Abstract
Objectives, Methods:
Using pooled multivariable adjusted rate ratios (RR), we explored relationships between prediagnostic body-mass-index (BMI), waist-to-hip-ratio (WHR), and weight-gain during adulthood, and ALS in 419,894 women and 148,166 men from ten community-based cohorts in US, Europe and Australia; 428 ALS deaths were documented in women and 204 in men.
Results:
Higher mid-to-later adulthood BMI was associated with lower ALS mortality rates. For 5 kg/m2 increased BMI, the rate was 15% lower (95% confidence interval[CI]: 5%−24%;p=0.005). Although a clear linear trend was not evident for WHR at enrolment, individuals in the highest cohort-specific quartile had 35% (95%CI: 7%−55%;p=0.02) lower ALS compared to those in the lowest. BMI in early adulthood did not predict ALS; fewer than 10% of participants had early adulthood BMI>25kg/m2, limiting power. Weight-gain during adulthood was strongly associated with lower ALS; for an additional 1kg gain in weight/year the RR=0.37 (95%CI: 0.23–0.59;p<0.001). Associations persisted when adjusted for diabetes at enrollment, restricted to never-smokers, and ALS deaths in the five years after enrollment were excluded (accounting for recent weight loss).
Conclusion:
These findings confirm somewhat conflicting, underpowered evidence that adiposity is inversely associated with ALS. We newly demonstrate that weight-gain during adulthood is strongly predictive of lower ALS risk.
Keywords: Amyotrophic lateral sclerosis, Body mass index, Waist-to-hip ratio, Weight gain
Introduction
ALS, a neurodegenerative disease affecting 1.5–3 in 100,000 people per year, is characterized by progressive wasting and death within 2–3 years of diagnosis.(1) While the main pathological basis of ALS is degeneration of motor neurons, other systemic changes occur. In particular, patients exhibit increased energy metabolism(2–7) contrary to expectation based on continued muscle-wasting. SOD1 mutant mice similarly exhibit hypermetabolism and leanness, even several weeks before onset, and high-energy diets delayed onset and improved motor neuron survival.(6) Whether leanness or hypermetabolism is part of the disease pathology, is a risk factor for ALS (in other words, could obesity be a protective factor or hypermetabolism detrimental), or whether leanness or hypermetabolism and ALS are independently triggered by other factor(s) remains to be fully elucidated. Prior prospective studies suggest mid-life BMI is related to ALS risk, but findings are somewhat inconsistent. (8) (9) (10) It remains unclear whether early-adulthood BMI or weight gain in adulthood predict ALS, and data on prediagnostic waist and hip circumference and waist-to-hip ratio (WHR) are limited. Some previous studies have not completely adjusted for smoking, (10) a very strong predictor of BMI and risk factor for ALS.
We aim to further clarify the relation between body size in early- and mid-life and ALS death in a larger study of 419,894 women and 148,166 men, 632 of whom died from ALS, and almost all of whom were recruited from the general population in the US, Europe and Australia. Specifically, we will evaluate the hypermetabolism hypothesis further by exploring propensity to gain weight since age 18 which may be more indicative of life-long leanness/hypermetabolism than either BMI at age 18 or BMI at the particular age the participant was recruited (generally, middle-age). We have adequate power to consider these anthropometric predictors of ALS among never smokers and excluding ALS deaths during the seven years after enrollment (accounting for recent weight loss).
Methods
Participants
The Pooling Project of Prospective Studies of Diet and Cancer (DCPP) is an ongoing collaboration with the primary goals of assembling sufficient data to examine nutrition and cancer associations with standardized analyses of primary data across cohorts. We invited all cohorts participating in DCPP to extend their collaboration to include ALS; many of these cohorts had insufficient cases to independently investigate ALS epidemiology with precision. Five cohorts that participate in DCPP are already in an established ALS collaboration.(9, 11, 12) In addition, the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort has sufficient numbers for independent analyses;(8, 13) excluding these six DCPP cohorts from the current project facilitated the establishment of an independent data set that could validate results from the other two prospective projects (EPIC and 5-cohort ALS study). Ten prospective cohorts with 568,070 participants from the general populations of Europe, US and Australia are included in our analyses (Table 1).
Table 1:
Enrollment Characteristics of Men and Women in Ten Cohorts Worldwide Followed for Death due to ALS
| Cohort (follow-up years) | Sex | Baseline cohort size | Age at enrollment range (yrs) | Number of ALS deaths | Age at ALS death (yrs) | BMI at enrollment (kg/m2) | BMI at age 18/20 (kg/m2) | Waist circumference at enrollment (cm) | Hip Circumference at enrollment (cm) | Height (m) |
|---|---|---|---|---|---|---|---|---|---|---|
| Median (10th and 90th percentile) | ||||||||||
| BCDDP (1987–2005) | F | 38,950 | 40–93 | 52 | 73 (65–82) | 23.8 (20.0–30.4) | / | 81.3 (68.6–96.5) | 101.6 (91.4–114.3) | 1.63 (1.55–1.70) |
| CTS (1995–2009) | F | 102,607 | 22–104 | 63 | 73 (54–84) | 23.6 (19.8–31.4) | 20.8 (18.3–25.0) | / | / | / |
| COSM (1998–2010) | M | 43,010 | 45–79 | 70 | 70 (58–84) | 25.4 (22.0–30.0) | 21.8 (19.2–24.6) | 95.0 (85.0–108.0) | 101.0 (94.0–110.0) | 1.77 (1.69–1.86) |
| IWHS (1986–2009) | F | 34,540 | 55–69 | 91 | 75 (67–84) | 25.2 (20.8–32.4) | 20.5 (17.8–24.4) | 86.1 (71.1–106.7) | 102.9 (92.7–119.1) | 1.63 (1.55–1.70) |
| MCCS (1990–2006) | F | 22,803 | 31–75 | 15 | 70 (56–79) | 25.8 (21.3–32.9) | 21.1 (18.2–25.0) | 78.0 (66.0–95.0) | 100.0 (90.1–114) | 1.60 (1.51–1.69) |
| M | 14,895 | 27–72 | 15 | 65 (55–78) | 26.8 (22.9–31.5) | 22.4 (19.4–26.0) | 92.5 (81.0–105.5) | 100.5 (92.5–109.5) | 1.73 (1.63–1.82) | |
| NLCS (1987–2003) (# in the subcohort) | F | 62,573 (2,367) | 55–70 | 61 | 73 (64–80) | 24.6 (21.1–29.7) | 21.3 (18.0–24.6) | / | / | 1.65 (1.58–1.73) |
| M | 58,279 (2,244) | 55–70 | 81 | 71 (63–77) | 24.8 (21.8–28.1) | 21.7 (18.9–24.5) | 1.76 (1.68–1.85) | |||
| PLCO (1993–2009) | F | 28,115 | 55–74 | 27 | 71 (58–85) | 25.9 (21.3–34.1) | 20.8 (18.3–24.3) | / | / | 1.63 (1.55–1.70) |
| M | 29,310 | 55–74 | 32 | 71 (58–80) | 27.0 (23.1–32.8) | 22.9 (19.4–26.6) | 1.78 (1.70–1.85) | |||
| SMC (1997–2010) | F | 35,944 | 48–83 | 51 | 75 (63–84) | 24.5 (20.6–30.1) | 20.3 (17.6–23.6) | 82.0 (71.0–98.0) | 102.0 (92.0–115.0) | 1.64 (1.57–1.72) |
| WHS (1993–2009) | F | 37,570 | 38–89 | 40 | 65 (57–80) | 24.9 (20.8–32.6) | / | 87.6 (72.4–109.2) | 105.4 (94.0–122.6) | 1.65 (1.57–1.73) |
| WLHS (1991–2009) | F | 45,739 | 30–50 | 18 | 60 (52–65) | 22.8 (19.8–28.0) | 20.2 (17.6–23.7) | 75.0 (67.0–89.0) | 98.0 (90.0–108.0) | 1.66 (1.59–1.73) |
| Total Women | 408,841 | 418 | ||||||||
| Total Men | 145,494 | 198 | ||||||||
Cohort size is determined after applying cohort-specific exclusion criteria and further excluding participants with energy intakes beyond 3 SDs of their loge-transformed cohort-specific mean energy intake and BMI below 14 and above 50.
The NLCS was analyzed as case–cohort study; above exclusions were applied to the analysis data set, and not applied to the baseline cohort size presented in the table.
Abbreviations: Female (F), Male (M); Breast Cancer Detection Demonstration Project Follow-up Study (BCDDP); California Teachers Study (CTS); Cohort of Swedish Men (COSM); Iowa Women’s Health Study (IWHS); Melbourne Collaborative Cohort Study (MCCS); The Netherlands Cohort Study (NLCS); Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO); the Swedish Mammography Cohort (SMC); the Women’s Health Study (WHS); and the Women’s Lifestyle and Health Study (WLHS)
Cohort Participant Descriptions:
The DCCP studies are prospective cohorts or randomized trials analyzed as prospective cohorts. The main disadvantage to this study design is the possibility that the self-selecting subjects who chose to enroll in each study do not represent the population base. While this may affect external generalizability, it will not affect internal validity. Each study has been escribed previously and is briefly outlined.
Breast Cancer Detection Demonstration Project Follow-up Study (BCDDP) During 1973–1981 over a quarter of a million women at 29 U.S. centers participated in a mammography screening program. In 1987–1989 a subset completed an FFQ and remain followed for subsequent outcomes.(14)
California Teachers Study (CTS) is a cohort of female public-school teachers and administrators that began enrollment in 1995. Over 130,000 teachers answered a questionnaire on risk factors for breast cancer including anthropometrics and diet. (15)
Cohort of Swedish Men (COSM) is a population-based cohort comprising 48,850 men aged 45–79 years who were residents in central Sweden. In 1997, they completed a comprehensive questionnaire on lifestyle factors, diet, and medical history.(16)
Iowa Women’s Health Study (IWHS) is a population-based prospective cohort of that enrolled 41,836 women age 55–69 years in 1986. Women were invited to complete a 16-page mailed questionnaire if they held an Iowa driver’s license in 1985.(17)
Melbourne Collaborative Cohort Study (MCCS) is a cohort study of 24,479 women and 17,049 men mostly aged 40–69, with oversampling of Southern European migrants (30%), in Melbourne between 1990 and 1994. Detailed information on lifestyle factors as well as blood samples and direct physical measurements was collected in face-to-face interviews.(18)
The Netherlands Cohort Study (NCLS) comprises 58,279 and 62,573 Dutch men and women aged 55–69 years old. Baseline questionnaires were self-administered in 1986. Follow-up is ongoing. NCLS uses a case-cohort design where all cases of interest and deaths are enumerated while the non-case experience is is estimated using a sub-cohort. (19)
Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) is a two-armed trial set in multiple centers across the U.S. that was designed to evaluate whether a screening test reduces risk of death from the named cancers. Over 150,000 men and women aged 55 to 74 participated between 1992 and 2001.(20)
Swedish Mammography Cohort (SMC) comprises 66,651 women born between 1914 and 1948 who returned a completed 6-page questionnaire between 1987 and 1990 in response to an invitation to participate in mammography screening.(21)
The Women’s Health Study (WHS) is a 2×2×2 randomized double-blind, placebo controlled clinical trial of aspirin, beta-carotene and/or vitamin E that enrolled almost 40,000 U.S. women over 45 years during 1992 to 1995. Enrollees completed questionnaires prior to randomization-phase.(22)
Women’s Lifestyle and Health Study (WLHS) In 1991, 50,000 premenopausal (30–49 years old) women returned comprehensive questionnaires on lifestyle, diet and reproductive factors.(23)
Exposure and Covariate Assessment
Each cohort collected information on height and current weight by self-report on baseline questionnaires, except MCCS which were directly measured. Weight during early adulthood (asked on the questionnaires as weight at age 18 or at 20) was also collected at enrollment in seven cohorts. Baseline BMI (mid-to-late adulthood) and BMI during early adulthood were calculated using weight at baseline and recalled weight from early adulthood, respectively, divided by height reported at baseline squared (kg/m2). In a U.S. national health survey, correlations between current self-reported and technician measured BMI values were very high (0.90 to 0.95 across groups).(24) Weight is reasonably well-remembered over time: correlation was 0.80 for middle-aged men who recalled weight at age 25 compared to weight recorded on military records, and 0.87 for middle-aged women who recalled weight at age 18 compared to weight recorded at entry to nursing school.(25, 26) In men and women over 70 recalled BMI and measured BMI in adolescence was 0.63 and 0.82, respectively.(27) WHR was available in seven cohorts and was calculated using self-assessed waist and hip tape measurements at baseline. Self-measured WHR has moderate validity; for example, in one study correlations between self-reported waist circumferences and the average of two technician-measured waist circumferences were 0.95 for men and 0.89 for women, hip measurements were 0.88 for men and 0.84 for women, and WHR were 0.69 for men and 0.70 for women.(28) The yearly rate of change in weight was calculated comparing weight in early adulthood and weight at time of enrollment, divided by age at enrollment less 18 or 20 years. Information on dietary and other factors, including smoking and education attained, was also collected on baseline questionnaires.
Exclusions
Individuals with missing or implausible BMI (<14 kg/m2 or >50 kg/m2) were excluded from all analyses shown here (3% of the overall study population; ranging from none in MCCS to 8% in BCDDP). When we analyzed other parameters of adiposity, individuals were excluded when that parameter was missing.
Outcome
A participant was considered to have died from ALS if his or her death certificate recorded motor neuron disease (International Classification for Disease, version 9 (ICD-9) 335.2 or ICD-10 G12.2) as an intermediate, underlying, or other cause of death.
Statistical Analysis
Anthropometric measures were modeled continuously, as predefined categories, or as study- and gender-specific quartiles. Baseline BMI was classified as follows: <18.5, 18.5–<23, 23–<25, 25–<30, and 30+ kg/m2 following the World Health Organization’s (WHO) definitions of underweight, low-normal weight, high-normal weight, overweight, and obese. BMI in early adulthood was classified into the following categories: <18.5, 18.5–<21, 21–<23, 23–<25, and 25+ kg/m2 reflecting leaner body mass at this age. WHR, height and rate of weight change during adulthood were categorized as quartiles.
Relative rates (RR) and their 95% confidence intervals (95% CI) were calculated using Cox proportional hazards models for each gender within each cohort. The model included stratification by baseline age (years) and year the baseline questionnaire was returned, follow-up time (months) was the timescale, resulting in a time metric that simultaneously accounted for age, calendar time, and time since entry into the study. Multivariable RRs were further adjusted for smoking status (ever smoking, smoking pack years <10, 10–<20, 20–<30, 30–<40, 40+), education attained (<high-school, high-school, >high-school), physical activity (low, moderate, high), and race (if appropriate to the cohort: overall >90% of participants are Caucasian). In the analyses of waist and hip circumferences, WHR, and BMI in early adulthood, the effect of adjustment for baseline BMI was examined. The missing indicator method (assigning the same value to all missings within a covariate, and adding a further stratification (dummy) variable indicating the value was originally missing=yes/no to the model) was used within a cohort, if needed. In general, data on covariates were missing for <10% of each study population.(29) NLCS was analyzed as a case-cohort study(30) because questionnaires were processed for only a random sample of the cohort in addition to all ALS deaths.(19) Cohort-specific results were pooled using a random-effects model,(31) weighted by the inverse of their variance. Between-studies heterogeneity was investigated using the Q test statistic.(31)
Results
Ten participating cohorts comprised 419,894 women and 148,166 men, 428 and 204 of whom, respectively, had ALS listed as their cause of death. After excluding participants with missing or implausible baseline BMI, 408,841 women (418 ALS deaths) and 145,494 men (198 ALS deaths) remained for the main analyses. The cohort-specific follow-up ranged from 12 to 23 years. Median baseline BMI ranged from 23.6 to 27.0 kg/m2 across studies; average baseline BMI was at least 2.5 kg/m2 higher than BMI in early adulthood (table 1).
BMI at enrollment: Overall individuals with higher BMI at the time of enrollment had lower rates of ALS mortality compared to individuals with lower BMI. For each 5 kg/m2 higher increment in baseline BMI the pooled age-adjusted rate of ALS mortality was 13% lower (95% CI: 2% to 22%; p-for-heterogeneity=0.34) and the multivariable-adjusted was 15% lower (95% CI: 5% to 24%; p-for-heterogeneity=0.49) for men and women combined (figure 1); among women, the multivariable-adjusted rate was 13% (95% CI: 1% to 23%) lower for each 5 kg/m2 higher baseline BMI while there was a borderline statistically significant lower rate among men (25% [95% CI: −1% to 44%] for the same increment; p-for-heterogeneity due to gender>0.9). Tests for deviation from linearity were not statistically significant (p-for-nonlinearity >0.05; data not shown).
Figure 1:
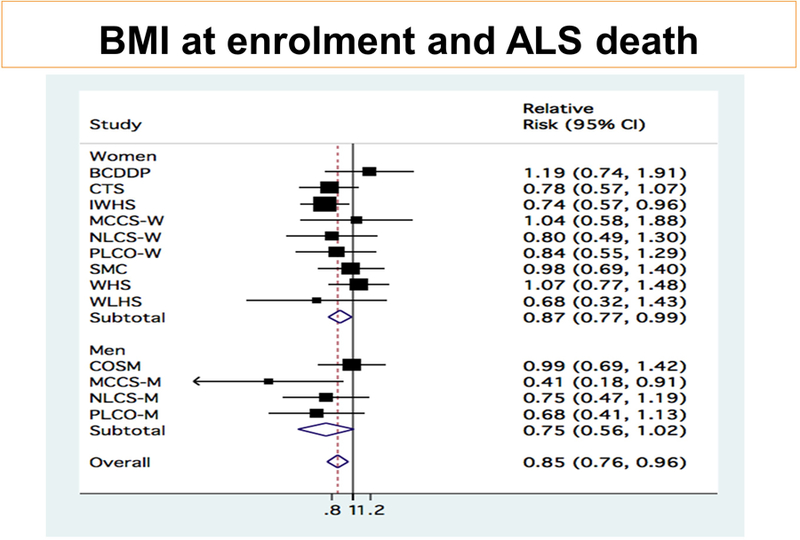
Pooled results for 5kg/m2 increase in BMI at enrollment. Pooled RR=0.85 (95% CI: 0.76 to 0.95); p=0.005). Among women, pooled RR=0.87 (95% CI: 0.77 to 0.99); p=0.036). Among men, pooled RR=0.75 (95% CI: 0.56 to 1.01); p=0.062)
BMI at enrollment categorized according to WHO definitions of underweight (<18.5 kg/m2), overweight (25–<30 kg/m2) and obesity (30+ kg/m2): Compared to men and women in the low-normal range at baseline (18.5–<23 kg/m2) the multivariable-adjusted RR of ALS death was 2.56 (95% CI: 1.38–4.77) for underweight individuals, RR=0.74 (95% CI: 0.58–0.95; p=0.016) for high-normal, RR=0.75 (95% CI: 0.59–0.95; p=0.017) for overweight, and 0.73 (95% CI: 0.53–1.00; p=0.053) for obese (table 2).
Table 2:
Analyses of Body Mass Index at Enrollment and ALS Death in Men and Women
| Continuous BMI per 5 kg/m2 Increment | World Health Organization Categories of BMI (kg/m2) | ||||||
|---|---|---|---|---|---|---|---|
| 14–<18.5ǂ | 18.5–<23 (reference) | 23–<25 | 25–<30 | 30+ | |||
| All | No. of cases | 616 | 14 | 178 | 127 | 214 | 67 |
| RR (95% CI) | 0.85 (0.76 – 0.95) | 2.56 (1.38 – 4.77) | 1.00 | 0.74 (0.58 – 0.95) | 0.75 (0.58 – 0.95) | 0.73 (0.53 – 1.00) | |
| p-value | 0.005 | 0.003 | 0.016 | 0.017 | 0.053 | ||
| p-H between studies | 0.486 | 0.411 | 0.533 | 0.294 | 0.368 | ||
| p-H due to sex | 0.417 | 0.285 | 0.179 | 0.172 | 0.662 | ||
| Three year lag | No. of cases | 556 | 8 | 161 | 112 | 196 | 64 |
| RR (95% CI) | 0.90 (0.80 – 1.01) | 1.81 (0.87 – 3.77) | 1.00 | 0.74 (0.57 – 0.95) | 0.79 (0.63 – 0.98) | 0.79 (0.58 – 1.09) | |
| p-value | 0.081 | 0.112 | 0.020 | 0.035 | 0.146 | ||
| p-H between studies | 0.617 | 0.621 | 0.705 | 0.731 | 0.629 | ||
| p-H due to sex | 0.978 | 0.566 | 0.201 | 0.336 | 0.965 | ||
| Five year lag | No. of cases | 497 | 8 | 142 | 103 | 173 | 56 |
| RR (95% CI) | 0.89 (0.79 – 1.01) | 2.16 (1.03 – 4.52) | 1.00 | 0.76 (0.58 – 1.00) | 0.79 (0.62 – 1.00) | 0.79 (0.56 – 1.11) | |
| p-value | 0.06 | 0.042 | 0.047 | 0.05 | 0.176 | ||
| p-H between studies | 0.680 | 0.616 | 0.645 | 0.689 | 0.504 | ||
| p-H due to sex | 0.872 | 0.666 | 0.201 | 0.211 | 0.915 | ||
| Seven year lag | No. of cases | 414 | 7 | 117 | 86 | 145 | 47 |
| RR (95% CI) | 0.89 (0.78 – 1.02) | 2.24 (1.02 – 4.93) | 1.00 | 0.80 (0.58 – 1.10) | 0.78 (0.60 – 1.02) | 0.83 (0.57 – 1.20) | |
| p-value | 0.091 | 0.045 | 0.168 | 0.07 | 0.320 | ||
| p-H between studies | 0.872 | 0.700 | 0.393 | 0.662 | 0.608 | ||
| p-H due to sex | 0.966 | 0.534 | 0.238 | 0.359 | 0.836 | ||
| Non-smokers | No. of cases | 486 | 11 | 137 | 96 | 177 | 65 |
| RR (95% CI) | 0.87 (0.76 – 1.00) | 2.91 (1.19 – 7.14) | 1.00 | 0.69 (0.52 – 0.91) | 0.73 (0.55 – 0.95) | 0.74 (0.50 – 1.10) | |
| p-value | 0.05 | 0.020 | 0.009 | 0.020 | 0.140 | ||
| p-H between studies | 0.292 | 0.187 | 0.675 | 0.269 | 0.185 | ||
| p-H due to sex | 0.327 | 0.091 | 0.111 | 0.069 | 0.478 | ||
Multivariable RRs were further adjusted for smoking status (ever smoking and smoking pack years <10, 10–<20, 20–<30, 30–<40, 40+), level of education attained (<high-school, high-school, >high-school), physical activity (low, moderate, high), and race (if appropriate to the cohort); age in years and year of questionnaire return were included as stratification variables.
MCCS-females are not part of the categorical analyses because no cases occurred among participants in the reference range of BMI 18.5 to <25 kg/m2. COSM, CTS, IOWA, NLCS-females, SMC, WHS are included in the analyses of BMI <18.5kg/m2; no cases occurred in the remaining cohorts. Including the non-cases in these cohorts (1.5% of overall populations) with BMI<18.5kg/m2 in the reference category did not change the results.
Abbreviations: P value for the test for between-studies heterogeneity (p-H between studies); P value for the test for between-studies heterogeneity due to sex (p-H due to sex).
Findings were similar for women (compared to low-normal weight, RR=2.21 (95% CI: 1.13–4.34; p=0.021) for underweight, RR=0.83 (95% CI: 0.62–1.12; p=0.226) for high-normal, RR=0.84 (95% CI: 0.63–1.13; p=0.244) for overweight and RR=0.75 (95% CI: 0.52–1.08; p=0.12) for those who were overweight), and men (only one study could contribute to the multivariate categorical analysis of BMI<18.5 kg/m2 due to small numbers; compared to low-normal, the RR=0.59 (95% CI: 0.38–0.89; p=0.013) for high-normal, RR=0.61 (95% CI: 0.42–0.88; p=0.008) for overweight and RR=0.57 (95% CI: 0.23 to 1.41; p=0.225) for obese). Five cohorts of women (CTS, IWHS, NLCS-w, PLCO-w, WHS) had adequate case numbers for analyses in individuals with baseline BMI of 35+ kg/m2; compared to women with low-normal BMI, RR=0.63 (95% CI: 0.29 to 1.37).
In sensitivity analyses excluding ALS deaths occurring 3, 5 and 7 years after baseline to exclude weight loss from pre-clinical disease, findings were only very slightly attenuated: for a 5 kg/m2 higher increment in baseline BMI, RRs were 0.90 (95% CI: 0.80 to 1.01; p=0.081), 0.89 (95% CI: 0.79 to 1.01; p=0.06) and 0.89 (95% CI: 0.78 to 1.02 p=0.091) for 3, 5 and 7 year lags, respectively. Because smokers weigh less than non-smokers on average and smoking may be a risk factor for ALS, analyses were repeated in non-smokers at baseline; findings were not materially changed (Table 2). In addition, the associations persisted when adjusted for self-reported diabetes at enrollment (for a 5 kg/m2 higher increment in baseline BMI, RR=0.85 (95% CI: 0.76 to 0.95; p=0.006; p-for-heterogeneity=0.51).
BMI in early adulthood: Weight in early adulthood was collected in eight cohorts (145,494 men and 332,321 women [198 and 326 cases, respectively]; table 1). BMI in early adulthood was not associated with ALS mortality (multivariable-adjusted RR=1.04; 95% CI: [0.88–1.22] per 5 kg/m2 increase). However, within each cohort fewer than 10% of participants were overweight or obese in early adulthood, except 23% of the men in PLCO and 17% of the men in MCCS.
Weight gain during adulthood: The median weight gained from early adulthood to baseline was 11.4 kg for men and 11.2 kg for women. For every additional 1kg gain in yearly weight gain, RR=0.37 (95% CI: 0.23–0.59; p<0.001) overall, 0.32 (95% CI: 0.13–0.83; p=0.018) among men, and 0.38 (95% CI: 0.21–0.68; p=0.001) among women (table 3). Tests for deviation from linearity were not significant (not shown). Risk estimates were similar when analyses were not adjusted for BMI in early adulthood (e.g., RR=0.42 (95% CI: 0.28–0.64; p<0.001) for men and women for every additional 1kg yearly weight gain). Excluding the first 5 years of follow-up (to reduce misclassification of yearly weight gain by recent weight loss) slightly attenuated the association (RR=0.43 (95% CI: 0.26–0.72; p=0.001) for each additional 1 kg increase yearly weight gain). When restricted to never-smokers to exclude those who did not gain weight due to smoking at any time during adulthood the RR=0.50 (95% CI: 0.21–1.21; p=0.125) for each additional 1 kg increase yearly weight gain.
Table 3:
Yearly Rate of Weight (kg) Gain from Early Adulthood to Enrollment by Cohort and Risk of ALS Death
| Cohort | Continuous yearly weight gain per 1kg Increment |
Quartile 1 Median (10th–90th percentile) RR (95% CI) |
Quartile 2 Median (10th–90th percentile) RR (95% CI) |
Quartile 3 Median (10th–90th percentile) RR (95% CI) |
Quartile 4 Median (10th–90th percentile) RR (95% CI) |
|---|---|---|---|---|---|
| Women | |||||
| IWHS | RR=0.29 (95% CI: 0.12, 0.71), p=0.007 |
0.03 (−1.17, 0.13) Ref |
0.21 (0.15, 0.27) RR=0.82 (95% CI:0.49, 1.39) |
0.37 (0.30, 0.45) RR=0.51 (95% CI:0.28, 0.93) |
0.63 (0.50, 0.96) RR=0.43 (95% CI:0.21, 0.80) |
| MCCS | RR=0.51 (95% CI: 0.06, 4.35), p=0.54 |
0.04 (−0.18, 0.13) Ref |
0.24 (0.17, 0.31) RR=1.63 (95% CI:0.43, 6.13) |
0.42 (0.35, 0.51) RR=1.30 (95% CI:0.29, 5.83) |
0.74 (0.57, 1.18) RR=0.47 (95% CI:0.05, 4.68) |
| NLCS | RR=0.36 (95% CI: 0.06, 2.11), p=0.260 |
0 (−0.21, 0.08) Ref |
0.16 (0.11, 0.21) RR=0.49 (95% CI:0.22, 1.07) |
0.29 (0.23, 0.35) RR=0.29 (95% CI:0.12, 0.70) |
0.49 (0.38, 0.73) RR=0.58 (95% CI:0.25, 1.35) |
| PLCO | RR=0.47 (95% CI: 0.10, 2.18), p=0.333 |
0.06 (−0.11, 0.14) Ref |
0.23 (0.17, 0.29) RR=1.32 (95% CI:0.51, 3.14) |
0.40 (0.32, 0.48) RR=0.67 (95% CI:0.21, 2.13) |
0.68 (0.53, 1.07) RR=0.64 (95% CI:0.18, 2.25) |
| SMC | RR=0.47 (95% CI: 0.10, 2.26), p=0.344 |
0.03 (−0.61, 0.11) Ref |
0.20 (0.14, 0.25) RR=1.21 (95% CI:0.50, 2.92) |
0.34 (0.28, 0.42) RR=1.20 (95% CI:0.47, 3.04) |
0.57 (0.45, 0.89) RR=0.70 (95% CI:0.23, 2.19) |
| WLHS | RR=0.55 (95% CI: 0.11, 2.77), p=0.467 |
0 (−0.38, 0.11) Ref |
0.23 (0.16, 0.31) RR=1.75 (95% CI: 0.42, 7.27) |
0.43 (0.35, 0.53) RR=1.95 (95% CI: 0.47, 1.21) |
0.78 (0.60, 1.33) RR=0.34 (95% CI: 0.03, 3.45) |
|
Pooled |
RR=0.38 (95% CI: 0.21, 0.68), p=0.001 |
Ref |
RR=0.91 (95% CI:0.65, 1.28), p=0.59 P for heterogeneity=0.4 |
RR=0.70 (95% CI:0.41, 1.21), p=0.20 P for heterogeneity=0.13 |
RR=0.51 (95% CI:0.33–0.78), p=0.002 P for heterogeneity=0.92 |
| Men | |||||
| COSM | RR=0.65 (95% CI: 0.20, 2.18), p=0.487 |
0.04 (−0.12, 0.12) Ref |
0.20 (0.15, 0.26) RR=0.74 (95% CI:0.37, 1.46) |
0.35 (0.29, 0.43) RR=0.57 (95% CI:0.26, 1.25) |
0.60 (0.47, 0.92) RR=1.03 (95%CI: 0.49, 2.16) |
| MCCS | RR=0.04 (95% CI: 0.00, 0.52), p=0.013 |
0.06 (−0.13, 0.15) Ref |
0.26 (0.19, 0.33) RR=1.11 (95% CI: 0.34, 3.66) |
0.44 (0.36, 0.53) RR=0.15 (95% CI: 0.02, 1.38) |
0.74 (0.58, 1.13) RR=0.17 (95% CI:0.02, 1.71) |
| NLCS | RR=0.43 (95% CI: 0.09, 2.12), p=0.303 |
0 (−0.15, 0.08) Ref |
0.16 (0.11, 0.21) RR=0.67 (95% CI:0.32, 1.43) |
0.28 (0.23, 0.33) RR=0.74 (95% CI:0.33, 1.66) |
0.49 (0.38, 0.71) RR=0.66 (95% CI:0.25, 1.74) |
| PLCO | RR=0.23 (95% CI: 0.05, 1.13), p=0.070 |
0.05 (−0.13, 0.13) Ref |
0.22 (0.16, 0.28) RR=0.86 (95% CI:0.36, 2.03) |
0.38 (0.31, 0.45) RR=0.49 (95% CI:0.18, 1.39) |
0.63 (0.50, 0.97) RR=0.35 (95% CI:0.10, 1.17) |
|
Pooled |
RR=0.32 (95% CI: 0.13, 0.83), p=0.018 |
Ref |
RR=0.78 (95% CI:0.52, 1.18), p=0.24 P for heterogeneity=0.91 |
RR=0.56 (95% CI:0.34, 0.90), p=0.017 P for heterogeneity=0.59 |
RR=0.63 (95% CI:0.37, 1.09), p=0.10 P for heterogeneity=0.37 |
|
All pooled |
RR=0.37 (95% CI: 0.23, 0.59), p<0.001 |
Ref |
RR=0.85 (95% CI:0.66, 1.11), p=0.24 P for heterogeneity=0.75 |
RR=0.62 (95% CI:0.44, 0.86), p=0.005 P for heterogeneity=0.30 |
RR=0.56 (95% CI:0.40, 0.78), p=0.001 P for heterogeneity=0.86 |
Multivariable RRs were adjusted for smoking status (ever smoking and smoking pack years <10, 10–<20, 20–<30, 30–<40, 40+), level of education attained (<high-school, high-school, >high-school), physical activity (low, moderate, high), and race (if appropriate to the cohort); age in years and year of questionnaire return were included as stratification variables.
Waist, hip and WHR at enrollment: Seven cohorts collected baseline waist and hip circumference (57,905 men and 215,546 women [85 and 267 cases, respectively]; table 1). Among men and women in the fourth quartile of WHR, there was a 35% (95% CI: 7% to 55%; p=0.020) reduction in ALS mortality compared to those in the first. (Figure 2) Findings were virtually unchanged when adjusted for baseline BMI. Among women, rates were similarly lowered comparing the fourth to the first quartile, but were borderline statistically significant (40% [95% CI: −6% to 66%]; p=0.079). In the two male cohorts with WHR, there was no relationship with ALS, perhaps due to inadequate power. (figures 2b–c). Results were similar when WHR was categorized as WHO sex-specific cut-points: among women, RR=0.76 (95% CI: 0.55–1.05; p=0.096) for WHR above 0.85 compared to 0.85 or below; among men, RR=0.57 (95% CI: 0.25–1.33; p=0.195) for WHR above 0.90 compared to 0.90 or below. Height was not associated with ALS (RR=1.04; 95% CI: 0.77–1.39 comparing men and women in the top quartile to the bottom).
Figure 2:
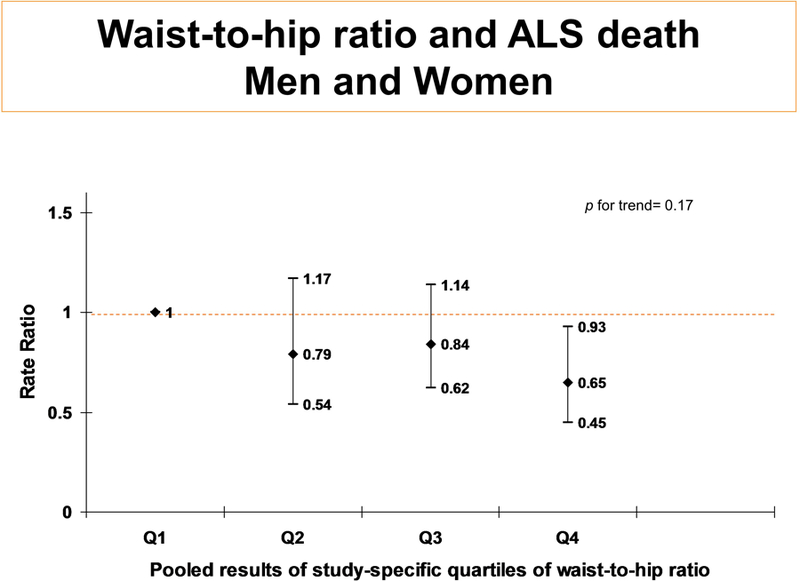
BCDDP (w), SMC (w), WHS (w), IWHS (w), MCCS (w & m), WLHS (w) and COSM (m) had waist and hip measurements, however, WLHS was not included in the analyses because of small case numbers. Quartiles are cohort-specific (and gender specific in MCCS), then pooled. All tests of heterogeneity had p-values >0.05. Analyses are adjusted for age, education, race, smoking, and physical activity, Further adjustment for BMI at baseline changed effect sizes minimally.
No significant heterogeneity across studies or gender was observed in any of the results above.
Discussion
In ten existing cohorts, BMI and WHR at enrollment were inversely associated with ALS mortality during follow-up. There was no association with BMI in early adulthood; this may be partly explained by the low prevalence of overweight and obese in the decades the participants reached adulthood. Among these mostly lean young adults, those with a propensity to gain weight during adulthood had substantially lower ALS death in later life.
Our findings extend observations of two prospective analyses. In a pan-European prospective cohort(8) (EPIC) of 152,368 men and 366,040 women with 222 ALS deaths during a 13-year follow-up findings were suggestive of an association between anthropometrics and subsequent ALS death. Briefly, while a clear pattern did not emerge, perhaps due to low case numbers across categories in analyses stratified on sex, there was evidence that ALS rates were lower among men with higher enrollment BMI. Among women, those who were underweight had higher ALS but rates among normal weight, overweight, and obese individuals did not differ. All findings in the European study were materially unchanged when ALS deaths during the first 3 years of follow-up were excluded. In a prospective project of five US cohorts with a total of 1153 ALS deaths during 14–28 years of follow-up among 537,968 women and 562,942 men, higher BMI was associated with lower risk of ALS.(9) Compared to individuals with a BMI of 18.5–<25 kg/m2, ALS rates were significantly lower among overweight and obese men and women. Finer categorization of the obese category revealed that rates were further lowered as the degree of excess weight increased. These findings persisted among non-smokers and when up-to the first 7 years of follow-up were excluded. The apparent inconsistent findings for BMI at enrollment among women in EPIC compared to the five-cohort US study would appear to be due to low power given that in the current study, with 410 ALS deaths among women, the association was manifest. Further, there is no significant heterogeneity in our results, which included 4 European studies, one Australian study, and several US studies.
The association between baseline BMI and ALS in this report was not attenuated when adjusted for diabetes in contrast to a finding among Danish nationals (odds ratio [OR]=0.72 (0.50–1.02) versus OR=0.81 (95% CI: 0.57–1.16)). While ascertainment of incident ALS using the national registries was excellent, obesity was defined as a hospital admission listed as ICD-8 code 277.99 and ICD-10 codes E65.0 to E66.9 because BMI was unknown, and COPD was used in place of smoking status. (10) The question of whether BMI or diabetes, or both, are predicting lower ALS rates cannot be fully addressed by the current (diabetes only at baseline) or the Danish study (the definition of obesity used means that many individuals with BMI 25–<30kg/m2 or 30+kg/m2 will be considered non-obese, and smoking is not fully adjusted for) but could be explored in a prospective cohort study with routinely updated diabetes ascertainment and confounder assessment.
ALS patients exhibit hypermetabolism at diagnosis with a resting energy expenditure that is higher than expected.(2–7) If hypermetabolism is an early symptom of ALS, then it is unclear when in the preclinical disease stage it begins. In an animal model of ALS, hypermetabolism and subsequent lower body mass are observed during the asymptomatic phase.(6) The finding of the current study that weight gain is perhaps less rapid in those who subsequently get ALS does not contradict the notion that hypermetabolism begins early in the disease process. Alternatively, additional weight or body-fat itself could be protective of ALS. A higher BMI at diagnosis (up to 35kg/m2) predicts better survival.(32) A third explanation for the observed association is confounding by a factor related to both adiposity/weight maintenance and ALS. Thus far a common genetic cause has not been identified. Strenuous physical activity, either as sport or occupation, is a potential environmental factor(33–37), although a considerable number of studies do not find an increased risk with physical activity.(38–40) There are insufficient data to fully address this hypothesis in the current study, with few cohorts with adequate case numbers in the high (according to the cohort-specific questionnaire) physical activity category. The interplay between cardiometabolic health, obesity, type-2 diabetes, and physical activity remain to be untangled in relation to ALS.
The present study takes advantage of a pre-existing database of ten cohorts worldwide, individually underpowered to examine the epidemiology of ALS. In each cohort adiposity parameters were measured at baseline, and therefore bias from differential recall in individuals with ALS compared to those without was minimized. Further, with the closed cohort study design, the non-case participants are representative of the ALS case population reducing internal selection bias. The present study was well powered for sensitivity analyses including lagged analyses and restriction to non-smokers.
A potential disadvantage of this study is the use of ALS on death certificates to approximate incident disease. Underreporting of ALS on death certificates would bias findings only if the probability of ALS diagnosis and of report in death certificates were related to BMI. For example, ALS may be less likely to be recorded on death certificates of those overweight with weight-related comorbidities. Strong bias from this source seems unlikely because the factors associated with BMI that would be expected to affect the accuracy of death certificates (age, smoking, and education) were included in the models. With evidence of improved survival with higher BMI at diagnosis, it could be argued that using ALS death will underrepresent incidence where there is longer survival.(32) This scenario is unlikely to fully explain our findings given the long follow-up in each cohort and their persistence in lagged analyses. Using death certificates may increase the likelihood that the baseline BMI measurement has been affected by as of yet undiagnosed ALS. Materially unchanged associations in lagged analyses suggest that these findings cannot be fully attributed to reverse causation. Another possibility is overreporting of bulbar and/or pyramidal ALS as follow-up progresses and the participants age when symptoms are due to non-diagnosed cerebrovascular disorders. Where this to occur then the true association of BMI and ALS in this study would be attenuated because higher BMI is a risk factor for cerebrovascular events.(41)
In summary, the present study confirms previously somewhat conflicting and underpowered evidence for an inverse relation between adiposity and future ALS risk. In particular, we newly demonstrate that the rate of weight gain during adulthood is strongly statistically correlated with ALS risk.
Acknowledgments
Funding: This work was supported by NIH grant R01-NS072494–01A1 awarded to EOR and NCI grant CA55075 awarded to SSW. The COSM and SMC cohorts are supported by the Swedish Research Council and by Strategic funds at Karolinska Institutet, Stockholm, Sweden. TK has received within the last 2 years investigator-initiated research funding from the French National Research Agency, the US National Institutes of Health, and the Parkinson’s Research Foundation. Further, he has received honoraria from the American Academy of Neurology and Allergan for educational lectures and from the BMJ and Cephalalgia for editorial services.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- BMI
body mass index
- WHR
waist-to-hip ratio
- WHO
World Health Organization
- EPIC
European prospective investigation into cancer and nutrition
- DCPP
Diet and Cancer Pooling Project
Footnotes
Conflicts of Interest: None declared
REFERENCES
- 1.Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nature reviews Neurology 2013;9(11):617–28. Epub 2013/10/16. [DOI] [PubMed] [Google Scholar]
- 2.Dupuis L, Pradat PF, Ludolph AC, Loeffler JP. Energy metabolism in amyotrophic lateral sclerosis. The Lancet Neurology 2011;10(1):75–82. Epub 2010/11/03. [DOI] [PubMed] [Google Scholar]
- 3.Cistaro A, Valentini MC, Chio A, Nobili F, Calvo A, Moglia C, et al. Brain hypermetabolism in amyotrophic lateral sclerosis: a FDG PET study in ALS of spinal and bulbar onset. European journal of nuclear medicine and molecular imaging 2012;39(2):251–9. Epub 2011/11/18. [DOI] [PubMed] [Google Scholar]
- 4.Bouteloup C, Desport JC, Clavelou P, Guy N, Derumeaux-Burel H, Ferrier A, et al. Hypermetabolism in ALS patients: an early and persistent phenomenon. J Neurol 2009;256(8):1236–42. Epub 2009/03/24. [DOI] [PubMed] [Google Scholar]
- 5.Desport JC, Torny F, Lacoste M, Preux PM, Couratier P. Hypermetabolism in ALS: correlations with clinical and paraclinical parameters. Neuro-degenerative diseases 2005;2(3–4):202–7. Epub 2006/08/16. [DOI] [PubMed] [Google Scholar]
- 6.Dupuis L, Oudart H, Rene F, Gonzalez de Aguilar JL, Loeffler JP. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc Natl Acad Sci U S A 2004;101(30):11159–64. Epub 2004/07/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desport JC, Preux PM, Magy L, Boirie Y, Vallat JM, Beaufrere B, et al. Factors correlated with hypermetabolism in patients with amyotrophic lateral sclerosis. Am J Clin Nutr 2001;74(3):328–34. Epub 2001/08/28. [DOI] [PubMed] [Google Scholar]
- 8.Gallo V, Wark PA, Jenab M, Pearce N, Brayne C, Vermeulen R, et al. Prediagnostic body fat and risk of death from amyotrophic lateral sclerosis: the EPIC cohort. Neurology 2013;80(9):829–38. Epub 2013/02/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Reilly EJ, Wang H, Weisskopf MG, Fitzgerald KC, Falcone G, McCullough ML, et al. Premorbid body mass index and risk of amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis & frontotemporal degeneration 2013;14(3):205–11. Epub 2012/11/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kioumourtzoglou MA, Rotem RS, Seals RM, Gredal O, Hansen J, Weisskopf MG. Diabetes Mellitus, Obesity, and Diagnosis of Amyotrophic Lateral Sclerosis: A Population-Based Study. JAMA neurology 2015;72(8):905–11. Epub 2015/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, O’Reilly EJ, Weisskopf MG, Logroscino G, McCullough ML, Schatzkin A, et al. Vitamin E intake and risk of amyotrophic lateral sclerosis: a pooled analysis of data from 5 prospective cohort studies. Am J Epidemiol 2011;173(6):595–602. Epub 2011/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, O’Reilly EJ, Weisskopf MG, Logroscino G, McCullough ML, Thun MJ, et al. Smoking and risk of amyotrophic lateral sclerosis: a pooled analysis of 5 prospective cohorts. Arch Neurol 2011;68(2):207–13. Epub 2011/02/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallo V, Bueno-De-Mesquita HB, Vermeulen R, Andersen PM, Kyrozis A, Linseisen J, et al. Smoking and risk for amyotrophic lateral sclerosis: analysis of the EPIC cohort. Ann Neurol 2009;65(4):378–85. Epub 2009/04/29. [DOI] [PubMed] [Google Scholar]
- 14.Mai V, Flood A, Peters U, Lacey JV Jr., Schairer C, Schatzkin A Dietary fibre and risk of colorectal cancer in the Breast Cancer Detection Demonstration Project (BCDDP) follow-up cohort. Int J Epidemiol 2003;32(2):234–9. Epub 2003/04/26. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein L, Allen M, Anton-Culver H, Deapen D, Horn-Ross PL, Peel D, et al. High breast cancer incidence rates among California teachers: results from the California Teachers Study (United States). Cancer Causes Control 2002;13(7):625–35. Epub 2002/09/26. [DOI] [PubMed] [Google Scholar]
- 16.Larsson SC, Rutegard J, Bergkvist L, Wolk A. Physical activity, obesity, and risk of colon and rectal cancer in a cohort of Swedish men. Eur J Cancer 2006;42(15):2590–7. Epub 2006/08/18. [DOI] [PubMed] [Google Scholar]
- 17.Folsom AR, Kushi LH, Anderson KE, Mink PJ, Olson JE, Hong CP, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med 2000;160(14):2117–28. Epub 2000/07/25. [DOI] [PubMed] [Google Scholar]
- 18.Giles GG, English DR. The Melbourne Collaborative Cohort Study. IARC Sci Publ 2002;156:69–70. Epub 2002/12/18. [PubMed] [Google Scholar]
- 19.van den Brandt PA, Goldbohm RA, van ‘t Veer P, Volovics A, Hermus RJ, Sturmans F. A large-scale prospective cohort study on diet and cancer in The Netherlands. J Clin Epidemiol 1990;43(3):285–95. Epub 1990/01/01. [DOI] [PubMed] [Google Scholar]
- 20.Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials 2000;21(6 Suppl):273S–309S. Epub 2001/02/24. [DOI] [PubMed] [Google Scholar]
- 21.Holmberg L, Ohlander EM, Byers T, Zack M, Wolk A, Bergstrom R, et al. Diet and breast cancer risk. Results from a population-based, case-control study in Sweden. Arch Intern Med 1994;154(16):1805–11. Epub 1994/08/22. [DOI] [PubMed] [Google Scholar]
- 22.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women’s Health Study. Journal of women’s health & gender-based medicine 2000;9(1):19–27. Epub 2000/03/16. [DOI] [PubMed] [Google Scholar]
- 23.Hjartaker A, Adami HO, Lund E, Weiderpass E. Body mass index and mortality in a prospectively studied cohort of Scandinavian women: the women’s lifestyle and health cohort study. Eur J Epidemiol 2005;20(9):747–54. Epub 2005/09/20. [DOI] [PubMed] [Google Scholar]
- 24.McAdams MA, Van Dam RM, Hu FB. Comparison of self-reported and measured BMI as correlates of disease markers in US adults. Obesity (Silver Spring) 2007;15(1):188–96. Epub 2007/01/18. [DOI] [PubMed] [Google Scholar]
- 25.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord 1995;19(8):570–2. Epub 1995/08/01. [PubMed] [Google Scholar]
- 26.Rhoads GG, Kagan A. The relation of coronary disease, stroke, and mortality to weight in youth and in middle age. Lancet 1983;1(8323):492–5. Epub 1983/03/05. [DOI] [PubMed] [Google Scholar]
- 27.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol 1993;138(1):56–64. Epub 1993/07/01. [DOI] [PubMed] [Google Scholar]
- 28.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1(6):466–73. Epub 1990/11/01. [DOI] [PubMed] [Google Scholar]
- 29.Smith-Warner SA, Spiegelman D, Ritz J, Albanes D, Beeson WL, Bernstein L, et al. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol 2006;163(11):1053–64. Epub 2006/04/21. [DOI] [PubMed] [Google Scholar]
- 30.Prentice RL. On the design of synthetic case-control studies. Biometrics 1986;42(2):301–10. Epub 1986/06/01. [PubMed] [Google Scholar]
- 31.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7(3):177–88. Epub 1986/09/01. [DOI] [PubMed] [Google Scholar]
- 32.Paganoni S, Deng J, Jaffa M, Cudkowicz ME, Wills AM. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve 2011;44(1):20–4. Epub 2011/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang F, Hallmarker U, James S, Ingre C, Michaelsson K, Ahlbom A, et al. Amyotrophic lateral sclerosis among cross-country skiers in Sweden. Eur J Epidemiol 2015. Epub 2015/07/30. [DOI] [PubMed]
- 34.Ascherio A, O’Reilly EJ. New insights on physical activity and amyotrophic lateral sclerosis. Eur J Epidemiol 2016. Epub 2016/03/10. [DOI] [PubMed]
- 35.Gallo V, Vanacore N, Bueno-de-Mesquita HB, Vermeulen R, Brayne C, Pearce N, et al. Physical activity and risk of Amyotrophic Lateral Sclerosis in a prospective cohort study. Eur J Epidemiol 2016. Epub 2016/03/13. [DOI] [PMC free article] [PubMed]
- 36.Harwood CAD, Westgate KM, Gunstone SM, Brage SD, Wareham NJP, McDermott CJD, et al. Long-term physical activity: an exogenous risk factor for sporadic amyotrophic lateral sclerosis? Amyotrophic lateral sclerosis & frontotemporal degeneration 2016:1–8. Epub 2016/03/22. [DOI] [PMC free article] [PubMed]
- 37.Eaglehouse YL, Talbott EO, Chang Y, Kuller LH. Participation in Physical Activity and Risk for Amyotrophic Lateral Sclerosis Mortality Among Postmenopausal Women. JAMA neurology 2016:1–9. Epub 2016/01/20. [DOI] [PMC free article] [PubMed]
- 38.Huisman MH, Seelen M, de Jong SW, Dorresteijn KR, van Doormaal PT, van der Kooi AJ, et al. Lifetime physical activity and the risk of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2013;84(9):976–81. Epub 2013/02/19. [DOI] [PubMed] [Google Scholar]
- 39.Veldink JH, Kalmijn S, Groeneveld GJ, Titulaer MJ, Wokke JH, van den Berg LH. Physical activity and the association with sporadic ALS. Neurology 2005;64(2):241–5. Epub 2005/01/26. [DOI] [PubMed] [Google Scholar]
- 40.Pupillo E, Messina P, Giussani G, Logroscino G, Zoccolella S, Chio A, et al. Physical activity and amyotrophic lateral sclerosis: a European population-based case-control study. Ann Neurol 2014;75(5):708–16. Epub 2014/04/08. [DOI] [PubMed] [Google Scholar]
- 41.Strazzullo P, D’Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke 2010;41(5):e418–26. Epub 2010/03/20. [DOI] [PubMed] [Google Scholar]


