Abstract
The influence of the microbiota on behavior and stress responses is poorly understood. Zebrafish larvae have unique characteristics that are advantageous for neuroimmune research, however, they are currently underutilized for such studies. Here, we used germ-free zebrafish to determine the effects of the microbiota on behavior and stress testing. The absence of a microbiota dramatically altered locomotor and anxiety-related behavior. Additionally, characteristic responses to an acute stressor were also obliterated in larvae lacking exposure to microbes. Lastly, treatment with the probiotic Lactobacillus plantarum was sufficient to attenuate anxiety-related behavior in conventionally-raised zebrafish larvae. These results underscore the importance of the microbiota in communicating to the CNS via the microbiome-gut-brain axis and set a foundation for using zebrafish larvae for neuroimmune research.
Keywords: Zebrafish, Microbiota, Microbiome, Germ-free, Gnotobiotic, Anxiety, Stress, Behavior, Gut-brain axis
1. Introduction
Zebrafish (Danio rerio) larvae are an emerging high-throughput model system for neurobehavioral studies. After hatching from their chorion between 2 and 3 days post fertilization (dpf), the larvae inflate their swim bladder and exhibit numerous neuro-behavioral phenotypes by day 4–5 dpf [1]. Many of these larval behaviors have been correlated with those seen in human neurological processes and disorders, such as anxiety [2], learning [3], fear [4], sociability[5], and psychosis[6]. One of the best characterized behaviors in zebrafish larvae is anxiety-related behavior, often measured by thigmotaxis or the tendency to remain close to vertical surfaces (i.e., “wall-hugging”). This behavior is evolutionarily conserved and exhibited by a wide range of species, including rodents [7], fish [8–11], and humans [12]. Thigmotactic behavior is a well-validated index of anxiety in zebrafish larvae since anxiolytic and anxiogenic drugs significantly attenuate and enhance this behavior, respectively [2,13–17]. Zebrafish larvae also exhibit physiological responses to stress similar to those seen in mammals, wherein responses to stress rely heavily on the hypothalamic-pituitary-adrenocortical (HPA) axis and its synthesis of glucocorticoids. Reports have demonstrated functional and anatomical parallels between the zebrafish hypothalamic-pituitary-interrenal (HPI) axis and the mammalian HPA axis [18–20]. Cortisol is the primary corticosteroid in both zebrafish and humans, unlike rodents wherein corticosterone is the main corticosteroid. During a stress response, the HPI axis is activated and there is an elevation of plasma cortisol which is mediated via activation of melanocortin type 2 receptor (mc2r) by ACTH binding [21]. Steroidogenic acute regulatory protein (StAR), and 11β-hydroxylase, both involved in the final steps of cortisol synthesis, are also increased in response to acute stressors [21,22].
Factors that play a role in the development of the corticoid system and stress responses during early life of vertebrates are not well understood. Recently, there have been many studies suggesting bidirectional influences between the microbiota and neurological development and stress-related behavior [23–26]. For example, social stress can alter the structure of the intestinal microbiota [27] while alterations in the microbiota can affect models of neurological disorders [28] and behavior [29]. Clarke et al. have also shown that the early life microbiota plays a critical role in affecting CNS signaling [30]. Similarly, long-lasting differences in the microbiota have been identified in rodents exposed to early life stress [31]. Furthermore, anxiety-related behavior and symptoms of stress have been shown to decrease in both mammals and zebrafish following the ingestion of particular probiotic Lactobacillus strains [24,32]. For example, Lactobacillus plantarum has been shown to reduce anxiety-related behavior and reduce stress-associated inflamma-tory cytokine levels in mice [33]. Interestingly, this particular strain has been shown to be highly adherent to gut epithelium during colonization of zebrafish [34,35].
The microbiota signals to the central nervous system (CNS) via several potential pathways. Likely mechanisms of communication include production of various metabolites that pass through the intestinal barrier into the circulatory system, and/or metabolites produced by microbes that can signal through the immune system [36]. Moreover, afferent pathways of the vagus nerve from the enteric nervous system (ENS) to the CNS have been implicated as a major route of communication between the microbiota and CNS [24]. Within the ENS, enteric glial cells outnumber enteric neurons by 4:1, and they are thought to play crucial roles in maintaining the intestinal epithelial barrier and regulating immune responses in the mucosa. Kabouridis et al. showed that the postnatal arrangement and ongoing supply of glial cells in the intestinal mucosa are regulated by the microbiota in mice, further indicating that this is a key pathway in the microbiota-gut-brain axis [37]. The development of intestinal innervation follows characteristic steps in zebrafish as in other vertebrates. Neural crest cells give rise to the bilateral vagal pathway through various signals, including hormonal cues, neurotrophins, and direct interactions with other cells. Development of the vagal extrinsic innervation of the gut occurs well before the onset of feeding which is comparable to other vertebrate species [38]. Despite architectural differences between the ENS of zebrafish and mammals, most of the molecular mechanisms underlying ENS development and function are conserved among species [39]. These functional similarities have allowed human ENS disorders (such as Hirschsprung’s disease, Goldberg-Shprintzen Syndrome, and Bardet-Biedl syndrome) to be modeled in zebrafish as they have been modeled in rodents [40,41].
Zebrafish larvae have unique characteristics that could help elucidate mechanisms involved in the microbiota-gut-brain axis. The optical transparency of zebrafish allows for in vivo visualization of labeled bacteria interacting with host cells [42]. Furthermore, the ex utero development of zebrafish allows for easy manipulation of microbial contact and investigation of microbial involvement throughout development. Lastly, the neurobehavioral similarities with mammals and the ability to readily produce and control gnotobiotic zebrafish larvae make them a valuable model for neuroimmune studies.
Here, to investigate the ability of the microbiota to influence anxiety-related behavior and stress responses in zebrafish larvae, thigmotaxis (a measure of anxiety-related behavior in zebrafish larvae) was assessed in larvae raised in germ-free or conventional environments, and stress responses were evaluated via cortisol production in reaction to an osmotic stress challenge. Zebrafish larvae were examined at 6 dpf, an age at which high-throughput behavioral screening can be accomplished using multi-well plates. It has been shown that enteric innervation is well-developed before the onset of feeding (5–6 dpf) [38], allowing for gnotobiotic studies to be easily controlled without the complications of maintaining sterility during feeding. Additionally, effects of probiotics were examined in zebrafish larvae in conjunction with anxiety-related behavior and stress responses. The current data demonstrate that the microbiota modulates locomotor behavior and thigmotactic behavior in larvae, and that the stress response of zebrafish larvae relies heavily on the presence of the microbiota. Moreover, supplementation with the probiotic Lactobacillus plantarum was sufficient to mitigate thigmotactic behavior. These results underscore the importance of microbes in gut-brain signaling to modulate behavior and appropriate responses to stress, and provide a foundation for the use of zebrafish larvae in neuroimmune studies.
2. Methods
2.1. Animals
Wild-type zebrafish purchased from Aquatica BioTech (Sun City Center, FL) were used for this study. Multiple breeders were placed into a breeding tank overnight to spawn. Eggs were collected immediately after fertilization and evenly divided into separate groups for subsequent treatment. Due to the high number of zebrafish larvae that can be collected at one time, sufficient numbers of embryos were collected for all treatment groups for any given experiment on the same day. This is ideal for eliminating variation between clutches and for eliminating variation in the microbial content of the fish water used for non-sterile larvae groups. Germ-free (GF) embryos were generated by following a previously published method [43]. Briefly, embryos were collected in sterile fish water containing 250 mg/mL amphotericin B, 5 μg/mL kanamycin, and 100 μg/mL ampicillin (AB-fish water). After sorting to remove unfertilized embryos, viable embryos were transferred to a tissue culture hood and gently washed 3 times in AB-fish water. Embryos were immersed in 0.1% PVP-Iodine solution for 2 min, and then immediately washed 3 times with sterile fish water. After washing, the embryos were immersed in 0.003% bleach solution for 1 h before being washed an additional 3 times with sterile fish water. Finally, the embryos were transferred into sterile tissue culture flasks and maintained in a 28.5 °C incubator. Conventionalized (CV) embryos followed the same sterilization process as GF embryos with the exception of being housed in conventional fish water rather than sterile fish water. Conventionally-raised (CR) embryos were collected and maintained in conventional fish water without undergoing the sterilization process. Zebrafish larvae were raised in their respected environment at a density of ~1 larvae/mL until 6 days post fertilization (dpf), when all tests were performed. Sterility monitoring was conducted according to previously reported procedures for generating gnotobiotic zebrafish larvae [43]. Lacto-bacillus administration was done following a previously published protocol [44]. Briefly, probiotic bacteria were grown for 24 h in MRS media at 37 °C, centrifuged at 4000g for 5 min, and then washed with sterile fish water. At 4 dpf, zebrafish larvae were exposed to 2 × 107 CFU/mL of Lactobacillus plantarum (USDA-ARS, Washington DC) by injecting the bacteria directly into the water of the larvae housing flask. Zebrafish larvae were allowed to be exposed to L. plantarum for 2 days (until testing at 6 dpf).
2.2. Microbial DNA extraction and quantification
Microbial DNA was extracted according to an adapted previously published protocol [45]. Immediately following euthanasia, 12 zebrafish larvae were aseptically collected into 800 μL of lysis buffer (500 mM NaCl, 50 mM tris-HCl, 50 mM EDTA, and 4% SDS), homogenized for 3 min in a Qiagen Tissuelyser II, and incubated at 70 °C for 20 min. Following centrifugation at 5000g for 5 min at room temperature, the supernatant was mixed with 200 μL of 10 mM ammonium acetate, incubated on ice for 5 min, and then centrifuged at 16,000g for 10 min at room temperature. 750 μL of supernatant was then mixed with an equal volume of chilled isopropanol, and incubated for 30 min on ice. The contents of the tube were then centrifuged at 16,000g at 4 °C for 15 min to pellet DNA. The pellet was rinsed twice with 70% EtOH and re-suspended in 150 μL of tris-EDTA. 15 μL of proteinase-K and 200 μL of buffer AL (DNeasy kit, Qiagen, Valencia, CA) were then added and tubes were incubated at 70 °C for 10 min. 200 μL of 100% EtOH was then added and the entire contents of the tube were transferred to a Qiagen spin column before continuing with the manufacturer’s instructions for DNA purification (DNeasy Kit, Qiagen). DNA was eluted in 50 μL of EB buffer (Qiagen). Yield of double-stranded DNA was determined via fluorometry (Qubit 2.0, Life Technologies, Carlsbad, CA) using Qubit® dsDNA BR assay kits (Life Technologies).
2.3. Metagenomic library preparation and sequencing
Sequencing of the V4 region of the 16S rRNA gene was performed on the Illumina MiSeq platform. Bacterial 16S rRNA amplicons were constructed by amplification of the V4 hypervariable region of the 16S rRNA gene with single-indexed primers flanked by Illumina standard adapter sequences. Universal primers (U515F/806R) previously developed against the V4 region were used for generating amplicons. Oligonucleotide sequences were obtained at proBase. A single forward primer and reverse primers with unique 12-base indices were used in all reactions. PCR reactions (50 μL) contained 100 ng of genomic DNA, forward and reverse primers (0.2 μM each), dNTPs (200 μM each), and Phusion High-Fidelity DNA Polymerase (1U). PCR amplification was performed as follows: amplification at 98 °C for 3 min, and 25 cycles at 98 °C for denaturation for 15 s, annealing at 50 °C for 30 s, and extension at 72 °C for 30 s, then a final extension at 72 °C for 7 min. Amplified product (5 μL) from each reaction was combined and thoroughly mixed; pooled amplicons were purified by addition of Axygen AxyPrep MagPCR Clean-up beads (50 μL) to an equal volume of 50 μL of amplicons and incubated at room temperature for 15 min. Products were washed multiple times with 80% EtOH and the dried pellet resuspended in Qiagen EB Buffer (32.5 μL), incubated at room temperature for 2 min, and then placed on a magnetic stand for 5 min. Supernatant (30 μL) was transferred to a low-binding microcentrifuge tube for storage. The final amplicon pool was evaluated using the Advanced Analytical Fragment Analyzer automated electrophoresis system, quantified with the Qubit flourometer using the quant-iT HS dsDNA reagent kit, and diluted according to the manufacturer’s protocol.
2.4. Bioinformatics analysis
Assembly, binning, and annotation of DNA sequences were performed at the MU Informatics Research Core Facility (IRCF, Columbia, MO). Briefly, contiguous sequences of DNA were assembled using FLASH software [46] and contigs were culled if found to be short after trimming for a base quality less than 31. Qiime v1.7 [47] software was used to perform de novo and reference-based chimera detection and removal, and remaining contigs were assigned to operational taxonomic units (OTUs) using a criterion of 97% nucleotide identity. Taxonomy was assigned to selected OTUs using BLAST [48] against the Greengenes database [49] of 16 S rRNA sequences and taxonomy.
2.5. Quantitative RT-PCR
The mRNA abundance of melanocortin type 2 receptor (mc2r), 11β-hydroxylase, and steroidogenic acute regulatory protein (StAR) was examined in larvae following a modification of a previously published method [21]. Briefly, RNA was extracted from pools of 14 larvae and cDNA synthesized using an EasyScript Plus™ cDNA Synthesis kit (Lambda Biotech, Ballwin, MO). Samples were then analyzed in triplicate and target mRNA expression was normalized to β-actin expression. Every 10 μL reaction contained 1 × SsoAdvanced universal SYBR® Green supermix (BioRad, Hercules, CA), 0.3 μM forward and reverse primers, and 100 ng cDNA template. PCR parameters were: amplification at 95 °C for 3 min, and 50 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 20 s, and extension at 72 °C for 20 s, with a plate read after each cycle using a BioRad CFX384 real-time system (BioRad, Hercules, CA).
2.6. Locomotor activity and thigmotaxis assay
Locomotor activity was assessed according to an adaptation of a published protocol [50]. Briefly, zebrafish larvae were individually placed in a sterile 24-well plate containing 1 mL/well of media from their home flask. The plate was then positioned atop a transmitted LED light stage to maximize contrast and facilitate tracking. The larvae were acclimated to the recording arena for 10 min prior to the start of video acquisition. After the acclimation period, total distance traveled, average speed, and mobility time were recorded during 10 min of spontaneous free swimming. To examine anxiety-related behavior, thigmotaxis was also calculated during the behavior trial. It is well defined that anxiolytic and anxiogenic agents dramatically alter the location of which a zebrafish larvae will spend its time during the assay [2,13–17]. Thigmotactic behavior was defined as a larva being within 4 mm of the side of its well; therefore, larvae that spent more time in the center zone or had more entries to the center zone were noted as being less thigmotactic. A new 24-well plate was used after every trial to eliminate any alarm cues from previously tested larvae.
2.7. Osmotic stress treatment
An osmotic shock stress test was performed according to an adaptation of previously published protocols [19,51]. Briefly, zebrafish larvae were incubated in fish water containing 100 mM NaCl at 28.5 °C for 10 min. Larvae were then immobilized in ice-cold fish water and immediately collected for further analysis. For RNA extractions, 14 larvae were collected in 500 μL of RNAlater® (ThermoFisher Scientific, Waltham, MA) per tube, and frozen in an ethanol/dry-ice bath. Larvae used for cortisol extraction were placed into a dry tube (exactly 14 larvae per tube) and frozen in an ethanol/dry-ice bath. All samples were stored at −80 °C until used.
2.8. Cortisol extraction and ELISA
Cortisol extraction was accomplished via a published protocol [19]. Briefly, tubes containing the larvae were thawed and 150 μL of water added to them. Samples were then homogenized for 30 s with a Qiagen Tissuelyser II. Following homogenization, 1 mL of ethyl acetate was added into the sample, mixed well, and centrifuged at 3000g at 4 °C for 5 min. The aqueous layer was then frozen in an ethanol/dry-ice bath and the solvent layer was transferred into a new tube. Next, the solvent was evaporated for 30 min at 30 °C in a vacuum centrifuge (Sorvall SpeedVac, Thermo Scientific). Lastly, the cortisol was dissolved in 60 μL of 0.2% BSA in PBS and used immediately for ELISA. Cortisol concentrations were determined by using a cortisol ELISA kit (Salimetrics, Carlsbad, CA) according to the manufacturer’s instructions. The sensitivity of the assay is less than 0.007 μg/dL, and cortisol concentrations were read on a plate reader (SpectraMax M3, Molecular Devices, Sunnyvale, CA).
2.9. Statistics
The recorded data were graphed and analyzed by GraphPad Prism 6.0. Bacterial 16 S rRNA qPCR data was analyzed using a one-way analysis of variances (ANOVA). Principal component analysis was performed using a non-linear iterative partial least squares algorithm implemented in an Excel macro kindly provided by Hiroshi Tsugawa of the Riken Institute (Wako, Japan) to evaluate β-diversity and its association with larvae treatment. A one-way ANOVA was used as the statistical test for most behavior data, with the exception of a student’s t-test for probiotic behavior tests. A multi-factorial ANOVA was used for osmotic stress test data with stress-treatment as one factor and microbiota-status as the second factor. Student-Newman-Keuls post-hoc testing was performed on all ANOVA analyses.
3. Results
3.1. Characterization of gnotobiotic zebrafish larvae
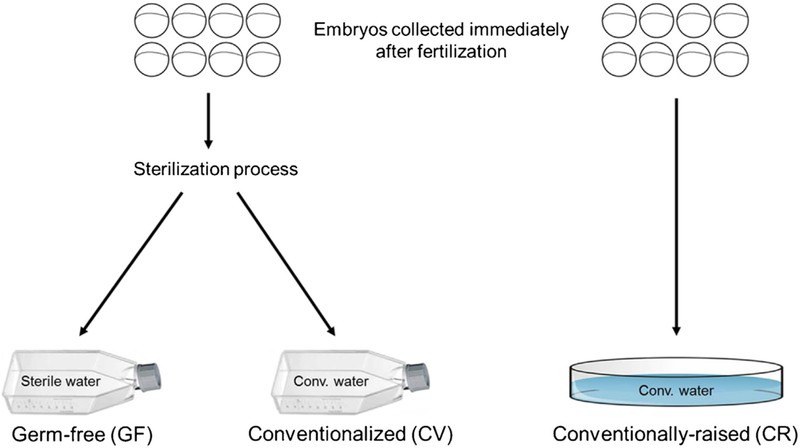
To investigate the influence of the microbiota on behavior, three separate groups of zebrafish larvae were generated. As illustrated in Fig. 1, embryos were collected immediately after fertilization and randomly divided into three groups. One group was put directly into conventional fish water (conventionally-raised; CR group), while the other two group were subjected to a sterilization process. After sterilization, the embryos were collected into tissue culture flasks containing either sterile fish water (germ-free; GF group) or tissue culture flasks containing conventional fish water (conventionalized; CV group).
Fig. 1.
Schematic illustrating the development of gnotobiotic zebrafish groups. Immediately after fertilization, zebrafish embryos were collected and randomly split into 3 groups. One group was collected directly into a sterile petri dish containing conventional fish water (CR group). The other 2 groups of embryos were sterilized and then collected into tissue culture flasks containing either sterile fish water (GF group) or conventional fish water (CV group). Embryos were maintained at ~1 embryo/mL until testing at 6 dpf.
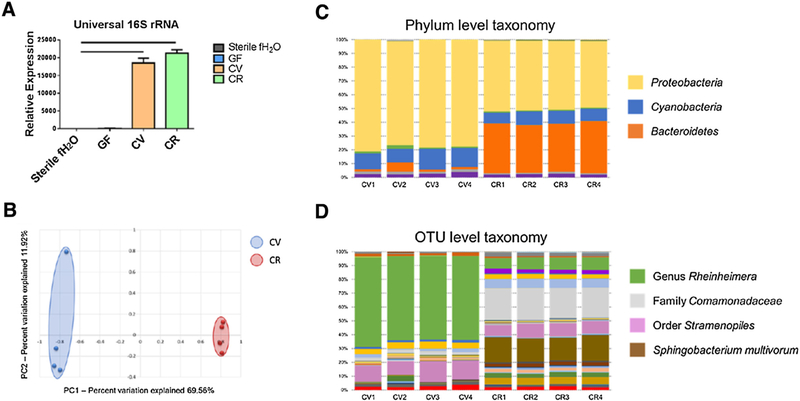
To confirm the microbial status of each group, five independent tests were performed to assure sterility and/or characterize microbiota of the zebrafish larvae groups. Blood agar plates were inoculated each day prior to behavior testing with 10 μL of water from each larval culture flask and cultured at 28.5 °C under aerobic conditions (Supp. Fig. 1A). At the end of the experiment (6 dpf), sterility was also tested by culturing water from the larvae flasks under anaerobic conditions at 28.5 °C in brain/heart infusion broth (which allows cultivation of a wide variety of fastidious microorganisms) (Supp. Fig. 1B). Culture techniques revealed no bacterial growth in any of the GF or media only flasks (Supp. Fig. 1A,B). In addition to traditional culture methods, molecular testing was conducted to assay for uncultivable bacteria. Microbial DNA was extracted from pooled zebrafish larvae and used as a template for quantitative real-time PCR (qPCR) using primers targeting universal 16 S rRNA bacterial genes. Since this approach is susceptible to false-positive results from free nucleic acid being detected in the absence of living microbes [43], all data were compared to autoclaved fish water that was never exposed to zebrafish larvae as a control. qPCR indicated no significant differences between GF larvae and autoclaved fish water, while CV and CR larvae had significantly greater abundance of the 16 S rRNA gene (Fig. 2A). Microbial DNA templates were also used for 16 S rRNA amplicon sequencing to determine which microbial taxa were present in the CV and CR groups. The relative abundance of the dominant taxa at both the phylum and operational taxonomic unit (otu) level demonstrate that even though similar taxa were detected, each group contained a distinct microbiota profile (Fig. 2C,D). Similarly, principal component analysis indicated that these groups harbored distinct, but consistent, microbial populations (Fig. 2B). This divergence of the microbiota is likely due to microbes colonizing the chorion which are washed away during sterilization of the CV embryos. A complete list and relative abundance of OTUs detected can be found in Table 1 of Ref. [52]. Lastly, a viability stain (ViaGram™, Molecular Probes) was performed according to manufacturer’s instruction on homogenized zebrafish larvae to inspect for viable microbial cells. In agreement with culture methods, viable bacteria were identified in CV and CR larvae, however, no viable bacteria were detected in GF larvae (Supp. Fig. 2).
Fig. 2.
Characterization of germ-free and conventionalized zebrafish larvae at 6 dpf. (A) Conventionalized (CV) and conventionally-raised (CR) larvae exhibit significantly increased 16 S rRNA levels relative to germ-free (GF) larvae and sterile fish water control levels. (B) Principal component analysis reveals distinct separation in microbial profiles between CV and CR larvae. (C,D) Relative abundances of phylum level and OTU level taxonomy illustrate compositional similarities within each group, and differences between CV and CR groups (legend at right). Data shown are represented by mean ± SEM (n = 12 larvae/sample from separate flasks for both qPCR and microbiota analysis). Bars denote p values ≤0.05 (one way ANOVA). A complete list of OTUs detected in each group is provided in Supplementary Table 1.
3.2. Microbiota modulates locomotor activity and anxiety-related behavior
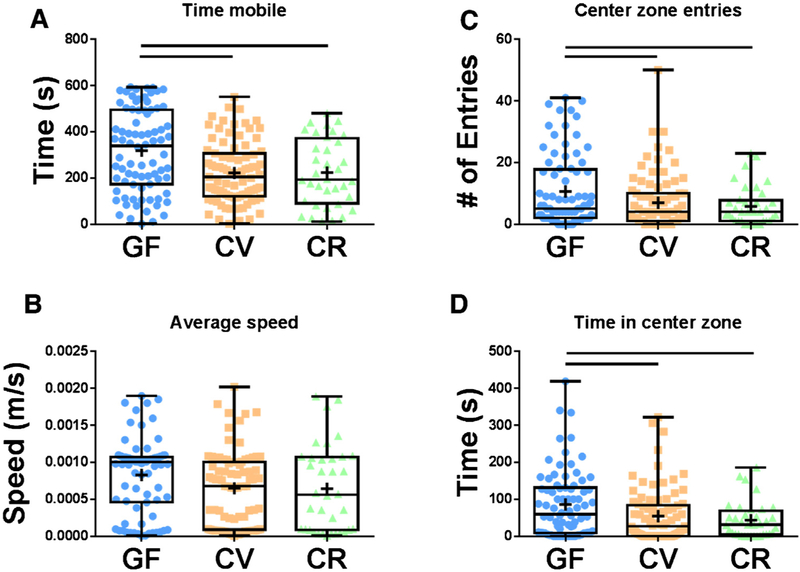
To determine if the microbiota influences anxiety-related behavior in zebrafish larvae, locomotor activity testing was performed, and thigmotactic behavior was assessed in larvae raised under conventional or sterile conditions. GF zebrafish displayed hyper-locomotor activity compared to CV and CR fish as measured by total time mobile, although no significant difference in average locomotion speed was observed (Fig. 3A,B). GF larvae also exhibited significantly less thigmotactic behavior than larvae reared under non-sterile conditions. This was evaluated by number of entries into the center zone and time spent in the center zone during the locomotor activity test (Fig. 3C,D). No differences were detected between the CR and CV cohorts indicating that although they have distinct microbiota profiles, the specific compositional differences between the groups do not drive behavioral differences. These results suggest that the microbiota plays an important role in loco-motor activity and anxiety-related behavior in zebrafish larvae.
Fig. 3.
Microbial modulation of locomotor activity and anxiety-related behavior. (A,B) At 6 dpf, germ-free (GF) zebrafish larvae display increased locomotor activity compared to larvae raised in a conventional environment (CV and CR). (C,D) GF larvae exhibit less thigmotactic behavior than CV and CR larvae as indicated by number of entries into the center zone (C) and time spent in the center zone (D). Data shown are represented by box and whisker plots showing individual data points (n = 36–87 larvae/group). Bars denote p values ≤0.05 (one way ANOVA).
3.3. The HPA (HPI) axis response to stress is influenced by the microbiota
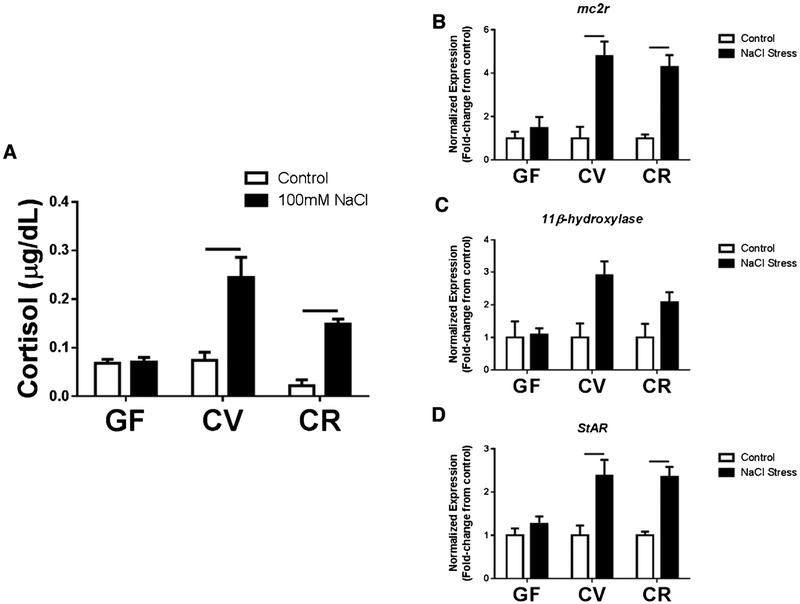
To investigate if the microbiota affects physiological responses to stress in zebrafish larvae, an osmotic stress test was performed and factors related to the HPI axis were measured. CV and CR larvae demonstrated the expected response to stress with a significant elevation in cortisol relative to untreated controls (Fig. 4A). Interestingly, GF larvae did not display an elevation in cortisol production in response to the stress test (Fig. 4A). As a secondary means of validating the physiological response to osmotic stress, qPCR was used to evaluate the expression of three genes associated with stress responses in both zebrafish and mammals, including melanocortin type 2 receptor (mc2r), 11β-hydroxylase, and steroidogenic acute regulatory protein (StAR). In agreement with cortisol production, larvae raised in non-sterile environments (i.e., CV and CR) had increased mc2r, 11β-hydroxylase, and StAR gene expression in response to stress, while GF fish displayed no signifi-cant changes relative to controls (Fig. 4 B–D). To determine if there is a critical time window during early life in which microbes are able to alter the stress response, zebrafish larvae were conventionalized at 0 dpf, 2 dpf (just before hatching), and 5 dpf, and then subjected to the stress test at 6 dpf. Although there was a slight trend toward decreased cortisol production in larvae conventionalized at 5 dpf, no significant difference was observed between larvae conventionalized at these later time points and CR larvae (Supp. Fig. 3). These results underscore the importance of exposure to microbes in mounting an appropriate response to stress, and suggest that microbial colonization within as little as 24 h prior to testing can revert the cortisol response phenotype.
Fig. 4.
Exposure to microbes allows for characteristic activation of HPI axis in response to a stressor in 6 dpf larvae. (A) Conventionally-raised (CV and CR) larvae demonstrate characteristic elevations of cortisol following an osmotic stress challenge whereas germ-free (GF) larvae exhibit a blunted response. (B,D) Expression of genes involved in activation of the HPI axis and cortisol production are also elevated in CV and CR larvae following a stressor while there is no change in GF larvae. Target gene expression were normalized to β-actin expression levels. Data shown are represented by mean ± SEM (n = 4 replicates with 14 pooled larvae/replicate for both ELISA data and qPCR data). Bars denote p values ≤ 0.05 (two way ANOVA).
3.4. Treatment with L. plantarum attenuates anxiety-related behavior in zebrafish larvae
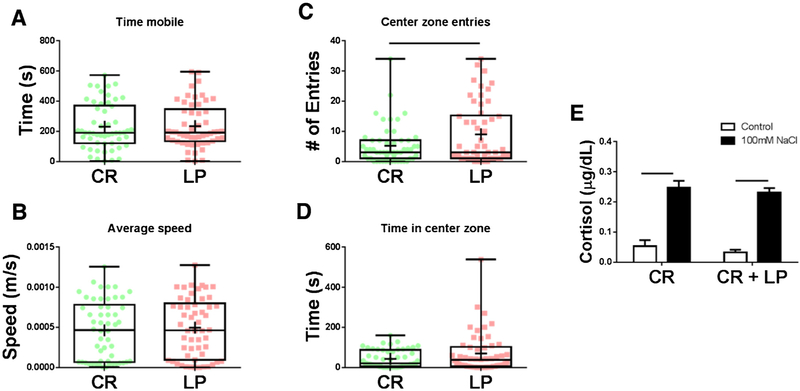
Certain probiotic Lactobacillus strains are known to reduce anxiety-related behavior and signs of stress in both mammals and zebrafish [24,32]. L. plantarum was of particular interest due to its ability to attenuate anxiety-related behavior in mice and due to its highly adherent colonization of zebrafish [33–35]. Supplementation with individual microbes known to alter behavior and stress responses is important to determine if microbial-induced behavior differences can be detected in larvae with an already developed and complex microbiota. To determine if L. plantarum has the ability to influence anxiety-related behavior in zebrafish larvae, CR fish were supplemented with either broth (CR) or L. plantarum (CR + LP), and thigmotactic behavior was assessed. Larvae colonized with L. plantarum exhibited significantly less thigmotactic behavior than broth-treated CR controls, with no differences detected in mobility time or average speed (Fig. 5A–D). These findings support the notion that specific commensal microbes have the ability to alleviate anxiety-related behavior. However, larvae treated with L. plantarum did not exhibit a significant difference in cortisol produced in response to osmotic stress relative to broth-treated CR fish (Fig. 5E).
Fig. 5.
L. plantarum reduces anxiety-related behavior in 6 dpf zebrafish larvae. (A–D) Treatment with L. plantarum did not alter basic locomotor activity (A,B), however, zebrafish larvae treated with L. plantarum (CR + LP) exhibited significantly less thigmotactic behavior (C,D) than broth-treated controls (CR). (E) L. plantarum treatment did not alter cortisol production following an osmotic stress challenge. Behavior data shown are represented by box and whisker plots showing individual data points, ELISA data are represented by mean ± SEM (n = 54 larvae/group for behavior data and n = 4 replicates with 14 pooled larvae/replicate for ELISA data). Bars denote p values ≤ 0.05 (student’s t-test for behavioral test data, two way ANOVA for ELISA data).
To investigate the effects on behavior attributable specifically to L. plantarum, GF zebrafish larvae were monoassociated with the probiotic bacterium (ML). Addition of L. plantarum alone completely diminished the hyper-locomotor phenotype displayed by the GF larvae (Supp. Fig. 4A– B). Anxiety-related behavior was similar comparing ML larvae and GF larvae, however, ML zebrafish larvae exhibited significantly less thigmotaxic behavior compared to CR zebrafish (Supp. Fig. 4C,D). Interestingly, ML zebrafish larvae seem to display an additive effect of the anxiolytic behaviors demonstrated by the LP and GF groups (Supp. Fig. 4C,D). Moreover, ML zebrafish larvae demonstrated a trend toward restoration of the blunted cortisol phenotype observed in GF larvae, although this was not statistically significant (Supp. Fig. 4E).
4. Discussion
The current study provides proof-of-principle that zebrafish are a valuable model in studies of microbial-mediated effects on neurological development and behavior. Similar to mammals that develop within the axenic environment of the uterus, zebrafish develop in an axenic environment within the protective chorion. At approximately 3 dpf, zebrafish larvae hatch from the chorion and begin to be colonized by microbes [43]. Microbiome studies of zebrafish have shown, similar to mice, that different microbial profiles are acquired from different institutions and vendors [45,53,54]. The microbiota of mice and humans is dominated by the phyla Firmicutes and Bacteroidetes, whereas the core micro-biota of adult zebrafish predominantly consists of bacteria from the Proteobacteria and Fusobacteria [55] phyla. However, studies have also shown similarities of the zebrafish microbiota and that of mammals, such as colonization of species from the genus Lacto-bacillus [32,34]. To our knowledge, the data in this study are the first to assess the microbiota of zebrafish larvae. Moreover, while the microbiota associated with the entire larvae was examined in the current study rather than the gut microbiota specifically, our data are consistent with previous studies investigating the gut micro-biota of adult zebrafish (i.e., consisting primarily of Proteobacteria and Fusobacteria) [53].
The recent use of gnotobiotic and germ-free (GF) animals has provided persuasive evidence of microbial involvement in brain signaling. Key findings demonstrate that the microbiota is essential for normal stress responsivity, anxiety-related behaviors, and many other factors that are associated with CNS signaling [56]. The data shown are congruent with previous research performed in mice showing that GF mice exhibit increased locomotor activity compared to conventionally-raised mice [23]. Microbiota modulation of anxiety-related behavior is also supportive of previous studies using rodent species. In the current studies, GF zebrafish larvae displayed less thigmotactic behavior than conventionally-raised larvae. This is similar to rodent work that revealed GF mice display reduced anxiety-related behavior relative to conventionally housed mice [23,30,57,58]. However, opposing data exist indicating that GF mice of particular inbred strains exhibit increased anxiety-like behavior [58,59]. This discrepancy may be due to genetic influences since there appears to be a correlation between the behavioral phenotype and whether the tests were performed in outbred stocks or inbred strains of mice. Alternatively, differential effects of the microbiota on host anxiety-related behavior may be due to differences in the composition or function of microbiota colonizing the host.
Anxiety is known to activate the HPA axis, involved in responding to psychological and physical stressors. Here, GF zebrafish larvae exhibited a significantly reduced cortisol response to an osmotic stress challenge when compared to conventionally-raised larvae. These results are inconsistent with previous mouse studies demonstrating that GF mice have an elevation in hypothalamic corticosterone in response to restraint stress [60]. These differences are again possibly due to genetic confounds, or due to the nature of the type of stressors used in each study. Dissimilarity could also be attributed to the HPI axis in zebrafish giving rise to the gluco-corticoid cortisol, whereas rodents utilize the HPA axis to produce corticosterone during a stress response. Other potential explanations for the incongruities with rodent studies could be related to the differences in taxa that colonize zebrafish larvae versus those that predominate in mammals as noted above or due to the developmental stages at which the larvae were tested. Nonetheless, our results are congruent with the hypothesis that a normal microbiota is required for characteristic responses to stress.
Manipulation of a well-established, complex microbiota has also been shown to alter stress-related physiology and behavior. Many probiotic studies have showed that treatment with various Lacto-bacillus and/or Bifidobacterium strains can alleviate anxiety- and depressive-like behavior and mitigate stress responses [24,61–63]. Zebrafish, being an ideal model for high-throughput drug screening, have also been used to evaluate the effects of particular probiotic strains[32,34,64,65]. However, the influence of probiotics on stress responses and behavior in zebrafish larvae is not yet well-characterized. Probiotic administration into zebrafish larvae is achieved by injecting the bacteria directly into the water of the housing flasks. Although larvae at this age survive on nutrients from their yolk sac and do not actually eat, bacteria from the environment readily make their way to the GI tract during development. Previous studies have shown that L. plantarum and other lactic acid bacteria can colonize the gut of larvae well before the onset of feeding [66]. In the present studies, larvae supplemented with L. plantarum showed decreased anxiety-related behavior relative to conventionally-raised fish. These results support previous rodent and human studies suggesting that Lactobacillus reduces anxiety-related behavior. In conclusion, the results of this study strengthen previous findings showing that the microbiota plays a major role in responding to stress and stress-related behavior. Further investigation is required to elucidate the exact mechanisms through which microbes communicate to the CNS to modulate behavior and stress responses. However, the present findings set a foundation for the use of zebrafish larvae in neuroimmune studies.
Supplementary Material
HIGHLIGHTS.
Microbiota modulates anxiety-related behavior in zebrafish larvae.
Larval stress responses are dramatically blunted in absence of microbiota.
L. plantarum attenuates anxiety-related behavior in conventionally-raised larvae.
Zebrafish larvae are a valuable tool for research on the gut-brain axis.
Acknowledgements
The authors would like to thank Holly M. Doerr, Julia E. Karpinski, and Agata K. Grzelak for assistance with experimental procedures, and Miriam Hankins for assistance with animal husbandry.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bbr.2016.05.040.
References
- [1].Richendrfer H, Pelkowski SD, Colwill RM, Creton R, On the edge: pharmacological evidence for anxiety-related behavior in zebrafish larvae, Behav. Brain Res 228 (2012) 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Li Q, Lin J, Zhang Y, Liu X, Chen XQ, Xu MQ, et al. , Differential behavioral responses of zebrafish larvae to yohimbine treatment, Psychopharmacology 232 (2015) 197–208. [DOI] [PubMed] [Google Scholar]
- [3].Andersson MA, Ek F, Olsson R, Using visual lateralization to model learning and memory in zebrafish larvae, Sci. Rep 5 (2015) 8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Colwill RM, Creton R, Imaging escape and avoidance behavior in zebrafish larvae, Rev. Neurosci 22 (2011) 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Engeszer RE, Barbiano LA, Ryan MJ, Parichy DM, Timing and plasticity of shoaling behaviour in the zebrafish, Danio rerio, Anim. Behav 74 (2007) 1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Giacomini NJ, Rose B, Kobayashi K, Guo S, Antipsychotics produce locomotor impairment in larval zebrafish, Neurotoxicol. Teratol 28 (2006) 245–250. [DOI] [PubMed] [Google Scholar]
- [7].Treit D, Fundytus M, Thigmotaxis as a test for anxiolytic activity in rats, Pharmacol. Biochem. Behav 31 (1988) 959–962. [DOI] [PubMed] [Google Scholar]
- [8].Sharma S, Coombs S, Patton P, Burt de Perera T, The function of wall-following behaviors in the Mexican blind cavefish and a sighted relative, the Mexican tetra (Astyanax), J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol 195 (2009) 225–240. [DOI] [PubMed] [Google Scholar]
- [9].Champagne DL, Hoefnagels CC, de Kloet RE, Richardson MK, Translating rodent behavioral repertoire to zebrafish (Danio rerio): relevance for stress research, Behav. Brain Res 214 (2010) 332–342. [DOI] [PubMed] [Google Scholar]
- [10].Colwill RM, Creton R, Locomotor behaviors in zebrafish (Danio rerio) larvae, Behav. Process 86 (2011) 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lopez-Patino MA, Yu L, Cabral H, Zhdanova IV, Anxiogenic effects of cocaine withdrawal in zebrafish, Physiol. Behav 93 (2008) 160–171. [DOI] [PubMed] [Google Scholar]
- [12].Kallai J, Makany T, Csatho A, Karadi K, Horvath D, Kovacs-Labadi B, et al. , Cognitive and affective aspects of thigmotaxis strategy in humans, Behav. Neurosci 121 (2007) 21–30. [DOI] [PubMed] [Google Scholar]
- [13].Schnorr SJ, Steenbergen PJ, Richardson MK, Champagne DL, Measuring thigmotaxis in larval zebrafish, Behav. Brain Res 228 (2012) 367–374. [DOI] [PubMed] [Google Scholar]
- [14].Peng X, Lin J, Zhu Y, Liu X, Zhang Y, Ji Y, et al. , Anxiety-related behavioral responses of pentylenetetrazole-treated zebrafish larvae to light-dark transitions, Pharmacol. Biochem. Behav (2016). [DOI] [PubMed] [Google Scholar]
- [15].Baiamonte M, Parker MO, Vinson GP, Brennan CH, Sustained effects of developmental exposure to ethanol on zebrafish anxiety-like behaviour, PLoS One 11 (2016) e0148425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu X, Lin J, Zhang Y, Peng X, Guo N, Li Q, Effects of diphenylhydantoin on locomotion and thigmotaxis of larval zebrafish, Neurotoxicol. Teratol 53 (2016) 41–47. [DOI] [PubMed] [Google Scholar]
- [17].Richendrfer H, Pelkowski SD, Colwill RM, Creton R, Developmental sub-chronic exposure to chlorpyrifos reduces anxiety-related behavior in zebrafish larvae, Neurotoxicol. Teratol 34 (2012) 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Alsop D, Vijayan MM, Molecular programming of the corticosteroid stress axis during zebrafish development, Comp. Biochem. Physiol. Part A Mol. Integ. Physiol 153 (2009) 49–54. [DOI] [PubMed] [Google Scholar]
- [19].Yeh CM, Glock M, Ryu S, An optimized whole-body cortisol quantification method for assessing stress levels in larval zebrafish, PLoS One 8 (2013) e79406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].To TT, Hahner S, Nica G, Rohr KB, Hammerschmidt M, Winkler C, et al. , Pituitary-interrenal interaction in zebrafish interrenal organ development, Mol. Endocrinol 21 (2007) 472–485. [DOI] [PubMed] [Google Scholar]
- [21].Alsop D, Vijayan MM, Development of the corticosteroid stress axis and receptor expression in zebrafish, Am. J. Physiol. Regul. Integr. Comp. Physiol 294 (2008) R711–R719. [DOI] [PubMed] [Google Scholar]
- [22].Aluru N, Vijayan MM, Aryl hydrocarbon receptor activation impairs cortisol response to stress in rainbow trout by disrupting the rate-limiting steps in steroidogenesis, Endocrinology 147 (2006) 1895–1903. [DOI] [PubMed] [Google Scholar]
- [23].Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. , Normal gut microbiota modulates brain development and behavior, Proc. Natl. Acad. Sci. U. S. A 108 (2011) 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. , Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve, Proc. Natl. Acad. Sci. U. S. A 108 (2011) 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cryan JF, Dinan TG, Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour, Nat. Rev. Neurosci 13 (2012) 701–712. [DOI] [PubMed] [Google Scholar]
- [26].Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF, Microbiota and neurodevelopmental windows: implications for brain disorders, Trends Mol. Med 20 (2014) 509–518. [DOI] [PubMed] [Google Scholar]
- [27].Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M, Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation, Brain Behav. Immun 25 (2011) 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].de Theije CG, Wopereis H, Ramadan M, van Eijndthoven T, Lambert J, Knol J, et al. , Altered gut microbiota and activity in a murine model of autism spectrum disorders, Brain Behav. Immun 37 (2014) 197–206. [DOI] [PubMed] [Google Scholar]
- [29].Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. , Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders, Cell 155 (2013) 1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. , The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner, Mol. Psychiatry 18 (2013) 666–673. [DOI] [PubMed] [Google Scholar]
- [31].Cryan JF, O’Mahony SM, The microbiome-gut-brain axis: from bowel to behavior, Neurogastroenterol. Motil 23 (2011) 187–192. [DOI] [PubMed] [Google Scholar]
- [32].Gioacchini G, Giorgini E, Olivotto I, Maradonna F, Merrifield DL, Carnevali O, The influence of probiotics on zebrafish Danio rerio innate immunity and hepatic stress, Zebrafish 11 (2014) 98–106. [DOI] [PubMed] [Google Scholar]
- [33].Liu YW, Liu WH, Wu CC, Juan YC, Wu YC, Tsai HP, et al. , Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naive adult mice, Brain Res 1631 (2016) 1–12. [DOI] [PubMed] [Google Scholar]
- [34].Zhou ZG, Wang WW, Liu WS, Gatlin DM, Zhang YT, Yao B, et al. , Identification of highly-adhesive gut Lactobacillus strains in zebrafish (Danio rerio) by partial rpoB gene sequence analysis, Aquaculture 370 (2012) 150–157. [Google Scholar]
- [35].Wang Y, Ren Z, Fu L, Su X, Two highly adhesive lactic acid bacteria strains are protective in zebrafish infected with Aeromonas hydrophila by evocation of gut mucosal immunity, J. Appl. Microbiol 120 (2016) 441–451. [DOI] [PubMed] [Google Scholar]
- [36].Sampson TR, Mazmanian SK, Control of brain development, function, and behavior by the microbiome, Cell host Microbe. 17 (2015) 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kabouridis PS, Lasrado R, McCallum S, Chng SH, Snippert HJ, Clevers H, et al. , Microbiota controls the homeostasis of glial cells in the gut lamina propria, Neuron 85 (2015) 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Olsson C, Holmberg A, Holmgren S, Development of enteric and vagal innervation of the zebrafish (Danio rerio) gut, J. Comp. Neurol 508 (2008) 756–770. [DOI] [PubMed] [Google Scholar]
- [39].Shepherd I, Eisen J, Development of the zebrafish enteric nervous system, Methods Cell Biol 101 (2011) 143–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lyons DA, Naylor SG, Mercurio S, Dominguez C, Talbot WS, KBP is essential for axonal structure, outgrowth and maintenance in zebrafish, providing insight into the cellular basis of Goldberg-Shprintzen syndrome, Development 135 (2008) 599–608. [DOI] [PubMed] [Google Scholar]
- [41].Tobin JL, Di Franco M, Eichers E, May-Simera H, Garcia M, Yan J, et al. , Inhibition of neural crest migration underlies craniofacial dysmorphology and Hirschsprung’s disease in Bardet-Biedl syndrome, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 6714–6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jemielita M, Taormina MJ, Burns AR, Hampton JS, Rolig AS, Guillemin K, et al. , Spatial and temporal features of the growth of a bacterial species colonizing the zebrafish gut, mBio 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pham LN, Kanther M, Semova I, Rawls JF, Methods for generating and colonizing gnotobiotic zebrafish, Nat. Protoc 3 (2008) 1862–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rendueles O, Ferrieres L, Fretaud M, Begaud E, Herbomel P, Levraud JP, et al. , A new zebrafish model of oro-intestinal pathogen colonization reveals a key role for adhesion in protection by probiotic bacteria, PLoS Pathog. 8 (2012) e1002815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ericsson AC, Davis JW, Spollen W, Bivens N, Givan S, Hagan CE, et al. , Effects of vendor and genetic background on the composition of the fecal microbiota of inbred mice, PLoS One 10 (2015) e0116704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Magoc T, Salzberg SL, FLASH: fast length adjustment of short reads to improve genome assemblies, Bioinformatics 27 (2011) 2957–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kuczynski J, Stombaugh J, Walters WA, Gonzalez A, Caporaso JG, Knight R, Using QIIME to Analyze 16 S rRNA Gene Sequences from Microbial Communities. Current Protocols in Bioinformatics/Editoral Board, Andreas D. Baxevanis et al. Chapter 10: Unit 10, 2011, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. , Gapped BLAST and PSI-BLAST: a new generation of protein database search programs, Nucleic Acids Res. 25 (1997) 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. , Greengenes, a chimera-checked 16 S rRNA gene database and workbench compatible with ARB, Appl. Environ. Microbiol 72 (2006) 5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ingebretson JJ, Masino MA, Quantification of locomotor activity in larval zebrafish: considerations for the design of high-throughput behavioral studies, Front. Neural Circuits 7 (2013) 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].De Marco RJ, Groneberg AH, Yeh CM, Trevino M, Ryu S, The behavior of larval zebrafish reveals stressor-mediated anorexia during early vertebrate development, Front. Behav. Neurosci 8 (2014) 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Davis DJ, Bryda EC, Gillespie CH, Ericsson AC, Microbiota variation between conventionalized and conventionally raised zebrafish larvae, Data in Brief (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hart ML, Meyer A, Johnson PJ, Ericsson AC, Comparative evaluation of DNA extraction methods from feces of multiple host species for downstream next-generation sequencing, PLoS One 10 (2015) e0143334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zac Stephens W, Burns AR, Stagaman K, Wong S, Rawls JF, Guillemin K, et al. , The composition of the zebrafish intestinal microbial community varies across development, ISME J. 10 (2016) 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, et al. , Evidence for a core gut microbiota in the zebrafish, ISME J. 5 (2011) 1595–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Luczynski P, McVey Neufeld KA, Oriach CS, Clarke G, Dinan TG, Cryan JF, Growing up in a bubble: using germ- free animals to assess the influence of the gut microbiota on brain and behavior, Int. J. Neuropsychopharmacol (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Neufeld KM, Kang N, Bienenstock J, Foster JA, Reduced anxiety-like behavior and central neurochemical change in germ-free mice, Neurogastroenterol. Motil 23 (2011) 119–255 [DOI] [PubMed] [Google Scholar]
- [58].Selkrig J, Wong P, Zhang X, Pettersson S, Metabolic tinkering by the gut microbiome: implications for brain development and function, Gut Microbes. 5 (2014) 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. , The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice, Gastroenterology 141 (2011) 599–609 [DOI] [PubMed] [Google Scholar]
- [60].Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. , Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice, J. Physiol 558 (2004) 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, et al. , Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects, Bri J. Nutr 105 (2011) 755–764. [DOI] [PubMed] [Google Scholar]
- [62].Savignac HM, Kiely B, Dinan TG, Cryan JF, Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice, Neurogastroenterol. Motil 26 (2014) 1615–1627. [DOI] [PubMed] [Google Scholar]
- [63].Savignac HM, Tramullas M, Kiely B, Dinan TG, Cryan JF, Bifidobacteria modulate cognitive processes in an anxious mouse strain, Behav. Brain Res 287 (2015) 59–72. [DOI] [PubMed] [Google Scholar]
- [64].Carnevali O, Avella MA, Gioacchini G, Effects of probiotic administration on zebrafish development and reproduction, Gen. Comp. Endocrinol 188 (2013) 297–302. [DOI] [PubMed] [Google Scholar]
- [65].Gioacchini G, Maradonna F, Lombardo F, Bizzaro D, Olivotto I, Carnevali O, Increase of fecundity by probiotic administration in zebrafish (Danio rerio), Reproduction 140 (2010) 953–959. [DOI] [PubMed] [Google Scholar]
- [66].Russo P, Iturria I, Mohedano ML, Caggianiello G, Rainieri S, Fiocco D, et al. , Zebrafish gut colonization by mCherry-labelled lactic acid bacteria, Appl. Microbiol. Biotechnol 99 (2015) 3479–3490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.