emmert-Buck MR, Nykter M, Foster C, Kote-Jarai Z, Easton D, Whitaker HC, ICGC Prostate UK Group, Neal DE, Cooper CS, Eeles RA, Visakorpi T, Campbell PJ, McDermott U, Wedge DC, Bova GS, University of Washington-Urology, Seattle, WA. Nature . 2015; 520(7547):353–7. doi: 10.1016/j.urolonc.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montanari M, Rossetti S, Cavaliere C, D’Aniello C, Malzone MG, Vanacore D, et al. Epithelial-mesenchymal transition in prostate cancer: an overview. Oncotarget. 2017;8:35376–89. doi: 10.18632/oncotarget.15686. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol. 2012;22:396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347–76. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- 6.Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012;139:3471–86. doi: 10.1242/dev.071209. [DOI] [PubMed] [Google Scholar]
- 7.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. doi: 10.1038/nrm3758. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisermann K, Fraizer G. The Androgen Receptor and VEGF: Mechanisms of Androgen-Regulated Angiogenesis in Prostate Cancer. Cancers (Basel) 2017;9 doi: 10.3390/cancers9040032. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melotte V, Qu X, Ongenaert M, van Criekinge W, de Bruine AP, Baldwin HS, et al. The N-myc downstream regulated gene (NDRG) family: diverse functions, multiple applications. FASEB J. 2010;24:4153–66. doi: 10.1096/fj.09-151464. [DOI] [PubMed] [Google Scholar]
- 10.Hu W, Fan C, Jiang P, Ma Z, Yan X, Di S, et al. Emerging role of N-myc downstream-regulated gene 2 (NDRG2) in cancer. Oncotarget. 2016;7:209–23. doi: 10.18632/oncotarget.6228. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu C, Wu G, Li R, Gao L, Yang F, Zhao Y, et al. NDRG2 acts as a negative regulator downstream of androgen receptor and inhibits the growth of androgen-dependent and castration-resistant prostate cancer. Cancer Biol Ther. 2015;16:287–96. doi: 10.1080/15384047.2014.1002348. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao L, Wu GJ, Liu XW, Zhang R, Yu L, Zhang G, et al. Suppression of invasion and metastasis of prostate cancer cells by overexpression of NDRG2 gene. Cancer Lett. 2011;310:94–100. doi: 10.1016/j.canlet.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alizadeh Zarei M, Takhshid MA, Behzad Behbahani A, Hosseini SY, Okhovat MA, Rafiee Dehbidi GR, et al. Synergistic Effects of NDRG2 Overexpression and Radiotherapy on Cell Death of Human Prostate LNCaP Cells. J Biomed Phys Eng. 2017;7:257–64. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SJ, Kim HS, Yu R, Lee K, Gardner TA, Jung C, et al. Novel prostate-specific promoter derived from PSA and PSMA enhancers. Mol Ther. 2002;6:415–21. doi: 10.1006/mthe.2002.0682. [DOI] [PubMed] [Google Scholar]
- 16.Faraji SN, Mojtahedi Z, Ghalamfarsa G, Takhshid MA. N-myc downstream regulated gene 2 overexpression reduces matrix metalloproteinase-2 and -9 activities and cell invasion of A549 lung cancer cell line in vitro. Iran J Basic Med Sci. 2015;18:773–9. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 17.Farokhinejad F, Behbahani AB, Rafiei Dehbidi GR, Takhshid MA. Expression and purification of TAT-NDRG2 recombinant protein and evaluation of its anti-proliferative effect on LNCaP cell line. Protein Expr Purif. 2017;138:25–33. doi: 10.1016/j.pep.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Golestan AM, Mojtahedi ZP, Ghalamfarsa GP, Hamidinia MM, Takhshid MAP. The Effects of NDRG2 Overexpression on Cell Proliferation and Invasiveness of SW48 Colorectal Cancer Cell Line. Iran J Med Sci. 2015;40:430–9. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye X, Weinberg RA. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015;25:675–86. doi: 10.1016/j.tcb.2015.07.012. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montanari M, Rossetti S, Cavaliere C, D’Aniello C, Malzone MG, Vanacore D, et al. Epithelial-mesenchymal transition in prostate cancer: an overview. Oncotarget. 2017;8:35376–89. doi: 10.18632/oncotarget.15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang SQ, Zhang GQ, Zhang L. Correlation between methylation of the E-Cadherin gene and malignancy of prostate cancer. Genet Mol Res. 2016;15 doi: 10.4238/gmr.15028046. [DOI] [PubMed] [Google Scholar]
- 22.Mao M, Zheng X, Jin B, Zhang F, Zhu L, Cui L. Effects of CD44 and E-cadherin overexpression on the proliferation, adhesion and invasion of ovarian cancer cells. Exp Ther Med. 2017;14:5557–63. doi: 10.3892/etm.2017.5259. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YJ, Kang HB, Yim HS, Kim JH, Kim JW. NDRG2 positively regulates E-cadherin expression and prolongs overall survival in colon cancer patients. Oncol Rep. 2013;30:1890–8. doi: 10.3892/or.2013.2642. [DOI] [PubMed] [Google Scholar]
- 24.Kim MJ, Lim J, Yang Y, Lee MS, Lim JS. N-myc downstream-regulated gene 2 (NDRG2) suppresses the epithelial-mesenchymal transition (EMT) in breast cancer cells via STAT3/Snail signaling. Cancer Lett. 2014;354:33–42. doi: 10.1016/j.canlet.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Shen L, Qu X, Ma Y, Zheng J, Chu D, Liu B, et al. Tumor suppressor NDRG2 tips the balance of oncogenic TGF-beta via EMT inhibition in colorectal cancer. Oncogenesis. 2014;3:e86. doi: 10.1038/oncsis.2013.48. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darrington E, Zhong M, Vo BH, Khan SA. Vascular endothelial growth factor A, secreted in response to transforming growth factor-beta1 under hypoxic conditions, induces autocrine effects on migration of prostate cancer cells. Asian J Androl. 2012;14:745–51. doi: 10.1038/aja.2011.197. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma J, Liu W, Yan X, Wang Q, Zhao Q, Xue Y, et al. Inhibition of endothelial cell proliferation and tumor angiogenesis by up-regulating NDRG2 expression in breast cancer cells. PLoS One. 2012;7:e32368. doi: 10.1371/journal.pone.0032368. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Qin W, Pan Y, Zheng X, Li D, Bu J, Xu C, et al. MicroRNA-124 regulates TGF-alpha-induced epithelial-mesenchymal transition in human prostate cancer cells. Int J Oncol. 2014;45:1225–31. doi: 10.3892/ijo.2014.2506. [DOI] [PubMed] [Google Scholar]
- 29.Krasnapolski MA, Todaro LB, de Kier Joffe EB. Is the epithelial-to-mesenchymal transition clinically relevant for the cancer patient? . Curr Pharm Biotechnol. 2011;12:1891–9. doi: 10.2174/138920111798377021. [DOI] [PubMed] [Google Scholar]