Abstract
Background:
The older patient with inflammatory bowel diseases (IBD) is particularly vulnerable to consequences of disease and therapy-related side effects but little is known about the best treatment options in this population.
Aim:
To compare safety and efficacy of tumor necrosis factor α antagonist (anti-TNF) or vedolizumab (VDZ) in patients with IBD ≥ 60 years of age.
Methods:
This retrospective study included patients with Crohn’s disease (CD) or ulcerative (UC) initiating anti-TNF or VDZ therapy at ≥ 60 years of age at 3 study sites. We examined occurrence of infection or malignancy within 1 year after therapy as our primary outcome. Our efficacy outcomes included clinical remission at 3, 6, and 12 months. Multivariable logistic regression models adjusting for relevant confounders estimated odds ratios (OR) and 95% confidence intervals.
Results:
The study included 131 anti-TNF and 103 VDZ initiated patients (age range 60 – 88 years). Approximately half had CD. At 1 year, there were no significant differences in safety profile between the two therapeutic classes. Infections were observed in 20% of anti-TNF treated and 17% of VDZ treated patients (p=0.54). Pneumonia was the most common infection in both groups. While more anti-TNF treated CD patients were in remission at 3 months compared to VDZ (OR 2.82, 95% CI 1.18 – 6.76), this difference was not maintained at 6 and 12 months suggesting similar efficacy of both classes.
Conclusions:
Both anti-TNF and VDZ therapy were similarly effective and safe in elderly IBD patients.
Keywords: infliximab, vedolizumab, elderly, Crohn’s disease, infections
INTRODUCTION
Inflammatory bowel diseases comprising Crohn’s disease (CD) and ulcerative colitis (UC) are chronic, immune-mediated diseases that often lead to hospitalization, surgery and impaired health-related quality of life1-3. As they fundamentally arise due to a dysregulated immune response to the gut microbiome, systemic or gut-selective immunosuppression forms the cornerstone of effective treatment for these diseases. However, such immunosuppression may be associated with rare but serious adverse effects including infections and treatment-related cancers. One population that is particularly vulnerable to such adverse effects but is frequently excluded from clinical trials and observational cohorts is the older IBD patient4, 5.
Up to one-fifth of patients with inflammatory bowel disease (IBD) may be diagnosed after the age of 60 years5-9. Owing to the aging of the population, patients older than age 60 years represent a growing subgroup of those with IBD globally4. Studies have demonstrated that IBD in older patients is associated with significant morbidity. As well, rates of surgery and hospitalization that are comparable to or even exceed that observed in younger patients10-13. Yet, use of immunosuppressive medications, and biologics in particular, remains infrequent in this population in large part due to concerns regarding safety and risk of infections and cancers10. Some initial studies confirmed this, demonstrating a higher risk of infections in older compared to younger individuals with IBD who are treated with monoclonal antibodies against tumor necrosis factor α (anti-TNF; infliximab (IFX), adalimumab (ADA), certolizumab pegol (CZP), golimumab(GLM)14-16. Gut-selective anti-integrin therapy with vedolizumab (VDZ) offers a safer and less systemic immunosuppressive option than other biologics. However, while the efficacy and safety of vedolizumab is well established in younger patients and in clinical trials17-19, the data on its use in older IBD patients are still scarce20. Furthermore, there are no data on the comparative efficacy and safety of anti-TNF agents compared to vedolizumab in the elderly IBD patients.
Thus, the aims of our study were (1) to examine the comparative effectiveness of anti-TNF biologics and vedolizumab in the treatment of CD and UC when initiated at age 60 years or older; and (2) to define the relative safety of both therapeutic classes with specific focus on the risk of infections and malignancy.
METHODS
Study Population
This retrospective cohort study included patients with established IBD initiating therapy with an anti-TNF or VDZ at age 60 years or older from three different sites – Massachusetts General Hospital (MGH) (Boston, MA), Mount Sinai Hospital (New York, NY), and Brooke Army Medical Center (BAMC) (Fort Sam Houston, TX). Patients were eligible for inclusion if they met the following criteria: (1) Established diagnosis of CD, UC, or IBD-unclassified (IBDU); and (2) initiating anti-TNF therapy (IFX, ADA, CZP, GLM) or VDZ at age 60 years or older. For patients initiating multiple sequential biologic agents after the age of 60 years, data regarding only the first anti-TNF or vedolizumab was used. Patients who initiated therapy prior to age 60 were not included.
Covariates
Through detailed review of the medical records, information was obtained about relevant covariates including age at diagnosis of IBD, duration of disease, prior treatment history, disease location and behavior per the Montreal classification21 and prior medical and surgical treatment for their IBD. Standard induction dosing was used for all patients; dose escalation was performed as clinically indicated. For both groups, we obtained information on whether the patient was on concomitant therapy with a thiopurine (azathioprine, mercaptopurine) or methotrexate, and whether the patient was naïve to that therapeutic class at initiation. Non-IBD comorbidity was quantified using the validated and widely used Charlson comorbidity index22.
Our primary outcomes pertained to both efficacy and safety. Our primary safety endpoint was development of any infectious complication within 1 year of therapy initiation. For significant infections (requiring antibiotic therapy, cessation or interruption of immunosuppression, or hospitalization), we noted type of infection and whether it led to therapy cessation. We also obtained information on new primary or recurrent skin cancers as well as other solid organ or hematologic cancers diagnosed within 1 year of therapy initiation. Our efficacy outcomes were clinical remission at 3, 6, and 12 months after initiation of therapy. Clinical remission status was defined based upon composite evaluation of clinical findings (absence of abdominal pain, diarrhea, or rectal bleeding), inflammatory markers, radiologic, and endoscopic findings obtained as part of standard of care. The team of investigators at each study site reviewed all the charts at their site and disagreements about remission status were resolved through consensus. None of the study sites prospectively obtained disease activity indices, patient-reported outcomes, serial C-reactive protein or fecal calprotectin measures for this study. Remission status at all sites was determined by a gastroenterologist reviewer. Secondary efficacy endpoints included hospitalization or surgery related to IBD at 1 year.
Statistical Analysis
The study was approved by the Institutional Review Board at all sites. Continuous variables were described using means and standard deviations and compared using the t-test. Categorical variables were compared using the chi-square test (with Fisher’s modification where relevant) and expressed as proportions. First, we performed univariate logistic regression for each of our primary and secondary safety and efficacy outcomes. Variables that were significant on this analysis at p < 0.2 were incorporated into a multivariable model to examine the independent effect of type of biologic on safety and efficacy in older patients with IBD. A two-sided p-value ≤ 0.05 on multivariable analysis indicated independent statistical significance. A priori specified subgroup analysis was performed stratifying by type of IBD, and by whether the biologic was in combination or monotherapy.
To account for the non-random allocation to treatment arm, we repeated the multivariable models adjusting for a propensity score assigning likelihood of receiving a particular treatment. This propensity score model used the treatment arm as the outcome and disease-related characteristics and demographics including age as predictive variables. The propensity score was then included as an independent variable in the multivariable model. Durability of treatment was assessed using survival analysis with time to cessation of therapy as the outcome. Survival curves for anti-TNF and VDZ were compared using the log-rank test and the proportion of patients still on treatment at 1 and 2 years after initiation was estimated.
RESULTS
Study Population
This study included 234 patients with age ≥ 60 years at the time of initiating a biologic among whom 131 and 103 initiated anti-TNF and VDZ respectively. Among the anti-TNF agents, IFX was the most common and used by 106 patients and ADA by 22. One and two patients used CZP and GLM respectively. Table 1 compares the characteristics of the two groups. The mean age at initiation of a biologic was 68 years in both groups (p=0.90) (range 60 – 88 years) with just over half being male. There were similar proportions of patients with CD or UC. There was no difference between the two groups in extent of colonic involvement in UC or distribution or behavior in CD. The mean Charlson comorbidity index at biologic initiation was similar between the two groups. Among patients in the anti-TNF arm, nearly 86% were previously naïve to another anti-TNF agent and 94% were naïve to VDZ. In contrast, among the VDZ group, only 40% were naïve to anti-TNF therapy at initiation. Approximately one-third of patients in both groups were on combination therapy with a nearly equal proportion on thiopurines (54%) or methotrexate (46%). There was no difference in the proportion of patients using steroids at initiation of either anti-TNF (60%) or vedolizumab (69%) therapy (p=0.14).
Table 1:
Characteristic of study population of patients older than 60 years with inflammatory bowel diseases initiating vedolizumab or anti-TNF
| Characteristics | Anti-TNF (n=131) [N(%)] |
Vedolizumab (n=103) [N(%)] |
p-value |
|---|---|---|---|
| Age (at biologic initiation) (in years) (mean (SD)) | 68 (6) | 68 (6) | 0.90 |
| Male | 76(58%) | 60(58%) | 0.97 |
| Hispanic ethnicity | 10(8%) | 10(10%) | 0.42 |
| Race – White | 105(80%) | 77(75%) | 0.32 |
| Type of IBD | 0.48 | ||
| Crohn’s disease | 68(52%) | 51(50%) | |
| Ulcerative colitis | 59(45%) | 51(50%) | |
| IBDU | 4(3%) | 1(1%) | |
| Mean age at diagnosis (in years) (mean (SD)) | 55 (16) | 52 (15) | 0.26 |
| UC – Extent – Pancolitis | 34(54%) | 29(55%) | 0.58 |
| Location – Crohn’s disease | 0.91 | ||
| Ileal (L1) | 14(21%) | 10(20%) | |
| Colonic (L2) | 18(27%) | 12(24%) | |
| Ileocolonic (L3) | 36(53%) | 29(57%) | |
| Behavior – Crohn’s disease | 0.51 | ||
| Non-penetrating, not stricturing (B1) | 23(34%) | 22(43%) | |
| Stricturing (B2) | 23(34%) | 13(25%) | |
| Penetrating (B3) | 22(32%) | 16(32%) | |
| Perianal disease | 17(25%) | 7(14%) | 0.02 |
| Extraintestinal manifestations | 29(22%) | 19(18%) | 0.49 |
| Prior cancer diagnosis | 35(27%) | 27(26%) | 0.86 |
| Mean Charlson comorbidity [mean(SD)] | 4 (2) | 4 (2) | 0.32 |
| Past IBD related surgery | 42(32%) | 32(31%) | 0.78 |
| Type of biologic | |||
| Infliximab | 106(81%) | ||
| Adalimumab | 22(17%) | ||
| Certolizumab | 1(1%) | ||
| Golimumab | 3(2%) | ||
| Vedolizumab | 103(100%) | ||
| Disease duration at biologic start (in years) (mean (SD)) | 13 (15) | 16 (14) | 0.21 |
| Anti-TNF naïve | 113(86%) | 41(40%) | < 0.001 |
| Vedolizumab naïve | 123(94%) | - | 0.04 |
| Concomitant combination therapy | 47(36%) | 34(33%) | 0.65 |
| Steroid use at biologic initiation | 79(60%) | 82(70%) | 0.14 |
| Triple immunosuppression (immunomodulator + steroid use) | 27 (21%) | 24 (23%) | 0.62 |
Safety
Significant infections were observed in 20% of patients on anti-TNF therapy within 1 year of initiation compared to 17% of those on vedolizumab (p=0.54) (Absolute difference 3.2, 95% CI −7.9 to 13.3) (Table 2). Adjusting for age, comorbidity and use of combination immunomodulator therapy, there was no statistically significant difference between the two groups (OR 1.29, 95% CI 0.63 – 2.67). Pneumonia was the most common serious infection, occurring in 29% and 24% of patients experiencing an infection in the anti-TNF and vedolizumab groups respectively (Table 2). Specifically, there was no difference between the two groups for C difficile or gastrointestinal infections (21% vs. 18%, p=0.57). Malignancies were infrequent in both groups. A new primary cancer (excluding skin cancer) or recurrence of prior cancer was noted in 3% of anti-TNF and 1% of vedolizumab treated patients (p=0.27).
Table 2:
Frequency of study outcomes with anti-TNF or vedolizumab in patients with inflammatory bowel diseases 60 years or older
| Outcome | Anti-TNF (n=131) | Vedolizumab (n=103) | p-value |
|---|---|---|---|
| Remission: | |||
| At 3 months | 65(50%) | 39(38%) | 0.07 |
| At 6 months | 65(54%) | 46(45%) | 0.23 |
| At 12 months | 61(58%) | 47(54%) | 0.63 |
| Need for IBD surgery at 1 year | 14(11%) | 10(10%) | 0.78 |
| Need for IBD hospitalization at 1 year | 26(20%) | 13(13%) | 0.12 |
| Infection (within 1 year) | 20% | 17% | 0.54 |
| Type of infection | (n = 24) | (n = 17) | |
| Pneumonia | 7(29%) | 4(24%) | |
| Sepsis | 2(8%) | 2(12%) | |
| UTI | 3(13%) | 0% | |
| Abscess | 4(17%) | 3(18%) | |
| C difficile | 5 (21%) | 1 (6%) | |
| CMV | 1 (4%) | 0 (0%) | |
| Dental infection | 1 (4%) | 0 (0%) | |
| Shingles | 1 (4%) | 0 (0%) | |
| URI | 0 (0%) | 5 (29%) | |
| Fever (unspecified) | 0 (0%) | 1 (6%) | |
| Gastroenteritis | 0 (0%) | 2 (12%) | |
| Skin cancer | 1(0.8%) | 1(1.0%) | 0.86 |
| Other cancers | 4(3%) | 1(1%) | 0.27 |
| Reason for cessation | (n = 80) | (n = 37) | |
| Infusion / Allergic reaction | 16(20%) | 2(5%) | |
| Infection | 9(11%) | 5(14%) | |
| Cancer | 4(5%) | 1(3%) | |
| Heart failure | 1(1%) | 0(0%) | |
| Other | 50(63%) | 29(78%) |
Among all patients initiating therapy, 80 anti-TNF and 37 vedolizumab treated patients eventually ceased the biologic therapy. Apart from loss of response, the most common reason for ceasing anti-TNF therapy was infusion/allergic reactions (20%) and infections (11%), while infection (14%) was the most common reason for stopping vedolizumab treatment.
Efficacy and durability of treatment
We observed no clear evidence favoring efficacy of one therapeutic class over the other in elderly IBD cohort. While there was a larger proportion of anti-TNF users with IBD in remission at 3 months compared to the vedolizumab arm (50% vs. 38%, p=0.07), there was no difference between the two groups at 6 months (54% vs 45%) and 12 months (58% vs. 54%, p=0.63), suggesting that the early difference may relate to timing of onset of benefit rather than overall efficacy (Table 2). 0.62 – 2.07). Importantly, this difference was only noted among patients with CD and not UC. At 3 months, use of anti-TNF (compared to vedolizumab) was associated with higher odds of remission in CD (56% vs. 41%, OR 2.82, 95% CI 1.18 – 6.76) but not UC (43% vs. 35%, OR 1.74, 95% CI 0.74 – 4.13) (Table 3). No difference was seen between anti-TNF and vedolizumab therapy at 6 and 12 months in either CD or UC.
Table 3:
Multivariable analysis of odds of study outcomes for anti-TNF compared to vedolizumab)
| (a) Crohn’s disease | |||
|---|---|---|---|
| Outcome | Multivariable odds ratio+ | 95% confidence interval | P-value |
| Remission at 3 months | 2.82 | 1.18 – 6.76 | 0.03 |
| Remission at 6 months | 1.34 | 0.62 – 2.88 | 0.58 |
| Remission at 12 months | 0.79 | 0.35 – 1.79 | 0.57 |
| Infection at 1 year | 1.00 | 0.37 – 2.73 | 0.89 |
| (b) Ulcerative colitis | |||
| Outcome | Multivariable odds ratio+ | 95% confidence interval | P-value |
| Remission at 3 months | 1.74 | 0.74 – 4.13 | 0.29 |
| Remission at 6 months | 1.69 | 0.73 – 3.91 | 0.38 |
| Remission at 12 months | 1.68 | 0.67 – 4.18 | 0.27 |
| Infection at 1 year | 1.89 | 0.61 – 5.78 | 0.31 |
Adjusted for type of IBD, combination immunomodulator use, race/ethnicity, and site of recruitment
For the entire cohort, remission rates at 3, 6, and 12 months were numerically higher among patients who were biologic naïve compared to those who were experienced, though these differences did not reach statistical significance at any of the time points (p > 0.2 for all). At 3 months, 47% of biologic naïve patients achieved remission compared to 40% of controls. Consequently, biologic exposure was not included in our final multivariable model. Forcing inclusion of prior biologic use in our multivariable model resulted in wider confidence intervals and the difference between anti-TNF and vedolizumab at 3 months was no longer statistically significant among patients with CD (OR 2.42, 95% CI 0.90 – 6.47).
Pooling both populations together, no clinical parameter was an independent predictor of remission at 3 or 6 months. Age (for each 1 year increase in age: OR 0.94, 95% CI 0.89 – 0.99) was associated with a lower likelihood of remission. Within each therapeutic class, combination therapy with thiopurine/methotrexate was not associated with higher rates of remission at 3, 6, or 12 months (p > 0.10) for all comparisons. One-in-five (20%) of older IBD patients initiating anti-TNF therapy required an IBD related hospitalization in the year following initiation compared to 13% of patients treated with vedolizumab (absolute difference 7%, −0.4% to 16%) but this difference was not significant on multivariable analysis. A similar proportion of patients in both groups underwent IBD-related surgery within 1 year (11% vs. 10%, p=0.78). Propensity-score adjusted models yielded similar results with comparable efficacy at 6 and 12 months between the two populations.
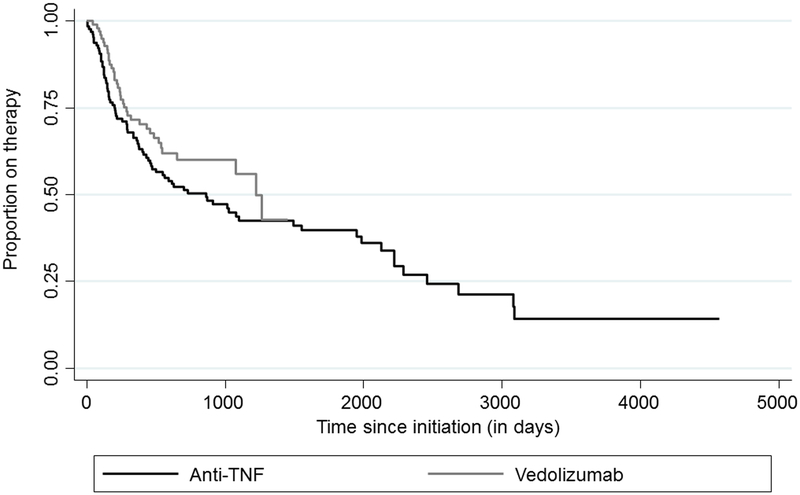
Figure 1 presents a survival curve analysis of time to cessation of anti-TNF or vedolizumab therapy. Type of biologic did not influence durability of agent, with similar rates of therapy cessation across both groups (log-rank p-value =0.17).
Figure 1: Durability of anti-TNF and vedolizumab therapy.
Log-rank p-value = 0.17
Stratified analysis
Stratifying by whether the medications were used in monotherapy or combination therapy demonstrated that the higher rates of remission at 3 months with anti-TNF was statistically significant for those on monotherapy (OR 2.35, 95% CI 1.12 – 4.94) but not in those on combination therapy (OR 1.92, 95% CI 0.63 – 5.87). There was a trend towards greater rates of remission at 6 months with anti-TNF therapy among combination therapy users (OR 2.45, 95% CI 0.86 – 6.97, p=0.09). There was no difference in safety of the two therapeutic classes either among those on mono- or combination therapy.
DISCUSSION
With the availability of therapies that differ in their mechanisms of action, studies of comparative effectiveness and safety are important to appropriately position them within the therapeutic algorithm. This is of great importance in older individuals with IBD, a growing patient subgroup, who are particularly vulnerable to both the consequences of active disease but also to therapy-related adverse events, particularly infections from systemic immunosuppression. Using a multi-center retrospective cohort of patients initiating biologic therapy, we demonstrate that both systemically acting anti-TNF therapy and gut-selective anti-integrin therapy with vedolizumab are similarly safe and durable in elderly IBD patients.
Many observational cohorts6, 7, 11, 23-25, summarized in a recent meta-analysis10, have demonstrated that biologic therapies are used infrequently in elderly IBD patients, primarily owing to concerns regarding safety. Several initial reports from North America15 and Europe14, 16
demonstrated that elderly IBD patients on biologics had significantly increased risk of infections compared to younger patients receiving the same therapy. In an Italian multi-center cohort, 11% of patients older than 65 years of age who received anti-TNF agents developed severe infections and 10% died, compared to only 2.6% and 1% of those younger than age 65 years respectively14. Similarly, Desai et al. identified higher rate of discontinuation of therapy in older anti-TNF users, particularly in those on combination therapy, and often due to infections15. In contrast, post-hoc analysis of vedolizumab clinical trials that included a small group of older patients did not identify an increase in infections with this gut-selective therapy20. A large pooled analysis of VDZ users from the pivotal clinical trials revealed no significant increase in risk of infections in contrast to anti-TNF therapy where a modest increase in risk was noted in some populations17, 26, 27. Comparative safety of the two classes of therapy have been examined mostly in the post-operative setting where there may be a modest but inconsistent increase in risk of post-operative infections with VDZ28, 29. In an analysis of the US multi-center VICTORY consortium that included 872 patients (436 on vedolizumab), VDZ-treated patients had a numerical but statistically insignificant lower rate of serious infections (6.9% vs 10.1%)30. Our findings of similar safety at 1 year between both therapeutic classes in elderly IBD patients provides reassurance to clinicians considering use of either anti-TNF or VDZ for the treatment of IBD. Notably, the most common infection in both groups was pneumonia. While we did not have information on the etiologic organisms, this emphasizes the need for minimizing preventable infections through appropriate vaccination strategies, particularly in older IBD patients. Given the gut-targeted nature of vedolizumab, it is reassuring that there was no difference in the rates of gastrointestinal infections between the two groups, consistent with observations from the GEMINI trials. Rates of malignancy (skin and non-skin) were low and similar in both groups, consistent with the published literature where an increase in risk of primary or recurrent malignancy has not been noted with either of the two therapeutic classes31.
A second important finding from our study was that both therapies were similarly effective when examined over the first year of treatment. While among patients with CD, there were more patients in remission at the earlier time point of 3 months with anti-TNF compared to vedolizumab, there was no difference in the rates of remission at 6 and 12 months, and overall durability of treatment was similar. In addition, over the first year, there were numerically more IBD related hospitalizations in the anti-TNF compared to vedolizumab group. Together, these data suggest that both treatments are similar in their benefit, with perhaps an earlier onset of action with anti-TNF therapy in CD consistent with clinical observations from the randomized controlled trials. There exist few head-to-head comparisons of VDZ and anti-TNF therapy in IBD. A single center small study by Allamneni et al. that included 59 patients suggested higher clinical response rates after induction with VDZ compared to IFX32; however the number of patients included was small and robust conclusions could not be drawn. Network meta-analysis in UC suggested similar efficacy of infliximab and vedolizumab33, 34, however vedolizumab had a superior safety profile in some33 but not all analyses35. In a multi-center propensity adjusted analysis, in CD, there was no significant difference in clinical remission rates at 12 months in the vedolizumab (38%) compared to anti-TNF (34%), while VDZ treated patients had higher rates of endoscopic healing36. In contrast, among 334 UC patients (167 on vedolizumab), VDZ treated patients had higher rates of clinical remission (54% vs. 37%) and endoscopic healing (50% vs. 42%) at 1 year37. Our findings of higher earlier remission rates with anti-TNF therapy is supported by our understanding of the mechanism of action of these therapies. Through its systemic effect neutralizing circulating and membrane bound tumor necrosis factor α, anti-TNF therapies have a relatively quick onset of action38. Among the 70-80% of patients who achieve an initial response, two-thirds will note a benefit within the first two doses of treatment, particularly for IFX. In contrast, as VDZ inhibits tracking of activated lymphocytes to the site of inflammation in the gut but may not neutralize pro-inflammatory cells already present in the gut39, the median onset of action is longer. Analysis of data from observational cohorts and clinical trials suggest that the median time to respond may be 10 weeks in UC and as much as 14 weeks in CD18, 19. Consistent with this, the difference in early remission was noted primarily among patients with CD in our cohort.
We readily acknowledge several limitations to our analysis. First, owing to the retrospective design of the cohort, assessment of clinical efficacy was based upon global clinical impression rather than prospectively ascertained using validated disease activity indices. Second, selection of vedolizumab or an anti-TNF agent for treatment was non-random and at the discretion of the treating clinician. Though both groups were similar in most characteristics and our findings were unchanged on multivariable and propensity-adjusted analysis, there were fewer anti-TNF naïve patients in the VDZ group. Third, assessment of adverse events may have been incomplete but missing information is unlikely to be systematically biased towards one agent. As data was from referral medical centers, our findings may not be generalizable to population-based IBD cohorts with milder severity of disease.
In conclusion, we present a large multi-center retrospective cohort demonstrating that both anti-TNF and VDZ therapy are comparably safe and effective in older patients with IBD. With the growing burden of IBD in older individuals, there is an important need both for prospective studies in elderly IBD patients as well as an effort to include them in clinical trials so that safety and efficacy can be robustly estimated.
Acknowledgments
Source of funding: Ananthakrishnan is supported in part by grants from the National Institutes of Health (R03 DK112909), Pfizer, the Chleck Family Foundation, and the Crohn’s and Colitis Foundation.
Footnotes
Conflicts of Interest: Ananthakrishnan has served on scientific advisory boards for Abbvie, Takeda, Gilead, and Merck and has received research support from Pfizer. Patel has served on scientific advisory boards for Abbvie, Janssen, and Takeda.
REFERENCES
- 1.Abraham C, Cho J. inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres J, Mehandru S, Colombel JF, et al. Crohn’s disease. Lancet 2017;389:1741–1755. [DOI] [PubMed] [Google Scholar]
- 3.Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet 2017;389:1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ananthakrishnan AN, Donaldson T, Lasch K, et al. Management of Inflammatory Bowel Disease in the Elderly Patient: Challenges and Opportunities. Inflamm Bowel Dis 2017;23:882–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Val JH. Old-age inflammatory bowel disease onset: a different problem? World J Gastroenterol 2011;17:2734–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease A population-based cohort study. Gut. 2014;63:423–432. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Saverymuttu SH, Keshavarzian A, et al. Is the pattern of inflammatory bowel disease different in the elderly? Age and Ageing 1985;14:366–370. [DOI] [PubMed] [Google Scholar]
- 8.Ha CY, Katz S. Clinical implications of ageing for the management of IBD. Nat Rev Gastroenterol Hepatol 2014;11:128–38. [DOI] [PubMed] [Google Scholar]
- 9.Jason H, Linda F, Akbar W. Characteristics and behavior of elderly-onset inflammatory bowel disease: A multi-center U.S. Study. Inflammatory Bowel Diseases 2014;20:S40–S41. [DOI] [PubMed] [Google Scholar]
- 10.Ananthakrishnan AN, Shi HY, Tang W, et al. Systematic Review and Meta-analysis: Phenotype and Clinical Outcomes of Older-onset Inflammatory Bowel Disease. J Crohns Colitis 2016;10:1224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen GC, Sheng L, Benchimol EI. Health Care Utilization in Elderly Onset Inflammatory Bowel Disease: A Population-based Study. Inflammatory Bowel Diseases 2015;21:777–782. [DOI] [PubMed] [Google Scholar]
- 12.Ananthakrishnan AN, McGinley EL, Binion DG. Inflammatory bowel disease in the elderly is associated with worse outcomes: a national study of hospitalizations. Inflamm Bowel Dis 2009;15:182–9. [DOI] [PubMed] [Google Scholar]
- 13.Manosa M, Calafat M, de Francisco R, et al. Phenotype and natural history of elderly onset inflammatory bowel disease: a multicentre, case-control study. Aliment Pharmacol Ther 2018;47:605–614. [DOI] [PubMed] [Google Scholar]
- 14.Cottone M, Kohn A, Daperno M, et al. Advanced Age Is an Independent Risk Factor for Severe Infections and Mortality in Patients Given Anti-Tumor Necrosis Factor Therapy for Inflammatory Bowel Disease. Clinical Gastroenterology and Hepatology 2011;9:30–35. [DOI] [PubMed] [Google Scholar]
- 15.Desai A, Zator ZA, de Silva P, et al. Older age is associated with higher rate of discontinuation of anti-TNF therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis 2013;19:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lobaton T, Ferrante M, Rutgeerts P, et al. Efficacy and safety of anti-TNF therapy in elderly patients with inflammatory bowel disease. Aliment Pharmacol Ther 2015;42:441–51. [DOI] [PubMed] [Google Scholar]
- 17.Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017;66:839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 19.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 20.Yajnik V, Khan N, Dubinsky M, et al. Efficacy and Safety of Vedolizumab in Ulcerative Colitis and Crohn’s Disease Patients Stratified by Age. Adv Ther 2017;34:542–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 23.Heresbach D, Alexandre JL, Bretagne JF, et al. Crohn’s disease in the over-60 age group: A population based study. European Journal of Gastroenterology and Hepatology 2004;16:657–664. [DOI] [PubMed] [Google Scholar]
- 24.Juneja M, Baidoo L, Schwartz MB, et al. Geriatric inflammatory bowel disease: phenotypic presentation, treatment patterns, nutritional status, outcomes, and comorbidity. Dig Dis Sci 2012;57:2408–15. [DOI] [PubMed] [Google Scholar]
- 25.Kariyawasam VC, Huang TD, Lunney PC, et al. Natural history of elderly onset inflammatory bowel disease-sydney IBD cohort (1942-2012). Gastroenterology 2013;1):S634–S635. [Google Scholar]
- 26.Shah ED, Farida JP, Siegel CA, et al. Risk for Overall Infection with Anti-TNF and Anti-integrin Agents Used in IBD: A Systematic Review and Meta-analysis. Inflamm Bowel Dis 2017;23:570–577. [DOI] [PubMed] [Google Scholar]
- 27.Bonovas S, Fiorino G, Allocca M, et al. Biologic Therapies and Risk of Infection and Malignancy in Patients With Inflammatory Bowel Disease: A Systematic Review and Network Meta-analysis. Clin Gastroenterol Hepatol 2016;14:1385–1397 e10. [DOI] [PubMed] [Google Scholar]
- 28.Lightner AL, McKenna NP, Tse CS, et al. Postoperative outcomes in vedolizumab-treated Crohn’s disease patients undergoing major abdominal operations. Aliment Pharmacol Ther 2018;47:573–580. [DOI] [PubMed] [Google Scholar]
- 29.Law CCY, Narula A, Lightner AL, et al. Systematic Review and Meta-Analysis: Preoperative Vedolizumab Treatment and Postoperative Complications in Patients with Inflammatory Bowel Disease. J Crohns Colitis 2018;12:538–545. [DOI] [PubMed] [Google Scholar]
- 30.Lukin DJ, Weiss A, Aniwan S, et al. Comparative safety profile of vedolizumab and tumor necrosis factor-antagnost therapy for inflammatory bowel disease: A multicenter consortium propensity score-matched analysis. 2018:277. [Google Scholar]
- 31.Shelton E, Laharie D, Scott FI, et al. Cancer Recurrence Following Immune-Suppressive Therapies in Patients With Immune-Mediated Diseases: A Systematic Review and Meta-analysis. Gastroenterology 2016;151:97–109 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allamneni C, Venkata K, Yun H, et al. Comparative Effectiveness of Vedolizumab vs. Infliximab Induction Therapy in Ulcerative Colitis: Experience of a Real-World Cohort at a Tertiary Inflammatory Bowel Disease Center. Gastroenterology Res 2018;11:41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonovas S, Lytras T, Nikolopoulos G, et al. Systematic review with network meta-analysis: comparative assessment of tofacitinib and biological therapies for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther 2018;47:454–465. [DOI] [PubMed] [Google Scholar]
- 34.Cholapranee A, Hazlewood GS, Kaplan GG, et al. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther 2017;45:1291–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danese S, Fiorino G, Peyrin-Biroulet L, et al. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Ann Intern Med 2014;160:704–11. [DOI] [PubMed] [Google Scholar]
- 36.Bohm M, Sagi S, Fischer M, et al. Comparative effectiveness of vedolizumab and tumor necrosis factor-antagonist therapy in Crohn’s disease: A multicenter consortium propensity score-matched analysis. 2018:Sa1723. [Google Scholar]
- 37.Faleck D, Shashi P, Meserve J, et al. Comparative effectiveness of vedolizumab and tumor-necrosis factor antagonist therapy in ulcerative colitis: A multicenter consortium propensity score-matched analysis. 2018:328. [Google Scholar]
- 38.Levin AD, Wildenberg ME, van den Brink GR. Mechanism of Action of Anti-TNF Therapy in Inflammatory Bowel Disease. J Crohns Colitis 2016;10:989–97. [DOI] [PubMed] [Google Scholar]
- 39.Raine T Vedolizumab for inflammatory bowel disease: Changing the game, or more of the same? United European Gastroenterol J 2014;2:333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]