Abstract
Background:
Clinician-reported toxicity grading through Common Terminology Criteria for Adverse Events (CTCAE) stages dysphagia based on symptoms, diet, and tube dependence. The new Dynamic Imaging Grade of Swallowing Toxicity (DIGEST) tool offers a similarly scaled 5-point ordinal summary grade of pharyngeal swallowing as determined through results of a modified barium swallow (MBS) study. This study aims to inform clinicians on the similarities and differences between dysphagia severity according to clinical CTCAE and MBS-derived DIGEST grading.
Methods:
A cross-sectional sample of 95 MBS studies was randomly selected from a prospectively- acquired MBS database among patients treated with organ preservation strategies for head and neck cancer. MBS DIGEST and clinical CTCAE dysphagia grades were compared.
Results:
DIGEST and CTCAE dysphagia grades had “fair” agreement per weighted ĸ of 0.358 (95% CI.231-.485). Using a threshold of DIGEST ≥3 as reference, CTCAE had an overall sensitivity of 0.50, specificity of 0.84, and area under the curve (AUC) of 0.67 to identify severe MBS-detected dysphagia. At less than 6 months, sensitivity was 0.72, specificity was 0.76, and AUC was 0.75 while at greater than 6 months, sensitivity was 0.22, specificity was 0.90, and AUC was 0.56 for CTCAE to detect dysphagia as determined by DIGEST.
Conclusions:
Classification of pharyngeal dysphagia on MBS using DIGEST augments our understanding of dysphagia severity according to the clinically derived CTCAE while maintaining the simplicity of an ordinal scale. DIGEST likely complements CTCAE toxicity grading through improved specificity for physiologic dysphagia in the acute-phase and improved sensitivity for dysphagia in the late-phase.
Keywords: Deglutition and deglutition disorders, Head and Neck Cancer, Toxicity Grading, Clinical Trials
Introduction
Dysphagia is arguably the most important long-term toxicity of patients after treatment for head and neck cancer (1, 2). This is particularly true among HPV-associated oropharyngeal carcinoma patient who, generally speaking, have favorable disease specific outcomes compared to non-HPV associated cancer, which in turn necessitates more careful consideration of treatment toxicities many years into survivorship (3). As efforts continue to establish the optimal methods to treat these cancers while preserving high rates of survival and potential reduction of toxicities, accurate quantification of these toxicities (i.e. dysphagia) is paramount (4, 5). When grading outcomes such as oncologic treatment toxicity, whether in the context of designing a clinical trial or in routine clinical practice, both the timing of assessment(s) and type of information provided by a particular metric or diagnostic test acquired must be carefully weighed (6). Alteration of eating and swallowing in the acute phase of radiotherapy can be a manifestation of many issues including mucositis, thick secretions, and dysgeusia (7). Surgery has its own acute impact on swallowing, largely dependent on the extent and location of tumor in addition to the surgical approach (8–11). While these acute toxicities can co-exist with pharyngeal dysphagia, they are certainly distinct from long-term sequelae such as soft tissue fibrosis, xerostomia, stricture, and late neuropathies (4, 12, 13). Nevertheless, a single tool, the Common Terminology Criteria of Adverse Events (CTCAE), is typically used to classify toxicity throughout the continuum of care, despite the fact that different tools may possess a unique ability to understand the severity of dysfunction or symptomatology.
Prior literature and clinical practice both support the notion that comprehensive assessment of a complex problem such as pharyngeal dysphagia may be best captured through a composite index that includes various metrics (3, 6, 13). Clinical trials have historically measured dysphagia toxicity principally through clinician determined means, namely the CTCAE, and through surrogate measures of swallowing dysfunction such as gastrostomy tube presence (14–17). Nevertheless, prior work demonstrates that clinician-reported dysphagia through CTCAE or other clinically-derived information may not accurately characterize the impact, nature, or severity of swallowing dysfunction compared to MBS (4, 18–21). Rather than symptomatology, diet alterations, and feeding tube dependence (i.e., the criteria for clinical CTCAE stage), MBS measures physiologic swallowing dysfunction and can identify dysphagia in asymptomatic patients. Despite the ability of MBS to identify silent aspirators in addition to providing specific anatomic/physiologic information that can guide efforts to maximize safer and more efficient swallowing during treatment and into survivorship, the information provided through MBS can be challenging to summarize using common toxicity reporting mechanisms and, therefore, less easily described in oncology clinical trials and by less familiar clinicians (22–24). As such, an effort was made to standardize reporting of MBS findings with regard to the safety and efficiency profiles of pharyngeal swallowing using the ordinal toxicity grading framework of the CTCAE. The Dynamic Imaging Grade of Swallowing Toxicity (DIGEST) tool addresses this need, offering a 5-point ordinal summary grade to standardize reporting of MBS findings using benchmarks of safety and efficiency of bolus clearance through the pharynx (25). In an effort to understand how DIGEST reporting compares to traditional CTCAE quantification of swallowing toxicity, this study analyzes the similarities and differences between these metrics and their comparative diagnostic impressions.
Methods
Eligibility and Design
With approval from the institutional review board, prospectively collected records of MBS conducted at MD Anderson Cancer Center between 2005 and 2013 were retrospectively identified. MBS from patients with a history of laryngeal and/or pharyngeal carcinoma were eligible for inclusion. Swallow studies in patients with history of “open” aerodigestive tract surgery (i.e. transmandibular or transcervical including total laryngectomy) were excluded as were those with known recurrent or second primary malignancy of the head and neck at the time of MBS. Patient who underwent neck dissection without entrance into the aerodigestive tract were not excluded. Indications for MBS in these patients included evaluation of clinical concern for dysphagia in addition to those performed as part of routine treatment pathways. Ninety-five MBS were then randomly selected from eligible cases, of which 10% represented swallow studies prior to treatment. Additionally, MBS were proportionally sampled 2:1 for abnormal Penetration Aspiration Scale (PAS) scores, defined as a score greater than or equal to three. The standard MBS protocol included in the following order: 2 trials each of 5-mL, 10-mL, and self-administered cup sip volumes of Varibar thin liquid barium (40% weight/volume), Varibar barium pudding, and a saltine cracker coated in barium paste in lateral plane video-recorded at 30 frames per second. As detailed in the DIGEST validation study, two blinded, trained laboratory raters who previously met 80% agreement on a training set of MBS independently scored MBS with random re-sampling and re-rating of 32 MBS to ensure inter- and intra-rater reliability (inter-rater weight-κ 0.67, intra-rater weight-κ 0.82–0.84) (25). Given the combination of surgical and non-surgical treatments, classification of tumor and nodal stage was completed clinically using pre-treatment imaging.
CTCAE
The most recent CTCAE (version 4) was published in 2009 by the National Cancer Institute as part of an ongoing effort to standardize adverse event (AE) reporting within oncology (26). AEs are graded according to an ordinal scale where zero represents no toxicity and five represents death from the AE. In between, grade one is mild, two is moderate, three is severe, and four is life-threatening. With regard to dysphagia, the CTCAE uses the descriptive terminology listed in Table 1, columns 1 and 2. CTCAE dysphagia grades are based on symptoms, diet, and tube dependency. These grades were assigned on the MBS date based on a standardized interview by the speech pathologist with normalcy of diet grading per Performance Status Scale of Head and Neck Cancer (PSS-HN) and a single question, “do you have difficulty swallowing?”, asked of each patient (27). CTCAE grades were assigned independently (without knowledge) of DIGEST scores using clinical documentation from the MBS encounter.
Table 1:
CTCAE Dysphagia Description and Patient Proportion in this Study
| Grade | Description | No. of Patients (%) |
|---|---|---|
| 0 | Asymptomatic | 29 (31) |
| 1 | Symptomatic, able to eat regular diet | 18 (19) |
| 2 | Symptomatic and altered eating/swallowing | 25 (26) |
| 3 | Severely altered eating/swallowing; tube feeding or TPN or hospitalization indicated | 23 (24) |
| 4 | Life-threatening consequences; urgent intervention indicated | 0(0) |
DIGEST
DIGEST combines an interaction of the 1) safety and 2) efficiency profiles of pharyngeal bolus transport on MBS to determine an overall rating of pharyngeal swallow function in a manner analogous to the American Joint Cancer Committee staging system’s combination of tumor, nodal, and metastatic information to quantify disease stage. In short, a patient’s safety profile is derived by assigning the maximum Penetration-Aspiration Scale (PAS) observed across a series of standard bolus trials with modifiers to account for the frequency and quantity of high grade penetration/aspiration events (PAS≥5). The efficiency profile is assigned through estimation of the maximum percentage of pharyngeal residue (<10%, 10–49%, 50–90%, and >90%) also with modifiers to account for variations across bolus types. Final DIGEST ratings combine the safety and efficiency profiles, which are demarcated using a similar scale as the CTCAE (0=no pharyngeal dysphagia, 1=mild, 2=moderate, 3=severe, 4=life threatening). Specific descriptions of the grading criteria and cross validation of the DIGEST rating are detailed elsewhere (25). Similar to CTCAE grading, DIGEST scores were assigned without knowledge of CTCAE grade.
Statistical methods
Patient, tumor, and treatment characteristics as well as CTCAE grades and DIGEST ratings were summarized using descriptive statistics. Agreement between DIGEST score and clinical CTCAE dysphagia grade was plotted by histogram with determination of the level of agreement between the two scales using the weighted Cohen’s kappa index. Diagnostic accuracy was determined by sensitivity, specificity, and area under the curve (AUC). Statistical analyses were performed using using STATA v14.0 (StataCorp LP, College Station, TX) and JMP, version 12.1 (SAS Institute Inc, Cary NC).
Results
A cross-sectional sample of 95 patients referred for MBS were included in the analysis. Patient demographics, primary tumor location, tumor stage, treatment type, and timing of MBS are detailed in Table 2.
Table 2:
Patient Characteristics (n = 95)
| No. of Patients (%) | |
|---|---|
| Age (mean(SD)) | 64.5 (8.0) |
| Sex | |
| Male | 78 (82) |
| Female | 17 (18) |
| Primary Tumor Site | |
| Nasopharynx | 6 (6) |
| Oropharynx | 59 (62) |
| Hypopharynx | 6 (6) |
| Larynx | 20 (21) |
| Unknown Primary | 4 (4) |
| T Classification | |
| 0 | 4 (4) |
| 1 | 16 (17) |
| 2 | 41 (43) |
| 3 | 22 (23) |
| 4 | 12 (13) |
| N Classification | |
| 0 | 26 (27) |
| 1-2a | 8 (8) |
| 2b | 23 (24) |
| 2c | 31 (33) |
| 3 | 7 (7) |
| Treatment Combination | |
| Surgery alonea | 9 (9) |
| Surgery + adjuvantb | 14 (15) |
| Radiation alone | 5 (5) |
| Radiation + chemotherapyc | 67 (71) |
| Timing of CTCAE/DIGEST | |
| Pre-treatment | 10 (10) |
| 0-3 monthsd | 20 (21) |
| 4-6monthsd | 13 (14) |
| 7-12monthsd | 29 (31) |
| >12monthsd | 23 (24) |
Includes induction chemotherapy in one patient
Includes post-operative radiation and chemoradiation
Includes induction with or without concurrent chemotherapy
Post-treatment
MBS derived DIGEST and clinical CTCAE dysphagia grades had a weighted ĸ of 0.358 (95% CI .231, .485), which is generally considered as a “fair” level of agreement (28). Figure 1 plots this relationship. Relative to MBS (DIGEST), clinical CTCAE grades over-estimated dysphagia severity in 25% (i.e., CTCAE higher than DIGEST) of exams and under-estimated dysphagia severity in 39% (i.e., CTCAE lower than DIGEST). Considering a threshold of severe dysphagia as determined by the CTCAE (grade ≥3, i.e., tube-fed patients), 17.4% (4/23) of patients had a DIGEST rating of no or mild dysphagia (DIGEST <2) and all were less than 6 months from completion of treatment. Conversely, 50% (10/20) of patients with a severe dysphagia per DIGEST (MBS grade≥3, i.e., severe inefficiency or chronic aspiration on MBS) had a lower CTCAE grade (<3), of which 80% (8/10) were 6 months or more beyond completion of therapy. Two of these latter patients were eating a regular diet with mild or no reported symptomatology on CTCAE despite severe dysphagia on MBS per DIGEST.
Figure 1:
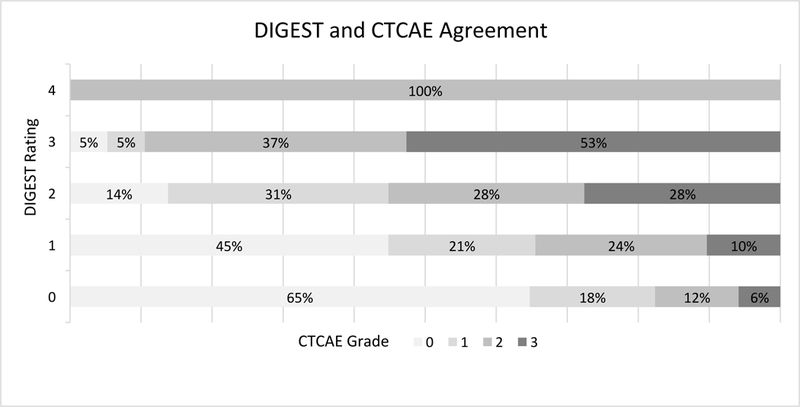
Graphical representation of agreement between MBS-derived DIGEST score and clinical CTCAE dysphagia grade
In terms of diagnostic accuracy, using a threshold of DIGEST ≥3 as reference, CTCAE had an overall sensitivity of 0.50, specificity of 0.84, and area under the curve (AUC) of 0.67 to identify severe MBS-detected dysphagia. For patients less than 6 months after treatment, sensitivity was 0.72, specificity was 0.76, and AUC was 0.75 whereas for greater than 6 months, sensitivity was 0.22, specificity was 0.90, and AUC was 0.56 for CTCAE to detect dysphagia as determined by MBS-derived DIGEST ratings.
Discussion
In this cohort of surgically and non-surgically treated patients, 85% of whom received multimodality organ preservation treatment, agreement between clinical CTCAE staging and MBS DIGEST is fair but agreement varies depending on the severity of dysphagia and length of follow-up. It is well-established that MBS provides distinct information in comparison to diagnostic observations from clinical examination and patient-reported symptoms (23, 29–32). Both CTCAE and MBS DIGEST grading were developed for the explicit purpose of grading the severity of swallowing toxicity in cancer patients. However, since DIGEST summarizes MBS findings and CTCAE grade relates to a clinical assessment of patient function, these measures report correlated and complementary rather than duplicative information. Indeed, a high degree of alignment between CTCAE and DIGEST would be unusual and supports our finding of fair agreement between them. Precisely because difficulty swallowing may represent the interplay of symptomatology, physiologic dysfunction, and anatomic alternations in addition to being influenced by patient adaptations over time that make clinical assessments less sensitive, swallowing dysfunction may best be encapsulated through a combination of metrics (33–36). It is the authors’ recommendation to include a combination of clinician-derived symptom reporting (CTCAE), radiographic data on swallowing via MBS (e.g., DIGEST), and validated, dysphagia-specific patient-reported outcome (PRO) measures to provide a broad representation of the patient’s swallowing function when planning toxicity analyses in oncology.
The relationship between CTCAE and MBS DIGEST graded dysphagia severity appears to vary over time. This study aggregated patients from all treatment times, but the sensitivity and specificity of CTCAE to detect MBS-derived dysphagia differed according to the post-treatment interval timing, broadly defined by less than or greater than 6 months (i.e. acute- vs late-phase). The results suggest that MBS-derived DIGEST adds specificity for dysphagia compared to CTCAE in the short term perhaps explained by the impact of acute toxicities of treatment on oral intake that necessitate alternate alimentation but do not represent true pharyngeal dysphagia (i.e., in acute phase DIGEST avoids false positives when a patient is not eating regular foods due to acute toxicities other than pharyngeal dysphagia). That is, the presence of a feeding tube (the most common reason for CTCAE grade ≥3 dysphagia), particularly in patients <6 months post-treatment, does not necessarily mean a patient has pharyngeal dysphagia on MBS.
Furthermore, DIGEST may be more sensitive to clinically-meaningful pharyngeal dysphagia, particularlyin later follow-up (>6 months post-treatment), by identifying patients who maintain a full oral diet (asymptomatic on CTCAE) but with a severely dysfunctional swallow on MBS (i.e., in the chronic phase, DIGEST avoids false negatives of clinical CTCAE grading). In other words, severe non-feeding tube dependent dysphagia detected by MBS-derived DIGEST was most commonly observed in this study in long-term follow-up, beyond 6 months. These time-dependent discrepancies between CTCAE and DIGEST grading demonstrate the ability of MBS graded measures of toxicity to provide sensitive, specific, and dynamic physiologic-based information on a patient’s ability to swallow along the continuum of survivorship.
All patients in this study had prospectively collected, standardized interviews incorporating a validated oral intake measure (PSS-HN) with uniform questions of feeding tube utilization and swallowing symptomology acquired at point of service at the time of each MBS study. This clinical practice allowed for tight control of CTCAE grading that is likely more sensitive to subtle low grade toxicity classifications (i.e., grade 1 or 2 CTCAE events) than unstructured toxicity reporting that is routine in many clinical settings. Similar standardization of clinical procedures, data collection, and interpretation occurred with MBS studies and DIGEST rating lending to a robust comparison of methods (25). Nevertheless, this is a retrospective analysis of prospectively acquired data on swallowing toxicity as determined by CTCAE grade and DIGEST rating. Though patients were systematically selected at random across the spectrum of treatment to allow for diversity in sampling during the validation study, this study is not sufficiently powered to analyze findings by timing before or after treatment. As a result, time-dependent factors that may affect swallowing must be taken into account secondarily as exploratory or hypothesis-generating observation. Prospective, longitudinal validation of these observations is underway. This analysis combines surgical and non-surgical patients with tumors from various upper aerodigestive subsites. Though this is representative of a contemporary head and neck cancer cohort in a large, North American center, combination of patients treated by different modalities may limit generalizability and the ability to identify subsets of patients where CTCAE and DIGEST may be either more or less complementary for the purposes of trial design and clinical decision-making.
In conclusion, use of a standardized metric for classification of pharyngeal dysphagia on MBS augments our understanding of dysphagia severity according to the clinically derived toxicity endpoints of the CTCAE while maintaining the simplicity of an ordinal, clinician-graded scale. DIGEST likely complements CTCAE toxicity grading through improved specificity for pharyngeal dysphagia (versus other toxicities impacting oral intake) in the acute-phase and through improved sensitivity for dysphagia in the late-phase (when gastrostomy-free does not equate to dysphagia-free). Though incorporation of this grading mechanism, MBS-derived data on pharyngeal dysphagia can be distilled into an intuitive, rank-based scale that harmonizes with ordinal standards for toxicity reporting in oncology trials.. This could improve advocacy for dysphagia management among all members of the multidisciplinary head and neck cancer team.
Table 3:
DIGEST Component Breakdown
| S0 | S1 | S2 | S3 | S4 | S Total | |
| E0 | 17 | 6 | 1 | 1 | 0 | 25 |
| E1 | 13 | 4 | 4 | 3 | 1 | 25 |
| E2 | 6 | 6 | 7 | 1 | 0 | 20 |
| E3 | 4 | 7 | 7 | 6 | 0 | 24 |
| E4 | 0 | 0 | 0 | 1 | 0 | 1 |
| E Total | 40 | 23 | 19 | 12 | 1 | |
| DIGEST Rating | No. of Patients (%) | |||||
| 0, no dysphagia | 17 (18) | |||||
| 1, mild | 29 (31) | |||||
| 2, moderate | 29 (31) | |||||
| 3, severe | 19 (20) | |||||
| 4, life-threatening | 1 (1) | |||||
No. of patients in each box.
Abbreviations: E, Efficiency profile; S, Safety profile
Acknowledgments
Author Funding Disclosures: This work with accomplished with support of the MD Anderson Institutional Research Grant Program. Dr. Hutcheson receives funding support from the National Cancer Institute (R03CA188162–01). Drs. Hutcheson, Lai, and Fuller receive funding support from the National Institutes of Health (NIH)/National Institute for Dental and Craniofacial Research (1R01DE025248– 01/R56DE025248–01). Dr. Fuller has received speaker travel funding from Elekta AB.
References
- 1.Wilson JA, Carding PN, Patterson JM: Dysphagia after nonsurgical head and neck cancer treatment: patients’ perspectives. Otolaryngol Head Neck Surg 145: 767–-771., 2011. [DOI] [PubMed] [Google Scholar]
- 2.Roe JW, Drinnan MJ, Carding PN, Harrington KJ, Nutting CM: Patient-reported outcomes following parotid-sparing intensity-modulated radiotherapy for head and neck cancer. How important is dysphagia? Oral Oncol 50: 1182–1187, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Feng FY, Kim HM, Lyden TH, Haxer MJ, Worden FP, Feng M, Moyer JS, Prince ME, Carey TE, Wolf GT, Bradford CR, Chepeha DB, Eisbruch A: Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: Clinical and functional results. J Clin Oncol 28: 2732–-2738., 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter KU, Schipper M, Feng FY, Lyden T, Haxer M, Murdoch-Kinch CA, Cornwall B, Lee CSY, Chepeha DB, Eisbruch A: Toxicities affecting quality of life after chemo-IMRT of oropharyngeal cancer: Prospective study of patient-reported, observer-rated, and objective outcomes. International Journal of Radiation Oncology Biology Physics 85: 935–-940., 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vainshtein JM, Moon DH, Feng FY, Chepeha DB, Eisbruch A, Stenmark MH: Long-term quality of life after swallowing and salivary-sparing chemo-intensity modulated radiation therapy in survivors of human papillomavirus-related oropharyngeal cancer. Int J Radiat Oncol Biol Phys 91: 925–933, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutcheson KA, Lewin JS: Functional outcomes after chemoradiotherapy of laryngeal and pharyngeal cancers. Curr Oncol Rep 14: 158–165, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moroney LB, Helios J, Ward EC, Crombie J, Wockner LF, Burns CL, Spurgin AL, Blake C, Kenny L, Hughes BGM: Patterns of dysphagia and acute toxicities in patients with head and neck cancer undergoing helical IMRT ± concurrent chemotherapy. Oral Oncol 64: 1–8, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Zuydam AC, Rogers SN, Brown JS, Vaughan ED, Magennis P: Swallowing rehabilitation after oro-pharyngeal resection for squamous cell carcinoma. Br J Oral Maxillofac Surg 38: 513–518, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Iseli TA, Kulbersh BD, Iseli CE, Carroll WR, Rosenthal EL, Magnuson JS: Functional outcomes after transoral robotic surgery for head and neck cancer. Otolaryngology - Head and Neck Surgery 141: 166–171, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Borggreven PA, Verdonck-De Leeuw IM, Rinkel RN, Langendijk JA, Roos JC, David EFL, De Bree R, Leemans CR: Swallowing after major surgery of the oral cavity or oropharynx: A prospective and longitudinal assessment of patients treated by microvascular soft tissue reconstruction. Head Neck 29:638–-647., 2007. [DOI] [PubMed] [Google Scholar]
- 11.Dale OT, Han C, Burgess CA, Eves S, Harris CE, White PL, Shah RT, Howard A, Winter SC: Long-term functional outcomes in surgically treated patients with oropharyngeal cancer. Laryngoscope 125:1637–1643, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal DI, Mendoza TR, Fuller CD, Hutcheson KA, Wang XS, Hanna EY, Lu C, Garden AS,Morrison WH, Cleeland CS, Gunn GB: Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: A prospective analysis using the University of Texas MD Anderson Cancer Center Symptom Inventory-Head and Neck Module. Cancer 120: 1975–-1984., 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenthal DI, Lewin JS, Eisbruch A: Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol 24: 2636–2643, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J, Youssoufian H, Rowinsky EK, Ang KK: Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 11: 21–28, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, Giralt J, Maingon P, Rolland F, Bolla M, Cognetti F, Bourhis J, Kirkpatrick A, Van Glabbeke M: Postoperative Irradiation with or without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. N Engl J Med 350: 1945–1952, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, Forastiere A, Ang KK: Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol 26: 3582–-3589., 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, Peters G, Lee D-J, Leaf A, Ensley J, Cooper J: Concurrent Chemotherapy and Radiotherapy for Organ Preservation in Advanced Laryngeal Cancer. N Engl J Med 349: 2091–2098, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Sinclair CF, McColloch NL, Carroll WR, Rosenthal EL, Desmond RA, Magnuson JS: Patient-perceived and objective functional outcomes following transoral robotic surgery for early oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 137: 1112–-1116., 2011. [DOI] [PubMed] [Google Scholar]
- 19.Rinkel RNPM, Verdonck-de Leeuw IM, de Bree R, Aaronson NK, Leemans CR: Validity of Patient-Reported Swallowing and Speech Outcomes in Relation to Objectively Measured Oral Function Among Patients Treated for Oral or Oropharyngeal Cancer. Dysphagia 30: 196–204, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Jensen K, Bonde Jensen A, Grau C: The relationship between observer-based toxicity scoring and patient assessed symptom severity after treatment for head and neck cancer. A correlative cross sectional study of the DAHANCA toxicity scoring system and the EORTC quality of life questionnaires. Radiother Oncol 78: 298–305, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Ding R, Logemann JA: Patient self-perceptions of swallowing difficulties as compared to expert ratings of videofluorographic studies. Folia Phoniatrica et Logopaedica 60: 142–150, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Eisbruch A, Schwartz M, Rasch C, Vineberg K, Damen E, Van As CJ, Marsh R, Pameijer FA, Balm AJ: Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys 60: 1425–1439, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Hunter KU, Lee OE, Lyden TH, Haxer MJ, Feng FY, Schipper M, Worden F, Prince ME, McLean SA, Wolf GT, Bradford CR, Chepeha DB, Eisbruch A: Aspiration pneumonia after chemo-intensity-modulated radiation therapy of oropharyngeal carcinoma and its clinical and dysphagia-related predictors. Head Neck 36: 120–-125., 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutcheson KA, Barringer DA, Rosenthal DI, May AH, Roberts DB, Lewin JS: Swallowing outcomes after radiotherapy for laryngeal carcinoma. Arch Otolaryngol Head Neck Surg 134: 178–183, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Hutcheson KA, Barrow MP, Barringer DA, Knott JK, Lin HY, Weber RS, Fuller CD, Lai SY, Alvarez CP, Raut J, Lazarus CL, May A, Patterson J, Roe JW, Starmer HM, Lewin JS: Dynamic Imaging Grade of Swallowing Toxicity (DIGEST): Scale development and validation. Cancer, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NCI N, DHHS: Common Terminology Criteria for Adverse Events v4.0. National Cancer Institute, May 29, 2009. [Google Scholar]
- 27.List MA, Ritter-Sterr C, Lansky SB: A performance status scale for head and neck cancer patients. Cancer 66: 564–-569., 1990. [DOI] [PubMed] [Google Scholar]
- 28.Landis JR, Koch GG: The measurement of observer agreement for categorical data. Biometrics 33: 159–174, 1977. [PubMed] [Google Scholar]
- 29.Rogus-Pulia NM, Pierce MC, Mittal BB, Zecker SG, Logemann JA: Changes in swallowing physiology and patient perception of swallowing function following chemoradiation for head and neck cancer. Dysphagia 29: 223–-233., 2014. [DOI] [PubMed] [Google Scholar]
- 30.Mann G, Hankey GJ, Cameron D: Swallowing disorders following acute stroke: Prevalence and diagnostic accuracy. Cerebrovasc Dis 10: 380–386, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Rosen A, Rhee TH, Kaufman R: Prediction of aspiration in patients with newly diagnosed untreated advanced head and neck cancer. Arch Otolaryngol Head Neck Surg 127: 975–979, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Splaingard ML, Hutchins B, Sulton LD, Chaudhuri G: Aspiration in rehabilitation patients: Videofluoroscopy vs bedside clinical assessment. Arch Phys Med Rehabil 69: 637–640, 1988. [PubMed] [Google Scholar]
- 33.Eisbruch A: Dysphagia and aspiration following chemo-irradiation of head and neck cancer: Major obstacles to intensification of therapy. Ann Oncol 15: 363–364, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Hutcheson KA, Lewin JS, Holsinger FC, Steinhaus G, Lisec A, Barringer DA, Lin HY, Villalobos S, Garden AS, Papadimitrakopoulou V, Kies MS: Long-term functional and survival outcomes after induction chemotherapy and risk-based definitive therapy for locally advanced squamous cell carcinoma of the head and neck. Head Neck 36: 474–-480., 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owadally W, Hurt C, Timmins H, Parsons E, Townsend S, Patterson J, Hutcheson K, Powell N, Beasley M, Palaniappan N, Robinson M, Jones TM, Evans M: PATHOS: a phase II/III trial of risk-stratified, reduced intensity adjuvant treatment in patients undergoing transoral surgery for Human papillomavirus (HPV) positive oropharyngeal cancer. BMC Cancer 15: 602, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson JM, McColl E, Carding PN, Hildreth AJ, Kelly C, Wilson JA: Swallowing in the first year after chemoradiotherapy for head and neck cancer: clinician- and patient-reported outcomes. Head Neck 36: 352–358, 2014. [DOI] [PubMed] [Google Scholar]


