Abstract
Background:
Insecticides may have negative effects on reproductive organs. Given the interaction between leptin and the hypothalamic-pituitary-gonadal (HPG) axis, we sought to investigate the changes in leptin and the HPG axis in adult male rats poisoned with Proteus and Biscaya insecticides.
Methods:
Our experimental subjects were 110 adult male Wistar rats (80-90 days of age; average weight=200-210 g). They were randomly split into 11 groups of 10 rats: control, sham, and 9 experimental groups namely treatment with 2.75, 5.5, and 11 mg/kg/BW of Proteus, treatment with 1.5, 3, and 6 mg/kg/BW of Biscaya, treatment with 2.75 mg/kg/BW of Proteus+1.5 mg/kg/BW of Biscaya, treatment with 5.5 mg/kg/BW of Proteus+3 mg/kg/BW of Biscaya, and treatment with 11 mg/kg/BW of Proteus+6 mg/kg/BW of Biscaya. Intraperitoneal injections were performed over a 14-day period. For bloodletting at the end of the experiment, blood samples were withdrawn from the rats in order to investigate the serum concentration of luteinizing hormone (LH), follicle-stimulating hormone (FSH), gonadotropin- releasing hormone (GnRH), testosterone, and leptin. The data were analyzed using SPSS, version 16, via ANOVA and the Duncan test. A P value equal to or less than 0.05 was considered statistically significant.
Results:
Our comparisons between the experimental groups (average and maximum compound concentrations of Proteus and Biscaya) and the control group showed a significant decrease in the mean serum levels of FSH (P=0.001), LH (P=0.001), GnRH (P=0.001), testosterone (P=0.005), and leptin (P=0.001) in all the experimental groups in a dose-dependent manner.
Conclusion:
Proteus and Biscaya decreased GnRH, LH, FSH, and testosterone by reducing the serum level of leptin in the hypothalamus in a dose-dependent manner.
Keywords: Proteus , Biscaya , Testosterone , Leptin , Rats
What’s Known
Proteus and Biscaya, insecticides with a widespread use in agriculture, cause various negative effects on human health such as reducing reproduction or increasing the risk of testicular cancer.
Effects of the simultaneous consumption of insecticides on sexual activity through impacts on the hypothalamic-pituitary-gonadal (HPG) axis have been discussed.
What’s New
Proteus and Biscaya decreased GnRH, LH, FSH, and testosterone by reducing the serum level of leptin in the hypothalamus in a dose-dependent manner.
Use of pesticides affects the HPG axis and, thus, damages the growth and development of reproductive tissues.
Introduction
Insecticides are amongst the chemicals which are extensively used for agricultural pest control.1 Acting as endocrine-disrupting chemicals, insecticides create negative effects on wildlife and humans2 and may reduce reproduction or increase the risk of testicular, prostate, or thyroid cancer in men.3
Thiacloprid is a neonicotinoid class and Deltamethrin is a pyrethroid class of synthetic insecticides which are mainly used either alone or in combination.4 Proteus (AKA Deltamethrin-Thiacloprid) and Biscaya (AKA Thiacloprid) are 2 insecticides which have been widely used in agriculture in recent years. The extensive use of these insecticides causes various negative effects on human health.5
Moreover, it has been found that these types of chemical compounds cause disturbances in the mechanism of testicular steroidogenesis.6 It has also been reported that endocrine-disrupting chemicals play a crucial role in the reduction of the number of sperm and reproduction in a hormonal manner. In other words, they are significantly implicated in hypospadias, cryptorchidism, and reproductive cancers.7
Several studies have shown that pyridaben pesticides create histomorphological and hormonal changes in the reproductive actions of BALB/C rats; reduce the levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone in a dose- and-time-dependent manner; and decrease the diameter of seminiferous tubules, thickness of epithelium, and distribution of the Leydig cells.8
There exist several reports on the effects of cyanotoxin on the hypothalamic-pituitary-gonadal (HPG) axis in adult male rats. In these experiments, the rats were treated with different concentrations of Microcystin-LR and it was indicated that the different concentrations of this toxin might directly or indirectly inhibit the synthesis of gonadotropin-releasing hormone (GnRH) in the hypothalamus, decrease the serum level of LH, and inhibit testosterone in testicles.9 Furthermore, an investigation demonstrated that pyrethroid insecticide cypermethrin accelerated pubertal onset in male mice.10 The results of another study revealed that neonatal exposure to estrogenic-disrupting compounds such as genistein advanced pubertal onset and induced premature anestrous in female rats.11 In this regard, leptin is considered an important controller in the secretion of LH by the pituitary gland (hypophysis).2,12 On the other hand, genetic studies have shown that leptin is needed for the initiation of puberty13 and it may act directly in the brain to initiate reproductive development.14 Additionally, previous research has shown that the combination of several classes of pesticides has more toxic effects than the compound of their own class alone.4,15
Given the increased consumption of toxins in contrasting reports on the effects of different toxins on body tissues and also the increase in puberty disorders, we conducted the present study with a view to controlling and reducing the negative effects of toxins on sexual activity and puberty. This is a comparative study on the toxic effects of Proteus and Biscaya, alone and in combination, on the HPG axis and GnRH, FSH, LH, testosterone, and leptin in adult male rats with regard to pertinent findings on humans. We also sought to investigate whether there is any significant relationship between leptin and changes in sex hormones.
Materials and Methods
The present study is a completely randomized and experimental research.
Animals and Their Classification
The current study complied with all the ethical issues vis-à-vis the care and use of laboratory animals during the research period. To that end, 110 adult male Wistar rats, aged between 80 and 90 days, with a mean weight of 200 g were selected for the current experimental study. The animals were kept in the Animal Breeding Room of Shiraz University of Medical Sciences for 1 week so that they could adapt themselves to the environment. The animals were kept on special shelves, made of transparent Poly Macrolone, under the conditions of 12 hours of light and 12 hours of dark at a room temperature of 23±2 ˚C and relative humidity of 50% to 55%. The rats had access to sufficient water and food. The effect and dose of each poison were determined via the LD50 test.16 The animals were randomly divided into 11 groups of 10 (Table 1).
Table 1.
Different groups of animals
| Group | Treatment |
|---|---|
| Control | Getting water and food naturally |
| Sham | Getting solvent of toxins (distilled water) |
| Experimental І | P 2.75 mg/kg/BW |
| Experimental П | P 5.5 mg/kg/BW |
| Experimental Ш | P 11 mg/kg/BW |
| Experimental ІV | B 1.5 mg/kg/BW |
| Experimental V | B 3 mg/kg/BW |
| Experimental VІ | B 6 mg/kg/BW |
| Experimental VП | B 1.5+P 2.75 mg/kg/BW |
| Experimental VШ | B 3+P 5.5 mg/kg/BW |
| Experimental ІХ | B 6+P 11 mg/kg/BW |
BW: Body weight; P: Proteus; B: Biscaya
All injections were intraperitoneal with insulin syringes; the injection volume was 0.44 cc in all the groups. It took 2 weeks to fulfill the experiment. The intended poisons were produced by Bayer Crop Science Company. The protocol of the study was established in accordance with the guideline of animal ethics and welfare17 and international regulations on the protection of laboratory animals and it was approved by the Ethics Committee of Islamic Azad University (IR.miau 13951206).
Bloodletting
Blood samples were withdrawn directly from the heart of the animals exactly 1 day after the last injection. Afterward, blood serums were collected via centrifuge (at 3000 rpm for 5 min) and stored at -20˚C until they were prepared for the experiment. The levels of FSH, LH, GnRH, testosterone, and leptin were measured using ELISA kits, specifically designed for rats. The ELISA kits were manufactured by Crystal Day Company in China (LOT=2015 10 14-2016 10 13).
Data Analysis
One-way ANOVA was used for data analysis. According to the Kolmogorov-Smirnov test, the data distribution was normal; hence, they were used in the next stages of the analysis of parametric tests. Additionally, the Duncan test was applied to determine the difference between the means if there was a statistically significant difference between the groups. The statistical analyses were done using SPSS, verison16, and the significance level was estimated at 5% (P<0.05). The data are presented as mean±standard error of the mean (SEM).
Results
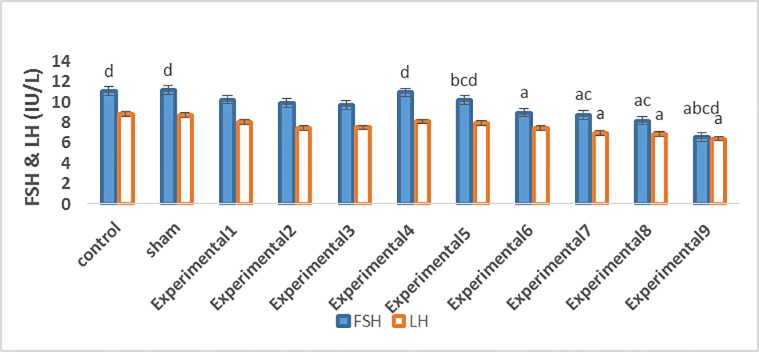
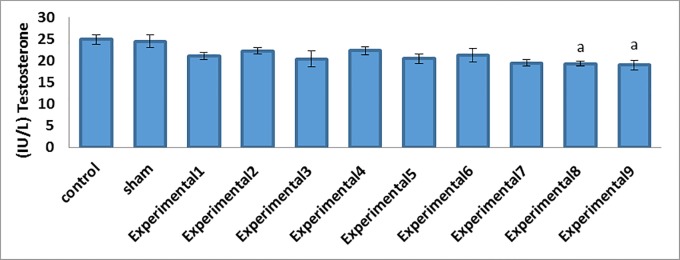
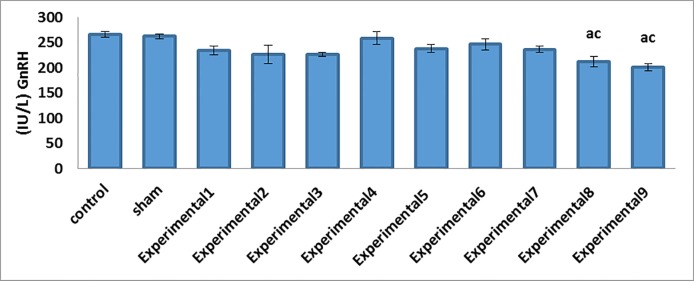
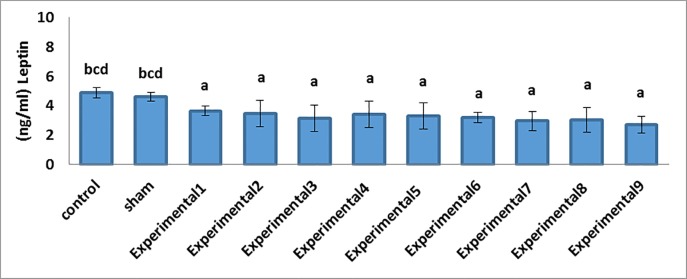
Our comparisons between the experimental groups (different compound concentrations of Proteus and Biscaya) and the control group showed that there was a significant decrease in the mean serum levels of FSH (P=0.001) and LH (P=0.001) in a dose-dependent manner (Table 2; figure 1). Furthermore, the comparisons between the experimental groups (average and maximum compound concentrations of Proteus and Biscaya) and the control group demonstrated a significant drop in the mean serum levels of GnRH (P=0.001) and testosterone (P=0.005) in a dose-dependent manner (Table 2; figure 2 and figure 3). Moreover, there was a significant drop in the serum level of leptin in the experimental groups, treated with different concentrations of Proteus and Biscaya alone and in combination, in comparison to the control group in a dose-dependent manner (P=0.001) (Table 2; figure 4).
Table 2.
Parameters in the different groups (mean±SEM)
| Group | Control | Sham | E-І | E-П | E-Ш | E-ІV | E-V | E-VІ | E-VП | E-VШ | E-ІХ | P value between Groups |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | ||||||||||||
| GnRH (IU/L) | 265.53±5.53 | 262.20±4.60 | 233.86±8.27 | 226.06±17.59 | 226.05±4.49 | 258.60±12.84 | 237.71±7.75 | 246.26±10.72 | 235.98±6.20 | 211.76±10.48 ac | 200.70 ±7.17 ac | 0.001 |
| FSH (IU/L) | 11.08±0.36 d | 11.16 ±0.33 d | 10.25±0.34 | 9.90±0.79 | 9.71±0.32 | 10.95±0.41 d | 10.20±0.42 bcd | 8.96±0.63 a | 8.76±0.13 ac | 8.18±0.17 ac | 6.58±0.46 abcd | 0.001 |
| LH (IU/L) | 8.83±0.22 | 8.71±0.14 | 8.03±0.44 | 7.48±0.17 | 7.51±0.45 | 8.11±0.25 | 7.96±0.46 | 7.43±0.20 | 6.96±0.32 a | 6.85±0.44 a | 6.41±0.58 a | 0.001 |
| Testosterone (IU/L) | 24.85±1.06 | 24.45±1.53 | 21.06±0.86 | 22.23±0.71 | 20.40±1.81 | 22.25±0.85 | 20.41±1.16 | 21.21±1.54 | 19.46±0.71 | 19.26±0.58 a | 19.00±1.10 a | 0.005 |
| Leptin (ng/mL) | 4.88±0.13 bcd | 4.61±0.11 bcd | 3.50±0.13 a | 3.56±0.41 a | 3.13±0.37 a | 3.43±0.36 a | 3.30±0.36 a | 3.18±0.14 a | 2.95±0.26 a | 3.05±0.34 a | 2.71±0.23 a | 0.001 |
E: Experimental; GnRH: Gonadotropin-releasing hormone; FSH: Follicle-stimulating hormone; LH: Luteinizing hormone
Anova test was used to compare each variable between the groups and a P value is generally given in the last column. The Duncan test was used for pairwisecomparisons.
a: Indicates a significant difference between the studied groups and the control group
b: Indicates a significant difference between the studied groups and the experimental E-І group
c: Indicates a significant difference between the studied groups and the experimental E-ІV group
d: Indicates a significant difference between the studied groups and the experimental E-VП group
Data means are presented as mean±SEM
Level of P<0.05 is considered statistically significant
Figure1.
Reduction in the concentration of FSH and LH in the studied groups has been compared. FSH: Follicle-stimulating hormone; LH: Luteinizing hormone a: Indicates a significant difference between the studied groups and the control group b: Indicates a significant difference between the studied groups and the experimental E-І group c: Indicates a significant difference between the studied groups and the experimental E-ІV group d: Indicates a significant difference between the studied groups and the experimental E-VП group Data means are presented as mean±SEM
Figure2.
Reduction in the testosterone concentrations in the studied groups in comparison with the control group. a: Indicates a significant difference between the studied groups and the control group Data means are presented as mean±SEM
Figure3.
Decrease in the GnRH concentrations in the studied groups by comparison with the control group. GnRH, Gonadotropin-releasing hormone a: Indicates a significant difference between the studied groups and the control group c: Indicates a significant difference between the studied groups and the experimental E-ІV group Data means are presented as mean±SEM
Figure4.
Decrease in the leptin concentrations in the studied groups in comparison to the control group. a: Indicates a significant difference between the studied groups and the control group b: Indicates a significant difference between the studied groups and the experimental E-І group c: Indicates a significant difference between the studied groups and the experimental E-ІV group d: Indicates a significant difference between the studied groups and the experimental E-VП group Data means are presented as mean±SEM
Discussion
According to the results of the present study, different doses of Biscaya and Proteus insignificantly decreased the mean serum levels of FSH, LH, GnRH, and testosterone. In contrast, the average and maximum compound doses of Proteus and Biscaya significantly reduced the mean serum levels of the aforesaid hormones. The more severe effects of the abovementioned toxins on the combined doses may have been due to the effects of Thiacloprid, which is in both types of insecticides.
The reduction in the serum levels of FSH, LH, GnRH, and testosterone in the present study is consistent with the findings of other researchers, while it does not chime in with the findings of some other investigators. Loza et al.11 studied the effects of genistein on pubertal development in female rats and found that genistein advanced pubertal onset and induced premature anestrous in the animals via the hypothalamic kisspeptin signaling pathways. Ye X et al.10 reported that early postnatal exposure to cypermethrin at environmentally relevant doses significantly accelerated the age of pubertal onset in their male mice. Elsewhere, Xiolu et al.9 and Ebadi et al.8 studied the effects of cyanotoxins on the HPG axis as well as the effects of pyridaben pesticide on reproductive actions in BALB/C rats and reported that different concentrations of Microcystin-LR reduced the serum level of LH and inhibited testosterone. Furthermore, previous research has shown that pyridaben pesticide creates histomorphological and hormonal changes in the reproductive actions of BALB/C rats and decreases FSH, LH, and testosterone in a dose-dependent manner.6,8 The reduction in FSH, LH, and testosterone is most probably in consequence of a drop in leptin levels.
There are several studies on the relationship between leptin and the secretion of GnRH and its effects on puberty. Ahmed Sayed et al.18 found that leptin facilitated the secretion of both GnRH through direct and indirect mechanisms and neuropeptide Y by neuropeptides. Previous research has also indicated that leptin, as an endogenous factor, expedites the onset of puberty and plays a crucial role in signaling the onset of puberty.19,20 The relationship between leptin and kisspeptin can directly activate the secretion of GnRH in GnRH neurons (i.e., GpR54 [G protein-coupled receptor-54] receptor in the preoptic area). 21,22 Kisspeptin has a crucial role in signaling the onset of puberty.21,23,24
There are several reports regarding the relationship between kisspeptin and GnRH. In this regard, Liu et al.25 concluded that the deficiency in the increase of GnRH might be associated with the downregulation of leptin receptors and the kisspeptin system (Kiss 1r) in the arcuate nucleus of adult male rates. Endocrine-disrupting chemicals such as estradiol benzoate and polychlorinated biphenyls can affect the kisspeptin system of GpR54 in rats and have significant effects on puberty.26
Joanne et al.27 found that kisspeptin acted as a stimulator in the secretion of GnRH through upregulation and concluded that a rise in kisspeptin receptors resulted in early puberty. Crofton et al.28 also highlighted the crucial role of GnRH in regulating the secretion of FSH, LH, and testosterone. Leptin acts as a pleiotropic regulator of several metabolic and neuroendocrine systems such as reproductive axis, mainly in the central area of the hypothalamus.29,30 There is also a study in the existing literature on the expression of leptin receptors and direct actions of leptin in male and female gonads.31 It is, therefore, probable that Proteus and Biscaya, in combination, reduce the secretion of GnRH in hypothalamic neurons and subsequently lessen the levels of FSH, LH, and testosterone by reducing the serum level of leptin in a dose-dependent manner.
It has been previously posited that leptin has the role of a biochemical message between fat deposits and the reproductive axis so that the superficial injection of recombinant leptin can restore fertility and reproduction in male and female OB/OB rats. A previous investigation demonstrated that an injection of leptin antiserum into the lateral ventricles of rats caused a decrease in LH pulse-shaped secretion and stopped estrus cycle.32 There is considerable evidence indicating that, like estrogen, leptin is regulated by sex hormones.33 A previous study showed that testosterone regulated the m-RNA expression and production of leptin in cultured human adipocytes.34 The relative amount of androgens plays a crucial role in determining the sensitivity of the brain to the catabolic actions of leptin.35 Leptin stimulates pituitary cells (hypophysis) and secretes FSH and LH, which accompany the onset of puberty.12,36 Not only does leptin enact its effects on the release of LH by increasing nitric oxide in the pituitary and hypothalamus,37 but also it has an important role in puberty regulation by controlling the secretion of neuropeptide Y from neuropeptide Y neurons.19
Research has shown that both exogenous melatonin and androgen inhibit leptin levels.38 Melatonin plays a significant role in controlling leptin production and regulating reproductive activities through testosterone concentration.39 Finally, there are several reports indicating that insecticides may poison reproductive organs through either direct poisoning or interface with hormonal actions.40 The other possible mechanism is that these poisons affect the secretion of GnRH through direct toxicity in reproductive organs or interface with hormonal action.
We recommended that further studies be undertaken on the effects of Proteus and Biscaya on the changes in kisspeptin in rats.
Conclusion
Our comparisons between the experimental groups (treated with different concentrations of Proteus and Biscaya) and the control group showed a significant decrease in mean serum levels of FSH, LH, and testosterone. Furthermore, there was a significant decrease in the mean serum level of GnRH in the experimental groups (treated with average and maximum compound concentrations of Proteus and Biscaya) by comparison with the control group in a dose-dependent manner. Additionally, the mean serum level of leptin was decreased in all the experimental groups compared to the control group. Consequently, it can be concluded that Proteus and Biscaya affect the HPG axis by reducing leptin and subsequently GnRH, as a result of which the mean serum levels of FSH, LH, and testosterone hormones are decreased in a dose-dependent manner.
Acknowledgement
A debt of gratitude is owed to the Vice Chancellor for Research and Technology, Animal House Laboratory of Shiraz University of Medical Sciences, and all those who help us in this project.
Conflict of Interest:None declared.
References
- 1.Ramazzini C. Collegium Ramazzini statement on the control of pesticides in the European Union: a call for action to protect human health. Am J Ind Med. 2009;52:176–7. doi: 10.1002/ajim.20654. [DOI] [PubMed] [Google Scholar]
- 2.Asawasinsopon R, Prapamontol T, Prakobvitayakit O, Vaneesorn Y, Mangklabruks A, Hock B. The association between organochlorine and thyroid hormone levels in cord serum: a study from northern Thailand. Environ Int. 2006;32:554–9. doi: 10.1016/j.envint.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Meeker JD, Barr DB, Hauser R. Pyrethroid insecticide metabolites are associated with serum hormone levels in adult men. Reprod Toxicol. 2009;27:155–60. doi: 10.1016/j.reprotox.2008.12.012. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekeroglu V, Sekeroglu ZA, Kefelioglu H. Cytogenetic effects of commercial formulations of deltamethrin and/or thiacloprid on Wistar rat bone marrow cells. Environ Toxicol. 2013;28:524–31. doi: 10.1002/tox.20746. [DOI] [PubMed] [Google Scholar]
- 5.Ghisari M, Bonefeld-Jorgensen EC. Impact of environmental chemicals on the thyroid hormone function in pituitary rat GH3 cells. Mol Cell Endocrinol. 2005;244:31–41. doi: 10.1016/j.mce.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Mixed LG. Pesticides that confuse hormones. Berkeley: Pesticides Action Network; 2002. pp. 1–6. [Google Scholar]
- 7.Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993;341:1392–5. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- 8.Ebadi*Manas G, Hasanzadeh S, Parivar K. The effects of pyridaben pesticide on the histomorphometric, hormonal alternations and reproductive functions of BALB/c mice. Iran J Basic Med Sci. 2013;16:1055–64. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong X, Zhong A, Xu H. Effect of cyanotoxins on the hypothalamic-pituitary-gonadal axis in male adult mouse. PLoS One. 2014;9:e106585. doi: 10.1371/journal.pone.0106585. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Ye X, Li F, Zhang J, Ma H, Ji D, Huang X, et al. Pyrethroid Insecticide Cypermethrin Accelerates Pubertal Onset in Male Mice via Disrupting Hypothalamic-Pituitary-Gonadal Axis. Environ Sci Technol. 2017;51:10212–21. doi: 10.1021/acs.est.7b02739. [DOI] [PubMed] [Google Scholar]
- 11.Losa SM, Todd KL, Sullivan AW, Cao J, Mickens JA, Patisaul HB. Neonatal exposure to genistein adversely impacts the ontogeny of hypothalamic kisspeptin signaling pathways and ovarian development in the peripubertal female rat. Reprod Toxicol. 2011;31:280–9. doi: 10.1016/j.reprotox.2010.10.002. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khodaii H, Chamani M, Sadeghi A, Hejazi H. Effects of conjugated linoleic acid on mouse factors and hormones in the process of ovulation in miceintint. J Fertil. 2009;2:101–9. [Google Scholar]
- 13.Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 14.Donato J, *Jr Cravo, RM Frazao, R Gautron, L Scott, MM Lachey, J et. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121:355–68. doi: 10.1172/JCI45106. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomizawa M, Casida JE. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Rev Entomol. 2003;48:339–64. doi: 10.1146/annurev.ento.48.091801.112731. [DOI] [PubMed] [Google Scholar]
- 16.El-Kashoury AA, Salama AF, Selim AI, Mohamed RA. Chronic exposure of dicofol promotes reproductive toxicity in male rats. Life Sci J. 2010;7:5–19. [Google Scholar]
- 17.Khodaparast AH, Abdolahzadeh A, Rasekh M. A Critical Study of the” Six Ethical Codes for Research” in Iran. J Reprod Infertil. 2008;8:365–79. [Google Scholar]
- 18.Sayed-Ahmed A, Abd-Elmaksoud A, Elnasharty M, El-Magd In situ hybridization and immunohistochemical localization of leptin hormone and leptin receptor in the seminal vesicle and prostate gland of adult rat. Acta Histochem. 2012;114:185–91. doi: 10.1016/j.acthis.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Gamba M, Pralong FP. Control of GnRH neuronal activity by metabolic factors: the role of leptin and insulin. Mol Cell Endocrinol. 2006;254-255:133–9. doi: 10.1016/j.mce.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 20.Nabi G, Amin M, Khan A, Kami M. Endogeneous signals and mammalian puberty onset: A review. Journal of Biology and Life Science. 2014;6:1–14. [Google Scholar]
- 21.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–43. doi: 10.1210/er.2009-0005. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sitticharoon C, Boonpuan V, Mitrpant C, Churintaraphan M. Determination of KISS1, KISS1R and Kisspeptin in fat tissue of normal weight and obese humans and correlations between Serum Kisspeptin and leptin. Siriraj Med J. 2017;65:112–6. [Google Scholar]
- 23.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–74. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 24.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–25. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu RR, Heng L, Zhang DM, LV YF, Lin XY, Zhang HQ. Expression of Kisspeptin/kiss1r System is Down-regulated in the Hypothalamic Arcuate Nucleus of Pubertal Male Rats with High-fat-diet. Reprod Contracept. 2014;25:1–11. [Google Scholar]
- 26.Bateman HL, Patisaul HB. Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology. 2008;29:988–97. doi: 10.1016/j.neuro.2008.06.008. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calley JL, Dhillo WS. Effects of the hormone kisspeptin on reproductive hormone release in humans. Advances in Biology. 2014 [Google Scholar]
- 28.Crofton PM, Evans AE, Wallace AM, Groome NP, Kelnar CJ. Nocturnal secretory dynamics of inhibin B and testosterone in pre- and peripubertal boys. J Clin Endocrinol Metab. 2004;89:867–74. doi: 10.1210/jc.2003-030884. [DOI] [PubMed] [Google Scholar]
- 29.Casanueva FF, Dieguez C. Neuroendocrine regulation and actions of leptin. Front Neuroendocrinol. 1999;20:317–63. doi: 10.1006/frne.1999.0187. [DOI] [PubMed] [Google Scholar]
- 30.Spicer Leptin: a possible metabolic signal affecting reproduction. Domest Anim Endocrinol. 2001;21:251–70. doi: 10.1016/s0739-7240(01)00120-5. [DOI] [PubMed] [Google Scholar]
- 31.Tena-Sempere M, Pinilla L, Gonzalez LC, Dieguez C, Casanueva FF, Aguilar E. Leptin inhibits testosterone secretion from adult rat testis in vitro. J Endocrinol. 1999;161:211–8. doi: 10.1677/joe.0.1610211. [DOI] [PubMed] [Google Scholar]
- 32.Caprio M, Isidori AM, Carta AR, Moretti C, Dufau ML, Fabbri A. Expression of functional leptin receptors in rodent Leydig cells. Endocrinology. 1999;140:4939–47. doi: 10.1210/endo.140.11.7088. [DOI] [PubMed] [Google Scholar]
- 33.Kimura M, Irahara M, Yasui T, Saito S, Tezuka M, Yamano S, et al. The obesity in bilateral ovariectomized rats is related to a decrease in the expression of leptin receptors in the brain. Biochem Biophys Res Commun. 2002;290:1349–53. doi: 10.1006/bbrc.2002.6355. [DOI] [PubMed] [Google Scholar]
- 34.Xue J, Dial GD, Pettigrew JE. Performance, carcass, and meat quality advantages of boars over barrows: A literature review. Journal of Swine Health and Production. 1997;5:21–8. [Google Scholar]
- 35.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–87. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 36.Tezuka M, Irahara M, Ogura K, Kiyokawa M, Tamura T, Matsuzaki T, et al. Effects of leptin on gonadotropin secretion in juvenile female rat pituitary cells. Eur J Endocrinol. 2002;146:261–6. doi: 10.1530/eje.0.1460261. [DOI] [PubMed] [Google Scholar]
- 37.Squires EY. Applied animal endocrinology. 1 St ed. Cambridge: CABI; 2003. [Google Scholar]
- 38.Canpolat S, Sandal S, Yilmaz B, Yasar A, Kutlu S, Baydas G, et al. Effects of pinealectomy and exogenous melatonin on serum leptin levels in male rat. Eur J Pharmacol. 2001;428:145–8. doi: 10.1016/s0014-2999(01)01230-4. [DOI] [PubMed] [Google Scholar]
- 39.Mahmud SA, Mahmud AM. Physiological effects of melatonin on leptin, testosterone and biochemical parameters in Albino rats. IOSR J Pharm. 2013;3:48–53. [Google Scholar]
- 40.Garcia AM. Occupational exposure to pesticides and congenital malformations: a review of mechanisms, methods, and results. Am J Ind Med. 1998;33:232–40. [PubMed] [Google Scholar]