Abstract
Anticipation of potentially threatening social situations is a key process in social anxiety disorder (SAD). In other anxiety disorders, recent research of neural correlates of anticipation of temporally unpredictable threat suggests a temporally dissociable involvement of amygdala and bed nucleus of the stria terminalis (BNST) with phasic amygdala responses and sustained BNST activation. However, the temporal profile of amygdala and BNST responses during temporal unpredictability of threat has not been investigated in patients suffering from SAD. We used functional magnetic resonance imaging (fMRI) to investigate neural activation in the central nucleus of the amygdala (CeA) and the BNST during anticipation of temporally unpredictable aversive (video camera observation) relative to neutral (no camera observation) events in SAD patients compared to healthy controls (HC). For the analysis of fMRI data, we applied two regressors (phasic/sustained) within the same model to detect temporally dissociable brain responses. The aversive condition induced increased anxiety in patients compared to HC. SAD patients compared to HC showed increased phasic activation in the CeA and the BNST for anticipation of aversive relative to neutral events. SAD patients as well as HC showed sustained activity alterations in the BNST for aversive relative to neutral anticipation. No differential activity during sustained threat anticipation in SAD patients compared to HC was found. Taken together, our study reveals both CeA and BNST involvement during threat anticipation in SAD patients. The present results point towards potentially SAD-specific threat processing marked by elevated phasic but not sustained CeA and BNST responses when compared to HC.
Keywords: FMRI, Threat anticipation, Social anxiety disorder, Bed nucleus of stria terminalis, Amygdala
Highlights
-
•
fMRI in SAD during anticipation of temporally unpredictable aversive events.
-
•
Anticipation of social observation induces increased anxiety in SAD patients.
-
•
SAD patients show elevated phasic activity in fundamental anxiety network regions.
-
•
Evidence of SAD-specific threat processing.
1. Introduction
Social anxiety disorder (SAD) is marked by persistent fear or anxiety regarding social or performance situations in which the individual is exposed to possible negative evaluation by others, e.g. meeting unfamiliar people, being observed while eating or giving a public speech (American Psychiatric Association, 2013; Heimberg et al., 2014). Furthermore, the mere anticipation of such situations can lead to restrictions in everyday life, avoidance behavior and impairment in social or occupational areas of functioning (American Psychiatric Association, 2013; Grupe and Nitschke, 2013). Because of the key role of maladaptive anticipation processes in the maintenance of the pathophysiology of SAD (Hofmann, 2007), insight into its underlying neural correlates is essential for a better understanding of this disorder and future directions in therapeutic interventions (Avery et al., 2016; Hammack et al., 2009).
Individuals with SAD seem to be characterized by a hyperactive fear circuitry (amygdala, insula, anterior cingulate [ACC] and prefrontal cortex [PFC]) and decoupled medial parietal and occipital regions (posterior cingulate cortex [PCC], precuneus, cuneus) during threat processing (Brühl et al., 2014; Miskovic and Schmidt, 2012). This neural pattern suggests a dysbalance between heightened threat processing on the one hand and disturbed cognitive control and emotion regulation on the other hand (Brühl et al., 2014; Cremers and Roelofs, 2016).
Only few studies investigated the neural correlates of anticipatory anxiety in SAD patients. Although partially afflicted with methodological limitations, findings of these studies point towards a maladaptive involvement of similar emotion processing and emotion regulation regions as during threat processing. An early positron emission tomography study compared SAD patients within a 2.5 min private speech, either performed before (anticipation condition) or after (control condition) a public speech. Enhanced activity was reported in the dorsolateral prefrontal cortex (dlPFC), inferior temporal cortex and in the amygdaloid-hippocampal region (Tillfors et al., 2002). However, conclusions drawn from this study are limited due to a small sample size (n = 9), the lack of a control group and the presence of actual speaking during data acquisition. Lorberbaum and colleagues (Lorberbaum et al., 2004) used functional magnetic resonance imaging (fMRI) to compare brain activity in SAD patients to that of HC during a 12 s public speech anticipation period relative to rest. Enhanced activity was found in the striatum, pons, amygdala/uncus/parahippocampus, insula, temporal pole and reduced activity in the dorsal ACC and PFC. Again, the small sample size (n = 8) and the usage of a rest period instead of a neutral anticipation control condition hamper firm conclusions. Another fMRI - study with a larger sample size (n = 17) investigated the anticipation of a public speech during a 40 s anticipation period compared to the anticipation of a “computer test” control condition (Boehme et al., 2014). SAD patients relative to HC showed increased activation of the insula and decreased activation in the ventral striatum. Solely for the first half (20s) of the anticipation period, increased amygdala activation was reported. The most recent fMRI study examining anticipation processes in SAD (Davies et al., 2017) used a 90 s public speech anticipation period compared to a 90 s control anticipation period. SAD patients relative to HC showed less variability in amygdala activity during speech anticipation but no mean differences in amygdala, insula, ventral striatum and dorsal ACC.
Besides these disorder-specific paradigms, another study (Brühl et al., 2011) investigated the anticipation (7 s) of disorder-unspecific negative, positive and neutral pictures in SAD patients. In addition to predictable positive, negative and neutral conditions announced by matching anticipation cues, an unpredictable “unknown” condition was introduced, in which the cue did not indicate the emotional valence of the following picture. Anticipation of negative compared to neutral pictures revealed higher activations for SAD patients relative to HC in medial and dorsolateral PFC and in occipitotemporal regions. When comparing the anticipation of unknown to neutral pictures, SAD patients showed higher activations in occipitotemporal regions.
To date, none of the existing studies investigating anticipatory processes in SAD patients varied the temporal predictability of threat. However, maladaptive and heightened responses under conditions of uncertainty about upcoming threat were highlighted as a key feature across anxiety disorders (Grupe and Nitschke, 2013). Unpredictability (or uncertainty) of threat is related to higher subjective and physiological stress responses (de Berker et al., 2016), increased threat attention, hypervigilance, heightened reactivity and biased expectancies of aversion (Grupe and Nitschke, 2011; Sarinopoulos et al., 2010). On a neural level, the amygdala (Herry et al., 2007; Whalen, 2007) and more specifically the central nucleus of the amygdala (CeA) and the BNST, as parts of the so-called extended amygdala (Alvarez et al., 2011; Davis et al., 2010; Grupe and Nitschke, 2013; Shackman and Fox, 2016) are supposed to be central in the response to threat uncertainty. Several recent studies provide concurrent evidence for hyperactivation of amygdala and BNST during anticipation of temporally unpredictable threat in healthy volunteers (Grupe et al., 2013; Herrmann et al., 2016), generalized anxiety disorder (Buff et al., 2017), panic disorder (Brinkmann et al., 2017a) and post-traumatic stress disorder (Brinkmann et al., 2017b). These findings strongly support the assumption of heightened responding to temporally unpredictable aversiveness for patients with anxiety- and stress-related disorders. Moreover, these studies revealed that both, the amygdala and the BNST, appear to follow a temporal dissociation of activity during threat anticipation, such that they consistently reported transient amygdala activity immediately after threat anticipation cue onset and sustained BNST activation over the course of the threat anticipation interval (Brinkmann et al., 2017a, Brinkmann et al., 2017b; Buff et al., 2017; Grupe et al., 2013; Herrmann et al., 2016). These findings were interpreted as transient vigilance and threat detection response through amygdala activity (Grupe and Nitschke, 2013; Phelps and LeDoux, 2005) as a reaction to the threat-indicating cue, turning into a longer-lasting state of sustained fear reflected by enhanced BNST activity (Davis et al., 2010). Especially CeA and BNST are supposed to be highly interconnected (Calhoon and Tye, 2015; Davis et al., 2010; Fox et al., 2015; Gungor and Paré, 2016; Shackman and Fox, 2016; Tovote et al., 2015; Walker et al., 2003). However, recent investigations question a strict temporal dissociation between phasic amygdala and sustained BNST responding and suggest that the BNST is equally involved in phasic threat processing (Brinkmann et al., 2018; Fox and Shackman, 2017; Gungor and Paré, 2016; Shackman and Fox, 2016).
Given this growing and highly relevant research field, studies investigating BNST responding especially during temporal unpredictability of threat and the temporal dissociation of amygdala and BNST during threat anticipation also in SAD patients are highly warranted. The present functional magnetic resonance imaging (fMRI) study investigated CeA and BNST activation during anticipation of temporally unpredictable aversive events in SAD patients compared to healthy controls (HC). The SAD-specific threat induction was operationalized via camera observation, a method known to induce anxiety, alertness and tension in socially anxious individuals and SAD patients (George and Stopa, 2008; Giménez et al., 2012). This passive method was favored over a speech anticipation paradigm (Boehme et al., 2014; Davies et al., 2017; Glassman et al., 2014; Lorberbaum et al., 2004; Tillfors et al., 2002) to avoid active motion throughout the experiment and thus to establish a better comparability to equally passive picture- or sound-based stimuli used in threat anticipation paradigms investigating other anxiety disorders (Brinkmann et al., 2017a, Brinkmann et al., 2017b; Buff et al., 2017). In order to create uncertainty about stimulus occurrences (also referred to as “manipulation of threat imminence”, (Fox and Shackman, 2017)), cue-stimulus-intervals in the present instructed threat anticipation paradigm varied in length. Following the idea of temporally dissociable roles for amygdala and BNST (Grupe et al., 2013; Herrmann et al., 2016) we modeled a phasic anticipation blood oxygenation level dependent (BOLD) signal time course immediately after cue onset and a sustained anticipation time course over the whole anticipation period. According to research in other anxiety disorders, we expected increased phasic activation in the CeA as well as increased sustained activation in the BNST for SAD versus healthy controls during anticipation of aversive relative to neutral events.
2. Methods
2.1. Subjects
In total 22 SAD patients and 22 HC were recruited via public advertisement and from a collaborating outpatient clinic. Three SAD patients with a comorbid major depression and a score > 30 on the Beck Depression Inventory (Hautzinger et al., 2006) indicating a severe depression as well as one HC with elevated scores in all SAD questionnaires (> M + 2 SD) were excluded from the study. Our final sample comprised 19 SAD patients (12 female) and 21 healthy controls (13 female) matched for gender (χ2(1) = 0.007, p = 1), age and education (Table 1). All participants were right-handed and had normal or corrected-to-normal vision and no history of neurological diseases or psychotic disorders. Participants meeting the general MRI requirements were screened by an experienced psychologist via standardized clinical interview (German version of the SCID; Wittchen et al., 1997). Included HC were free of any diagnosis. SAD patients met the criteria of a social anxiety disorder according to DSM-IV-R as main diagnosis.
Table 1.
Demographic and questionnaire data.
| SAD (M ± SD) | HC (M ± SD) | t-value | p-value | |
|---|---|---|---|---|
| Age | 29.47 ± 8.89 | 27.14 ± 5.93 | 0.98 | .33 |
| Education (years in school) | 12.83 ± 1.1 | 12.90 ± 0.77 | −0.24 | .81 |
| LSAS | 71.26 ± 13.19 | 9.48 ± 7.95 | 17.71 | ≤ .001 |
| SPS | 33.68 ± 11.64 | 2.62 ± 3.49 | 11.19 | ≤ .001 |
| SIAS | 47.32 ± 12.62 | 9.9 ± 5.92 | 11.80 | ≤ .001 |
| BDI | 12.05 ± 7.62 | 0.55 ± 0.89 | 6.54 | ≤ .001 |
| STAI-T | 52.63 ± 9.64 | 28.75 ± 4.01 | 10.00 | ≤ .001 |
Note: SAD, social anxiety disorder; HC, healthy controls; LSAS, Liebowitz Social Anxiety Scale (Stangier and Heidenreich, 2004); SPS, Social Phobia Scale (Stangier et al., 1999); SIAS, Social Interaction Anxiety Scale (Stangier et al., 1999); BDI, Beck Depression Inventory II (Hautzinger et al., 2006); STAI-T, State-Trait Anxiety Inventory (Spielberger et al., 1970).
Comorbid diagnoses (multiple entries possible) comprised current major depression (n = 5, BDI-score < 30), obsessive-compulsive disorder (n = 1), general anxiety disorder (n = 1) and specific phobia (n = 1). Three SAD patients took antidepressant medication. Liebowitz-Social-Anxiety-Scale (Stangier and Heidenreich, 2004), Social Interaction Anxiety Scale (Stangier et al., 1999), Social Phobia Scale (Stangier et al., 1999), and the State-Trait-Anxiety-Inventory (Spielberger et al., 1970) supported the diagnosis of SAD. Assessment of depression severity by the Beck Depression Inventory (Hautzinger et al., 2006) indicated minimal depressive symptoms in the patients sample. SAD patients scored higher than HC in all questionnaires listed (Table 1). Written informed consent was obtained from each participant prior to the experiment. The study was approved by the local ethics committee and conducted in compliance with the Declaration of Helsinki.
2.2. Experimental paradigm
The experiment consisted of 11 aversive and 11 neutral trials. Each trial started with the presentation of a cue (either “Δ” or “Ο”, counterbalanced across participants) either signaling that at any time in the following period the camera would switch on (aversive condition, e.g. signaled by “Δ”) or stay switched off (control condition, e.g. signaled by “Ο”). To induce temporal unpredictability, anticipatory intervals had variable durations (2 × 3 s, 1 × 5 s, 1 × 10 s, 7 × 16s). After the anticipatory interval, a symbol was presented to inform the participants that the camera was now switched on (camera symbol, 10 s) or that it remained switched off (crossed camera symbol, 10 s). Participants were briefed about the assignment of cues to conditions via standardized screen instructions before the experiment. Furthermore, they were informed that their faces would be recorded by a video camera when the camera symbol was presented and that two experimenters were going to judge their facial rubescence (Becker et al., 2017; Simon et al., 2014). For that purpose a camera dummy was installed on the MRI head coil.
2.3. Behavioral data
After fMRI scanning participants were requested to rate their feelings associated to both stimuli pairs shown during scanning procedure (“Δ” vs. “Ο” and “camera on” vs. “camera off”) on three nine-point Self-Assessment Manikin Scales (Bradley and Lang, 1994) regarding valence, arousal and anxiety (valence: 1 = very negative, 5 = neutral, 9 = very positive; arousal: 1 = not arousing, 9 = very arousing; anxiety: 1 = not anxious, 9 = very anxious). Behavioral data were analyzed by two-way mixed measures analysis of variance (ANOVA) and post-hoc t-tests including Welch's correction for unequal variances and Bonferroni correction for multiple comparisons (corrected significance level p < .004) using SPSS software (Version 23.0, IBM Corp., Armonk, N.Y., USA).
2.4. fMRI data
Anatomical and functional MRI was performed on a 3 T scanner (“Magnetom PRISMA”, Siemens Medical Systems, Erlangen, Germany) using a 20-channel head-neck coil. Following a high resolution T1-weighted anatomical scan with 192 slices, functional data were acquired with a T2*-weighted echo-planar sequence (TE = 30 ms, flip angle = 90°, matrix = 92 × 92, FOV = 208 mm2, TR = 2080 ms) consisting of 410 volumes with 36 axial slices (thickness = 3 mm, gap = 0.3 mm, in plane resolution = 2.26 × 2.26 mm).
Functional data were preprocessed and analyzed using BrainVoyager QX (Version 2.8.4; Brain Innovation, Maastricht, the Netherlands). The first four volumes were skipped from each run to allow for magnetization equilibrium before image acquisition. Data preprocessing comprised correction for slice time errors and movement artifacts, co-registration of anatomical and functional data, normalization to fit Talairach space (Talairach and Tournoux, 1988) and spatial smoothing (6 mm full-width at half maximum [FWHM] Gaussian kernel) as well as temporal filtering (high pass filter: 10 cycles per run; low pass filter: 2.8 s; linear trend removal).
The preprocessed data were analyzed using multiple linear regression of the signal time course at each voxel. The expected BOLD signal change for each condition was modeled with a canonical double-gamma hemodynamic response function (HRF). We calculated a general linear model (GLM) including two temporally distinct regressors for the anticipation interval. The HRF of the “phasic” regressor was limited to the onset (first second), whereas the “sustained” regressor was modeled over the whole anticipation interval. Additionally, the six movement parameters and the presentation of camera symbols were modeled as predictors of no interest. %-standardized predictor estimates based on voxel-wise statistical maps for each participant were calculated. Random effects analysis with adjustment for autocorrelation following a global AR(2) model across the individual predictor estimates for planned contrasts was performed. Average beta values were estimated for each subject within a-priori defined regions of interest (ROIs).
The bilateral centromedial amygdala ROI was defined on the basis of cytoarchitectonic probability maps (Amunts et al., 2005) provided by the Anatomy toolbox (Eickhoff et al., 2005). For the bilateral BNST ROI, we used a BNST mask based on a multisubject manual BNST segmentation method elaborated and provided on neurovault.org by Theiss and colleagues (Theiss et al., 2017). MNI-coordinates for both ROIs were transformed into Talairach space with ICBM2tal (Lancaster et al., 2007).
We calculated two-factor analysis of variance (ANOVA) with repeated measures including group affiliation (SAD patients/HC) and condition (aversive / neutral anticipation) separately for each ROI and each regressor of interest using SPSS software (Version 25.0, IBM Corp., Armonk, N.Y., USA). An alpha-level < .05 was considered statistically significant.
For subsequent whole brain analysis, we used a cluster-based permutation (CBF) approach based on an in-house Matlab script (Release 2014a; The MathWorks, Inc., Natick, Massachusetts) to correct for multiple comparisons. This non-parametric method is assumed to result in adequate control over the familywise alpha level (Eklund et al., 2016). We analyzed the interaction effect of group (SAD, HC) by anticipation condition (aversive, neutral) separately for phasic and sustained regressors. Voxel-level threshold was set to p < .005. Testing was performed with 1000 permutations. For each permutation, individual beta maps were randomly assigned without replacement to one of the groups. Cluster mass was assessed by adding the absolute values of all t-values in neighboring significant voxels. The observed cluster mass was compared with the distribution of the maximum cluster mass observed in each of the 1000 permutations. Cluster masses larger or equal to the 95th percentile of the permutation distribution were considered as statistically significant (p < .05). For SAD patients pearson correlation coefficients were calculated for the differences between aversive minus neutral condition mean beta estimates and the differences between aversive minus neutral ratings on a familywise error level of p < .05 using SPSS software (Version 25.0, IBM Corp., Armonk, N.Y., USA).
3. Results
3.1. Behavioral data
3.1.1. Anticipation of “camera on” vs. “camera off”
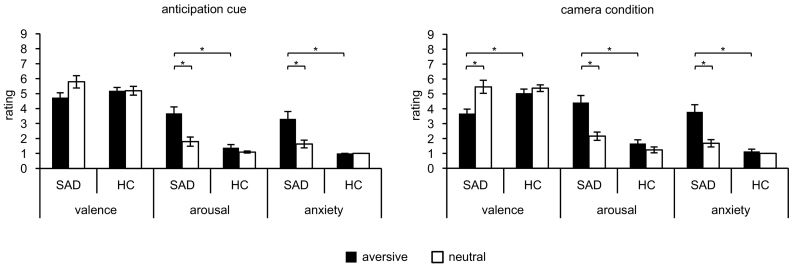
Analyses of arousal, valence and anxiety ratings revealed significant main effects of group (arousal: F[1,38] = 21.03, p < .001; anxiety: F[1,38] = 23.36, p < .001) and condition (arousal: F[1,38] = 25.72, p < .001; valence: F[1,38] = 4.83, p = .034; anxiety: F[1,38] = 16.77, p < .001). Interaction effects of Group by Condition were found regarding valence (F[1,38] = 4.83, p = .034), arousal (F[1,38] = 14.00, p = .001) and anxiety (F[1,38] = 16.77, p < .001). Post-hoc independent t-tests showed that the aversive anticipation cue was rated as more arousing (t[27] = 4.84, p < .001) and more anxiety-inducing (t[18] = 4.78, p < .001) by SAD patients compared to HC. There were no significant group differences for the neutral anticipation cue. Within-subject effects were analyzed by post-hoc paired t-tests revealing that SAD patients rated the aversive relative to the neutral anticipation cue as more arousing (t[18] = 4.87, p < .001) and more anxiety-inducing (t[18] = 3.89, p = .001), whereas HC showed no differences (Fig. 1).
Fig. 1.
Mean ratings on a 9-point Self-assessment manikin scale (Bradley and Lang, 1994). Ratings for valence (1 = very unpleasant, 9 = very pleasant, with 5 = neutral), arousal (1 = not arousing, 9 = highly arousing), and anxiety (1 = not anxiety-inducing, 9 = highly anxiety-inducing) for anticipation cues (aversive = symbol associated with “camera on” condition; neutral = symbol associated with “camera off” condition) and camera condition (aversive = camera on; neutral = camera off). *p < .004.
3.1.2. Camera on vs. camera off
Analyses of arousal, valence and anxiety ratings each revealed significant main effects of group (arousal: F[1,38] = 25.28, p < .001; anxiety: F[1,38] = 38.47, p < .001) and condition (arousal: F[1,38] = 32.38, p < .001; valence: F[1,38] = 17.46, p < .001; anxiety: F[1,38] = 18.01, p < .001). Interaction effects of Group by Condition were found regarding valence (F[1,38] = 8.22, p = .007), arousal (F[1,38] = 15.04, p < .001) and anxiety (F[1,38] = 13.72, p = .001). Post-hoc independent t-tests showed that SAD patients compared to HC rated the “Camera on” symbol as more negative (t[38] = −3.40, p = .002), more arousing (t[28] = 5.19, p < .001) and more anxiety-inducing (t[21] = 5.27, p < .001). There were no significant group differences for the “Camera off” symbol. Furthermore, SAD patients rated the “Camera on” symbol relative to the “Camera off” symbol as more negative (t[18] = −3.77, p = .001), more arousing (t[18] = 4.94, p < .001) and more anxiety-inducing (t[18] = 3.94, p = .001). HC showed no differences (Fig. 1).
3.2. fMRI data
3.2.1. Phasic response average ROI analysis
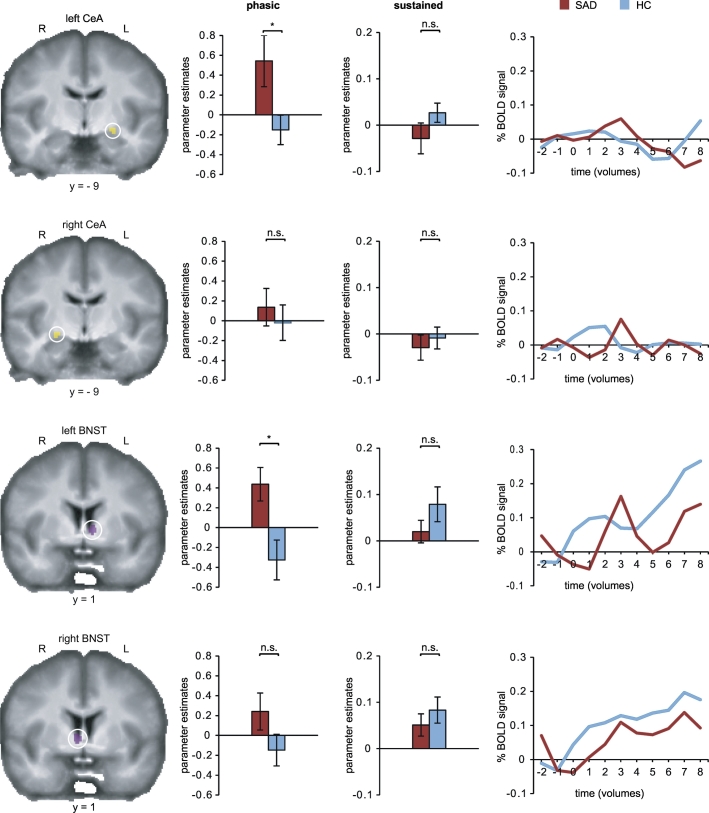
A significant group by condition interaction was revealed for the left CeA (F[1,38] = 5.67, p = .022) and the left BNST (F[1,38] = 8.27, p = .007), indicating higher activation for SAD patients than HC for aversive relative to neutral threat anticipation. All other comparisons were not significant (Table 2 and Fig. 2).
Table 2.
Phasic response two-way repeated measures ROI ANOVA.
| ROI | df | mean square | F-value | p-value | |
|---|---|---|---|---|---|
| CeA left | condition | 1 | 0.77 | 1.82 | .186 |
| group | 1 | 0.87 | 3.43 | .072 | |
| condition x group | 1 | 2.40 | 5.67 | .022⁎ | |
| CeA right | condition | 1 | 0.07 | 0.20 | .655 |
| group | 1 | 0.41 | 1.28 | .265 | |
| condition x group | 1 | 0.12 | 0.36 | .552 | |
| BNST left | condition | 1 | 0.06 | 0.18 | .676 |
| group | 1 | 0.49 | 0.58 | .451 | |
| condition x group | 1 | 2.90 | 8.27 | .007⁎ | |
| BNST right | condition | 1 | 0.04 | 0.15 | .704 |
| group | 1 | 0.04 | 0.08 | .777 | |
| condition x group | 1 | 0.75 | 2.53 | .120 |
Note: CeA, central nucleus of the amygdala; BNST, bed nucleus of the stria terminalis; Factor condition includes two levels (aversive and neutral anticipation); Factor group includes two levels (SAD and HC).
p < 0.05.
Fig. 2.
Phasic and sustained responses during anticipation.
Social anxiety disorder (SAD) patients compared to healthy controls (HC) showed increased phasic activation for anticipation of “camera on” relative to “camera off” in the left central nucleus of the amygdala (CeA) and the left bed nucleus of the stria terminalis (BNST). Participants across both groups showed higher sustained responses for anticipation of “camera on” relative to “camera off” in the right and left BNST. In the left column CeA and BNST ROIs are overlaid on an averaged T1 scan. Bar graphs in the second column display contrasts of parameter estimates for phasic, bar graphs in the third column for sustained anticipation (anticipation of “Camera on” vs. “Camera off”; mean ± SE; *p ≤ .05; n.s., not significant). Line charts in the right column represent time courses of the percentage blood oxygenation level dependent (BOLD) signal change for anticipation of “Camera on” vs. “Camera off” averaged across all 16 s-trials and across participants per group. Shorter trials were excluded for the analysis of time course data.
3.2.2. Sustained response average ROI analysis
For the left BNST (F[1,38] = 4.67, p = .037) and the right BNST (F[1,38] = 12.79, p = .001) significant main effects of condition were revealed, indicating higher activation for SAD patients as well as HC for aversive compared to neutral antcipation. All other comparisons were not significant (Table 3 and Fig. 2).
Table 3.
Sustained response two-way repeated measures ROI ANOVA.
| ROI | df | Mean square | F-value | p-value | |
|---|---|---|---|---|---|
| CeA left | Condition | 1 | < 0.001 | 0.002 | .961 |
| Group | 1 | 0.04 | 3.00 | .091 | |
| Condition × group | 1 | 0.01 | 2.07 | .158 | |
| CeA right | Condition | 1 | 0.01 | 1.15 | .289 |
| Group | 1 | 0.04 | 2.22 | .144 | |
| Condition × group | 1 | 0.002 | 0.33 | .567 | |
| BNST left | Condition | 1 | 0.05 | 4.67 | .037⁎ |
| Group | 1 | 0.01 | 0.49 | .490 | |
| Condition × group | 1 | 0.02 | 1.66 | .206 | |
| BNST right | Condition | 1 | 0.09 | 12.79 | .001⁎ |
| Group | 1 | 0.001 | 0.46 | .831 | |
| Condition × group | 1 | 0.005 | 0.73 | .398 |
Note: CeA, central nucleus of the amygdala; BNST, bed nucleus of the stria terminalis; Factor condition includes two levels (aversive and neutral anticipation); Factor group includes two levels (SAD and HC).
p < 0.05.
3.2.3. Whole brain analysis and correlations of fMRI and behavioral data
Whole brain analysis of phasic and sustained responses did not reveal any significant clusters when comparing SAD patients to HC for the contrast anticipation of aversive minus neutral events.
For SAD patients there were no significant correlations between aversive minus neutral condition mean beta estimates and the differences between aversive minus neutral ratings concerning valence, anxiety and arousal.
4. Discussion
In the present study, we investigated BNST and CeA activity in SAD patients compared to HC during the anticipation of temporally unpredictable aversive (camera monitoring) versus neutral events. For phasic anticipatory response modeling we used the first second after cue onset and found hyperactivation of the CeA as well as the BNST for SAD patients compared to HC. Contrary to our hypothesis, there was no differential activity in the BNST during sustained threat anticipation in SAD patients compared to HC. However, we detected increased sustained activation in the BNST for aversive relative to neutral anticipation across groups.
We revealed an elevated phasic CeA response for SAD patients compared to HC when anticipating social observation. The amygdala is thought to function as a hub region (Pessoa, 2017) relevant in vigilance, arousal, threat detection and interpretation processes (Calhoon and Tye, 2015; Pessoa, 2010; Whalen, 1998). In SAD patients, increased threat attention and vigilance (Grupe and Nitschke, 2013), reflected by a hyperresponsive emotion processing network including the amygdala, are supposed to be essential in the presence of potentially threatening social situations (Brühl et al., 2014; Cremers and Roelofs, 2016; Duval et al., 2015; Miskovic and Schmidt, 2012). Thus, the present result might be interpreted as SAD patients perceiving the signal that a video camera is going to be switched on as more salient and arousing than HC, resulting in an initial hypervigilant reaction following cue onset. To date, none of the existing studies on threat anticipation in SAD examined a phasic anticipatory time interval as modeled in our paradigm. However, the present finding of elevated phasic amygdala activity to threat anticipation in SAD patients is in line with previous studies investigating panic disorder (Brinkmann et al., 2017a), post-traumatic stress disorder (Brinkmann et al., 2017b) and generalized anxiety disorder (Buff et al., 2017) pointing towards a transdiagnostically shared neural response to threatening stimuli in anxiety disorders.
Interestingly, we additionally revealed a higher phasic BNST activation in SAD patients compared to HC during anticipation of aversive compared to neutral events. Among other functions, enhanced BNST activity is supposed to reflect heightened reactivity to threat uncertainty associated with hypervigilance and increased arousal (Avery et al., 2016; Grupe and Nitschke, 2013). Thus, SAD patients seem to be more affected by states of uncertainty than HC, especially when confronted with unpredictable aversive events. This is in line with theoretical models for SAD suggesting a higher Intolerance of Uncertainty (IU) level for such patients (Carleton et al., 2012; Grupe and Nitschke, 2013; Whiting et al., 2014) paired with heightened vigilance and increased processing of social threat (Brühl et al., 2014; Cremers and Roelofs, 2016; Duval et al., 2015; Miskovic and Schmidt, 2012). Originally, the function of the BNST was assumed to be restricted to longer lasting, more diffuse and unpredictable threatening situations (Davis, 1998) and to be more relevant later in time course (McMenamin et al., 2014; Walker et al., 2009). However, recent reviews (Avery et al., 2016; Fox and Shackman, 2017; Gungor and Paré, 2016; Shackman and Fox, 2016) questioned the strict temporal dissociation of amygdala and BNST and suggest instead an involvement of the BNST in both, longer lasting responses during sustained threat, and transient responses during immediate threat. In line with this suggested role of the BNST in short-term responses to threat, a recent study found phasic BNST activation during processing of short threat stimuli in a large sample of healthy volunteers (Brinkmann et al., 2018). Additionally, phasic BNST activation has been found during onset of an aversive anticipation context (Alvarez et al., 2011). In the present study, SAD patients compared to HC showed an elevated phasic BNST response in the first second following anticipation onset for the aversive relative to neutral condition. This finding strongly supports the hypothesis of an early, short-term BNST involvement in threat processing and suggests that SAD patients might be hyperresponsive regarding cue onset during anticipation of aversive events.
During sustained threat anticipation, contrary to our hypothesis we did not reveal a BNST activation difference for individuals with SAD compared to HC. Across groups however, we found a higher BNST response during aversive relative to neutral anticipation. These findings suggest that both, SAD and HC respond similarly with BNST activation to threat uncertainty when regarding the whole anticipation interval. Previous studies investigating other anxiety disorders consistently reported an elevated sustained BNST activation for patients compared to HC in aversive relative to neutral situations for spider phobia (Münsterkötter et al., 2015; Straube et al., 2007), generalized anxiety disorder (Buff et al., 2017), post-traumatic stress disorder (Brinkmann et al., 2017b) and panic disorder (Brinkmann et al., 2017a). Thus, the present findings provide preliminary evidence for SAD specific BNST responding to threat anticipation suggesting a fast salience detection reaction (Blackford et al., 2009; Kim and Yoon, 2017) mediated by phasic BNST and CeA responses following threat-indicating cues but a less pronounced sustained response compared to HC.
To summarize, we investigated BNST and CeA involvement during anticipation of temporally unpredictable aversive (camera switched on) relative to neutral (camera switched off) events. Compared to HC, SAD patients showed elevated phasic CeA as well as BNST responses in the first second after cue onset. However, both groups did not differ regarding a more sustained time period. For the first time, we could reveal a BNST involvement during threat anticipation in SAD patients. Additionally, present results point towards a SAD-specific threat processing marked by elevated phasic but not sustained CeA and BNST responses when compared to HC.
Conflict of interest
The authors report no conflicts of interest and no financial relationships with commercial interests.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Acknowledgment
This work was supported by the German Research Foundation (DFG: SFB/TRR 58: C07).
References
- Alvarez R.P., Chen G., Bodurka J., Kaplan R., Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage. 2011;55:389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; Arlington: 2013. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [Google Scholar]
- Amunts K., Kedo O., Kindler M., Pieperhoff P., Mohlberg H., Shah N.J., Habel U., Schneider F., Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. (Berlin) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Avery S.N., Clauss J.A., Blackford J.U. The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology. 2016;41:126–141. doi: 10.1038/npp.2015.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M.P.I., Simon D., Miltner W.H.R., Straube T. Altered activation of the ventral striatum under performance-related observation in social anxiety disorder. Psychol. Med. 2017;47:2502–2512. doi: 10.1017/S0033291717001076. [DOI] [PubMed] [Google Scholar]
- Blackford J.U., Avery S.N., Shelton R.C., Zald D.H. Amygdala temporal dynamics: temperamental differences in the timing of amygdala response to familiar and novel faces. BMC Neurosci. 2009;10(145) doi: 10.1186/1471-2202-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme S., Ritter V., Tefikow S., Stangier U., Strauss B., Miltner W.H.R., Straube T. Brain activation during anticipatory anxiety in social anxiety disorder. Soc. Cogn. Affect. Neurosci. 2014;9:1413–1418. doi: 10.1093/scan/nst129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J. Measuring emotion: the self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Brinkmann L., Buff C., Feldker K., Tupak S.V., Becker M.P.I., Herrmann M.J., Straube T. Distinct phasic and sustained brain responses and connectivity of amygdala and bed nucleus of the stria terminalis during threat anticipation in panic disorder. Psychol. Med. 2017:1–14. doi: 10.1017/S0033291717001192. [DOI] [PubMed] [Google Scholar]
- Brinkmann L., Buff C., Neumeister P., Tupak S.V., Becker M.P.I., Herrmann M.J., Straube T. Dissociation between amygdala and bed nucleus of the stria terminalis during threat anticipation in female post-traumatic stress disorder patients. Hum. Brain Mapp. 2017;38:2190–2205. doi: 10.1002/hbm.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann L., Buff C., Feldker K., Neumeister P., Heitmann C.Y., Hofmann D., Bruchmann M., Herrmann M.J., Straube T. Inter-individual differences in trait anxiety shape the functional connectivity between the bed nucleus of the stria terminalis and the amygdala during brief threat processing. Neuroimage. 2018;166:110–116. doi: 10.1016/j.neuroimage.2017.10.054. [DOI] [PubMed] [Google Scholar]
- Brühl A.B., Rufer M., Delsignore A., Kaffenberger T., Jäncke L., Herwig U. Neural correlates of altered general emotion processing in social anxiety disorder. Brain Res. 2011;1378:72–83. doi: 10.1016/j.brainres.2010.12.084. [DOI] [PubMed] [Google Scholar]
- Brühl A.B., Delsignore A., Komossa K., Weidt S. Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neurosci. Biobehav. Rev. 2014;47:260–280. doi: 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Buff C., Brinkmann L., Bruchmann M., Becker M.P.I., Tupak S., Herrmann M.J., Straube T. Activity alterations in the bed nucleus of the stria terminalis and amygdala during threat anticipation in generalized anxiety disorder. Soc. Cogn. Affect. Neurosci. 2017 doi: 10.1093/scan/nsx103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoon G.G., Tye K.M. Resolving the neural circuits of anxiety. Nat. Neurosci. 2015;18:1394–1404. doi: 10.1038/nn.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton R.N., Mulvogue M.K., Thibodeau M.A., McCabe R.E., Antony M.M., Asmundson G.J.G. Increasingly certain about uncertainty: intolerance of uncertainty across anxiety and depression. J. Anxiety Disord. 2012;26:468–479. doi: 10.1016/j.janxdis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Cremers H.R., Roelofs K. Social anxiety disorder: a critical overview of neurocognitive research. Wiley Interdiscip. Rev. Cogn. Sci. 2016;7:218–232. doi: 10.1002/wcs.1390. [DOI] [PubMed] [Google Scholar]
- Davies C.D., Young K., Torre J.B., Burklund L.J., Goldin P.R., Brown L.A., Niles A.N., Lieberman M.D., Craske M.G. Altered time course of amygdala activation during speech anticipation in social anxiety disorder. J. Affect. Disord. 2017;209:23–29. doi: 10.1016/j.jad.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol. Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M., Walker D.L., Miles L., Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Berker A.O., Rutledge R.B., Mathys C., Marshall L., Cross G.F., Dolan R.J., Bestmann S. Computations of uncertainty mediate acute stress responses in humans. Nat. Commun. 2016;7 doi: 10.1038/ncomms10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval E.R., Javanbakht A., Liberzon I. Neural circuits in anxiety and stress disorders: a focused review. Ther. Clin. Risk Manag. 2015;11:115–126. doi: 10.2147/TCRM.S48528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K., Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U. S. A. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A.S., Shackman A.J. The central extended amygdala in fear and anxiety: closing the gap between mechanistic and neuroimaging research. Neurosci. Lett. 2017 doi: 10.1016/j.neulet.2017.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A.S., Oler J.A., Tromp D.P.M., Fudge J.L., Kalin N.H. Extending the amygdala in theories of threat processing. Trends Neurosci. 2015;38:319–329. doi: 10.1016/j.tins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George L., Stopa L. Private and public self-awareness in social anxiety. J. Behav. Ther. Exp. Psychiatry. 2008;39:57–72. doi: 10.1016/j.jbtep.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Giménez M., Pujol J., Ortiz H., Soriano-Mas C., López-Solà M., Farré M., Deus J., Merlo-Pich E., Martín-Santos R. Altered brain functional connectivity in relation to perception of scrutiny in social anxiety disorder. Psychiatry Res. Neuroimaging. 2012;202:214–223. doi: 10.1016/j.pscychresns.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Glassman L.H., Herbert J.D., Forman E.M., Bradley L.E., Izzetoglu M., Ruocco A.C., Goldstein S.P. Near-infrared spectroscopic assessment of in vivo prefrontal activation in public speaking anxiety: a preliminary study. Psychol. Conscious. Theory Res. Pract. 2014;1:271–283. [Google Scholar]
- Grupe D.W., Nitschke J.B. Uncertainty is associated with biased expectancies and heightened responses to aversion. Emotion. 2011;11:413–424. doi: 10.1037/a0022583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe D.W., Nitschke J.B. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat. Rev. Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe D.W., Oathes D.J., Nitschke J.B. Dissecting the anticipation of aversion reveals dissociable neural networks. Cereb. Cortex. 2013;23:1874–1883. doi: 10.1093/cercor/bhs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor N.Z., Paré D. Functional heterogeneity in the bed nucleus of the Stria terminalis. J. Neurosci. 2016;36:8038–8049. doi: 10.1523/JNEUROSCI.0856-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack S.E., Guo J.-D., Hazra R., Dabrowska J., Myers K.M., Rainnie D.G. The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2009;33:1309–1320. doi: 10.1016/j.pnpbp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautzinger M., Keller F., Kühner C. 2006. BDI II Beck Depressions-Inventar (2.Revision). Harcourt Test Services, Frankfurt/Main. [Google Scholar]
- Heimberg R.G., Hofmann S.G., Liebowitz M.R., Schneier F.R., Smits J.A.J., Stein M.B., Hinton D.E., Craske M.G. Social anxiety disorder in Dsm-5. Depress. Anxiety. 2014;31:472–479. doi: 10.1002/da.22231. [DOI] [PubMed] [Google Scholar]
- Herrmann M.J., Boehme S., Becker M.P.I., Tupak S.V., Guhn A., Schmidt B., Brinkmann L., Straube T. Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Hum. Brain Mapp. 2016;37:1091–1102. doi: 10.1002/hbm.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C., Bach D.R., Esposito F., Salle F.D., Perrig W.J., Scheffler K., Lüthi A., Seifritz E. Processing of temporal unpredictability in human and animal amygdala. J. Neurosci. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S.G. Cognitive factors that maintain social anxiety disorder: a comprehensive model and its treatment implications. Cogn. Behav. Ther. 2007;36:193–209. doi: 10.1080/16506070701421313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-K., Yoon H.-K. Common and distinct brain networks underlying panic and social anxiety disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017 doi: 10.1016/j.pnpbp.2017.06.017. [DOI] [PubMed] [Google Scholar]
- Lancaster J.L., Tordesillas-Gutiérrez D., Martinez M., Salinas F., Evans A., Zilles K., Mazziotta J.C., Fox P.T. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorberbaum J.P., Kose S., Johnson M.R., Arana G.W., Sullivan L.K., Hamner M.B., Ballenger J.C., Lydiard R.B., Brodrick P.S., Bohning D.E., George M.S. Neural correlates of speech anticipatory anxiety in generalized social phobia. NeuroReport. 2004;15:2701–2705. [PubMed] [Google Scholar]
- McMenamin B.W., Langeslag S.J.E., Sirbu M., Padmala S., Pessoa L. Network organization unfolds over time during periods of anxious anticipation. J. Neurosci. 2014;34:11261–11273. doi: 10.1523/JNEUROSCI.1579-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V., Schmidt L.A. Social fearfulness in the human brain. Neurosci. Biobehav. Rev. 2012;36:459–478. doi: 10.1016/j.neubiorev.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Münsterkötter A.L., Notzon S., Redlich R., Grotegerd D., Dohm K., Arolt V., Kugel H., Zwanzger P., Dannlowski U. Spider or no spider? neural correlates of sustained and phasic fear in spider phobia. Depress. Anxiety. 2015;32:656–663. doi: 10.1002/da.22382. [DOI] [PubMed] [Google Scholar]
- Pessoa L. Emotion and cognition and the amygdala: from “what is it?” to “what's to be done?”. Neuropsychologia. 2010;48:3416–3429. doi: 10.1016/j.neuropsychologia.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. A network model of the emotional brain. Trends Cogn. Sci. 2017;21:357–371. doi: 10.1016/j.tics.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Sarinopoulos I., Grupe D.W., Mackiewicz K.L., Herrington J.D., Lor M., Steege E.E., Nitschke J.B. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb. Cortex. 2010;20:929–940. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman A.J., Fox A.S. Contributions of the central extended amygdala to fear and anxiety. J. Neurosci. 2016;36:8050–8063. doi: 10.1523/JNEUROSCI.0982-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D., Becker M.P.I., Mothes-Lasch M., Miltner W.H.R., Straube T. Effects of social context on feedback-related activity in the human ventral striatum. NeuroImage. 2014;99:1–6. doi: 10.1016/j.neuroimage.2014.05.071. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R.E. Consulting Psychologists Press; Palo Alto, Ca: 1970. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Stangier U., Heidenreich T. Die Liebowitz Soziale Angst-Skala (LSAS) [Liebowitz Social Anxiety Scale] In: Psychiatrie Internationale Skalen Für., editor. Collegium Internationale Psychiatriae Scalarum. Beltz; Weinheim: 2004. International Psychiatry Scales. [Google Scholar]
- Stangier U., Heidenreich T., Berardi A., Golbs U., Hoyer J. Die Erfassung sozialer Phobie durch die social interaction anxiety scale (SIAS) und die social phobia scale (SPS) Z. Für Klin. Psychol. Psychother. 1999;28:28–36. [Google Scholar]
- Straube T., Mentzel H.-J., Miltner W.H.R. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme; New York: 1988. Co-Planar Stereotaxic Atlas of the Human Brain. 3-Dimensional Proportional System: An Approach to Cerebral Imaging. [Google Scholar]
- Theiss J.D., Ridgewell C., McHugo M., Heckers S., Blackford J.U. Manual segmentation of the human bed nucleus of the stria terminalis using 3T MRI. NeuroImage. 2017;146:288–292. doi: 10.1016/j.neuroimage.2016.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillfors M., Furmark T., Marteinsdottir I., Fredrikson M. Cerebral blood flow during anticipation of public speaking in social phobia: a PET study. Biol. Psychiatry. 2002;52:1113–1119. doi: 10.1016/s0006-3223(02)01396-3. [DOI] [PubMed] [Google Scholar]
- Tovote P., Fadok J.P., Lüthi A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- Walker D.L., Toufexis D.J., Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur. J. Pharmacol. Anim. Model. Anxiety Disord. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Walker D.L., Miles L.A., Davis M. Selective participation of the bed nucleus of the Stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen P.J. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Curr. Dir. Psychol. Sci. 1998;7:177–188. [Google Scholar]
- Whalen P.J. The uncertainty of it all. Trends Cogn. Sci. 2007;11:499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Whiting S.E., Jenkins W.S., May A.C., Rudy B.M., Davis T.E., Reuther E.T. The role of intolerance of uncertainty in social anxiety subtypes. J. Clin. Psychol. 2014;70:260–272. doi: 10.1002/jclp.22024. [DOI] [PubMed] [Google Scholar]
- Wittchen H.-U., Zaudig M., Fydrich T. SKID. Strukturiertes Klinisches Interview für DSM-IV Achse I und II. Göttingen. 1997;28:68–70. [Google Scholar]